Introduction
As the development of sequencing technologies
advances, personalized medicine has become increasingly available,
particularly for the treatment of cancer (1,2). This
offers customized treatment for patients based upon the molecular
analysis of tumor-associated biomarkers (3). The identification of driver genes and
genomic aberrations, including KRAS proto-oncogene GTPase (KRAS)
and epidermal growth factor receptor (EGFR) (4–8), as
oncogenes for lung cancer has driven personalized medicine. Based
on the number and specific somatic mutations detected in a patient,
one could be diagnosed more precisely and treated with targeted
drugs (9).
Tumor tissue biopsies offer the ideal sample for
molecular analysis to target personalized cancer medicine (10). However, limitations exist due to the
level of invasiveness involved in obtaining a biopsy for certain
tumor types, the limited feasibility of this technique and tumor
heterogeneity, which results in a number of potential false
negatives (11). Circulating tumor
DNA (ctDNA), which is comprised of small fragments of DNA, has been
identified in the blood of patients. Typically, tumor cells
undergoing apoptosis or necrosis release ctDNA into the circulatory
system (12–14). Indeed, previous studies have detected
mutations in driver genes via examination of liquid biopsies
(15,16). A previous study of patients with
non-small cell lung cancer (NSCLC), which analyzed blood samples
for genetic alterations in EGFR, demonstrated adequate sensitivity
and specificity for the approval of liquid biopsy testing in the
clinic (17).
Previous studies have reported that the average
concordance rate between tissue and plasma genotyping is ~70%
(range, 48–98%) (18–23). A clinical trial used Therascreen EGFR
detection kit, approved for diagnostic use in Europe, to detect
somatic mutations in tissue and liquid biopsies, demonstrated a
sensitivity of 65.7% for plasma genotyping and a concordance of
94.3% among plasma and tumor tissue samples (24). However, implementation of plasma
genotyping within the clinic is currently hindered by the number of
inconsistencies observed in results compared with tumor biopsies,
which is thought to be due to tumor heterogeneity (25). If this is the case, theoretically,
using tumor biopsies as a reference, more mutations are likely to
be identified in ctDNA compared with tissue DNA (11,26).
However, in practice, plasma tests often fail to detect numerous
mutations, including genomic aberrations in the driver genes
(27). Consequently, within clinical
practice, despite the ability to sensitively detect EGFR mutations
within ctDNA samples, tissue biopsy is recommended for all patients
with negative results regarding the T790M mutation in this gene, in
order to eliminate the potential for false negative results from
plasma genotyping (26).
Furthermore, it is unclear which patient sub-populations may be
more likely to carry specific mutations. This means that currently
all patients need to undergo costly and invasive procedures to
determine diagnosis, prognosis and optimal treatment
strategies.
In the present study, targeted sequencing was
performed using 47 pairs of blood and tumor tissue biopsies from
patients with advanced lung cancer. By comparing the paired genetic
profiles of tumors and plasma samples, a parameter was derived to
indicate the sensitivity of the plasma test, termed the plasma
maximum allelic fraction (Max AF). Max AF is defined as the maximum
mutant allele fraction in a plasma sample. Patients with a lower
plasma Max AF (≤2.2%) were more likely to carry tissue-specific
mutations that are not identifiable through plasma genotyping;
Therefore, tumor tissue biopsy may be necessary for these patients
for a complete knowledge of their tumor genotype. Such a parameter
may prove useful for the accurate diagnosis and personalized
medical treatment of patients with lung cancer.
Materials and methods
Patient and sample collection
Patients ≥18-years-old diagnosed with any type of
lung cancer at the General Hospital of Southern Theater Command in
Guangzhou (China) between January 2015 and December 2016 were
eligible for the present study. The inclusion criteria for the
study were as follows: i) Sufficient tissue and plasma samples were
available for targeted sequencing; and ii) intervals between the
tissue biopsy and blood sample collection were ≤14 days. Blood (~10
ml) was obtained and stored in an EDTA-coated tube at 4°C until DNA
extraction. In total, 47 patients with advanced lung cancer were
enrolled in the current study and paired biopsy and blood samples
were obtained from each patient. Patients with no genetic mutations
observed in tumor tissues were excluded from further analysis. The
maximum allelic fraction (Max AF) was defined as the highest mutant
allele fraction detected in a particular sample, regardless of gene
or mutation site. The present study was approved by the Ethical
Committee at the General Hospital of Southern Theater Command, PLA
(Guangzhou, China). All patients provided written informed consent
for their participation in the study.
DNA extraction for tissue and plasma
samples
Tissue DNA was extracted using the commercially
available QIAamp DNA formalin-fixed paraffin-embedded tissue kit
(Qiagen, Inc.), according to the manufacturer's protocol.
Similarly, blood DNA was extracted using a QIAamp Circulating
Nucleic Acid kit (Qiagen, Inc.), as previously described (28). Briefly, plasma were removed from
whole blood samples and transferred to separate tubes prior to
centrifugation at 16,000 × g for 10 min at 4°C to remove debris.
Circulating DNA was extracted from the plasma, according to the
manufacturer's protocol. Quantification of both tissue DNA and
plasma DNA were performed using a Qubit 2.0 fluorometer (Thermo
Fisher Scientific, Inc.).
Next generation sequencing (NGS)
library preparation and sequencing
The NGS library was prepared as described previously
(29). Briefly, nucleotide fragments
of 200–400 base pairs were selected using Agencourt AMPure beads
(Beckman Coulter, Inc.). The quality of the DNA fragments was
evaluated using a Qubit 2.0 fluorometer (Thermo Fisher Scientific,
Inc.), following hybridization and amplification. Paired samples of
tissue and plasma samples were sequenced using a capture-based
targeted sequencing panel (Burning Rock Biotech). A sequencing
panel consisting of 56lung cancer-associated genes was used to
detect and quantify genomic aberrations.
Statistical analysis
Statistical analysis was performed using SPSS 20.0
software (IBM Corp., Armonk, NY, USA). Analysis was performed with
unpaired t-test. Box and whisker plots were generated to present
the median values and 95% confidence intervals. Correlation between
plasma Max AF and the number of tissue mutations was analyzed by
Spearman's correlation. Correlation between the tissue Max AF and
the plasma Max AF was analyzed using a linear regression model.
Receiver operating characteristic (ROC) analysis was performed to
quantify the extent to which Max AF can discriminate patients based
on their likelihood to harbor tissue-specific mutations. P<0.05
was considered to indicate a statistically significant
difference.
Results
Patient characterization
Patients diagnosed with advanced lung cancer at the
General Hospital of Guangzhou Military Command of PLA (Guangzhou,
China) between 2015 and 2016 were enrolled in the present study
(n=47) with a median age of 59. All 47 patients underwent a biopsy
examination and blood test. Both a tumor tissue sample and plasma
sample were collected from each patient. Of the 29 patients with
known sex information, 13 were male and 16 were female. A total of
45 patients had been diagnosed with NSCLC and two with small cell
lung cancer. All patients had been diagnosed with stage IV
disease.
Genomic mutations in tissue and plasma
samples
Genomic alterations detected in tumor tissues were
considered a reference for comparison between tumor and plasma
samples. A sequencing panel consisting of 56 lung cancer-associated
genes was used to detect and quantify genomic aberrations. The
panel included driver genes and genes to which targeted therapies
exist, both in development and in the clinic. Sequencing was
performed on all 47 tumor biopsy samples and their patient-matched
plasma samples. Of the 47 tissue biopsy samples analyzed, 40
patients (87%) presented with mutations within the sequencing panel
used in the present study. Collectively, 116 variants were
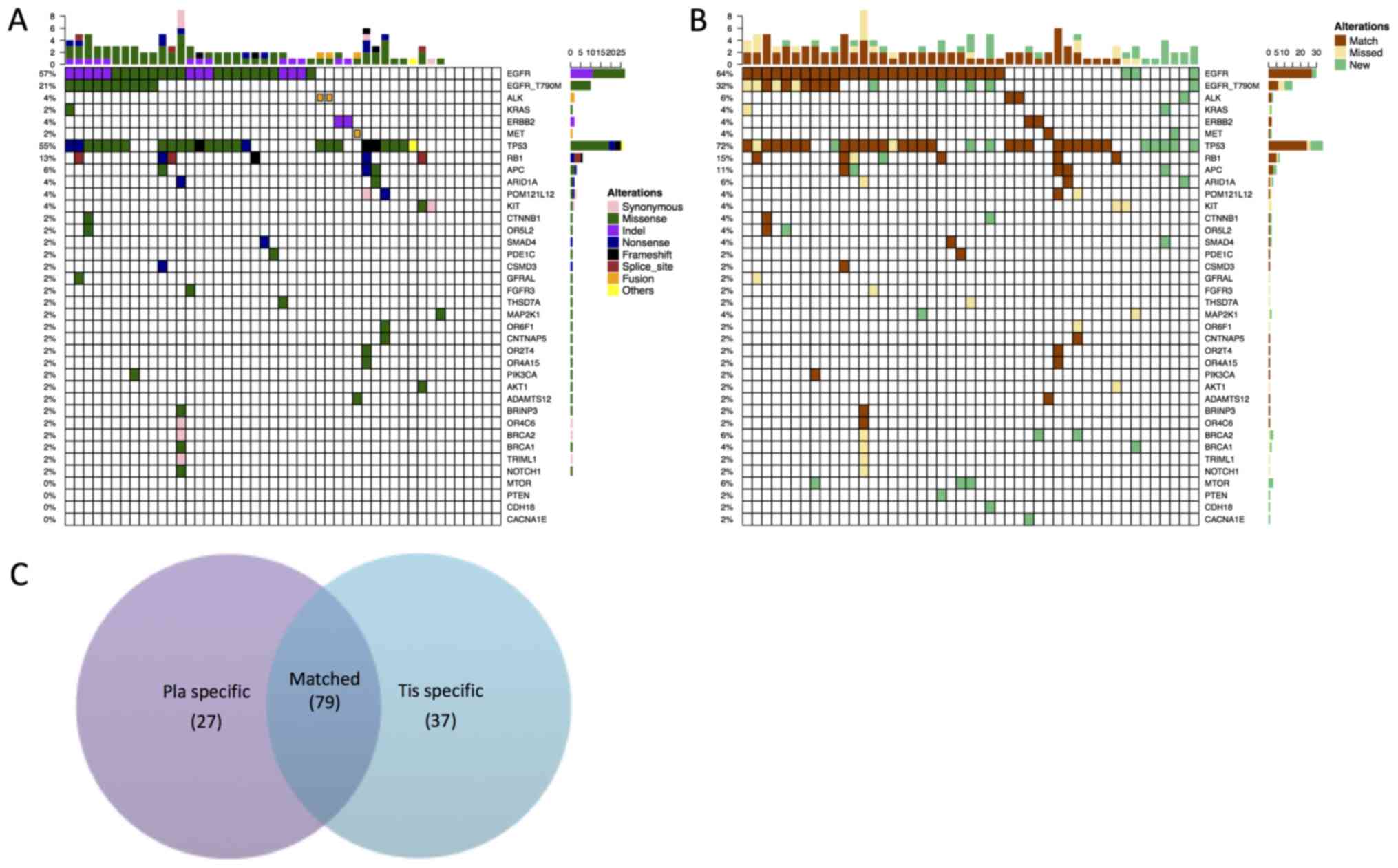
identified, spanning 33 genes (Fig.
1A). EGFR was the most frequently mutated gene, with mutations
observed in 57% of patients, followed by tumor protein p53 (TP53)
and RB transcriptional corepressor 1, with mutations in 56 and 13%
of patients, respectively. In addition to carrying EGFR as a driver
mutation, two patients presented with ALK receptor tyrosine kinase
(ALK) rearrangements, two presented with ERBB2 receptor tyrosine
kinase 2 mutations and one with a MET proto-oncogene receptor
tyrosine kinase (MET) mutation. One patient with an EGFR mutation
also tested positive for a KRAS mutation. In addition, 10 patients
carried the EGFR T790M mutation (Fig.
1A).
Subsequently, the spectrum of mutations across the
tissue and plasma samples was compared. Collectively, 106 genomic
alterations spanning 37 genes within the plasma samples were
identified. Using the mutations detected within the patient-matched
tumor samples as a reference, a by-variant association of 68.1% was
achieved between plasma and tumor samples, with 79 matched, 37
missed and 27 new genomic aberrations that were only present in
plasma samples (Fig. 1B), while the
by-patient sensitivity was calculated as 83%. Among the 37
mutations uniquely detected within plasma samples, a number of them
were classic NSCLC drivers, including EGFR (L858R, 19 del and
T790M) and ALK rearrangements. Additionally, four patients carried
the EGFR T790M mutation that was only detectable in the tissue
biopsy samples, whilst EGFR L858R appeared to be tissue-specific
for three patients. The tissue-specific mutations of 19 del and a
rearrangement in ALK were also observed only in a single patient
for each mutation. Notably, the present data revealed 27 mutations
that were only present in plasma samples. This could potentially be
due to tumor heterogeneity. The majority of the plasma-specific
mutations were either in driver genes or TP53 mutations, including
eight EGFR mutations (including five EGFR T790M), one ALK, one
KRAS, one MET and eight TP53 mutations. The Venn diagram presented
in Fig. 1C summarizes all of the
genomic alterations detected across all analyzed samples. In
summary, the present data demonstrate a concordance between tissue
and plasma samples.
Patients with tissue-specific
mutations exhibit a low plasma Max AF
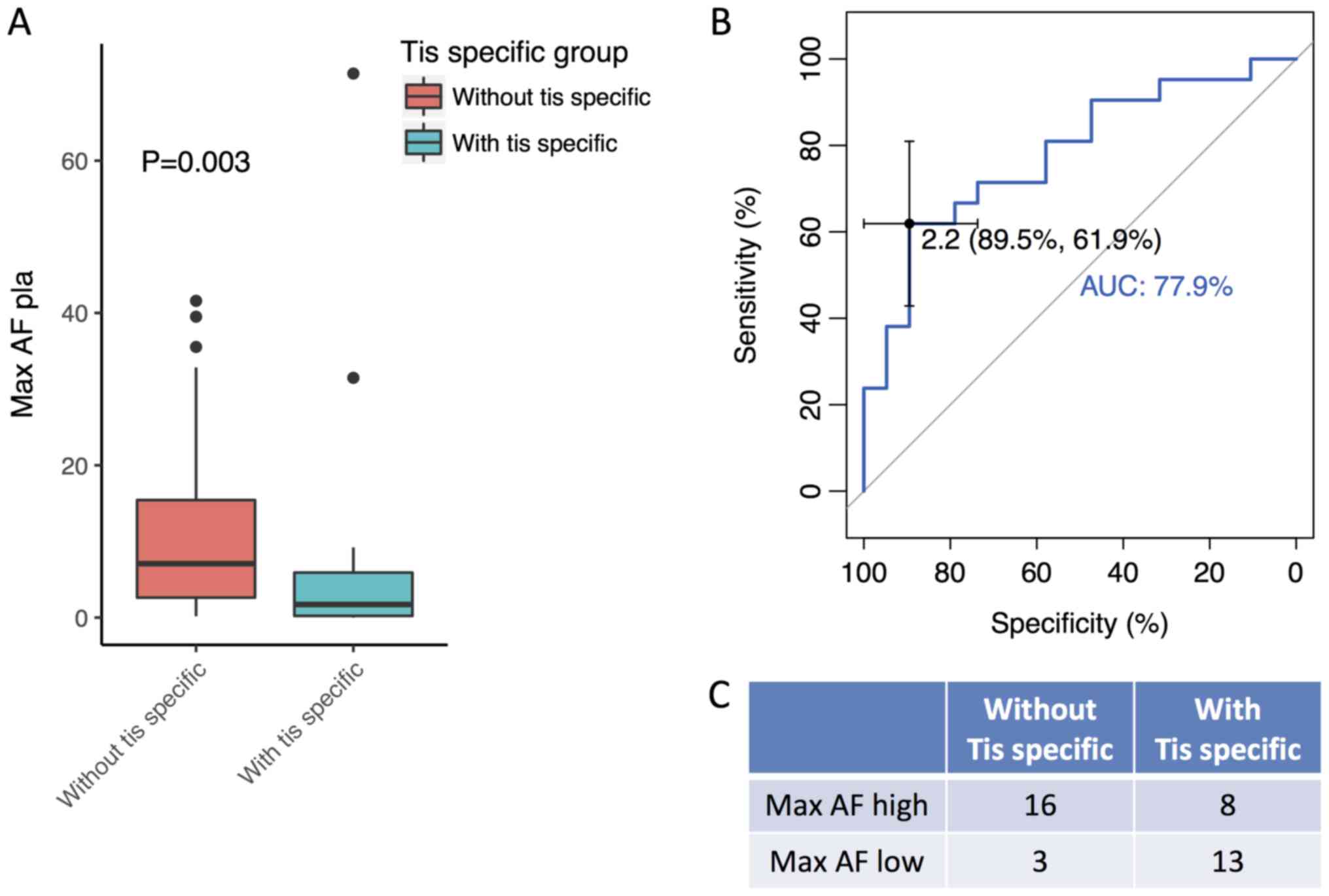
The plasma Max AF of patients with and without
tissue-specific mutations was compared to determine whether there
was a difference in these patient sub-groups. The plasma Max AF of
patients without tissue-specific mutations were observed to be
significantly higher compared with patients with tissue specific
mutations (P=0.003; Fig. 2A). A
receiver operating characteristic analysis was subsequently
performed to derive plasma Max AF as a percentage that could be
used to differentiate patients on the likelihood of them having
tissue-specific mutations. The analysis revealed that patients with
a plasma Max AF ≤2.2% were more likely to have tissue-specific
mutations, achieving an area under curve of 78% (specificity,
89.5%; sensitivity, 61.9%; Fig. 2B).
Utilizing 2.2% as a cutoff, 24 patients were classified as having a
high plasma Max AF (>2.2%) and the remaining 16 patients were
classified as having a low Max AF (≤2.2%). Among the 24 patients
with a high Max AF, 16 possessed no tissue-specific mutations,
while 8 exhibited tissue-specific mutations. Among the 16 patients
with a low Max AF, 13 possessed tissue-specific mutations and 3
demonstrated no tissue-specific mutations (Fig. 2C). Further analysis revealed that the
detection rate of tissue-specific mutations was 81.3% (13/16) in
patients with a plasma Max AF <2.2%, while the rate was 33.3%
(8/24) in patients with a Max AF ≥2.2 (Fig. 2C). In summary, these results
demonstrate that the tumor tissue mutation profile is more likely
to be comprehensively reflected by plasma in patients with a high
plasma Max AF (>2.2%). In such patients, liquid biopsy may have
the potential to replace tissue biopsy.
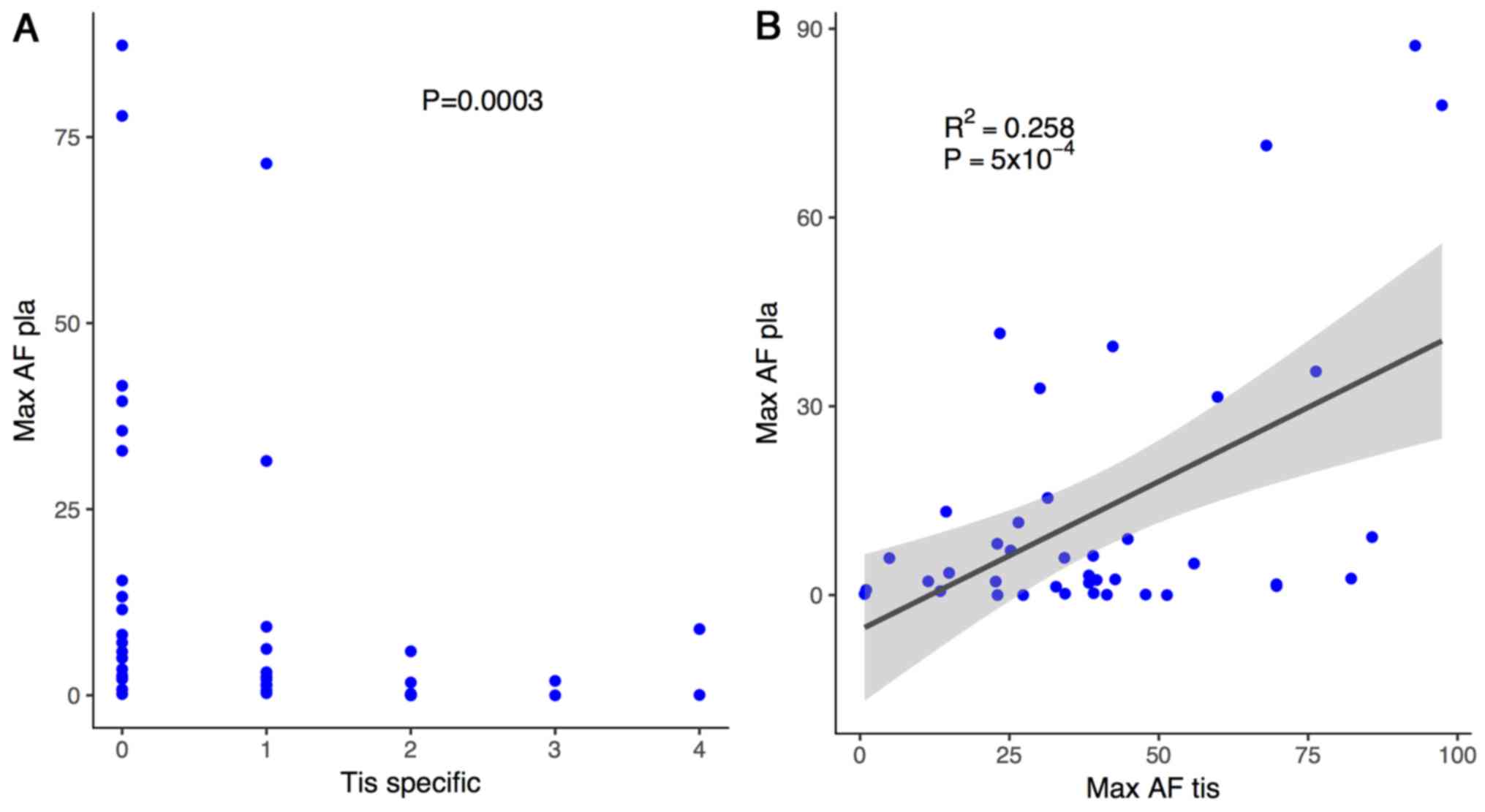
To further confirm the current finding, the number
of tissue-specific mutations was plotted against plasma Max AF for
each patient. Patients with more than one tissue-specific mutation
consistently demonstrated a lower plasma Max AF (P=0.0003; Fig. 3A). The correlation between the tissue
Max AF and the plasma Max AF was also analyzed for each patient
using a linear regression model, which revealed a significant
positive correlation between these factors (r2=0.285;
P<0.001; Fig. 3B). Collectively,
these data confirm an inverse association between plasma Max AF and
the likelihood of harboring tissue-specific mutations. Furthermore,
a positive correlation between tissue Max AF and plasma Max AF was
noted in this patient population.
Discussion
The present study derived a parameter, termed Max
AF, to differentiate patients with lung cancer with tissue-specific
mutations from those without tissue-specific mutations. The
sensitivity of plasma genotyping, which is reported to be ~70%
accurate compared with tissue biopsy analysis (18–23,25),
remains a challenge for the use of this method in clinical
settings. The current study revealed an inverse correlation between
a patient's plasma Max AF and the likelihood of that patient
possessing tissue-specific mutations. Furthermore, a plasma Max AF
of 2.2% was derived as a binary classifier to differentiate between
patient with and without tissue-specific mutations. Plasma
genotyping results of patients with plasma Max AF >2.2% were
more likely to reflect the mutation profile in tumor tissue
samples.
One possible explanation for missing mutations not
detected plasma genotyping is the low levels of ctDNA (30). The ctDNA concentration is
significantly diluted by DNA released from normal cells. Another
possibility is tumor heterogeneity, where very few clones with
certain mutations exist, resulting in the low levels of this
genomic aberration present in the ctDNA sample (31–33). In
any case, a high sequencing depth is required for the detection of
mutations in plasma samples. In the present study, the average
sequencing depths were ~1,000X for tissue samples and ~10,000X for
the ctDNA of plasma. However, the sensitivity of the plasma test is
still at an unsatisfactory level for accurate prognostic and
treatment use within the clinic. A recent study reported that
although the detection sensitivity of EGFR mutations in plasma
samples was comparable to that of tumor tissue, the detection rate
of KRAS mutations was much lower, and this may be due to the
limited number of tumor sub-clones in plasma samples (34). The low concordance between tissue and
plasma for the additional coexisting mutations in EGFR-mutant
NSCLCs is clinically relevant. It has been demonstrated that
patients with an EGFR mutant that carry additional mutations in
other genes, such as KRAS, are more likely to exhibit worse
outcomes when treated with EGFR tyrosine kinase inhibitor drugs
(35–37). Consequently, it is important to
identify new methods of analysis for plasma samples that reduce the
incidence of false negative results.
The present study derived plasma Max AF as a
biomarker to reflect plasma test sensitivity. The majority of
patients (81%) with a plasma Max AF <2.2% presented with
tissue-specific mutations, suggesting that they may benefit from
additional tissue biopsy due to a limited amount of ctDNA present
in plasma samples. Abundant ctDNA is the key characteristic that
determines high sensitivity in liquid biopsy examination (27,38). The
concentration of ctDNA can be assessed through measurement of the
cell free DNA concentration; however, this further increases the
cost of molecular analysis (38).
Using the cutoff value of Max AF, patients that are more likely to
have false negative results in plasma tests can be easily
identified. Therefore, a parameter indicating liquid biopsy
sensitivity could contribute to improved clinical practice in the
future.
The newly identified mutations in plasma samples not
present in tumor samples are largely due to tumor heterogeneity
(39,40). In an individual tumor, cells from
different regions could have different genetic characteristics
largely due to the presence of sub-clones (41). A single biopsy of tumor tissue cannot
represent the comprehensive features of the tumor (42). As such, it is possible that more
mutations are identified in plasma-derived ctDNA compared with
alterations in tissue DNA. As it is difficult to obtain a complete
analysis of the molecular features of a tumor through a single
biopsy test, it can be recommended that a more comprehensive
examination of tissue biopsy samples could potentially extend these
findings in the future.
Acknowledgements
Not applicable.
Funding
This study was supported by the Science and
Technology Program of Guangzhou, China (grant no.
201607010117).
Availability of data and materials
The data and materials are available upon reasonable
request from the corresponding author.
Authors' contributions
YT, XL and GQ worked on the conception and design of
the study. YT, XL, ZO, ZH, QZ, YW and MY collected the data. JY
assisted with the statistical analysis. YT, XL, ZO, ZH, QZ, YW, MY,
JY, HHZ and GQ analyzed and interpreted the data. YT, XL and HHZ
wrote the manuscript in consultation with GQ. All the authors
approved the manuscript and are accountable for the content of the
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethical
Committee at the General Hospital of Southern Theater Command of
PLA (Guangzhou, China). All patients provided written informed
consent for their participation in the study.
Patient consent for publication
Not applicable.
Competing interests
All the authors declare no conflict of interest.
References
|
1
|
Morash M, Mitchell H, Beltran H, Elemento
O and Pathak J: The role of next-generation sequencing in precision
medicine: A review of outcomes in oncology. J Pers Med. 8:E302018.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ziogas DE, Kyrochristos ID and Roukos DH:
Next-generation sequencing: From conventional applications to
breakthrough genomic analyses and precision oncology. Expert Rev
Med Devices. 15:1–3. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Horak P, Fröhling S and Glimm H:
Integrating next-generation sequencing into clinical oncology:
Strategies, promises and pitfalls. ESMO Open. 1:e0000942016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Maemondo M, Inoue A, Kobayashi K, Sugawara
S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I,
et al: Gefitinib or chemotherapy for non-small-cell lung cancer
with mutated EGFR. N Engl J Med. 362:2380–2388. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mazières J, Zalcman G, Crinò L, Biondani
P, Barlesi F, Filleron T, Dingemans AM, Léna H, Monnet I,
Rothschild SI, et al: Crizotinib therapy for advanced lung
adenocarcinoma and a ROS1 rearrangement: Results from the EUROS1
cohort. J Clin Oncol. 33:992–999. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ohtsuka K, Ohnishi H, Furuyashiki G,
Nogami H, Koshiishi Y, Ooide A, Matsushima S, Watanabe T and Goya
T: Clinico-pathological and biological significance of tyrosine
kinase domain gene mutations and overexpression of epidermal growth
factor receptor for lung adenocarcinoma. J Thorac Oncol. 1:787–795.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shaw AT, Ou SH, Bang YJ, Camidge DR,
Solomon BJ, Salgia R, Riely GJ, Varella-Garcia M, Shapiro GI, Costa
DB, et al: Crizotinib in ROS1-rearranged non-small-cell lung
cancer. N Engl J Med. 371:1963–1971. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tan CS, Gilligan D and Pacey S: Treatment
approaches for EGFR-inhibitor-resistant patients with
non-small-cell lung cancer. Lancet Oncol. 16:e447–e459. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Garraway LA: Genomics-driven oncology:
Framework for an emerging paradigm. J Clin Oncol. 31:1806–1814.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim HK, Ku BM, Lee H, Heo MH, Hong JH, Sun
J-M, Lee SH, Ahn JS, Park K and Ahn M-J: The feasibility of using
small biopsy samples from lung cancer for targeted next-generation
sequencing. J Clin Oncol. 35:e20584. 2017. View Article : Google Scholar
|
|
11
|
Sundaresan TK, Sequist LV, Heymach JV,
Riely GJ, Jänne PA, Koch WH, Sullivan JP, Fox DB, Maher R,
Muzikansky A, et al: Detection of T790M, the acquired resistance
EGFR mutation, by tumor biopsy versus noninvasive blood-based
analyses. Clin Cancer Res. 22:1103–1110. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Alix-Panabières C and Pantel K: Clinical
applications of circulating tumor cells and circulating tumor DNA
as liquid biopsy. Cancer Discov. 6:479–491. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Brugger W, Triller N, Blasinska-Morawiec
M, Curescu S, Sakalauskas R, Manikhas GM, Mazieres J, Whittom R,
Ward C, Mayne K, et al: Prospective molecular marker analyses of
EGFR and KRAS from a randomized, placebo-controlled study of
erlotinib maintenance therapy in advanced non-small-cell lung
cancer. J Clin Oncol. 29:4113–4120. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Diaz LA Jr and Bardelli A: Liquid
biopsies: Genotyping circulating tumor DNA. J Clin Oncol.
32:579–586. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee J, Cho SM, Kim MS, Lee SH, Chung YJ
and Jung SH: Circulating tumor DNA in a breast cancer patient's
plasma represents driver alterations in the tumor tissue. Genomics
Inform. 15:48–50. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schwaederle M, Husain H, Fanta PT,
Piccioni DE, Kesari S, Schwab RB, Patel SP, Harismendy O, Ikeda M,
Parker BA and Kurzrock R: Use of liquid biopsies in clinical
oncology: Pilot experience in 168 patients. Clin Cancer Res.
22:5497–5505. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fenizia F, De Luca A, Pasquale R, Sacco A,
Forgione L, Lambiase M, Iannaccone A, Chicchinelli N, Franco R,
Rossi A, et al: EGFR mutations in lung cancer: From tissue testing
to liquid biopsy. Future Oncol. 11:1611–1623. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Karlovich C, Goldman JW, Sun JM, Mann E,
Sequist LV, Konopa K, Wen W, Angenendt P, Horn L, Spigel D, et al:
Assessment of EGFR mutation status in matched plasma and tumor
tissue of NSCLC patients from a phase I study of rociletinib
(CO-1686). Clin Cancer Res. 22:2386–2395. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Thress KS, Brant R, Carr TH, Dearden S,
Jenkins S, Brown H, Hammett T, Cantarini M and Barrett JC: EGFR
mutation detection in ctDNA from NSCLC patient plasma: A
cross-platform comparison of leading technologies to support the
clinical development of AZD9291. Lung Cancer. 90:509–515. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ishii H, Azuma K, Sakai K, Kawahara A,
Yamada K, Tokito T, Okamoto I, Nishio K and Hoshino T: Digital PCR
analysis of plasma cell-free DNA for non-invasive detection of drug
resistance mechanisms in EGFR mutant NSCLC: Correlation with paired
tumor samples. Oncotarget. 6:30850–30858. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chai YJ, Song J, Kang J, Woo JW, Song RY,
Kwon H, Kim SJ, Choi JY and Lee KE: A comparative study of
postoperative pain for open thyroidectomy versus bilateral
axillo-breast approach robotic thyroidectomy using a self-reporting
application for iPad. Ann Surg Treat Res. 90:239–245. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Han JY, Lee KH, Kim SW, Min YJ, Cho E, Lee
Y, Lee SH, Kim HY, Lee GK, Nam BH, et al: A Phase II study of
poziotinib in patients with epidermal growth factor receptor
(EGFR)-mutant lung adenocarcinoma who have acquired resistance to
EGFR-tyrosine kinase inhibitors. Cancer Res Treat. 49:10–19. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Takahama T, Sakai K, Takeda M, Azuma K,
Hida T, Hirabayashi M, Oguri T, Tanaka H, Ebi N, Sawa T, et al:
Detection of the T790M mutation of EGFR in plasma of advanced
non-small cell lung cancer patients with acquired resistance to
tyrosine kinase inhibitors (West Japan oncology group 8014LTR
study). Oncotarget. 7:58492–58499. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Douillard JY, Ostoros G, Cobo M, Ciuleanu
T, McCormack R, Webster A and Milenkova T: First-line gefitinib in
caucasian EGFR mutation-positive NSCLC patients: A phase-IV,
open-label, single-arm study. Br J Cancer. 110:55–62. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Perdigones N and Murtaza M: Capturing
tumor heterogeneity and clonal evolution in solid cancers using
circulating tumor DNA analysis. Pharmacol Ther. 174:22–26. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Oxnard GR, Thress KS, Alden RS, Lawrance
R, Paweletz CP, Cantarini M, Yang JC, Barrett JC and Jänne PA:
Association between plasma genotyping and outcomes of treatment
with osimertinib (AZD9291) in advanced non-small-cell lung cancer.
J Clin Oncol. 34:3375–3382. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cheung AH, Chow C and To KF: Latest
development of liquid biopsy. J Thorac Dis. 10 (Suppl
14):S1645–S1651. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Diehl F, Schmidt K, Choti MA, Romans K,
Goodman S, Li M, Thornton K, Agrawal N, Sokoll L, Szabo SA, et al:
Circulating mutant DNA to assess tumor dynamics. Nat Med.
14:985–990. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mao X, Zhang Z, Zheng X, Xie F, Duan F,
Jiang L, Chuai S, Han-Zhang H, Han B and Sun J: Capture-based
targeted ultradeep sequencing in paired tissue and plasma samples
demonstrates differential subclonal ctDNA-releasing capability in
advanced lung cancer. J Thorac Oncol. 12:663–672. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Crowley E, Di Nicolantonio F, Loupakis F
and Bardelli A: Liquid biopsy: Monitoring cancer-genetics in the
blood. Nat Rev Clin Oncol. 10:472–484. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Belchis DA, Tseng LH, Gniadek T, Haley L,
Lokhandwala P, Illei P, Gocke CD, Forde P, Brahmer J, Askin FB, et
al: Heterogeneity of resistance mutations detectable by
nextgeneration sequencing in TKI-treated lung adenocarcinoma.
Oncotarget. 7:45237–45248. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chabon JJ, Simmons AD, Lovejoy AF,
Esfahani MS, Newman AM, Haringsma HJ, Kurtz DM, Stehr H, Scherer F,
Karlovich CA, et al: Circulating tumour DNA profiling reveals
heterogeneity of EGFR inhibitor resistance mechanisms in lung
cancer patients. Nat Commun. 7:118152016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Morii E: Heterogeneity of tumor cells in
terms of cancer-initiating cells. J Toxicol Pathol. 30:1–6. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rachiglio AM, Esposito Abate R, Sacco A,
Pasquale R, Fenizia F, Lambiase M, Morabito A, Montanino A, Rocco
G, Romano C, et al: Limits and potential of targeted sequencing
analysis of liquid biopsy in patients with lung and colon
carcinoma. Oncotarget. 7:66595–66605. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mao C, Qiu LX, Liao RY, Du FB, Ding H,
Yang WC, Li J and Chen Q: KRAS mutations and resistance to
EGFR-TKIs treatment in patients with non-small cell lung cancer: A
meta-analysis of 22 studies. Lung Cancer. 69:272–278. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Metro G, Chiari R, Duranti S, Siggillino
A, Fischer MJ, Giannarelli D, Ludovini V, Bennati C, Marcomigni L,
Baldi A, et al: Impact of specific mutant KRAS on clinical outcome
of EGFR-TKI-treated advanced non-small cell lung cancer patients
with an EGFR wild type genotype. Lung Cancer. 78:81–86. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bria E, Pilotto S, Amato E, Fassan M,
Novello S, Peretti U, Vavalà T, Kinspergher S, Righi L, Santo A, et
al: Molecular heterogeneity assessment by next-generation
sequencing and response to gefitinib of EGFR mutant advanced lung
adenocarcinoma. Oncotarget. 6:12783–12795. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jung A and Kirchner T: Liquid biopsy in
tumor genetic diagnosis. Dtsch Arztebl Int. 115:169–174.
2018.PubMed/NCBI
|
|
39
|
Hiley C, de Bruin EC, McGranahan N and
Swanton C: Deciphering intratumor heterogeneity and temporal
acquisition of driver events to refine precision medicine. Genome
Biol. 15:4532014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yates LR, Gerstung M, Knappskog S, Desmedt
C, Gundem G, Van Loo P, Aas T, Alexandrov LB, Larsimont D, Davies
H, et al: Subclonal diversification of primary breast cancer
revealed by multiregion sequencing. Nat Med. 21:751–759. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang J, Fujimoto J, Zhang J, Wedge DC,
Song X, Zhang J, Seth S, Chow CW, Cao Y, Gumbs C, et al: Intratumor
heterogeneity in localized lung adenocarcinomas delineated by
multiregion sequencing. Science. 346:256–259. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Taniguchi K, Okami J, Kodama K,
Higashiyama M and Kato K: Intratumor heterogeneity of epidermal
growth factor receptor mutations in lung cancer and its correlation
to the response to gefitinib. Cancer Sci. 99:929–935. 2008.
View Article : Google Scholar : PubMed/NCBI
|