Introduction
Lung cancer is one of the most lethal diseases
worldwide, and is responsible for ~1.2 million cases of mortality
each year (1). According to the
pathological type, lung cancer can be divided into small cell lung
cancer and non-small cell lung cancer (NSCLC). NSCLC accounts for
85% of all lung cancer cases. Squamous cell carcinoma represents
~30% of small cell lung cancer cases, and non-squamous cell
carcinoma accounts for ~70%. NSCLC is the leading cause of
tumor-associated mortality in the United States and Europe. The
majority of patients (~60%) are diagnosed with locally advanced or
metastatic NSCLC, and the 5-year survival rate is only ~5%.
Vascular endothelial growth factor (VEGF) (2) and epidermal growth factor receptor
(3) are currently the main
therapeutic targets in NSCLC. However, >40% of patients with
NSCLC develop tumor recurrence, even when they have received early
treatment (4). Therefore, the
specific mechanisms of NSCLC development, and novel therapeutic
targets, require further investigation.
Apoptosis is a type of gene-encoded spontaneous cell
death that occurs during cell growth, differentiation, development
and pathology. It is also known as programmed cell death (5). Normal epithelial or endothelial cells
are adhesive-dependent, and their survival depends on cell-cell and
cell-matrix signaling (6). However,
tumor cells can survive without adhesion, which is named anchorage
independence (7). Anoikis is a
specific form of programmed cell death induced by loss of contact
between cells and extracellular matrices or other cells. Progress
on the determination of anoikis-related genes has been made. It has
been reported that the decrease of B-cell lymphoma-extra large
(Bcl-XL) protein expression induces a significant increase in
ovarian cancer cell anoikis; however, it has no effect on cells
that are attached to other cells (8). Mutation or loss of heterozygosis of the
phosphatase and tension homolog (PTEN) gene is closely
associated with the occurrence and development of tumors, including
glioblastoma and prostate cancer. It can also mediate the
regulation of anoikis signaling (9).
In addition, integrins serve a crucial role in anchorage
independence as a central signaling pathway between cells and
matrix (10).
Interleukin enhancer binding factor 2 (ILF2) is also
known as nuclear factor 45. In the past few years, ILF2 has been
extensively studied in tumors. For example, ILF2 is overexpressed
in various types of malignancy, including glioma, NSCLC and
esophageal cancer, and promotes their development (11–13).
Therefore ILF2 upregulation may be necessary for cancer cell
progression. In addition, recent studies reported that ILF2
expression is associated with tumor size in pancreatic ductal
carcinoma (PDAC) (14,15). Furthermore, ILF2 can be a valuable
prognostic indicator of PDAC survival (15). Lee et al (16) reported that ILF2 expression is high
in liver cancer tissues by using immunohistochemistry and western
blotting. However, the specific function of ILF2 and its mechanism
underlying tumorigenesis require further investigation.
PTEN was the first tumor suppressor gene with
phosphatase activity to be discovered (17). Numerous degrees of PTEN gene
mutation or loss exist in various types of tumor tissues, cell
lines and xenografts. PTEN is involved in many important
intracellular pathways and serves therefore crucial roles in
suppressing the occurrence and development of tumors. PTEN blocks
the phosphoinositide 3-kinase (PI3K)/protein kinase B signaling
pathway by decreasing phosphatidylinositol (3,4,5)-trisphosphate (PIP3) levels to promote
apoptosis (18). PTEN is a
diphosphatase, which activates PI3K to dephosphorylate PIP3. This
reaction blocks PI3K-regulated growth factor signaling pathways and
maintains cell growth during normal growth cycles (19). PTEN can also inhibit focal adhesion
kinase phosphorylation to suppress cell migration (20). In addition, PTEN inhibits tumor
angiogenesis through regulation of VEGF expression (21).
Although ILF2 has been previously reported in NSCLC,
the present study aimed to examine the function of ILF2 in a
completely novel way. The results from this study demonstrated that
ILF2 was highly expressed in NSCLC cell lines and was associated
with poor patient prognosis, according to an online database. In
addition, ILF2 reduced cell-matrix adhesion and promoted anchorage
independence. Further analyses revealed that ILF2 directly bound to
the PTEN gene and regulated its expression. The results
suggested that ILF2 may achieve these functions through PTEN
regulation.
Materials and methods
Cell culture
HUVEC-C, HBEC-5i, BEAS-2B, A549, H460, H1155 and
H1299 cell lines were obtained from the American Type Culture
Collection (Manassas, VA, USA). HUVEC-C is a human umbilical vein
endothelium cell line. HBEC-5i is a human cerebral microvascular
endothelium cell line. BEAS-2B is a human normal lung epithelial
cell line. A549, H460, H1155 and H1299 are NSCLC cell lines.
HUVEC-C cells were maintained in Kaighn's Modification of Ham's
F-12 medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo
Fisher Scientific, Inc.), 0.1 mg/ml heparin (cat. no. H3393;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and 40 µg/ml
endothelial cell growth supplement (ECGS; cat. no. 354006; BD
Biosciences, San Jose, CA, USA). HBEC-5i cells were maintained in
Dulbecco's modified Eagle's medium/F12 (Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% FBS and 40 µg/ml ECGS.
BEAS-2B, A549, H460, H1155 and H1299 cells were maintained in
RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% FBS. All cells were cultured at 37°C in a
humidified incubator containing 5% CO2.
Adhesion assay
Cell culture dishes (diameter, 60-mm) were covered
with fibronectin (500 µl, 10 µg/ml; Sigma-Aldrich; Merck KGaA) and
incubated in a cell incubator overnight. HUVEC-C, HBEC-5i, A549 or
H460 were transfected with control, shILF2, ILF2, shPTEN and PTEN.
Dishes were washed twice with PBS (Thermo Fisher Scientific, Inc.),
and 2×105 HUVEC-C, HBEC-5i, A549 or H460 cells in normal
medium were seeded in each dish and incubated for 30 min at 37°C in
a cell culture incubator. The media was discarded, and cells were
washed twice with PBS. Crystal violet [0.05% (w/v)] was used to
stain adhered cells for 10 min at room temperature, and plates were
imaged by light microscopy (magnification, ×100). Finally, the
number of cells that were stained were counted.
Cell death detection by ELISA
To examine the ability of anchorage independence,
cells transfected with control, shILF2, ILF2, shPTEN and PTEN were
seeded (~2×105) in low-attachment (Corning Inc.,
Corning, NY, USA) and normal surface 24-well plates at 37°C cell
culture incubator. Cells that normally grow adherently cannot
adhere to low-attachment (PolyHEMA-coated) 24-well plates. This can
be used to simulate the detachment of cells from the matrix.
According to the manufacturer's protocol, a Cell Death Detection
ELISA PLUS kit (cat. no. 11774425001; Roche Diagnostics, Basel,
Switzerland) was used to assess cell apoptosis. Apoptotic cells
were measured using a microplate reader at 405 nm. The ELISA kit
was used to detect the level of apoptosis. It determines the level
of apoptosis by detecting the DNA-ladder produced by endogenous
restriction endonuclease cleavage during apoptosis (22,23).
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated from cells with
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) and reverse transcribed with oligo dT (Takara Biotechnology
Co., Ltd., Dalian, China). The reverse transcription temperature
protocol was 1 h at 42°C and 10 min at 72°C according to the
manufacturer's protocol. RT-qPCR was performed with Ex
Taq® polymerase (Takara Biotechnology Co., Ltd.), and
the cycling conditions were as follows: Initial denaturation for 3
min at 95°C, followed by 31 cycles of 15 sec at 95°C, 15 sec at
55°C and 30 sec at 72°C, with a final extension for 7 min at 72°C.
The sequences of the primers were designed as follows: ILF2 (gene
ID: 3608), forward 5′-AGGCCCTTTGTACCACATATC-3′, reverse
5′-ATCCTGTGCTCTTAGGCTTTC-3′ (reverse); and GAPDH (gene ID: 2597),
forward 5′-GATTCCACCCATGGCAAATTC-3′ and reverse
5′-GTCATGAGTCCTTCCACGATAC-3′. The 2−∆∆Cq method was used
to normalize the expression to GAPDH (24).
Western blotting
For protein extraction, 2×106 cells were
incubated in lysis buffer that was composed of 100 µl 50 mM
Tris-HCl (pH 8.0) containing 1% NP-40, 150 mM NaCl, 100 µg/ml
phenylmethylsulfonyl fluoride and 0.1% SDS. Lysis was performed on
ice and lysates were subsequently denatured at 100°C for 10 min. A
Pierce bicinchoninic acid protein assay kit (Invitrogen; Thermo
Fisher Scientific, Inc.) was used to measure the protein
concentration and 30-µg protein was loaded into each well. Proteins
were separated by 10% SDS-PAGE and transferred to polyvinylidene
fluoride membranes. The membranes were blocked in 3% (w/v) bovine
serum albumin for 1 h at room temperature. The primary antibodies
used were as follows: Rabbit polyclonal ILF2 (cat. no. ab154791;
1:5,000), the rabbit polyclonal PTEN (cat. no. ab31392; 1:1,000)
and the mouse monoclonal GAPDH (cat. no. ab8245; 1:5,000; all from
Abcam, Cambridge, UK), which were all dissolved in 5% bovine serum
albumin (w/v). The primary antibodies were incubated with the
membranes at 4°C overnight. The secondary antibodies used were the
goat anti-rabbit immunoglobulin (Ig) G H&L (cat. no. ab6721;
1:1,000) and the goat anti-mouse IgG H&L (cat. no. ab6789;
1:1,000; all from Abcam), which were all dissolved in 3% bovine
serum albumin (w/v). The secondary antibodies were incubated with
the blots at room temperature for 2 h. Bands were visualized with
using chemiluminescent horse radish peroxidase substrate (EMD
Millipore, Billerica, MA, USA). X-ray films (Beijing Solarbio
Science & Technology Co., Ltd., Beijing, China) and a GE
Amersham Imager 600 (GE Healthcare, Chicago, IL, USA) were used for
signal detection.
Cloning and transfection
Human ILF2 and PTEN were amplified
from H460 and HUVEC-C cDNA using Platinum® Taq DNA
Polymerase (Invitrogen; Thermo Fisher Scientific, Inc.),
respectively. The thermocycling conditions were as follows: Initial
denaturation for 5 min at 95°C; followed by 35 cycles of 30 sec at
95°C, 30 sec at 55°C and 2 min at 72°C; and a final extension for 5
min at 72°C. ILF2 and PTEN were ligated into the
lentiviral shuttle pCCL.PPT.hPGK.IRES.GFP/pre. The sequences of the
primers used for amplification were as follows: ILF2-ORF, forward
5′-CGCGGATCCATGAGGGGTGACAGAGGCCG-3′, reverse
5′-CGCGGATCCTCACTCCTGAGTTTCCATGC-3′; and PTEN-ORF, forward
5′-CGCGGATCCATGACAGCCATCATCAAAGA-3′ and reverse
5′-CGCGGATCCTCAGACTTTTGTAATTTGTG-3′. Oligos encoding short hairpin
(sh)RNA specific for ILF2 were ligated into pSUPER.retro.puro, and
the fragment containing the H1 promoter and hairpin sequences was
subcloned into the lentiviral shuttle pCCL.PPT.hPGK.GFP.Wpre. The
shRNA sequences were as follows: shRNA-1 targeting ILF2,
GGCCTTGCTGAAGAGGAATCA; shRNA-2 targeting ILF2,
CTGTGATGAACAACCCCACCA; shRNA-1 targeting PTEN,
GAAAGGGACGAACTGGTGTAA; and shRNA-2 targeting PTEN,
GGCGTATACAGGAACAATATT. HEK293T was used for lentiviral packaging. A
total of 15 ng target plasmid was transfected into HEK293T with a
confluence of 80% in 100 mm dishes. Polyethylenimine (Polysciences,
Inc., Mount Arlington, NJ, USA) was used as the transfection
reagent and a 1:3 transfection reagent: Plasmid ratio was used.
Cells were transfected for 4 h in DMEM, after which the media was
replaced. Cells were incubated at 37°C after transfection and the
lentivirus was collected 24 h after transfection. Lentivirus was
used to infect the corresponding target cells for 24 h.
Chromatin immunoprecipitation
(ChIP)
ChIP was performed as described by Liu and Garrard
(25). The primary antibody against
ILF2 was used at a 1:50 dilution.
Luciferase reporter gene
technology
DNA fragments of the PTEN upstream regulatory
region were amplified from HUVEC-C DNA using Platinum®
Taq DNA Polymerase (Invitrogen; Thermo Fisher Scientific, Inc.).
Cycling conditions were as follows: Initial denaturation for 5 min
at 95°C, followed by 35 cycles of 30 sec at 95°C, 30 sec at 55°C
and 2 min at 72°C, with a final extension for 5 min at 72°C. They
were inserted into the XhoI and the HindIII sites of
pGL3-basic with firefly luciferase. A Renilla reniformis
luciferase plasmid (pRL-TK) was used for normalization. A549 and
H460 cells (1×105) were plated in 24-well plates and
were transfected after ~24 h, once they reached ~80% confluence.
Cells were co-transfected with 900 ng target plasmid and 15 ng
pRL-TK using 1 µl Lipofectamine® 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.) at 37°C in a cell culture
incubator. After 24 h, the Dual-Luciferase Reporter assay system
(Promega Corporation, Madison, WI, USA) was used to lyse the cells
and measure the luciferase activity with a Promega GloMax 20/20
Luminometer in Eppendorf Tubes according to the manufacturer's
protocol.
Statistical analysis
SPSS v.19.0 software (IBM Corp., Armonk, NY, USA)
was used to perform statistical analyses. Data are presented as the
means ± standard deviation. Comparison analysis was performed using
the Student's t-test and one-way analysis of variance. Dunnett's
test was used for pairwise comparisons of multiple treatment groups
with a single control group. All experimental groups were compared
to the control groups. P<0.05 was considered to indicate a
statistically significant difference. Kaplan-Meier survival
analysis of ILF2 was performed with an online tool (http://kmplot.com/analysis/) and the log rank test was
used to generate P-values.
Results
ILF2 is highly expressed in NSCLC cell
lines and is associated with poor patient outcomes
ILF2 expression levels were detected in the human
normal cell lines HUVEC-C, HBEC-5i and BEAS-2B, and in the NSCLC
cell lines A549, H460, H1155 and H1299. High transcriptional and
translational levels of ILF2 were detected in all NSCLC cell lines
according to the results from RT-qPCR and western blotting
experiments, respectively (Fig. 1A and
B).
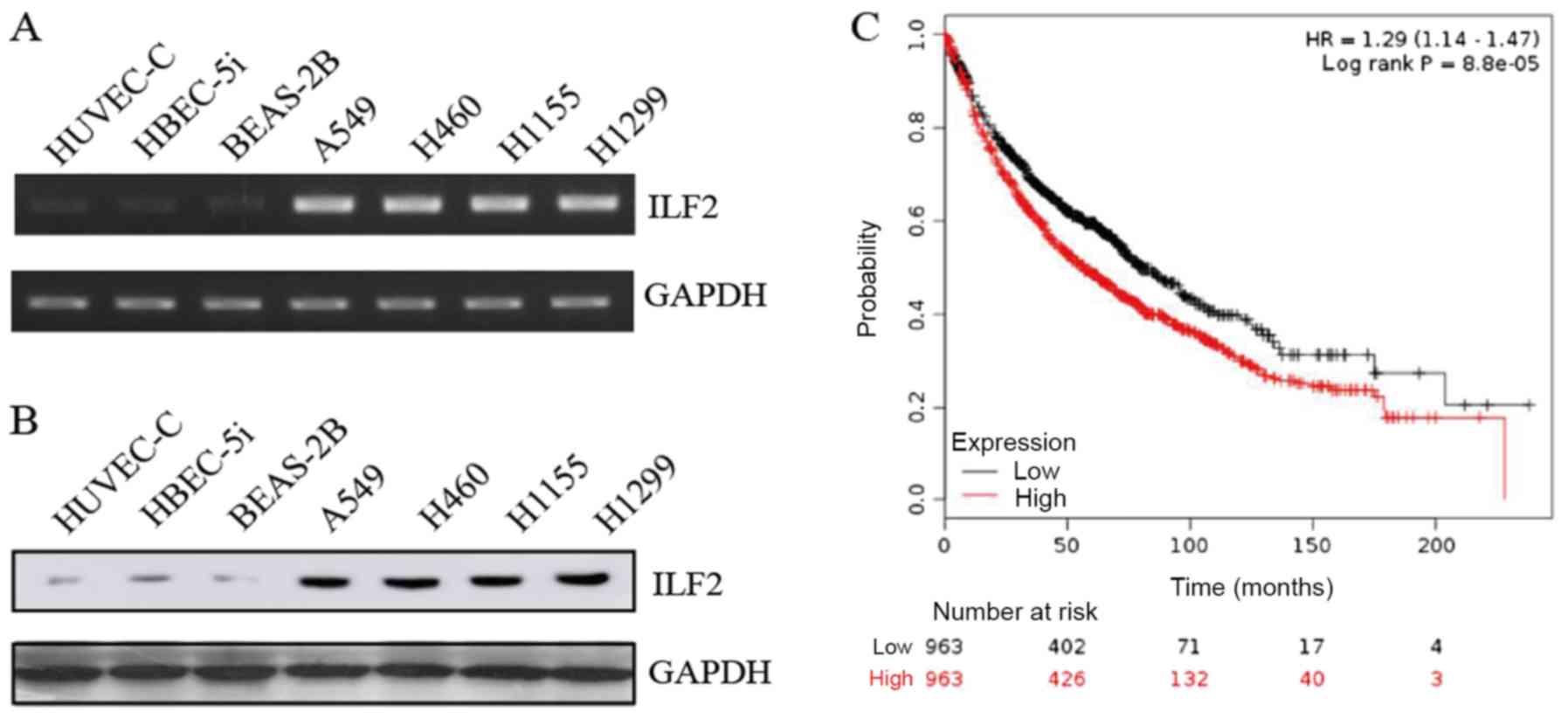 | Figure 1.ILF2 is highly expressed in NSCLC
cell lines and is associated with poor patient outcome. (A)
Representative gel presenting mRNA expression of ILF2 in HUVEC-C,
HBEC-5i, BEAS-2B, A549, H460, H1155 and H1299 cell lines. (B)
Protein levels of ILF2 were measured by western blotting in
HUVEC-C, HBEC-5i, BEAS-2B, A549, H460, H1155 and H1299 cell lines.
(C) Kaplan-Meier survival analysis of the association between
survival time and ILF2 signature in lung cancer using an online
tool (http://kmplot.com/analysis/). ILF2,
interleukin enhancer binding factor 2. |
In order to investigate the effect of ILF2 in lung
cancer, Kaplan-Meier survival analysis was used to determine the
association between ILF2 expression and the survival time of
patients with lung cancer using an online tool (http://kmplot.com/analysis/) (26). The results demonstrated that
increased ILF2 expression was significantly associated with a worse
overall survival rate of patients with lung cancer (n=2,437;
P=0.000088; Fig. 1C), which
suggested that ILF2 may serve a crucial role in lung cancer
malignancy progression.
ILF2 reduces cell-matrix adhesion and
promotes anchorage independence
To determine the role of ILF2 in the development of
NSCLC, a series of cytology tests were conducted. Although it has
been reported that ILF2 affects NSCLC cell proliferation (12), the results from the present study
demonstrated a different effect. HUVEC-C and HBEC-5i were the
normal cell lines used. A previous study demonstrated that, HUVEC-C
and HBEC-5i are sensitive to anoikis (22), which may aid determining the function
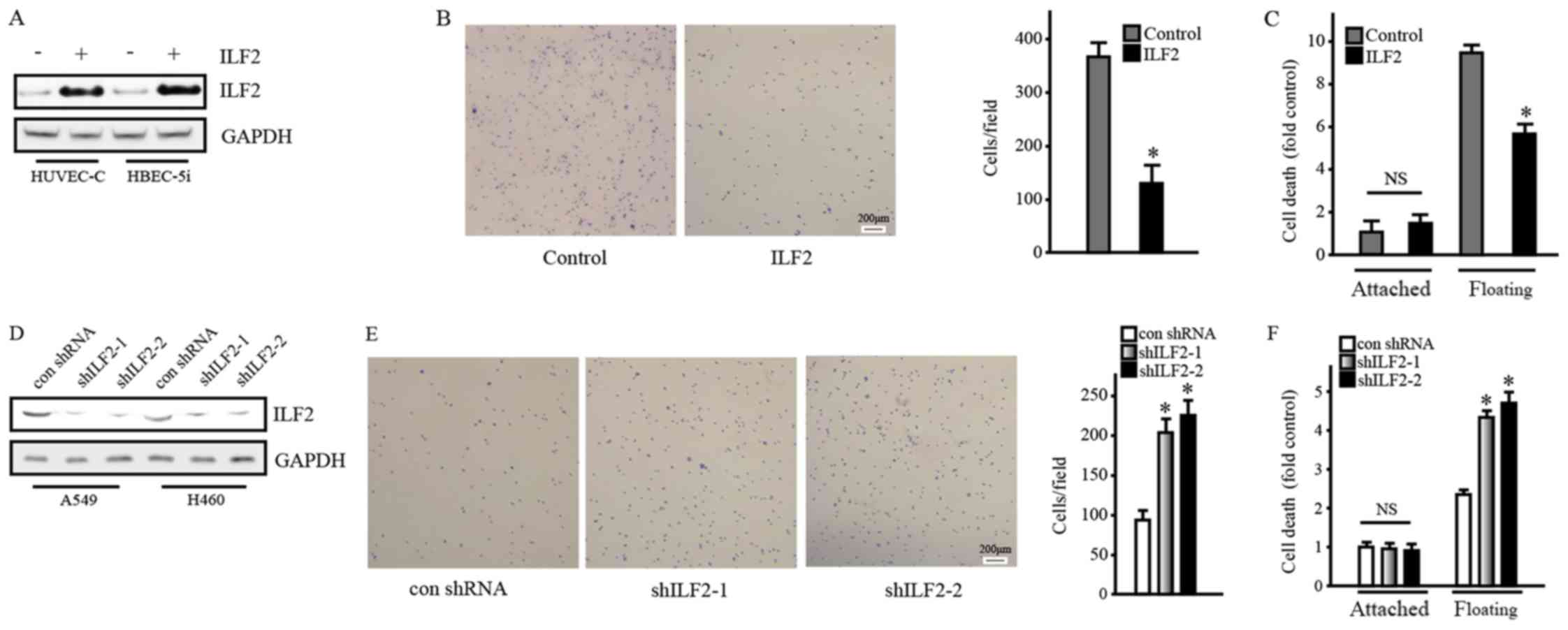
and underlying mechanism of ILF2. Western blotting demonstrated
that ILF2 overexpression in HUVEC-C and HBEC-5i cells using the
lentivirus system was successful (Fig.
2A). In addition, the adhesion assay reported that ILF2
significantly reduced cell adhesion (Fig. 2B). Furthermore, following 24 h of
suspension culture, the survival rate of floating cells
overexpressing ILF2 decreased compared with the control cells, as
measured with the Cell Death Detection ELISA PLUS kit (Fig. 2C). The results obtained from HBEC-5i
cell line were similar (data not shown). Furthermore, ILF2
knockdown in A549 and H460 cells confirmed these phenomena
(Fig. 2D-F). Western blotting
confirmed ILF2 knockdown in A549 and H460 cell lines (Fig. 2D). In addition, ILF2 knockdown
significantly increased cell adhesion and reduced cell survival
after suspension culture (Fig. 2E and
F, respectively). The results obtained from the H460 cell line
were similar (data not shown). Taken together, these results
suggested that ILF2 may reduce cell-cell and cell-matrix adhesions
and promote anchorage independence.
ILF2 promotes anchorage independence
by inhibiting PTEN expression
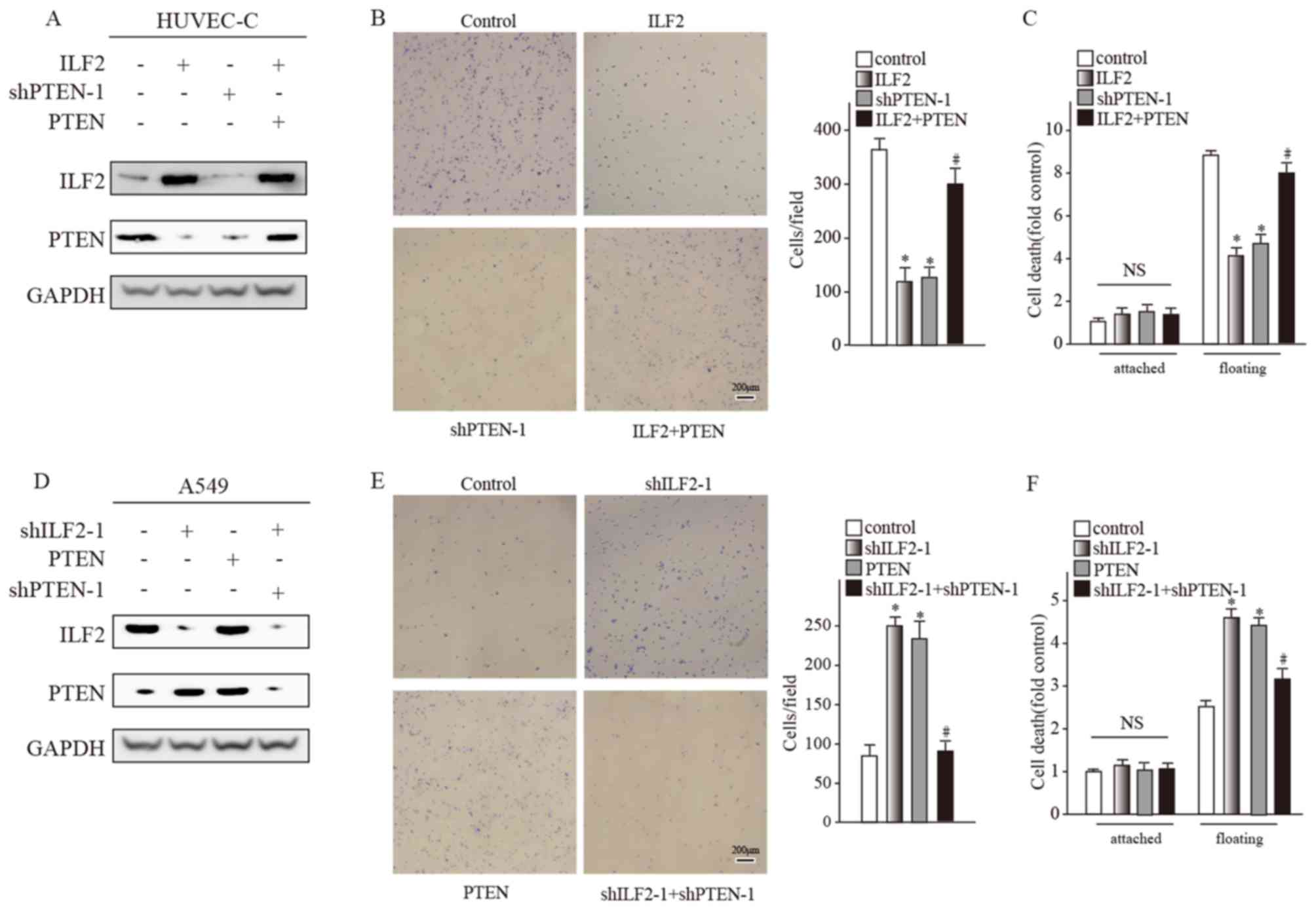
The results demonstrated that ILF2 overexpression in
HUVEC-C and HBEC-5i cell lines decreased the PTEN protein level
(Fig. 3A). It has been reported that
PTEN affects many aspects of tumor progression, including apoptosis
promotion (27). Following PTEN
knockdown by shRNA in HUVEC-C, the cell phenotype was similar
(Fig. 3B and C) to the one observed
following ILF2 overexpression. The results obtained from HBEC-5i
cell line were similar (data not shown). These results suggested
that ILF2 may reduce cell adhesion and promote anchorage
independence by inhibiting PTEN expression. To test this
hypothesis, the PTEN expression decrease caused by ILF2
overexpression was recovered by overexpressing PTEN. Cell adhesion
and apoptosis after suspension culture recovered to some degree
(Fig. 3B and C).
ILF2 knockdown in A549 and H460 cell lines led to
PTEN upregulation (Fig. 3D). PTEN
recovery reduced cell-matrix adhesion and stimulated cell survival
following 24 h of suspension culture (Fig. 3E and F). The results obtained from
the H460 cell line were similar (data not shown). These data
indicated that ILF2 reduced cell adhesion and promoted anchorage
independence by regulating PTEN expression.
ILF2 can directly bind to PTEN gene to
regulate its transcription
The specific mechanism of ILF2 on PTEN regulation
was further explored. Since ILF2 is a transcription factor, it was
hypothesized that it could bind to the PTEN gene upstream to
regulate its transcription. It has been reported that ILF2
interacts with the TGACAA motif of IL-2 proximal promoter (28). According to DNase I hypersensitive
sites analyzed by the Encyclopedia of DNA Elements at the UCSC
database (http://genome.ucsc.edu/ENCODE/), a series of primers
for ChIP assays in the range of 10 kb upstream and downstream of
the PTEN transcription start site were designed (Fig. 4A). In HUVEC-C and HBEC-5i cells
overexpressing ILF2, the results from the ChIP assay demonstrated
that ILF2 could bind to the R3 region of PTEN (Fig. 4B and C). A series of luciferase
reporter vectors containing fragments of different lengths upstream
of PTEN were then constructed. A549 and H460 cells highly
expressing ILF2 were used to conduct the luciferase reporter gene
assays. The results demonstrated that the activity of the
luciferase reporter containing the −2,956 to −2,642 bp fragment was
very high (Fig. 4D). Furthermore,
the position of the −2,956 to −2,642 bp fragment overlaps with the
R3 position. These results indicated that the transcription factor
ILF2 bound directly to the upstream region of the PTEN gene
to affect its expression.
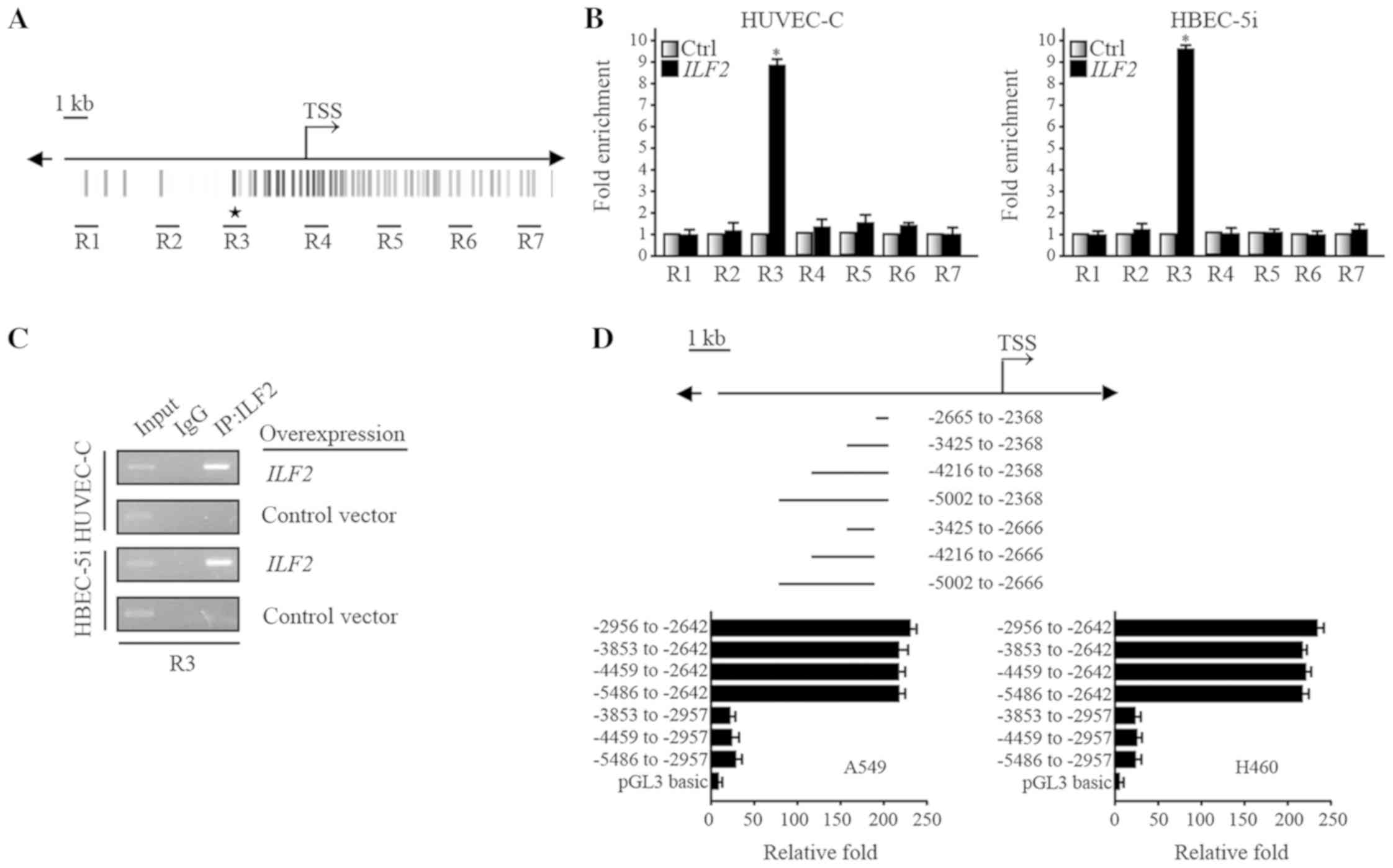 | Figure 4.ILF2 can directly bind to the
PTEN gene to regulate its transcription. (A) The seven
regions designed for ChIP are highlighted. (B) ChIP analysis
demonstrated that ILF2 antibody was enriched in the region R3 of
PTEN in HUVEC-C and HBEC-5i cell lines following ILF2
overexpression, according to RT-qPCR results. (C) Representative
gel for R3 of PTEN following ChIP analysis of HUVEC-C and
HBEC-5i cell lines that overexpressed ILF2, according to RT-qPCR
results. (D) Luciferase reporter studies indicated that the region
of PTEN regulated by ILF2 may be located within the −2956 to
−2642 bp fragment. Asterisks indicate site with ILF2 consensus
sequences. ChIP, chromatin immunoprecipitation; Ctrl, control; IgG,
immunoglobulin G; ILF2, interleukin enhancer binding factor 2;
PTEN, phosphatase and tensin homolog deleted on chromosome ten; R3,
region 3; TSS, transcriptional start site; Ctrl, control. |
Discussion
Lung cancer is one of the most common malignancies
worldwide. NSCLC includes squamous cell carcinoma, adenocarcinoma
and large cell carcinoma. In comparison with small cell carcinoma,
NSCLC cells have slower growth and division rates, and diffusion
and metastasis occur relatively late. NSCLC accounts for ~80% of
all lung cancers. At the time of diagnosis, ~75% of patients with
NSCLC are in advanced stages and have a poor 5-year survival rate
of ~16%. The majority of patients with NSCLC are elderly, and ~50%
of patients with lung cancer are >65 years old (29). Early diagnosis of NSCLC is
essentially based on sputum cytology examination, chest X-ray and
other related examinations (30).
Patients are conventionally treated by chemotherapy combined with
surgery; however, the overall efficiency remains poor (31). With the major breakthrough and rapid
development of the Human Genome Project, the diagnosis and
treatment of lung tumors has reached the molecular level. Due to
genetic engineering, novel discoveries have been made at the gene
level, which has attracted the attention of the medical community
(31).
ILF2 is a transcription factor that contains an
internal ribosome entry site. ILF2 interacts with ILF3 to form a
complex that affects the nuclear redistribution of mRNA, repairs
non-homologous end-linked DNA damage, negatively regulates the
processing of microRNA and affects the expression of downstream
genes (32–34). The main biological function of ILF2
is the control of cell cycle and apoptosis. However, these
biological roles are cell type-specific. For example, ILF2 is lowly
expressed in the liver, heart, lung, skeletal muscle and spleen,
whereas it is highly expressed in kidney, testis, thymus and brain
(35). The results from the present
study demonstrated that ILF2 high expression was associated with
NSCLC cell detachment and survival.
Previous studies reported abnormal ILF2 expression
in cervical and colorectal cancers (36,37). By
analyzing Kaplan-Meier prognosis data of NSCLC, it was demonstrated
that high ILF2 expression was associated with poor prognosis in the
present study. A series of experiments were conducted to examine
the specific functions of ILF2 in NSCLC cell lines here. The
results demonstrated that ILF2 was highly expressed in NSCLC cells
and reduced adhesion in cells, and promoted cell survival
independently of anchorage. In addition, ILF2 achieved these
functions by binding to the upstream regulatory region of the
PTEN gene to inhibit its expression.
Although the present study was not the first to
explore the function of ILF2 in NSCLC cells, novel features have
been demonstrated. To the best of our knowledge, this study was the
first to explore the association between the transcription factor
ILF2 and the tumor suppressor gene PTEN in NSCLC cell lines.
These results may aid scientists and clinicians to better
understand NSCLC in order to provide novel molecular targets for
diagnosis and treatment.
The present study demonstrated that high expression
of ILF2 could cause weak cell adhesion to the extracellular matrix
and anoikis resistance. These two phenomenons may be influenced by
the ILF2/PTEN pathway. A previous study revealed that cells reduced
adhesion and bypassed anoikis by down-regulating the
integrin-signaling pathway (38).
ILF2/PTEN pathway may also achieve these functions by affecting the
integrin signaling pathway, which requires further investigation.
Integrin expression and related signaling pathways will be examined
in a future study.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors contributions
TL designed the present study, and prepared, edited
and reviewed the manuscript. NL, HL and LFZ performed the
experiments. NL, LPC and MYG acquired the data. NL, WKH and QZQ
analyzed the data. QGM, JHZ and JZ performed the statistical
analysis.
Ethics approval and consent to
participate
Not applicable.
Patients consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
NSCLC
|
non-small cell lung cancer
|
|
PTEN
|
phosphatase and tensin homolog deleted
on chromosome ten
|
|
ILF2
|
interleukin enhancer-binding factor
2
|
|
ChIP
|
chromatin immunoprecipitation
|
|
ENCODE
|
Encyclopedia of DNA Elements
|
References
|
1
|
Fernandez LE, Gabri MR, Guthmann MD, Gomez
RE, Gold S, Fainboim L, Gomez DE and Alonso DF: NGcGM3 ganglioside:
A privileged target for cancer vaccines. Clin Dev Immunol.
2010:8143972010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ladanyi M and Pao W: Lung adenocarcinoma:
Guiding EGFR-targeted therapy and beyond. Mod Pathol. 21 (Suppl
2):S16–S22. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhan P, Wang J, Lv XJ, Wang Q, Qiu LX, Lin
XQ, Yu LK and Song Y: Prognostic value of vascular endothelial
growth factor expression in patients with lung cancer: A systematic
review with meta-analysis. J Thorac Oncol. 4:1094–1103. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wyllie AH: ‘Where, O death, is thy sting?’
A brief review of apoptosis biology. Mol Neurobiol. 42:4–9. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ruoslahti E and Reed JC: Anchorage
dependence, integrins, and apoptosis. Cell. 77:477–478. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cheng TL, Symons M and Jou TS: Regulation
of anoikis by Cdc42 and Rac1. Exp Cell Res. 295:497–511. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Frankel A, Rosen K, Filmus J and Kerbel
RS: Induction of anoikis and suppression of human ovarian tumor
growth in vivo by down-regulation of Bcl-X(L). Cancer Res.
61:4837–4841. 2001.PubMed/NCBI
|
|
9
|
Yamada KM and Araki M: Tumor suppressor
PTEN: Modulator of cell signaling, growth, migration and apoptosis.
J Cell Sci. 114:2375–2382. 2001.PubMed/NCBI
|
|
10
|
Zhan M, Zhao H and Han ZC: Signalling
mechanisms of anoikis. Histol Histopathol. 19:973–983.
2004.PubMed/NCBI
|
|
11
|
Huang Q, He X, Qiu X, Liu X, Sun G, Guo J,
Ding Z, Yang L, Ban N, Tao T and Wang D: Expression of NF45
correlates with malignant grade in gliomas and plays a pivotal role
in tumor growth. Tumour Biol. 35:10149–10157. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ni T, Mao G, Xue Q, Liu Y, Chen B, Cui X,
Lv L, Jia L, Wang Y and Ji L: Upregulated expression of ILF2 in
non-small cell lung cancer is associated with tumor cell
proliferation and poor prognosis. J Mol Histol. 46:325–335. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ni S, Zhu J, Zhang J, Zhang S, Li M, Ni R,
Liu J, Qiu H, Chen W, Wang H and Guo W: Expression and clinical
role of NF45 as a novel cell cycle protein in esophageal squamous
cell carcinoma (ESCC). Tumour Biole. 36:747–756. 2015. View Article : Google Scholar
|
|
14
|
Haselmann V, Kurz A, Bertsch U, Hübner S,
Olempska-Müller M, Fritsch J, Häsler R, Pickl A, Fritsche H,
Annewanter F, et al: Nuclear death receptor TRAIL-R2 inhibits
maturation of let-7 and promotes proliferation of pancreatic and
other tumor cells. Gastroenterology. 146:278–290. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wan C, Gong C, Ji L, Liu X, Wang Y, Wang
L, Shao M, Yang L, Fan S, Xiao Y, et al: NF45 overexpression is
associated with poor prognosis and enhanced cell proliferation of
pancreatic ductal adenocarcinoma. Mol Cell Biochem. 410:25–35.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee YY, McKinney KQ, Ghosh S, Iannitti DA,
Martinie JB, Caballes FR, Russo MW, Ahrens WA, Lundgren DH, Han DK,
et al: Subcellular tissue proteomics of hepatocellular carcinoma
for molecular signature discovery. J Proteome Res. 10:5070–5083.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li J, Yen C, Liaw D, Podsypanina K, Bose
S, Wang SI, Puc J, Miliaresis C, Rodgers L, McCombie R, et al:
PTEN, a putative protein tyrosine phosphatase gene mutated in human
brain, breast, and prostate cancer. Science. 275:1943–1947. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Datta SR, Brunet A and Greenberg ME:
Cellular survival: A play in three Akts. Genes Dev. 13:2905–2927.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sulis ML and Parsons R: PTEN: From
pathology to biology. Trends Cell Biol. 13:478–483. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tamura M, Gu J, Matsumoto K, Aota S,
Parsons R and Yamada KM: Inhibition of cell migration, spreading,
and focal adhesions by tumor suppressor PTEN. Science.
280:1614–1617. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhong H, Chiles K, Feldser D, Laughner E,
Hanrahan C, Georgescu MM, Simons JW and Semenza GL: Modulation of
hypoxia-inducible factor 1alpha expression by the epidermal growth
factor/phosphatidylinositol 3-kinase/PTEN/AKT/FRAP pathway in human
prostate cancer cells: Implications for tumor angiogenesis and
therapeutics. Cancer Res. 60:1541–1545. 2000.PubMed/NCBI
|
|
22
|
Li X, Xu Z, Du W, Zhang Z, Wei Y, Wang H,
Zhu Z, Qin L, Wang L, Niu Q, et al: Aiolos promotes anchorage
independence by silencing p66Shc transcription in cancer cells.
Cancer Cell. 25:575–589. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu CY, Takemasa A, Liles WC, Goodman RB,
Jonas M, Rosen H, Chi E, Winn RK, Harlan JM and Chuang PI:
Broad-spectrum caspase inhibition paradoxically augments cell death
in TNF-alpha-stimulated neutrophils. Blood. 101:295–304. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu Z and Garrard WT: Long-range
interactions between three transcriptional enhancers, active Vkappa
gene promoters, and a 3′ boundary sequence spanning 46 kilobases.
Mol Cell Biol. 25:3220–3231. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Győrffy B, Surowiak P, Budczies J and
Lánczky A: Online survival analysis software to assess the
prognostic value of biomarkers using transcriptomic data in
non-small-cell lung cancer. PLoS One. 8:e822412013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Di Cristofano A and Pandolfi PP: The
multiple roles of PTEN in tumor suppression. Cell. 100:387–390.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shi L, Qiu D, Zhao G, Corthesy B,
Lees-Miller S, Reeves WH and Kao PN: Dynamic binding of Ku80, Ku70
and NF90 to the IL-2 promoter in vivo in activated T-cells. Nucleic
Acids Res. 35:2302–2310. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Reck M, Heigener DF, Mok T, Soria JC and
Rabe KF: Management of non-small-cell lung cancer: Recent
developments. Lancet. 382:709–719. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Goldstraw P, Ball D, Jett JR, Le Chevalier
T, Lim E, Nicholson AG and Shepherd FA: Non-small-cell lung cancer.
Lancet. 378:1727–1740. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tan WL, Jain A, Takano A, Newell EW, Iyer
NG, Lim WT, Tan EH, Zhai W, Hillmer AM, Tam WL and Tan DSW: Novel
therapeutic targets on the horizon for lung cancer. Lancet Oncol.
17:e347–e362. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Karmakar S, Mahajan MC, Schulz V, Boyapaty
G and Weissman SM: A multiprotein complex necessary for both
transcription and DNA replication at the β-globin locus. EMBO J.
29:3260–3271. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shamanna RA, Hoque M, Lewis-Antes A, Azzam
EI, Lagunoff D, Pe'ery T and Mathews MB: The NF90/NF45 complex
participates in DNA break repair via nonhomologous end joining. Mol
Cell Biol. 31:4832–4843. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Volk N and Shomron N: Versatility of
MicroRNA biogenesis. PLoS One. 6:e193912011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhao G, Shi L, Qiu D, Hu H and Kao PN:
NF45/ILF2 tissue expression, promoter analysis, and interleukin-2
transactivating function. Exp Cell Res. 305:312–323. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shim C, Zhang W, Rhee CH and Lee JH:
Profiling of differentially expressed genes in human primary
cervical cancer by complementary DNA expression array. Clin Cancer
Res. 4:3045–3050. 1998.PubMed/NCBI
|
|
37
|
Chung FH, Lee HH and Lee HC: ToP: A
trend-of-disease- progression procedure works well for identifying
cancer genes from multi-state cohort gene expression data for human
colorectal cancer. PLoS One. 8:e656832013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Overholtzer M, Mailleux AA, Mouneimne G,
Normand G, Schnitt SJ, King RW, Cibas ES and Brugge JS: A
nonapoptotic cell death process, entosis, that occurs by
cell-in-cell invasion. Cell. 131:966–979. 2007. View Article : Google Scholar : PubMed/NCBI
|