Introduction
Gastric cancer is one of the most common causes of
cancer-associated mortality (1).
Despite significant improvements in therapy, including neoadjuvant
chemoradiotherapy, advanced surgical techniques and novel target
drugs, gastric cancer has a high tendency for recurrence (2). Furthermore, the progression and genetic
characteristics of gastric cancer remain unclear. In recent
decades, it has been revealed that gastric cancer results from
multi-gene alterations in a number of signaling pathways. A deeper
understanding of gastric cancer-associated genes may improve the
diagnosis and treatment of the disease.
The gap junction α (GJA) genes encode connexin (Cx)
proteins. Cxs compose cell gap junctions, facilitating
intercellular communication to regulate a number of cell functions,
including cell death, proliferation and differentiation. To date,
12 Cx proteins, GJA1-GJA12, have been identified. These proteins
are expressed in different tissues to varying degrees (3,4), and the
genetic and biological features of each Cx can differ. These
proteins have been demonstrated to be involved in cancer
development, metastasis and the regulation of cancer cell survival
during radiotherapy (5–9). Several studies have indicated that the
GJA family serve an important role in gastric cancer, particularly
in Helicobacter pylori infection-associated and
intestinal-type gastric cancer (5,6).
However, these studies focused primarily on in vitro
investigations of the molecular mechanisms of these genes in
gastric cancer; their roles in clinical practice are largely
unknown.
In the present study, the prognostic roles of GJA
mRNA expression levels were assessed in patients with gastric
cancer using the Kaplan-Meier plotter (KM plotter, http://kmplot.com/analysis/index.php?p=service&cancer=gastric)
and Gene expression profiling interactive analysis (GEPIA,
http://gepia.cancer-pku.cn) platforms.
The Kaplan-Meier plotter is an open database within the Gene
Expression Omnibus (www.ncbi.nlm.nih.gov/geo/) that collects gene
expression and survival data from patients with gastric, lung and
breast cancer. GEPIA is an online tool for extracting RNA
sequencing data from The Cancer Genome Atlas (TCGA) and
Genotype-Tissue Expression (GTEx) databases. The use of these
online tools revealed a significant association between GJA mRNA
expression levels and the survival of patients with gastric
cancer.
Materials and methods
The association between GJA mRNA expression level
and patient overall survival (OS) was determined using the
Kaplan-Meier plotter (http://kmplot.com/analysis/index.php?p=service&cancer=gastric)
(10), which includes the data of
876 patients with gastric cancer. A total of 593 patients from the
GSE14210, GSE15459, GSE22377, GSE29272 and GSE51105 datasets were
assessed; the GSE62254 database, which exhibited markedly different
patient characteristics to the other datasets, was excluded. Using
the Kaplan-Meier plotter, the data associated with different
clinicopathological variables, including sex, tumor stage, Lauren
classification, tumor differentiation and human epidermal growth
factor receptor 2 (Her-2) status were collected. Data regarding GJA
gene family members, including GJA1, GJA3, GJA4, GJA10 and GJA12
was also assessed, and the mRNA expression levels of these members
were analyzed. P<0.05 was considered to indicate a statistically
significant difference. All possible cutoff values between the
lower and upper quartiles were determined, and the best performing
threshold used as the cutoff.
The mRNA expression levels of tumor and normal
tissues were analyzed using the GEPIA database (http://gepia.cancer-pku.cn), which contains the data
of 9,736 tumors and 8,587 normal samples from TCGA and GTEx
datasets. The expression levels of GJA1, GJA3, GJA4 and GJA12 in
619 patients with gastric cancer were assessed using GEPIA. The
expression levels in tumor and normal tissues at different disease
stages were displayed as boxplots and stage plots, for which the
method of differential analysis was one-way ANOVA. For the
boxplots, disease state (tumor or normal) was used as the variable
for calculating differential expression; pathological stage was
used for the stage plots. P<0.05 was considered to indicate a
statistically significant difference.
Results
Differential expression of GJA members
between gastric cancer and normal tissues
In the present study, the prognostic value of GJAs
in gastric cancer was assessed. The GEPIA was used to determine the
mRNA expression levels of GJA1, GJA3, GJA4, GJA10 and GJA12 in
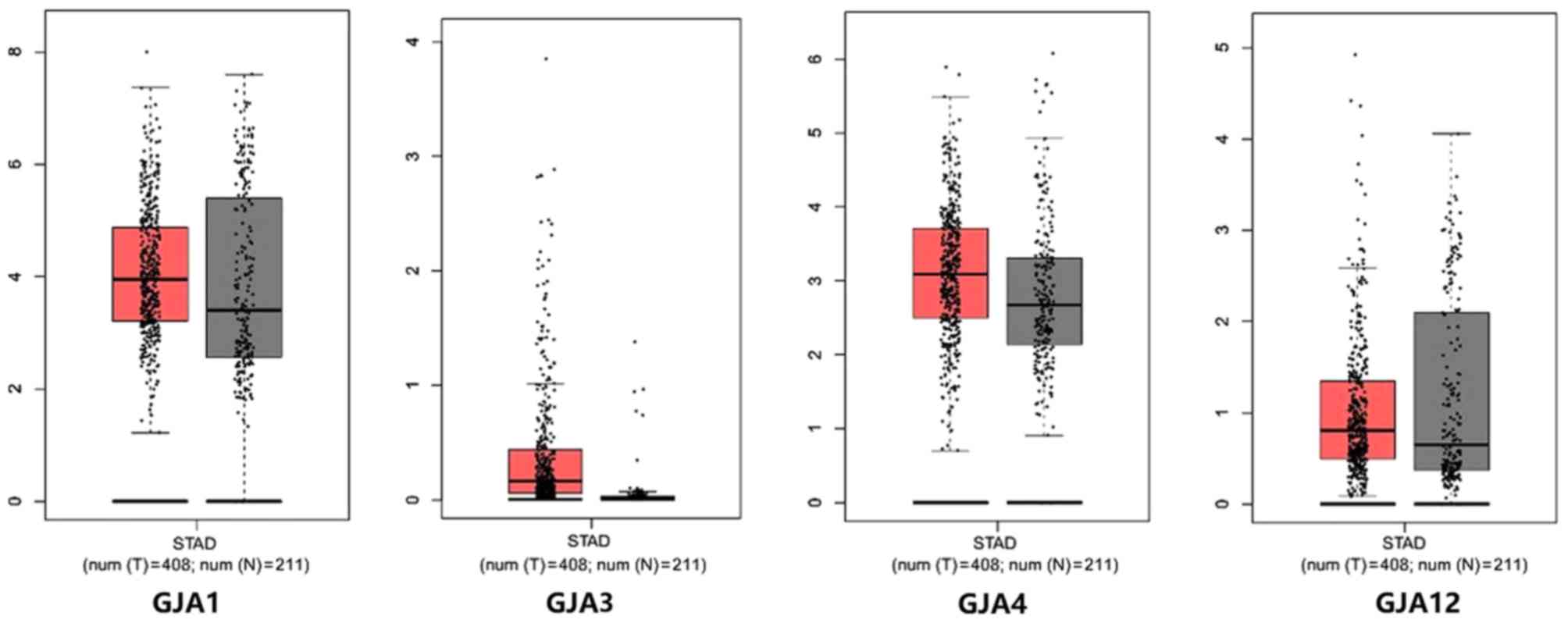
patients with gastric cancer. The median expression levels of GJA1,
GJA3, GJA4 and GJA12 were determined (Fig. 1). However, the expression data for
GJA10 was not collected from the GEPIA database. Notably, GJA3 and
GJA4 exhibited a higher expression level in gastric cancer tissues
(Fig. 1). Furthermore, the mRNA
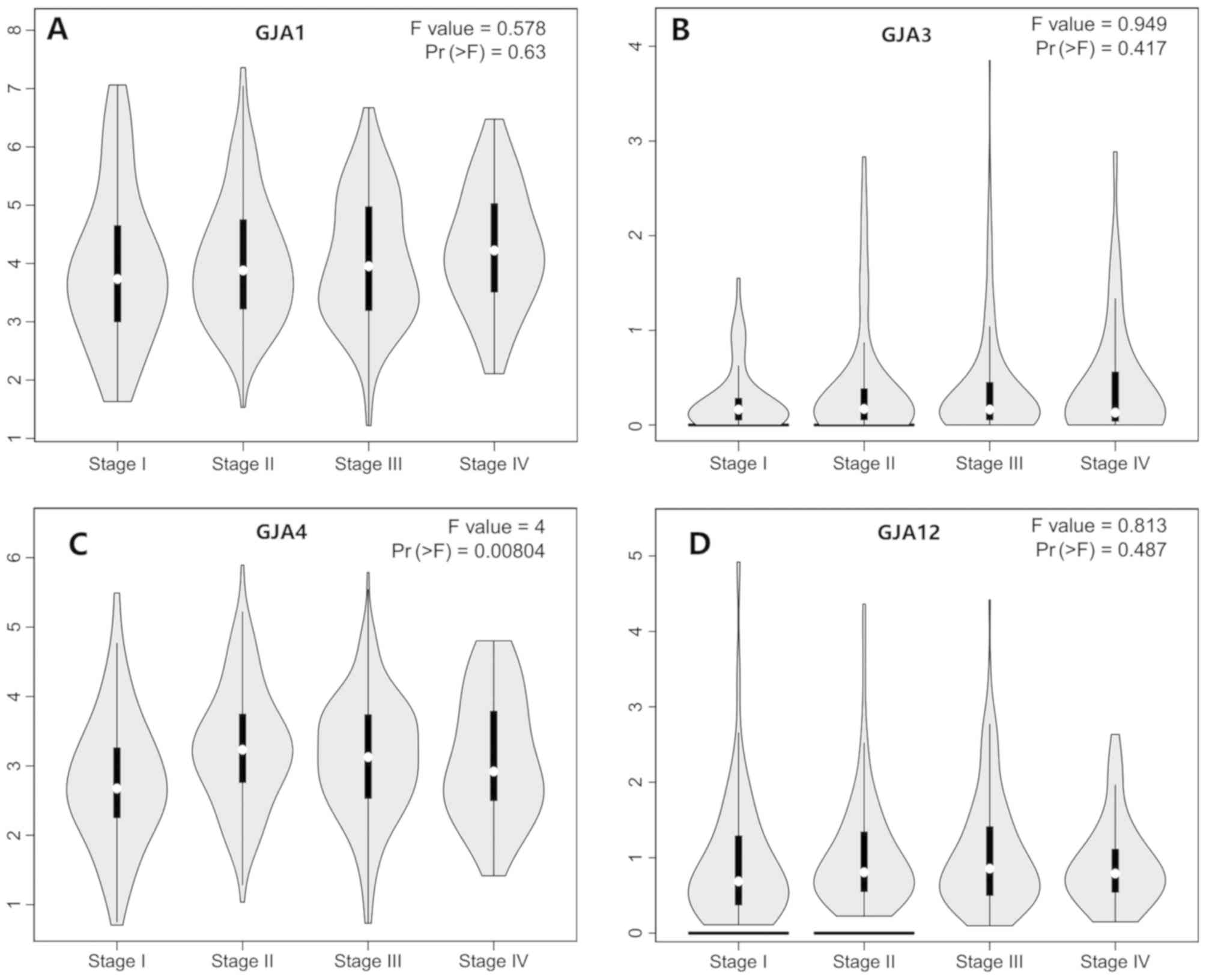
expression levels of GJAs at different tumor stages were assessed
and are indicated as stage plots (Fig.
2). Notably, there was a statistically significant association
between GJA4 expression and the different pathological stages
(Fig. 2C). No significant
association was revealed for the other family members (Fig. 2A, B and D). These findings indicated
that specific GJA members were differentially expressed in gastric
cancer tissues compared with normal tissues.
Prognostic value of GJA members in
gastric cancer
The Kaplan-Meier plotter was used to assess the
prognostic value of GJA members in gastric cancer; patient
characteristics are listed in Table
I. The data revealed that the mRNA expression levels of GJA1,
GJA4, GJA10 and GJA12 were significantly associated with OS in
patients with gastric cancer (Fig.
3). High mRNA expression levels of GJA1 and GJA10 were
associated with poorer OS (Fig. 1A and
C), whereas high mRNA expression levels of GJA4 and GJA12 were
associated with improved OS (Fig. 1B and
D). Regarding GJA3, no significant difference was indicated
between mRNA expression level and OS (HR=1.27; 95% CI=0.92–1.75;
P=0.14). In conclusion, GJA1, GJA4, GJA10 and GJA12 were associated
with the survival outcome of patients with gastric cancer.
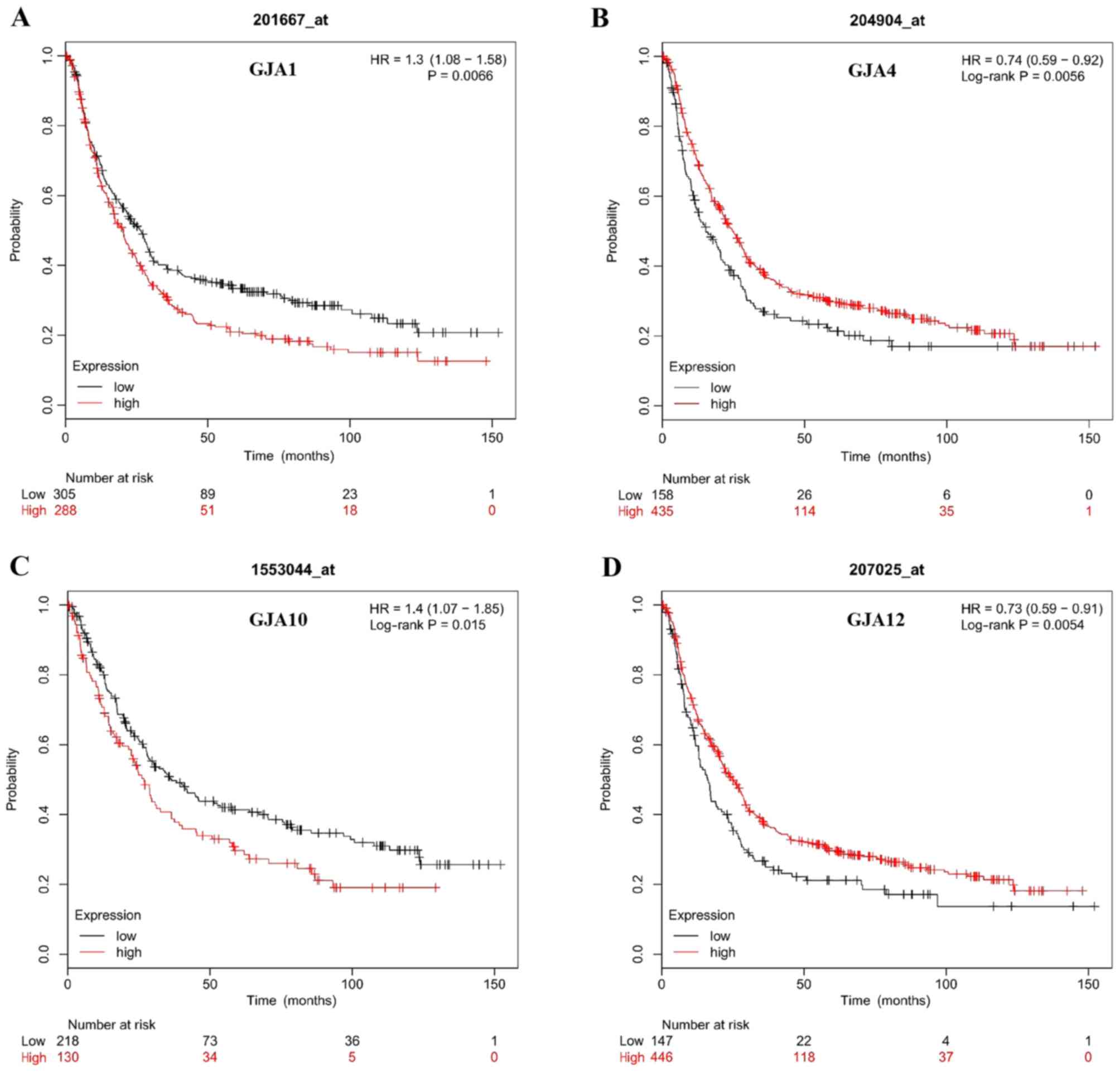 | Figure 3.Association between GJA members and
the occurrence of gastric cancer. (A) Survival curve of GJA1
(Affymetrix IDs: 20167_at, HR=1.3; 95% CI, 1.08–1.58; P=0.0066).
(B) Survival curve of GJA4 (Affymetrix IDs: 204904_at, HR=0.74; 95%
CI, 0.59–0.92; P=0.0056). (C) Survival curve of GJA10 (Affymetrix
IDs: 1553044_at, HR=1.4; 95% CI, 1.07–1.85; P=0.015). (D) Survival
curve of GJA12 (Affymetrix IDs: 207025_at, HR=0.73; 95% CI,
0.59–0.91; P=0.0054). GJA, gap junction α; HR, hazard ratio; CI,
confidence interval. |
 | Table I.Characteristics of patients with
gastric cancer in the Kaplan-Meier plotter database. |
Table I.
Characteristics of patients with
gastric cancer in the Kaplan-Meier plotter database.
|
Characteristics | Patients, n | Ratio |
|---|
| Age |
|
<65 | 83 | 0.43 |
|
≥65 | 109 | 0.57 |
| Sex |
|
Male | 567 | 0.69 |
|
Female | 244 | 0.31 |
| Stage |
| I | 69 | 0.10 |
| II | 145 | 0.21 |
|
III | 319 | 0.47 |
| IV | 152 | 0.22 |
| T Stage |
| 1 | 14 | 0.03 |
| 2 | 253 | 0.49 |
| 3 | 208 | 0.40 |
| 4 | 39 | 0.08 |
| N Stage |
| 0 | 76 | 0.15 |
| 1 | 232 | 0.45 |
| 2 | 129 | 0.25 |
| 3 | 76 | 0.15 |
| M Stage |
| 0 | 459 | 0.74 |
| 1 | 58 | 0.26 |
| Her-2 status |
|
Positive | 425 | 0.40 |
|
Negative | 641 | 0.60 |
| Lauren
classification |
|
Intestinal | 336 | 0.54 |
|
Diffuse | 248 | 0.40 |
|
Mixed | 33 | 0.06 |
|
Differentiation |
|
Poor | 166 | 0.63 |
|
Moderate | 67 | 0.25 |
|
Well | 32 | 0.12 |
Prognostic value of GJA members in
patients with different pathological characteristics
To further understand the association between the
mRNA expression levels of GJAs and survival outcome, the OS of
patients with different pathological characteristics was evaluated,
including Her-2 status, Lauren classification, tumor
differentiation status and tumor-node-metastasis (TNM) stage. As
indicated in Fig. 4, the prognostic
value of GJAs with Her-2 status was determined. A high GJA1 and
GJA10 mRNA expression level was associated with poorer OS in
Her-2-positive patients (Fig. 4A and
C), whilst low GJA4 and GJA12 expression levels were associated
with reduced survival time in Her-2-negative patients (Fig. 4E and F).
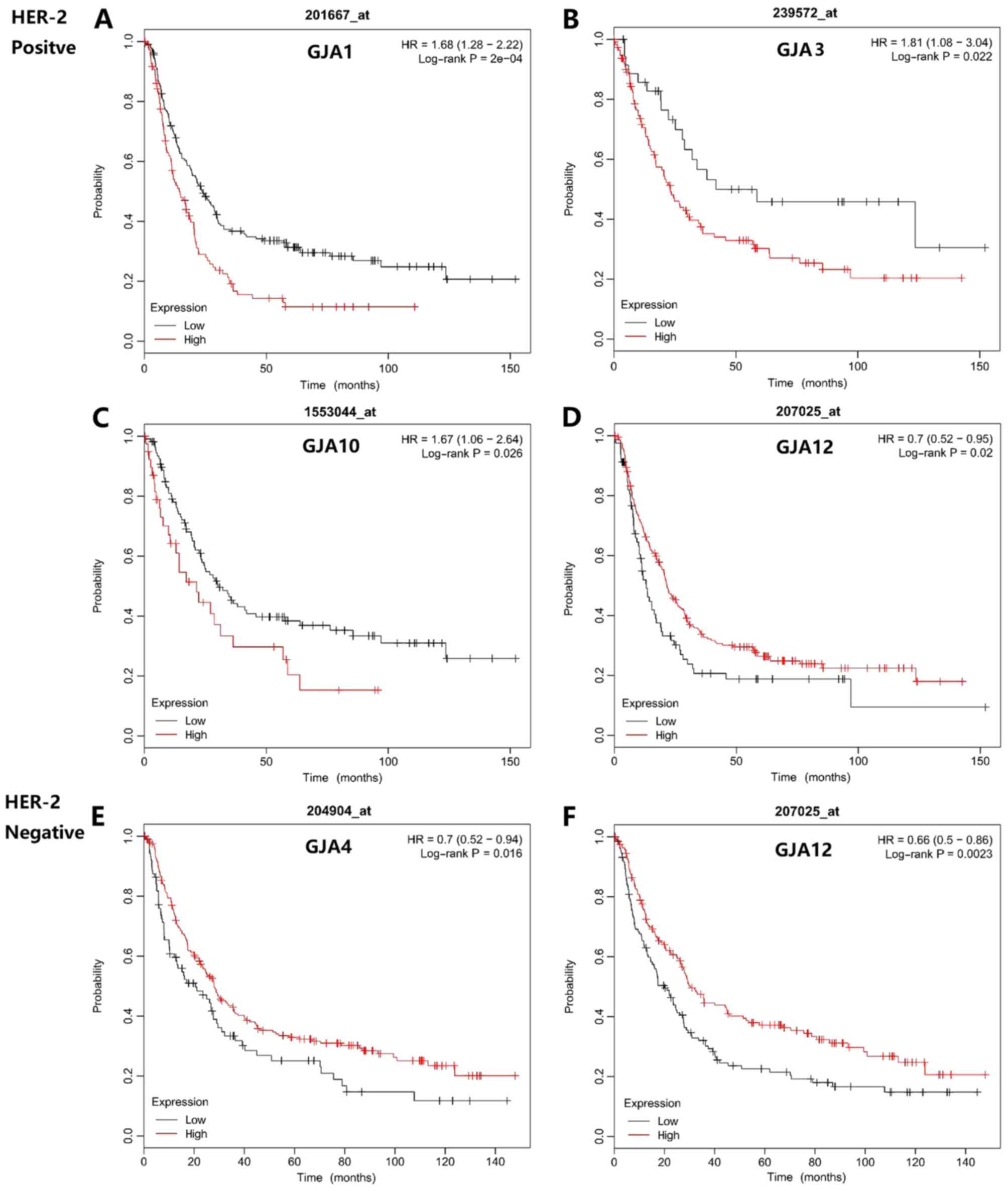 | Figure 4.Association between mRNA expression
levels of GJA members and the overall survival of Her-2-positive
and -negative patients with gastric cancer. For Her-2-positive
patients, the prognostic relevance was revealed for (A) GJA1
(HR=1.68; 95% CI, 1.28–2.22; P=0.0002), (B) GJA3 (Affymetrix IDs:
239572_at, HR=1.81; 95% CI, 1.08–3.04; P=0.022), (C) GJA10
(HR=1.67; 95% CI, 1.06–2.64; P=0.026) and (D) GJA12 (HR=0.7; 95%
CI, 0.52–0.95; P=0.02) expression. For (E) GJA4 (HR=0.70; 95% CI,
0.52–0.94; P=0.016) and (F) GJA12 (HR=0.66; 95% CI, 0.5–0.86;
P=0.0023) expression, the differences in prognosis were presented
in Her-2-negative patients. GJA, gap junction α; HR, hazard ratio;
CI, confidence interval; Her-2, human epidermal growth factor
receptor 2. |
The Lauren classification is used to define gastric
cancer into intestinal, diffuse and mixed subtypes. The prognostic
value of GJA members in different Lauren classifications was
revealed (Fig. 5). A high GJA1 mRNA
expression level was associated with poorer OS in patients with
intestinal and diffuse type gastric cancer (Fig. 5A and B). An elevated GJA3 mRNA
expression level was associated with poorer OS in patients with
intestinal type gastric cancer (Fig.
5C). Furthermore, high GJA10 mRNA expression level was also
associated with poorer OS time in patients with intestinal or
diffuse type gastric cancer (Fig. 5D and
E).
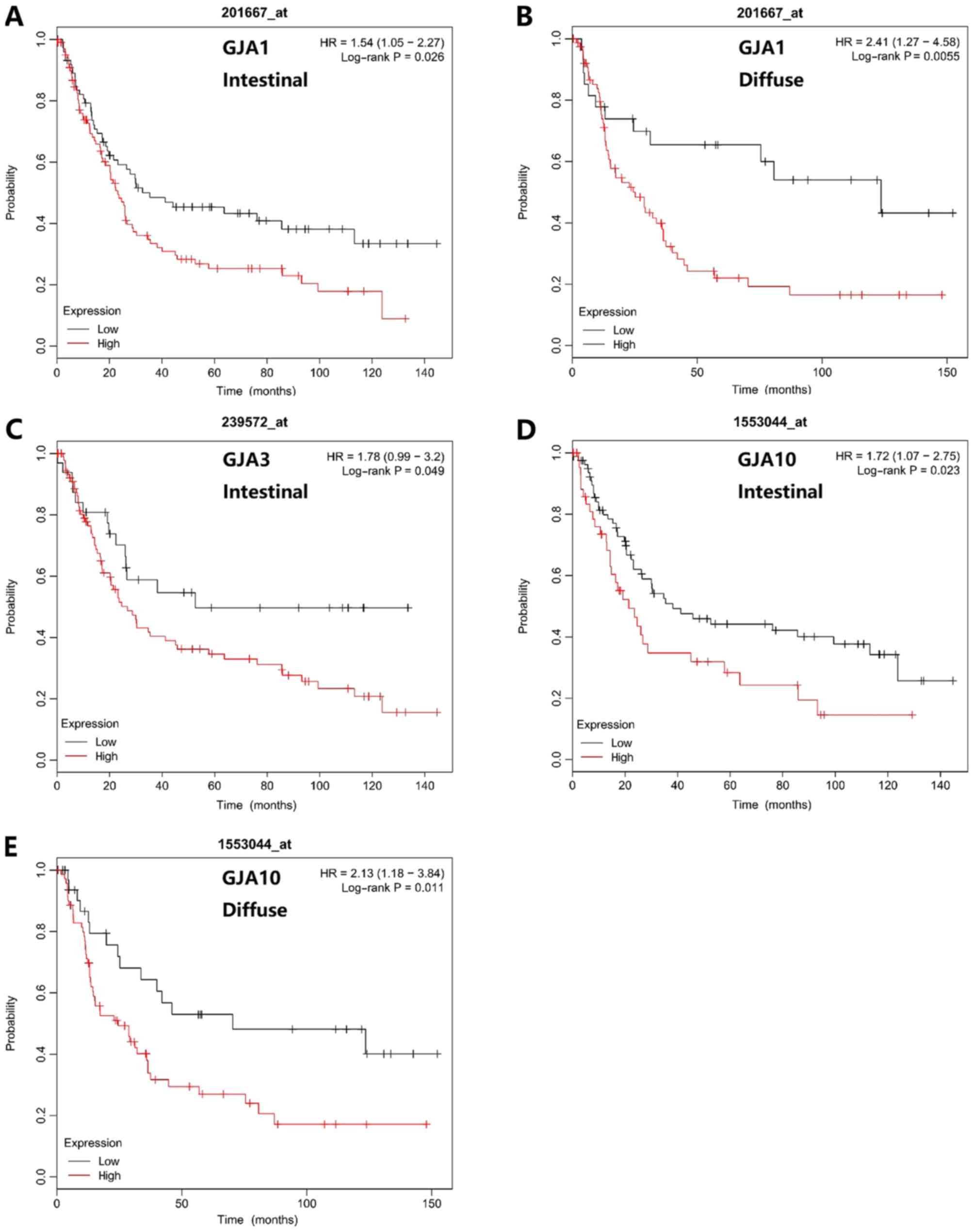 | Figure 5.Association between mRNA expression
levels of GJA members and the overall survival of patients with
different Lauren classifications. (A and B) GJA1 (HR=1.54; 95% CI,
1.05–2.27; P=0.026; and HR=2.41; 95% CI, 1.27–4.58; P=0.0055), (C)
GJA3 (HR=1.78; 95% CI, 0.99–3.2; P=0.049) and (D and E) GJA10
(HR=1.72; 95% CI, 1.07–2.75; P=0.023, and HR=2.13; 95% CI,
1.18–3.84; P=0.011) mRNA expression levels exhibited prognostic
values for different Lauren differentiations. GJA, gap junction α;
HR, hazard ratio; CI, confidence interval. |
Regarding tumor differentiation, GJA mRNA expression
level also demonstrated prognostic value. As indicated in Fig. 6, High GJA1 mRNA expression was
associated with poorer OS in patients with well- or
moderately-differentiated gastric cancer (Fig. 6A and B). High GJA10 expression level
was associated with poorer OS times in patients with moderately or
poorly differentiated gastric cancer (Fig. 6C and D). GJA12 expression was
significantly associated with the OS in patients with moderately
differentiated tumors (Fig. 6E). All
other conditions not indicated in the figure. were analyzed, but
had no statistical significance in terms of prognostic value.
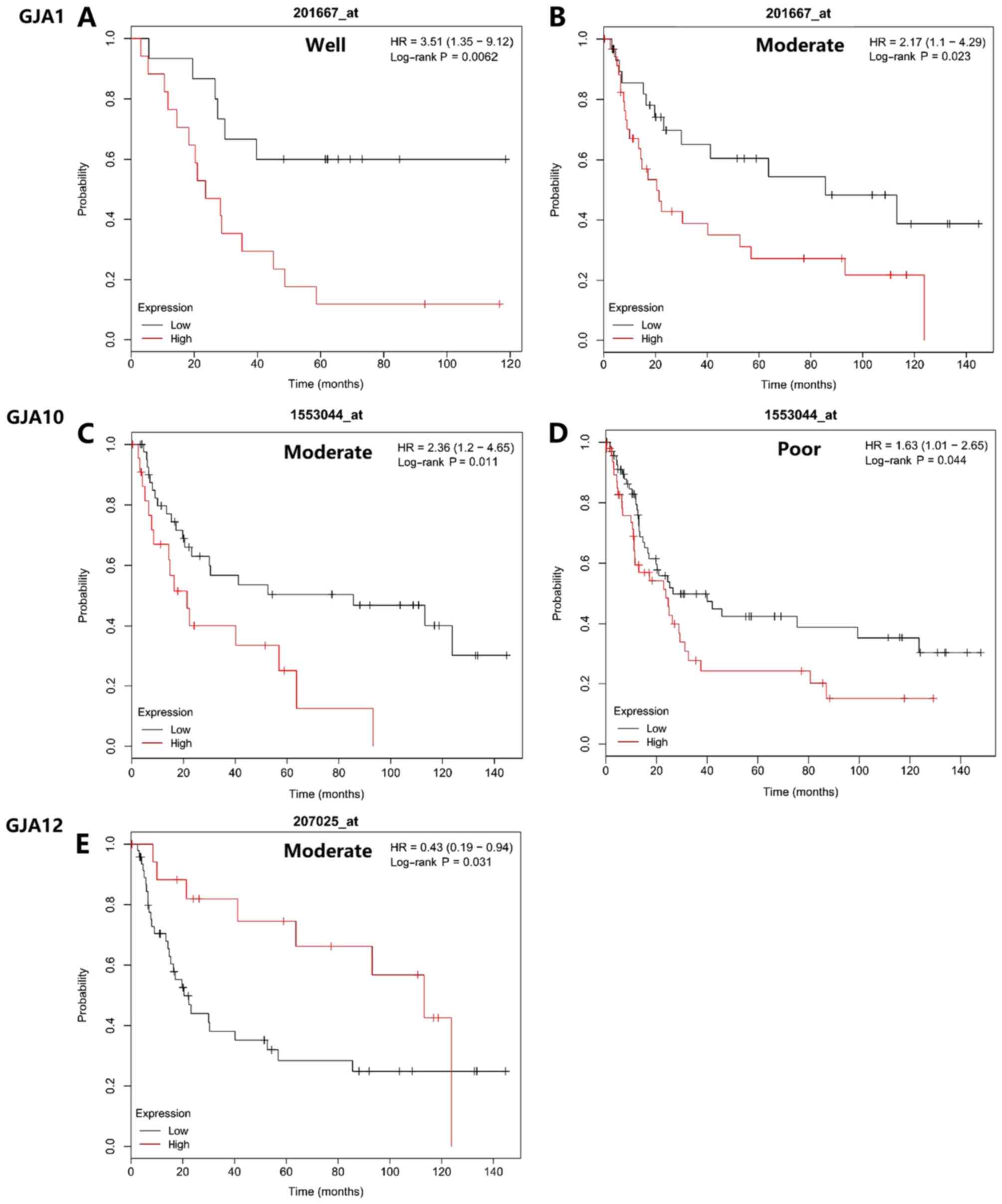 | Figure 6.Association between mRNA expression
levels of GJA members and the overall survival of patients with
different pathological differentiations. Statistically significant
differences were revealed regarding (A and B) GJA1 (HR=3.51; 95%
CI, 1.35–9.12; P=0.0062, and HR=2.17; 95% CI, 1.1–4.29; P=0.023),
(C and D) GJA10 (HR=2.36; 95% CI, 1.2–4.65; P=0.011, and HR=1.63;
95% CI, 1.01–2.65; P=0.044) and (E) GJA12 (HR=0.43; 95% CI,
0.19–0.94; P=0.031). GJA, gap junction α; HR, hazard ratio; CI,
confidence interval. |
For different gastric cancer TNM stages, the GJA
mRNA expression level was of prognostic value on a number of
levels. As indicated in Table II,
some of the GJA members were associated with prognosis in different
stages of gastric cancer. Among those listed, GJA1, GJA10 and GJA12
demonstrated the most significant association with gastric cancer
survival outcome. Notably, the number of stage T1 and T4 patients
was too small to be valid for analysis in the KM plotter
database.
 | Table II.Association between mRNA expression
levels of GJA members and the overall survival of patients with
gastric cancer at different Tumor-Node-Metastasis stages. |
Table II.
Association between mRNA expression
levels of GJA members and the overall survival of patients with
gastric cancer at different Tumor-Node-Metastasis stages.
| Variable | mRNA | HR | 95% CI | Log rank
P-value |
|---|
| T2 (n=66) | GJA1 | 2.34 | 1.04–5.27 | 0.04a |
|
| GJA3 | 1.85 | 0.9–3.8 | 0.09a |
|
| GJA4 | 1.71 | 0.82–3.56 | 0.15 |
|
| GJA10 | 2.5 | 1.2–5.22 | 0.01a |
|
| GJA12 | 0.41 | 0.19–0.86 | 0.02a |
| T3 (n=117) | GJA1 | 1.43 | 0.93–2.2 | 0.11 |
|
| GJA3 | 0.69 | 0.44–1.1 | 0.12 |
|
| GJA4 | 0.66 | 0.43–1.03 | 0.06 |
|
| GJA10 | 1.71 | 1.09–2.7 | 0.02a |
|
| GJA12 | 0.43 | 0.27–0.7 |
<0.01b |
| N0 (n=38) | GJA1 | 1.56 | 0.44–5.48 | 0.49 |
|
| GJA3 | 0.53 | 0.19–1.49 | 0.22 |
|
| GJA4 | 2.89 | 0.65–12.77 | 0.14 |
|
| GJA10 | 4.3 | 1.58–11.75 |
<0.01b |
|
| GJA12 | 0.5 | 0.18–1.41 | 0.18 |
| N1+2+3 (n=175) | GJA1 | 2.16 | 1.43–3.27 |
<0.01b |
|
| GJA3 | 1.38 | 0.92–2.07 | 0.11 |
|
| GJA4 | 0.79 | 0.52–1.2 | 0.27 |
|
| GJA10 | 1.25 | 0.85–1.84 | 0.25 |
|
| GJA12 | 0.61 | 0.41–0.92 | 0.02a |
| M0 (n=186) | GJA1 | 1.93 | 1.27–2.92 |
<0.01b |
|
| GJA3 | 1.29 | 0.87–1.9 | 0.2 |
|
| GJA4 | 0.68 | 0.43–1.09 | 0.11 |
|
| GJA10 | 1.49 | 1–2.22 | 0.05 |
|
| GJA12 | 0.47 | 0.31–0.72 |
<0.01b |
| M1 (n=31) | GJA1 | 1.4 | 0.63–3.11 | 0.4 |
|
| GJA3 | 0.58 | 0.26–1.29 | 0.18 |
|
| GJA4 | 1.61 | 0.73–3.54 | 0.23 |
|
| GJA10 | 0.47 | 0.19–1.16 | 0.09 |
|
| GJA12 | 1.97 | 0.73–5.31 | 0.17 |
Discussion
The GJA gene family encodes Cxs, which compose cell
hemi-channels, connexons and gap junction channels (11–13). It
has been reported that these gap junction structures are involved
in several cellular responses under physiological and pathological
states (14). GJA-encoded Cxs are
widely distributed in the human body and are abundantly expressed
in gastrointestinal cells. Among the GJA family members, GJA1
(Cx43) and GJA4 (Cx37) have been confirmed to be expressed in
normal gastrointestinal cells (15).
However, Cx isotypes not identified in normal tissue may be
detected in associated cancers (16). In gastric cancer cells, GJA has been
demonstrated to be associated with intestinal type gastric cancer
(17) and Helicobacter
pylori-associated gastric tumorigenesis (18,19);
however, the functional mechanism remains unclear. According to a
recent study on the GJA family members, the majority of studies
focus on the molecular mechanisms and pathological progression of
GJA. However, the number of studies on the clinical value of GJA
was limited.
Among the GJA members investigated in the present
study, GJA1 was the most widely reported. GJA1-encoded Cx43 is the
most abundant cx in gastric epithelial and intestinal epithelial
cells (15). Cx43 is a component of
cell gap junctions that serves an important role in intercellular
communication, cell-cell channel formation and the exchange of
signaling molecules (20). Several
hypotheses and potential mechanisms of Cx43-associated gastric
cancer have been reported. Cx43 may induce Helicobacter
pylori-associated gastric cancer via VacA-induced cell death
(20,21). Additionally, Tang et al
(22,23) reported that the abnormal expression
of Cx43 may promote lymph node metastasis and peritoneal metastasis
of gastric cancer through Cx43-mediated heterocellular
gap-junctional intercellular communication. Furthermore, Liu et
al (24) suggested that Cx43 may
increase the chemotherapeutic sensitivity of gastric cancer cells.
These in vitro studies suggest an association between GJA1
and gastric cancer. In the present study, a high GJA1 mRNA
expression level was associated with a poorer OS in patients with
gastric cancer, particularly in those that were also
Her-2-positive. Previous results have indicated that GJA1 enhances
tumor proliferation and migration in Her-2-positive patients with
breast cancer (25), which suggests
a mutual effect between Her-2 and GJA1. In intestinal and diffuse
types of gastric cancer, GJA1 was also associated with a poorer
patient outcome.
GJA3 is primarily distributed in heart and
testicular tissues but is also expressed at low levels in the
stomach. It is associated with various types of breast cancer and
cataract (26–29), and also has a distinct prognostic
value for breast cancer (26,29).
However, no scientific study has been published concerning its
association with gastric cancer. GJA4 has been reported to
participate in tumor proliferation (30) and growth suppression (31); Jing et al (32) reported that GJA4 was correlated with
gastric cancer and that small interfering RNA inhibition of GJA4 in
gastric tumors in mice could promote tumor cell apoptosis. Notably,
there is limited research regarding GJA10. It has been suggested
that the GJA10 (Cx62) is mainly distributed in the retina (33) and may be involved in visual
perception functions (34). To the
best of our knowledge, no study has reported the role of GJA10 in
gastric cancer. GJA12 is methylated in the carcinogenesis of
Barrett's esophagus, which indicates its potential function in
tumor progression (35). GJA12 is
also associated with an increased risk of secondary lymphedema
following breast cancer treatment (36).
GJA family members predominantly mediate
gap-junctional intercellular communication (GJIC), which is the
only way for adjacent cells to exchange signals internally
(37). Gap junctions also serve an
important role in maintaining tissue homeostasis. Deletion of Cx
genes may result in disorders such as cancer and cardiac
malformation (38). Several studies
have indicated reduced GJIC among neighboring cells in malignant
tumor tissues (39–41). Additionally, Ruch et al
(40) suggested that reduced GJIC
promoted cancer cell growth and tumorigenicity, and Trosko et
al (42) demonstrated that one
of the differences between a cancer cell and its normal parental
cell is GJIC-competence. These results suggest the importance of
GJIC in carcinogenesis and regulation of the cell cycle. However,
Yamasaki et al (38)
indicated that the expression of Cxs, rather than GJIC level, is
more closely associated with the control of tumor growth, which
suggests a GJIC-independent function for GJA members.
Although the relatively well-studied GJA1 is
reportedly associated with the carcinogenesis of gastric cancer, in
the present study there were no significant differences in the
expression levels of GJA1 in tumor tissues compared with normal
tissues. Yamasaki et al (39)
identified that Cxs were normally expressed, but aberrantly
localized in many tumor cells, indicating that the dysregulation of
GJIC may be caused by the abnormal expression and localization of
Cxs. Therefore, it was speculated that the differential expression
of GJA members was not the only mechanism in gap
junction-associated carcinogenesis. Tang et al (22) suggested that GJA1-mediated
heterocellular GJIC between gastric cancer cells serves an
essential role in metastasis. These reports indicated that GJA
members were not only involved in cell cycle control of individual
malignant cells, but also neighboring cells that may promote tumor
proliferation and metastasis.
Among the GJA members, it was revealed that GJA1
(Cx43) was most obviously associated with gastric cancer. Her-2
expression in gastric cancer is associated with a poor outcome
(43), and for Her-2-positive
patients with gastric cancer, high GJA1, GJA3 and GJA10 mRNA
expression levels were predictive of poorer OS times, whereas high
GJA12 mRNA expression levels were associated with an improved
outcome. Although several studies have aimed to determine the link
between GJA mRNA expression and Her-2 status in other cancer types
(25,27), there is still a lack of evidence to
explain the potential mechanism in gastric cancer.
For different tumor stages, the prognostic value of
GJA also generated some significant results. It has been indicated
that gap junctions act as suppressors in the early stages of tumor
development; however, in the advanced stages, they may facilitate
tumor progression by promoting the invasion of migrating tumor
cells into the surrounding tissues (5,8,9). These 2 functions are considered to be
the result of different gap junction mechanisms at different tumor
stages (8). In the present study,
the expression of GJA at N and M stage exhibited a significant
difference between N0 and N1+2+3/M0 and M1, which supports previous
research. Several studies have also indicated that Cxs may be
associated with lymph node metastasis (22,36,44).
Kanczuga-Koda et al (44)
suggested that the expression of GJA1 was increased in metastatic
lymph nodes compared with primary breast tumors, and Ezumi et
al (45) found that
GJA1-negative primary colorectal tumors could metastasis into
GJA1-positive tumors in the lymph nodes. The potential role of GJAs
in lymph node metastasis may also explain their differential
expression at different N stages.
The present study had a number of limitations.
Previous studies have confirmed that the relationship between
specific GJA family members and gastric cancer remains unclear.
However, in the present study, the potential joint influence of
these members on patient survival was not analyzed. Additionally,
it is a bioinformatics-based analysis of GEO and TCGA datasets,
with a lack of in vitro and in vivo validation
experiments.
The present study focused on the abnormal expression
of GJAs and their prognostic value in gastric cancer. The findings
suggested that GJA family members are associated with the survival
outcome of patients with gastric cancer; specifically, high GJA1
and GJA10 expression levels were associated with poorer outcomes,
whilst high GJA4 and GJA12 were associated with improved outcomes.
Moreover, for different pathological features, including Her-2
status, Lauren classification, tumor differentiation and TNM stage,
GJA mRNA expression level also exhibited predictive value. These
results indicated the potential of GJA members as gene targets for
the prognosis and treatment of gastric cancer, though further
insights are required to determine their clinical applications and
mechanisms.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81402423, 81572818
and 81871984) and the Shanghai Municipal Commission of Health and
Family Planning (grant no. 2017YQ062).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors contributions
XZ analyzed the mRNA expression and the prognosis
data, and contributed to the writing of the manuscript. CRY
analyzed the bioinformatics data and constructed the figures and
tables. JS and MHZ instructed the whole research study and revised
the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
No applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Balakrishnan M, George R, Sharma A and
Graham DY: Changing trends in stomach cancer throughout the world.
Curr Gastroenterol Rep. 19:362017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Goodenough DA and Paul DL: Gap junctions.
Cold Spring Harb Perspect Biol. 1:a0025762009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sohl G and Willecke K: Gap junctions and
the connexin protein family. Cardiovasc Res. 62:228–232. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Naoi Y, Miyoshi Y, Taguchi T, Kim SJ, Arai
T, Tamaki Y and Noguchi S: Connexin26 expression is associated with
lymphatic vessel invasion and poor prognosis in human breast
cancer. Breast Cancer Res Treat. 106:11–17. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Elzarrad MK, Haroon A, Willecke K,
Dobrowolski R, Gillespie MN and Al-Mehdi AB: Connexin-43
upregulation in micrometastases and tumor vasculature and its role
in tumor cell attachment to pulmonary endothelium. BMC Med.
6:202008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Laird DW: The gap junction proteome and
its relationship to disease. Trends Cell Biol. 20:92–101. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Naus CC and Laird DW: Implications and
challenges of connexin connections to cancer. Nat Rev Cancer.
10:435–441. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Czyz J: The stage-specific function of gap
junctions during tumourigenesis. Cell Mol Biol Lett. 13:92–102.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Szasz AM, Lánczky A, Nagy A, Förster S,
Hark K, Green JE, Boussioutas A, Busuttil R, Szabo A and Gyorffy B:
Cross-validation of survival associated biomarkers in gastric
cancer using transcriptomic data of 1,065 patients. Oncotarget.
7:49322–49333. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jiang JX and Gu S: Gap junction- and
hemichannel-independent actions of connexins. Biochim Biophys Acta.
1711:208–214. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Beyer EC and Berthoud VM: Gap junction
structure: Unraveled, but not fully revealed. F1000Res. 6:5682017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Puebla C, Cisterna BA, Salas DP,
Delgado-Lopez F, Lampe PD and Saez JC: Linoleic acid permeabilizes
gastric epithelial cells by increasing connexin 43 levels in the
cell membrane via a GPR40- and Akt-dependent mechanism. Biochim
Biophys Acta. 1861:439–448. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fasciani I, Temperan A, Perez-Atencio LF,
Escudero A, Martinez-Montero P, Molano J, Gomez-Hernandez JM, Paino
CL, Gonzalez-Nieto D and Barrio LC: Regulation of connexin
hemichannel activity by membrane potential and the extracellular
calcium in health and disease. Neuropharmacology. 75:479–490. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Maes M, Cogliati B, Crespo Yanguas S,
Willebrords J and Vinken M: Roles of connexins and pannexins in
digestive homeostasis. Cell Mol Life Sci. 72:2809–2821. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Carette D, Gilleron J, Chevallier D,
Segretain D and Pointis G: Connexin a check-point component of cell
apoptosis in normal and physiopathological conditions. Biochimie.
101:1–9. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu X, Furuya T, Li D, Xu J, Cao X, Li Q,
Xu J, Xu Z, Sasaki K and Liu X: Connexin 26 expression correlates
with less aggressive phenotype of intestinal type-gastric
carcinomas. Int J Mol Med. 25:709–716. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang Y, Huang LH, Xu CX, Xiao J, Zhou L,
Cao D, Liu XM and Qi Y: Connexin 32 and 43 promoter methylation in
Helicobacter pylori-associated gastric tumorigenesis. World J
Gastroenterol. 20:11770–11779. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu X, Cao K, Xu C, Hu T, Zhou L, Cao D,
Xiao J, Luo L, Guo Y and Qi Y: GATA-3 augmentation down-regulates
Connexin43 in Helicobacter pylori associated gastric
carcinogenesis. Cancer Biol Ther. 16:987–996. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yahiro K, Hirayama T, Moss J and Noda M:
Helicobacter pylori VacA toxin causes cell death by inducing
accumulation of cytoplasmic connexin 43. Cell Death Dis.
6:e19712015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Radin JN, Gonzalez-Rivera C, Frick-Cheng
AE, Sheng J, Gaddy JA, Rubin DH, Algood HM, McClain MS and Cover
TL: Role of connexin 43 in Helicobacter pylori VacA-induced cell
death. Infect Immu. 82:423–432. 2014. View Article : Google Scholar
|
|
22
|
Tang B, Peng ZH, Yu PW, Yu G and Qian F:
Expression and significance of Cx43 and E-cadherin in gastric
cancer and metastatic lymph nodes. Med Oncol. 28:502–508. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tang B, Peng ZH, Yu PW, Yu G, Qian F, Zeng
DZ, Zhao YL, Shi Y, Hao YX and Luo HX: Aberrant expression of Cx43
is associated with the peritoneal metastasis of gastric cancer and
Cx43-mediated gap junction enhances gastric cancer cell diapedesis
from peritoneal mesothelium. PLoS One. 8:e745272013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu D, Zhou H, Wu J, Liu W, Li Y, Shi G,
Yue X, Sun X, Zhao Y, Hu X, et al: Infection by Cx43 adenovirus
increased chemotherapy sensitivity in human gastric cancer BGC-823
cells: Not involving in induction of cell apoptosis. Gene.
574:217–224. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yeh ES, Williams CJ, Williams CB, Bonilla
IV, Klauber-DeMore N and Phillips SL: Dysregulated connexin 43 in
HER2-positive drug resistant breast cancer cells enhances
proliferation and migration. Oncotarget. 8:109358–109369. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Teleki I, Szasz AM, Maros ME, Gyorffy B,
Kulka J, Meggyeshazi N, Kiszner G, Balla P, Samu A and Krenacs T:
Correlations of differentially expressed gap junction connexins
Cx26, Cx30, Cx32, Cx43 and Cx46 with breast cancer progression and
prognosis. PLoS One. 9:e1125412014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Teleki I, Krenacs T, Szasz MA, Kulka J,
Wichmann B, Leo C, Papassotiropoulos B, Riemenschnitter C, Moch H
and Varga Z: The potential prognostic value of connexin 26 and 46
expression in neoadjuvant-treated breast cancer. BMC Cancer.
13:502013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Banerjee D, Gakhar G, Madgwick D, Hurt A,
Takemoto D and Nguyen TA: A novel role of gap junction connexin46
protein to protect breast tumors from hypoxia. Int J Cancer.
127:839–848. 2010.PubMed/NCBI
|
|
29
|
Yuan L, Guo Y, Yi J, Xiao J, Yuan J, Xiong
W, Xu H, Yang Z, Zhang J and Deng H: Identification of a novel GJA3
mutation in congenital nuclear cataract. Optom Vis Sci. 92:337–342.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Morel S, Burnier L, Roatti A, Chassot A,
Roth I, Sutter E, Galan K, Pfenniger A, Chanson M and Kwak BR:
Unexpected role for the human Cx37 C1019T polymorphism in tumour
cell proliferation. Carcinogenesis. 31:1922–1931. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Good ME, Nelson TK, Simon AM and Burt JM:
A functional channel is necessary for growth suppression by Cx37. J
Cell Sci. 124:2448–2456. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jing Y, Guo S, Zhang X, Sun A, Tao F, Ju H
and Qian H: Effects of small interfering RNA interference of
connexin 37 on subcutaneous gastric tumours in mice. Mol Med Rep.
10:2955–2960. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sohl G, Joussen A, Kociok N and Willecke
K: Expression of connexin genes in the human retina. BMC
Ophthalmol. 10:272010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shen B, Fang T, Dai M, Jones G and Zhang
S: Independent losses of visual perception genes Gja10 and Rbp3 in
echolocating bats (Order: Chiroptera). PLoS One. 8:e688672013.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Alvi MA, Liu X, O'Donovan M, Newton R,
Wernisch L, Shannon NB, Shariff K, di Pietro M, Bergman JJ,
Ragunath K and Fitzgerald RC: DNA methylation as an adjunct to
histopathology to detect prevalent, inconspicuous dysplasia and
early-stage neoplasia in Barrett's esophagus. Clin Cancer Res.
19:878–888. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Finegold DN, Baty CJ, Knickelbein KZ,
Perschke S, Noon SE, Campbell D, Karlsson JM, Huang D, Kimak MA,
Lawrence EC, et al: Connexin 47 mutations increase risk for
secondary lymphedema following breast cancer treatment. Clin Cancer
Res. 18:2382–2390. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kang KS, Yun JW, Yoon B, Lim YK and Lee
YS: Preventive effect of germanium dioxide on the inhibition of gap
junctional intercellular communication by TPA. Cancer Lett.
166:147–153. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yamasaki H, Krutovskikh V, Mesnil M,
Tanaka T, Zaidan-Dagli ML and Omori Y: Role of connexin (gap
junction) genes in cell growth control and carcinogenesis. C R Acad
Sci III. 322:151–159. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yamasaki H, Omori Y, Zaidan-Dagli ML,
Mironov N, Mesnil M and Krutovskikh V: Genetic and epigenetic
changes of intercellular communication genes during multistage
carcinogenesis. Cancer Detect Prev. 23:273–279. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ruch RJ, Porter S, Koffler LD, Dwyer-Nield
LD and Malkinson AM: Defective gap junctional intercellular
communication in lung cancer: Loss of an important mediator of
tissue homeostasis and phenotypic regulation. Exp Lung Res.
27:231–243. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Trosko JE: The role of stem cells and gap
junctional intercellular communication in carcinogenesis. J Biochem
Mol Biol. 36:43–48. 2003.PubMed/NCBI
|
|
42
|
Trosko JE and Ruch RJ: Cell-cell
communication in carcinogenesis. Front Biosci. 3:d208–d236. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Gravalos C and Jimeno A: HER2 in gastric
cancer: A new prognostic factor and a novel therapeutic target. Ann
Oncol. 19:1523–1529. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kanczuga-Koda L, Sulkowski S, Lenczewski
A, Koda M, Wincewicz A, Baltaziak M and Sulkowska M: Increased
expression of connexins 26 and 43 in lymph node metastases of
breast cancer. J Clin Pathol. 59:429–433. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ezumi K, Yamamoto H, Murata K, Higashiyama
M, Damdinsuren B, Nakamura Y, Kyo N, Okami J, Ngan CY, Takemasa I,
et al: Aberrant expression of connexin 26 is associated with lung
metastasis of colorectal cancer. Clin Cancer Res. 14:677–684. 2008.
View Article : Google Scholar : PubMed/NCBI
|