Introduction
Thyroid cancer is a class of heterogeneous diseases
that have various subtypes with different biological behaviors.
Papillary thyroid carcinoma (PTC) is the most common subtype of
thyroid cancer, and its incidence has been increasing considerably
in recent years (1). A majority of
PTCs are considered to have a good prognosis, whereas others may
behave more aggressively (2). The
risk stratification in patients with PTC has important clinical
implications for the selection of therapeutic strategies.
Therefore, novel biomarkers are urgently required for predicting
the malignant potential of primary lesions and clinical outcome. To
the best of our knowledge, the present study is the first to
provide an epigenetic biomarker to help identify the differences
between benign and malignant lesions and to assist in predicting
their biological behaviors.
Epigenetic modifications serve a key role in
numerous biological and pathological processes. DNA methylation at
the 5 position of cytosine (5-mC) acts as a key epigenetic marker
that serves essential roles in genomic imprinting and regulation of
gene expression (3). Aberrant DNA
methylation is frequently observed in cancer. Hypermethylation of
tumor suppressors and hypomethylation of oncogenes triggers
tumorigenesis and tumor development (4). Disrupted DNA methylation patterns at
the 5 position of cytosine have been substantiated as
mechanism-based evidence in cancer (5). 5-mC can become oxidized to
5-hydroxymethylcytosine (5-hmC) by the ten-eleven translocation
(TET) family of 5-mC hydroxylases, including TET1, 2 and 3
(6,7). Accumulating evidence has suggested that
loss of 5-hmC is an epigenetic hallmark in various types of cancer,
with diagnostic and prognostic implications (5,8–10). Aberrant methylation of various
candidate genes have been reported potentially associated with
thyroid carcinogenesis (11).
However, the role of 5-hmC with regard to thyroid cancer remains
largely unknown. In the present study, it was observed that the
global level of 5-hmC is significantly decreased in PTC compared
with benign thyroid disease. Furthermore, 5-hmC reduction is
associated with potential malignant biological behavior,
contributing to our present understanding of thyroid cancer
epigenetics.
Materials and methods
Ethics statement
Thyroid cancer and nodular goiter samples were
obtained from the archives of the Pathology Department of the First
Affiliated Hospital of Dalian Medical University (Dalian, China),
following the approval of the institutional review board. The
Committee of Research Ethics waived the requirement of informed
consent for the tissue sections used in this study, as patient data
were anonymized.
Patient cohorts and tissue
samples
Cancer tissue and adjacent matching healthy tissue
samples from 88 patients with PTC and 20 patients with nodular
goiter (NG) treated at the First Affiliated Hospital of Dalian
Medical University (Dalian, China) between January 2015 and
September 2017 were included in the present study. The cohort had
an age range of 23–75 years with 26 males and 82 females. All
tissue sections were reviewed by an expert pathologist for
verification of the clinical diagnosis. Each tissue sample had at
least three independent tissue sections for the following
analyses.
Immunohistochemistry (IHC)
staining
IHC was employed to analyze 5 mm tissue sections,
which had been fixed in 10% formalin for 24 h at room temperature
and paraffin-embedded. Sections were dewaxed and rehydrated
following standard protocols (12).
Subsequently, antigen retrieval was performed by boiling the
sections for 5 min in citric acid. The sections were dipped in the
endogenous peroxidase blocker (cat. no. PV-6000D; OriGene
Technologies, Inc., Beijing, China) for 10 min at room temperature.
For immunolabeling of 5-hmC and 5-mC, a rabbit monoclonal
5-hydroxymethylcytosine antibody (cat. no. ab214728; Abcam,
Cambridge, UK) and a mouse monoclonal 5-methylcytosine antibody
(cat. no. ab10805; Abcam) were applied at 1:200 and 1:100 dilution,
respectively for 1 h at room temperature. After the sections were
washed with PBS, they were incubated with peroxidase-conjugated
anti-rabbit/mouse immunoglobulin G (cat. no. PV-6000D; OriGene
Technologies, Inc.; undiluted) at room temperature for 20 min,
followed by DAB chromogenic reaction performed according to the
protocol of the DAB chromogenic reagent kit (cat. no. ZLI-9019;
OriGene Technologies, Inc.). The sections were then counterstained
with hematoxylin at room temperature for 1 min, dehydrated with
graded alcohol and xylene, and mounted onto coverslips. The stained
cell images were captured under the light microscope (Olympus,
Tokyo, Japan), and cells were counted and assessed at a
magnification of ×100.
Scoring system
Immunoreactivity of the nucleus was evaluated for
each tissue section. In the case of NG, sections that indicated
strong immunostaining in follicular cell nuclei were further
evaluated. In the case of PTC, adjacent non-neoplastic cells were
used as internal controls to evaluate the immunoreactivity.
Thereafter, the H-score system was applied to evaluate positive
immunoreactivity (9). Briefly,
nuclei staining intensity (0, 1, 2 or 3) was first determined for
each cell in a fixed field corresponding to the presence of
negative, weak, intermediate and strong staining, respectively. The
percentage of cells at each staining intensity level was
subsequently calculated, and an H-score (0–300) was assigned using
the following formula: H-score=0 × (% of cells staining 0) + 1 × (%
of cells staining 1) + 2 × (% of cells staining 2) + 3 × (% of
cells staining 3). H-scores for each case were calculated as the
mean score of at least three individual section scores for each
case, from which the mean score of all the individual field scores
of each section was derived. In total 330 sections across 108
samples were analyzed.
Statistical analysis
The data are presented as either median ±
interquartile range or the mean ± standard deviation and were
analyzed using GraphPad Prism 7.00 software (GraphPad Software,
Inc., La Jolla, CA, USA). The χ2 test was used to
analyze differences of clinical parameters between two groups of
patients. A Student's t-test was used to analyze differences in
5-hmC level (H-scores). Pearson or Spearman's rank correlation
analysis was used to evaluate the correlation between 5-hmC level
and pathological parameters. P<0.05 was considered to indicate a
statistically significant difference.
Results
5-hmC expression level is high in NG
and lost in PTC
To evaluate the global 5-hmC landscape, 5-hmC levels
in the thyroid tissues were examined by IHC staining with a
specific anti-5-hmC antibody in two representative cohorts
(Table I), including PTC (n=88) and
NG (n=20). IHC analysis of NG tissues indicated robust 5-hmC
staining of the nucleus, whereas the cancer cells in PTC tissues
exhibited reduced 5-hmC staining by comparison (Fig. 1A). The H-scoring indicated that the
5-hmC level was significantly decreased in PTC tissues compared
with NG tissues (P<0.0001; Fig.
1B). Low nuclear 5-hmC staining was also observed in thyroid
cancer tissues compared with the adjacent non-neoplastic tissues
(Fig. 1C and D). However, there was
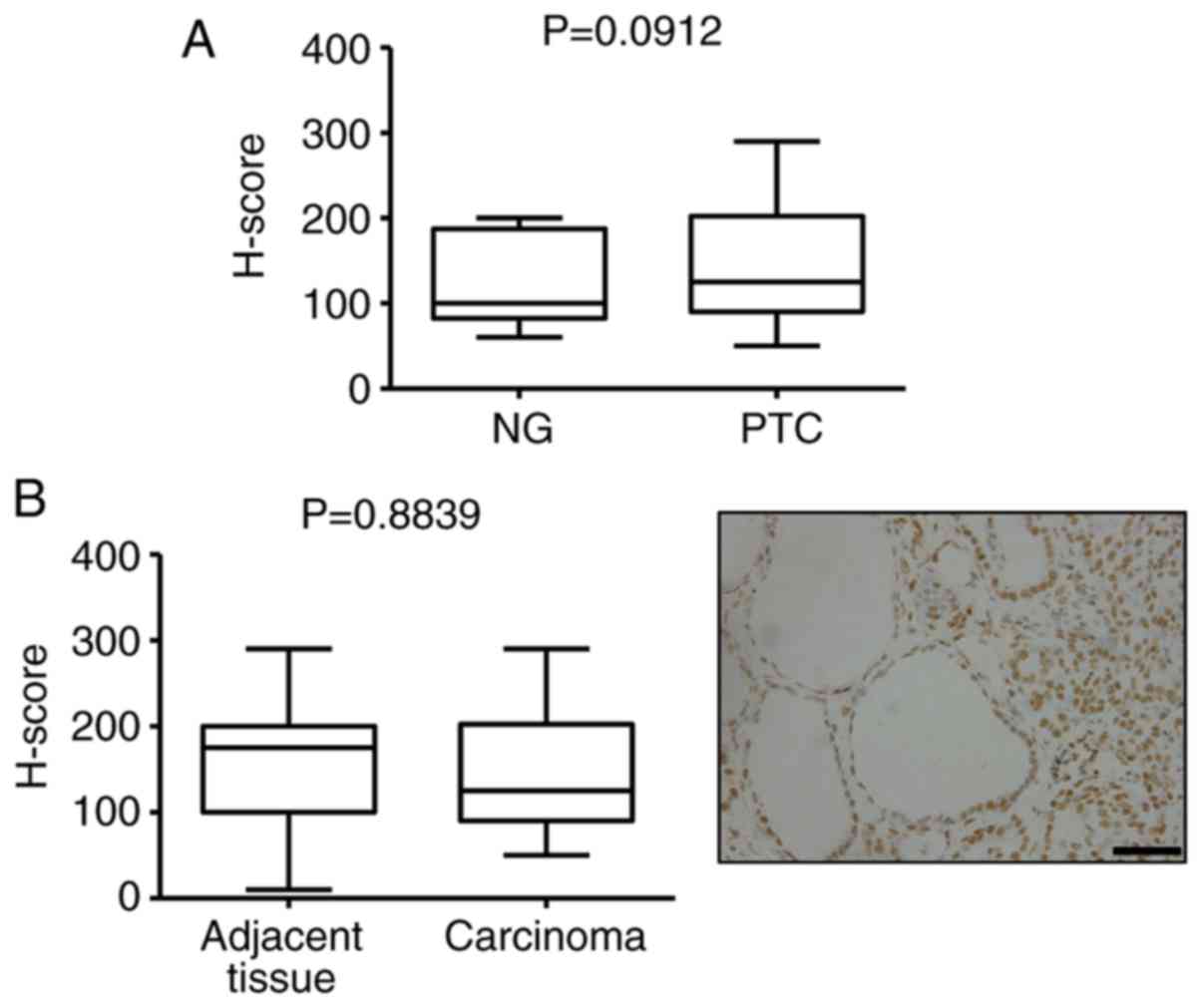
no difference in 5-mC levels between NG and PTC, as well as cancer
and adjacent normal tissue (Fig. 2).
These data indicate that a high level of 5-hmC is a distinct
epigenetic signature in benign thyroid disease, and a significant
loss of 5-hmC is a distinctive hallmark of thyroid cancer. In
addition, the 5-hmC level has a more sensitive diagnostic value
compared with 5-mC in PTC.
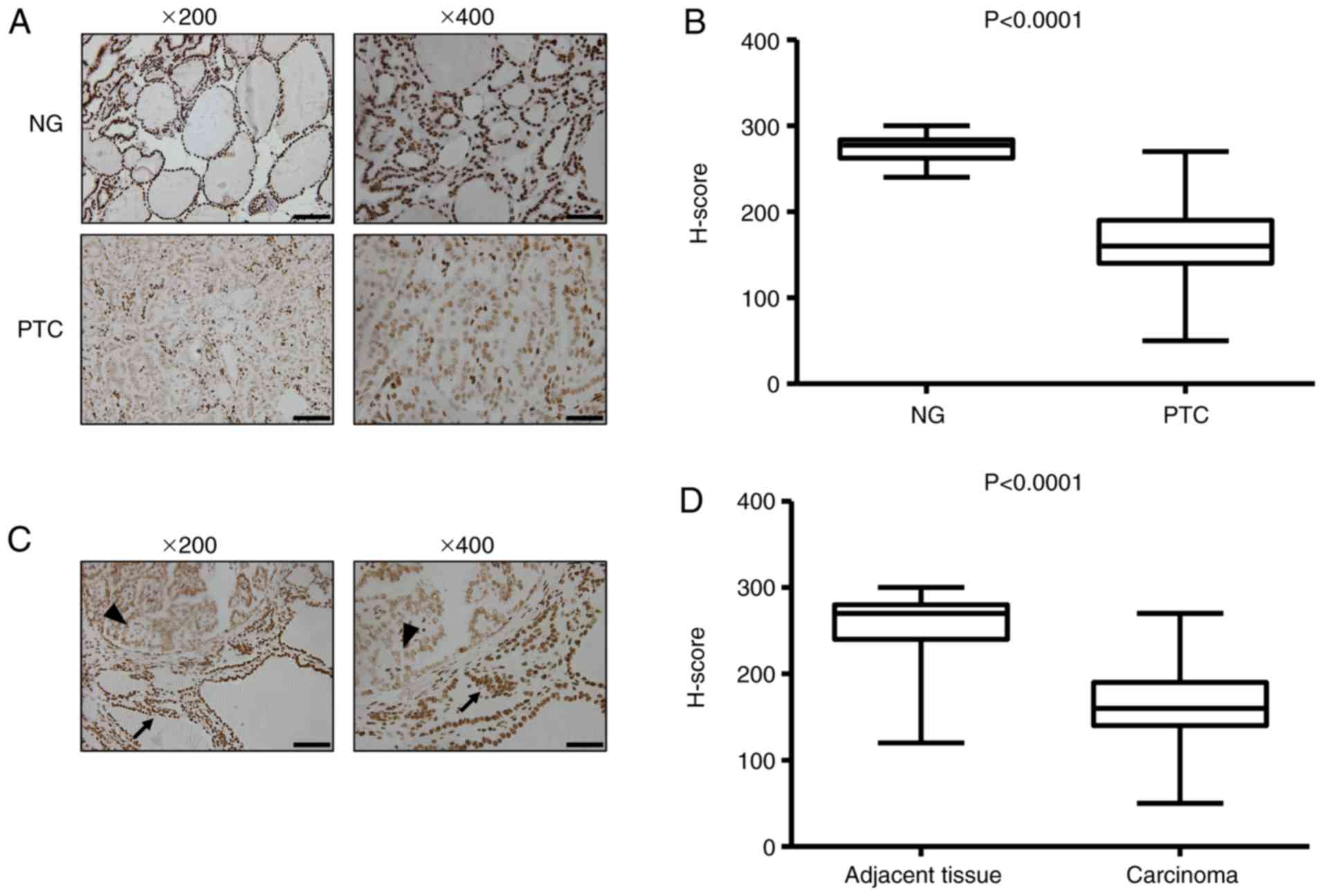 | Figure 1.The level of 5-hmC is reduced in PTC.
(A) Representative histology for immunohistochemistry staining of
5-hmC in representative cases of benign and malignant thyroid
disease. (B) Boxplot depicting 5-hmC H-score distribution in
patients with NG (n=20) and PTC (n=88). Each case has duplicated
tissue cores. (C) Normal thyroid follicular epithelium indicates
strong nuclear 5-hmC staining (arrow); cancer cells exhibit reduced
staining intensities (arrowhead). (D) Boxplot depicting 5-hmC
H-score distribution in adjacent non-neoplastic tissue and
carcinoma (n=88). Each case has duplicated tissue cores. The
boxplot lines from top to bottom represent maximum, 75th
percentile, median, 25th percentile and minimum, respectively.
Magnification, ×200; scale bar, 100 µm. Magnification, ×400; scale
bar, 50 µm. 5-hmC, 5-hydroxymethylcytosine; NG, nodular goiter;
PTC, papillary thyroid carcinoma. |
 | Table I.Clinical characteristics of NG and
PTC cohort. |
Table I.
Clinical characteristics of NG and
PTC cohort.
| Parameter | NG (%), n=20 | PTC (%), n=88 | P-value
(χ2 test) |
|---|
| Age at surgery
(years) |
|
| 0.354 |
|
<50 | 8 (40) | 48 (54.5) |
|
|
≥50 | 12 (60) | 40 (45.5) |
|
| Sex (%) |
|
| 0.329 |
|
Male | 7 (35) | 19 (21.6) |
|
|
Female | 13 (65) | 69 (78.4) |
|
| Nodule size,
cm |
|
| 0.068 |
| ≤1 | 6 (30) | 49 (55.7) |
|
|
>1 | 14 (70) | 39 (44.3) |
|
| Location |
|
| 0.183 |
| Left
lobe | 7 (35) | 48 (54.5) |
|
| Right
lobe | 13 (65) | 40 (45.5) |
|
5-hmC is a useful molecular hallmark
of potential PTC
5-hmC levels between PTCs with lymph node metastases
(N+) and PTCs without lymph node metastases
(N−) were analyzed by IHC in order to evaluate if 5-hmC
level correlates with malignancy of PTC. H-score indicated that the
5-hmC level was significantly lower in PTCs with N+
(n=45) compared with PTCs with N− (n=43; P=0.0039;
Fig. 3A and B). Consistently, there
was a negative correlation between 5-hmC staining and the number of
metastatic lymph nodes, a predictor of malignant potential
(Fig. 3A; r2=0.2104;
P<0.0001). A correlation analysis was subsequently conducted
comparing the 5-hmC level to tumor size (the largest diameter the
tumor), with no correlation indicated, while the 5-hmC levels of
micro-carcinomas with N+ (n=22) were lower compared than
those of macro-carcinomas with N− (n=21; P=0.0086;
Fig. 3C). In addition, in the
limited number of patients that exhibited tumor development during
the follow-up duration (12–42 months), no significant correlation
between cancer 5-hmC levels and cancer recurrence was indicated
(P=0.3679; data not shown). Therefore, these results support the
conclusion that a reduction in 5-hmC serves as a distinctive
epigenetic event in the initiation and progression of PTC,
suggesting it may represent a novel epigenetic hallmark for PTC
recognition and prediction of malignancy.
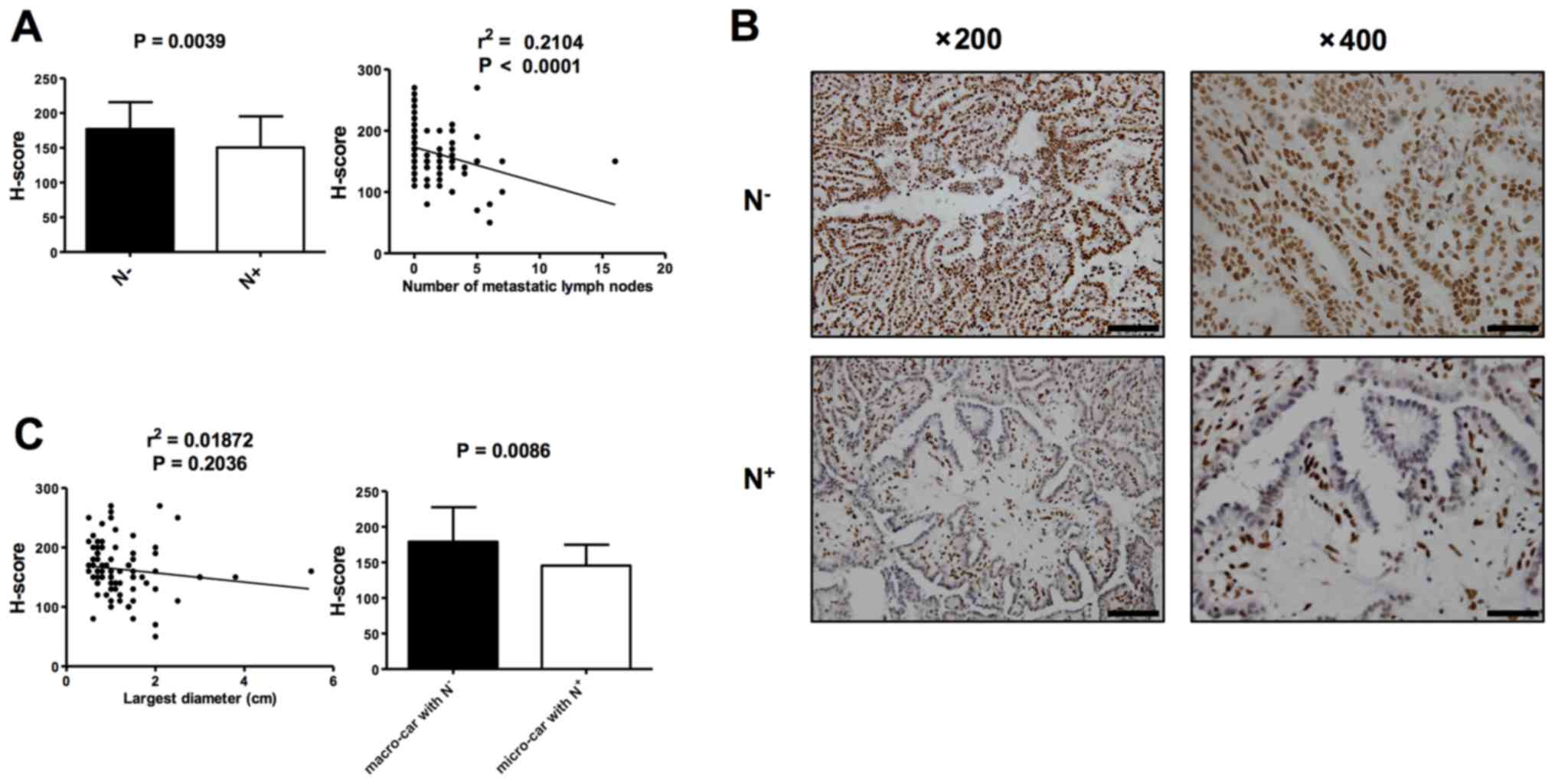 | Figure 3.Loss of 5-hmC is associated with
malignant potential of PTC. (A) Analysis of 5-hmC levels between
PTCs with N+ (n=45) and N− (n=43),
represented by H-score (left). Spearman correlation analysis
between 5-hmC staining and the number of metastatic lymph nodes
(right). At least three tissue sections were analyzed for each
case. (B) Representative IHC staining of 5-hmC in the individual
cases of PTC with N− and PTC with N+. Left:
Low-power images (magnification, ×200); scale bars, 100 µm. Right:
High-power images (magnification, ×400); scale bar, 50 µm. (C)
Pearson correlation analysis between 5-hmC staining and tumor size
(left). Analysis of 5-hmC levels between macro-carcinoma with
N− (n=21) and micro-carcinoma with N+ (n=22),
represented by H-score (right). At least three tissue sections were
analyzed for each case. Data are presented as the mean ± standard
error. 5-hmC, 5-hydroxymethylcytosine; PTC, papillary thyroid
carcinoma; 5- N+, lymph node metastases; N−,
no lymph node metastases. |
Discussion
DNA methylation is an essential epigenetic
modification that is often altered in cancer (13). In general, global 5-mC reduction at
specific sites of the genome is associated with cancer progression
(14,15). Current evidence suggests that the
oxidation of 5-mC to 5-hmC serves a critical role in epigenetic
plasticity (16–18). The family of TET enzymes are
5-methylcytosine oxidases that transform 5-mC to 5-hmC (19). 5-hmC is a key intermediate of DNA
demethylation that can affect global gene expression in mammals
(20). A number of previous studies
have reported that the amount of 5-hmC is substantially decreased
in various types of cancer, including in brain, lung, breast,
liver, pancreatic, colon and prostate cancer, and myeloid leukemia
(5,21–26).
However, the 5-hmC level in PTC remains unknown. In the present
study, it was indicated that reduced 5-hmC is a characteristic
epigenetic event in PTCs that is associated with malignant
biological behavior. Given that 5-hmC is oxidized from 5-mC, and
hypomethylation is an acknowledged epigenetic alteration in cancer
(27), it was hypothesized that the
underlying mechanism for the global decrease in 5-hmC levels in PTC
is due to a reduction in 5-mC. However, it was indicated that 5-mC
levels were similar between PTC and NG, therefore the mechanism is
derived from the oxidation process rather than 5-mC loss. As TET
enzymes mediate the oxidation process of 5-mC to 5-hmC, and partial
or complete loss of 5-hmC is frequently associated with
significantly decreased expression of the three TET genes,
suggesting a potential mechanism to explain the loss of 5-hmC in
cancer cells (21). In addition, the
catalytic oxidation reaction through TET enzyme requires the
cofactor α-ketoglutarate (6,7), which is mainly controlled by isocitrate
dehydrogenase (IDH). Therefore, understanding the role of 5-hmC,
TET and IDH involved in PTC initiation and progression is crucial.
Further examination of the underlying mechanism of this epigenetic
marker is required, in addition to the role of the associated
enzymes involved in PTC.
PTC is a unique cancer, and the prognosis varies
widely. PTCs measuring <10 mm at the largest diameter are termed
micro-carcinomas (28). Due to the
advent of high-resolution ultrasound detection and increasing
regular fine-needle aspiration biopsies, the incidence of
micro-carcinomas has increased worldwide (29). Micro-carcinomas are often considered
as indolent tumors and have a good prognosis; however, a small
proportion of these tumors are capable of metastasizing when the
volume of the original tumor is estimated to be as little as 1
mm3 (29). Accumulating
evidence indicates that surgical excision is not recommended for
micro-carcinomas, particularly indolent micro-carcinomas (30,31).
Radiofrequency ablation can effectively eliminate low-risk
micro-carcinomas with a low complication rate (32,33).
Therefore, distinguishing aggressive from indolent micro-carcinomas
has important clinical implications for the selection of
therapeutic strategies. Current routine characteristics, based on
clinical assessment and pathology, cannot explicitly distinguish
aggressive from indolent PTCs at an early phase (34), and, therefore, uncovering novel
prognostic biomarkers is required. In the present study, it was
indicated that 5-hmC expression levels in micro-carcinomas with
metastasis (high risk) is lower compared with macro-carcinomas
without metastasis (low risk), which suggests that the level of
5-hmC may serve as a valuable biomarker for predicting the
malignant potential of PTC and assist in the selection of
therapeutic strategies.
This study also endeavors to evaluate the patients'
survival data by directly associating the low 5-hmC levels with PTC
prognosis. However, recurrence of PTC following optimized surgery
is uncommon, and the latent durations prior to detection of distant
and lethal metastases may vary from years to decades (35). The follow-up duration of the current
study is not long enough to observe the prognostic value of 5-hmC
in PTC. Studies of hallmarks used for predicting clinical outcome
of thyroid cancer also have this limitation, as investigation is
hindered by the barriers of the follow-up interviews. Consequently,
clinically annotated biospecimen archives can serve as valuable
substitutes for theoretical and impractical prospective
strategies.
The present study demonstrates that global 5-hmC
levels are greatly diminished in the majority of PTCs. Ongoing
mechanistic investigations and identification of target genes
through comprehensive genome-wide mapping will shed further light
on cancer epigenetics (5,36–38). In
the present study, 5-hmC loss in PTC was a basic epigenetic event
that suggested that fundamental molecules in the 5-hmC biological
pathway can be therapeutically targeted to re-establish the level
of 5-hmC, and therefore reveal novel approaches in the therapy of
aggressive PTC. With the significance of clinical therapeutics, the
current study provides a novel direction for cancer prevention and
treatment, by targeting the cellular molecules and biochemical
pathways that restore the landscape of 5-hmC in PTC.
Acknowledgements
The authors acknowledge Dr Shuang Li from the
Clinical Laboratory of Integrative Medicine of the First Affiliated
Hospital of Dalian Medical University (Dalian, China) and Dr
Yandong Zhang from the Chinese Academy of Medical Sciences for
their help with IHC staining processing, and Dr Liang Wang from the
Central Laboratory of the First Affiliated Hospital of Dalian
Medical University for the helpful comments.
Funding
The present study was funded by The Natural Science
Foundation of Liaoning Province (grant no. 201602221; Dalian,
China).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the author on reasonable request.
Authors' contributions
MT, SG and WQ performed experiments, analyzed the
data and wrote the paper. MQ, ZS, CS, ZW, FY, HL and SB carried out
additional analyses and supported the study. YC and LW jointly
designed, oversaw and directed the study. All authors read and
approved the final version of this manuscript.
Ethics approval and consent to
participate
Thyroid cancer and nodular goiter samples were
obtained from the archives of the Pathology Department of the First
Affiliated Hospital of Dalian Medical University (Dalian, China)
following the approval of the committee of research ethics. The
committee of research ethics of the First Affiliated Hospital of
Dalian Medical University (Dalian, China) waived the requirement of
informed consent for the tissue sections used in this study as
patient data were anonymized.
Patient consent for publication
Not applicable
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Vigneri R, Malandrino P and Vigneri P: The
changing epidemiology of thyroid cancer: Why is incidence
increasing? Curr Opin Oncol. 27:1–7. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mazeh H and Chen H: Advances in surgical
therapy for thyroid cancer. Nat Rev Endocrinol. 7:581–588. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Suzuki MM and Bird A: DNA methylation
landscapes: Provocative insights from epigenomics. Nat Rev Genet.
9:465–476. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wu YC and Ling ZQ: The role of TET family
proteins and 5-hydroxymethylcytosine in human tumors. Histol
Histopathol. 29:991–997. 2014.PubMed/NCBI
|
|
5
|
Lian CG, Xu Y, Ceol C, Wu F, Larson A,
Dresser K, Xu W, Tan L, Hu Y, Zhan Q, et al: Loss of
5-hydroxymethylcytosine is an epigenetic hallmark of melanoma.
Cell. 150:1135–1146. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ito S, D'Alessio AC, Taranova OV, Hong K,
Sowers LC and Zhang Y: Role of Tet proteins in 5mC to 5hmC
conversion, ES-cell self-renewal and inner cell mass specification.
Nature. 466:1129–1133. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tahiliani M, Koh KP, Shen Y, Pastor WA,
Bandukwala H, Brudno Y, Agarwal S, Iyer LM, Liu DR, Aravind L and
Rao A: Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in
mammalian DNA by MLL partner TET1. Science. 324:930–935. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu X, Zhang G, Yi Y, Xiao L, Pei M, Liu
S, Luo Y, Zhong H, Xu Y, Zheng W and Shen J: Decreased
5-hydroxymethylcytosine levels are associated with TET2 mutation
and unfavorable overall survival in myelodysplastic syndromes. Leuk
Lymphoma. 54:2466–2473. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Munari E, Chaux A, Vaghasia AM, Taheri D,
Karram S, Bezerra SM, Gonzalez Roibon N, Nelson WG,
Yegnasubramanian S, Netto GJ and Haffner MC: Global
5-hydroxymethylcytosine levels are profoundly reduced in multiple
genitourinary malignancies. PLoS One. 11:e01463022016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Qiu L, Liu F, Yi S, Li X, Liu X, Xiao C,
Lian CG, Tu P and Wang Y: Loss of 5-hydroxymethylcytosine is an
epigenetic biomarker in cutaneous T cell lymphoma. J Invest
Dermatol. 138:2388–2397. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mancikova V, Buj R, Castelblanco E,
Inglada-Pérez L, Diez A, de Cubas AA, Curras-Freixes M, Maravall
FX, Mauricio D, Matias-Guiu X, et al: DNA methylation profiling of
well-differentiated thyroid cancer uncovers markers of recurrence
free survival. Int J Cancer. 135:598–610. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Paulsen IM, Dimke H and Frische S: A
single simple procedure for dewaxing, hydration and heat-induced
epitope retrieval (HIER) for immunohistochemistry in formalin fixed
paraffin-embedded tissue. Eur J Histochem. 59:25322015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bhutani N, Burns DM and Blau HM: DNA
demethylation dynamics. Cell. 146:866–872. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Esteller M: Epigenetics in cancer. N Engl
J Med. 358:1148–1159. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jones PA and Baylin SB: The epigenomics of
cancer. Cell. 128:683–692. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hemberger M, Dean W and Reik W: Epigenetic
dynamics of stem cells and cell lineage commitment: Digging
Waddington's canal. Nat Rev Mol Cell Biol. 10:526–537. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kohli RM and Zhang Y: TET enzymes, TDG and
the dynamics of DNA demethylation. Nature. 502:472–479. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shen L and Zhang Y:
5-Hydroxymethylcytosine: Generation, fate, and genomic
distribution. Curr Opin Cell Biol. 25:289–296. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rasmussen KD and Helin K: Role of TET
enzymes in DNA methylation, development, and cancer. Genes Dev.
30:733–750. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li W, Zhang X, Lu X, You L, Song Y, Luo Z,
Zhang J, Nie J, Zheng W, Xu D, et al: 5-Hydroxymethylcytosine
signatures in circulating cell-free DNA as diagnostic biomarkers
for human cancers. Cell Res. 27:1243–1257. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang H, Liu Y, Bai F, Zhang JY, Ma SH, Liu
J, Xu ZD, Zhu HG, Ling ZQ, Ye D, et al: Tumor development is
associated with decrease of TET gene expression and
5-methylcytosine hydroxylation. Oncogene. 32:663–669. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Haffner MC, Chaux A, Meeker AK, Esopi DM,
Gerber J, Pellakuru LG, Toubaji A, Argani P, Iacobuzio-Donahue C,
Nelson WG, et al: Global 5-hydroxymethylcytosine content is
significantly reduced in tissue stem/progenitor cell compartments
and in human cancers. Oncotarget. 2:627–637. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jin SG, Jiang Y, Qiu R, Rauch TA, Wang Y,
Schackert G, Krex D, Lu Q and Pfeifer GP: 5-Hydroxymethylcytosine
is strongly depleted in human cancers but its levels do not
correlate with IDH1 mutations. Cancer Res. 71:7360–7365. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kudo Y, Tateishi K, Yamamoto K, Yamamoto
S, Asaoka Y, Ijichi H, Nagae G, Yoshida H, Aburatani H and Koike K:
Loss of 5-hydroxymethylcytosine is accompanied with malignant
cellular transformation. Cancer Sci. 103:670–676. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ko M, Huang Y, Jankowska AM, Pape UJ,
Tahiliani M, Bandukwala HS, An J, Lamperti ED, Koh KP, Ganetzky R,
et al: Impaired hydroxylation of 5-methylcytosine in myeloid
cancers with mutant TET2. Nature. 468:839–843. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kraus TF, Globisch D, Wagner M, Eigenbrod
S, Widmann D, Münzel M, Müller M, Pfaffeneder T, Hackner B, Feiden
W, et al: Low values of 5-hydroxymethylcytosine (5hmC), the ‘sixth
base,’ are associated with anaplasia in human brain tumors. Int J
Cancer. 131:1577–1590. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kanwal R and Gupta S: Epigenetic
modifications in cancer. Clin Genet. 81:303–311. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zafon C, Baena JA, Castellvi J, Obiols G,
Monroy G and Mesa J: Differences in the form of presentation
between papillary microcarcinomas and papillary carcinomas of
larger size. J Thyroid Res. 2011:6391562010.PubMed/NCBI
|
|
29
|
Schneider DF and Chen H: New developments
in the diagnosis and treatment of thyroid cancer. CA Cancer J Clin.
63:374–394. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ito Y, Miyauchi A, Inoue H, Fukushima M,
Kihara M, Higashiyama T, Tomoda C, Takamura Y, Kobayashi K and Miya
A: An observational trial for papillary thyroid microcarcinoma in
Japanese patients. World J Surg. 34:28–35. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Londero SC, Krogdahl A, Bastholt L,
Overgaard J, Trolle W, Pedersen HB, Bentzen J, Schytte S,
Christiansen P and Godballe C; Danish Thyroid Cancer Group, :
Papillary thyroid microcarcinoma in Denmark 1996–2008: A national
study of epidemiology and clinical significance. Thyroid.
23:1159–1164. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jeong SY, Baek JH, Choi YJ, Chung SR, Sung
TY, Kim WG, Kim TY and Lee JH: Radiofrequency ablation of primary
thyroid carcinoma: Efficacy according to the types of thyroid
carcinoma. Int J Hyperthermia. 34:611–616. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang M, Luo Y, Zhang Y and Tang J:
Efficacy and safety of ultrasound-guided radiofrequency ablation
for treating low-risk papillary thyroid microcarcinoma: A
prospective study. Thyroid. 26:1581–1587. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Haugen BR, Alexander EK, Bible KC, Doherty
GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM,
Schlumberger M, et al: 2015 American thyroid association management
guidelines for adult patients with thyroid nodules and
differentiated thyroid cancer: The American thyroid association
guidelines task force on thyroid nodules and differentiated thyroid
cancer. Thyroid. 26:1–133. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chereau N, Oyekunle TO, Zambeli-Ljepović
A, Kazaure HS, Roman SA, Menegaux F and Sosa JA: Predicting
recurrence of papillary thyroid cancer using the eighth edition of
the AJCC/UICC staging system. Br J Surg. 106:889–897. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Figueroa ME, Abdel-Wahab O, Lu C, Ward PS,
Patel J, Shih A, Li Y, Bhagwat N, Vasanthakumar A, Fernandez HF, et
al: Leukemic IDH1 and IDH2 mutations result in a hypermethylation
phenotype, disrupt TET2 function, and impair hematopoietic
differentiation. Cancer Cell. 18:553–567. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Plongthongkum N, Diep DH and Zhang K:
Advances in the profiling of DNA modifications: Cytosine
methylation and beyond. Nat Rev Genet. 15:647–661. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Shim EH, Livi CB, Rakheja D, Tan J, Benson
D, Parekh V, Kho EY, Ghosh AP, Kirkman R, Velu S, et al:
L-2-Hydroxyglutarate: An epigenetic modifier and putative
oncometabolite in renal cancer. Cancer Discov. 4:1290–1298. 2014.
View Article : Google Scholar : PubMed/NCBI
|