Introduction
Ovarian cancer (OC) is the most lethal gynecological
malignancy (1). Surgical resection
remains the primary option for patients with OC (2). High mortality rates (5-year survival
rate, ~30%) of OC are based on late diagnosis, distant metastasis
within 5 years after surgical treatment and chemotherapeutic
resistance (3,4). Patients with different pathological
stages of OC remain a considerable prognostic challenge (5). In a clinical setting, same stage tumors
can lead to different outcomes (6).
While mucin 16, cell surface associated and WAP four-disulfide core
domain 2 levels exhibited great potential in the detection of
high-grade serous ovarian carcinoma at later stages, the levels are
not sensitive enough for early stage detection of this disease
(7). Consequently, further
understanding of the molecular mechanisms associated with the
progression of OC is required.
Various cell adhesion molecules are located at
synapses, but only few are considered synaptic cell adhesion
molecules (8). Synaptic cell
adhesion molecules (SynCAMs/CADMs) are a subfamily of the
immunoglobulin superfamily of cell adhesion molecules (8). SynCAMs are single transmembrane
proteins that were discovered in the central nervous system, due to
their ability to induce synapse formation (9,10).
SynCAMs are true synaptic cell adhesion molecules and are crucial
for synapse formation and plasticity (11,12).
SynCAM in the cytoplasmic domain contains the binding motifs that
connect to actin fibers (8). SynCAMs
are involved in synapse formation, neuronal connectivity,
myelination and cerebellum morphogenesis (13–15).
Furthermore, it was suggested that SynCAMs may contribute to autism
spectrum disorder (16), glioma
generation, non-small cell lung cancer and hepatocarcinogenesis
(17,18). However, the prognostic importance of
SynCAM expression and the associated underlying mechanisms has not
fully been elucidated.
A biomarker can be defined as any measurable
characteristic that provides an indication of the biological state
of a patient (19). In the present
study, SynCAM expression was investigated in 74 patients with OC
using immunohistochemistry. In addition, membrane palmitoylated
protein 6 (MPP6), a member of the palmitoylated membrane protein
subfamily of the peripheral membrane-associated guanylate kinases
(MAGUK), levels were investigated in OC cells.
Materials and methods
Patients and tissue samples
The inclusion criteria were as follows: i) Patients
agreed to surgical resection or chemotherapy; and ii) the all
ovarian tumor was primary. The exclusion criteria were as follows:
i) Patients that had refused any surgical resection or
chemotherapy; ii) ovarian metastatic tumor; and iii) there was no
sufficient tissue specimen for the immunohistochemical and western
blot analyses. A total of 74 OC tissue specimens were derived from
patients in The First Hospital of Lanzhou University (Lanzhou,
China) between January 2011 and December 2012. None of the patients
with OC had received chemo or radiotherapy prior to surgery. All
patients underwent radical resection and were regularly followed
up. The median age was 53 years (range, 17–75 years). The median
follow-up time was 33 months (range, 6–60 months). The study was
approved by the Ethics Committee of the First Hospital of Lanzhou
University (Lanzhou, China) and written informed consent was
obtained from the patients or their families. Borderline ovarian
tumors (BOT; n=24) and benign ovarian tumor tissues (BEOT; n=34)
were obtained from patients with ovarian tumors. Parts of the tumor
specimens were frozen in liquid nitrogen after collection and
stored at −80°C until use and other parts were formalin-fixed for 1
week at room temperature and paraffin-embedded.
Immunohistochemical staining
The specimens were cut into 4 µm sections. The
deparaffinized specimens were washed twice with distilled water, 5
min at a time. Citrate buffer (pH 6.0) was used in antigen
retrieval at 121°C for 20 min. After retrieval, the tissue sections
were cooled for 20 min at room temperature. Finally, 0.01 mol/l PBS
buffer was used to wash the sections twice for 5 min each time, and
distilled water was then used to wash the sections three times for
3 min each time. To block endogenous peroxidase activity, 3%
hydrogen peroxide was used for 15 min at 37°C. The slides were
blocked with 10% normal goat serum (OriGene Technologies, Inc.) in
PBS for 30 min at room temperature, and further incubated with
rabbit anti-human primary monoclonal antibodies against SynCAM
(1:500; cat. no. GR3184359-5; Abcam) and MPP6 (1:500; cat. no.
5324; Signalway Antibody LLC) at 4°C overnight. The following day
the slides were incubated with ultraView universal HRP Multimer
secondary antibody (1:1,000; cat. no. TA130015; anti-rabbit IgG
Detection System; OriGene Technologies, Inc.) for 30 min at 37°C to
assess protein expression, according to the manufacturer's
protocol. Hematoxylin was used to stain cell nuclei for 1–2 min at
37°C. PBS was used as a negative control.
The tissue sections were assessed under a light
microscope (Olympus Corporation) at ×10 magnification using the
Allred scoring system (20).
Brown-yellow staining was considered as positive protein
expression. For the semi-quantitative evaluation of protein levels
in the tissues, an immunoreactivity-scoring system was used as
previously described (21). The
staining intensity was graded (0, no stain; 1+, weak stain; 2+,
moderate stain; and 3+, strong stain). High expression of SynCAM
and MPP6 was defined as detectable immunoreactions in cytoplasm and
membranes with scores ≥2.
Western blot analysis
Ovarian tumor tissues were homogenized in cold NP40
buffer (50 mM Tris (pH 7.4), 150 mM NaCl, 1% NP-40; Beyotime
Institute of Biotechnology), sonicated (20 KHz, 300 W, 5 min) on
ice and lysates were centrifuged at 10,000 × g for 15 min at 4°C to
collect the supernatant. The protein concentration was determined
using a bicinchoninic acid assay (Beijing Solarbio Science &
Technology Co., Ltd.). A total of 30 µg of protein per lane were
separated on 10% SDS-PAGE gels and transferred to polyvinylidene
fluoride membranes (OriGene Technologies, Inc.). Membranes were
incubated with primary antibodies for SynCAM (dilution, 1:1,000;
cat. no. GR3184359-5; Abcam), MPP6 (dilution, 1:1,000; cat. no.
5324; Signalway Antibody LLC) and β-actin (dilution, 1:5,000; cat.
no. 14395-1-AP; Proteintech Group, Inc.) for 24 h at 4°C following
a blocking step with 5% fat-free milk in TBST for 2 h at room
temperature. Membranes were then incubated with secondary goat
anti-rabbit IgG H&L antibody (1:1,000; cat. no. TA100015;
OriGene Technologies, Inc.) for 1 h at room temperature.
Immunoreactive bands were visualized using enhanced
chemiluminescence (Thermo Fisher Scientific, Inc.). Densitometry of
the bands was performed using Quantity One software version 4.5.5
(Bio-Rad Laboratories, Inc.).
Statistical analysis
All data are presented as the mean ± standard
deviation. Student's t-test was used for statistical analysis
between two groups. P<0.05 considered to indicate a
statistically significant difference. Positive expression rates and
clinicopathological factors in the low and high SynCAM expression
patients were compared using a χ2 test with a threshold
value of P<0.05. Survival curves were plotted using the
Kaplan-Meier method and were compared using the log-rank test.
One-way ANOVA followed by Tukey post-hoc test were used to analysis
of variance between groups. All statistical analyses were performed
using the SPSS statistical software package (version 19.0; IBM,
Corp.).
Results
Clinicopathological
characteristics
Demographic, clinical and histopathological
variables are presented in Table I.
The current study included 74 patients with OC. The median age was
53 years (range, 17–75 years) and the cohort comprised 16 (21.62%)
cases diagnosed at T1 and 12 (16.22%), 40 (54.05%) and 6 (8.11%)
cases diagnosed at stages T2, T3 and T4 (TNM staging system)
(22), respectively. The median
follow-up time was 33 months (range, 6–60 months).
 | Table I.Association between SynCAM expression
and clinicopathological characteristics in patients with ovarian
cancer. |
Table I.
Association between SynCAM expression
and clinicopathological characteristics in patients with ovarian
cancer.
|
|
| SynCAM expression
(n=74) |
|
|---|
|
|
|
|
|
|---|
| Variables | Number (%) | Low expression | High
expression | P-value |
|---|
| Age (years) |
|
|
| 0.7886 |
|
<60 | 48 (64.86) | 28 | 20 |
|
|
≥60 | 26 (35.14) | 16 | 10 |
|
| Tumor size
(cm) |
|
|
| 0.0308a |
|
<8 | 50 (67.60) | 34 | 16 |
|
| ≥8 | 24 (32.40) | 10 | 14 |
|
| T stage |
|
|
| 0.1772 |
| T1 | 16 (21.62) | 10 | 6 |
|
| T2 | 12 (16.22) | 10 | 2 |
|
| T3 | 40 (54.05) | 22 | 18 |
|
| T4 | 6 (8.11) | 2 | 4 |
|
| N stage |
|
|
| 0.2509 |
| N0 | 50 (67.57) | 32 | 18 |
|
| N1 | 24 (32.43) | 12 | 12 |
|
| M stage |
|
|
| 0.5639 |
| M0 | 66 (89.19) | 40 | 26 |
|
| M1 | 8 (10.81) | 4 | 4 |
|
| Differentiation
level of tumor cells |
|
|
|
<0.0001a |
|
Low | 18 (24.32) | 2 | 16 |
|
|
High | 56 (75.68) | 42 | 14 |
|
Association between SynCAM expression
and clinicopathological characteristics
In the present study, immunohistochemical analysis
of 132 human ovarian tumor specimens was used evaluate SynCAM
protein expression. SynCAMs exhibited cytolymph or cytoplasmic
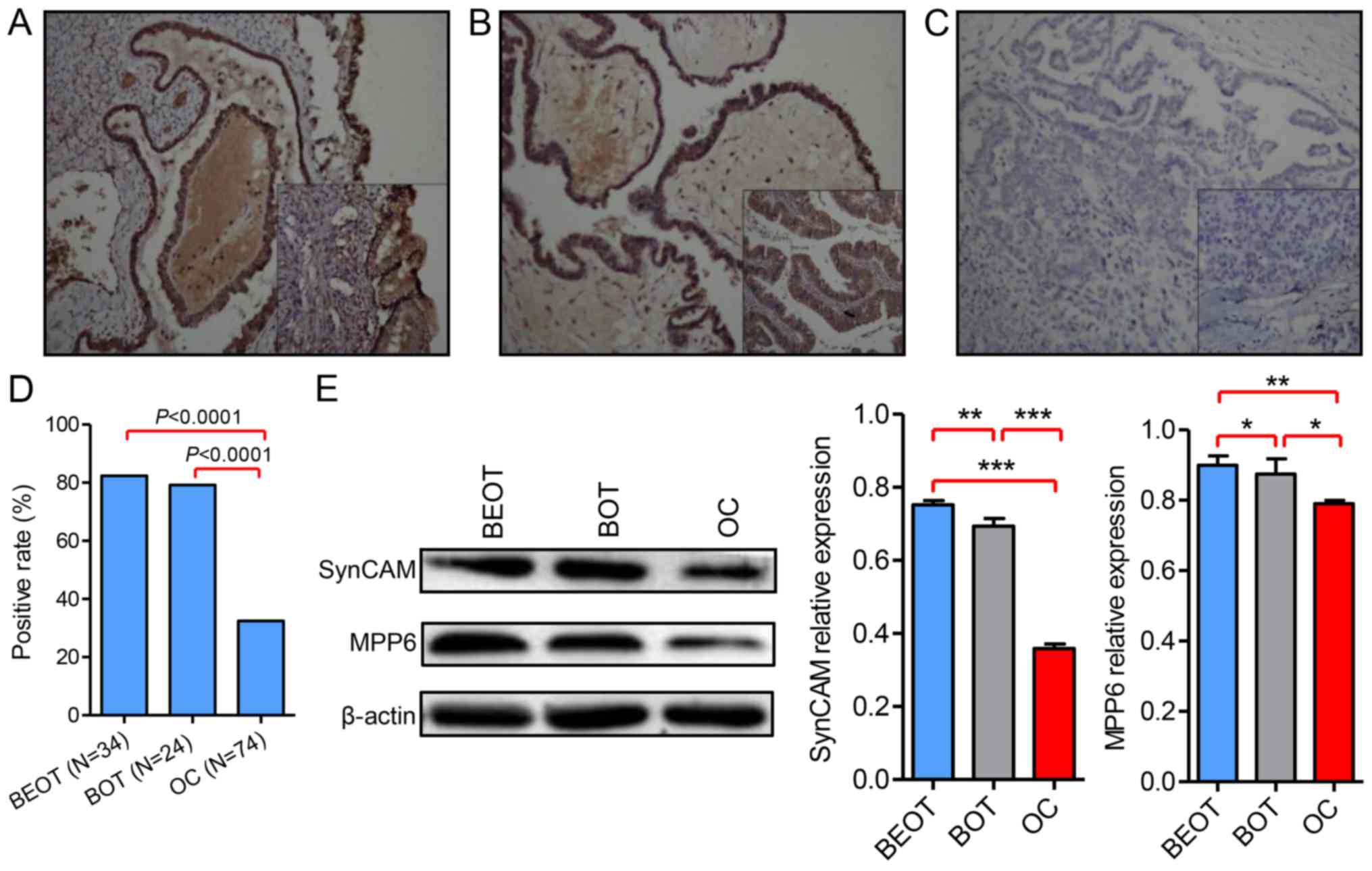
expression in ovarian tumor specimen. Representative images
demonstrating SynCAM expression by staining BEOT, BOT and OC
specimen were indicated (Fig. 1).
Increased SynCAM expression was detected in BEOT (Fig. 1A) and BOT (Fig. 1B) compared with OC tissues (Fig. 1C). A total of 28/34 BEOT specimens
exhibited positive SynCAM expression, representing a positive
expression rate of 82.35% and 19/24 BOT cases exhibited positive
SynCAM expression, representing a positive expression rate of
79.17%. Out of 74 OC specimen only 30 cases exhibited positive
SynCAM expression, representing a positive expression rate of
40.54%. The majority of OC tissues exhibited no evidence for SynCAM
staining. The positive expression rate was significantly increased
in BEOT and BOT compared with OC (P<0.0001; Fig. 1D). The results suggested that SynCAM
downregulation occurs in human ovarian tumor tissues. To further
investigate the expression pattern of SynCAM in ovarian tumor
tissues, SynCAM expression was examined by western blot analysis.
The results showed that SynCAM levels were significantly decreased
in OC and BOT compared with BEOT (P<0.001; Fig. 1E). Results demonstrated that SynCAM
expression was decreased in all ovarian tumor tissues compared with
normal tissues (Fig. 1E). The data
suggested that SynCAM may function as a tumor suppressor in ovarian
tumor tissues. SynCAM expression and the clinicopathological
characteristics are presented in Table
I. SynCAM expression was correlated with the tumor diameter
(P=0.0308) and differentiation level of tumor cells
(P<0.0001).
MPP6 protein expression is
downregulated in OC
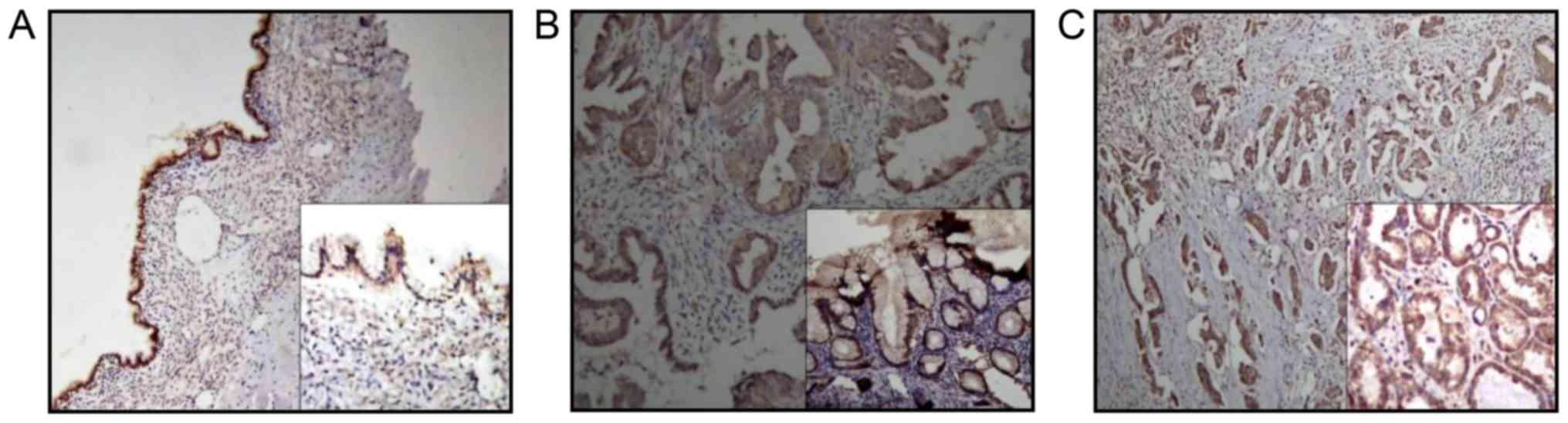
Immunohistochemical analysis was used to evaluate
MPP6 protein expression in ovarian tumor tissues. The MPP family
belongs to the MAGUK family. MPP6 exhibited cytomembrane and
cytoplasm expression in ovarian tumor tissues (Fig. 2). Fig.
2A-C are representative microphotographs of immunostained
tissue sections of BEOT, BOT and OC, respectively, highlighting
MPP6 staining. MPP6 expression in ovarian tumor samples was further
determined by western blot analysis. The results suggested that
MPP6 expression was significantly downregulated in OC compared with
BEOT and BOT specimen (P<0.01 and P<0.05, respectively;
Fig. 1E).
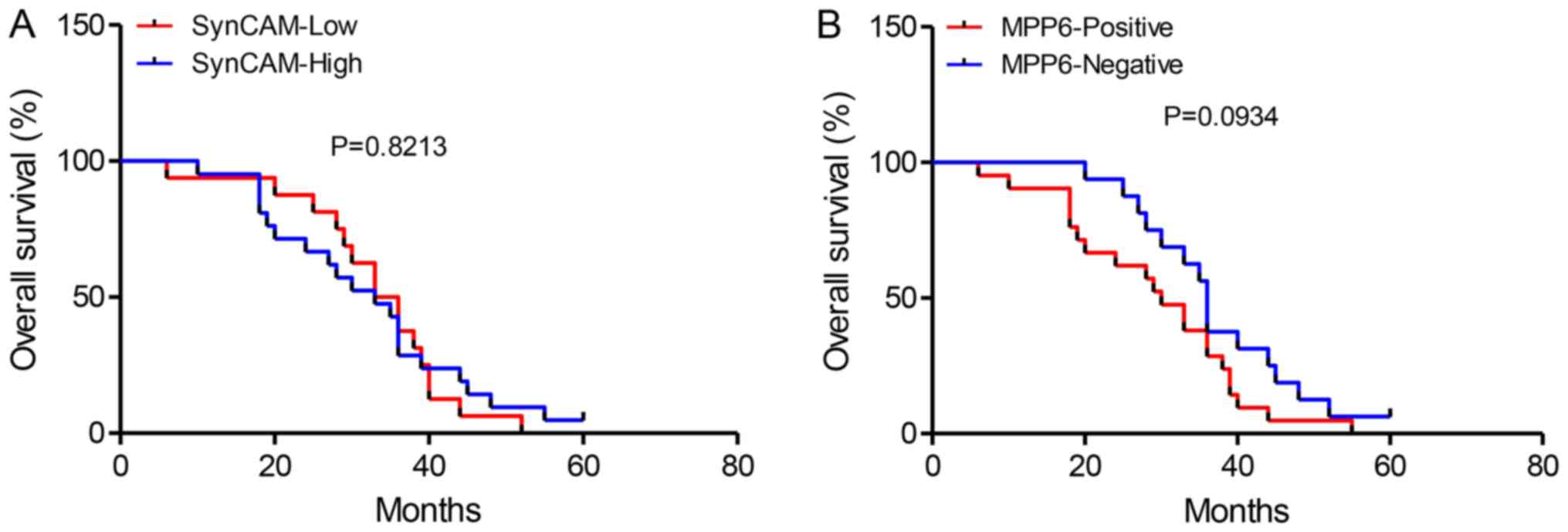
Association between survival and
expression of SynCAM and MPP6
SynCAM expression was not associated with overall
survival (OS; n=74; P=0.8213; Fig.
3A). MPP6 expression was associated with poorer OS time;
however not significantly (n=74; P=0.0934; Fig. 3B).
Discussion
Metastasis, recurrence and chemotherapeutic
resistance occur in patients with OC following radical resection
are leading causes of mortality worldwide (23,24).
SynCAMs, members of the immunoglobulin superfamily, encode for
membrane glycoproteins and participate in cell adhesion (25). SynCAMs associate with different
intracellular binding partners, including proteins of the MAGUK
family (26,27). SynCAMs act as tumor suppressors in
various types of human cancer including lung, prostate, pancreas
and breast cancer, and are preferentially inactivated in invasive
cancer (28,29). At present, to the best of our
knowledge, little is known about the functional role of SynCAMs in
OC. This study investigated the potential role of SynCAMs as tumor
suppressors in OC.
SynCAMs are tumor suppressors in non-small cell lung
cancer, hepatic cell carcinoma and pancreatic cancer (30,31). To
the best of our knowledge, SynCAM expression in OC has not yet been
reported. In the present study, it was demonstrated that SynCAM
expression was downregulated in human OC tumor tissues compared
with normal tissues, using immunohistochemical and western blot
analyses. The majority of paraffin-embedded human OC tissues
exhibited no evidence for SynCAM staining. The loss of SynCAM
expression was correlated with increased tumor size and
differentiation levels of tumor cells. SynCAM was expressed at
higher levels in normal ovarian tissue compared with OC tissue.
These findings demonstrated that SynCAMs may serve a tumor
suppressive role in human OC. It is suggested that OC cell
proliferation may be affected by downregulating SynCAM expression.
Loss of SynCAMs expression is observed in non-small cell lung
cancer, and could cause morphological transformation leading cancer
cells to invasion and/or metastasis (28). The results of the present study are
consistent with this finding.
Downregulation of SynCAM expression may further be
associated with silenced methylation (32). SynCAM mRNA levels were described to
be increased after demethylation in glioblastoma cell lines
(32). However, the mechanism of
SynCAM inactivation by promoter hypermethylation remains to be
elucidated. MPP6, a member of the palmitoylated membrane protein
subfamily of peripheral MAGUK, is primarily involved in controlling
epithelial cell polarity (33). MPPs
further function in tumor suppression and receptor clustering by
forming multiprotein complexes containing distinct sets of
transmembrane, cytoskeletal and cytoplasmic signaling proteins
(34). A direct association between
tumor suppression and cell polarity proteins is equivocal in
mammals (35). Disruption of cell
polarity and function causes abnormalities in vertebrates (35). Similar to E-cadherin, MPP loss can
disturb cell-cell junctions and the mechanical integrity of
epithelial cells, resulting in defective branching morphogenesis
and maintenance of epithelial tubular structures (36–38).
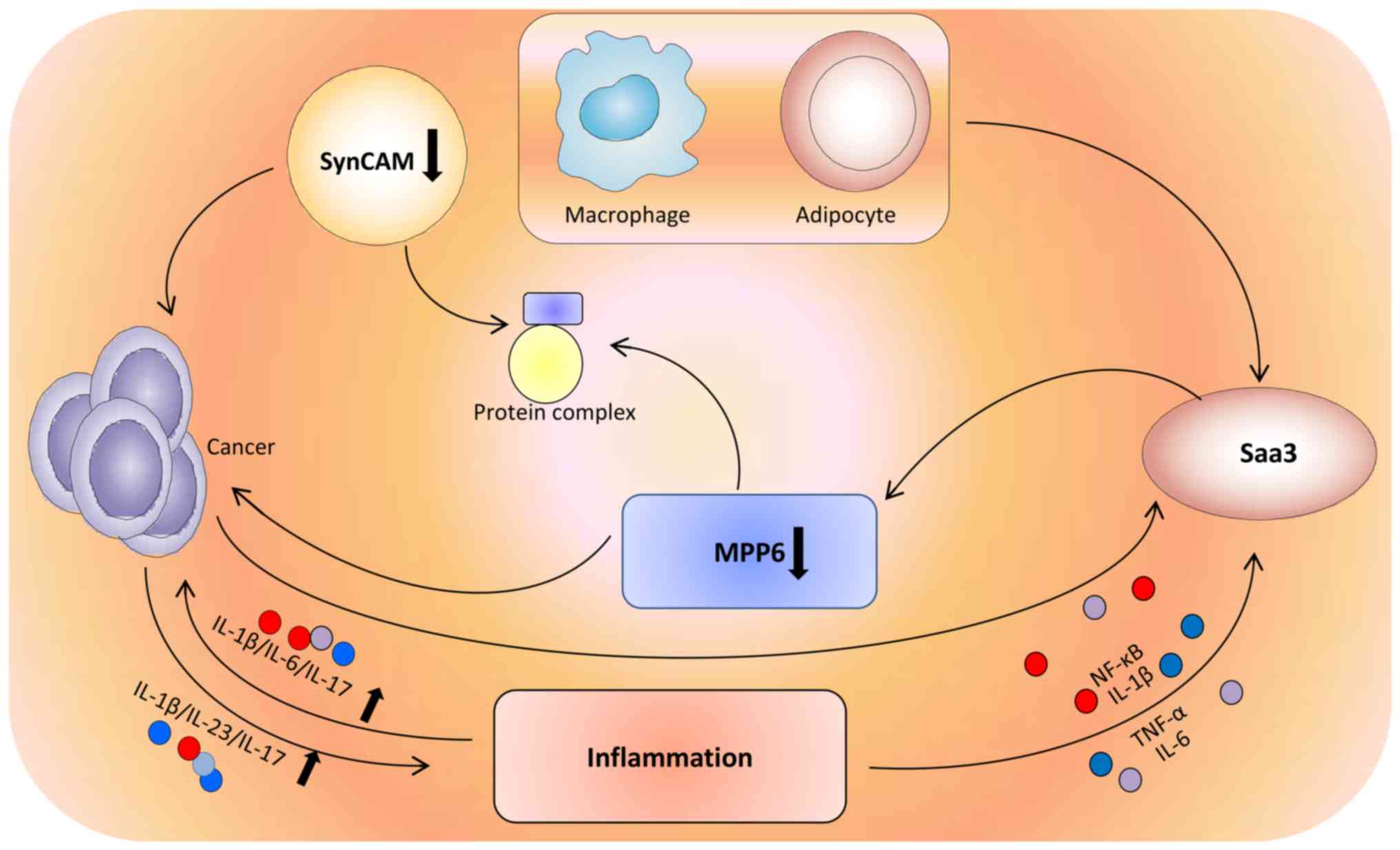
Saa3 is a member of the acute-phase serum amyloid A (SAA)
apolipoprotein family. Murine Saa3 has been shown to be expressed
in macrophages (39) and adipose
tissue (40). Accumulation of SAA
proteins in the blood is observed during chronic inflammation and
cancer (39). Saa3 expression is
effectively induced by interleukin (IL)-1β, tumor necrosis factor
(TNF)-α, and IL-6 through NF-κB signaling in inflammatory processes
(41). Inflammatory diseases greatly
increase the risk of cancer, due to elevated expression of
inflammatory cytokines, including IL-6, TNF, and IL-1β (42). Stimulating IL-23 and IL-17 production
can promote cancer development and progression (42). MPPs can interact with SynCAM and form
a protein complex (28). The
protumorigenic activity of Saa3 can be regulated by MPP6. MPP6
overexpression is responsible for the loss of the protumorigenic
effect of Saa3 in cancer-associated fibroblasts (41) (Fig.
4). In the present study, MPP6 immunolocalization was examined
in OC tissues. MPP6 exhibited cytomembrane and cytoplasm staining
and expression was significantly downregulated in ovarian tumor
tissues compared with normal tissues. SynCAM and MPP6 expression
may be used as prognostic factors to predict OS for individual
patients with OC. However, SynCAM was determined not to be a
prognostic factor and MPP6 expression was not significantly
associated with worse OS, which may be due to the small sample size
of OC in the present study. Further experiments are necessary to
elucidate the potential association between SynCAM and MPP6.
In summary, the data suggested that human OC cells
proliferate through downregulation of SynCAM expression. It is
suggested that decreased expression of SynCAM was associated with
downregulation of the tight junction protein MPP6. Therefore, a
SynCAM-regulated pathway may be an important molecular target in
the treatment of ovarian cancer and the associated biomarkers may
be used in a diagnostic clinical setting.
Acknowledgements
The authors would like to thank the research
assistance provided by Gansu Key Laboratory of Gynecologic Oncology
(Lanzhou, China).
Funding
The present study was supported by the Talent
Training Plan of Gansu Province.
Availability of data and materials
The datasets used or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
FXX and YY conceived and designed the study. Data
collection and experiments were performed by FXX, XS, JD and FHX.
AY, CZ and XZ analyzed the data. FX and YY wrote the manuscript.
All authors have read and approved of the final version of the
manuscript.
Ethics approval and consent to
participate
The research program used in the study was approved
by the Ethics Committee of the First Hospital of Lanzhou University
(Lanzhou, China), and written informed consent was obtained from
patients or patients' family.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jin M, Cai J, Wang X, Zhang T and Zhao Y:
Successful maintenance therapy with apatinib inplatinum-resistant
advanced ovarian cancer and literature review. Cancer Biol Ther.
19:1088–1092. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tang G, Guo J, Zhu Y, Huang Z, Liu T, Cai
J, Yu L and Wang Z: Metformin inhibits ovarian cancer via
decreasing h3k27 trimethylation. Int J Oncol. 52:1899–1911.
2018.PubMed/NCBI
|
|
4
|
Cannistra SA: Cancer of the ovary. N Engl
J Med. 351:2519–2529. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Winter WE III, Maxwell GL, Tian C, Carlson
JW, Ozols RF, Rose PG, Markman M, Armstrong DK, Muggia F and
McGuire WP; Gynecologic Oncology Group Study, : Prognostic factors
for stage III epithelial ovarian cancer: A Gynecologic Oncology
Group Study. J Clin Oncol. 25:3621–3627. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhuo C, Wu X, Li J, Hu D, Jian J, Chen C,
Zheng X and Yang C: Chemokine (C-X-C motif) ligand 1 is associated
with tumor progression and poor prognosis in patients with
colorectal cancer. Biosci Rep. 38:BSR201805802018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Áyen Á, Jiménez Martínez Y Marchal JA and
Boulaiz H: Recent progress in gene therapy for ovarian cancer. Int
J Mol Sci. 19:E19302018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Takai Y, Ikeda W, Ogita H and Rikitake Y:
The immunoglobulin-like cell adhesion molecule nectin and its
associated protein afadin. Annu Rev Cell Dev Biol. 24:309–342.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Biederer T, Sara Y, Mozhayeva M, Atasoy D,
Liu X, Kavalali ET and Südhof TC: SynCAM, a synaptic adhesion
molecule that drives synapse assembly. Science. 297:1525–1531.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gomyo H, Arai Y, Tanigami A, Murakami Y,
Hattori M, Hosoda F, Arai K, Aikawa Y, Tsuda H, Hirohashi S, et al:
A 2-Mb sequenceready contig map and a novel immunoglobulin
superfamily gene IGSF4 in the LOH region of chromosome 11q23.2.
Genomics. 62:139–146. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Frei JA and Stoeckli ET: SynCAMs-From axon
guidance to neurodevelopmental disorders. Mol Cell Neurosci.
81:41–48. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Frei JA, Andermatt I, Gesemann M and
Stoeckli ET: The SynCAM synaptic cell adhesion molecules are
involved in sensory axon pathfinding by regulating axon-axon
contacts. J Cell Sci. 127:5288–5302. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fowler DK, Peters JH, Williams C and
Washbourne P: Redundant postsynaptic functions of SynCAMs 1–3
during synapse formation. Front Mol Neurosci. 10:242017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Stagi M, Fogel AI and Biederer T: SynCAM 1
participates in axo-dendritic contact assembly and shapes neuronal
growth cones. Proc Natl Acad Sci USA. 107:7568–7573. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Maurel P, Einheber S, Galinska J, Thaker
P, Lam I, Rubin MB, Scherer SS, Murakami Y, Gutmann DH and Salzer
JL: Nectin-like proteins mediate axon Schwann cell interactions
along the internode and are essential for myelination. J Cell Biol.
178:861–874. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Casey JP, Magalhaes T, Conroy JM, Regan R,
Shah N, Anney R, Shields DC, Abrahams BS, Almeida J, Bacchelli E,
et al: A novel approach of homozygous haplotype sharing identifies
candidate genes in autism spectrum disorder. Hum Genet.
131:565–579. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Du H, Li W, Wang Y, Chen S and Zhang Y:
Celecoxib induces cell apoptosis coupled with up-regulation of the
expression of VEGF by a mechanism involving ER stress in human
colorectal cancer cells. Oncol Rep. 26:495–502. 2011.PubMed/NCBI
|
|
18
|
Wakayama Y, Inoue M, Kojima H, Murahashi
M, Shibuya S and Oniki H: Localization of sarcoglycan, neuronal
nitric oxide synthase, beta-dystroglycan, and dystrophin molecules
in normal skeletal myofiber: Triple immunogold labeling electron
microscopy. Microsc Res Tech. 55:154–163. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ahmadzada T, Kao S, Reid G, Boyer M, Mahar
A and Cooper WA: An update on predictive biomarkers for treatment
selection in non-small cell lung cancer. J Clin Med. 7:E1532018.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liang C, Wang S, Qin C, Bao M, Cheng G,
Liu B, Shao P, Lv Q, Song N, Hua L, et al: TRIM36, a novel
androgen-responsive gene, enhances anti-androgen efficacy against
prostatecancer by inhibiting MAPK/ERK signaling pathways. Cell
Death Dis. 9:1552018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu D, Zhang XX, Li MC, Cao CH, Wan DY, Xi
BX, Tan JH, Wang J, Yang ZY, Feng XX, et al: C/EBPβ enhances
platinum resistance of ovarian cancer cells by reprogramming H3K79
methylation. Nat Commun. 9:17392018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Webber C, Gospodarowicz M, Sobin LH,
Wittekind C, Greene FL, Mason MD, Compton C, Brierley J and Groome
PA: Improving the TNM classification: Findings from a 10-year
continuous literature review. Int J Cancer. 135:371–378. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gao Y, Foster R, Yang X, Feng Y, Shen JK,
Mankin HJ, Hornicek FJ, Amiji MM and Duan Z: Up-regulation of CD44
in the development of metastasis, recurrence and drug resistance of
ovarian cancer. Oncotarget. 6:9313–9326. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Vaughan S, Coward JI, Bast RC Jr, Berchuck
A, Berek JS, Brenton JD, Coukos G, Crum CC, Drapkin R,
Etemadmoghadam D, et al: Rethinking ovarian cancer: Recommendations
for improving outcomes. Nat Rev Cancer. 11:719–725. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Galuska SP, Rollenhagen M, Kaup M, Eggers
K, Oltmann- Norden I, Schiff M, Hartmann M, Weinhold B, Hildebrandt
H, Geyer R, et al: Synaptic cell adhesion molecule SynCAM 1 is a
target for polysialylation in postnatal mouse brain. Proc Natl Acad
Sci USA. 107:10250–10255. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Frei JA and Stoeckli ET: SynCAMs extend
their functions beyond the synapse. Eur J Neurosci. 39:1752–1760.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cheadle L and Biederer T: The novel
synaptogenic protein Farp1 links postsynaptic cytoskeletal dynamics
and transsynaptic organization. J Cell Biol. 199:985–1001. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sakurai-Yageta M, Masuda M, Tsuboi Y, Ito
A and Murakami Y: Tumor suppressor CADM1 is involved in epithelial
cell structure. Biochem Biophys Res Commun. 390:977–982. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kuramochi M, Fukuhara H, Nobukuni T, Kanbe
T, Maruyama T, Ghosh HP, Pletcher M, Isomura M, Onizuka M, Kitamura
T, et al: TSLC1 is a tumor-suppressor gene in human non-small-cell
lung cancer. Nat Genet. 27:427–430. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Watabe K, Ito A, Koma YI and Kitamura Y:
IGSF4: A new intercellular adhesion molecule that is called by
three names, TSLC1, SgIGSF and SynCAM, by virtue of its diverse
function. Histol Histopathol. 18:1321–1329. 2003.PubMed/NCBI
|
|
31
|
Tsujiuchi T, Sugata E, Masaoka T, Onishi
M, Fujii H, Shimizu K and Honoki K: Expression and DNA methylation
patterns of Tslc1 and Dal-1 genes in hepatocellular carcinomas
induced by N-nitrosodiethylamine in rats. Cancer Sci. 98:943–948.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang X, Li W, Kang Y, Zhang J and Yuan H:
SynCAM, a novel putative tumor suppressor, suppresses growth and
invasiveness of glioblastoma. Mol Biol Rep. 40:5469–5475. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Quinn BJ, Welch EJ, Kim AC, Lokuta MA,
Huttenlocher A, Khan AA, Kuchay SM and Chishti AH: Erythrocyte
scaffolding protein p55/MPP1 functions as an essential regulator of
neutrophil polarity. Proc Natl Acad Sci USA. 106:19842–19847. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tseng TC, Marfatia SM, Bryant PJ, Pack S,
Zhuang Z, O'Brien JE, Lin L, Hanada T and Chishti AH: VAM-1: A new
member of the MAGUK family binds to human Veli-1 through a
conserved domain. Biochim Biophys Acta. 1518:249–259. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Saito Y, Desai RR and Muthuswamy SK:
Reinterpreting polarity and cancer: The changing landscape from
tumor suppression to tumor promotion. Biochim Biophys Acta Rev
Cancer. 1869:103–116. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mahoney ZX, Sammut B, Xavier RJ,
Cunningham J, Go G, Brim KL, Stappenbeck TS, Miner JH and Swat W:
Discs-large homolog 1 regulates smooth muscle orientation in the
mouse ureter. Proc Natl Acad Sci USA. 103:19872–19877. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Nechiporuk T, Fernandez TE and Vasioukhin
V: Failure of epithelial tube maintenance causes hydrocephalus and
renal cysts in Dlg5-/- mice. Dev Cell. 13:338–350. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Schneider MR, Dahlhoff M, Horst D, Hirschi
B, Trülzsch K, Müller-Hücker J, Vogelmann R, Allgäuer M, Gerhard M,
Steininger S, et al: A key role for E-cadherin in intestinal
homeostasis and Paneth cell maturation. PLoS One. 5:e143252010.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ather JL, Ckless K, Martin R, Foley KL,
Suratt BT, Boyson JE, Fitzgerald KA, Flavell RA, Eisenbarth SC and
Poynter ME: Serum amyloid A activates the NLRP3 inflammasome and
promotes Th17 allergic asthma in mice. J Immunol. 187:64–73. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sommer G, Weise S, Kralisch S, Scherer PE,
Lössner U, Blüher M, Stumvoll M and Fasshauer M: The adipokine SAA3
is induced by interleukin-1beta in mouse adipocytes. J Cell
Biochem. 104:2241–2247. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Djurec M, Graña O, Lee A, Troulé K,
Espinet E, Cabras L, Navas C, Blasco MT, Martín-Díaz L, Burdiel M,
et al: Saa3 is a key mediator of the protumorigenic properties of
cancer-associated fibroblasts in pancreatic tumors. Proc Natl Acad
Sci USA. 115:E1147–E1156. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhong Z, Sanchez-Lopez E and Karin M:
Autophagy, inflammation, and immunity: A troika governing cancer
and its treatment. Cell. 166:288–298. 2016. View Article : Google Scholar : PubMed/NCBI
|