Introduction
The BCR-ABL oncoprotein is the culprit of chronic
myeloid leukemia (CML) as it transforms the hematopoietic stem cell
altering its survival, proliferation and interaction with both the
cell cytoskeleton and the bone marrow microenvironment (1–6). In the
last 15 years, multiple tyrosine kinase inhibitors (TKIs) have been
approved for the first line treatment of the disease including
imatinib (IM), dasatinib (DAS), nilotinib (NIL) and bosutinib (BOS)
(7–12). From a genetic standpoint, the
BCR-ABL1 chimeric oncogene derives from the t(9;22)
reciprocal translocation that generates the Philadelphia (Ph)
chromosome (13). Most patients
express one of three fusion transcripts juxtaposing BCR
exons 1, 13 or 14 with exon 2 of ABL1 (14). However, several alternative
breakpoints involving different BCR and/or ABL1 exons
have been previously described. Specifically, BCR exons 1,
6, 8, 13, 14 and 19 can rearrange with ABL1 exons 2 or 3,
generating e6a2, e8a2, e1a3, e13a3, e14a3, e19a3 fusions (15).
BCR-ABL1 fusions involving ABL1 exon 3
are extremely rare (0.3%) and are usually associated with
contrasting clinical outcome (16,17). In
the present study we report a CML patient expressing an atypical
e14a3 BCR-ABL1 transcript that was successfully treated with
NIL.
Case report
In October 2016 a 52 year-old male (Table I) with a history of neutrophilia in
the absence of thrombocytosis was admitted to the Hematology ward
and subjected to a bone marrow aspirate in order to perform a
karyotype analysis by G-banding. His cytogenetic profile showed the
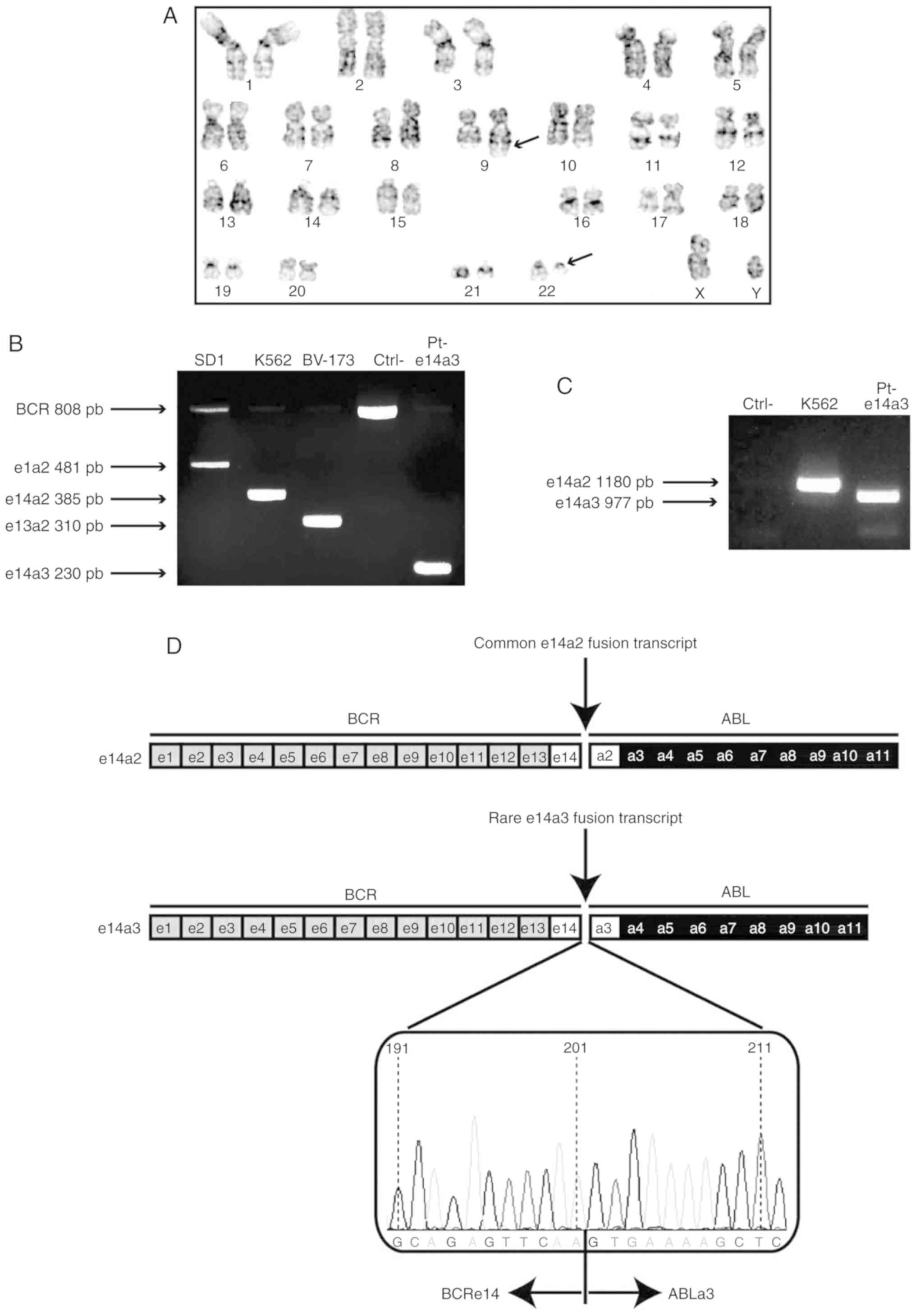
t(9;22) translocation in 100% of the analyzed metaphases (20/20)
(Fig. 1A). At this time, his white
blood cells (WBCs) were lysed and used to extract total messenger
RNA (mRNA) that was reverse transcribed in cDNA and employed to
perform a multiplex RT-PCR in order to determine his
BCR-ABL1 fusion transcript variant (18). An atypical band of approximately 230
base pairs was detected (Fig. 1B)
with no amplification of the common BCR-ABL1 variants in
real-time PCR, performed as previously described (19,20).
However, the detection of the Ph chromosome by G-banding led to
exclude that this 230 base pair band was a nonspecific RT-PCR
product. Hence, we employed the cDNA for a new PCR reaction using
forward (BCR-10 5′-TATGACTGCAAATGGTACATTCC-3′) and reverse
(ABL-4 5′-TCGTAGTTGGGGGACACACC-3′) primers recognizing exons
10 and 4 of BCR and ABL1, respectively. The 977 bp
PCR product (Fig. 1C) generated by
the Pfx platinum DNA polymerase enzyme was then cloned in the
pcr4-TOPO-TA vector according to the manufacture's protocol (all
from Thermofisher Scientific). Plasmid DNA obtained from ten
individual bacterial colonies was then subjected to Sanger
sequencing and each colony showed the e14a3 fusion transcript. One
representative pherogram displaying the BCRe14 and
ABL1a3 gene exons rearrangement is depicted in Fig. 1D. Finally, Sokal (0.79, low), Hasford
(949, intermediate), EUTOS (11, low) and ELTS (1.39, low) scores
were calculated as indicated in Table
I. In conclusion, the patient was diagnosed with chronic phase
CML expressing a rare e14a3 BCR-ABL1 variant. Although IM
represents an excellent first line treatment for CML, previous data
show that not all patients expressing the e14a3 isoform benefit
from this drug (16,21). Thus, the patient, with no additional
medication, received NIL 300 mg bis in die (BID), as this
compound is a more potent inhibitor of ABL1 catalytic activity.
 | Table I.Patient characteristics at the time
of diagnosis. |
Table I.
Patient characteristics at the time
of diagnosis.
| Patient
characteristic | Values |
|---|
| Complete blood
count |
|
|
Platelets (µl) | 207.000 |
| WBCs
(µl) |
53910×103 |
|
Neutrophils | 64% |
|
Eosinophils | 3% |
|
Basophils | 1% |
|
Lymphocytes | 10% |
|
Monocytes | 1% |
|
Metamyelocytes | 5% |
|
Myelocytes | 10% |
|
Promyelocytes | 4% |
|
Myeloblasts | 2% |
|
Haemoglobin (g/dl) | 12.5 |
| Cytogenetic
analysis |
|
|
Karyotype | 46, XY,100% |
|
|
(9;22)(q34;q11) |
| Fusion
transcript |
|
|
BCR-ABL1 | e14a3 |
|
Relative risk |
|
|
Sokal | 0.79 (Low) |
|
Hasford | 949
(Intermediate) |
|
EUTOS | 11 (Low) |
|
ELTS | 1.39 (Low) |
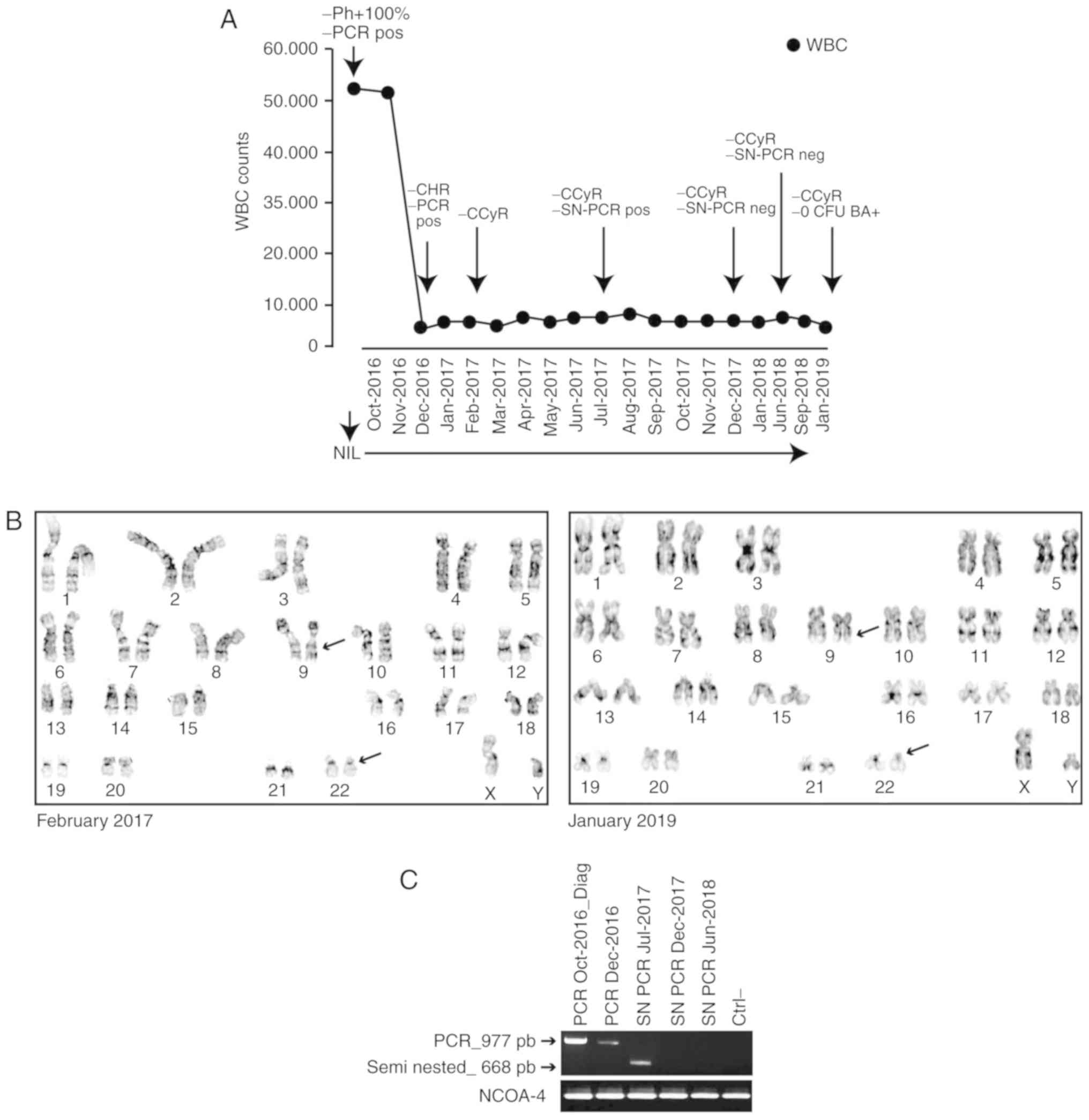
Disease evolution was monitored measuring both
hematological and cytogenetic parameters. After two months of NIL,
the patient achieved a complete hematological response (CHR) and,
in February 2017, a new cytogenetic analysis failed to detect Ph+
metaphases suggesting a complete cytogenetic response (CCyR).
During the following months, the patient maintained both his CHR
and CCRy (Fig. 2A and B).
To molecularly monitor the disease, we evaluated the
presence of the e14a3 BCR-ABL1 gene by PCR and the negative
reaction was confirmed by semi nested-PCR (SN-PCR) at the time
points indicated in Fig. 2A. A first
round of PCR was performed using the primers specified above while
for the SN-PCR we employed a Fw primer recognizing exon 12 of the
BCR gene (BCR-12 5′-GTGCAGAGTGGAGGGAGAACA−3′)
obtaining a PCR product of approximately 668 bp. In December 2016
the PCR detected the e14a3 fusion transcript. However, in July
2017, BCR-ABL1 mRNA-reverse transcribed by Superscript III
One-Step RT-PCR (Thermofisher Scientific)-was detected only after
SN-PCR and by December 2017 and June 2018 neither reaction detected
the e14a3 fusion transcript suggesting a strong reduction in the
overall number of BCR-ABL1-positive cells (Fig. 2B).
In order to evaluate the depth of the response to
NIL, in January 2019 we isolated CD34+ cells from peripheral blood
performing a colony forming units (CFU) assay as previously
described (22). Ten CD34+-derived
colonies were collected and total RNA extracted using the Trizol
reagent (Life Technologies). We subsequently employed the
Superscript III One-Step RT-PCR (Life Technologies) to detect the
e14a3 BCR-ABL1 fusion. Negative colonies for the first
BCR-ABL1 amplification were subjected to a semi-nested PCR
as described above. Interestingly, we failed to detect the fusion
transcript in any of the analyzed colonies (data not show).
At the last control (January 2019), the patient is
still receiving NIL with no clinical, hematological, cytogenetic or
molecular signs of disease progression. Furthermore, he has not
reported side effects to NIL treatment during the course of his
regular outpatient visits.
Discussion
BCR-ABL1 transcripts displaying breakpoints
lacking ABL1 exon 2 comprise e1a3, e13a3 and e14a3
rearrangements and represent infrequently occurring oncogenic
isoforms (21). A methodological
issue may have contributed to the uncommon detection of these
transcripts as multiplex RT-PCR reactions can generate atypical PCR
fragments often interpreted as nonspecific products. In our study,
the detection of the t(9;22) translocation by G-banding indicated
the presence of a Ph chromosome. We therefore decided to employ
primers recognizing more distant exons from the common
BCR-ABL1 breakpoints and successfully identified the
atypical BCR-ABL1 e14a3 rearrangement. To date, different
cases of CML patients expressing this isoform have been reported
(16,21,23,24).
Although IM represents an excellent first line therapy for most CML
patients (7), extensive published
data suggest that patients receiving this drug may more frequently
develop both BCR-ABL-dependent and BCR-ABL-independent resistance
to therapy (8,19,25)
requiring alternative treatments (26). Furthermore, an inverse correlation
has been reported between the size of the BCR portion retained in
the oncogenic fusion protein and CML aggressiveness (27). Indeed, complex variant
translocations, intron-derived insertions/truncations in the
BCR-ABL1 kinase domain or hyperdiploidy suggest that CML patients
displaying these genetic alterations often present an unfavorable
clinical outcome and inferior IM responses (13,16,21,28,29).
The ABL1 a2 exon encodes for a Src homology
domain 3 (SH3) and, although its loss may lead to reduced
leukemogenesis with a benign clinical course, its role as a
negative regulator of the ABL kinase domain (SH1) may explain the
reportedly more aggressive CML phenotype (30,31).
Moreover, while the e14a3 BCR-ABL1 breakpoint preserves the
ATP binding domain, the lack of the SH3 domain may modify the SH1
domain tertiary structure thus affecting drug response (32).
On the base of these findings and the controversial
data on IM efficacy in subjects displaying the e14a3 transcript
(16), we wanted to employ a
second-generation TKI (2G-TKI) as first line treatment. Nilotinib
was selected for his treatment because DAS was temporarily
unavailable in our pharmacy at the time. Since the patient is an
accountant who works from home, he has shown excellent compliance
with the administration of the drug 1 h before meals.
Using NIL, a more potent ABL1 inhibitor (9), we observed a rapid decline in Ph
metaphases that generated a CCyR after 4 months of NIL. Finally,
employing a semi-nested RT-PCR we failed to detect BCR-ABL1
transcripts both in peripheral WBCs and in CFUs grown in
methylcellulose, indicating that the drug induced a rapid decline
in the overall number of leukemic cells.
In summary, CML patients expressing the e14a3
BCR-ABL1 fusion transcript may be undiagnosed because this
rearrangement generates an atypical PCR product often
misinterpreted as a nonspecific band that is then coupled to a
negative real-time PCR result. Hence, performing a cytogenetic
analysis is critical to identify these CML patients. Moreover, the
difficulty to perform a precise quantitative molecular monitoring
and the rare incidence of atypical BCR-ABL1 fusion
transcripts does not allow the design of randomized clinical trials
that may compare the efficacy of IM vs. 2G-TKIs.
We conclude that the BCR-ABL1 oncoprotein, derived
from BCRe14 and ABL1a3 exons rearrangement, is highly
sensitive to NIL, suggesting that chronic phase CML patients
exhibiting this rare rearrangement may quickly achieve CCyR
followed by strong reductions in the size of the leukemic
clone.
Acknowledgements
Not applicable.
Funding
Piano Sanitario Nazionale 2015 (grant no. Linea
Progettuale 6-Azione 6.3).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
MM drafted the manuscript; MM, ET and SS were
responsible for study conception, and designed and performed the
experiments; MM, ET, SS, MSP, AP, SRV and CR analyzed and
interpreted the data; MLC performed cytogenetic analysis; FS and VZ
made a critical revision of the paper and managed the patient; FDR
supervised the project and contributed to study design; LM
conceived the original idea and supervised the project. All authors
approved the final version of the manuscript to be published.
Ethics approval and consent to
participate
The patient provided written informed consent to
participate. The study adheres to the declaration of Helsinki and
the biological samples were collected following an institutionally
approved protocol at the Azienda Ospedaliero-Universitaria
‘Policlinico-Vittorio Emanuele’ (Catania, Italy).
Patient consent for publication
Written informed consent obtained from patient for
publication of this report.
Competing interest
The authors declare that they have no competing
interests.
References
|
1
|
Ren R: Mechanisms of BCR-ABL in the
pathogenesis of chronic myelogenous leukaemia. Nat Rev Cancer.
5:172–183. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stella S, Tirrò E, Conte E, Stagno F, Di
Raimondo F, Manzella L and Vigneri P: Suppression of survivin
induced by a BCR-ABL/JAK2/STAT3 pathway sensitizes
imatinib-resistant CML cells to different cytotoxic drugs. Mol
Cancer Ther. 12:1085–1098. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ishii Y, Nhiayi MK, Tse E, Cheng J,
Massimino M, Durden DL, Vigneri P and Wang JY: Knockout serum
replacement promotes cell survival by preventing BIM from inducing
mitochondrial cytochrome C release. PLoS One. 10:e01405852015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Manzella L, Tirrò E, Pennisi MS, Massimino
M, Stella S, Romano C, Vitale SR and Vigneri P: Roles of interferon
regulatory factors in chronic myeloid leukemia. Curr Cancer Drug
Targets. 16:594–605. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Preyer M, Vigneri P and Wang JY: Interplay
between kinase domain autophosphorylation and F-actin binding
domain in regulating imatinib sensitivity and nuclear import of
BCR-ABL. PLoS One. 6:e170202011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Giallongo C, Tibullo D, La Cava P, Branca
A, Parrinello N, Spina P, Stagno F, Conticello C, Chiarenza A,
Vigneri P, et al: BRIT1/MCPH1 expression in chronic myeloid
leukemia and its regulation of the G2/M checkpoint. Acta Haematol.
126:205–210. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stagno F, Stella S, Spitaleri A, Pennisi
MS, Di Raimondo F and Vigneri P: Imatinib mesylate in chronic
myeloid leukemia: Frontline treatment and long-term outcomes.
Expert Rev Anticancer Ther. 16:273–278. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hochhaus A, Larson RA, Guilhot F, Radich
JP, Branford S, Hughes TP, Baccarani M, Deininger MW, Cervantes F,
Fujihara S, et al: Long-term outcomes of imatinib treatment for
chronic myeloid leukemia. N Engl J Med. 376:917–927. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hochhaus A, Rosti G, Cross NC, Steegmann
JL, le Coutre P, Ossenkoppele G, Petrov L, Masszi T, Hellmann A,
Griskevicius L, et al: Frontline nilotinib in patients with chronic
myeloid leukemia in chronic phase: Results from the European
ENEST1st study. Leukemia. 30:57–64. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cortes JE, Saglio G, Kantarjian HM,
Baccarani M, Mayer J, Boqué C, Shah NP, Chuah C, Casanova L,
Bradley-Garelik B, et al: Final 5-year study results of DASISION:
The dasatinib versus imatinib study in treatment-naïve chronic
myeloid leukemia patients trial. J Clin Oncol. 34:2333–2340. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Stagno F, Vigneri P, Cupri A, Stella S and
Di Raimondo F: Personalized strategies for CML patients considering
discontinuation of tyrosine kinase inhibitors treatment. Leuk Res.
36:1208–1209. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cortes JE, Gambacorti-Passerini C,
Deininger MW, Mauro MJ, Chuah C, Kim DW, Dyagil I, Glushko N,
Milojkovic D, le Coutre P, et al: Bosutinib versus imatinib for
newly diagnosed chronic myeloid leukemia: Results from the
randomized BFORE trial. J Clin Oncol. 36:231–237. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Stagno F, Vigneri P, Del Fabro V, Stella
S, Cupri A, Massimino M, Consoli C, Tambè L, Consoli ML, Antolino
A, et al: Influence of complex variant chromosomal translocations
in chronic myeloid leukemia patients treated with tyrosine kinase
inhibitors. Acta Oncol. 49:506–508. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Laurent E, Talpaz M, Kantarjian H and
Kurzrock R: The BCR gene and philadelphia chromosome-positive
leukemogenesis. Cancer Res. 61:2343–2355. 2001.PubMed/NCBI
|
|
15
|
Burmeister T and Reinhardt R: A multiplex
PCR for improved detection of typical and atypical BCR-ABL fusion
transcripts. Leuk Res. 32:579–585. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gui X, Zhang Y, Pan J, Qiu H, Cen J, Xue
Y, Chen S, Shen H, Yao L, Zhang J, et al: Chronic myeloid leukemia
with e14a3 BCR-ABL transcript: Analysis of characteristics and
prognostic significance. Leuk Lymphoma. 56:3343–3347. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chisti MM and Sanders DS: Chronic myeloid
leukemia with b3a3 (e14a3) fusion: A rare BCR/ABL rearrangement
presenting with thrombocytosis-does MTHFR polymorphism matter. Case
Rep Oncol. 11:485–492. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cross NCP: Detection of BCR-ABL in
hematological malignancies by RT-PCR. Methods Mol Med. 6:25–36.
1996.PubMed/NCBI
|
|
19
|
Vigneri P, Stagno F, Stella S, Cupri A,
Forte S, Massimino M, Antolino A, Siragusa S, Mannina D, Impera SS,
et al: High BCR-ABL/GUSIS levels at diagnosis of chronic
phase CML are associated with unfavorable responses to
standard-dose imatinib. Clin Cancer Res. 23:7189–7198. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vella V, Puppin C, Damante G, Vigneri R,
Sanfilippo M, Vigneri P, Tell G and Frasca F: DeltaNp73alpha
inhibits PTEN expression in thyroid cancer cells. Int J Cancer.
124:2539–2548. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jinawath N, Norris-Kirby A, Smith BD,
Gocke CD, Batista DA, Griffin CA and Murphy KM: A rare e14a3 (b3a3)
BCR-ABL fusion transcript in chronic myeloid leukemia: Diagnostic
challenges in clinical laboratory practice. J Mol Diagn.
11:359–363. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Massimino M, Consoli ML, Mesuraca M,
Stagno F, Tirrò E, Stella S, Pennisi MS, Romano C, Buffa P, Bond
HM, et al: IRF5 is a target of BCR-ABL kinase activity and reduces
CML cell proliferation. Carcinogenesis. 35:1132–1143. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hu LH, Pu LF, Yang DD, Zhang C, Wang HP,
Ding YY, Li MM, Zhai ZM and Xiong S: How to detect the rare BCR-ABL
(e14a3) transcript: A case report and literature review. Oncol
Lett. 14:5619–5623. 2017.PubMed/NCBI
|
|
24
|
Cai H, Yang L, Shen K, Zhang W, Xiong J,
Zhang M, Mao X, Wang Y and Xiao M: A rare e14a3 BCR/ABL fusion
transcript in acute lymphoblastic leukemia patient treated with
CAR-modified T-cell therapy. Oncol Lett. 15:2491–2494.
2018.PubMed/NCBI
|
|
25
|
Buffa P, Romano C, Pandini A, Massimino M,
Tirrò E, Di Raimondo F, Manzella L, Fraternali F and Vigneri PG:
BCR-ABL residues interacting with ponatinib are critical to
preserve the tumorigenic potential of the oncoprotein. FASEB J.
28:1221–1236. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Massimino M, Stella S, Tirrò E, Romano C,
Pennisi MS, Puma A, Manzella L, Zanghì A, Stagno F, Di Raimondo F
and Vigneri P: Non ABL-directed inhibitors as alternative treatment
strategies for chronic myeloid leukemia. Mol Cancer. 17:562018.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yao J, Douer D, Wang L, Arcila ME, Nafa K
and Chiu A: A case of acute myeloid leukemia with e6a2 BCR-ABL
fusion transcript acquired after progressing from chronic
myelomonocytic leukemia. Leuk Res Rep. 7:17–19. 2017.PubMed/NCBI
|
|
28
|
Cayuela JM, Rousselot P, Nicolini F,
Espinouse D, Ollagnier C, Bui-Thi MH, Chabane K, Raffoux E,
Callet-Bauchu E, Tigaud I, et al: Identification of a rare e8a2
BCR-ABL fusion gene in three novel chronic myeloid leukemia
patients treated with imatinib. Leukemia. 19:2334–2336. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Stagno F, Vigneri P, Consoli ML, Cupri A,
Stella S, Tambè L, Massimino M, Manzella L and Di Raimondo F:
Hyperdiploidy associated with a high BCR-ABL transcript level may
identify patients at risk of progression in chronic myeloid
leukemia. Acta Haematol. 127:7–9. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fujisawa S, Nakamura S, Naito K, Kobayashi
M and Ohnishi K: A variant transcript, e1a3, of the minor BCR-ABL
fusion gene in acute lymphoblastic leukemia: Case report and review
of the literature. Int J Hematol. 87:184–188. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Snyder DS, McMahon R, Cohen SR and Slovak
ML: Chronic myeloid leukemia with an e13a3 BCR-ABL fusion: Benign
course responsive to imatinib with an RT-PCR advisory. Am J
Hematol. 75:92–95. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Smith KM, Yacobi R and Van Etten RA:
Autoinhibition of Bcr-Abl through its SH3 domain. Mol Cell.
12:27–37. 2003. View Article : Google Scholar : PubMed/NCBI
|