Introduction
Clear-cell renal cell carcinoma (ccRCC) comprises
the majority of malignant tumors in the kidneys (1) and inactivation of the von Hippel-Lindau
(VHL) gene is the driving force in ccRCC tumorigenesis. The VHL
gene is somatically mutated in up to 80% of sporadic ccRCCs and is
causative for the familial cancer syndrome VHL disease that
predisposes affected patients to hereditary ccRCC (2). VHL inactivation results in
stabilization of hypoxia-inducible factor (HIF), which activates
many downstream hypoxia-driven genes, including vascular
endothelial growth factor and other genes involved in angiogenesis
(3). In addition, there is now a
greater appreciation of HIF-independent VHL functions. For
instance, VHL loss triggers HIF-independent senescence that is
mediated by retinoblastoma protein (Rb) and p400 (4,5).
Consistently, senescence occurs in VHL-deficient renal epithelial
cells in vivo (6). The
induction of senescence via the inactivation of tumor-suppressor
genes has also been shown for neurofibromin 1 and
PTEN, and also via the activation of oncogenes such as
RAS and BRAF (7–10). This
mechanism is considered important in order to limit tumor
development in mammalian cells (11).
Senescence induced by oncogene activation decreases
the expression of Lamin-B1 (LMNB1) (12), a nuclear intermediate filament
protein that plays a vital role in DNA replication, the formation
of the mitotic spindle, gene transcription and the maintenance of
cell proliferation (13).
LMNB1 is an E2F target gene (14) and its down regulation during
senescence is mediated by Rb. LMNB1 downregulation has been
reported to be a key event following senescence induction and is a
trigger of local chromatin changes that result in an impact on
global gene expression (15). In
addition, silencing LMNB1 expression decreases proliferation and
induces premature senescence in human diploid fibroblasts (12). Furthermore, adult-onset autosomal
dominant leukodystrophy, a slow progressive neurological disorder
characterized by symmetrical widespread myelin loss in the central
nervous system, is caused by LMNB1 duplications resulting in
increased gene expression in brain tissue (16).
Little is known about LMNB1 in cancer development
and progression. However, LMNB1 expression has been shown to be
reduced in gastrointestinal tract neoplasms (17) and lung cancer (18). On the other hand, increased
LMNB1 expression has been observed in prostate (19) and liver cancers (20), and is associated with a poorer
clinical outcome in patients with colon (21) and pancreatic cancers (22).
Very little is known about LMNB1 expression
in RCC. Therefore, the present study examined the protein
expression of LMNB1 in a large, hospital cohort of patients with
RCC with long-term follow-up information to determine if LMNB1 may
serve as a novel biomarker in RCC.
Materials and methods
Patients
Tissue samples from 932 patients with primary RCCs
treated at the Department of Urology at the University of
Heidelberg (Heidelberg, Germany) between 1987 and 2005 were
provided by the Tissue Bank of the National Centre for Tumor
Diseases Heidelberg (Table I) and
used retrospectively in the present study after approval was
obtained from the Ethics Committee of the University of Heidelberg.
Further details regarding patient evaluation for clinical follow-up
have been described previously (23).
 | Table I.Clinical and pathological features of
the study population (n=622). |
Table I.
Clinical and pathological features of
the study population (n=622).
| Feature | Total n, (%) |
|---|
| Fuhrman grade |
|
| 1 | 170 (27.3) |
| 2 | 345 (55.5) |
| 3 | 107 (17.2) |
| Tumor grade |
|
| 1 | 334 (53.7) |
| 2 | 49 (7.9) |
| 3 | 217 (34.9) |
| 4 | 22 (3.5) |
| Local lymph node
metastasis |
|
| No | 588 (94.5) |
|
Yes | 34 (5.5) |
| Distant
metastasis |
|
| No | 521 (83.8) |
|
Yes | 101 (16.2) |
| Sex |
|
|
Female | 249 (40.0) |
|
Male | 373 (60.0) |
| Age at surgery,
years |
|
|
≤65 | 353 (56.8) |
|
>65 | 269 (43.2) |
| ECOG |
|
| 0 | 381 (61.3) |
|
>0 | 241 (38.7) |
Tissue microarray
A tissue microarray containing 932 primary tumors
and the corresponding matched normal tissue samples of the 932
patients was created. The tumors were graded according to the
3-tiered nuclear grading system (24) and pathologically staged based on the
Tumor-Node-Metastasis classification of 2009 (25).
Immunohistochemistry
Tissues were buffered in 4% formalin overnight in
Sakura VIP 2000 (cat. no. 5217031; Sakura Finetek Europe B.V.) at
37°C followed by paraffin-embedding at 60°C. In the next step
tissues were cut into 2 µm thick slices using a microtome (Hyrax
M55; Carl Zeiss AG). Target retrieval solution (cat. no. K8005;
DakoCytomation; Dako; Agilent Technologies, Inc.) was used for
antigen retrieval. Briefly, following heat-induced antigen
retrieval, which was comprised of initial heating at 94–98°C for 5
min, slides were washed with the kit's washing buffer, rehydrated
in descending alcohol series with ethanol concentrations ranging
from 50–100% and xylene, and blocked with the kit's blocking
solution (Envision Flex Peroxidase-Blocking Reagent) at room
temperature for 5 min. Tissue microarray slides were stained with a
polyclonal anti-LMNB1 rabbit antiserum (1:1,000) for 30 min at room
temperature (Abcam; cat. no. ab16048). For Ki-67, sections were
incubated with Flex monoclonal Mouse anti-human Ki-67 Clone MIB-1
(dilution: ready to use solution; EnVision FLEX; Agilent
Technologies, Inc.; cat. no. IR626) at room temperature for 20 min
according to the manufacturer's protocol.
Staining was performed at room temperature using an
automated staining system (Autostainer Plus; Dako; Agilent
Technologies, Inc.) in accordance with the manufacturer's
instructions using the following solutions: Flex Envision (cat. no.
8002); Envision Flex DAB+ Chromogen (cat. no. 20052435), Envision
Flex Antibody Diluent (cat. no. K8006), Envision Flex Rabbit
(Linker; cat. no. K8019) and Dako Real Hematoxylin (cat. no.
S2020). LMNB1 expression was independently scored by light
microscopy with magnification up to ×400 by two of the authors who
were blinded to tissue annotations and patient outcomes. For the
cell line experiments, digital image analysis was performed.
Cell lines and cell culture
experiments
The RCC cell lines, ACHN, 769-P, 786-O and Caki-2
were purchased from American Type Culture Collection. All cell
lines were maintained in RPMI-1640 medium (Thermo Fisher
Scientific, Inc.) supplemented with 10% fetal calf serum, 1 mM
glutamine, 25 mM glucose and 1% penicillin-streptomycin (all Thermo
Fisher Scientific, Inc.) and cultured at 37°C in a 5%
CO2 atmosphere. After recovery from storage in liquid
nitrogen, the cells were cultured for no more than 3 months.
Senescence was induced using a low dose etoposide (Selleck
Chemicals) treatment protocol (769-P cells: 5 µM; ACHN cells: 1 µM;
768-O cells: 2 µM; Caki-2 cells: 20 µM) for 24 h, followed by a 3–5
day recovery phase in normal RPMI culture medium. After this, cell
pellets were prepared from exponentially growing cells harvested
using accutase (Sigma Aldrich; Merck KGaA; cat. no. A6964).
Following centrifugation at 300 × g at 4°C for 5 min, the cells
were resuspended, washed in phosphate-buffered saline and fixed in
4% formalin at 37°C for 15 min (Sigma-Aldrich; Merck KGaA). This
was followed by another wash step with PBS and then the cells were
transferred into 100% alcohol and precipitated with 30% bovine
serum albumin (Carl Roth GmbH & Co. KG). For immunostaining
followed by digital image analysis, cell lines were additionally
fixed in formalin, paraffin embedded and sectioned into 2 µm slices
according to standard protocols that were also used for tissue
samples as described previously (5).
Digital image analysis
Prior to image analysis, slides were digitalized
using the NanoZoomer-Series Digital slide scanner (Hamamatsu
Photonics). Digital image analysis was performed using the
HALO® platform from Indica Labs with the CytoNuclear
v1.4 module as described previously (26).
The Cancer Genome Atlas (TCGA)
data
Disease specific data was generated by the TCGA
Research Network (cancergenome.nih.gov/) and the cohort used was
KIRC-TCGA from 2016-01-28. A self-made R script was utilized to
retrieve, process and visualize the relevant data or outcome,
respectively.
Statistical analysis
Survival analysis was conducted for 622 patients
with a mean survival time of 72.6 months using the functions coxph
and survfit from the R package survival (cran.r-project.org/web/packages/survival/index.html;
version 2.41–3). LMNB1 protein expression was dichotomized
utilizing the Charité Cut-off finder functions to provide a
significant distinction between the high and low LMNB1 protein
expression levels based on survival outcome (27). Associations between survival times
and LMNB1 expression were firstly assessed by log-rank tests and
presented as Kaplan-Meier plots. In order to account for the
influence of established prognostic factors, hazard ratios (HRs)
and 95% confidence intervals (CIs) were adjusted for patient gender
and age, tumor extent, lymph node metastasis, distant metastasis,
grade of malignancy, and Eastern Cooperative Oncology Group (ECOG)
Performance Status in a multiple Cox proportional hazard regression
(28). Efron was used as a method
for handling tied death times (29)
in multivariate analysis of the Cox proportional hazard model
(semiparametric and estimation of the HR, CI). Statistical tests
were performed using R-Statistical Software (www.rproject.org; version 3.4.3) and R-Studio (version
1.1.383; www.rstudio.com). All plots were created
using the following R-packages (cran.r-project.org/): ggplot2 (version 2.2.1.9000),
survminer (version 0.4.1), ggpubr (version 0.1.6) and survival.
P≤0.05 was considered to indicate a statistically significant
difference.
Results
Immunohistochemistry
Immunostaining was performed on tissue microarrays
containing tumor and the corresponding normal renal tissues from
932 patients with primary RCCs. The present study is focused on 763
cases with ccRCC, the most common kidney cancer subtype. In total,
the cancer tissues of 622 ccRCCs were successfully scored for LMNB1
expression by immunohistochemistry. The remaining cases were
excluded from further analyses either due to insufficient tumor
tissues, poor tissue preservation or missing patient information.
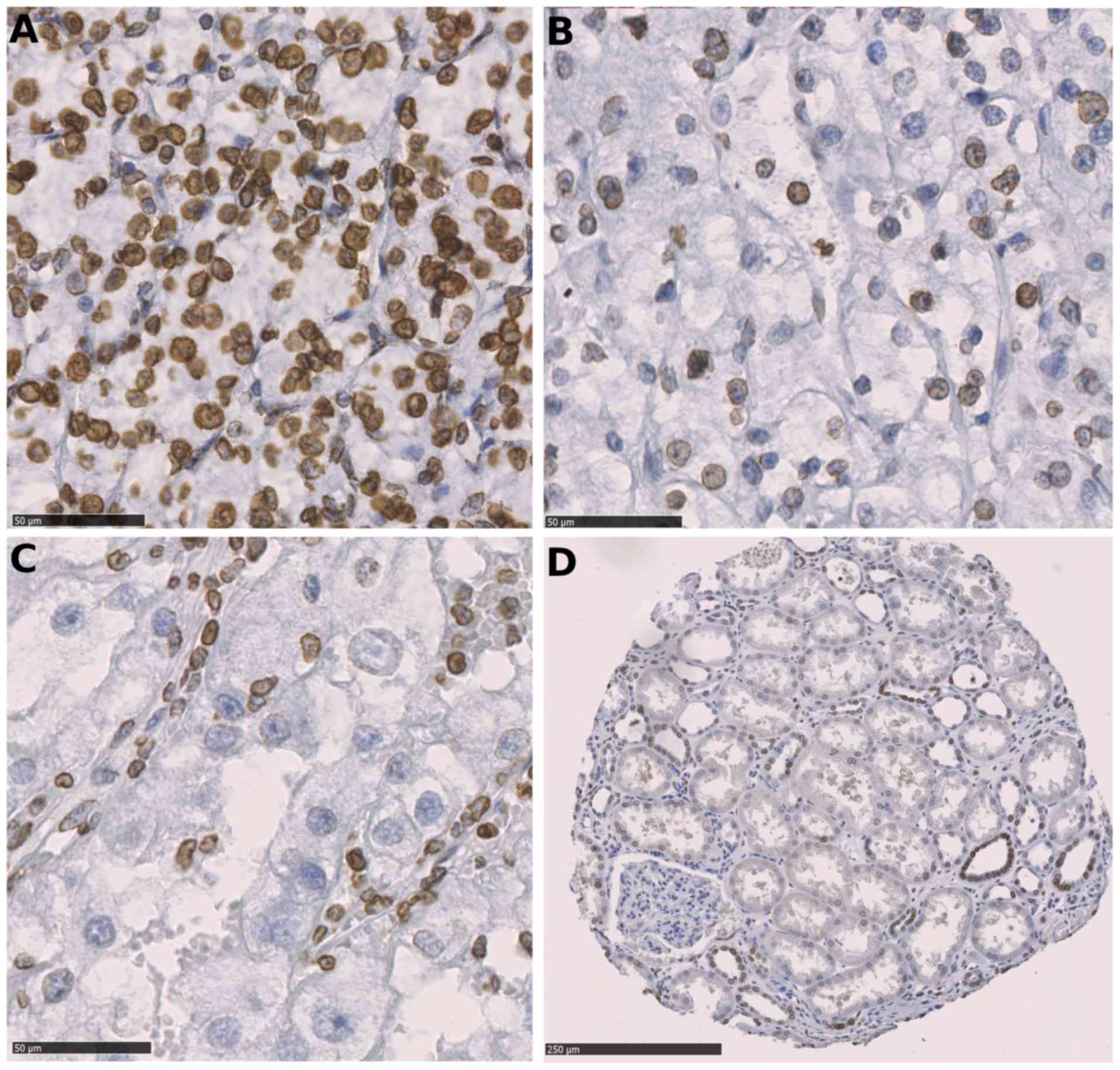
Fig. 1 depicts the
immunohistochemical LMNB1 expression in tumor cells; LMNB1 was
expressed in distal tubuli, and infrequently and less intensely in
proximal tubuli.
Clinical characteristics of the
patients
The median time of follow-up was 5.4 years (mean,
6.05 years; maximum, 19.76 years). At the end of the follow-up
period, 209 patients had succumbed to RCC; the median follow-up
time among these patients was 1.53 years (mean, 2.72 years;
maximum, 17.7 years). The clinical and pathological features of the
study population are summarized in Table
I.
LMNB1 expression and patient
prognosis
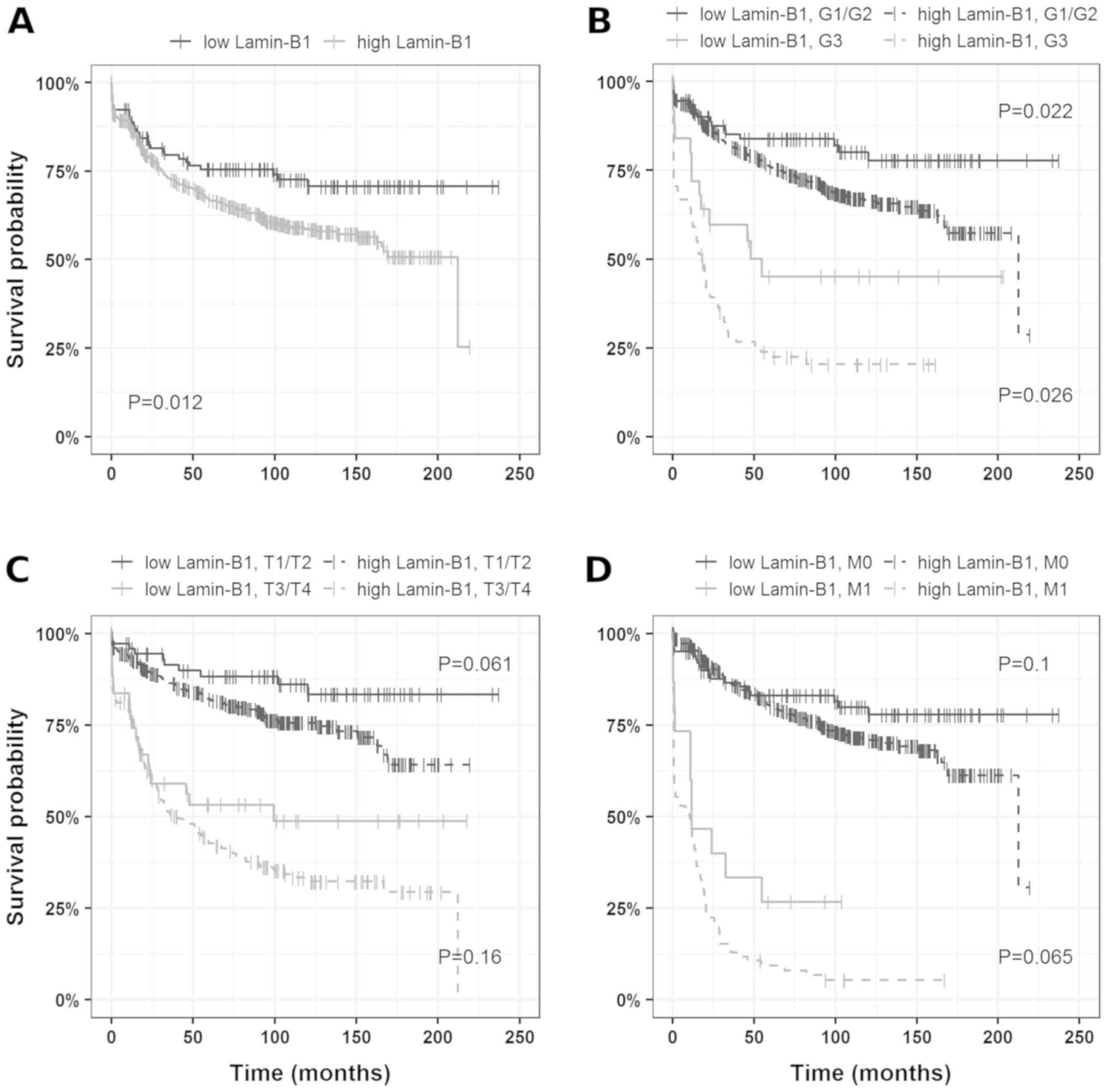
Based on the Charité Cut-off finder functions
(27), 60% positive tumor cells were
determined as the cutoff. When tumors were grouped according to
LMNB1 expression (>60%, LMNB1-high; ≤60%, LMNB1-low) univariate
survival analysis revealed a decrease in cancer-specific survival
(HR, 1.63; 95% CI, 1.11–2.41) in patients harboring tumors with
high LMNB1 expression compared to tumors with low/no LMNB1
expression. The corresponding Kaplan-Mayer plots are depicted in
Fig. 2.
Comparison of LMNB1 expression with
clinical and pathological features
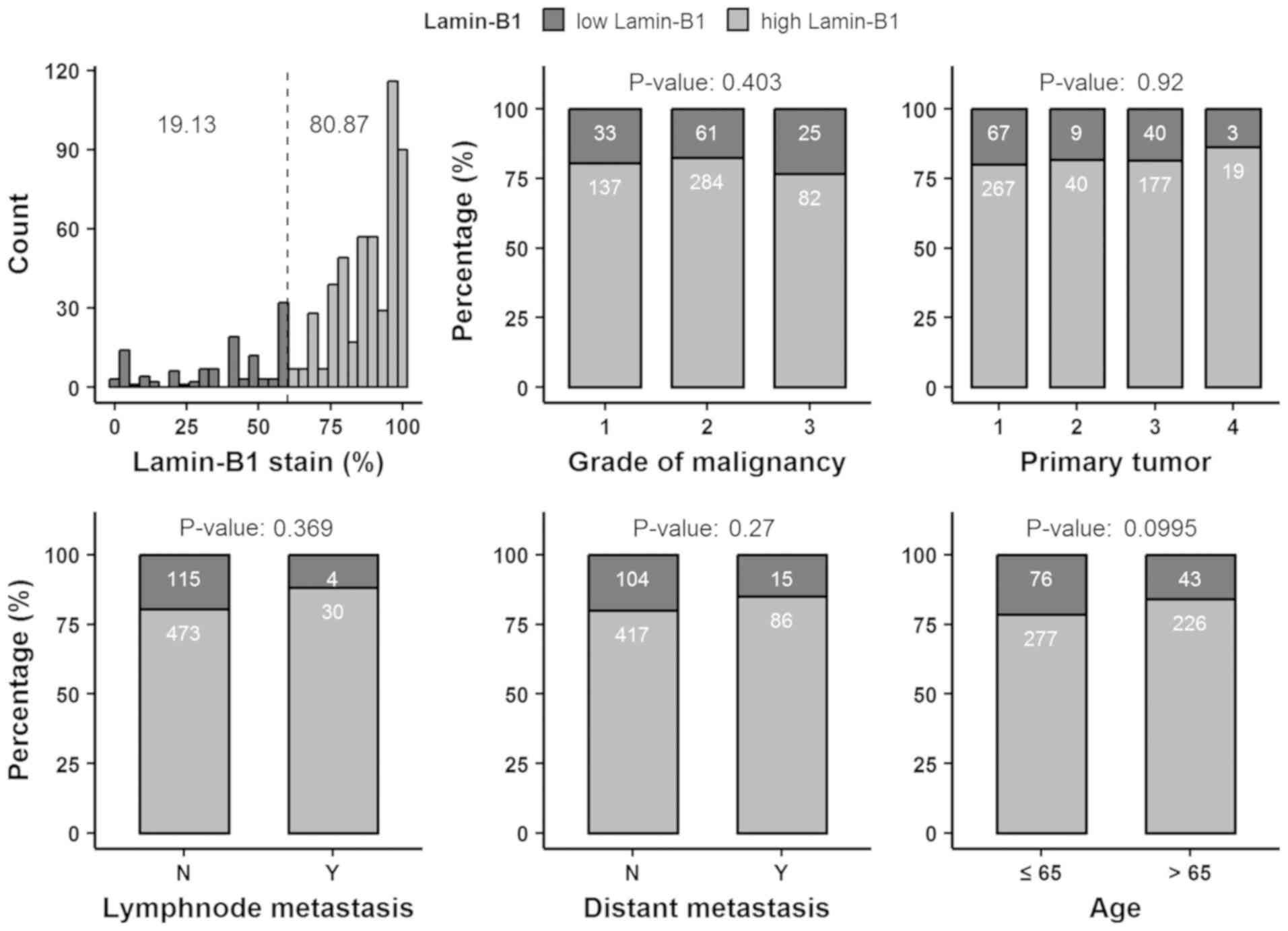
No consistent association between LMNB1 expression
and differentiation, tumor extent, lymph node metastasis, distant
metastasis or patient age was observed (Fig. 3; Table
SI).
Multivariate analysis of LMNB1
expression and clinical and pathological features
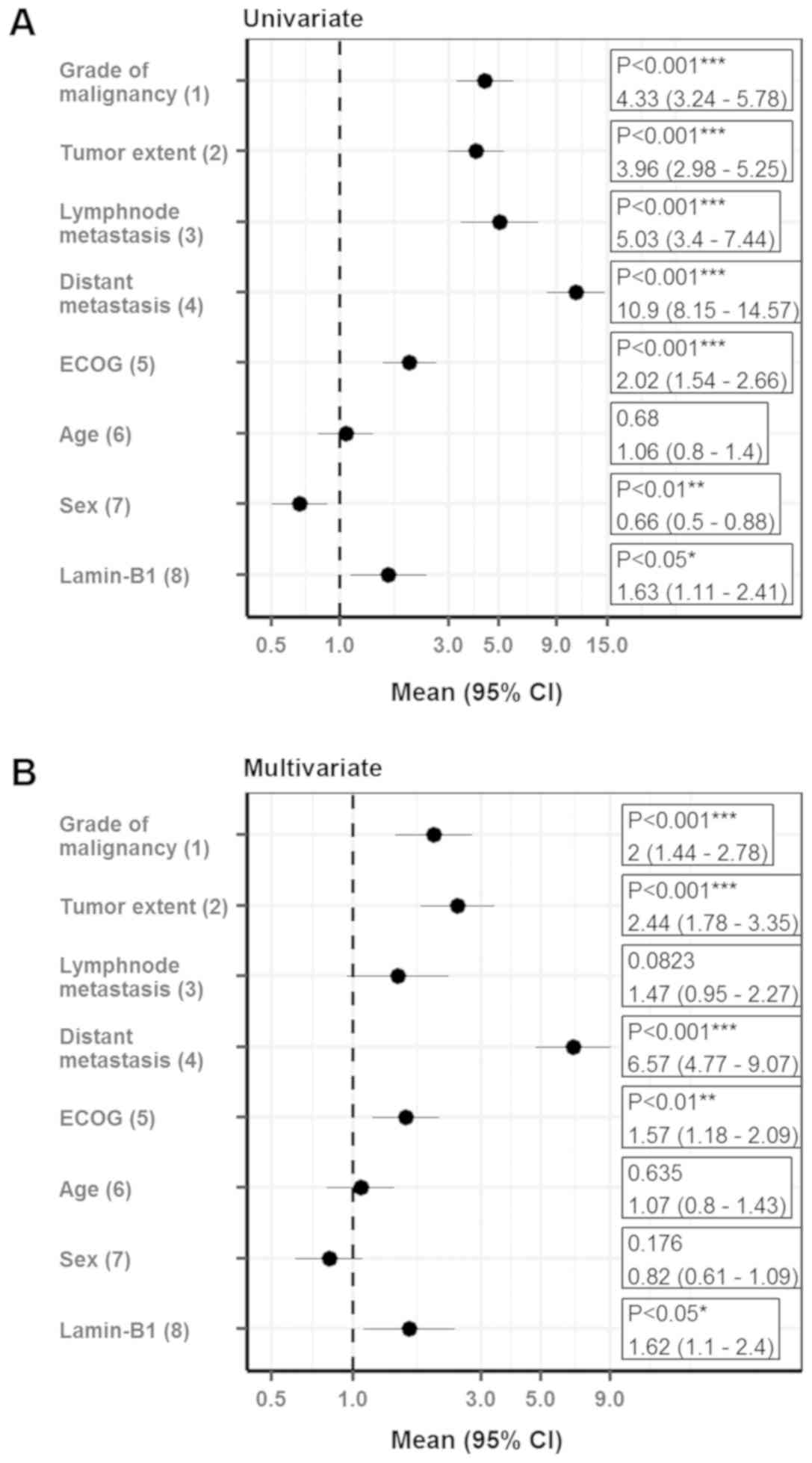
To further validate the results, multivariate
analysis was performed using the Cox proportional hazards model.
Notably, LMNB1 expression qualified as an independent prognostic
marker for cancer-specific survival after adjustments for
established prognostic factors (grade of malignancy, tumor extent,
lymph node metastasis, distant metastasis, ECOG performance status
and gender) in the multiple regression analysis for ccRCC patients
(HR, 1.62; 95% CI, 1.1–2.4; Fig. 4).
After adjustment for prognostic factors, LMNB1 expression also
remained statistically significant for patients with localized
disease (HR, 1.82; 95% CI, 1.09–3.02; Fig. S1).
To further elucidate the relationship between LMNB1
expression and patient prognosis, the present study utilized TCGA
data on ccRCC. When tumors were grouped according to LMNB1 mRNA
levels (a Z-score >1.96 indicated upregulation and a Z-Score
≤1.96 and >-1.96 indicated no regulation when considering that a
Z-score of 1.96 presents the approximate value of the 97.5
percentile point of the normal distribution; 95% of the area under
a normal curve lies within roughly 1.96 standard deviations of the
mean), univariate survival analysis revealed that 77 out of 176
patients (43.75%) with increased LMNB1 mRNA levels were deceased
whereas only 83 out of 357 (23.25%) of the patients without
elevated mRNA levels were deceased (P≤0.0001); the Kaplan-Meier
curves are depicted in Fig. S2.
LMNB1 is regulated during
etoposide-induced senescence
LMNB1 downregulation is known as a senescence
effector (15). To investigate
whether the downregulation of LMNB1 plays a role in cellular
senescence in RCC the present study conducted in-vitro
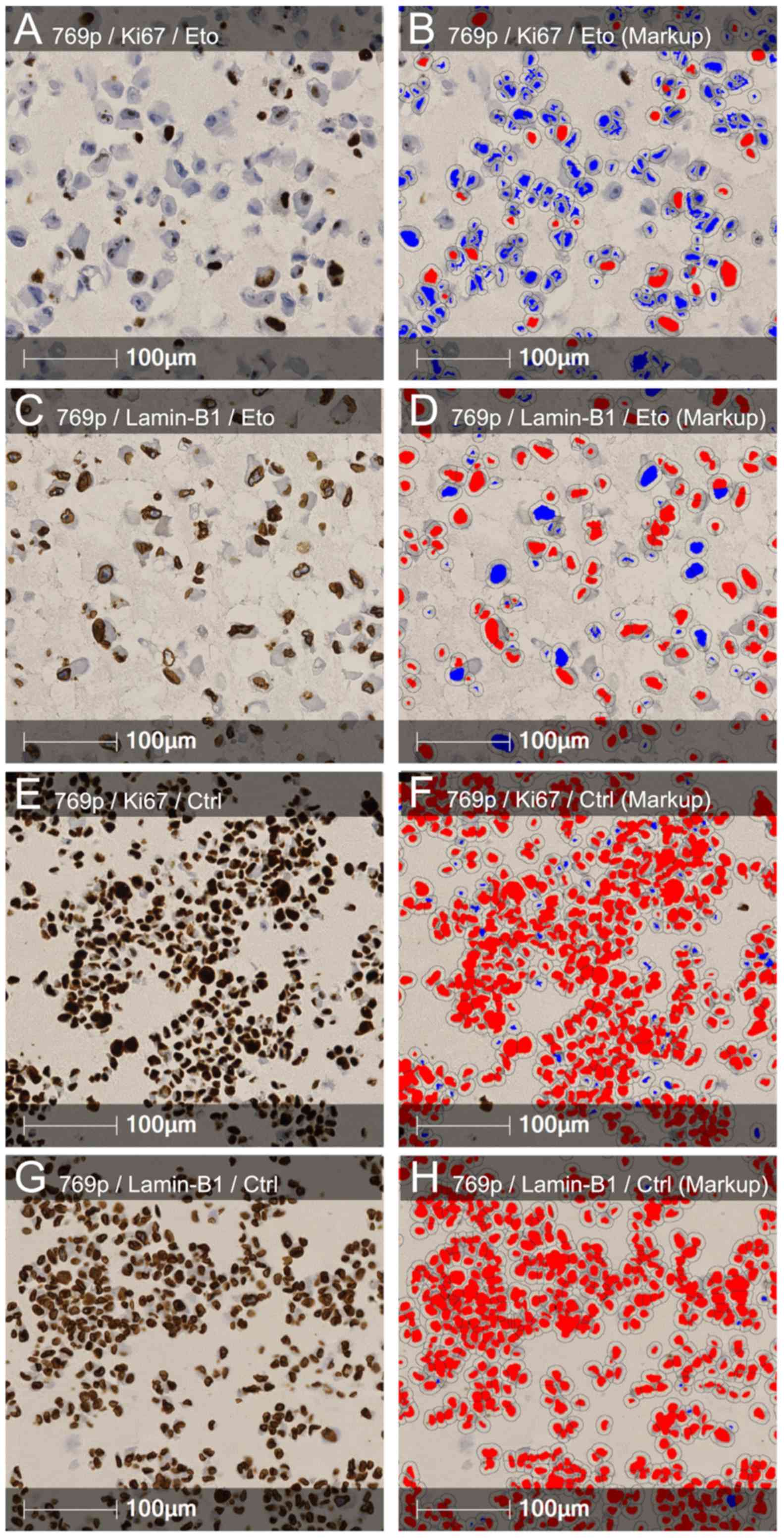
studies. A total of 4 different RCC cell lines (ACHN, Caki-2, 786-O
and 769-P) were subjected to etoposide-induced senescence as
described by Nagano et al (30). As expected, treatment reduced the
proliferation rate; however, the effects varied widely between the
different cell lines. Notably, a strong reduction in LMNB1
expression was accompanied by a stronger decrease in proliferation
in 3 of the 4 cell lines (Caki-2, 786-O and 769-P), and the cell
line ACHN, with the lowest effects on LMNB1 expression, also
exhibited a reduced effect of etoposide treatment on the
proliferation rate (Fig. 5). ACHN,
786-O and 769-P are ccRCC cell lines. Caki-2 was originally defined
as a ccRCC cell line, however, a growing body of evidence has
suggested that this cell line may actually be papillary RCC
(31,32). This suggests that LMNB1 is functional
not only in ccRCC but also in other subtypes of RCC.
Discussion
RCCs are known to be resistant to conventional
radio- and chemotherapy, therefore, traditional immunotherapy, such
as high-dose interleukin-2 and interferon-α, has been the standard
treatment in patients with systemic disease in the past. Currently,
targeted antiangiogenic therapies and novel immunotherapy agents,
such as checkpoint inhibitors, have become an integral part of the
management of metastatic RCC (33).
However, the development of resistance often occurs and the chance
of recovery is marginal. Therefore, new therapeutic targets are
urgently needed to develop novel drugs with different mechanisms of
action (34).
The results of the present study have revealed a
high LMNB1 expression in 80% of the analyzed ccRCC tumors, without
pre-selecting for a specific tumor or patients parameter. LMNB1 is
a senescence effector and triggers widespread changes in gene
expression upon down regulation (15). In agreement with this biological
function, the present study identified high LMNB1 expression as an
unfavorable prognostic marker in ccRCC patients. This indicated
that the VHL loss-induced senescence program (4) may be bypassed in RCC development by a
currently unknown mechanism, resulting in continuous LMNB1
expression. However, in vitro experiments demonstrated that
etoposide-induced senescence may be associated with LMNB1 reduction
in ccRCC and papillary RCC cell lines. Notably, it appears that
there is a link between LMNB1 expression and the cell proliferation
rate (5). RCCs with high LMNB1
expression predominantly exhibited higher proliferation rates when
compared with those with low LMNB1 expression (Fig. S3). This indicated that LMNB1 may be
a functional downstream senescence effector in RCC and may serve as
a potential predictive biomarker for innovative senescence-based
therapeutic approaches in ccRCC.
Therapy-induced senescence represents a novel
functional target that may improve cancer therapy (35). In recent years, therapeutic
approaches such as p53 reactivation, inhibition of c-MYC in p53- or
c-MYC-driven tumors, or treatment with cyclin-dependent kinase
inhibitors have proven effective by invoking a senescence response
(36). Zhu et al (37) reported that the tyrosine kinase
inhibitor sunitinib used as a first-line therapeutic agent against
metastatic RCC induced cellular senescence in renal cell carcinoma
cells and exhibited similar effects in vivo following
xenograft experiments in nude mice. In addition,
immunohistochemical studies demonstrated a senescence-phenotype in
tumor tissues from patients with RCC after neoadjuvant sunitinib
treatment (37). Thus, reinforcing a
therapy-induced senescence therapy by adding a novel synthetic
compound such as STK899704, which suppresses the proliferation of a
broad range of cancer cell types, may offer a novel therapeutic
strategy for patients with advanced RCC (38).
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Mrs. Jutta Richter,
Mrs. Silke Mitschke and Mrs. Bonny Adami (Institute of Pathology,
University Medical Center Mainz, Mainz, Germany) for their
excellent technical assistance. Aspects of this trial are part of
the MD thesis of the author, MMR.
Funding
The present study was supported by the tissue bank
of the University Medical Center Mainz and the Tissue Bank of the
National Center for Tumor Diseases Heidelberg (Heidelberg,
Germany).
Availability of data and materials
The datasets generated and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
MMR, MS, PS, SF, KET and SMG conceived and designed
the experiments. EH, MH, GH and WR obtained the patients' data and
the corresponding tissue specimens. MMR, MS and SMG wrote the
manuscript with input from all authors. All authors discussed the
results and contributed to the final manuscript.
Ethics approval and consent to
participate
The human tissue samples were provided by the Tissue
Bank of the National Centre for Tumor Diseases Heidelberg
(Heidelberg, Germany) and used retrospectively with approval from
the Ethics Committee of the University of Heidelberg (Heidelberg,
Germany).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Moch H, Humphrey PA, Ulbright T and Reuter
V: WHO Classification of Tumours of the Urinary System and Male
Genital Organs. 4th. IARC; Lyon: 2016
|
|
2
|
Jonasch E, Futreal PA, Davis IJ, Bailey
ST, Kim WY, Brugarolas J, Giaccia AJ, Kurban G, Pause A, Frydman J,
et al: State of the science: An update on renal cell carcinoma. Mol
Cancer Res. 10:859–880. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kaelin WG Jr: The von Hippel-Lindau tumour
suppressor protein: O2 sensing and cancer. Nat Rev Cancer.
8:865–873. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Young AP, Schlisio S, Minamishima YA,
Zhang Q, Li L, Grisanzio C, Signoretti S and Kaelin WG Jr: VHL loss
actuates a HIF-independent senescence programme mediated by Rb and
p400. Nat Cell Biol. 10:361–369. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Macher-Goeppinger S, Bermejo JL,
Schirmacher P, Pahernik S, Hohenfellner M and Roth W:
Senescence-associated protein p400 is a prognostic marker in renal
cell carcinoma. Oncol Rep. 30:2245–2253. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Welford SM, Dorie MJ, Li X, Haase VH and
Giaccia AJ: Renal oxygenation suppresses VHL loss-induced
senescence that is caused by increased sensitivity to oxidative
stress. Mol Cell Biol. 30:4595–4603. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Courtois-Cox S, Genther Williams SM,
Reczek EE, Johnson BW, McGillicuddy LT, Johannessen CM, Hollstein
PE, MacCollin M and Cichowski K: A negative feedback signaling
network underlies oncogene-induced senescence. Cancer Cell.
10:459–472. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Michaloglou C, Vredeveld LC, Soengas MS,
Denoyelle C, Kuilman T, van der Horst CM, Majoor DM, Shay JW, Mooi
WJ and Peeper DS: BRAFE600-associated senescence-like cell cycle
arrest of human naevi. Nature. 436:720–724. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen Z, Trotman LC, Shaffer D, Lin HK,
Dotan ZA, Niki M, Koutcher JA, Scher HI, Ludwig T, Gerald W, et al:
Crucial role of p53-dependent cellular senescence in suppression of
Pten-deficient tumorigenesis. Nature. 436:725–730. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Serrano M, Lin AW, McCurrach ME, Beach D
and Lowe SW: Oncogenic ras provokes premature cell senescence
associated with accumulation of p53 and p16INK4a. Cell. 88:593–602.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kuilman T, Michaloglou C, Mooi WJ and
Peeper DS: The essence of senescence. Genes Dev. 24:2463–2479.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shimi T, Butin-Israeli V, Adam SA,
Hamanaka RB, Goldman AE, Lucas CA, Shumaker DK, Kosak ST, Chandel
NS and Goldman RD: The role of nuclear lamin B1 in cell
proliferation and senescence. Genes Dev. 25:2579–2593. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jung HJ, Lee JM, Yang SH, Young SG and
Fong LG: Nuclear lamins in the brain-new insights into function and
regulation. Mol Neurobiol. 47:290–301. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hallstrom TC, Mori S and Nevins JR: An
E2F1-dependent gene expression program that determines the balance
between proliferation and cell death. Cancer Cell. 13:11–22. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shah PP, Donahue G, Otte GL, Capell BC,
Nelson DM, Cao K, Aggarwala V, Cruickshanks HA, Rai TS, McBryan T,
et al: Lamin B1 depletion in senescent cells triggers large-scale
changes in gene expression and the chromatin landscape. Genes Dev.
27:1787–1799. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Padiath QS, Saigoh K, Schiffmann R,
Asahara H, Yamada T, Koeppen A, Hogan K, Ptácek LJ and Fu YH: Lamin
B1 duplications cause autosomal dominant leukodystrophy. Nat Genet.
38:1114–1123. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Moss SF, Krivosheyev V, de Souza A, Chin
K, Gaetz HP, Chaudhary N, Worman HJ and Holt PR: Decreased and
aberrant nuclear lamin expression in gastrointestinal tract
neoplasms. Gut. 45:723–729. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Broers JL, Raymond Y, Rot MK, Kuijpers H,
Wagenaar SS and Ramaekers FC: Nuclear A-type lamins are
differentially expressed in human lung cancer subtypes. Am J
Pathol. 143:211–220. 1993.PubMed/NCBI
|
|
19
|
Coradeghini R, Barboro P, Rubagotti A,
Boccardo F, Parodi S, Carmignani G, D'Arrigo C, Patrone E and Balbi
C: Differential expression of nuclear lamins in normal and
cancerous prostate tissues. Oncol Rep. 15:609–613. 2006.PubMed/NCBI
|
|
20
|
Sun S, Xu MZ, Poon RT, Day PJ and Luk JM:
Circulating Lamin B1 (LMNB1) biomarker detects early stages of
liver cancer in patients. J Proteome Res. 9:70–78. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Izdebska M, Gagat M and Grzanka A:
Overexpression of lamin B1 induces mitotic catastrophe in colon
cancer LoVo cells and is associated with worse clinical outcomes.
Int J Oncol. 52:89–102. 2018.PubMed/NCBI
|
|
22
|
Li L, Du Y, Kong X, Li Z, Jia Z, Cui J,
Gao J, Wang G and Xie K: Lamin B1 is a novel therapeutic target of
betulinic acid in pancreatic cancer. Clin Cancer Res. 19:4651–4661.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Macher-Goeppinger S, Aulmann S, Tagscherer
KE, Wagener N, Haferkamp A, Penzel R, Brauckhoff A, Hohenfellner M,
Sykora J, Walczak H, et al: Prognostic value of tumor necrosis
factor-related apoptosis-inducing ligand (TRAIL) and TRAIL
receptors in renal cell cancer. Clin Cancer Res. 15:650–659. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gringon D, Eble J, Bonsib SM and Moch H:
Pathology and Genetics of Tumors of the Urinary System & Male
Genital Organs. Eble JN, Sauter G, Epstein J and Sesterhenn I:
Clear cell renal cell carcinoma. IARC; Lyon, France: pp. 23–25.
2004
|
|
25
|
Sobin L, Gospodarowicz M and Wittekind C:
TNM Classification of Malignant Tumours. (7th). 2009.
|
|
26
|
Foersch S, Schindeldecker M, Keith M,
Tagscherer KE, Fernandez A, Stenzel PJ, Pahernik S, Hohenfellner M,
Schirmacher P, Roth W and Macher-Goeppinger S: Prognostic relevance
of androgen receptor expression in renal cell carcinomas.
Oncotarget. 8:78545–78555. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Budczies J, Klauschen F, Sinn BV, Győrffy
B, Schmitt WD, Darb-Esfahani S and Denkert C: Cutoff Finder: A
comprehensive and straightforward Web application enabling rapid
biomarker cutoff optimization. PLoS One. 7:e518622012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shvarts O, Lam JS, Kim HL, Han KR, Figlin
R and Belldegrun A: Eastern Cooperative Oncology Group performance
status predicts bone metastasis in patients presenting with renal
cell carcinoma: Implication for preoperative bone scans. J Urol.
172:867–870. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hertz-Picciotto I and Rockhill B: Validity
and efficiency of approximation methods for tied survival times in
Cox regression. Biometrics. 53:1151–1156. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nagano T, Nakano M, Nakashima A, Onishi K,
Yamao S, Enari M, Kikkawa U and Kamada S: Identification of
cellular senescence-specific genes by comparative transcriptomics.
Sci Rep. 6:317582016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Brodaczewska KK, Szczylik C, Fiedorowicz
M, Porta C and Czarnecka AM: Choosing the right cell line for renal
cell cancer research. Mol Cancer. 15:832016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Furge KA, Chen J, Koeman J, Swiatek P,
Dykema K, Lucin K, Kahnoski R, Yang XJ and Bin TT: Detection of DNA
copy number changes and oncogenic signaling abnormalities from gene
expression data reveals MYC activation in high-grade papillary
renal cell carcinoma. Can Res. 67:3171–3176. 2007. View Article : Google Scholar
|
|
33
|
Barata PC and Rini BI: Treatment of renal
cell carcinoma: Current status and future directions. CA Cancer J
Clin. 67:507–524. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Choueiri TK and Motzer RJ: Systemic
therapy for metastatic renal-cell carcinoma. N Engl J Med.
376:354–366. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ewald JA, Desotelle JA, Wilding G and
Jarrard DF: Therapy-induced senescence in cancer. J Natl Cancer
Inst. 102:1536–1546. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Acosta JC and Gil J: Senescence: A new
weapon for cancer therapy. Trends Cell Biol. 22:211–219. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhu Y, Xu L, Zhang J, Hu X, Liu Y, Yin H,
Lv T, Zhang H, Liu L, An H, et al: Sunitinib induces cellular
senescence via p53/Dec1 activation in renal cell carcinoma cells.
Cancer Sci. 104:1052–1061. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Park CW, Bak Y, Kim MJ, Srinivasrao G,
Hwang J, Sung NK, Kim BY, Yu JH, Hong JT and Yoon DY: The novel
small molecule STK899704 promotes senescence of the human A549
NSCLC cells by inducing DNA damage responses and cell cycle arrest.
Front Pharmacol. 9:1632018. View Article : Google Scholar : PubMed/NCBI
|