Introduction
Osteosarcoma (OS), one of the most prevalent types
of malignancies of the bone that predominantly affects children and
adolescents, was the second leading cause of cancer-associated
mortality in this group of individuals in the USA between 1973 and
2004 (1,2). Despite advances in therapeutic
strategies (including chemotherapy and surgical resection), the
5-year survival rate of patients with OS who are resistant to
treatment or have metastases remains low (3). Moreover, due to poor overall prognosis,
>40% of patients develop recurrent or progressive disease
following traditional first-line therapy (4). Due to the highly aggressive nature of
these tumors, the poor therapeutic outcome and the development of
chemoresistance, the exploration of novel and efficient treatment
strategies for patients with OS is required (5).
Growing evidence indicates the potential of natural
compounds as successful anticancer agents (6). Baicalin (baicalein
7-O-β-D-glucuronide), an important flavonoid, is found in the roots
of the Chinese herb Huang Qin (Scutellaria baicalensis
Georgi) (7). Baicalin exhibits a
wide range of pharmacological properties, including against
oxidation, tumor, inflammation and proliferation (8–11).
Studies on the effect of this compound on various types of cancer
cells indicated significantly suppressed tumor growth (12), induction of cell apoptosis and
senescence (13–15), as well as inhibition of metastasis
(16,17). This is mediated by the suppression of
multiple signaling pathways, including ERK, STAT3, β-catenin and
p38 mitogen-activated protein kinase (MAPK) (16–19).
Treatment of OS cells with baicalin significantly induces apoptosis
and inhibits metastasis, through reactive oxygen species-mediated
mitochondrial and TGF-β pathways (20,21).
However, the role of baicalin in OS and its underlying mechanism
has not been fully evaluated.
The AKT pathway, one of the most important
intracellular signaling pathways, has been reported to involve a
cascade of events that play an essential role in a variety of
physiological and pathological processes, including proliferation,
migration, cell growth and metabolism (22,23). In
most types of cancer in humans, the AKT pathway has been identified
as one of the most important oncogenic pathways (24). In OS, the AKT pathway is frequently
hyperactivated, playing a critical role in the initiation and
development of OS, including tumorigenesis, proliferation,
apoptosis and metastasis (25–28).
Activation of the AKT pathway contributes to these processes in
cancer by mediating the expression of its downstream genes,
including cyclin D1, CDK4, anti-apoptotic protein Bcl-2 and
pro-apoptotic protein Bax (29–32). Due
to the essential role of the AKT pathway in OS, the inhibition of
the AKT pathway has therapeutic potential in OS (25,26,33–35) and
natural compounds that target this pathway safely and effectively
need to be further investigated.
In the present study, the role of baicalin on U-2 OS
cell growth was assessed by cell confluence observation and
performing cell number counts. The cell viability, cell survival,
cell cycle and cell apoptosis of U-2 OS cells, following baicalin
treatment, were further assessed. Moreover, the underlying
mechanism of baicalin was explored by investigating the activation
of the AKT pathway and its downstream effectors using western
blotting.
Materials and methods
Antibodies and chemicals
McCoy's 5A medium, fetal bovine serum (FBS) and the
cell cycle determination kit [FxCycle™ Propidium Iodide (PI)/RNase
Staining solution; cat. no. F10797] were all purchased from Thermo
Fisher Scientific, Inc. The mixture of penicillin and streptomycin
was obtained from Hyclone; GE Healthcare Life Sciences. The Annexin
V staining kit was provided by Nanjing KeyGen Biotech Co., Ltd. The
Cell Counting kit-8 (CCK-8) was provided by Abbkine Scientific Co.,
Ltd. Baicalin and DMSO were obtained from Beijing Solarbio Science
& Technology Co., Ltd. The antibodies against GAPDH (cat. no.
5174), AKT (cat. no. 4691s), phosphorylated (p)-AKT (cat. no.
4060s), cyclin D1 (cat no. 2978s), CDK4 (cat. no. 12790), Bax (cat.
no. 5023) and Bcl-2 (cat. no. 15071) were all purchased from Cell
Signaling Technology, Inc.
Cell culture and baicalin
treatment
U-2 OS cells were obtained from the Type Culture
Collection of the Chinese Academy of Sciences. The cells were
cultured in McCoy's 5A medium, supplemented with 10% FBS and a
mixture of 100 U/ml penicillin and 100 mg/ml streptomycin, in a
humidified atmosphere of 37°C and 5% CO2. The cells were
cultured to 80–90% confluence and were not used after >20
passages. The cells were seeded in multiple plates and treated with
various concentrations of baicalin (25, 50 or 100 µM; dissolved in
DMSO). The concentrations were selected based on a preliminary
study (data not shown). Equal volume of DMSO (≤0.5%) was added to
wells as a control treatment.
Cell confluence observation
U-2 OS cells (0.4×105 cells/well) were
seeded on a 6-well plate for 24 h, followed by treatment with 0,
25, 50 or 100 µM baicalin for 24 h. The confluence of cells was
observed and images of each well were captured using a
phase-contrast inverted light microscope (Leica Microsystems GmbH)
at a magnification of ×100.
Cell number counts
Following the observation of cell confluence, the
cells were trypsinized and diluted with fresh medium. An equal
volume of medium containing cells was mixed with 0.2% trypan blue
solution (Sigma Aldrich; Merck KGaA) and the cell number was
counted using the Countstar Automated Cell Counter (ALIT Life
Science Co., Ltd.).
CCK-8 assay
U-2 OS cells (2×103 cells/well) were
seeded on a 96-well plate for 24 h and then treated with various
concentrations of baicalin (0, 25, 50 or 100 µM). Following
treatment for 24, 48 and 72 h, 10 µl CCK-8 reagent (Abbkine
Scientific Co., Ltd.) was added to each well and the cells were
incubated at 37°C for an additional 2 h. The absorbance was
measured at 450 nm using an Infinite 200 Pro microplate reader
(Tecan Group, Ltd.).
Cell colony-formation analysis
Following the cell number counts for each treatment
group, U-2 OS cells treated with or without baicalin were seeded on
12-well plates (500 cells/well), and the medium was changed every
2–3 days. After culture for 8–10 days, the colonies formed were
washed with PBS twice and fixed with 4% paraformaldehyde for 15 min
at room temperature (RT), followed by 0.01% crystal violet staining
for 15 min at RT. Images were captured using an electronic camera
(cat. no. DS126201; Canon, Inc.) and the numbers of colonies were
recorded. The number of colonies formed was normalized to the
control cells.
Cell cycle analysis
U-2 OS cells (0.4×105 cells/well) were
seeded on a 6-well plate, incubated for 24 h, and treated with 0,
25, 50 or 100 µM baicalin for 24 h. The cells were harvested and
fixed with 70% ethanol at 4°C overnight. The fixed cells were
washed twice with cold PBS and incubated for 30 min with PI/RNase
at RT. The fluorescence signal was detected by the FL2 channel of a
flow cytometer and the proportion of DNA at each phase was analyzed
using ModFit LT software version 3.0 (Verity Software House,
Inc.).
Annexin V staining and cell apoptosis
analysis
U-2 OS cells (0.4×105 cells/well) were
seeded on a 6-well plate and incubated for 24 h, after treatment
with 0, 25, 50 or 100 µM baicalin for 24 h. The cells were
harvested with trypsin without EDTA and washed twice with PBS. The
collected cells were incubated with Annexin V-phycoerythrin
solution for 15 min at RT. The cells were sorted using a flow
cytometer (FACSCalibur™; Becton Dickinson Company). The percentage
of cells at the early stage of apoptosis was calculated according
to Annexin V-positivity using Cell Quest Pro software (version 6.0;
Becton, Dickinson and Company).
Western blotting
U-2 OS cells were cultured in 25-cm2
flasks at a density of 1.5×106 cells/flask in 5 ml
medium for 24 h, and then treated with 0, 25, 50 or 100 µM baicalin
for 24 h. After harvesting, the cells were lysed with ice-cold RIPA
lysis buffer (Beyotime Institute of Biotechnology), containing
protease and phosphatase inhibitor cocktails (Roche Diagnostics)
for 30 min, and centrifuged at 18,894 × g at 4°C for 10 min to
remove sediment. The concentration of soluble protein was
determined by BCA assay (Thermo Fisher Scientific, Inc.). Protein
samples (50 µg) were separated by 10% SDS-PAGE and transferred to
PVDF membranes (EMD Millipore). The PVDF membranes were blocked
with 5% skimmed milk at RT for 1 h. The membranes were then
incubated with primary antibodies (1:1,000 dilution) against
phosphorylated (p-)AKT, AKT, cyclin D1, CDK4, Bax and Bcl-2
overnight at 4°C. The blots were then probed with a goat
anti-rabbit horseradish peroxidase-conjugated secondary antibody
(1:2,000 dilution) at RT for 2 h, followed by detection using
enhanced chemiluminescence (Thermo Fisher Scientific, Inc.) with
the ChemiDoc XRS+ imaging system (Bio-Rad Laboratories, Inc.). The
intensities of bands were quantified relative to the intensity of
GAPDH bands using the ImageJ software (version 1.46; National
Institutes of Health). The levels of target proteins were expressed
relative to the levels in untreated cells, defined as 1.00.
Statistical analysis
All statistical analyses were conducted using the
SPSS version 20.0 statistical software (IBM Corp.). The data are
presented as the mean ± standard deviation of three independent
experiments. The significance of the differences between the groups
(>3 groups) was tested using ANOVA, followed by the Least
Significant Difference test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Baicalin suppresses the growth of U-2
OS cells
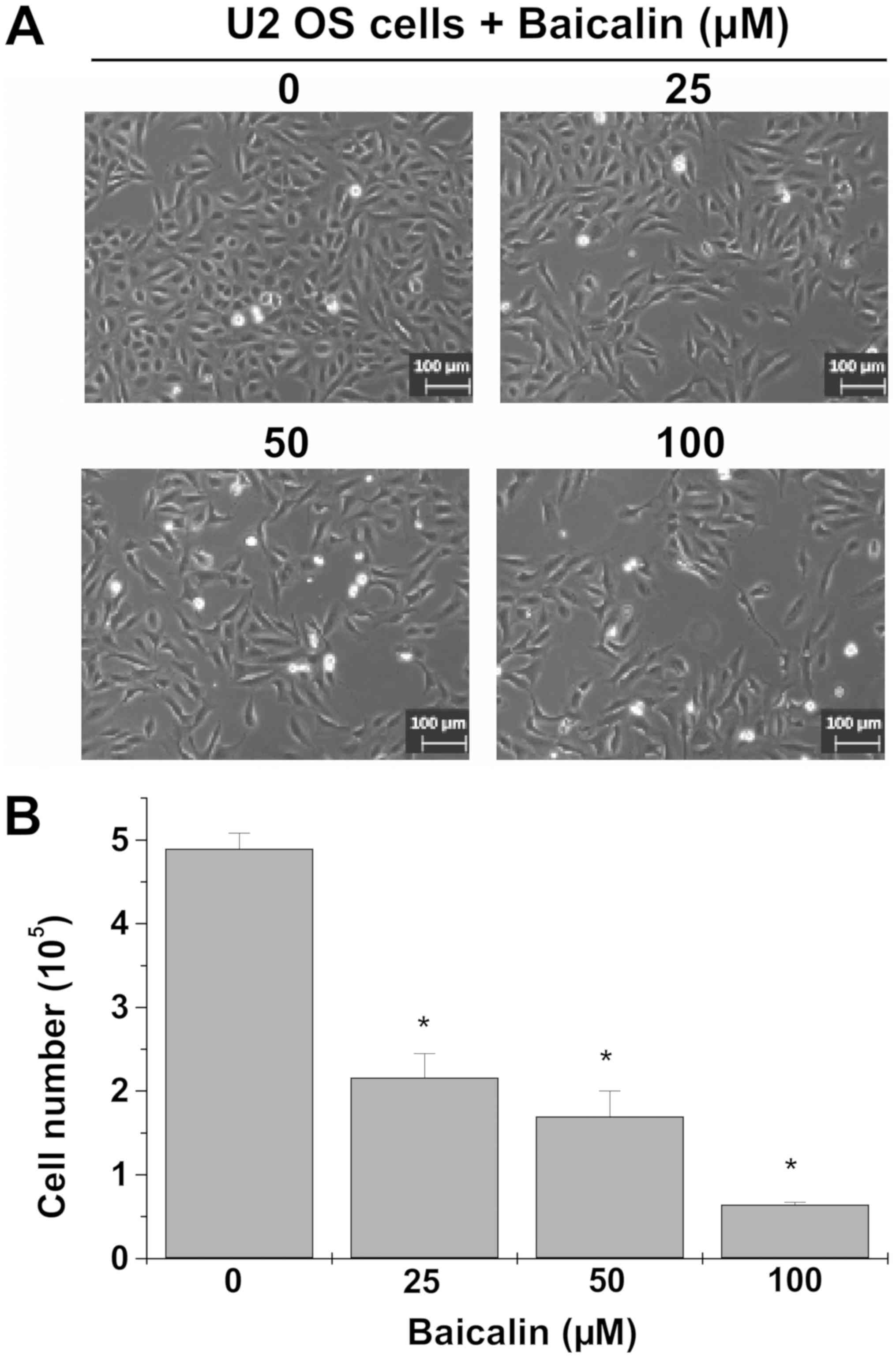
Cell confluence observation by microscopy revealed
decreased cell confluence of cultured U-2 OS cells that were
treated at various concentrations of baicalin (25, 50 or 100 µM;
Fig. 1A). Moreover, cell number
counts, using trypan blue staining, demonstrated significantly
decreased number of live cells following treatment with 25–100 µM
baicalin (P<0.05 vs. untreated U-2 OS cells; Fig. 1B). Thus, baicalin suppresses the
growth of U-2 OS cells.
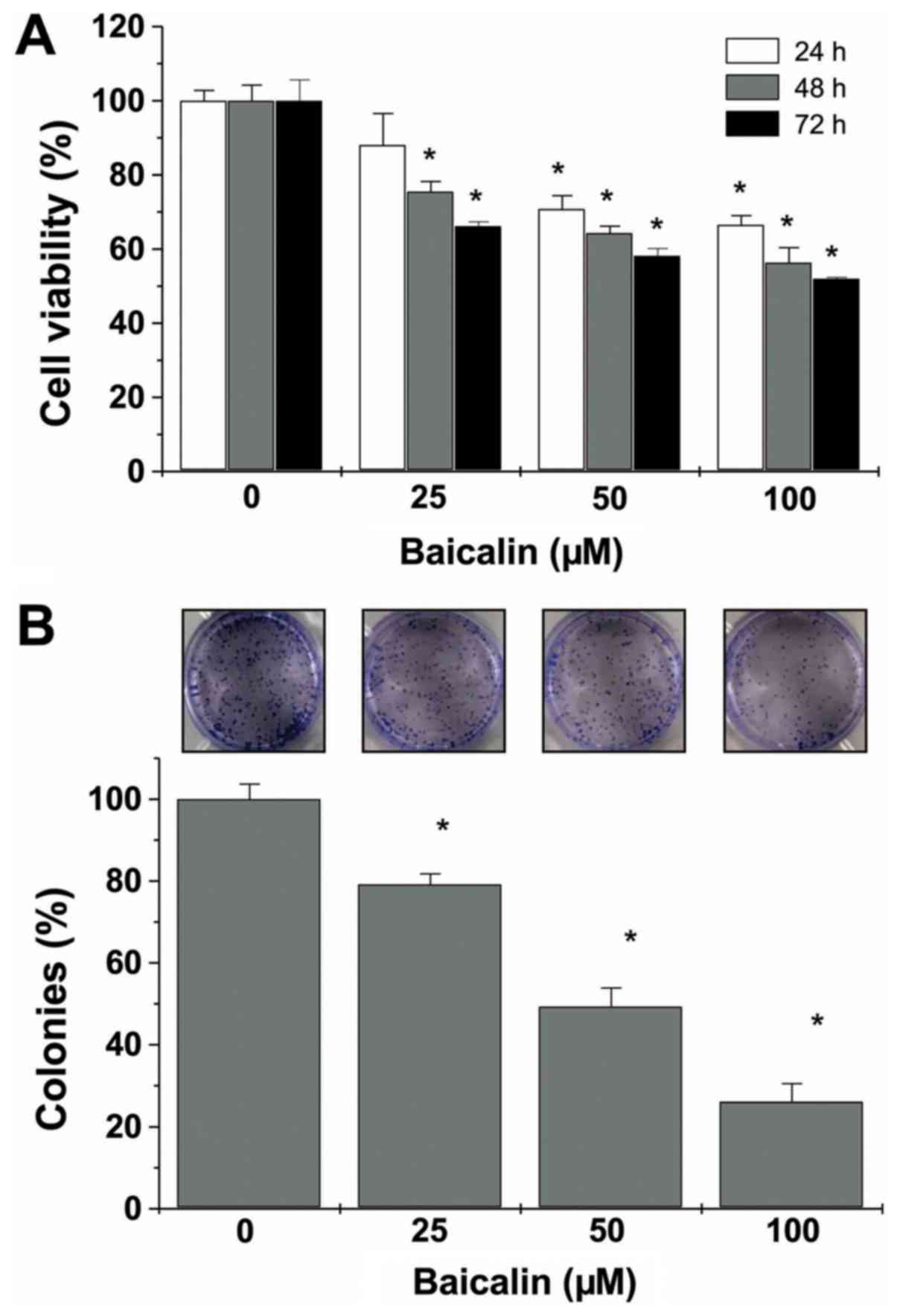
Baicalin decreases cell viability and
cell survival of U-2 OS cells
The cell viability of U-2 OS cells was determined
using the CCK-8 assay. This demonstrated markedly decreased cell
viability of U-2 OS cells that were treated with baicalin (25, 50
or 100 µM) at various time points (24, 48 or 72 h) compared with
untreated cells (all P<0.05, except 25 µM baicalin treatment for
24 h; Fig. 2A). The images and
calculations of the colonies revealed significantly decreased
numbers of colonies at the various concentrations of baicalin (all
P<0.05 vs. untreated cells; Fig.
2B).
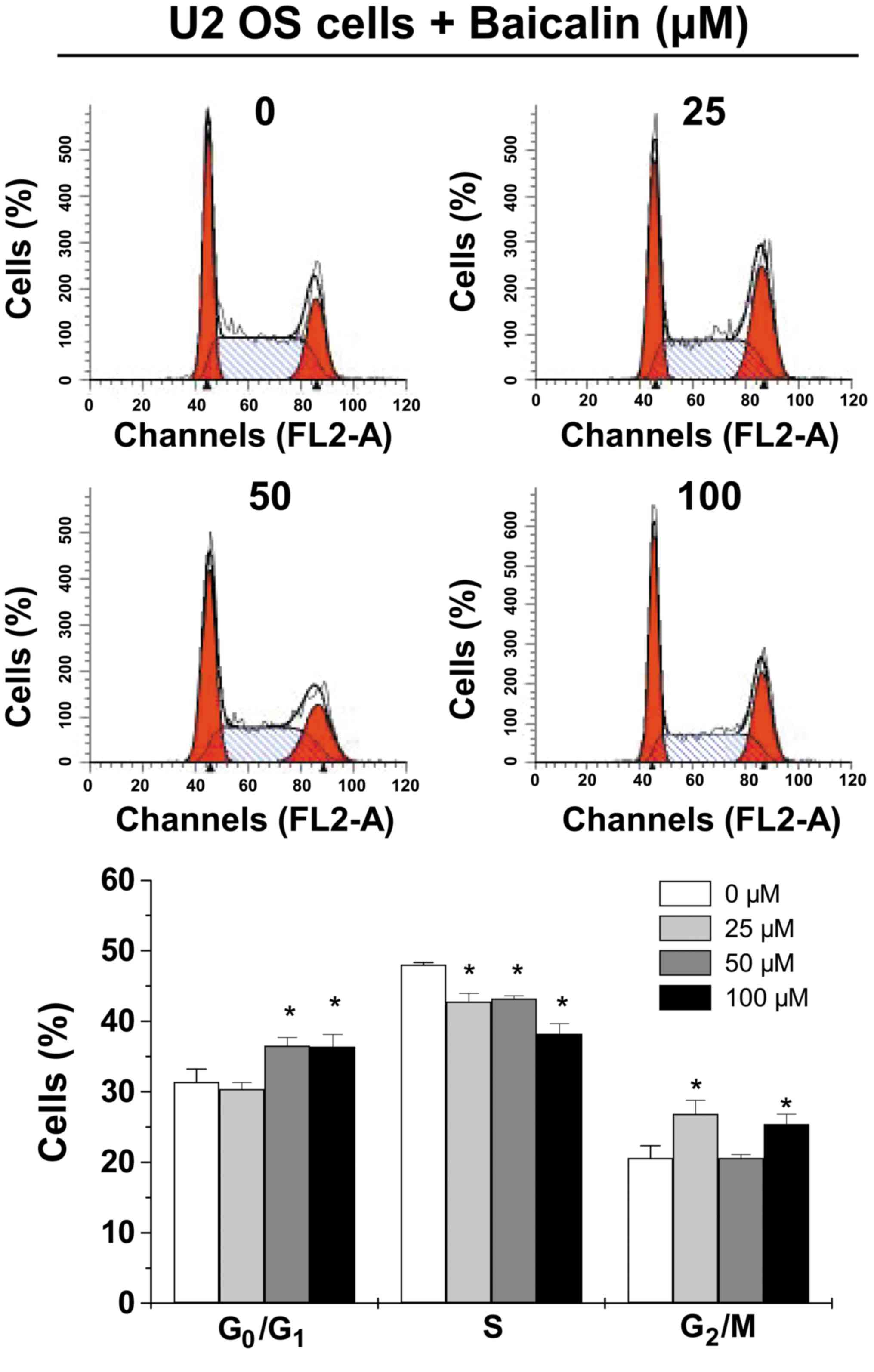
Baicalin induces the entry of U-2 OS
cells into the G0/G1 phase
In order to explore the underlying mechanism of the
effect of baicalin on cell cycle progression, cell cycle analysis
was conducted (Fig. 3). PI staining
followed by FACS analysis indicated a significantly increased
percentage of U-2 OS cells at the G0/G1 phase
after 50-µM (36.55±1.16%) and 100-µM (36.37±1.77%) baicalin
treatment compared with untreated U-2 OS cells (31.39±1.84%) (both
P<0.05). The percentage of U-2 OS cells at the S phase was
significantly decreased following treatment with 25 (42.79±1.18%),
50 (43.20±0.42%) and 100 µM (38.24±1.43) baicalin compared with
untreated U-2 OS cells (48.03±0.32%) (all P<0.05). In addition,
the percentage of cells at the G2/M phase was increased
following treatment with 25 and 100 µM baicalin (P<0.05),
whereas 50 µM baicalin treatment had no effect.
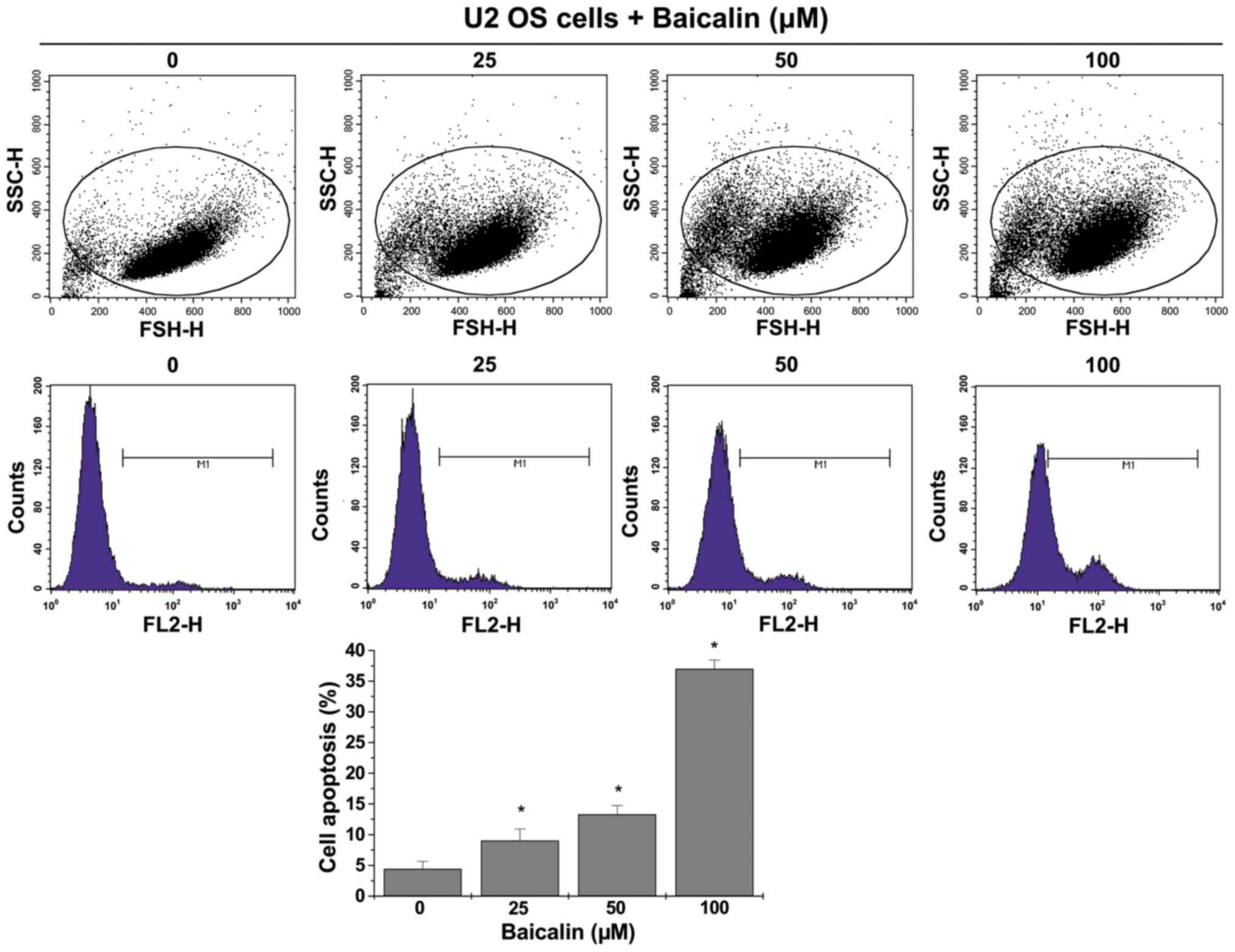
Baicalin induces apoptosis of U-2 OS
cells
Annexin V staining followed by FACS analysis
revealed a significant shift in the peak of fluorescence to the
right and significantly increased percentage of U-2 OS cells that
were positively stained with Annexin V following treatment with 25,
50 and 100 µM baicalin (9.03±1.90, 13.28±1.44 and 37.00±1.42%,
respectively), compared with untreated cells (all P<0.05;
Fig. 4).
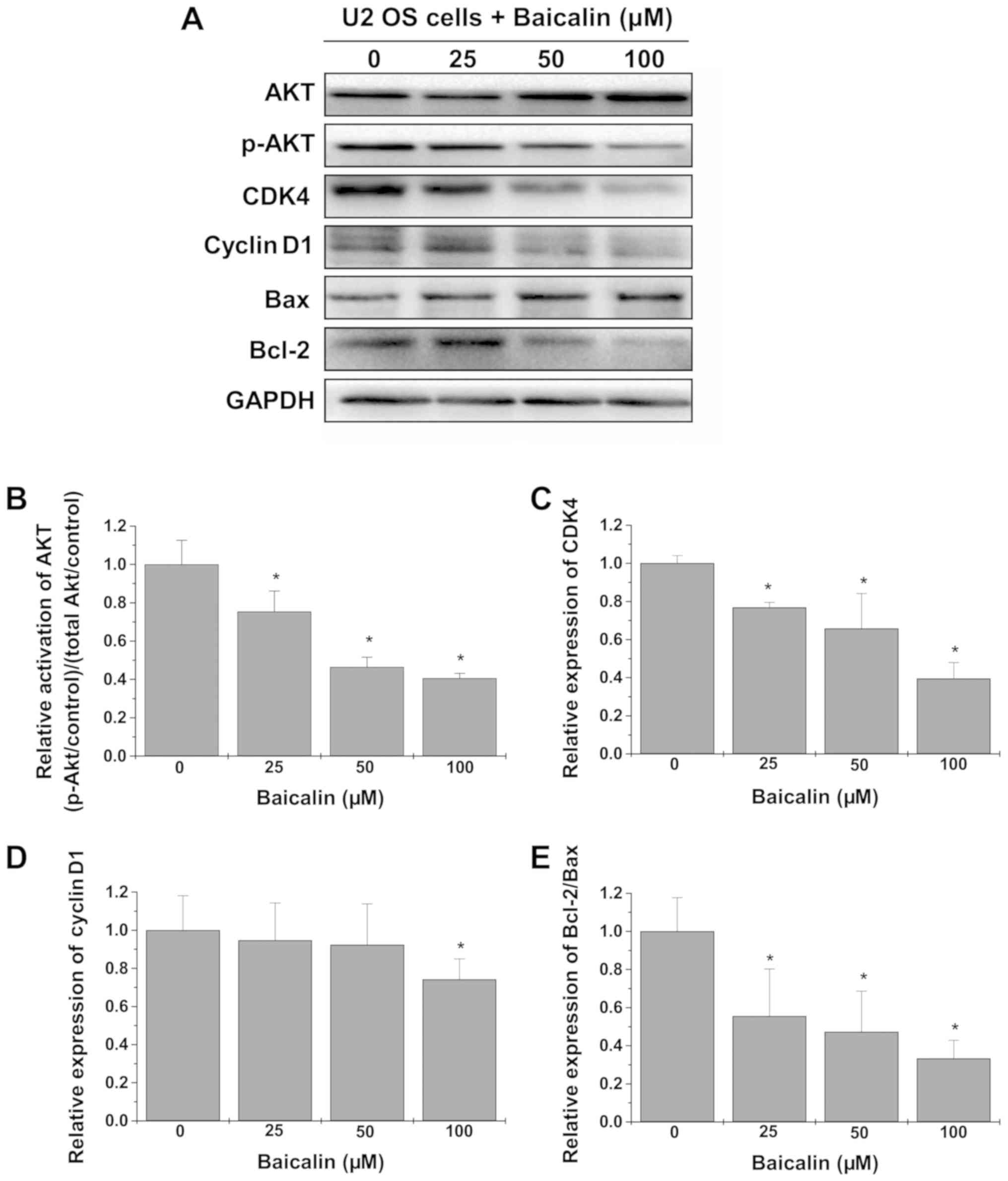
Baicalin treatment suppresses the
activation of the AKT pathway
The underlying mechanism of the antitumor effect of
baicalin was further investigated by western blot analysis
(Fig. 5A). As shown in Fig. 5B, treatment with baicalin
significantly decreased the levels of p-AKT relative to total AKT
(all P<0.05 vs. untreated cells). Determination of the protein
levels of its downstream effectors revealed significantly
downregulated expression of CDK4 at all tested concentrations, and
cyclin D1 at 100 µM only, and decreased Bcl-2/Bax ratio in U-2 OS
cells treated with baicalin (all P<0.05 vs. untreated cells;
Fig. 5C-E).
Discussion
Treatment of OS by chemotherapy and surgical
resection is limited, and patients who are resistant to treatment
or have metastasis have poor overall survival (3). Thus, the development of effective
therapeutic approaches for OS, with low toxicity and minimal side
effects, is required. A number of natural compounds, including
kaempferol and oleanolic acid, exhibit potential as anticancer
agents with low toxicity (36,37).
Baicalin is a naturally bioactive compound extracted from the
Chinese herb Huang Qin (Scutellaria baicalensis Georgi)
(7). Previous studies demonstrated
significant effects of baicalin, such as those against oxidation,
tumor, inflammation and proliferation (8–11).
Moreover, treatment of various cancer cells with baicalin
significantly suppresses tumor growth and metastasis, via multiple
downstream signaling pathways (13–19).
Other studies on OS revealed significant induction of cell
apoptosis and inhibition of metastasis following baicalin treatment
(20,21). In the present study, the role of
baicalin on the growth of OS cells was further explored. This
revealed significantly decreased cell confluence and number of OS
cells. Moreover, the CCK-8 and colony-formation assays revealed
significantly decreased cell viability of OS cells treated with
baicalin, suggesting the potential of baicalin as an anticancer
therapy for OS. However, the effect of baicalin treatment on tumor
growth, metastasis and chemotherapy resistance in OS should be
further assessed in vivo.
As with most cancer cells, OS cells are
characterized by imbalanced cell proliferation and apoptosis.
Therefore, the present study determined the progression of the cell
cycle in OS cells and found that treatment with baicalin
significantly decreased the percentage of cells at the S phase,
whereas as an increased percentage at the
G0/G1 phase was observed. Cyclin D1, together
with specific kinases (CDK4 and CDK6), plays an essential role in
the regulation of the cell cycle at the G0/G1
restriction points. Therefore, western blot analysis of cyclin D1
and CDK4 was performed on U-2 OS cells treated with baicalin,
revealing their downregulation. However, other regulators
(including p21, p27 and CDK6) that are involved in the control of
cell cycle checkpoints at the G0/G1 and S
phases should be further evaluated in future studies. Moreover, the
percentage of cells at the G2/M phase was increased
following treatment with 25 and 100 µM baicalin, whereas 50 µM
baicalin had no effect. This observation can be further explored in
future studies.
Evading apoptosis is another hallmark of cancer
cells, which is mediated by anti-proliferative proteins (such as
Bcl-2) and anti-apoptotic proteins (such as Bax). Analysis of the
fragmented DNA (a characteristic of late apoptosis) by PI staining
revealed a low percentage of cells at the Sub-G1 phase. This was in
agreement with the finding of an increased percentage of apoptotic
cells by Annexin V staining. However, the role of baicalin in the
apoptotic status of cells should be further investigated using
double staining of Annexin V-allophycocyanin with PI or
7-aminoactinomycin D. Furthermore, western blot analysis revealed
enhanced expression of the anti-apoptotic protein Bax, whereas
expression of the anti-apoptotic protein Bcl-2 was decreased, in
U-2 OS cells treated with baicalin. These findings thus suggest
induced cell cycle arrest and cell apoptosis via the downregulation
of cell cycle regulators cyclin D1 and CDK4, and anti-apoptotic
protein Bcl-2. However, the underlying mechanism of baicalin needs
to be further investigated with omics technologies, including cDNA
arrays and sequencing.
Multiple signaling pathways (including ERK, p38 MAPK
and STAT3) have been reported to play a critical role in the
development and prognosis of OS, which has been reported to be
suppressed by baicalin, leading to the inhibition of tumor growth
and metastasis (12–19). However, to the best of our knowledge,
the regulatory effect of baicalin on the AKT pathway has never been
evaluated in OS. In OS, hyperactivation of the AKT pathway is
critical in tumorigenesis, proliferation, cell cycle, apoptosis and
metastasis, by regulating the expression of its downstream
effectors (such as cyclin D1, CDK4, Bcl-2 and Bax) (25–32).
Thus, the downregulation of cyclin D1, CDK4, Bcl-2 expression and
upregulation of Bax expression observed in OS cells after baicalin
treatment prompts further exploration of the role of this compound
on the activation of the AKT pathway.
In conclusion, baicalin significantly suppressed the
growth of OS cells, inhibited cell viability and survival, and
induced cell cycle arrest and apoptosis. Mechanistic studies
revealed suppression of the AKT pathway and decreased protein
expression of cyclin D1, CDK4 and Bcl-2/Bax ratio as the possible
mechanism of the baicalin antitumor effect in OS. The present study
provides a basis to further explore the treatment of OS with
baicalin in the future, using in vitro and in vivo
studies.
Acknowledgements
Not applicable.
Funding
This study was supported by the National Nature
Science Foundation of China (grant no. 81373659).
Availability of data and materials
The datasets generated and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
YL and JH conceived and designed the experiments.
YL, PC and JW performed the experiments on cells. ZH and YZ
conducted the western blot analysis. YL and JW conducted the data
analysis. YL and PC produced and revised the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mirabello L, Troisi RJ and Savage SA:
Osteosarcoma incidence and survival rates from 1973 to 2004: Data
from the surveillance, epidemiology, and end results program.
Cancer. 115:1531–1543. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dong F, Liu T, Jin H and Wang W:
Chimaphilin inhibits human osteosarcoma cell invasion and
metastasis through suppressing the TGF-β1-induced
epithelial-to-mesenchymal transition markers via PI-3K/Akt, ERK1/2,
and Smad signaling pathways. Can J Physiol Pharmacol. 96:1–7. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang Y, Li L, Shao N, Hu Z, Chen H, Xu L,
Wang C, Cheng Y and Xiao J: Triazine-modified dendrimer for
efficient TRAIL gene therapy in osteosarcoma. Acta Biomater.
17:115–124. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bienemann K, Staege MS, Howe SJ,
Sena-Esteves M, Hanenberg H and Kramm CM: Targeted expression of
human folylpolyglutamate synthase for selective enhancement of
methotrexate chemotherapy in osteosarcoma cells. Cancer Gene Ther.
20:514–520. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wu G, Liang Q and Liu Y: Primary
osteosarcoma of frontal bone: A case report and review of
literature. Medicine (Baltimore). 96:e93922017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu J, Zhang L, Ren Y, Gao Y, Kang L and
Qiao Q: Anticancer and immunoregulatory activity of Gynostemma
pentaphyllum polysaccharides in H22 tumor-bearing mice. Int J Biol
Macromol. 69:1–4. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li B, Wan L, Li Y, Yu Q, Chen P, Gan R,
Yang Q, Han Y and Guo C: Baicalin, a component of Scutellaria
baicalensis, alleviates anorexia and inhibits skeletal muscle
atrophy in experimental cancer cachexia. Tumour Biol.
35:12415–12425. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gao Z, Huang K and Xu H: Protective
effects of flavonoids in the roots of Scutellaria
baicalensis Georgi against hydrogen peroxide-induced oxidative
stress in HS-SY5Y cells. Pharmacol Res. 43:173–178. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ikezoe T, ChenS S, Heber D, Taguchi H and
Koeffler HP: Baicalin is a major component of PC-SPES which
inhibits the proliferation of human cancer cells via apoptosis and
cell cycle arrest. Prostate. 49:285–292. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shen YC, Chiou WF, Chou YC and Chen CF:
Mechanisms in mediating the anti-inflammatory effects of baicalin
and baicalein in human leukocytes. Eur J Pharmacol. 465:171–181.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dong LH, Wen JK, Miao SB, Jia Z, Hu HJ,
Sun RH, Wu Y and Han M: Baicalin inhibits PDGF-BB-stimulated
vascular smooth muscle cell proliferation through suppressing
PDGFRβ-ERK signaling and increase in p27 accumulation and prevents
injury-induced neointimal hyperplasia. Cell Res. 20:1252–1262.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen WC, Kuo TH, Tzeng YS and Tsai YC:
Baicalin induces apoptosis in SW620 human colorectal carcinoma
cells in vitro and suppresses tumor growth in vivo. Molecules.
17:3844–3857. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yu Y, Pei M and Li L: Baicalin induces
apoptosis in hepatic cancer cells in vitro and suppresses tumor
growth in vivo. Int J Clin Exp Med. 8:8958–8967. 2015.PubMed/NCBI
|
|
14
|
Wang H, Li H, Chen F, Luo J, Gu J, Wang H,
Wu H and Xu Y: Baicalin extracted from Huangqin (Radix
Scutellariae Baicalensis) induces apoptosis in gastric cancer
cells by regulating B cell lymphoma (Bcl-2)/Bcl-2-associated X
protein and activating caspase-3 and caspase-9. J Tradit Chin Med.
37:229–235. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dou J, Wang Z, Ma L, Peng B, Mao K, Li C,
Su M, Zhou C and Peng G: Baicalein and baicalin inhibit colon
cancer using two distinct fashions of apoptosis and senescence.
Oncotarget. 9:20089–20102. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou T, Zhang A, Kuang G, Gong X, Jiang R,
Lin D, Li J and Li H, Zhang X, Wan J and Li H: Baicalin inhibits
the metastasis of highly aggressive breast cancer cells by
reversing epithelial-to-mesenchymal transition by targeting
β-catenin signaling. Oncol Rep. 38:3599–3607. 2017.PubMed/NCBI
|
|
17
|
Wang XF, Zhou QM, Du J, Zhang H, Lu YY and
Su SB: Baicalin suppresses migration, invasion and metastasis of
breast cancer via p38MAPK signaling pathway. Anticancer Agents Med
Chem. 13:923–931. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang Z, Ma L, Su M, Zhou Y, Mao K, Li C,
Peng G, Zhou C, Shen B and Dou J: Baicalin induces cellular
senescence in human colon cancer cells via upregulation of DEPP and
the activation of Ras/Raf/MEK/ERK signaling. Cell Death Dis.
9:2172018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang Q, Xu H and Zhao X: Baicalin inhibits
human cervical cancer cells by suppressing protein kinase C/signal
transducer and activator of transcription (PKC/STAT3) signaling
pathway. Med Sci Monit. 24:1955–1961. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wan D and Ouyang H: Baicalin induces
apoptosis in human osteosarcoma cell through ROS-mediated
mitochondrial pathway. Nat Prod Res. 32:1996–2000. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang Y, Wang H, Zhou R, Zhong W, Lu S, Ma
Z and Chai Y: Baicalin inhibits human osteosarcoma cells invasion,
metastasis, and anoikis resistance by suppressing the transforming
growth factor-β1-induced epithelial-to-mesenchymal transition.
Anticancer Drugs. 28:581–587. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Abeyrathna P and Su Y: The critical role
of Akt in cardiovascular function. Vascul Pharmacol. 74:38–48.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Martini M, De Santis MC, Braccini L,
Gulluni F and Hirsch E: PI3K/AKT signaling pathway and cancer: An
updated review. Ann Med. 46:372–383. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Porta C, Paglino C and Mosca A: Targeting
PI3K/Akt/mTOR signaling in cancer. Front Oncol. 4:642014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang J, Yu XH, Yan YG, Wang C and Wang
WJ: PI3K/Akt signaling in osteosarcoma. Clin Chim Acta.
444:182–192. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhou Y, Zhu LB, Peng AF, Wang TF, Long XH,
Gao S, Zhou RP and Liu ZL: LY294002 inhibits the malignant
phenotype of osteosarcoma cells by modulating the
phosphatidylinositol 3-kinase/Akt/fatty acid synthase signaling
pathway in vitro. Mol Med Rep. 11:1352–1357. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang B, Li YL, Zhao JL, Zhen O, Yu C,
Yang BH and Yu XR: Hypoxia-inducible factor-1 promotes cancer
progression through activating AKT/Cyclin D1 signaling pathway in
osteosarcoma. Biomed Pharmacother. 105:1–9. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ren L, Hong ES, Mendoza A, Issaq S, Tran
Hoang C, LeBlanc A and Khanna C: Metabolomics uncovers a link
between inositol metabolism and osteosarcoma metastasis.
Oncotarget. 8:38541–38553. 2017.PubMed/NCBI
|
|
29
|
Zhang Y, Hu H, Song L, Cai L, Wei R and
Jin W: Epirubicin-mediated expression of miR-302b is involved in
osteosarcoma apoptosis and cell cycle regulation. Toxicol Lett.
222:1–9. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang K, Tian F, Zhang Y, Zhu Q, Xue N,
Zhu H, Wang H and Guo X: MACC1 is involved in the regulation of
proliferation, colony formation, invasion ability, cell cycle
distribution, apoptosis and tumorigenicity by altering Akt
signaling pathway in human osteosarcoma. Tumour Biol. 35:2537–2548.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li G, Cai M, Fu D, Chen K, Sun M, Cai Z
and Cheng B: Heat shock protein 90B1 plays an oncogenic role and is
a target of microRNA-223 in human osteosarcoma. Cell Physiol
Biochem. 30:1481–1490. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Atif F, Yousuf S and Stein DG: Anti-tumor
effects of progesterone in human glioblastoma multiforme: Role of
PI3K/Akt/mTOR signaling. J Steroid Biochem Mol Biol. 146:62–73.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhuo B, Li Y, Li Z, Qin H, Sun Q, Zhang F,
Shen Y, Shi Y and Wang R: PI3K/Akt signaling mediated Hexokinase-2
expression inhibits cell apoptosis and promotes tumor growth in
pediatric osteosarcoma. Biochem Biophys Res Commun. 464:401–406.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang Y, Wan D, Zhou R, Zhong W, Lu S and
Chai Y: Geraniin inhibits migration and invasion of human
osteosarcoma cancer cells through regulation of PI3K/Akt and ERK1/2
signaling pathways. Anticancer Drugs. 28:959–966. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Angulo P, Kaushik G, Subramaniam D,
Dandawate P, Neville K, Chastain K and Anant S: Natural compounds
targeting major cell signaling pathways: A novel paradigm for
osteosarcoma therapy. J Hematol Oncol. 10:102017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lee J and Kim JH: Kaempferol inhibits
pancreatic cancer cell growth and migration through the blockade of
EGFR-related pathway in vitro. PLoS One. 11:e01552642016.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li L, Wei L, Shen A, Chu J, Lin J and Peng
J: Oleanolic acid modulates multiple intracellular targets to
inhibit colorectal cancer growth. Int J Oncol. 47:2247–2254. 2015.
View Article : Google Scholar : PubMed/NCBI
|