Breast cancer is the most common cancer type in
females, and the incidence rate has been steadily increasing
worldwide over the past decade (1,2). Breast
cancer-associated mortality typically results from distant
metastasis, rather than from the primary tumor (3). Despite recent advances in the
application of targeted therapeutic strategies, no significant
improvements in the prognosis of patients with metastatic breast
cancer have been achieved due to the incomplete understanding of
the molecular mechanisms governing the metastatic process.
Metastasis is a complex cascade involving
interactions between cancer cells and surrounding
microenvironmental components, including mesenchymal cells, immune
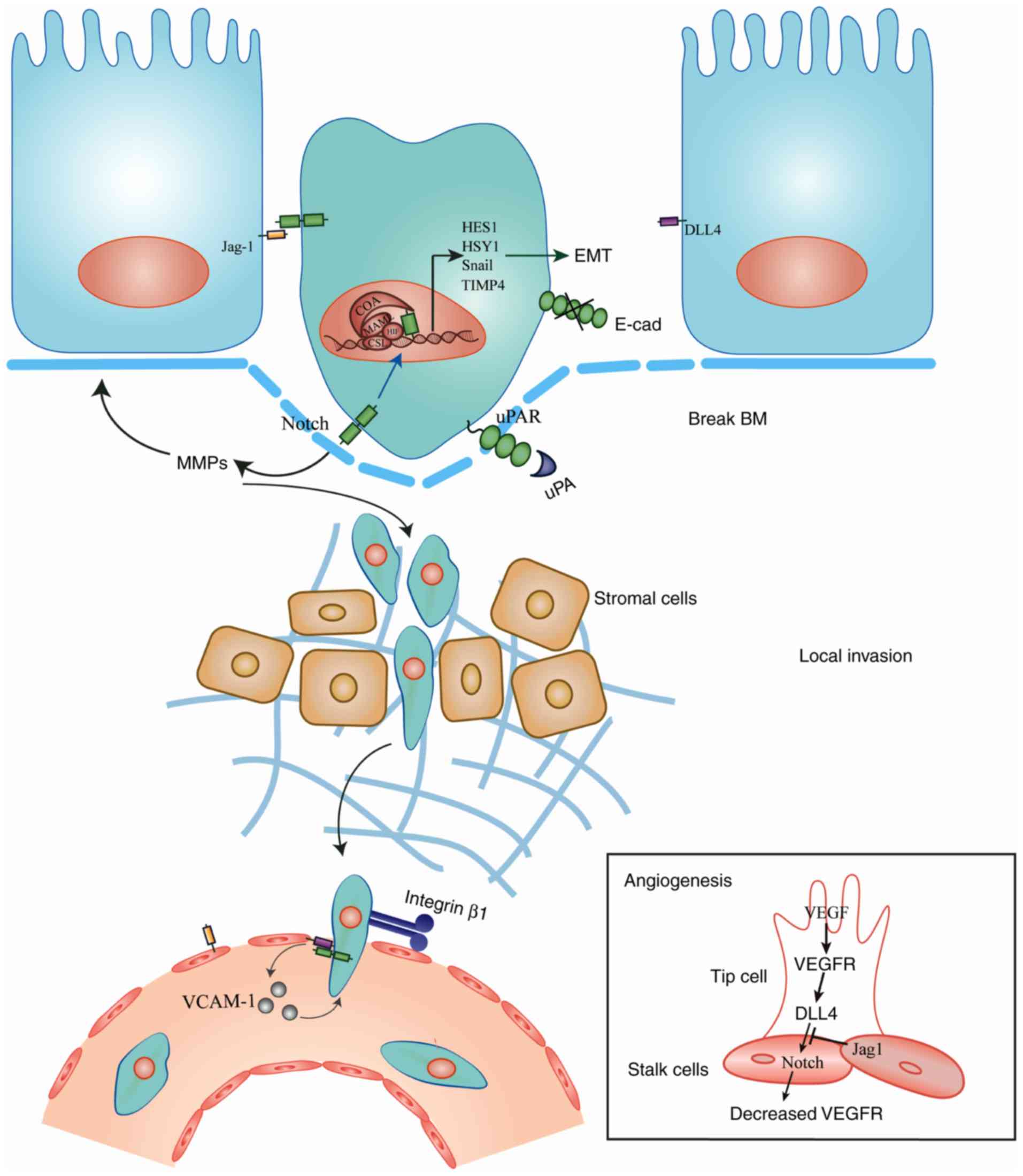
cells and the extracellular matrix (4). The first stage of breast cancer
metastasis is characterized by an invasion of the basement membrane
by primary tumor cells, which then become disseminated tumor cells
(DTCs) (5). These cells then promote
abnormal angiogenesis, intravasate into the circulatory or
lymphatic system, migrate to distant organs and establish secondary
tumors (6).
Accumulating evidence has indicated the important
role of Notch, a highly conserved family of signaling molecules, in
breast cancer metastasis. The deregulation of Notch signaling is
reflected in all aspects of the metastatic processes and its role
in breast cancer appears to be highly context-dependent.
Activation of Notch signaling requires interactions
between ligands on the surface of signal-sending cells and Notch
receptors (Notch1-4) on the surface of signal-receiving cells.
Mammalian Notch signaling comprises two pathways: The canonical
pathway and the non-canonical pathway (7).
However, the understanding of non-canonical Notch
signaling is primitive compared with that of the canonical one.
Non-canonical Notch signaling has its distinctive ligands,
including Delta-like 1, an integral membrane protein,
microfibril-associated glycoprotein and a secreted ligand (12). Of note, activation of non-canonical
Notch signaling does not require the participation of CSL; after
the binding of the ligand and receptor, NICD is released and can
therefore enter the nucleus directly (12).
In the orderly development of mammary tissues, the
balance between differentiation and division is achieved by
asymmetric divisions (ACD), which is controlled by several
lineage-specific differentiation-inducing transcription factors
(13). Through ACD, the bi-potent
mammary stem cells (MaSCs) divide into basal or luminal stem cells,
and then become myoepithelial or ductal/alveolar cells respectively
(14).
Notch signaling acts as an intrinsic regulator in
the biological behavior of normal MaSCs (15). Notch signaling has been hypothesized
to promote self-renewal proliferation and facilitate the
myoepithelial lineage-specific commitment of MaSCs during the
development of mammary glands (16).
The cell fate developmental decisions of Notch signaling are
negatively controlled by Numb, a protein asymmetrically located in
dividing progenitor cells (17).
Numb facilitates Notch ubiquitination at the membrane, promotes
degradation of NICD, circumvents its nuclear translocation and
inhibits activation of signaling downstream of Notch (18). However, in the case of overexpression
of Notch components, the steady-state number of MaSCs may be
disrupted, allowing mutant stem cells/breast cancer stem cells
(BCSCs) to arise. These poorly differentiated BCSCs exhibit a high
level of CD44; however, little or very low levels of CD24,
resulting in a CD44+/CD24−/lo phenotype
(19). CD44 is a cell surface
adhesion molecule that is enriched in basal-like breast cells
(20). CD44 binds to hyaluronate and
is associated with metastasis (21).
While CD24 is a cell surface marker of differentiated breast
luminal cells, cells with low expression of CD24 are usually
basal-like (22). BCSCs are
heterogeneous and can be subtyped into
CD44+/CD24lo progeny and
CD44+/CD24− progeny regarding CD24 expression
(23). Compared with
CD44+/CD24− cells,
CD44+/CD24lo cells acquire significantly
overexpressed Notch signaling components and upregulated embryonic
stem cell transcription programs such as Notch1-mediated embryonic
transcription factor Sox2 activation, which may aid in explaining
why the CD44+/CD24lo progeny exhibits greater
tumor initiating ability compared with
CD44+/CD24− progeny (23). Notch1 overexpression also helps
CD44+/CD24− cells convert into
CD44+/CD24lo cells, and Notch4 signaling has
exhibited greater efficacy, when compared with Notch1 in the
formation and maintenance of BCSCs, as Notch4-knockdown completely
suppresses the tumor formation while Notch1-knockdown only reduces
the tumor size and number (23).
The heterogeneity of BCSCs also confers it with
drug-resistant ability. Notch inhibition had little effect in the
CD44+/CD24− subpopulation. However, peptides
derived from Notch and Numb can activate cell-toxic lymphocytes to
eliminate BCSCs, which provides a novel insight into breast cancer
treatment (23).
ELF5 regulates MaSCs differentiation into the
alveolar and luminal lineages through the Notch signaling pathway
by binding to the responsive elements within the Notch gene
(24). ELF5 may also inhibit breast
cancer metastasis by suppressing the activation of Slug, a
transcription factor in the epithelial-mesenchymal transition (EMT)
process, and loss of ELF5 provides a basis for tumorigenesis
(27). NICD1 and NICD4 are
hyperactivated in ELF5-null mammary epithelial cells, which may be
a strong initiator for the ELF5-null breast cancer phenotype
(24). RNF8 affects breast cancer
development and can also regulate the basal-to-luminal cell fate as
well. It has observed to interact with Notch signaling by
ubiquitylating NICD1 (25). Loss of
RNF8 leads to the upregulation of Notch target genes and aberrant
luminal progenitor cell expansion, resulting in an increased risk
of mammary tumorigenesis (25). In
addition, another differentiation-inducing factor GATA3, is also
directly regulated by Notch3, through the CSL-binding motif in the
GATA3 promoter (26,28).
Specific gene programs equip cancer cells with
increased mobility, which drives their migration away from primary
sites (29). EMT constitutes the
basis of the regulation of epithelial plasticity and cancer cell
mobility. During this cascade process, epithelial cells lose their
adhesion junctions and cellular polarity while acquiring
mesenchymal characteristics (30).
In vitro studies (31,32) have
suggested that Notch1-knockdown reverses the Jagged1-induced EMT.
These Notch1-silenced cells are capable of a less aggressive form
of invasion, and may be characterized by a cobblestone-shaped
phenotype rather than a spindle-like mesenchymal phenotype. Of
note, numerous studies (32–37) have demonstrated that Jagged1-mediated
Notch activation suppresses the levels of E-cadherin and increases
the levels of the mesenchymal markers N-cadherin and vimentin, the
transcription factors Slug, Snail and zinc finger E-box binding
homeobox 1 (Zeb1), as well as β-catenin in breast cancer cells to
promote migration and invasion. However, to the best of our
knowledge, the involvement of DLL in EMT has not been reported.
Signal transducer and activator of transcription 3
(STAT3) is an important pro-EMT transcription factor mediated by
Notch (38). Notch1 has been
hypothesized to activate EMT by inducing STAT3 and upregulating the
expression of p65 and interleukin (IL)-1 (38). Notch2 has also been identified to
promote EMT via the IL-6/Janus kinase (JAK)/STAT3 pathway in a
radiation-driven model of breast cancer EMT (39). Notably, non-canonical Notch signaling
was also identified to be involved in this pathway, with
upregulation of IL-6 in breast cancer cells leading to the
activation of JAK/STAT3 signaling (40). In the more aggressive triple-negative
breast cancer, the loss of Numb leads to the activation of Notch
signaling, and induces EMT and the acquirement of cancer stem
cell-like properties, culminating in early relapse and metastasis
(41,42). Other mobility-promoting programs,
including F-actin polymerization, may also be induced by Notch1
(43).
However, Notch3 serves the opposite role in EMT by
regulating estrogen receptor α (ERα). ERα is characteristic of
luminal epithelial phenotype in breast cancer cells, the loss of
which causes EMT and metastasis (44). Notch3 stimulates ERα expression not
only by directly binding to CSL-binding elements in ERα promoter,
but also indirectly by upregulating GATA-3 (an activator of ERα)
(28). These two patterns result in
ERα overexpression and thus suppress EMT.
Enhanced migratory ability alone, however, is
insufficient to drive metastasis. Disseminating cancer cells must
also invade the surrounding complex network, which primarily
consists of extracellular matrix (ECM), basement membrane and
mesenchyme (5). The matrix
metallopeptidase (MMP) family is known to degrade the ECM and
promote cancer cell invasion and metastasis (45). In breast cancer, Notch1 activation
promotes the expressions of MMP-2 and −9 to break down the ECM
components (46). Notch has also
been demonstrated to be associated with urokinase-type plasminogen
activator (uPA), which is an ECM-degradation enzyme associated with
poor outcome, and a high risk of metastasis and recurrence
(47). Under normal conditions, uPA
induces a plasminogen proteolytic sequence. However, in breast
cancer, uPA works with MMPs to erode the microvasculature and
degrade the ECM to facilitate tumor cell metastasis. uPA receptor
(uPAR) is highly expressed in malignant tissues and tends to be
located at the leading edge or invasion front (48). Upon binding to uPA, the receptor
converts plasminogen to plasmin, then degrades ECM through MMP
(48). A precious study placed Notch
upstream of the uPA cascade (49). A
positive association has been observed between Jagged1 and uPA in
various breast cancer cell lines, and Notch1-knockdown reduced uPA
levels (49). Furthermore, Notch may
directly regulate uPA transcription via centromere-binding factor 1
binding sites within the uPA promoter and enhancer. The
subsequently activated uPAR then cleaves ECM-associated signaling
molecules, including fibronectin and the laminin receptor (49,50).
Hypoxia is a term for a low-oxygen environment, and
may be the result of leaky vasculature and a lack of blood supply,
and is important for tumor progression (51). Hypoxia-inducible factor 1α (HIF-1α)
promotes metastasis and is associated with poor prognosis (52–54).
Accumulation of HIF-1α and HIF-2α enhance Notch signaling (both
receptors and ligands) as well as the expression of the downstream
genes HES1 and HEY1; HIFs and mastermind-like protein (MAML)1, a
key Notch co-activator, form a complex with NICD to recruit other
Notch co-activators, including p300, indicating a HIF/MAML1/Notch
axis under hypoxia (33). Hypoxia
stabilizes HIFs through this signaling cascade, resulting in
elevated Notch (33).
The tumor microenvironment, which primarily consists
of mesenchymal cells and immune cells, is central to the
progression of breast cancer (60).
Solid experimental evidence has indicated that cancer-associated
fibroblasts (CAFs) secrete cytokines to support breast cancer cells
and protect them from host surveillance (61). CAFs secrete ADAM10-rich exosomes,
which in turn were recently identified to be associated with loss
of TIMP family member expression, to potentiate cell motility and
aldehyde dehydrogenase (ALDH) expression through Ras homolog family
member A and Notch, respectively (62). Silencing of Notch effector Rbp-Jκ,
combined with downregulation of the tumor suppressor p53, induces a
senescent phenotype and the expression of CAF effector genes
(63).
Immune regulation also serves an important role in
breast cancer progression. CD8+ T cell infiltration,
together with type 1 interferon, activates innate immunity, acting
as an anti-tumor mechanism in breast cancer (64). It has been reported that Notch
signaling controls CD8+ T cell activation through the
binding of DLL1 with Notch1 or Notch2 (65). Notch1 has a crucial role in the
immune-suppressive tumor microenvironment and the inhibition of
Notch1 leads to recruitment of active CD8+ T cells and a
decrease of immune suppressive cells, regulatory T cells (Tregs)
and myeloid-derived suppressor cells (MDSCs) (66). However, Notch2 contributes to the
anti-tumor response, and the deletion of CD8+ T cell
specific Notch2 in mice results in increased tumor size and
decreased survival in tumor-bearing mice (67). Thus, Notch signaling has a dual role
in regulating the tumor immune response, as it may exert oncogenic
and tumor suppressive functions. Notch signaling may also act as a
transcriptional regulator in the differentiation of
tumor-associated macrophages (TAMs). TAMs may recruit Tregs and
MDSCs and also suppress CD8+ T cells (68). CSL deletion in monocytes inhibits not
only differentiation, but also the antigen-presenting function of
TAMs, restraining the immune-suppressive function of TAMs (69). Of note, overexpression of NICD has
been reported to suppress the function of TAMs and then repress
tumor growth, indicating that the effects of Notch signaling on
TAMs may depend on the extent of Notch signaling (70).
Breast cancer cell multiplications requires a lot of
nutrition, as the original blood vessels at the site of the tumor
are insufficient to the amount of nutrition for the rapid growth of
breast cancer cells (71).
Therefore, breast cancer cells exhibit an angiogenic phenotype that
allows new blood vessels to branch and create a massed vascular
network (72). In addition to
transport nutrition, these immature and highly permeable new blood
vessels also provide an efficient route of exit for breast cancer
cells to leave the primary site and enter the circulation, which
can then elicit metastasis (73). In
the process of angiogenesis, Notch ligands, together with vascular
endothelial growth factor (VEGF), the strongest mitogenic factor,
stimulates the formation of vascular endothelial cells to establish
a neovasculature (74), which then
promotes breast cancer metastasis.
In vascular endothelial cells, the ends of vessel
sprouts are termed tip cells and the other cells are called stalk
cells (75). These cell types are
essential for vessel polarity and barrier function of vessels
(76). This endothelial cell
specification is regulated by Notch signaling during tumor
angiogenesis process, with the two types of Notch ligands exerting
the opposite effects (77).
In normal conditions, VEGF receptor (VEGFR)
signaling serves as an initiator in tip cell formation, while DLL4
serves an inhibitory role (78).
Upregulation of DLL4 by VEGF/VEGFR in endothelial tip cells
suppresses the tip-like phenotype; therefore, the single tip cell
can be selected from among many candidate vascular endothelial
cells and form the new vessel sprout (78–80). To
avoid the excessive tip cell formation and immoderate angiogenesis,
high level of DLL4 signals are sent to the adjacent cells (stalk
cells) through Notch1, which then inhibits the expression of VEGFR
in stalk cells and induces vascular network quiescence (81). Jagged-1, another type of ligand, is
not directly associated with sprouting angiogenesis, but shifts the
balance between DLL4/Notch and VEGFR signaling (82). Jagged-1 primarily exists in stalk
cells and can antagonize DLL4/Notch signaling in stalk cells to
ameliorate the low VEGF response, thus activating stalk cells to
promote angiogenesis (80). These
processes are mediated by the glycosyltransferase Fringe family,
which results in Notch binding to DLL4 more easily; however,
impedes its ability to bind to Jagged-1 (83). However, in metastatic breast cancer,
overexpression of Jagged-1 transforms angiogenesis from
physiological to pathological patterns that favors metastasis. This
causes excessive angiogenesis and even gives rise to a new hybrid
tip/stalk phenotype (84,85). Therefore, it is well demonstrated
that hybrid tip/stalk phenotype leads to the formation of new
sprouts; however, new blood vessels produced under these conditions
exhibit poor perfusion with high microvessel density, which is what
metastatic DTCs require. These pathological blood vessels confer
great plasticity to the leading cell that have are capable of
exchanging its position with adjacent stalk cells rapidly, thus
creating a fast but chaotic and dense vascular network route for a
large number of DTCs to exit the primary sites (84).
Demethylases and Notch-associated proteases also
dynamically participate in angiogenesis in breast cancer, and the
overexpression of the lysine demethylase 2A (KDM2A) in human breast
cancer is associated with a worse outcome. Jagged1 is essential for
KDM2A-driven tumor angiogenesis and acting as a direct target of
KDM2A (86). Inhibition of KDM2A in
breast cancer cells blocks Notch activation and endothelial cell
tube formation (87). Proteases
including MMPs are able to make space for angiogenesis and
lymphangiogenesis (45). In
addition, uPA and uPAR may combine to activate VEGF (49).
Notch signaling modulates the ability of breast
cancer cells to cross mesenchymal and endothelial barriers
(87). Integrins are associated with
normal mammary epithelial cells, as well as with breast cancer
cells (88). A feedback loop has
been reported between integrins and Notch, and is characterized by
activated Notch signaling controlling β1 integrin affinity, while
β1 integrin inhibits the expression of Notch (89,90). β1
integrin cooperates with Notch to promote the transendothelial
migration of breast cancer cells, which is characterized by
enhanced polarity reversal and adhesion to the blood vessel wall
(91,92).
Aberrant Notch activation stimulates endothelial
cells to promote breast cancer intravasation. Vascular cell
adhesion molecule-1 may be subverted by Notch1 to enhance the
adhesion of tumor cells and neutrophils to endothelial cells, thus
favoring the dissemination of tumor cells (93). As stated previously, breast cancer
cells may also recruit factors including MMPs that increase
vascular permeability and thus promote intravasation (Fig. 1).
Breast cancer cells detach from primary sites and
then enter the circulation, becoming circulating tumor cells (CTCs)
(94). CTCs must first survive in
the bloodstream prior to arriving at distant organs (95).
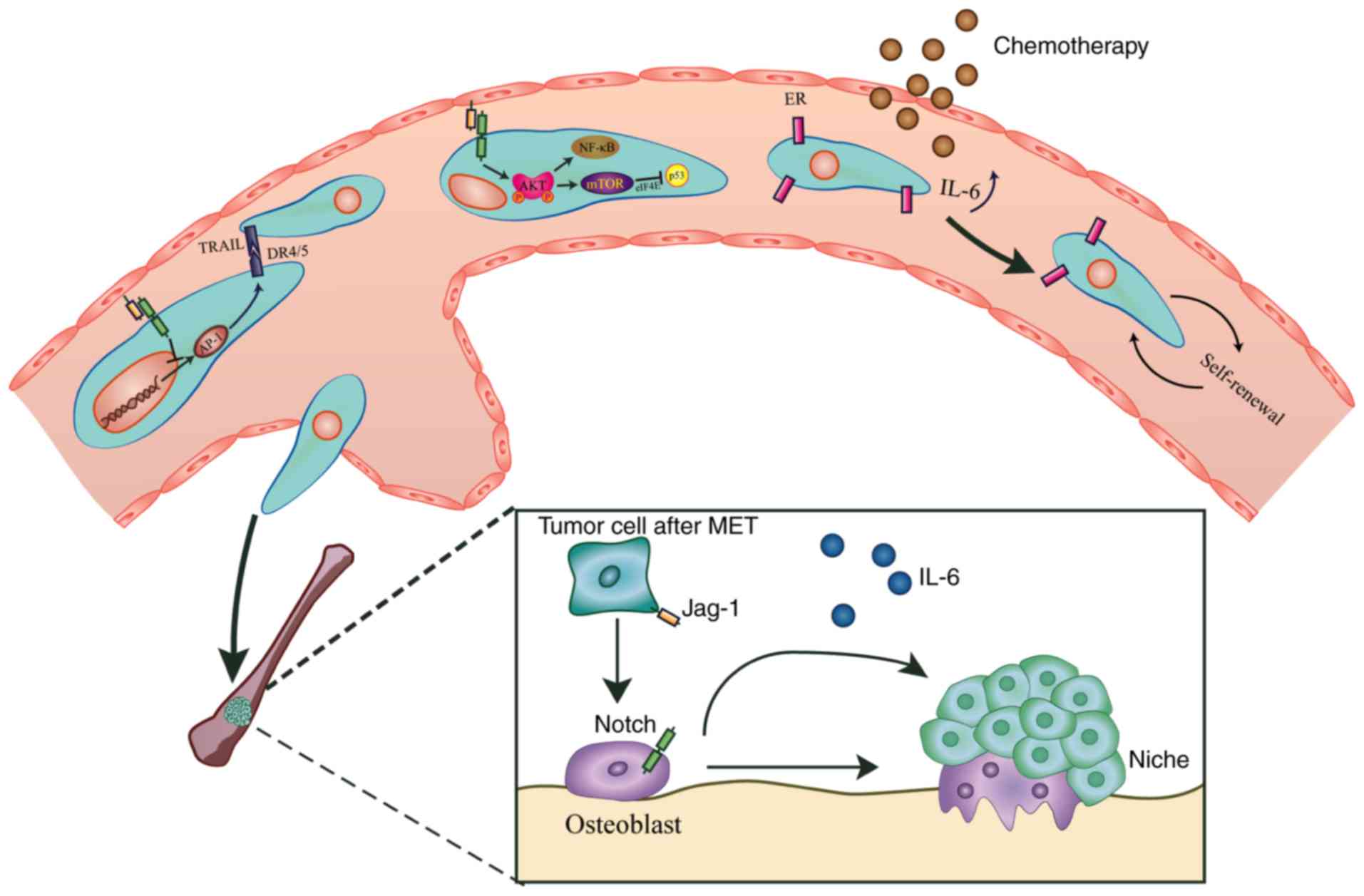
Apoptosis negatively regulates tumor progression by
preventing overgrowth. This process depends on the coordination of
numerous ligands and receptors, including tumor necrosis
factor-related apoptosis-inducing ligand (TRAIL)/TRAIL-receptor1
and 2, also termed DR4 and DR5 (96). Administration of γ-secretase
inhibitors (GSIs) may lead to a marked upregulation of DR4 and DR5,
increase the sensitization of breast cancer cells to TRAIL-mediated
apoptosis (96), activate the
caspase system (i.e. caspase-8) (97), promote mitochondrial membrane
leakiness and further induce apoptosis. This Notch-mediated
anti-apoptosis function may depend on activator protein (AP)1,
which is a dimeric transcription factor complex activated by c-Jun
N-terminal kinase (JNK) (96).
Blocked by GSIs, Notch fails to be activated, which increases the
levels of AP1 and JNK (96,98,99),
thus activating DR4 and DR5 (100).
A recent study indicated that Notch4, but not Notch1, is involved
in the sensitization of breast cancer cells to TRAIL-induced
apoptosis (101). Alternatively,
inhibition of β1 integrins may sensitize tumor cells to
TRAIL-induced apoptosis, which is mediated by Notch (102). The GSI/TRAIL combination also
decreases several survival factors, including survivin and B-cell
lymphoma 2 (96). Furthermore,
different types of breast cancer cell differ in their response to
such inhibition. For example, ER-negative breast cancer cells are
more sensitive to GSI/TRAIL synergism compared with ER-positive
cells (96).
AKT impedes DNA damage-induced apoptosis via
inhibition of apoptosis signal-regulating kinase 1, which in turn
prevents JNK-mediated activation of p53 (103), leading to an aberrant increase of
mammary progenitor cells (104).
Substantial evidence has demonstrated that impairment of Notch
signaling may inhibit AKT activity and sensitize cells to apoptosis
(103,105). It has also been reported that the
addition of DAPT, a GSI, improves the anti-tumor efficacy of
RY10-4, an anti-breast cancer drug, due to the accessorial
restraint on AKT phosphorylation exhibited by DAPT, which reduces
the survival of breast cancer cells (106).
Notch-mediated regulation of AKT contributes to
tumor cell survival through multiple pathways. AKT is hypothesized
to increase MMP production via several downstream target proteins,
including nuclear factor (NF)-κB and mammalian target of rapamycin
(mTOR) (106). Li et al
(46) suggested that Notch1
inhibition enhances protein phosphatase 2A (PP2A) activity and
downregulates NF-κB, which may be restored by the PP2A inhibitor
okadaic acid (OA). Treatment with OA also upregulates VEGF, MMP2
and MMP9, suggesting a key role of PP2A in the Notch/AKT/NF-κB axis
(107). mTOR also takes part in
AKT-mediated tumor cell survival, a mechanism contributing to
chemoresistance (108). Its
downstream effector, eukaryotic initiation factor 4E, is crucial
for mTOR-mediated inhibition of p53 and may reverse p53-mediated
cytotoxicity (109). Furthermore,
Notch activation enhances the activity of MDM2, an E3
ubiquitin-protein ligase, to also degrade p53 (110). Apart from these indirect Notch/p53
signaling pathways, Notch also directly binds to the amino terminus
of p53 without the presence of AKT, thus inhibiting p53
phosphorylation and DNA-binding activity (111).
To overcome the threat of chemotherapy, CTCs must
employ several sophisticated approaches. To contend with
chemotherapeutic agents for breast cancer, CTCs may acquire
morphological and functional endothelial features characteristic of
tumor vascularization (112). DLL3
and Notch4, with their downstream targets p65 (an NF-κB subunit)
and Zeb1, are overexpressed in tumor-derived endothelial cells
during chemotherapy (113).
Silencing of Notch4/DLL3 may decrease the functionality of
tumor-derived endothelial cells and endothelial markers (113). The expression of VEGFR3, an
important factor in tumor angiogenesis, is significantly
upregulated in patients receiving chemotherapy. Notch4/DLL3
silencing also suppress the expression of VEGFR3 transcripts,
indicating that breast cancer chemotherapy triggers the formation
of functional tumor-derived endothelial vessels by regulating Notch
and VEGF signaling (113).
Patients with ER-positive breast cancer with high
levels of ALDH1 and Notch4 exhibit poor prognosis following
anti-estrogen treatment (114).
Although short-term treatment suppresses tumor cell proliferation,
it also increases CTC activity through Jagged1/Notch4 activation,
as the administration of Notch inhibitors attenuates drug
resistance and improves patient outcomes (114). Long-term hormonal therapies may
reduce ERα expression and increase the levels of IL-6, thus
enhancing the self-renewal properties of hormonal therapy-resistant
ER-dependent, as well as ER-independent tumor cells. Subsequently,
IL-6 may cause a departure from metabolic dormancy induced by
mitochondrial activation through an IL-6/STAT3/Notch3 transduction
pattern, hence leading to the acquirement of resistance (115). Blocking IL-6 reduces the levels of
STAT3/Notch3 in breast cancer cells, resulting in increased
sensitivity to hormonal therapy such as tamoxifen (115). In addition, STAT1 may also
facilitate the expansion of therapy-resistant breast cancer cells
via Notch3 (116).
Of note, multiple courses of treatment for patients
with ER-positive/human epidermal growth factor receptor 2
(HER2)-negative breast cancer may endow their CTCs with HER2
expression (117). A further study
demonstrated that breast cancer CTCs that underwent this
transformation maintain discrete HER2-positive and HER2-negative
subpopulations (118). Of note,
HER2-positive and HER2-negative breast cancer CTCs may
spontaneously interconvert; however, have different functions.
HER2-positive CTCs acquire a stronger proliferation potential and a
higher lung metastasis frequency but are no more sensitive to
HER2-targeted therapy, while HER2-negative CTCs exhibit an
increased expression of Notch1 but a resistance to chemotherapy.
Therefore, dual treatment in Notch inhibitor-sensitive
HER2-negative/Notch-positive and chemotherapy-sensitive
HER2-positive/Notch1-negative CTCs may be a reasonable approach
(118).
Prior to establishing metastases in secondary
organs, breast cancer CTCs release factors, including MMPs, and
gather bone marrow-derived hematopoietic precursor cells to combine
with perivascular fibroblasts and fibronectin to form the
pre-metastatic niche (29). Once
CTCs extravasate and colonize the niche, they become
micrometastases (29).
The mechanism underlying metastatic organotropism
remains largely elusive; specifically, no rationale for the
propensity of breast cancer to metastasize to the bone, lung and
liver has been proven thus far. However, evidence has revealed that
bone metastasis of breast cancer may arise with the help of Notch.
In bone, breast cancer cells may employ numerous signaling
pathways, including Notch, to mediate osteoblast activation and
differentiation (119). Bone marrow
osteoblasts produce transforming growth factor-β, which increases
the levels of the Notch signaling proteins Notch3 and Jagged1, thus
promoting the secretion of osteoblast-derived IL-6 and osteoblast
differentiation (120,121). Inhibition of Notch signaling via
knockdown of Notch3 or treatment with a GSI markedly decreases
breast cancer bone metastasis (Fig.
2) (120,121).
KiSS1, a metastasis suppressor gene, is
downregulated in breast cancer secondary tumor sites (122). By enhancing the activation of
inhibitor of NF-κB, KiSS1 prevents NF-κB binding to the promoters
of pro-inflammatory and pro-metastatic genes, thus potentially
competing with Notch (122).
Furthermore, KiSS1 encodes a COOH-terminally amidated active
peptide, metastin (123). Of note,
metastin only affects secondary tumor sites but not primary lesions
(123).
Expression of tenascin C (TNC), an ECM protein
located in the stem cell niche, is an effective biomarker for
breast CTCs that have infiltrated the lung (128). TNC enhances the level of musashi
homolog 1 (MSI1), a regulator of Notch signaling, and thus confers
enhanced migratory and invasive properties to breast CTCs (128). High levels of MSI1 and Jagged1 are
indicative of a poor prognosis (129,130).
Notably, cancer-induced sprouting neovasculature may induce tip
cells to secret periostin [POSTN; Notch1 associates with POSTN at
epidermal growth factor repeats (131,132)]
to bind to TNC and then ECM, and facilitate TNC deposition on the
ECM and its incorporation into the ECM (133).
It is incumbent on DTCs to expand and establish new
colonies; otherwise, these cells enter dormancy, which is defined
as growth arrest, a balance between proliferation and apoptosis
(134). Dormant breast DTCs in the
lung may be experiencing an absence of uPA- and α5β1
integrin-triggered proliferative signaling (135). Re-activation of dormant cells calls
for increased uPAR-α5β1 integrin complexes and activation of
upstream Notch signaling (135).
Notch3 is responsible for the stability of mitogen-activated
protein kinase phosphatase-1 (MKP-1), a widely expressed
phosphatase (136). A previous
study demonstrated that the levels of Notch3 and MKP-1 are
relatively low in dormant tumors, resulting in high levels of
phosphorylated p38, a target of MKP-1 that contributes to the
maintenance of dormancy (137).
MicroRNAs (miRNAs) are a series of endogenous small
single-stranded non-coding RNAs that are ~18–24 nucleotides in
length (138). miRNAs regulate the
expression of endogenous genes by complementary base pairing at the
transcriptional or post-transcriptional levels (139). Over the past decade, the abnormal
expression of miRNAs has been observed in nearly all malignant
tumor types; therefore, miRNAs are considered to be an emerging
oncology research direction (140).
Certain miRNAs have been reported to be associated with Notch
signaling, while a number of them are aberrantly expressed in
breast cancer and are therefore associated with promoting
metastasis.
The miR-34 family member miR-34a is highly expressed
in normal mammary tissues; however, it is significantly
downregulated in breast cancer tissues (141). miR-34a has been demonstrated to
function as an important tumor suppressor by regulating a variety
of tumor progression steps, including cell proliferation, invasion
and apoptosis, and Notch, which is a target gene of miR-34a
(142). In metastatic breast cancer
cells, overexpression of miR-34a significantly increases the
protein level of tumor suppressor gene p53 and decreases the
expression of Notch1, thereby inhibiting cell proliferation,
invasion and inducing apoptosis (143,144).
Additionally, miR-34a can sensitize metastatic breast cancer cells
to paclitaxel and adriamycin (chemotherapeutic drugs for breast
cancer) partly by downregulating Notch1 expression (142,145).
miR-34a and miR-34c, another member of miR-34 family, have been
reported to prevent self-renewal and differentiation of BCSCs
(146,147). Their expression is also at a very
low level in BCSCs. Overexpression of miR-34a and miR-34c
suppresses stemness by targeting Notch1 and Notch4, respectively
(145,147). Two prognostic factors miR-1179 and
miR-3178 are downregulated in breast cancer and have both been
demonstrated to target Notch signaling. miR-1179 is a newly
identified miRNA in 2018 (148).
Clinicopathological analysis revealed that decreased miR-1179
expression in breast cancer was correlated with advanced clinical
stage and lymph node metastasis (149). Upregulated miR-1179 suppresses the
breast cancer vitality and motility, by inhibiting the expression
of Notch1, Notch4 and their downstream Hes1 (149). miR-3178 is a prognostic factor,
particularly in TNBC, and its ectopic overexpression can inhibit
metastasis by blocking Notch1-induced EMT (150). In addition, miR-9 can reduce
metastatic behaviors in TNBC by targeting Notch1 (151).
However, miRNAs do not all function as tumor
suppressors in breast cancer, some have been observed to also
promote metastasis. Notch3 can inhibit EMT in breast cancer and
directly target miR-221/222 (152).
By directly binding to the 3′-untranslated region of Notch3 and
inhibiting its translation, miR-221/222 exerts an oncogenic role by
promoting EMT (152). miR-146a is
also upregulated in BCSCs, and activates Notch signaling by
targeting the Notch suppressor Numb (153,154).
Current treatments for metastatic breast cancer
(BC) are predominantly palliative with little clinical efficacy
(155). Encouragingly, numerous
studies have focused on the treatment of metastatic BC via
targeting Notch signaling. γ-secretase inhibitors exhibit great
potential, for example, PF-03084014 (Pfizer Oncology), a small
molecule selective noncompetitive and reversible GSI, displays
synergistic activity with docetaxel and has demonstrated
significant antitumor activity in a patient with triple-negative BC
(156). In addition, a potent
non-competitive oral GSI, MK-0752 (MERK), has been evaluated for
the treatment of metastatic BC via induction of G0/G1 arrest
(157). Monoclonal antibodies
against DLL4, including REGN421/SAR153192 (Regeneron
Pharmaceuticals), OMP-21M18, OMP-59R5 and OPM-52M51 (OncoMed
Pharmaceuticals), which target Notch 2/3 and Notch 1 receptors,
have also been investigated in clinical trials (158). Additionally, BXL0124, a Gemini
vitamin D analog, has been demonstrated to be effective in
suppressing CD44+/CD24−/lo BCSCs in
basal-like BC through HES1-mediated Notch1 inhibition (159). Although numerous drugs are in
development, significant challenges still exist before a
Notch-targeted therapeutic strategy can be clinically applied. For
example, patients with BC receiving continuous doses of MK-0752 at
450 mg/daily present with symptoms of toxicity and fatigue
(160). Furthermore,
gastrointestinal toxicity is also a major side effect in patients
treated with GSIs (161).
In recent years, cancer immunotherapy has
demonstrated striking improvements in long-term survival (162), which has had a large impact on
conventional systemic cancer therapy. Studies have revealed a key
role of Notch in breast cancer immunotherapy. Notch1 depletion
improves the efficacy of anti-tumor drugs, nivolumab
(anti-programmed death-1 (PD-1) antibody) and ipilimumab (cytotoxic
T cell-associated antigen-4 (CTLA-4) antibody) (66). PD-1 and CTLA-4 are inhibitory
receptors on the surfaces of T cells, which can abrogate T cell
activation when binding to ligands (163), and BC cells can express their
ligands (PDL1 and B7 for PD-1 and CTLA-4, respectively), thereby
deactivating cytotoxic T cells and attenuating the immune
response.
Breast cancer is therapeutically challenging due to
its distant metastasis. Recurrence at distant organs suggests that
the dissemination of tumor cells may occur at very early, typically
asymptomatic stages. Notch signaling modulates breast cancer
metastasis in many links, and different receptors and ligands serve
distinct roles (32–34,36–43,46).
Notch3, however, can exert oncogenic or anti-oncogenic functions in
cancer progression in a context dependent manner. By modulating
GATA3 and ERα, Notch3 suppresses EMT and metastasis in breast
cancer (28,44). Notch3 is also negatively associated
with chemoresistance, and it has been reported that the
overexpression of Notch3 results in low degree of breast cancer
chemoresistance (164). Of note,
breast cancer metastasis exhibits organotropisms (165), and Notch3 has been reported to be
associated with bone metastasis. Notch3 enhances bone metastasis by
increasing the secretion of transforming growth factor β1 by
osteoblasts, thus activating the colony formation of breast cancer
cells (120).
In order to promote primary tumor cell
dissemination, Notch signaling can either trigger or inhibit EMT by
interacting with downstream effectors (28,38–44),
then regulating the invasion of breast cancer cells through the
mesenchyme and basement membrane (62–70).
With the help of the neovascular network, Notch signaling further
initiates anti-apoptotic (96–100)
and chemoresistant (114–116) characteristics in circulation and
secondary colonization, which facilitate metastasis to distant
organs. However, the mechanism of metastasis remains elusive. For
example, certain patients carrying DTCs never develop metastasis,
while other patients with large metastases do not present with DTCs
at the time of primary tumor detection.
Another topic of interest in breast cancer research
is exosomes, which are cell-derived vesicles that contain various
biomolecules of their cell origin, such as DNA, RNA and proteins
(166). It has been demonstrated
that exosomes can regulate therapy resistance of breast cancer via
exosomal RNA (exoRNA) transferring from stromal cells of the tumor
microenvironment to breast cancer cells (116). The exoRNA can activate the RIG-I
receptor (a subtype of pathogen recognition receptor) on breast
cancer cells to induce STAT1 expression, which then facilitates
Notch target genes expression in breast cancer cells, resulting in
the upregulation of Notch3 signaling and an increase in
chemoresistant BCSCs (116). In
addition, exosomes have also been observed to enable organotropic
metastasis by preparing a pre-metastatic niche, which is achieved
through the fusion of the specific integrin (ITG) and
organ-specific resident cells (167). Exosomes have also been shown to
correlate with immune modulation and apoptosis in breast cancer
(168). Therefore, the interaction
between exosomes and Notch should be the focus of additional
investigations, and exosomes may be a potential research target in
breast cancer in the future.
Investigation of only one signaling pathway is also
insufficient for the development of appropriate therapy, since the
activation of associated pathways as well as the cross-talk between
Notch and other signaling pathways are of critical importance in
breast cancer metastasis. Specific aspects that will be important
to consider include TNC stimulation of Notch and WNT signaling to
balance dormancy and activation (128). Furthermore, Notch is also
associated with the Hedgehog signaling pathway, which then
regulates the tumor immunity response (169,170).
However, the complicated mechanisms of metastasis reveal just the
tip of the iceberg, and the presently available knowledge of Notch
signaling and BC metastasis is insufficient.
Not applicable.
This study was funded by a grant from the National
Science Foundation of China (grant. no. 31860317).
Not applicable.
YZ was responsible for the conception and design of
the review, and the drafting of the manuscript. ZX and XG were
responsible for collecting the evidence and revising the
manuscript. XX and LX gave final approval of the present version of
the manuscript to be published. All authors read and approved the
manuscript and agreed to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
Statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wu Y, Shao A, Wang L, Hu K, Yu C, Pan C
and Zhang S: The role of lncRNAs in the distant metastasis of
breast cancer. Front Oncol. 9:4072019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Spill F, Reynolds DS, Kamm RD and Zaman
MH: Impact of the physical microenvironment on tumor progression
and metastasis. Curr Opin Biotechnol. 40:41–48. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wan L, Pantel K and Kang Y: Tumor
metastasis: Moving new biological insights into the clinic. Nat
Med. 19:1450–1464. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kozłowski J, Kozłowska A and Kocki J:
Breast cancer metastasis-insight into selected molecular mechanisms
of the phenomenon. Postepy Hig Med Dosw (Online). 69:447–451. 2014.
View Article : Google Scholar
|
|
7
|
Bray SJ: Notch signalling in context. Nat
Rev Mol Cell Biol. 17:722–735. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bray SJ: Notch signalling: A simple
pathway becomes complex. Nat Rev Mol Cell Biol. 7:678–689. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brou C, Logeat F, Gupta N, Bessia C,
LeBail O, Doedens JR, Cumano A, Roux P, Black RA and Israël A: A
novel proteolytic cleavage involved in Notch signaling: The role of
the disintegrin-metalloprotease TACE. Mol Cell. 5:207–216. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sprinzak D, Lakhanpal A, Lebon L, Santat
LA, Fontes ME, Anderson GA, Garcia-Ojalvo J and Elowitz MB:
Cis-interactions between Notch and Delta generate mutually
exclusive signalling states. Nature. 465:86–90. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wu L, Aster JC, Blacklow SC, Lake R,
Artavanis-Tsakonas S and Griffin JD: MAML1, a human homologue of
Drosophila mastermind, is a transcriptional co-activator for
NOTCH receptors. Nat Genet. 26:484–489. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
D'Souza B, Miyamoto A and Weinmaster G:
The many facets of Notch ligands. Oncogene. 27:5148–5167. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Visan I: Asymmetric division. Nature
Immunol. 13:1202012. View Article : Google Scholar
|
|
14
|
Santoro A, Vlachou T, Carminati M, Pelicci
PG and Mapelli M: Molecular mechanisms of asymmetric divisions in
mammary stem cells. EMBO Rep. 17:1700–1720. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bouras T, Pal B, Vaillant F, Harburg G,
Asselin-Labat ML, Oakes SR, Lindeman GJ and Visvader JE: Notch
signaling regulates mammary stem cell function and luminal
cell-fate commitment. Cell Stem Cell. 3:429–441. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dontu G, Jackson KW, Mcnicholas E,
Kawamura MJ, Abdallah WM and Wicha MS: Role of Notch signaling in
cell-fate determination of human mammary stem/progenitor cells.
Breast Cancer Res. 6:R605–R615. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rhyu MS, Jan LY and Jan YN: Asymmetric
distribution of numb protein during division of the sensory organ
precursor cell confers distinct fates to daughter cells. Cell.
76:477–491. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
McGill MA and McGlade CJ: Mammalian numb
proteins promote Notch1 receptor ubiquitination and degradation of
the Notch1 intracellular domain. J Biol Chem. 278:23196–23203.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Honeth G, Bendahl PO, Ringnér M, Saal LH,
Gruvberger-Saal SK, Lövgren K, Grabau D, Fernö M, Borg A and
Hegardt C: The CD44+/CD24-phenotype is enriched in
basal-like breast tumors. Breast Cancer Res. 10:R532008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Calaf GM, Ponce-Cusi R and Abarca-Quinones
J: Effect of curcumin on the cell surface markers CD44 and CD24 in
breast cancer. Oncol Rep. 39:2741–2748. 2018.PubMed/NCBI
|
|
21
|
Huiping L, Patel MR, Prescher JA,
Patsialou A, Qian D, Lin J, Wen S, Chang YF, Bachmann MH, Shimono
Y, et al: Cancer stem cells from human breast tumors are involved
in spontaneous metastases in orthotopic mouse models. Proc Natl
Acad Sci USA. 107:18115–18120. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shipitsin M, Campbell LL, Argani P,
Weremowicz S, Bloushtain-Qimron N, Yao J, Nikolskaya T,
Serebryiskaya T, Beroukhim R, Hu M, et al: Molecular definition of
breast tumor heterogeneity. Cancer Cell. 11:259–273. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Azzam DJ, Zhao D, Sun J, Minn AJ,
Ranganathan P, Drews-Elger K, Han X, Picon-Ruiz M, Gilbert CA,
Wander SA, et al: Triple negative breast cancer initiating cell
subsets differ in functional and molecular characteristics and in
γ-secretase inhibitor drug responses. EMBO Mol Med. 5:1502–1522.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chakrabarti R, Wei Y, Romano RA, DeCoste
C, Kang Y and Sinha S: Elf5 regulates mammary gland stem/progenitor
cell fate by influencing notch signaling. Stem Cells. 30:1496–1508.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li L, Guturi KKN, Gautreau B, Patel PS,
Saad A, Morii M, Mateo F, Palomero L, Barbour H, Gomez A, et al:
Ubiquitin ligase RNF8 suppresses Notch signaling to regulate
mammary development and tumorigenesis. J Clin Invest.
128:4525–4542. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kouros-Mehr H, Bechis SK, Slorach EM,
Littlepage LE, Egeblad M, Ewald AJ, Pai SY, Ho IC and Werb Z:
GATA-3 links tumor differentiation and dissemination in a luminal
breast cancer model. Cancer Cell. 13:141–152. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chakrabarti R, Hwang J, Andres Blanco M,
Wei Y, Lukačišin M, Romano RA, Smalley K, Liu S, Yang Q, Ibrahim T,
et al: Elf5 inhibits epithelial mesenchymal transition in mammary
gland development and breast cancer metastasis by transcriptionally
repressing Snail2. Nat Cell Biol. 14:1212–1222. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lin HY, Liang YK, Dou XW, Chen CF, Wei XL,
Zeng, Bai JW, Guo YX, Lin FF, Huang WH, et al: Notch3 inhibits
epithelial-mesenchymal transition in breast cancer via a novel
mechanism, upregulation of GATA-3 expression. Oncogenesis.
7:592018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mack GS and Marshall A: Lost in migration.
Nat Biotechnol. 28:214–229. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu X, Li J, Cadilha BL, Markota A, Voigt
C, Huang Z, Lin PP, Wang DD, Dai J, Kranz G, et al: Epithelial-type
systemic breast carcinoma cells with a restricted mesenchymal
transition are a major source of metastasis. Sci Adv.
5:eaav42752019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shao S and Zhao X, Zhang X, Luo M, Zuo X,
Huang S, Wang Y, Gu S and Zhao X: Notch1 signaling regulates the
epithelial-mesenchymal transition and invasion of breast cancer in
a Slug-dependent manner. Mol Cancer. 14:282015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Leong KG, Niessen K, Kulic I, Raouf A,
Eaves C, Pollet I and Karsan A: Jagged1-mediated Notch activation
induces epithelial-to-mesenchymal transition through Slug-induced
repression of E-cadherin. J Exp Med. 204:2935–2948. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen J, Imanaka N, Chen J and Griffin JD:
Hypoxia potentiates Notch signaling in breast cancer leading to
decreased E-cadherin expression and increased cell migration and
invasion. Br J Cancer. 102:351–360. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jian J, Yang Q, Shao Y, Axelrod D, Smith
J, Singh B, Krauter S, Chiriboga L, Yang Z, Li J and Huang X: A
link between premenopausal iron deficiency and breast cancer
malignancy. BMC Cancer. 13:3072013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu L, Chen X, Wang Y, Qu Z, Lu Q, Zhao J,
Yan X, Zhang H and Zhou Y: Notch3 is important for TGF-β-induced
epithelial-mesenchymal transition in non-small cell lung cancer
bone metastasis by regulating ZEB-1. Cancer Gene Ther. 21:364–372.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bolos V, Mira E, Martinez-Poveda B, Luxán
G, Cañamero M, Martínez-A C, Mañes S and de la Pompa JL: Notch
activation stimulates migration of breast cancer cells and promotes
tumor growth. Breast Cancer Res. 15:R542013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Brabletz S, Bajdak K, Meidhof S, Burk U,
Niedermann G, Firat E, Wellner U, Dimmler A, Faller G, Schubert J
and Brabletz T: The ZEB1/miR-200 feedback loop controls Notch
signalling in cancer cells. EMBO J. 30:770–782. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang X and Zhao X, Shao S, Zuo X, Ning Q,
Luo M, Gu S and Zhao X: Notch1 induces epithelial-mesenchymal
transition and the cancer stem cell phenotype in breast cancer
cells and STAT3 plays a key role. Int J Oncol. 46:1141–1148. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kim RK, Kaushik N, Suh Y, Yoo KC, Cui YH,
Kim MJ, Lee HJ, Kim IG and Lee SJ: Radiation driven
epithelial-mesenchymal transition is mediated by Notch signaling in
breast cancer. Oncotarget. 7:53430–53442. 2016.PubMed/NCBI
|
|
40
|
Jin S, Mutvei AP, Chivukula IV, Andersson
ER, Ramsköld D, Sandberg R, Lee KL, Kronqvist P, Mamaeva V, Ostling
P, et al: Non-canonical Notch signaling activates IL-6/JAK/STAT
signaling in breast tumor cells and is controlled by p53 and
IKKα/IKKβ. Oncogene. 32:4892–4902. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang J, Shao X, Sun H, Liu K, Ding Z,
Chen J, Fang L, Su W, Hong Y and Li H and Li H: NUMB negatively
regulates the epithelial-mesenchymal transition of triple-negative
breast cancer by antagonizing Notch signaling. Oncotarget.
7:61036–61053. 2016.PubMed/NCBI
|
|
42
|
Garcia-Heredia JM, Verdugo Sivianes EM,
Lucena-Cacace A, Molina-Pinelo S and Carnero A: Numb-like (NumbL)
downregulation increases tumorigenicity, cancer stem cell-like
properties and resistance to chemotherapy. Oncotarget.
7:63611–63628. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang J, Fu L, Gu F and Ma Y: Notch1 is
involved in migration and invasion of human breast cancer cells.
Oncol Rep. 26:1295–1303. 2011.PubMed/NCBI
|
|
44
|
Dou XW, Liang YK, Lin HY, Wei XL, Zhang
YQ, Bai JW, Chen CF, Chen M, Du CW, Li YC, et al: Notch3 maintains
luminal phenotype and suppresses tumorigenesis and metastasis of
breast cancer via trans-activating estrogen receptor-alpha.
Theranostics. 7:4041–4056. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kessenbrock K, Plaks V and Werb Z: Matrix
metalloproteinases: Regulators of the tumor microenvironment. Cell.
141:52–67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Li L, Zhao F, Lu J, Li T, Yang H, Wu C and
Liu Y: Notch-1 signaling promotes the malignant features of human
breast cancer through NF-κB activation. PLoS One. 9:e959122014.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Mahmood N, Mihalcioiu C and Rabbani SA:
Multifaceted role of the urokinase-type plasminogen activator (uPA)
and its receptor (uPAR): Diagnostic, Prognostic, and therapeutic
applications. Front Oncol. 8:242018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Heiss MM, Allgayer H, Gruetzner KU, Funke
I, Babic R, Jauch KW and Schildberg FW: Individual development and
uPA-receptor expression of disseminated tumour cells in bone
marrow: A reference to early systemic disease in solid cancer. Nat
Med. 1:1035–1039. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Shimizu M, Cohen B, Goldvasser P, Berman
H, Virtanen C and Reedijk M: Plasminogen activator uPA is a direct
transcriptional target of the JAG1-Notch receptor signaling pathway
in breast cancer. Cancer Res. 71:277–286. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Song J: Notch signaling mediates Tumor-CAF
crosstalk in basal-like breast cancer. 2014.
|
|
51
|
Liu ZJ, Lsemenza G and Zhang HF:
Hypoxia-inducible factor 1 and breast cancer metastasis. J Zhejiang
Univ Sci B. 16:32–43. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Gupta GP and Massagué J: Cancer
metastasis: Building a framework. Cell. 127:679–695. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Semenza GL: HIF-1 and tumor progression:
Pathophysiology and therapeutics. Trends Mol Med. 8 (Suppl
4):S62–S67. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Harris AL: Hypoxia-a key regulatory factor
in tumour growth. Nat Rev Cancer. 2:38–47. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
De EF, Maggiolini M and Musti AM:
Crosstalk between Notch, HIF-1α and GPER in Breast Cancer EMT. Int
J Mol Sci. 19:20112018. View Article : Google Scholar
|
|
56
|
Lim SO, Kim HS, Quan X, Ahn SM, Kim H,
Hsieh D, Seong JK and Jung G: Notch1 binds and induces degradation
of Snail in hepatocellular carcinoma. BMC Biol. 9:832011.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Boufraqech M, Zhang L, Nilubol N, Sadowski
SM, Kotian S, Quezado M and Kebebew E: Lysyl Oxidase (LOX)
Transcriptionally Regulates SNAI2 Expression and TIMP4 Secretion in
Human Cancers. Clin Cancer Res. 22:4491–4504. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Sahlgren C, Gustafsson MV, Jin S,
Poellinger L and Lendahl U: Notch signaling mediates
hypoxia-induced tumor cell migration and invasion. Proc Natl Acad
Sci USA. 105:6392–6397. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Villanueva MT: Metastasis: LOX does some
prepping. Nat Rev Cancer. 15:3842015. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Deshmukh SK, Srivastava SK, Tyagi N, Ahmad
A, Singh AP, Ghadhban AAL, Dyess DL, Carter JE, Dugger K and Singh
S: Emerging evidence for the role of differential tumor
microenvironment in breast cancer racial disparity: A closer look
at the surroundings. Carcinogenesis. 38:757–765. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Aboussekhra A: Role of cancer-associated
fibroblasts in breast cancer development and prognosis. Int J Dev
Biol. 55:841–849. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Shimoda M, Principe S, Jackson HW, Luga V,
Fang H, Molyneux SD, Shao YW, Aiken A, Waterhouse PD, Karamboulas
C, et al: Loss of the Timp gene family is sufficient for the
acquisition of the CAF-like cell state. Nat Cell Biol. 16:889–901.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Procopio MG, Laszlo C, Al Labban D, Kim
DE, Bordignon P, Jo SH, Goruppi S, Menietti E, Ostano P, Ala U, et
al: Combined CSL and p53 downregulation promotes cancer-associated
fibroblast activation. Nat Cell Biol. 17:1193–1204. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Gajewski TF, Schreiber H and Fu YX: Innate
and adaptive immune cells in the tumor microenvironment. Nat
Immunol. 14:1014–1022. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Cho OH, Shin HM, Miele L, Golde TE, Fauq
A, Minter LM and Osborne BA: Notch regulates cytolytic effector
function in CD8+ T cells. J Immunol. 182:3380–3389. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Qiu H, Zmina PM, Huang AY, Askew D and
Bedogni B: Inhibiting Notch1 enhances immunotherapy efficacy in
melanoma by preventing Notch1 dependent immune suppressive
properties. Cancer Lett. 434:144–151. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Sugimoto K, Maekawa Y, Kitamura A, Nishida
J, Koyanagi A, Yagita H, Kojima H, Chiba S, Shimada M and Yasutomo
K: Notch2 signaling is required for potent antitumor immunity in
vivo. J Immunol. 184:4673–4678. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Palaga T, Wongchana W and Kueanjinda P:
Notch signaling in macrophages in the context of cancer immunity.
Front Immunol. 9:6522018. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Franklin RA, Liao W, Sarkar A, Kim MV,
Bivona MR, Liu K, Pamer EG and Li MO: The cellular and molecular
origin of tumor-associated macrophages. Science. 344:921–925. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Zhao JL, Huang F, He F, Gao CC, Liang SQ,
Ma PF, Dong GY, Han H and Qin HY: Forced activation of notch in
macrophages represses tumor growth by upregulating miR-125a and
disabling tumor-associated macrophages. Cancer Res. 76:1403–1415.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Hanahan D and Folkman J: Patterns and
emerging mechanisms of the angiogenic switch during tumorigenesis.
Cell. 86:353–364. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Zhou Z, Yao H and Hu H: Disrupting Tumor
Angiogenesis and ‘the Hunger Games’ for Breast Cancer. Adv Exp Med
Biol. 1026:1712017. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Zetter BR: Angiogenesis and tumor
metastasis. Annu Rev Med. 49:407–424. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Cuervo H, Nielsen CM, Simonetto DA,
Ferrell L, Shah VH and Wang RA: Endothelial notch signaling is
essential to prevent hepatic vascular malformations in mice.
Hepatology. 64:1302–1316. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Kontomanolis EN, Kalagasidou S, Pouliliou
S, Anthoulaki X, Georgiou N, Papamanolis V and Fasoulakis ZN: The
notch pathway in breast cancer progression. ScientificWorldJournal.
2018:24154892018. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Blanco R and Gerhardt H: VEGF and Notch in
tip and stalk cell selection. Cold Spring Harb Perspect Med.
3:a0065692013. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Siekmann AF, Covassin L and Lawson ND:
Modulation of VEGF signalling output by the Notch pathway.
Bioessays. 30:303–313. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Mailhos C, Modlich U, Lewis J, Harris A,
Bicknell R and Ish-Horowicz D: Delta4, an endothelial specific
notch ligand expressed at sites of physiological and tumor
angiogenesis. Differentiation. 69:135–144. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Hellstrom M, Phng LK, Hofmann JJ, Wallgard
E, Coultas L, Lindblom P, Alva J, Nilsson AK, Karlsson L, Gaiano N,
et al: Dll4 signalling through Notch1 regulates formation of tip
cells during angiogenesis. Nature. 445:776–780. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Suchting S and Eichmann A: Jagged gives
endothelial tip cells an edge. Cell. 137:988–990. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Phng LK and Gerhardt H: Angiogenesis: A
team effort coordinated by notch. Dev Cell. 16:196–208. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Oon CE, Li JL, Sainson R, Sheldon H,
Turley H, Leek R and Harris A: 360 Role of DLL4 and JAG1 in tumour
angiogenesis. EJC Supplements. 8:92. 2010. View Article : Google Scholar
|
|
83
|
Panin VM, Papayannopoulos V, Wilson R and
Irvine KD: Fringe modulates Notch-ligand interactions. Nature.
387:908–912. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
84
|
Boareto M, Jolly MK, Ben-Jacob E and
Onuchic JN: Jagged mediates differences in normal and tumor
angiogenesis by affecting tip-stalk fate decision. Proc Natl Acad
Sci USA. 112:E3836–E3844. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Benedito R, Roca C, Sörensen I, Adams S,
Gossler A, Fruttiger M and Adams RH: The Notch Ligands Dll4 and
Jagged1 have opposing effects on Angiogenesis. Cell. 137:1124–1135.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Chen JY, Li CF, Chu PY, Lai YS, Chen CH,
Jiang SS, Hou MF and Hung WC: Lysine demethylase 2A promotes
stemness and angiogenesis of breast cancer by upregulating Jagged1.
Oncotarget. 7:27689–27710. 2016.PubMed/NCBI
|
|
87
|
Rodriguez-Vita J and Fischer A: Notch
signaling facilitates crossing of endothelial barriers by tumor
cells. Mol Cell Oncol. 4:e13118282017. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
White DE and Muller WJ: Multifaceted Roles
of integrins in breast cancer metastasis. J Mammary Gland Biol
Neoplasia. 12:135–142. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Deford P, Brown K, Richards RL, King A,
Newburn K, Westover K and Albig AR: MAGP2 controls Notch via
interactions with RGD binding integrins: Identification of a novel
ECM-integrin-Notch signaling axis. Exp Cell Res. 341:84–91. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Hodkinson PS, Elliott PA, Lad Y, McHugh
BJ, MacKinnon AC, Haslett C and Sethi T: Mammalian NOTCH-1
activates beta1 integrins via the small GTPase R-Ras. J Biol Chem.
282:28991–29001. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Liu B, Zheng X, Meng F, Han Y, Song Y, Liu
F, Li S, Zhang L, Gu F, Zhang X and Fu L: Overexpression of β1
integrin contributes to polarity reversal and a poor prognosis of
breast invasive micropapillary carcinoma. Oncotarget. 9:4338–4353.
2017.PubMed/NCBI
|
|
92
|
Stoletov K, Kato H, Zardouzian E, Kelber
J, Yang J, Shattil S and Klemke R: Visualizing extravasation
dynamics of metastatic tumor cells. J Cell Sci. 123:2332–2341.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Guo P and Rafii S: Dangerous liaisons:
Deviant endothelium NOTCHes toward tumor metastasis. Cancer Cell.
31:301–303. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Lianidou ES and Markou A: Circulating
tumor cells in breast cancer: Detection systems, molecular
characterization, and future challenges. Clin Chem. 57:1242–1255.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Boral D, Vishnoi M, Liu HN, Yin W, Sprouse
ML, Scamardo A, Hong DS, Tan TZ, Thiery JP, Chang JC and Marchetti
D: Molecular characterization of breast cancer CTCs associated with
brain metastasis. Nat Commun. 8:1962017. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Portanova P, Notaro A, Pellerito O,
Sabella S, Giuliano M and Calvaruso G: Notch inhibition restores
TRAIL-mediated apoptosis via AP1-dependent upregulation of DR4 and
DR5 TRAIL receptors in MDA-MB-231 breast cancer cells. Int J Oncol.
43:121–130. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Day TW, Huang S and Safa AR: c-FLIP
knockdown induces ligand-independent DR5-, FADD-, caspase-8-, and
caspase-9-dependent apoptosis in breast cancer cells. Biochem
Pharmacol. 76:1694–1704. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Kim JW, Kim MJ, Kim KJ, Yun HJ, Chae JS,
Hwang SG, Chang TS, Park HS, Lee KW, Han PL, et al: Notch
interferes with the scaffold function of JNK-interacting protein 1
to inhibit the JNK signaling pathway. Proc Natl Acad Sci USA.
102:14308–14313. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Archibald A, Mihai C, Macara IG and
McCaffrey L: Oncogenic suppression of apoptosis uncovers a Rac1/JNK
proliferation pathway activated by loss of Par3. Oncogene.
34:3199–3206. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Zou W, Liu X, Yue P, Zhou Z, Sporn MB,
Lotan R, Khuri FR and Sun SY: c-Jun NH2-terminal kinase-mediated
up-regulation of death receptor 5 contributes to induction of
apoptosis by the novel synthetic triterpenoid
methyl-2-cyano-3,12-dioxooleana-1, 9-dien-28-oate in human lung
cancer cells. Cancer Res. 64:7570–7578. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Naik S, MacFarlane M and Sarin A: Notch4
signaling confers susceptibility to TRAIL-Induced Apoptosis in
breast cancer cells. J Cell Biochem. 116:1371–1380. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Phipps LE, Hino S and Muschel RJ:
Targeting cell spreading: A method of sensitizing metastatic tumor
cells to TRAIL-induced apoptosis. Mol Cancer Res. 9:249–258. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Meurette O, Stylianou S, Rock R, Collu GM,
Gilmore AP and Brennan K: Notch activation induces Akt signaling
via an autocrine loop to prevent apoptosis in breast epithelial
cells. Cancer Res. 69:5015–5022. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Tao L, Roberts AL, Dunphy KA, Bigelow C,
Yan H and Jerry DJ: Repression of mammary stem/progenitor cells by
p53 is mediated by Notch and separable from apoptotic activity.
Stem Cells. 29:119–127. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Su F, Zhu S, Ruan J, Muftuoglu Y, Zhang L
and Yuan Q: Combination therapy of RY10-4 with the gamma-secretase
inhibitor DAPT shows promise in treating HER2-amplified breast
cancer. Oncotarget. 7:4142–4154. 2016.PubMed/NCBI
|
|
106
|
Hay N: The Akt-mTOR tango and its
relevance to cancer. Cancer Cell. 8:179–183. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Li L, Zhang J, Xiong N, Li S, Chen Y, Yang
H, Wu C, Zeng H and Liu Y: Notch-1 signaling activates NF-κB in
human breast carcinoma MDA-MB-231 cells via PP2A-dependent AKT
pathway. Med Oncol. 33:332016. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Guerrero-Zotano A, Mayer IA and Arteaga
CL: PI3K/AKT/mTOR: Role in breast cancer progression, drug
resistance, and treatment. Cancer Metastasis Rev. 35:515–524. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Mungamuri SK, Yang X, Thor AD and
Somasundaram K: Survival signaling by Notch1: Mammalian target of
rapamycin (mTOR)-dependent inhibition of p53. Cancer Res.
66:4715–4724. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Dotto GP: Crosstalk of Notch with p53 and
p63 in cancer growth control. Nat Rev Cancer. 9:587–595. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Kim SB, Chae GW, Lee J, Park J, Tak H,
Chung JH, Park TG, Ahn JK and Joe CO: Activated Notch1 interacts
with p53 to inhibit its phosphorylation and transactivation. Cell
Death Differ. 14:982–991. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Cherdyntseva NV, Litviakov NV, Denisov EV,
Gervas PA and Cherdyntsev ES: Circulating tumor cells in breast
cancer: Functional heterogeneity, pathogenetic and clinical
aspects. Exp Oncol. 39:2–11. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Zhang P, He D, Chen Z, Pan Q, Du F, Zang
X, Wang Y, Tang C, Li H, Lu H, et al: Chemotherapy enhances tumor
vascularization via Notch signaling-mediated formation of
tumor-derived endothelium in breast cancer. Biochem Pharmacol.
118:18–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Simões BM, O'Brien CS, Eyre R, Silva A, Yu
L, Sarmiento-Castro A, Alférez DG, Spence K, Santiago-Gómez A,
Chemi F, et al: Anti-estrogen resistance in human breast tumors is
driven by JAG1-NOTCH4-Dependent cancer stem cell activity. Cell
Rep. 12:1968–1977. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Sansone P, Ceccarelli C, Berishaj M, Chang
Q, Rajasekhar VK, Perna F, Bowman RL, Vidone M, Daly L, Nnoli J, et
al: Self-renewal of CD133(hi) cells by IL6/Notch3 signalling
regulates endocrine resistance in metastatic breast cancer. Nat
Commun. 7:104422016. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Boelens MC, Wu TJ, Nabet BY, Xu B, Qiu Y,
Yoon T, Azzam DJ, Twyman-Saint Victor C, Wiemann BZ, Ishwaran H, et
al: Exosome transfer from stromal to breast cancer cells regulates
therapy resistance pathways. Cell. 159:499–513. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Arteaga CL and Engelman JA: ERBB
receptors: From oncogene discovery to basic science to
mechanism-based cancer therapeutics. Cancer Cell. 25:282–303. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Jordan NV, Bardia A, Wittner BS, Benes C,
Ligorio M, Zheng Y, Yu M, Sundaresan TK, Licausi JA, Desai R, et
al: HER2 expression identifies dynamic functional states within
circulating breast cancer cells. Nature. 537:102–106. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Kang Y: Dissecting Tumor-Stromal
interactions in breast cancer bone metastasis. Endocrinol Metab
(Seoul). 31:206–212. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Zhang Z, Wang H, Ikeda S, Fahey F,
Bielenberg D, Smits P and Hauschka PV: Notch3 in human breast
cancer cell lines regulates osteoblast-cancer cell interactions and
osteolytic bone metastasis. Am J Pathol. 177:1459–1469. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Sethi N, Dai X, Winter CG and Kang Y:
Tumor-derived JAGGED1 promotes osteolytic bone metastasis of breast
cancer by engaging notch signaling in bone cells. Cancer Cell.
19:192–205. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Lee JH and Welch DR: Suppression of
metastasis in human breast carcinoma MDA-MB-435 cells after
transfection with the metastasis suppressor gene, KiSS-1. Cancer
Res. 57:2384–2387. 1997.PubMed/NCBI
|
|
123
|
Ohtaki T, Shintani Y, Honda S, Matsumoto
H, Hori A, Kanehashi K, Terao Y, Kumano S, Takatsu Y, Masuda Y, et
al: Metastasis suppressor gene KiSS-1 encodes peptide ligand of a
G-protein-coupled receptor. Nature. 411:613–617. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Leone A, Flatow U, Vanhoutte K and Steeg
PS: Transfection of human nm23-H1 into the human MDA-MB-435 breast
carcinoma cell line: Effects on tumor metastatic potential,
colonization and enzymatic activity. Oncogene. 8:2325–2333.
1993.PubMed/NCBI
|
|
125
|
Yu HG, Huang JA, Yang YN, Huang H, Luo HS,
Yu JP, Meier JJ, Schrader H, Bastian A, Schmidt WE and Schmitz F:
The effects of acetylsalicylic acid on proliferation, apoptosis,
and invasion of cyclooxygenase-2 negative colon cancer cells. Eur J
Clin Invest. 32:838–846. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Moon CM, Kwon JH, Kim JS, Oh SH, Jin Lee
K, Park JJ, Pil Hong S, Cheon JH, Kim TI and Kim WH: Nonsteroidal
anti-inflammatory drugs suppress cancer stem cells via inhibiting
PTGS2 (cyclooxygenase 2) and NOTCH/HES1 and activating PPARG in
colorectal cancer. Int J Cancer. 134:519–529. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Ignesti M, Barraco M, Nallamothu G,
Woolworth JA, Duchi S, Gargiulo G, Cavaliere V and Hsu T: Notch
signaling during development requires the function of awd, the
Drosophila homolog of human metastasis suppressor gene Nm23.
BMC Biol. 12:122014. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Oskarsson T, Acharyya S, Zhang XH,
Vanharanta S, Tavazoie SF, Morris PG, Downey RJ, Manova-Todorova K,
Brogi E and Massagué J: Breast cancer cells produce tenascin C as a
metastatic niche component to colonize the lungs. Nat Med.
17:867–874. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Wang XY, Penalva LO, Yuan H, Linnoila RI,
Lu J, Okano H and Glazer RI: Musashi1 regulates breast tumor cell
proliferation and is a prognostic indicator of poor survival. Mol
Cancer. 9:2212010. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Reedijk M, Odorcic S, Chang L, Zhang H,
Miller N, McCready DR, Lockwood G and Egan SE: High-level
coexpression of JAG1 and NOTCH1 is observed in human breast cancer
and is associated with poor overall survival. Cancer Res.
65:8530–8537. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Tanabe H, Takayama I, Nishiyama T,
Shimazaki M, Kii I, Li M, Amizuka N, Katsube K and Kudo A:
Periostin associates with Notch1 precursor to maintain Notch1
expression under a stress condition in mouse cells. PLoS One.
5:e122342010. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Zhou M, Kawashima N, Suzuk N, Yamamoto M,
Ohnishi K, Katsube K, Tanabe H, Kudo A, Saito M and Suda H:
Periostin is a negative regulator of mineralization in the dental
pulp tissue. Odontology. 103:152–159. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Kii I, Nishiyama T, Li M, Matsumoto K,
Saito M, Amizuka N and Kudo A: Incorporation of tenascin-C into the
extracellular matrix by periostin underlies an extracellular
meshwork architecture. J Biol Chem. 285:2028–2039. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Aguirre-Ghiso JA: Models, mechanisms and
clinical evidence for cancer dormancy. Nat Rev Cancer. 7:834–846.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Aguirre Ghiso JA, Kovalski K and Ossowski
L: Tumor dormancy induced by downregulation of urokinase receptor
in human carcinoma involves integrin and MAPK signaling. J Cell
Biol. 147:89–104. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Masiero M, Minuzzo S, Pusceddu I, Moserle
L, Persano L, Agnusdei V, Tosello V, Basso G, Amadori A and
Indraccolo S: Notch3-mediated regulation of MKP-1 levels promotes
survival of T acute lymphoblastic leukemia cells. Leukemia.
25:588–598. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Enderling H, Almog N and Hlatky L: Systems
biology of tumor dormancy. Springer; New York: 2013, View Article : Google Scholar
|
|
138
|
Zhang Y, Li J, Lai XN, Jiao XQ, Xiong JP
and Xiong LX: Focus on Cdc42 in Breast Cancer: New insights, target
therapy development and non-coding RNAs. Cells. 8(pii): E1462019.
View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Ors-Kumoglu G, Gulce-Iz S and Biray-Avci
C: Therapeutic microRNAs in human cancer. Cytotechnology.
71:411–425. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Hayes J, Peruzzi PP and Lawler S:
MicroRNAs in cancer: Biomarkers, functions and therapy. Trends Mol
Med. 20:460–469. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Wu MY, Fu J, Xiao X, Wu J and Wu RC:
MiR-34a regulates therapy resistance by targeting HDAC1 and HDAC7
in breast cancer. Cancer Lett. 354:311–319. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Li XJ, Ji MH, Zhong SL, Zha QB, Xu JJ,
Zhao JH and Tang JH: MicroRNA-34a modulates chemosensitivity of
breast cancer cells to adriamycin by targeting Notch1. Arch Med
Res. 43:514–521. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Rui X, Zhao H, Xiao X, Wang L, Mo L and
Yao Y: MicroRNA-34a suppresses breast cancer cell proliferation and
invasion by targeting Notch1. Exp Ther Med. 16:4387–4392.
2018.PubMed/NCBI
|
|
144
|
Lan L, Wang Y, Pan Z, Wang B, Yue Z, Jiang
Z, Li L, Wang C and Tang H: Rhamnetin induces apoptosis in human
breast cancer cells via the miR-34a/Notch-1 signaling pathway.
Oncol Lett. 17:676–682. 2019.PubMed/NCBI
|
|
145
|
Kang L, Mao J, Tao Y, Song B, Ma W, Lu Y,
Zhao L, Li J, Yang B and Li L: MiR-34a suppresses the breast cancer
stem cell-like characteristics by downregulating Notch1 pathway.
Cancer Sci. 106:700–708. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Park EY, Chang E, Lee EJ, Lee HW, Kang HG,
Chun KH, Woo YM, Kong HK, Ko JY, Suzuki H, et al: Targeting of
miR34a-NOTCH1 axis reduced breast cancer stemness and
chemoresistance. Cancer Res. 74:7573–7582. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Suman S, Das TP and Damodaran C: Silencing
NOTCH signaling causes growth arrest in both breast cancer stem
cells and breast cancer cells. Br J Cancer. 109:2587–2596. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Heller G, Altenberger C, Steiner I,
Topakian T, Ziegler B, Tomasich E, Lang G, End-Pfützenreuter A,
Zehetmayer S, Döme B, et al: DNA methylation of microRNA-coding
genes in non-small-cell lung cancer patients. J Pathol.
245:387–398. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Li WJ, Xie XX, Bai J, Wang C, Zhao L and
Jiang DQ: Increased expression of miR-1179 inhibits breast cancer
cell metastasis by modulating Notch signaling pathway and
correlates with favorable prognosis. Eur Rev Med Pharmacol Sci.
22:8374–8382. 2018.PubMed/NCBI
|
|
150
|
Kong P, Chen L, Yu M, Tao J, Liu J, Wang
Y, Pan H, Zhou W and Wang S: miR-3178 inhibits cell proliferation
and metastasis by targeting Notch1 in triple-negative breast
cancer. Cell Death Dis. 9:10592018. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Mohammadi-Yeganeh S, Mansouri A and Paryan
M: Targeting of miR9/NOTCH1 interaction reduces metastatic behavior
in triple-negative breast cancer. Chem Biol Drug Des. 86:1185–1191.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Liang YK, Lin HY, Dou XW, Chen M, Wei XL,
Zhang YQ, Wu Y, Chen CF, Bai JW, Xiao YS, et al: MiR-221/222
promote epithelial-mesenchymal transition by targeting Notch3 in
breast cancer cell lines. NPJ Breast Cancer. 4:202018. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Forloni M, Dogra SK, Dong Y, Conte D Jr,
Ou J, Zhu LJ, Deng A, Mahalingam M, Green MR and Wajapeyee N:
miR-146a promotes the initiation and progression of melanoma by
activating Notch signaling. Elife. 3:e014602014. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Kuang W, Tan J, Duan Y, Duan J, Wang W,
Jin F, Jin Z, Yuan X and Liu Y: Cyclic stretch induced miR-146a
upregulation delays C2C12 myogenic differentiation through
inhibition of Numb. Biochem Biophys Res Commun. 378:259–263. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Marino N, Woditschka S, Reed LT, Nakayama
J, Mayer M, Wetzel M and Steeg PS: Breast cancer metastasis: Issues
for the personalization of its prevention and treatment. Am J
Pathol. 183:1084–1095. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Locatelli MA, Aftimos P, Dees EC, LoRusso
PM, Pegram MD, Awada A, Huang B, Cesari R, Jiang Y, Shaik MN, et
al: Phase I study of the gamma secretase inhibitor PF-03084014 in
combination with docetaxel in patients with advanced
triple-negative breast cancer. Oncotarget. 8:2320–2328. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Piha-Paul SA, Munster PN, Hollebecque A,
Argilés G, Dajani O, Cheng JD, Wang R, Swift A, Tosolini A and
Gupta S: Results of a phase 1 trial combining ridaforolimus and
MK-0752 in patients with advanced solid tumours. Eur J Cancer.
51:1865–1873. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Takebe N, Nguyen D and Yang SX: Targeting
notch signaling pathway in cancer: Clinical development advances
and challenges. Pharmacol Ther. 141:140–149. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
So JY, Wahler J, Das Gupta S, Salerno DM,
Maehr H, Uskokovic M and Suh N: HES1-mediated inhibition of Notch1
signaling by a Gemini vitamin D analog leads to decreased
CD44+/CD24−/low tumor-initiating
subpopulation in basal-like breast cancer. J Steroid Biochem Mol
Biol. 148:111–121. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Krop IE, Kosh M, Fearen I, Savoie JG,
Dallob AL, Matthews CK, Stone J, Winer EP, Freedman SJ and LoRusso
PM: Phase I pharmacokinetic (PK), and pharmacodynamic (PD) trial of
the novel oral Notch inhibitor MK-0752 in patients (pts) with
advanced breast cancer (BC) and other solid tumors. Ann Oncol.
22:1413–1419. 2006.
|
|
161
|
Locatelli M and Curigliano G: Notch
inhibitors and their role in the treatment of triple negative
breast cancer: Promises and failures. Curr Opin Oncol. 29:411–427.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Sanchez L, Muchene L, Lorenzo-Luaces P,
Viada C, Rodriguez PC, Alfonso S, Crombet T, Neninger E, Shkedy Z
and Lage A: Differential effects of two therapeutic cancer vaccines
on short- and long-term survival populations among patients with
advanced lung cancer. Semin Oncol. 45:52–57. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Makhoul I, Atiq M, Alwbari A and
Kieber-Emmons T: Breast cancer immunotherapy: An update. Breast
Cancer (Auckl). May 30–2018.(Epud ahead of print). doi:
10.1177/1178223418774802. View Article : Google Scholar
|
|
164
|
Gu X, Lu C, He D, Lu Y, Jin J, Liu D and
Ma X: Notch3 negatively regulates chemoresistance in breast
cancers. Tumour Biol. Oct 14–2016.(Epub ahead of print). View Article : Google Scholar
|
|
165
|
Lu X and Kang Y: Efficient acquisition of
dual metastasis organotropism to bone and lung through stable
spontaneous fusion between MDA-MB-231 variants. Proc Natl Acad Sci
USA. 106:9385–9390. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Colombo M, Raposo G and Thery C:
Biogenesis, secretion, and intercellular interactions of exosomes
and other extracellular vesicles. Annu Rev Cell Dev Biol.
30:255–289. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Hoshino A, Costa-Silva B, Shen TL,
Rodrigues G, Hashimoto A, Tesic Mark M, Molina H, Kohsaka S, Di
Giannatale A, Ceder S, et al: Tumour exosome integrins determine
organotropic metastasis. Nature. 527:329–335. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Lowry MC, Gallagher WM and O'Driscoll L:
The role of exosomes in breast cancer. Clin Chem. 61:1457–1465.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Schreck KC, Taylor P, Marchionni L,
Gopalakrishnan V, Bar EE, Gaiano N and Eberhart CG: The Notch
target Hes1 directly modulates Gli1 expression and Hedgehog
signaling: A potential mechanism of therapeutic resistance. Clin
Cancer Res. 16:6060–6070. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Hanna A, Metge BJ, Bailey SK, Chen D,
Chandrashekar DS, Varambally S, Samant RS and Shevde LA: Inhibition
of Hedgehog signaling reprograms the dysfunctional immune
microenvironment in breast cancer. Oncoimmunology. 8:15482412018.
View Article : Google Scholar : PubMed/NCBI
|