Breast cancer (BC) is one of the most common
malignancies among women globally, with ~2.1 million newly
diagnosed cases of BC in 2018 and 626,679 mortalities worldwide
(1). On account of improvements in
treatment and early detection by mammography, the 5-year overall
survival (OS) rate has notably improved by 12% and the mortality
rate has declined by 40% over the past three decades (2). However, due to the multi-aspect
dysregulation of genes and the complicated mechanisms that involve
several molecular and pathological subtypes, the efficiency of
accurate target therapies for patients with BC remains unclear
(3). The examination of BC
pathogenesis and identifying novel targets for the treatment of BC
is required for the development of medical science. Therefore, in
order to reach the goal of individualized treatment, it is crucial
and urgent to identify novel biological markers in the
microenvironment in BC.
In invasive and metastatic tumors, the
zinc-dependent matrix metalloproteases (MMPs) are the most
plentiful class of non-serine proteases present, first identified
in 1962 by Gross and Lapiere (4).
They have the ability to degrade the components of the
extracellular matrix (5). At
present, >20 MMPs have been identified in humans (6), including collagenases, gelatinases,
stromelysins, and the transmembrane type MMPs (7). MMPs can regulate a number of changes in
the microenvironment during tumor progression (such as in signaling
pathways that control cell growth, inflammation or angiogenesis,
and may also function in a non-proteolytic manner) (8). Their enzymatic activity modulates the
activities of a wide range of extracellular and intracellular
proteins, including proliferation, migration, and adhesion
(9). A previous study (10) showed that enhanced expression of MMPs
is usually associated with BC invasion and metastasis, and acts as
an important prognostic indicator. Therefore, an in-depth
bioinformatics analysis associated with MMPs and patients with BC
is required. In order to clarify the prognostic and potential
therapeutic value of MMPs in BC treatment, this study analyzed the
clinicopathological and survival data associated with MMPs in
patients with BC, based on a number of large public databases.
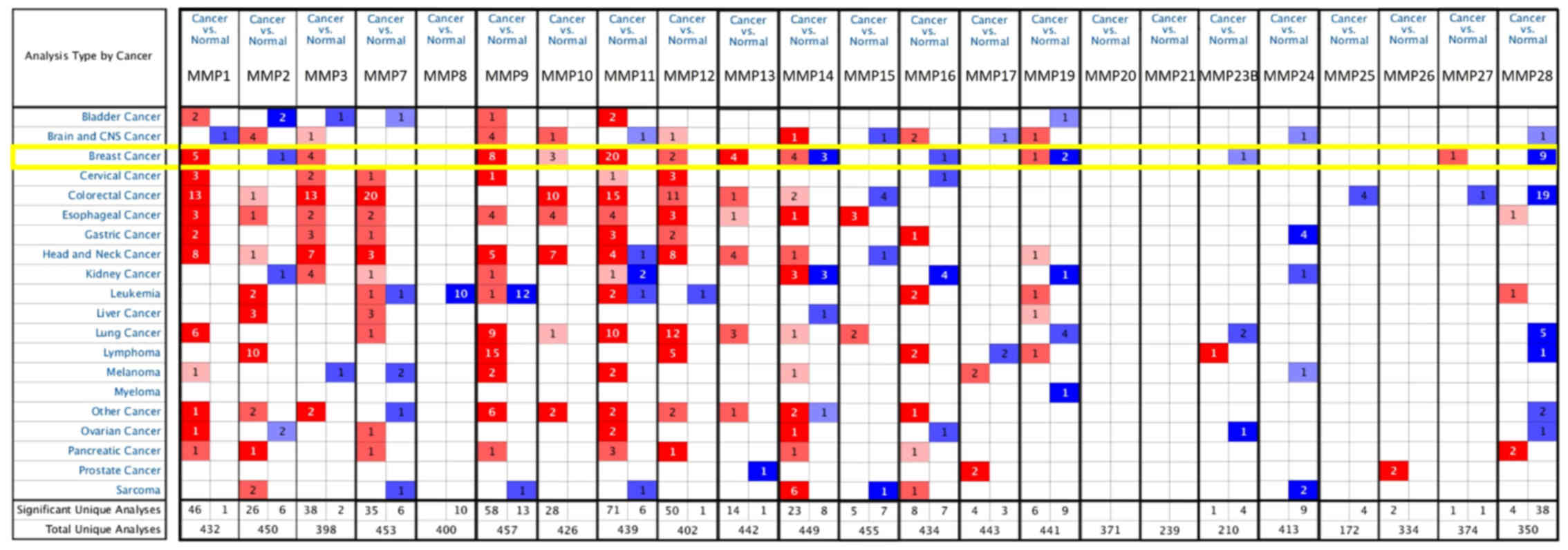
A total of 23 MMPs were identified using the
ONCOMINE database. The expression level of MMPs was analyzed in 20
types of cancer, and compared with that in normal individuals.
Fig. 1 shows the transcription
levels of MMPs in various types of cancers. High expression was
defined when MMPs expression was higher than that of adjacent
non-tumors, and vice versa. The mRNA expression of
MMP1/3/9/10/11/12/13 was upregulated in BC specimens in different
studies (Fig. 1). According to the
TCGA breast dataset, MMP1 was indicated to be highly expressed in
several types of BC compared with normal tissues and the
fold-change was 11.611 in invasive BC (Table SI). Richardson et al
(15) reported that the fold-change
of MMP1 in ductal BC was 21.13, whereas studies by Sorlie et
al (16,17) demonstrated fold changes of 2.963 and
2.482 for MMP1 in ductal BC. Compared with normal tissues, MMP3 was
overexpressed in some types of BC. The fold-change of invasive
lobular BC in TCGA data was 5.945, while Curtis et al
(18) reported that the fold-change
of MMP3 in invasive lobular BC was 2.008. According to the TCGA
dataset, MMP9 was overexpressed in almost all types of BC and
normal breast tissues, including intraductal cribriform BC with
fold-change=5.463, mucinous BC with fold-change=4.783 and invasive
BC with fold-change=3.245. Curtis et al (18) reported that the fold-changes of MMP9
in medullary BC and ductal BC in situ were 9.238 and 6.629,
respectively. In different datasets for MMP9, a fold-change of
4.378 in invasive ductal BC compared with normal breast was
observed (18). Radvanyi et
al (19) (fold-change=4.043) and
Turashvili et al (20)
(fold-change=3.640) found similar trends.
According to TCGA dataset, the transcriptional
levels of MMP10 showed fold-changes of 6.767, 4.265, and 4.130, in
invasive BC, invasive ductal BC and invasive lobular BC,
respectively. On the other hand, Turashvili et al (20) reported invasive ductal BC with an
MMP10 fold-change of 2.596. Radvanyi et al (19) and Karnoub et al (21) reported that MMP11 expression
increased significantly in ductal BC in situ and invasive
ductal BC stroma, with a fold-change of 2.639 and 188.233
respectively. Compared with normal breast tissue, upregulated
expression of MMP12 and MMP13 were also found in most types of BC
(Table SI).
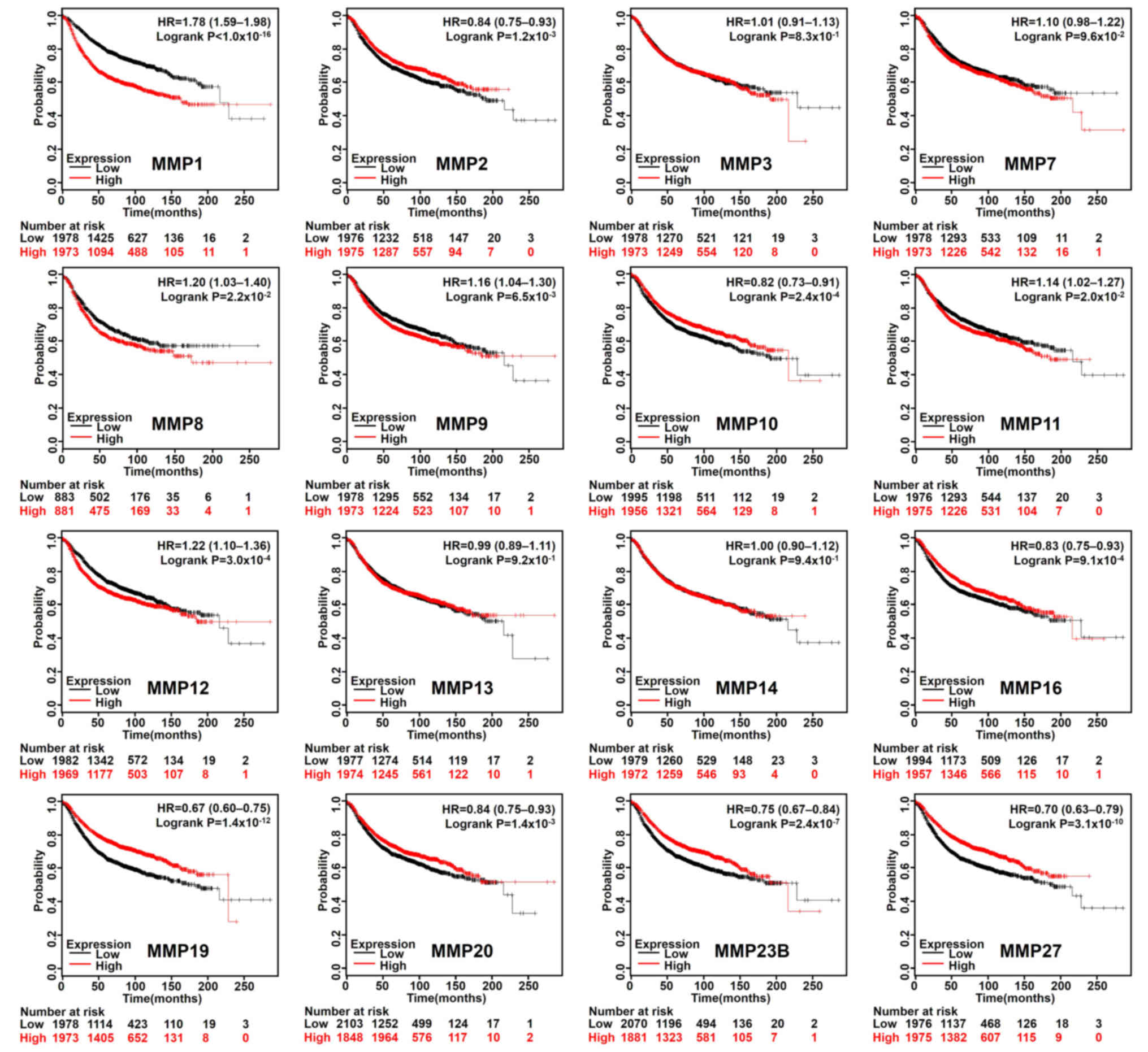
Based on the median expression value of each MMP in
all samples, all types of patients with BC were divided into two
groups to detect MMP expression (high expression vs. low
expression). The Kaplan-Meier curves of survival data showed that
increased expression of MMP 2/10/16/19/20/23B/27 and reduced
expression of MMP 1/8/9/11/12 were associated with better RFS rate
(P<0.05) in all BC types (Fig.
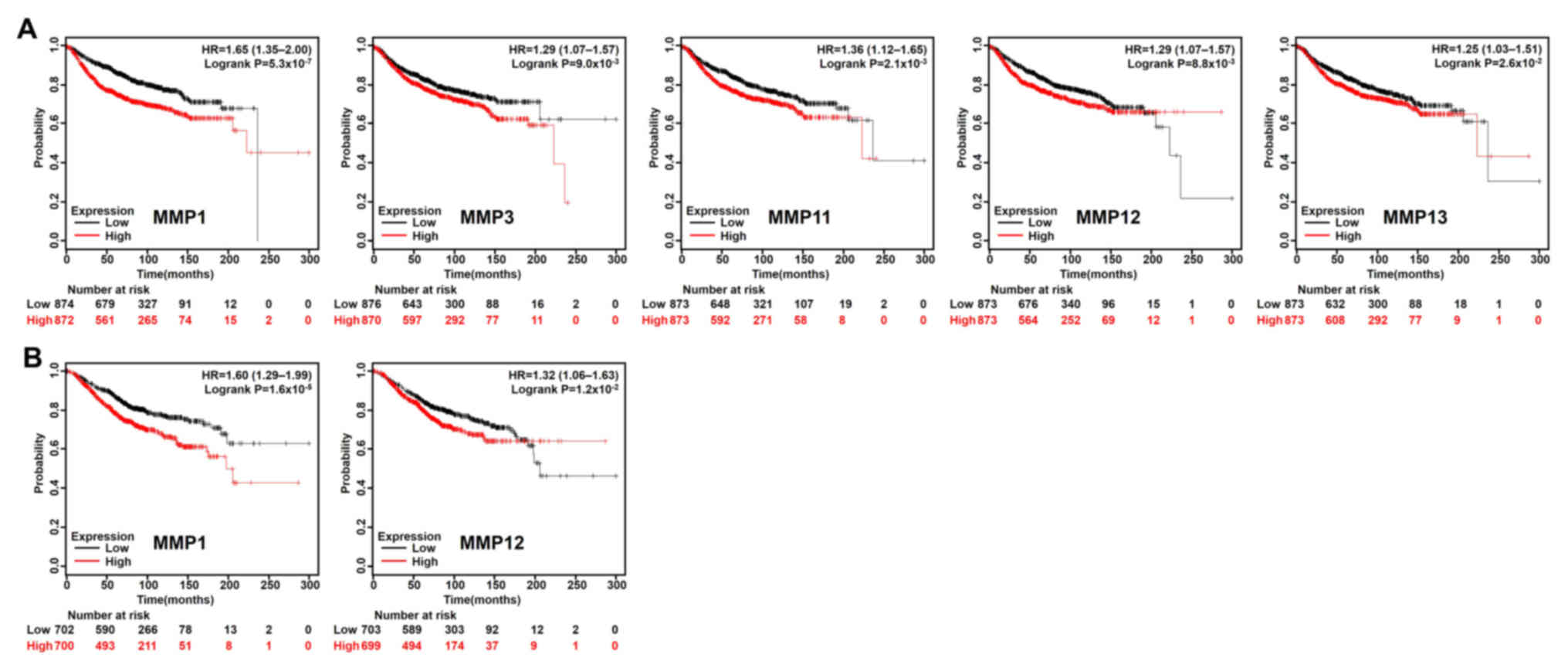
2). In addition, patients with BC with lower MMP1/3/11/12/13
transcriptional expression levels indicated better DMFS rate
(Fig. 3A), and lower mRNA levels of
MMP1/12 exhibited better OS rate than those with high expression
(Fig. 3B). To further examine the
role of MMPs in BC prognosis, the Bc-GenExMiner version 4.1 was
used to validate this research. MMP1/9/11/12/13/14/15 has a
significant negative impact on the prognosis of patients. High
expression of MMP16/20 was associated with better prognosis
(Table I).
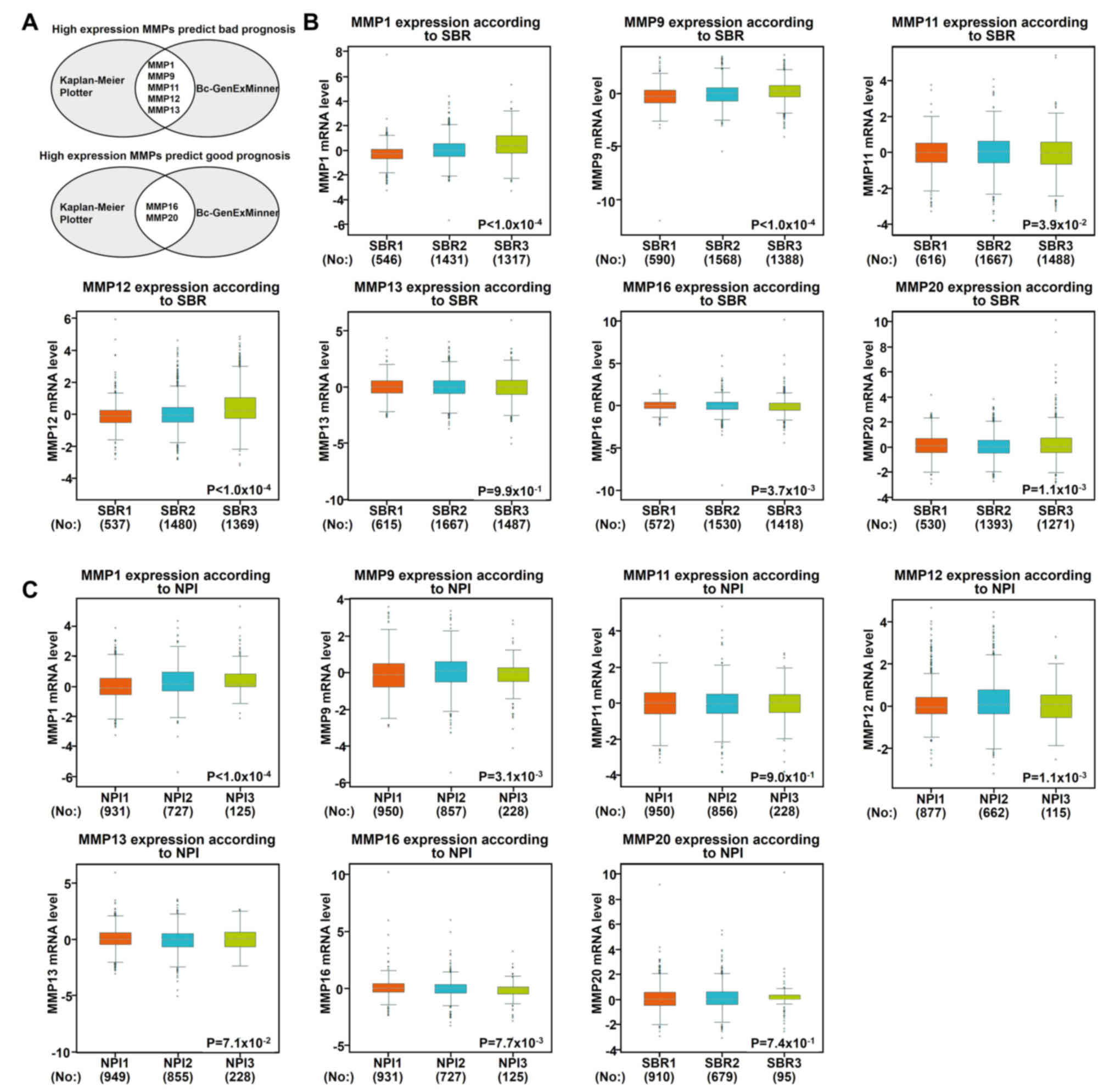
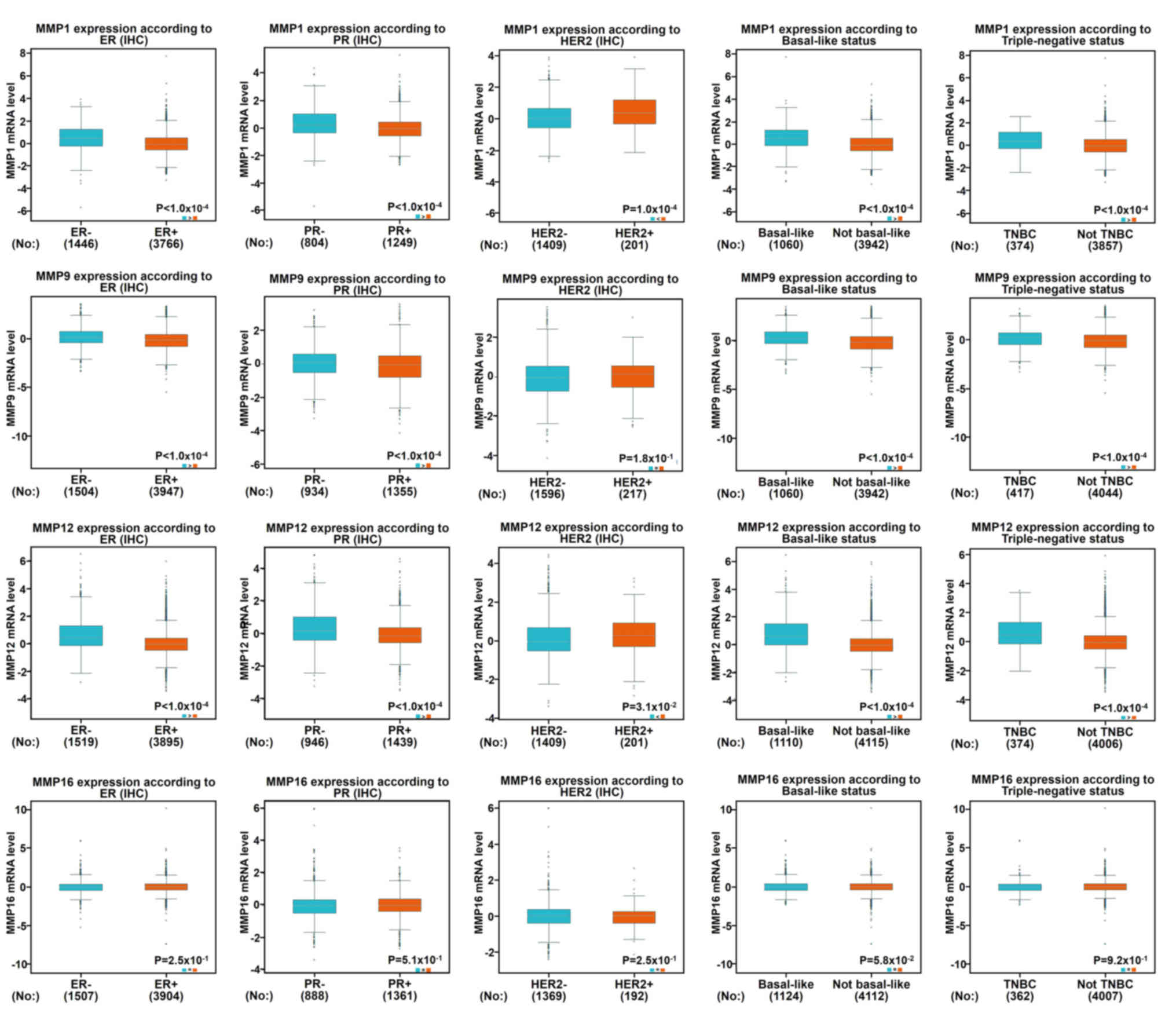
Using the aforementioned databases, this study
subsequently focused on whether MMP1/9/11/12/13 and MMP16/20 play a
key role in the progression of BC (Fig.
4A). Scarff, Bloom and Richardson grade (SBR) is one of the
important pathological parameters to be evaluated for the
management of breast carcinoma (22,23). The
survival rate of patients with poorly differentiated cancer (grades
II and III) was lower than that of patients with
well-differentiated cancer (grade I) (23). Using the Bc-GenExMiner software, it
was observed that patients with high-grade tumors (grades II and
III) appeared to exhibit high levels of MMP 1/9/11/12 and low
levels of MMP 16/20 (Fig. 4B). In
addition to molecular subtypes, the Nottingham prognostic index
(NPI) is also a helpful prognostic model for patients with BC
(24). In Fig. 4C, it was indicated that MMP1/9/12/16
were associated with NPI: Patients with high-grade tumors expressed
high levels of MMP1/9/12 and lower levels of MMP16, which was
consistent with our previous data mentioned above.
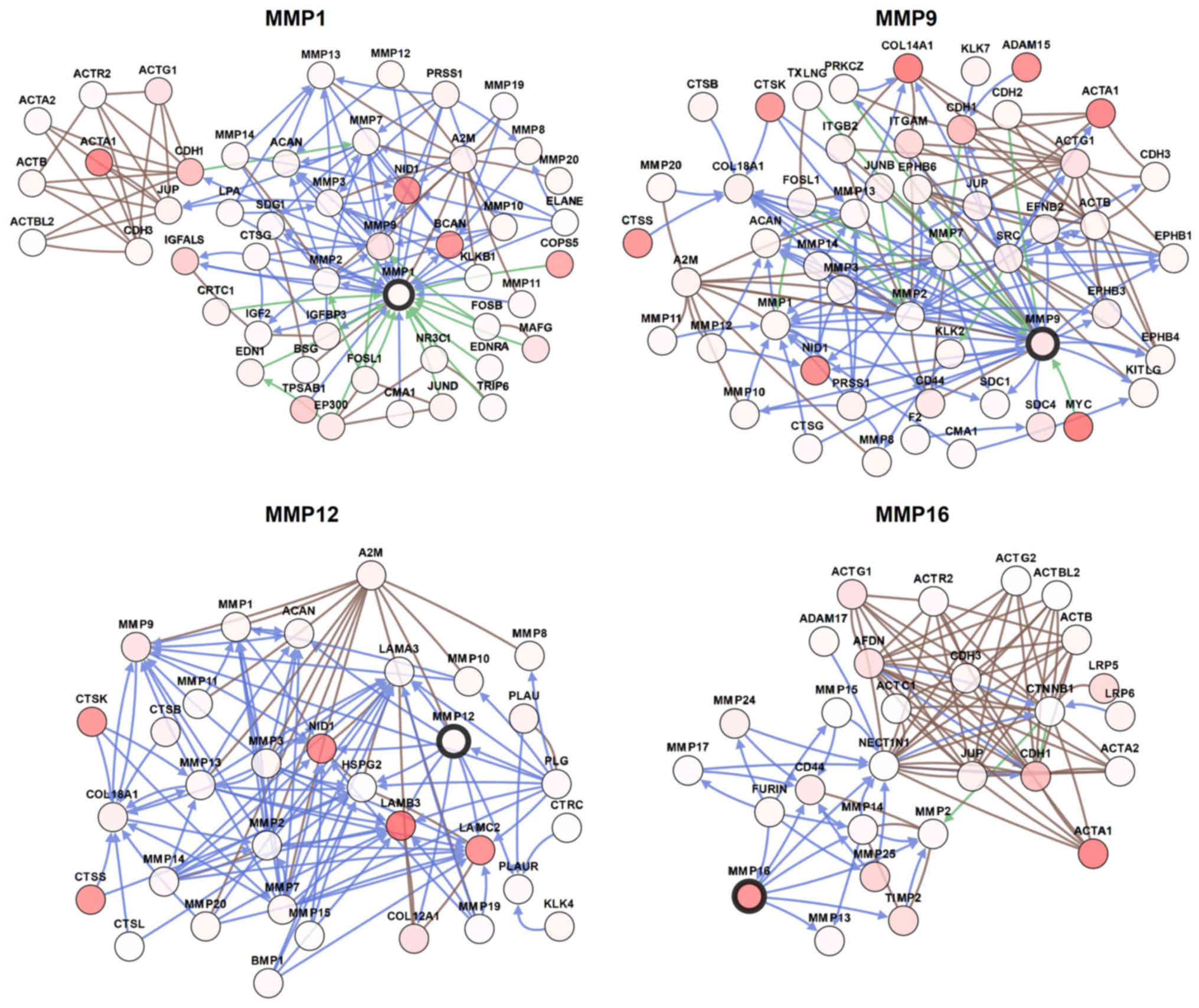
Combined with this information, multiple
bioinformatics analyses identified MMP1/9/12/16 as potential
therapeutic targets for patients with BC. In order to visualize the
potential genes co-expressed with MMP1/9/12/16, the gene network
was constructed as shown in Fig. 6.
The network was designed to recognize potential interactions
between MMP1/9/12/16 and a number of other key proteins. The
network analysis can provide a series of gene candidates to help
clarify the molecular mechanism of MMPs involvement in BC.
MMPs have been identified as an important family of
proteinases associated with tumorigenesis and as key mediators of
tumor progression (27). MMPs can
mediate various events in the microenvironment during the
progression of tumors, such as inflammation, angiogenesis,
proliferation, migration, and adhesion (27). Due to their role in cancer, MMPs have
been speculated to serve as an effective therapeutic target
(28). However, in clinical trials,
marimastat, a broad-spectrum MMPs inhibitor, failed to prolong
progression-free survival of patients with metastatic BC (28). Despite the recent research
illustrating the complex role of MMPs, the role of different MMPs
in the progression and metastasis of BC remains elusive (29). To the best of our knowledge, this
research is the first comprehensive bioinformatics study to
elucidate specific MMPs, and their prognostic value and biological
function in breast cancer.
A previous study demonstrated that MMP1 mRNA
expression in BC tissues is higher than in normal breast tissue
(30). In addition, previously
published data reported that patients with BC and high MMP1
expression are associated with poor prognoses, such as shorter
relapse-free-survival (31), which
is consistent with our findings. In contrast, Kulic et al
(32) reported that patients with
lower expression levels of MMP1 had significantly shorter 5-year
survival rates than those with higher levels of MMP1, suggesting a
poor prognostic role of low levels of serum MMP1. An animal
experiment verified that MMP2 plays a role in the development of BC
metastases, regulated by astrocyte factors and the ERK1/2 signaling
pathway (33). Results on whether
MMP2 is associated with worse prognosis in patients with BC have
been contradictory. High MMP2 expression was reported to be
indicative of relatively poor patient prognosis in BC (34), while a meta-analysis indicated that
worse OS rate was not linked to MMP2-positive patients (35).
As a direct downstream target of microRNA-519d, MMP3
acted as an executive molecule for the carcinogenic effect of
microRNA-519d in BC (36). A mouse
model revealed that peroxidases induced the transcription of the
MMP3 gene, in turn, MMP3 promoted tumor growth, invasion and
metastasis (37). MMP3 has been
reported to contribute to BC by participating in cancer invasion
and metastasis, especially in ER-/PR-tumors (38). Vizoso et al (39) found that the high expression of MMP7
was associated with shorter recurrence-free survival in patients
with BC. Kim et al (40)
reported that compared with luminal A and HER2-overexpressing
subtype, MMP7 expression was significantly higher in the basal-like
subtype. Furthermore, findings have identified MMP7 as a new
mechanism through which forkhead box C1 can regulate the aggressive
phenotype of basal-like breast cancer and the tendency of
metastasis of these cancers (41).
However, circulating MMP7 expression levels are poor predictors of
BC development (42); therefore, an
in-depth study analysis is required. MMP8 was initially thought to
be produced only by neutrophils, however it was subsequently found
to be expressed by a large variety of other cell types (43), including breast cancer cells
(44). It has been reported that
MMP8 has a suppressive effect on tumor progression and metastasis,
particularly in protecting against lymph node metastasis, while the
molecular mechanisms underlying these effects are unclear (45).
MMP9 has been reported to be mainly expressed in the
positive region of proliferating cell nuclear antigen, and mediates
different signaling pathways to promote or inhibit BC progression
or metastasis (46). Wang et
al (47) reported that
activation of MMP9 by Kruppel like factor 8 serves as a new signal
transduction mechanism for invasion and metastasis of human BC. In
another study, recombinant WNT-5A was demonstrated to activate CD42
in BC cells, whereas WNT-5A signaling and constitutively active
Cdc42 both decreased MMP9 activity, thereby inhibiting the
migration and metastasis of BC cells (48). MMP10 was first cloned from the human
adenocarcinoma cDNA library (49).
Previously, senescent cells were determined to secrete increased
levels of MMPs, whereas MMP10 is uniformly upregulated in
fibroblasts undergoing senescence (50). MMP11 is expressed in the stromal
fibroblasts associated with epithelial cancer cells, and the high
level of MMP11 has been linked to the progression of cancer and the
adverse prognosis of BC (51).
MMP11, a proliferation-related gene, is reported to be
significantly associated with DMFS rate in HR−/
HER2+ BC (52). A number
of previous studies have shown that MMP11 functioned in BC via
insulin-like growth factor-1 signaling or as a downstream target of
oncogene or tumor suppressor microRNA (53,54).
Several studies have reported that MMP12 has a dual
role in cancer, including anti-angiogenesis (55), or tumor invasion and metastasis
(56). An animal experiment showed
that overexpressed MMP12 converts plasminogen to angiostatin by
cleaving the plasminogen precursor molecule, which then inhibits
angiogenesis (55). Another study
has identified MMP12 as a potential mediator for CXCR4 to increase
invasion (57). A number of studies
have reported that MMP13 is highly overexpressed in BC tissue,
which is a potential tumor marker for BC diagnosis and serves as a
therapeutic target for bone metastasis of BC (58,59).
Membrane type I-MMP14 is known to be involved in the initiation and
progression of angiogenesis through multiple mechanisms (60,61). The
MMP14 inhibitor has been reported to impede angiogenesis, and delay
tumor progression and the formation of metastatic lesions;
therefore, a selective MMP14 inhibitor may be a potential
therapeutic strategy for the treatment of BC (62). To the best of our knowledge, there
are a limited number of studies reporting on the relevance between
MMP16 and BC.
In this study, MMP1/9/12 mRNA levels were indicated
to be significantly overexpressed in BC and increased in higher SBR
grades, which suggested a rapid growth of metastatic tumors. This
result is in line with the aforementioned studies. In addition,
high levels of MMP 1/9/12 indicated that RFS rate in all patients
with BC is relatively short; thus, these MMPs may be considered as
potential therapeutic targets. In the present study, the
transcriptional expression and prognostic value of all MMPs in BC
was systematically analyzed, providing a deeper understanding of
the complex mechanisms in the molecular biology of BC. Integrative
bioinformatics analysis showed that MMP1/9/12/16 could be potential
targets for the precise treatment of BC compared with other MMPs;
however, the lack of clinical samples is a limitation of the
present study. Additional clinical trials are required to validate
the diagnostic potentials of these MMPs. In addition, more in-depth
experiments, such as single-cell sequencing, are essential to
examine the latent interaction mechanism between cancer cells and
stromal cells in BC.
Not applicable.
No funding was received.
The datasets used and analyzed during the present
study are available from the corresponding author on reasonable
request.
YZ carried out the design of this study. YZ and HX
performed the statistical analysis and drafted the manuscript. WY
and ML made substantial contributions to acquisition of data and
helped to revise the manuscript. HL, WP and LW participated in its
design and coordination and also helped to draft the manuscript.
All authors read and approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Miller KD, Siegel RL, Lin CC, Mariotto AB,
Kramer JL, Rowland JH, Stein KD, Alteri R and Jemal A: Cancer
treatment and survivorship statistics, 2016. CA Cancer J Clin.
66:271–289. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen E, Qin X, Peng K, Xu X, Li W, Cheng
X, Tang C, Cui Y, Wang Z and Liu T: Identification of potential
therapeutic targets among CXC chemokines in breast tumor
microenvironment using integrative bioinformatics analysis. Cell
Physiol Biochem. 45:1731–1746. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gross J and Lapiere CM: Collagenolytic
activity in amphibian tissues: A tissue culture assay. Proc Natl
Acad Sci USA. 48:1014–1022. 1962. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Takahashi E, Tateyama H, Akatsu H,
Yamakawa Y, Fujii Y and Eimoto T: Expression of matrix
metalloproteinases 2 and 7 in tumor cells correlates with the World
Health Organization classification subtype and clinical stage of
thymic epithelial tumors. Hum Pathol. 34:1253–1258. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Page-McCaw A, Ewald AJ and Werb Z: Matrix
metalloproteinases and the regulation of tissue remodelling. Nat
Rev Mol Cell Biol. 8:221–233. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cui N, Hu M and Khalil RA: Biochemical and
biological attributes of matrix metalloproteinases. Prog Mol Biol
Transl Sci. 147:1–73. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kessenbrock K, Plaks V and Werb Z: Matrix
metalloproteinases: Regulators of the tumor microenvironment. Cell.
141:52–67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Alaseem A, Alhazzani K, Dondapati P,
Alobid S, Bishayee A and Rathinavelu A: Matrix metalloproteinases:
A challenging paradigm of cancer management. Semin Cancer Biol.
56:100–115. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fernandez-Garcia B, Eiro N, Marin L,
González-Reyes S, González LO, Lamelas ML and Vizoso FJ: Expression
and prognostic significance of fibronectin and matrix
metalloproteases in breast cancer metastasis. Histopathology.
64:512–522. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rhodes DR, Yu J, Shanker K, Deshpande N,
Varambally R, Ghosh D, Barrette T, Pandey A and Chinnaiyan AM:
ONCOMINE: A cancer microarray database and integrated data-mining
platform. Neoplasia. 6:1–6. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gyorffy B, Lanczky A, Eklund AC, Denkert
C, Budczies J, Li Q and Szallasi Z: An online survival analysis
tool to rapidly assess the effect of 22,277 genes on breast cancer
prognosis using microarray data of 1,809 patients. Breast Cancer
Res Treat. 123:725–731. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jezequel P, Campone M, Gouraud W,
Guérin-Charbonnel C, Leux C, Ricolleau G and Campion L:
bc-GenExMiner: An easy-to-use online platform for gene prognostic
analyses in breast cancer. Breast Cancer Res Treat. 131:765–775.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gao J, Aksoy BA, Dogrusoz U, Dresdner G,
Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, et al:
Integrative analysis of complex cancer genomics and clinical
profiles using the cBioPortal. Sci Signal. 6:pl12013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Richardson AL, Wang ZC, De Nicolo A, Lu X,
Brown M, Miron A, Liao X, Iglehart JD, Livingston DM and Ganesan S:
X chromosomal abnormalities in basal-like human breast cancer.
Cancer Cell. 9:121–132. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sorlie T, Perou CM, Tibshirani R, Aas T,
Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey
SS, et al: Gene expression patterns of breast carcinomas
distinguish tumor subclasses with clinical implications. Proc Natl
Acad Sci USA. 98:10869–10874. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sorlie T, Tibshirani R, Parker J, Hastie
T, Marron JS, Nobel A, Deng S, Johnsen H, Pesich R, Geisler S, et
al: Repeated observation of breast tumor subtypes in independent
gene expression data sets. Proc Natl Acad Sci USA. 100:8418–8423.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Curtis C, Shah SP, Chin SF, Turashvili G,
Rueda OM, Dunning MJ, Speed D, Lynch AG, Samarajiwa S, Yuan Y, et
al: The genomic and transcriptomic architecture of 2,000 breast
tumours reveals novel subgroups. Nature. 486:346–352. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Radvanyi L, Singh-Sandhu D, Gallichan S,
Lovitt C, Pedyczak A, Mallo G, Gish K, Kwok K, Hanna W, Zubovits J,
et al: The gene associated with trichorhinophalangeal syndrome in
humans is overexpressed in breast cancer. Proc Natl Acad Sci USA.
102:11005–11010. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Turashvili G, Bouchal J, Baumforth K, Wei
W, Dziechciarkova M, Ehrmann J, Klein J, Fridman E, Skarda J,
Srovnal J, et al: Novel markers for differentiation of lobular and
ductal invasive breast carcinomas by laser microdissection and
microarray analysis. BMC Cancer. 7:552007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Karnoub AE, Dash AB, Vo AP, Sullivan A,
Brooks MW, Bell GW, Richardson AL, Polyak K, Tubo R and Weinberg
RA: Mesenchymal stem cells within tumour stroma promote breast
cancer metastasis. Nature. 449:557–563. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Le Doussal V, Tubiana-Hulin V, Friedman S,
Hacene K, Spyratos F and Brunet M: Prognostic value of histologic
grade nuclear components of Scarff-Bloom-Richardson (SBR). An
improved score modification based on a multivariate analysis of
1262 invasive ductal breast carcinomas. Cancer. 64:1914–1921. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bansal C, Singh US, Misra S, Sharma KL,
Tiwari V and Srivastava AN: Comparative evaluation of the modified
Scarff-Bloom-Richardson grading system on breast carcinoma
aspirates and histopathology. Cytojournal. 9:42012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yu K, Lee CH, Tan PH, Hong GS, Wee SB,
Wong CY and Tan P: A molecular signature of the Nottingham
prognostic index in breast cancer. Cancer Res. 64:2962–2968. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gluck S: Extending the clinical benefit of
endocrine therapy for women with hormone receptor-positive
metastatic breast cancer: Differentiating mechanisms of action.
Clin Breast Cancer. 14:75–84. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dawood S: Triple-negative breast cancer:
Epidemiology and management options. Drugs. 70:2247–2258. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Javadian M, Gharibi T, Shekari N,
Abdollahpour-Alitappeh M, Mohammadi A, Hossieni A, Mohammadi H and
Kazemi T: The role of microRNAs regulating the expression of matrix
metalloproteinases MMPs) in breast cancer development, progression,
and meta stasis. J Cell Physiol. 234:5399–5412. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Coussens LM, Fingleton B and Matrisian LM:
Matrix metalloproteinase inhibitors and cancer: Trials and
tribulations. Science. 295:2387–2392. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shay G, Lynch CC and Fingleton B: Moving
targets: Emerging roles for MMPs in cancer progression and
metastasis. Matrix Biol. 44-46:200–206. 2005. View Article : Google Scholar
|
|
30
|
Kohrmann A, Kammerer U, Kapp M, Dietl J
and Anacker J: Expression of matrix metalloproteinases (MMPs) in
primary human breast cancer and breast cancer cell lines: New
findings and review of the literature. BMC Cancer. 9:1882009.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bostrom P, Soderstrom M, Vahlberg T,
Söderström KO, Roberts PJ, Carpén O and Hirsimäki P: MMP-1
expression has an independent prognostic value in breast cancer.
BMC Cancer. 11:3482011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kulic A, Dedic Plavetic N, Vrbanec J and
Sirotković-Skerlev M: Low serum MMP-1 in breast cancer: A negative
prognostic factor? Biomarkers. 17:416–421. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mendes O, Kim HT, Lungu G and Stoica G:
MMP2 role in breast cancer brain metastasis development and its
regulation by TIMP2 and ERK1/2. Clin Exp Metastasis. 24:341–351.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li H, Qiu Z, Li F and Wang C: The
relationship between MMP-2 and MMP-9 expression levels with breast
cancer incidence and prognosis. Oncol Lett. 14:5865–5870.
2017.PubMed/NCBI
|
|
35
|
Ren F, Tang R, Zhang X, Madushi WM, Luo D,
Dang Y, Li Z, Wei K and Chen G: Overexpression of MMP family
members functions as prognostic biomarker for breast cancer
patients: A Systematic review and meta-analysis. PLoS One.
10:e01355442015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chu C, Liu X, Bai X, Zhao T, Wang M, Xu R,
Li M, Hu Y, Li W, Yang LU, et al: MiR-519d suppresses breast cancer
tumorigenesis and metastasis via targeting MMP3. Int J Biol Sci.
14:228–236. 2014. View Article : Google Scholar
|
|
37
|
Panagopoulos V, Leach DA, Zinonos I,
Ponomarev V, Licari G, Liapis V, Ingman WV, Anderson P, DeNichilo
MO and Evdokiou A: Inflammatory peroxidases promote breast cancer
progression in mice via regulation of the tumour microenvironment.
Int J Oncol. 50:1191–1200. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Slattery ML, John E, Torres-Mejia G, Stern
M, Lundgreen A, Hines L, Giuliano A, Baumgartner K, Herrick J and
Wolff RK: Matrix metalloproteinase genes are associated with breast
cancer risk and survival: The Breast Cancer Health Disparities
Study. PLoS One. 8:e631652013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Vizoso FJ, Gonzalez LO, Corte MD,
Rodríguez JC, Vázquez J, Lamelas ML, Junquera S, Merino AM and
García-Muñiz JL: Study of matrix metalloproteinases and their
inhibitors in breast cancer. Br J Cancer. 96:903–911. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kim GE, Lee JS, Choi YD, Lee KH, Lee JH,
Nam JH, Choi C, Kim SS, Park MH, Yoon JH and Kweon SS: Expression
of matrix metalloproteinases and their inhibitors in different
immunohistochemical-based molecular subtypes of breast cancer. BMC
Cancer. 14:9592014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sizemore ST and Keri RA: The forkhead box
transcription factor FOXC1 promotes breast cancer invasion by
inducing matrix metalloprotease 7 (MMP7) expression. J Biol Chem.
287:24631–24640. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Aroner SA, Rosner BA, Tamimi RM, Tworoger
SS, Baur N, Joos TO and Hankinson SE: Plasma matrix
metalloproteinase 1, 3, and 7 levels and breast cancer risk in the
Nurses' Health study. Cancer Causes Control. 25:1717–1723. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Van Lint P and Libert C: Matrix
metalloproteinase-8: Cleavage can be decisive. Cytokine Growth
Factor Rev. 17:217–223. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Decock J, Long JR, Laxton RC, Shu XO,
Hodgkinson C, Hendrickx W, Pearce EG, Gao YT, Pereira AC, Paridaens
R, et al: Association of matrix metalloproteinase-8 gene variation
with breast cancer prognosis. Cancer Res. 67:10214–10221. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Decock J, Hendrickx W, Vanleeuw U, Van
Belle V, Van Huffel S, Christiaens MR, Ye S and Paridaens R: Plasma
MMP1 and MMP8 expression in breast cancer: Protective role of MMP8
against lymph node metastasis. BMC Cancer. 8:772008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Liu B, Cui J, Sun J, Li J, Han X, Guo J,
Yi M, Amizuka N, Xu X and Li M: Immunolocalization of MMP9 and MMP2
in osteolytic metastasis originating from MDA-MB-231 human breast
cancer cells. Mol Med Rep. 14:1099–1106. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wang X, Lu H, Urvalek AM, Li T, Yu L,
Lamar J, DiPersio CM, Feustel PJ and Zhao J: KLF8 promotes human
breast cancer cell invasion and metastasis by transcriptional
activation of MMP9. Oncogene. 30:1901–1911. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Prasad CP, Chaurasiya SK, Axelsson L and
Andersson T: WNT-5A triggers Cdc42 activation leading to an ERK1/2
dependent decrease in MMP9 activity and invasive migration of
breast cancer cells. Mol Oncol. 7:870–883. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Muller D, Quantin B, Gesnel MC,
Millon-Collard R, Abecassis J and Breathnach R: The collagenase
gene family in humans consists of at least four members. Biochem J.
253:187–192. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Thakur S, Nabbi A, Klimowicz A and
Riabowol K: Stromal ING1 expression induces a secretory phenotype
and correlates with breast cancer patient survival. Mol Cancer.
14:1642015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
de Vega RC, Sanchez MLF, Eiro N, Vizoso
FJ, Sperling M, Karst U and Medel AS: Multimodal laser
ablation/desorption imaging analysis of Zn and MMP-11 in breast
tissues. Anal Bioanal Chem. 410:913–922. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Han J, Choi YL, Kim H, Choi JY, Lee SK,
Lee JE, Choi JS, Park S, Choi JS, Kim YD, et al: MMP11 and CD2 as
novel prognostic factors in hormone receptor-negative,
HER2-positive breast cancer. Breast Cancer Res Treat. 164:41–56.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Kasper G, Reule M, Tschirschmann M,
Dankert N, Stout-Weider K, Lauster R, Schrock E, Mennerich D, Duda
GN and Lehmann KE: Stromelysin-3 over-expression enhances
tumourigenesis in MCF-7 and MDA-MB-231 breast cancer cell lines:
Involvement of the IGF-1 signalling pathway. BMC Cancer. 7:122007.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Kwon YJ, Hurst DR, Steg AD, Yuan K, Vaidya
KS, Welch DR and Frost AR: Gli1 enhances migration and invasion via
up-regulation of MMP-11 and promotes metastasis in ERalpha negative
breast cancer cell lines. Clin Exp Metastasis. 28:437–449. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Margheri F, Serrati S, Lapucci A,
Anastasia C, Giusti B, Pucci M, Torre E, Bianchini F, Calorini L,
Albini A, et al: Systemic sclerosis-endothelial cell antiangiogenic
pentraxin 3 and matrix metalloprotease 12 control human breast
cancer tumor vascularization and development in mice. Neoplasia.
11:1106–1115. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Delassus GS, Cho H and Eliceiri GL: New
signaling pathways from cancer progression modulators to mRNA
expression of matrix metalloproteinases in breast cancer cells. J
Cell Physiol. 226:3378–3384. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Hernandez L, Magalhaes MA, Coniglio SJ,
Condeelis JS and Segall JE: Opposing roles of CXCR4 and CXCR7 in
breast cancer metastasis. Breast Cancer Res. 13:R1282011.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Chang HJ, Yang MJ, Yang YH, Hou MF, Hsueh
EJ and Lin SR: MMP13 is potentially a new tumor marker for breast
cancer diagnosis. Oncol Rep. 22:1119–1127. 2009.PubMed/NCBI
|
|
59
|
Nannuru KC, Futakuchi M, Varney ML,
Vincent TM, Marcusson EG and Singh RK: Matrix metalloproteinase
(MMP)-13 regulates mammary tumor-induced osteolysis by activating
MMP9 and transforming growth factor-beta signaling at the
tumor-bone interface. Cancer Res. 70:3494–3504. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Alfranca A, Lopez-Oliva JM, Genis L,
López-Maderuelo D, Mirones I, Salvado D, Quesada AJ, Arroyo AG and
Redondo JM: PGE2 induces angiogenesis via MT1-MMP-mediated
activation of the TGFbeta/Alk5 signaling pathway. Blood.
112:1120–1128. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Turunen SP, Tatti-Bugaeva O and Lehti K:
Membrane-type matrix metalloproteases as diverse effectors of
cancer progression. Biochim Biophys Acta Mol Cell Res.
1864:1974–1988. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Ager EI, Kozin SV, Kirkpatrick ND, Seano
G, Kodack DP, Askoxylakis V, Huang Y, Goel S, Snuderl M, Muzikansky
A, et al: Blockade of MMP14 activity in murine breast carcinomas:
Implications for macrophages, vessels, and radiotherapy. J Natl
Cancer Inst. 107(pii): djv0172015.PubMed/NCBI
|
|
63
|
Ma XJ, Dahiya S, Richardson E, Erlander M
and Sgroi DC: Gene expression profiling of the tumor
microenvironment during breast cancer progression. Breast Cancer
Res. 11:R72009.(Table S1). View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Finak G, Bertos N, Pepin F, Sadekova S,
Souleimanova M, Zhao H, Chen H, Omeroglu G, Meterissian S, Omeroglu
A, et al: Stromal gene expression predicts clinical outcome in
breast cancer. Nat Med. 14:518–527. 2008.(Table S1). View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Perou CM, Sorlie T, Eisen MB, van de Rijn
M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA,
et al: Molecular portraits of human breast tumours. Nature.
406:747–752. 2000.(Table S1). View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Gluck S, Ross JS, Royce M, McKenna EF Jr,
Perou CM, Avisar E and Wu L: TP53 genomics predict higher clinical
and pathologic tumor response in operable early-stage breast cancer
treated with docetaxel-capecitabine ± trastuzumab. Breast Cancer
Res Treat. 132:781–791. 2012.(Table S1). View Article : Google Scholar : PubMed/NCBI
|