Introduction
Tumour metastasis is the primary cause of mortality
in patients with breast cancer (1),
and reports have indicated that mortality rates rise sharply from
10% for localized carcinoma, to 80% for metastatic breast cancer
(2,3). Previous studies have demonstrated that
metastasis involves tumour cells (the ‘seed’) detaching from the
primary tumour, migrating through lymphatic and blood vessels,
adhering to the target organ (the ‘soil’), forming a
micro-metastasis and ultimately developing into clinically
detectable metastases (4,5). However, due to the complexity of
metastasis and the heterogeneity of solid tumours, only a small
subset of cells can successfully navigate the entire metastatic
course and re-initiate tumour growth (6). Accumulating studies have reported that
metastatic cancer stem-like cells may represent the small subset of
cells that are responsible for tumour metastasis (7,8). As a
focus of current cancer research, breast cancer stem-like cells
(BCSLCs), or breast tumour initiating cells, are comparable to
dormant adult stem cells; they not only have the ability to
self-renew, maintain stemness and differentiate into tumour cells,
but are also able to transform into metastatic BCSLCs and promote
tumour metastasis when their epigenetics or resident
microenvironments are altered (9,10).
Cancer-testicular antigen (CTA) is a type of
embryonic antigen whose expression is limited to the testes,
ovaries and endometrium, and is notably re-expressed in metastatic
tumours (11). To date, New York
oesophageal squamous cell carcinoma-1 (NY-ESO-1) has been the most
promising and attractive CTA for immune-based therapy, with a clear
correlation between its expression in tumours and the induction of
an immune response in the majority of malignancies (12), in addition to limited off-target
toxicity (13). NY-ESO-1 is also
known as cancer-testis antigen 1B, which was cloned from a cDNA
library of oesophageal carcinoma using recombinant cDNA library
serological analysis technology in 1997 (14). NY-ESO-1 expression has been
demonstrated in a variety of tumour types, including bladder,
oesophageal, ovarian, prostate, gastrointestinal and
triple-negative breast cancers (TNBC) (15–20). A
number of studies have confirmed a greater prevalence of NY-ESO-1
in metastatic melanoma compared with primary lesions (19,21).
Furthermore, NY-ESO-1 possesses a putative role in the
proliferation of stem cells and cancer cells; as mesenchymal stem
cells differentiate, NY-ESO-1 expression is downregulated (22). Another report also verified that the
expression level of NY-ESO-1 was increased in glioma cancer stem
cells compared with differentiated cells (23). However, the protein expression level
and function of NY-ESO-1 in BCSLCs remain unclear, although studies
have reported the expression of NY-ESO-1 mRNA in 10–30% of patients
with breast cancer (19,24).
Considerable progress has been made in the early
diagnosis and treatment of breast cancer, but metastasis remains an
inevitable outcome for the majority of patients. Previous studies
have primarily focused on cancer stem-like cells (CSLCs) or
NY-ESO-1; the association between BCSLCs, NY-ESO-1 and metastasis
has seldom been described. Thus, the present study aimed to
investigate the correlation between NY-ESO-1+ and
CD44+/CD24−/low cells, and to analyse the
association between BCSLCs with increased expression of NY-ESO-1
and metastasis to provide a potential target for the treatment of
breast cancer.
Materials and methods
Patients and tumour samples
Formalin fixed paraffin-embedded (FFPE) human breast
cancer samples were obtained from 30 female patients (median age,
48.75 years; range, 20–81 years; average weight, 44 kg; range,
42–60 kg) attending The Second Affiliated Hospital of Guangzhou
Medical University (Guangzhou, China) between June 2016 and June
2017. The stage and grade of each sample were confirmed based on
the 2002 American Joint Committee on Cancer Tumour-Node-Metastasis
and World Health Organisation classifications by two independent
pathologists in a blinded manner. A total of 12 metastatic and 18
non-metastatic tissues were ultimately utilized. In addition, the
breast cancer type differed between the patients, with 7 cases of
luminal A, 14 of luminal B, 5 with human epidermal growth factor
receptor 2 overexpression and 4 cases of TNBC. All samples were
collected with informed patient consent, and the use of patient
tissues was approved by the Clinical Research and Application
Institutional Review Board of The Second Affiliated Hospital of
Guangzhou Medical University.
Reagents
Mouse monoclonal anti-CD44 (cat. no. 3570), rabbit
monoclonal anti-NY-ESO-1 (cat. no. 45437) and rabbit monoclonal
anti-GAPDH (cat. no. 2118) primary antibodies, horseradish
peroxidase (HRP)-conjugated goat anti-rabbit and horse anti-mouse
(cat. no. 7074 and 7076) IgG secondary antibodies, donkey
anti-mouse IgG H&L (Alexa Fluor® 488; cat. no. 4408)
and donkey anti-rabbit IgG H&L (Alexa Fluor® 555;
cat. no. 4413) were purchased from Cell Signaling Technology, Inc.,
and anti-mouse IgG H&L (Alexa Fluor® 594; cat. no.
987237) from Invitrogen; Thermo Fisher Scientific Inc. Rabbit
monoclonal anti-CD24 (cat. no. ab110448) and mouse monoclonal
anti-NY-ESO-1 (cat. no. ab139339) antibodies were purchased from
Abcam. The antibodies used for flow cytometry are described in the
corresponding methods section. Histostain UltraSensitive™-plus kits
(cat. no. 9730) were obtained from Fujian Maixin Biotechnology,
Inc. Other chemicals were purchased from Sigma-Aldrich; Merck KGaA,
unless otherwise stated.
Oncomine database and The Cancer
Genome Atlas (TCGA) analysis
In order to identify potential molecular markers and
therapeutic targets based on known gene-drug analysis, a cancer
microarray database and web-based data mining platform aimed to
analyse and compare the transcriptome data of target genes in
prominent tumour types, as well as their corresponding normal
tissues or subtypes (http://tcga-data.nci.nih.gov/tcga) (25). The individual gene expression levels
of CD44 and NY-ESO-1 were analysed using the Oncomine database
(https://www.oncomine.org/resource/main). The mRNA
levels of samples within invasive ductal breast carcinoma (IDBC)
and ductal breast carcinoma in situ (DBCS) datasets were compared.
‘ALL’ fold change and P=0.05 were selected, and the top 10% gene
rank was selected as the threshold. The median intensity and the
10th and 90th percentile data of the CD44 and NY-ESO-1 genes from
the Oncomine database were plotted using GraphPad Prism (version
5.0; GraphPad Software, Inc.).
Immunohistochemistry (IHC) and
immunofluorescence co-localisation staining
The procedure was performed using FFPE samples as
previously described (26). The
tissue sections were deparaffinised and rehydrated using an alcohol
gradient. After incubation with hydrogenase inhibitors (3%) for 15
min at room temperature, and the sections were blocked for 2 h in
washing buffer containing 5% normal goat serum (Sigma-Aldrich;
Merck KGaA) at room temperature. The primary monoclonal antibodies
(CD44, CD24 and NY-ESO-1) were diluted to the appropriate
concentration for IHC, according to the manufacturer's protocol.
Bound primary antibodies were detected using secondary,
biotinylated goat anti-mouse/rabbit antibodies and HRP-conjugated
streptavidin. The tissues were subsequently stained using
diaminobenzidine and the nuclei were counterstained with
haematoxylin. For the negative controls, an isotype mouse/rabbit
immunoglobulin was substituted for the primary antibody. The
stained slides were examined using an Olympus DP70 light microscope
(Olympus Corporation) at ×200 magnification by two experienced
pathologists from The Second Affiliated Hospital of Guangzhou
Medical University who were blinded to the clinical data.
The degree of staining was determined, of which CD44
was primarily expressed at the cytoplasmic membrane, and CD24 and
NY-ESO-1 in the cytoplasm (27–29); the
staining intensity was scored from 0–3 corresponding to negative,
weak, intermediate and strong staining, respectively. The number of
cells stained at each intensity was evaluated and the H-score
determined using the following formula: 1× (% of cells exhibiting
weak staining) + 2× (% of cells exhibiting moderate staining) + 3×
(% of cells exhibiting strong staining).
For immunofluorescence co-localization staining of
breast cancer tissues, the slides were incubated with primary
CD44-mouse and CD24-rabbit monoclonal antibodies (1:200) overnight
at 4°C. Subsequently, anti-mouse IgG H&L (Alexa
Fluor® 594) and anti-rabbit IgG H&L (Alexa
Fluor® 555) were added for 1 h and the slides were
washed ≥5 times. Mouse monoclonal anti- NY-ESO-1 (1:200) was then
added for 2 h at room temperature, prior to incubation with the
corresponding anti-mouse IgG H&L (Alexa Fluor® 488)
for 1 h in the dark. Cells were observed with an inverted
fluorescence microscope at ×1,000 magnification (Zeiss GmbH).
Cell and mammosphere suspension
culture
MCF-7 and SK-BR-3 breast cancer cells were purchased
from the American Type Culture Collection and cultured in
Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher
Scientific, Inc.) according to standard protocols. Mammospheres are
one of the most commonly used breast cancer spherical models and
are able to enrich CD44+/CD24−/low BCSLCs.
MCF-7 (1×103 cells/ml) and SK-BR-3 cells
(1×104 cells/ml) were cultured in suspension in
serum-free DMEM-F12 (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with B27 (1:50; Gibco; Thermo Fisher Scientific, Inc.;
cat. no. 17504-044), 20 ng/ml EGF recombinant human protein (Gibco;
Thermo Fisher Scientific, Inc.; cat. no. PHG0311), 0.4% BSA
(Sigma-Aldrich; Merck KGaA), 4 mg/ml insulin (Sigma-Aldrich; Merck
KGaA) and penicillin-streptomycin (100X; Invitrogen; Thermo Fisher
Scientific, Inc.). The cells were shaken twice a day, 20 times each
time, to prevent adhesion to the flask walls. Mammospheres was
apparent after 7–10 days (30,31).
Flow cytometric analysis
Stem-like mammosphere and parental cells were
collected by centrifugation, trypsinized, and resuspended in
serum-free medium to obtain a single-cell suspension. Anti-CD44-PE
(1:100; cat. no. 338808) and anti-CD24-Percp/cy5.5 (1:100; cat. no.
311116), in addition to the isotype control antibodies for CD44
(1:100; cat. no. 400114) and CD24 (1:100; cat. no. 400252) (all
from BioLegend, Inc.), were added to the appropriate sample tubes,
mixed and incubated at room temperature in the dark for 30 min. The
samples were washed and centrifuged at 400 × g for 5 min at room
temperature. For the intracellular detection of NY-ESO-1, the cells
were permeabilised using the FIX & PERM™ Cell Permeabilization
kit (Invitrogen; Thermo Fisher Scientific Inc.), and stained with
the NY-ESO-1 rabbit mAb (1:50) for 1 h at room temperature. The
samples were washed, and anti-rabbit IgG H&L (Alexa
Fluor® 488; 1:200; cat. no. 1583138; Thermo Fisher
Scientific Inc.,) was added for 30 min in the dark. Finally, 500 µl
PBS was added and the cells were analysed using a BD FACScalibur™
flow cytometer with CellQuest software (version 5.1; Beckman
Coulter, Inc.).
Immunofluorescence
The procedure was conducted as previously described
(32). Stem-like mammosphere and
parental cells (5.0×104 cells/glass slip) were fixed
with 0.4% polyoxymethylene, and blocked with 1% BSA (Guangzhou
Tianjun Biotechnology Co., Ltd.) for 2 h at room temperature.
Following incubation with primary antibody against NY-ESO-1-rabbit
(1:100) overnight at 4°C, the cells were incubated with anti-rabbit
IgG H&L (Alexa Fluor® 488; 1:200) in the dark for 1
h at room temperature. The cells were then counterstained with DAPI
and observed using an inverted fluorescence microscope (Olympus
Corporation) at ×400 magnification, with a core data acquisition
system (model no. IX71-A21PH; Applied Precision, Ltd.).
Western blotting
The procedure was conducted as previously described
(32). Stem-like mammosphere and
parental cells (2×106 cells/ml) were harvested and
washed in triplicate with cold PBS. The cells were lysed in
ice-cold lysis buffer (50 mM Tris-HCl; pH 7.4; 250 mM NaCl; 50 mM
NaF; 5 mM EDTA; 0.1% Triton X-100; and 0.1 mM Na3VO4). Following
centrifugation at 12,000 × g for 15 min at 4°C to remove all
organelles, the supernatants were removed, and the protein levels
were estimated using a Super-Bradford Protein Assay kit (CoWin
Biosciences Co., Ltd.), according to the manufacturer's protocol.
Equal amounts of proteins (25 µg/lane) were denatured in SDS-PAGE
buffer, subjected to SDS-PAGE on a 20% Tris-glycine gel and
transferred onto polyvinylidene fluoride membranes (EMD Millipore).
Membranes were blocked with 5% skimmed milk in TBS containing 0.1%
Tween-20 for 1 h at room temperature. After washing with TBST one
times, membranes were co-incubated with the primary antibodies
against NY-ESO-1-rabbit (1:1,000), CD44-mouse (1:1,000),
CD24-rabbit (1:500) and GAPDH-rabbit (1:1,000) overnight at 4°C in
TBST. Following incubation with horseradish peroxidase
(HRP)-conjugated goat anti-rabbit (1:5,000; cat. no. 7074) and
horse anti-mouse (1:5,000; cat. no. 7076) IgG secondary antibodies
in TBST at room temperature for 60 min, bands were detected using
enhanced chemiluminescence reagents (Thermo Fisher Scientific,
Inc.).
Cell migration and invasion
assays
The migration and invasion capacities of stem-like
mammosphere cells and parental cells were determined using 24-well
chambers with 8-µM inserts (Corning Inc.); 2×105
cells/well with 5% (MCF-7) or 1% (SK-BR-3) FBS were placed into the
upper bovine fibronectin (2:50; cat. no. 03-090-1-01; Biological
Industries) and Matrigel-coated wells (300 µg/ml; Corning Inc.,;
cat. no. 356234; invasion only), respectively. DMEM/F12 (BCSLCs) or
DMEM (BC) containing 20% FBS was added to the lower chambers. After
12 h (MCF-7) or 24 h (SK-BR-3) for migration, and 24 h (MCF-7) or
36 h (SK-BR-3) for invasion at 37°C, the migratory and invasive
cells were fixed for 30 min with 4% paraformaldehyde and stained
for 10 min with crystal violet (0.005%; Sigma-Aldrich; Merck KGaA)
at room temperature. Images were captured with a light microscope
(Olympus Corporation) at ×200 magnification, and the cells were
counted as previously described (32).
Statistics
All statistical analyses were performed using SPSS
for Windows version 16.0 (SPSS, Inc.). A t-test or non-parametric
rank test was used to analyse the differences between two
variables. Correlation analysis was performed with Spearman's rank
correlation. All experiments were performed in triplicate and
P<0.05 was considered to indicate a statistically significant
difference. Canvas 12 and Prism 5 (GraphPad Software, Inc.) were
used for image gathering and processing.
Results
Expression of NY-ESO-1, CD44 and CD24
is correlated with the metastasis of breast cancer
Surface markers such as CD44 and CD24 have been
reliably used to identify and isolate BCSLCs. In order to confirm
the expression of CD44, CD24 and NY-ESO-1, the mRNA expression
levels of NY-ESO-1+/CD44+ samples were
investigated using Oncomine analysis, comparing IDBC with DBCS
sample data. The GSE14548 dataset obtained by Ma et al
(33) (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE14548)
demonstrated that the mRNA expression level of CD44 in IDBC was
increased by 1.632-fold (P=0.047). The TCGA database demonstrated
that the NY-ESO-1 mRNA expression level in IDBC samples was
2.259-fold greater than that of DBCS samples
(P=7.76×10−6; Fig.
1A).
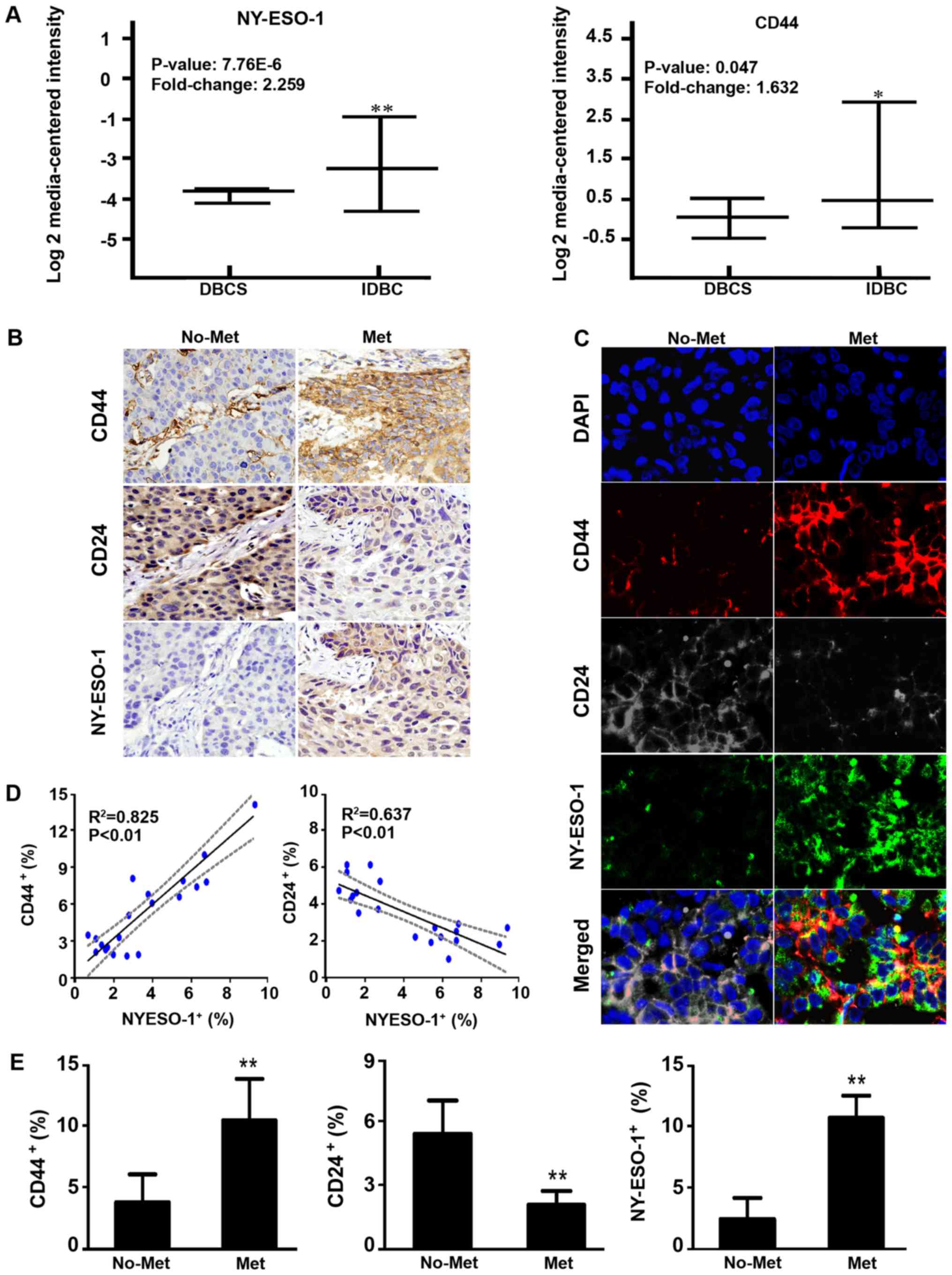 | Figure 1.Expression of NY-ESO-1, CD44 and CD24
in MBCT and non-MBCT. (A) NY-ESO-1 and CD44 mRNA expression
analysis of IDBC vs. DBCS data from the Oncomine database. (B) IHC
of NY-ESO-1, CD44 and CD24 in MBCT and non-MBCT. Magnification,
×400. (C) Co-localization of CD44 (red), CD24 (grey) and NY-ESO-1
(green) proteins in breast cancer tissues. Magnification, ×1,000.
Experiments were performed in triplicate. (D) Correlation between
the positive cell rate of NY-ESO-1 and that of CD44/CD24. (E) Error
bars correspond to the mean ± standard deviation of IHC.
**P<0.01, *P<0.05 compared with non-MBCT or DBCS. NY-ESO-1,
New York oesophageal squamous cell carcinoma 1; MBCT, metastatic
breast cancer tissue; IDBC, invasive ductal breast carcinoma; DBCS,
non-invasive ductal breast carcinoma; IHC, immunohistochemistry;
Met: metastatic breast cancer tissues; no-Met, non-metastatic
breast cancer tissues. |
Subsequently, IHC was performed on 12 metastatic and
18 non-metastatic tissue samples. Compared with the non-metastatic
tissues, the positive expression levels of NY-ESO-1 and CD44
increased 4.14- and 2.67-fold, respectively, while that of CD24
decreased 2.54-fold in metastatic tissues (P<0.01; Fig. 1B and E). Four-color immunofluorescent
staining illustrated the co-localization of CD44, CD24 and NY-ESO-1
proteins; this revealed that the expression level of CD44 and
NY-ESO-1 in metastatic tissues was greater than in non-metastatic
tissues, while that of CD24 was reduced (Fig. 1C). Spearman's rank correlation
indicated that the expression level of NY-ESO-1 significantly
correlated with that of CD44 (R2=0.825; P<0.01) and
CD24 (R2=0.637; P<0.01; Fig. 1D).
Collectively, the data suggested that CD44, CD24 and
NY-ESO-1 expression correlated with the metastasis of breast
cancer, and that NY-ESO-1 expression may be increased in
CD44+/CD24−/low BCSLCs.
CD44+/CD24−/low
BCSLCs exhibit increased expression of NY-ESO-1
In order to demonstrate the increased expression
level of NY-ESO-1 in CD44+/CD24−/low BCSLCs,
MCF-7 and SK-BR-3 cells were cultured in suspension to generate
mammospheres (Fig. 2A). The isotype
controls for CD44, CD24 and NY-ESO-1 gating are presented in
Figs. 2Ba and B3, the
non-CD44+ CD24− MCF-7 and SK-BR-3 cells were
gated to analyse the expression of NY-ESO-1 (left panel), while in
the right panel, CD44+/CD24− BCSLCs were
gated. Independent experiments were repeated three times. The
results revealed that 35.67±3.50% of the MCF-7, and 20.00±2.90% of
the SK-BR-3 spheres were CD44+/CD24−/low,
while 97.50±1.22% of parental MCF-7, and 97.90±0.15% of parental
SK-BR-3 cells were non-CD44+/CD24−/low
(Fig. 2Bb and Bd). In addition, flow
cytometric analysis demonstrated that the NY-ESO-1 expression level
was 40±12% (MCF-7) and 45.1±5.34% (SK-BR-3) in BCSLCs, while that
of the parental MCF-7 and SK-BR-3 cells was 19.1±13 and 8.5±2.76%,
respectively (Fig. 2Bb and Bd;
P<0.05). These data strongly suggested that mammospheres were
able to enrich CD44+/CD24−/low cells.
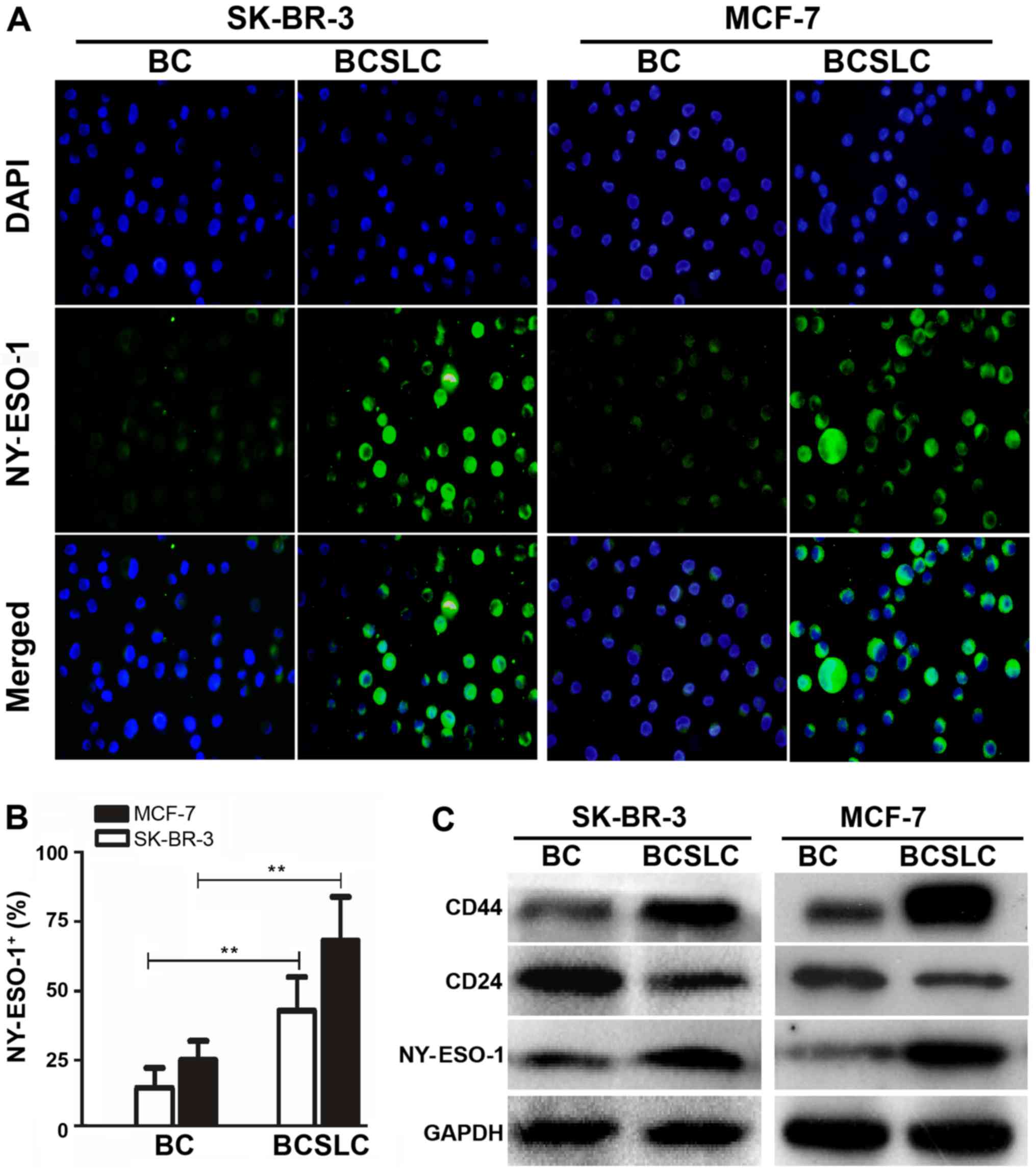
Furthermore, immunofluorescence indicated a 2.72- (MCF-7) and
2.87-fold (SK-BR-3) increase in NY-ESO-1 expression level in
stem-like mammosphere cells (Fig. 3A and
B; P<0.05). Western blotting also demonstrated that the CD44
and NY-ESO-1 expression level was increased, while that of CD24 was
decreased in stem-like mammosphere cells (Fig. 3C; P<0.05). Thus, these data
adequately demonstrated that the expression level of NY-ESO-1 was
increased in CD44+/CD24−/low BCSLCs.
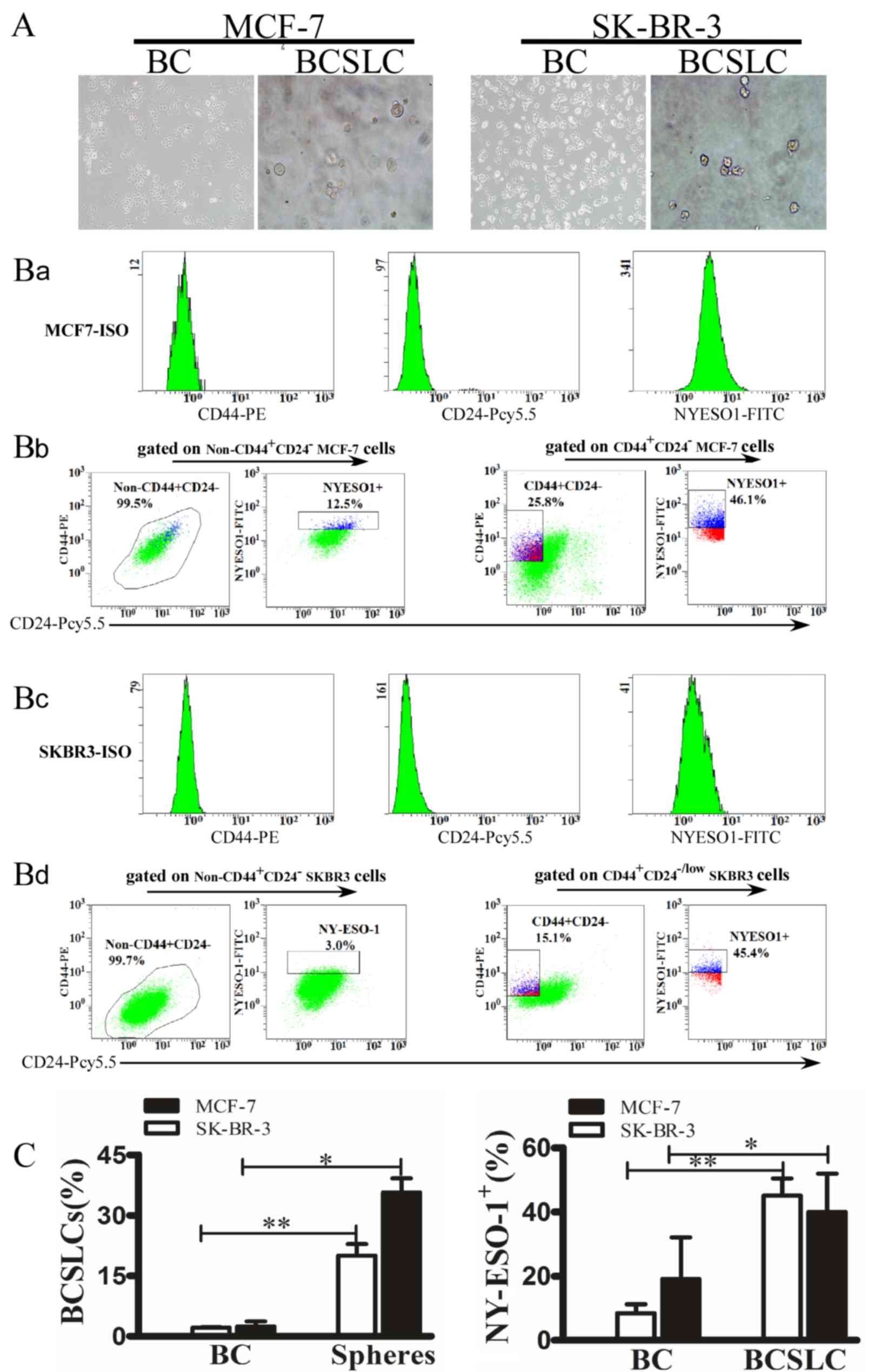 | Figure 2.Protein expression of NY-ESO-1 in
CD44+/CD24−/low BCSLCs and parental cells.
(A) Mammospheres generated from single-cell cultures of SK-BR-3 and
MCF-7 cells. Parental breast cancer cells. (left; magnification,
×100) and mammospheres (right; magnification, ×400). (B) Flow
cytometric analysis of the expression of NY-ESO-1 in SK-BR-3 and
MCF-7 BCSLCs. The isotype controls for CD44, CD24 and NY-ESO-1
gating are presented in Fig. 2Ba and
Bc. The non-CD44+CD24− MCF-7 and SK-BR-3
cells were gated to analyse the expression of NY-ESO-1 (left
panel), while in the right panel, CD44+/CD24−
BCSLCs were gated. The y and × axes represent the mean fluorescence
intensity of CD44, CD24 and NY-ESO-1 (Fig. 2Bb and Bd). (C) Error bars correspond
to the mean ± standard deviation. Percentage of mammospheres and
parental breast cancer cells expressing
CD44+/CD24−/low (left) or NY-ESO-1 (right).
*P<0.05, **P<0.01 vs. parental SK-BR-3 and MCF-7 cells. All
experiments were performed in triplicate. NY-ESO-1, New York
oesophageal squamous cell carcinoma 1; BC, breast cancer cells;
BCSLC, breast cancer stem-like cells. |
CD44+/CD24−/low
BCSLCs with higher expression levels of NY-ESO-1 promote the
invasive and migratory properties of breast cancer cells
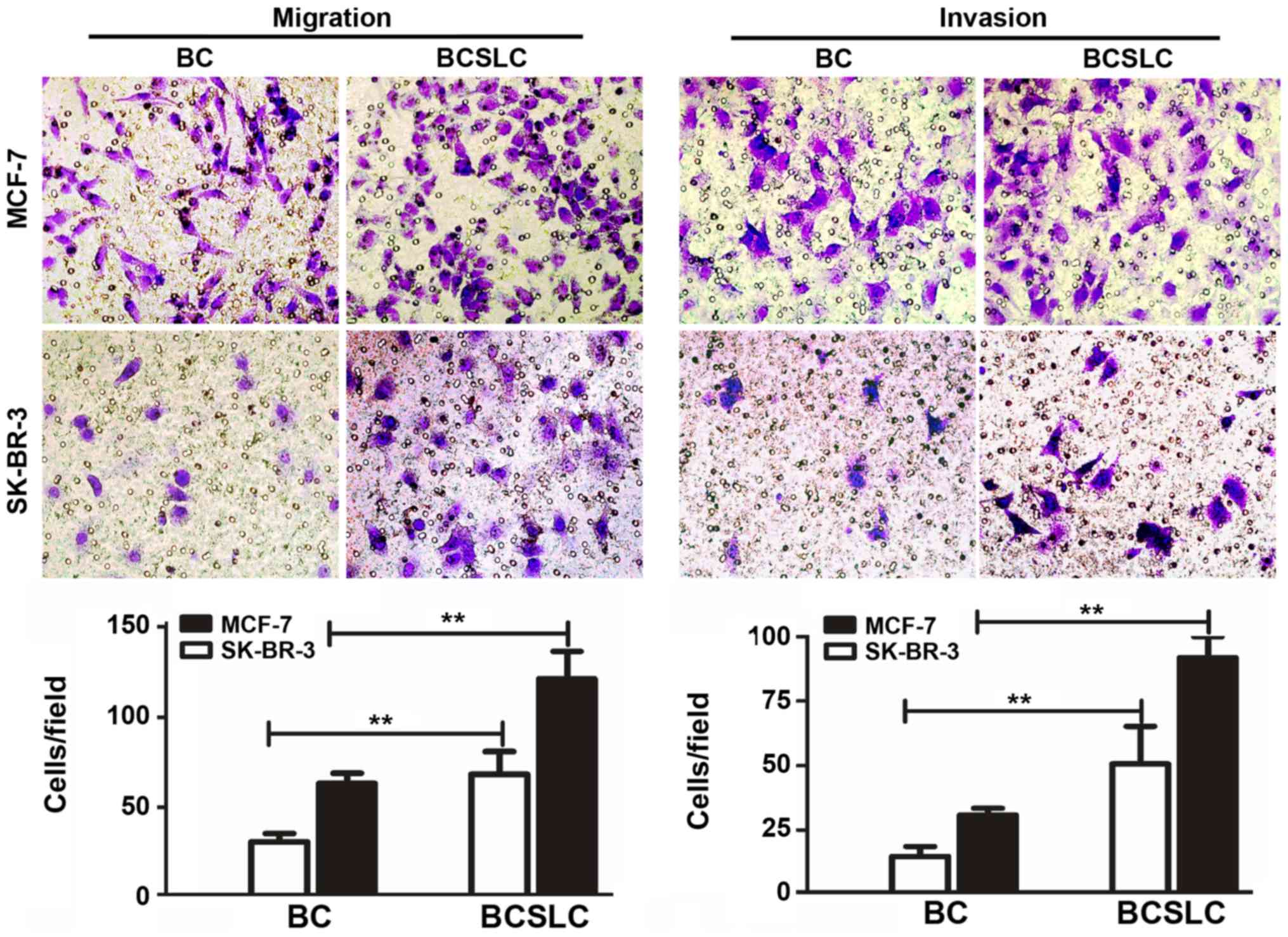
To verify whether
CD44+/CD24−/low BCSLCs with increased
NY-ESO-1 expression levels exhibited altered migration and invasion
capacities, the migration and invasion assays were performed. The
results demonstrated that compared with parental MCF-7 or SK-BR-3
cells, MCF-7 BCSLCs were increased by 1.78-fold (migration) and
1.81-fold (invasion), and SK-BR-3 BCSLCs by up to 2.05-fold
(migration) and 2.09-fold (invasion) (Fig. 4, P<0.05).
Collectively, the presented data indicated that
CD44+/CD24−/low BCSLCs with increased
expression levels of NY-ESO-1 were able to effectively promote the
invasiveness and metastasis of breast cancer cells.
Discussion
CSLCs were first identified in acute myeloid
leukaemia, and were termed leukaemia-initiating cells (34,35).
Subsequently, in 2003, CD44+/CD24− BCSLCs
were identified and isolated for the first time, and it was
verified that these cells possessed metastatic capabilities
(36). The same conclusion has also
been reached by other researchers (37,38).
Consistent with previous studies, the present data revealed that
CD44+/CD24−/low BCSLCs were involved in
cancer metastasis. These genetic signatures in CSLCs support the
concept that CSLCs may be metastatic precursors (10).
In addition, the altered expression levels of
certain proteins, including KLF49 (10) and phosphatase and tensin homolog
(39), are likely to contribute to
the maintenance of stem cell-like features (metastatic ability) in
breast cancer cells. A previous report suggested that specific CTAs
exhibit preferential re-expression in certain tumour types, taking
melanoma-associated antigen 1–4 in 70% of metastatic melanomas,
acrosin-binding protein in 70% of ovarian tumours, and NY-ESO-1 in
46% of breast tumours as examples (40). Comparably, the present study
demonstrated that the expression of the CTA NY-ESO-1 was increased
in metastatic breast cancer at the mRNA and protein levels.
Additionally, NY-ESO-1 was significantly correlated with the
expression of CD44 and CD24. Thus, it was preliminarily verified
that NY-ESO-1 may exhibit higher expression levels in
CD44+/CD24−/Low BCSLCs.
NY-ESO-1 is a representative CTA. Although NY-ESO-1
expression has been reported in a wide range of tumour types,
whether NY-ESO-1 was expressed in BCSLCs remained unknown. In the
present study, in vitro analyses revealed that NY-ESO-1 was
abundantly expressed on CD44+/CD24−/low
BCSLCs. Furthermore, CD44+/CD24−/low BCSLCs
with increased expression levels of NY-ESO-1 exhibited a stronger
capacity for invasion and migration compared with parental
cells.
In conclusion, NY-ESO-1 was confirmed to be highly
expressed in CD44+/CD24−/low BCSLCs, and for
the first time, those with increased expression levels of NY-ESO-1
were shown to effectively initiate breast cancer metastasis.
NY-ESO-1 may transform BCSLCs from a dormant to a metastatic state,
although further research is required to support this hypothesis.
NY-ESO-1, with its strong immunogenicity, unique expression pattern
and low toxicity, is a promising candidate target for breast cancer
immunotherapy, particularly with respect to BCSLCs. Single
(NY-ESO-1) or double-target (NY-ESO-1 and CD44) T-cell receptor
mimic mAbs may be a potential focus for future research.
Acknowledgments
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81172537, 81272900
and 81772828), and the Special Fund for Science and Technology
Development of Guangdong Province (grant no. 2016A020216028).
Availability of data and materials
All data generated or analysed during the present
study are included in this published article.
Authors' contributions
Study concept and design, or data acquisition,
analysis and interpretation were conducted by MYL, HS, HLH and JQC.
Manuscript drafting or revision for important intellectual content,
and approval of the final version of the submitted manuscript was
also conducted by MYL, HS, HLH and JQC. MYL and HS performed the
statistical analysis.
Ethics approval and consent to
participate
All patient tissues were collected with written
informed consent, and the study was approved by the Clinical
Research and Application Institutional Review Board of The Second
Affiliated Hospital of Guangzhou Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Marino N, Woditschka S, Reed LT, Nakayama
J, Mayer M, Wetzel M and Steeg PS: Breast cancer metastasis. Am J
Pathol. 183:1084–1095. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yin C, Li H, Zhang B, Liu Y, Lu G, Lu S,
Sun L, Qi Y, Li X and Chen W: Erratum to: RAGE-binding S100A8/A9
promotes the migration and invasion of human breast cancer cells
through actin polymerization and epithelial-mesenchymal transition.
Breast Cancer Res Treat. 156:407–408. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mukherjee D and Zhao J: The Role of
chemokine receptor CXCR4 in breast cancer metastasis. Am J Cancer
Res. 3:46–57. 2013.PubMed/NCBI
|
|
4
|
Paget S: The distribution of secondary
growths in cancer of the breast. 1889. Cancer Metastasis Rev.
8:98–101. 1989.PubMed/NCBI
|
|
5
|
Chambers AF, Groom AC and MacDonald IC:
Dissemination and growth of cancer cells in metastatic sites. Nat
Rev Cancer. 2:563–572. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Croker AK, Goodale D, Chu J, Postenka C,
Hedley BD, Hess DA and Allan AL: High aldehyde dehydrogenase and
expression of cancer stem cell markers selects for breast cancer
cells with enhanced malignant and metastatic ability. J Cell Mol
Med. 13:2236–2252. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sottoriva A, Verhoeff JJ, Borovski T,
McWeeney SK, Naumov L, Medema JP, Sloot PM and Vermeulen L: Cancer
stem cell tumor model reveals invasive morphology and increased
phenotypical heterogeneity. Cancer Res. 70:46–56. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lawson JC, Blatch GL and Edkins AL: Cancer
stem cells in breast cancer and metastasis. Breast Cancer Res
Treat. 118:241–254. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li F, Tiede B, Massagué J and Kang Y:
Beyond tumorigenesis: Cancer stem cells in metastasis. Cell Res.
17:3–14. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shiozawa Y, Nie B, Pienta KJ, Morgan TM
and Taichman RS: Cancer stem cells and their role in metastasis.
Pharmacol Ther. 138:285–293. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Salmaninejad A, Zamani MR, Pourvahedi M,
Golchehre Z, Hosseini Bereshneh A and Rezaei N: Cancer/testis
antigens: Expression, regulation, tumor invasion, and use in
immunotherapy of cancers. Immunol Invest. 45:619–640. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fratta E, Coral S, Covre A, Parisi G,
Colizzi F, Danielli R, Nicolay HJ, Sigalotti L and Maio M: The
biology of cancer testis antigens: Putative function, regulation
and therapeutic potential. Mol Oncol. 5:164–182. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Thomas R, Al-Khadairi G, Roelands J,
Hendrickx W, Dermime S, Bedognetti D and Decock J: NY-ESO-1 based
immunotherapy of cancer: Current perspectives. Front Immunol.
9:9472018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tavakoli Koudehi A, Mahjoubi B, Mirzaei R,
Shabani S and Mahjoubi F: AKAP4, SPAG9 and NY-ESO-1 in iranian
colorectal cancer patients as probable diagnostic and prognostic
biomarkers. Asian Pac J Cancer Prev. 19:463–469. 2018.PubMed/NCBI
|
|
15
|
Sharma P, Gnjatic S, Jungbluth AA,
Williamson B, Herr H, Stockert E, Dalbagni G, Donat SM, Reuter VE,
Santiago D, et al: Frequency of NY-ESO-1 and LAGE-1 expression in
bladder cancer and evidence of a new NY-ESO-1 T-cell epitope in a
patient with bladder cancer. Cancer Immun. 3:192003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Akcakanat A, Kanda T, Koyama Y, Watanabe
M, Kimura E, Yoshida Y, Komukai S, Nakagawa S, Odani S, Fujii H and
Hatakeyama K: NY-ESO-1 expression and its serum immunoreactivity in
esophageal cancer. Cancer Chemother Pharmacol. 54:95–100. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Odunsi K, Jungbluth AA, Stockert E, Qian
F, Gnjatic S, Tammela J, Intengan M, Beck A, Keitz B, Santiago D,
et al: NY-ESO-1 and LAGE-1 cancer-testis antigens are potential
targets for immunotherapy in epithelial ovarian cancer. Cancer Res.
63:6076–6083. 2003.PubMed/NCBI
|
|
18
|
Fossa A, Berner A, Fossa SD, Hernes E,
Gaudernack G and Smeland EB: NY-ESO-1 protein expression and
humoral immune responses in prostate cancer. Prostate. 59:440–447.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Raghavendra A, Kalita-de Croft P, Vargas
AC, Smart CE, Simpson PT, Saunus JM and Lakhani SR: Expression of
MAGE-A and NY-ESO-1 cancer/testis antigens is enriched in
triple-negative invasive breast cancers. Histopathology. 73:68–80.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang Y, Wu XJ, Zhao AL, Yuan YH, Chen YT,
Jungbluth AA, Gnjatic S, Santiago D, Ritter G, Chen WF, et al:
Cancer/testis antigen expression and autologous humoral immunity to
NY-ESO-1 in gastric cancer. Cancer Immun. 4:112004.PubMed/NCBI
|
|
21
|
Aung PP, Liu YC, Ballester LY, Robbins PF,
Rosenberg SA and Lee CC: Expression of New York esophageal squamous
cell carcinoma-1 in primary and metastatic melanoma. Hum Pathol.
45:259–267. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cronwright G, Le Blanc K, Götherström C,
Darcy P, Ehnman M and Brodin B: Cancer/testis antigen expression in
human mesenchymal stem cells: Down-regulation of SSX impairs cell
migration and matrix metalloproteinase 2 expression. Cancer Res.
65:2207–2215. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yawata T, Nakai E, Park KC, Chihara T,
Kumazawa A, Toyonaga S, Masahira T, Nakabayashi H, Kaji T and
Shimizu K: Enhanced expression of cancer testis antigen genes in
glioma stem cells. Mol Carcinog. 49:532–544. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Coombes RC, Caballero OL, Shousha S,
Ghaem-Maghami S, Woodley-Barker L, Wilhelm-Benartzi CS and Neville
AM: NY-ESO-1 expression in DCIS: A new predictor of good prognosis.
Oncoscience. 4:33–40. 2017.PubMed/NCBI
|
|
25
|
Hou GX, Liu P, Yang J and Wen S: Mining
expression and prognosis of topoisomerase isoforms in
non-small-cell lung cancer by using Oncomine and Kaplan-Meier
plotter. PLoS One. 12:e1745152017. View Article : Google Scholar
|
|
26
|
Choudhary D, Hegde P, Voznesensky O,
Choudhary S, Kopsiaftis S, Claffey KP, Pilbeam CC and Taylor JA
III: Increased expression of L-selectin (CD62L) in high-grade
urothelial carcinoma: A potential marker for metastatic disease.
Urol Oncol. 33:317–387. 2015. View Article : Google Scholar
|
|
27
|
Ademuyiwa FO, Bshara W, Attwood K,
Morrison C, Edge SB, Karpf AR, James SA, Ambrosone CB, O'Connor TL,
Levine EG, et al: NY-ESO-1 cancer testis antigen demonstrates high
immunogenicity in triple negative breast cancer. PLoS One.
7:e387832012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Theurillat JP, Ingold F, Frei C, Zippelius
A, Varga Z, Seifert B, Chen YT, Jäger D, Knuth A and Moch H:
NY-ESO-1 protein expression in primary breast carcinoma and
metastases: Correlation with CD8+ T-cell and CD79a+
plasmacytic/B-cell infiltration. Int J Cancer. 120:2411–2417. 2017.
View Article : Google Scholar
|
|
29
|
Shao J, Fan W, Ma B and Wu Y: Breast
cancer stem cells expressing different stem cell markers exhibit
distinct biological characteristics. Mol Med Rep. 14:4991–4998.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dontu G, Abdallah WM, Foley JM, Jackson
KW, Clarke MF, Kawamura MJ and Wicha MS: In vitro propagation and
transcriptional profiling of human mammary stem/progenitor cells.
Genes Dev. 17:1253–1270. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ponti D, Costa A, Zaffaroni N, Pratesi G,
Petrangolini G, Coradini D, Pilotti S, Pierotti MA and Daidone MG:
Isolation and in vitro propagation of tumorigenic breast cancer
cells with stem/progenitor cell properties. Cancer Res.
65:5506–5511. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li HY, Cui XY, Wu W, Yu FY, Yao HR, Liu Q,
Song EW and Chen JQ: Pyk2 and Src mediate signaling to
CCL18-induced breast cancer metastasis. J Cell Biochem.
115:596–603. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ma XJ, Dahiya S, Richardson E, Erlander M
and Sgroi DC: Gene expression profiling of the tumor
microenvironment during breast cancer progression. Breast Cancer
Res. 11:R72009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bonnet D and Dick JE: Human acute myeloid
leukemia is organized as a hierarchy that originates from a
primitive hematopoietic cell. Nat Med. 3:730–737. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lapidot T, Sirard C, Vormoor J, Murdoch B,
Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA and
Dick JE: A cell initiating human acute myeloid leukaemia after
transplantation into SCID mice. Nature. 367:645–648. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Al-Hajj M, Wicha MS, Benito-Hernandez A,
Morrison SJ and Clarke MF: Prospective identification of
tumorigenic breast cancer cells. Proc Natl Acad Sci USA.
100:3983–3988. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu H, Patel MR, Prescher JA, Patsialou A,
Qian D, Lin J, Wen S, Chang YF, Bachmann MH, Shimono Y, et al:
Cancer stem cells from human breast tumors are involved in
spontaneous metastases in orthotopic mouse models. Proc Natl Acad
Sci USA. 107:18115–18120. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu C, Kelnar K, Liu B, Chen X,
Calhoun-Davis T, Li H, Patrawala L, Yan H, Jeter C, Honorio S, et
al: The microRNA miR-34a inhibits prostate cancer stem cells and
metastasis by directly repressing CD44. Nat Med. 17:211–215. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhou J, Wulfkuhle J, Zhang H, Gu P, Yang
Y, Deng J, Margolick JB, Liotta LA, Petricoin E III and Zhang Y:
Activation of the PTEN/mTOR/STAT3 pathway in breast cancer
stem-like cells is required for viability and maintenance. Proc
Natl Acad Sci USA. 104:16158–16163. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Whitehurst AW: Cause and consequence of
cancer/testis antigen activation in cancer. Annu Rev Pharmacol
Toxicol. 54:251–272. 2014. View Article : Google Scholar : PubMed/NCBI
|