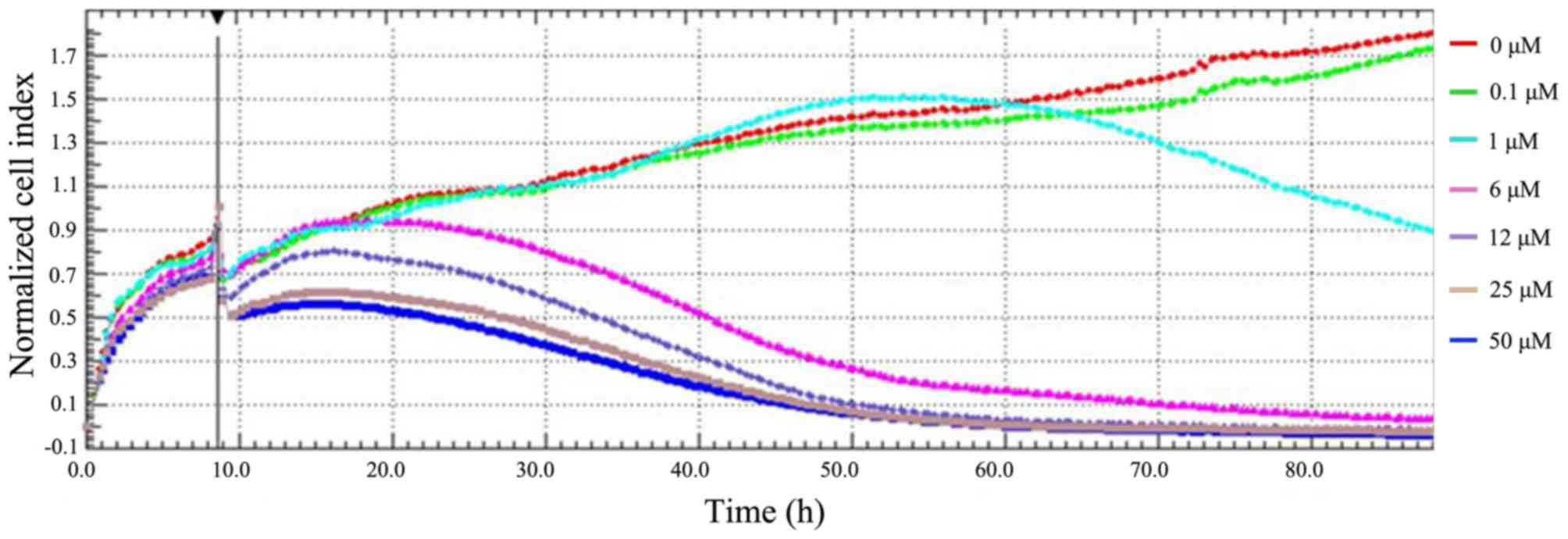
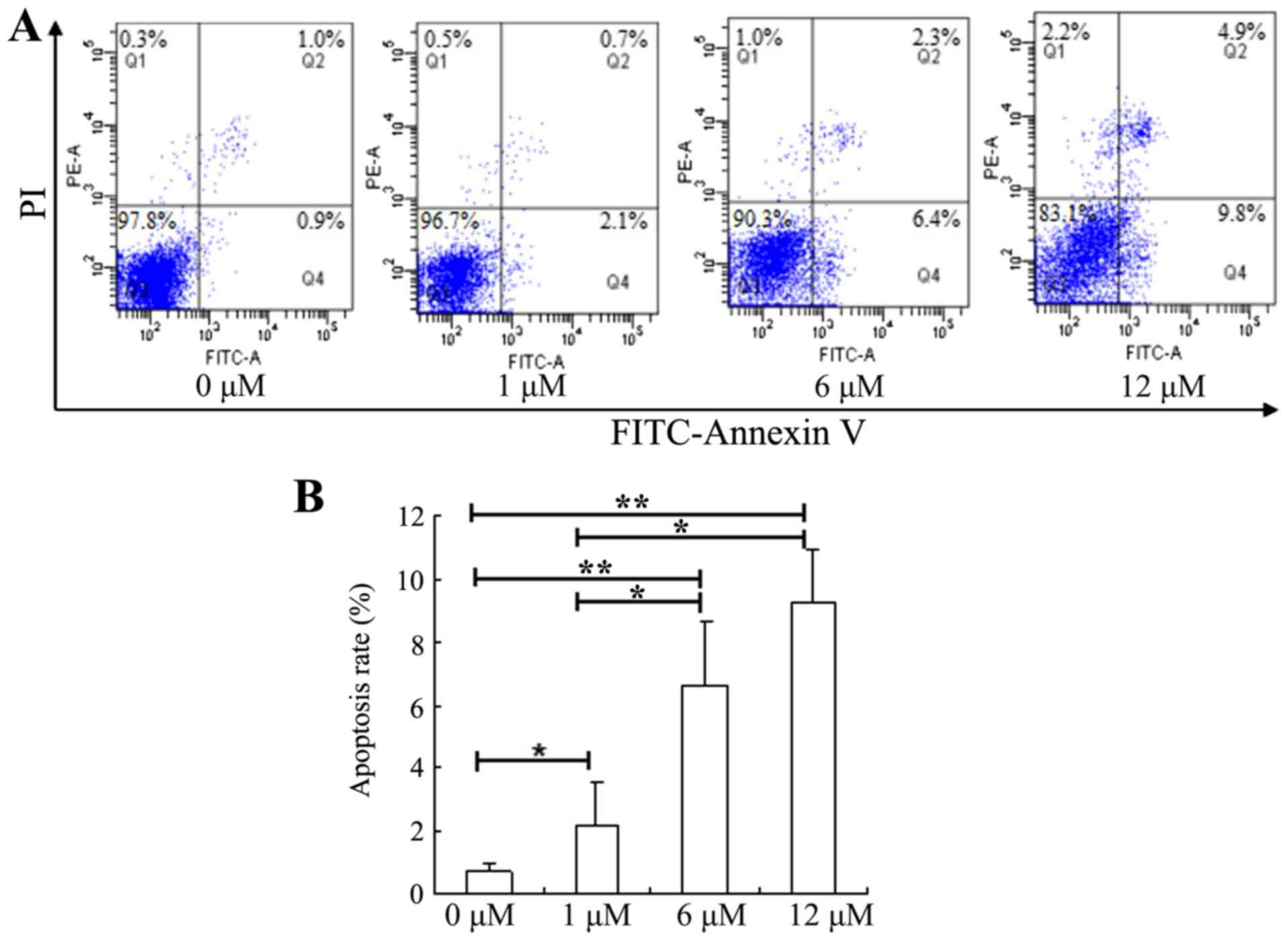
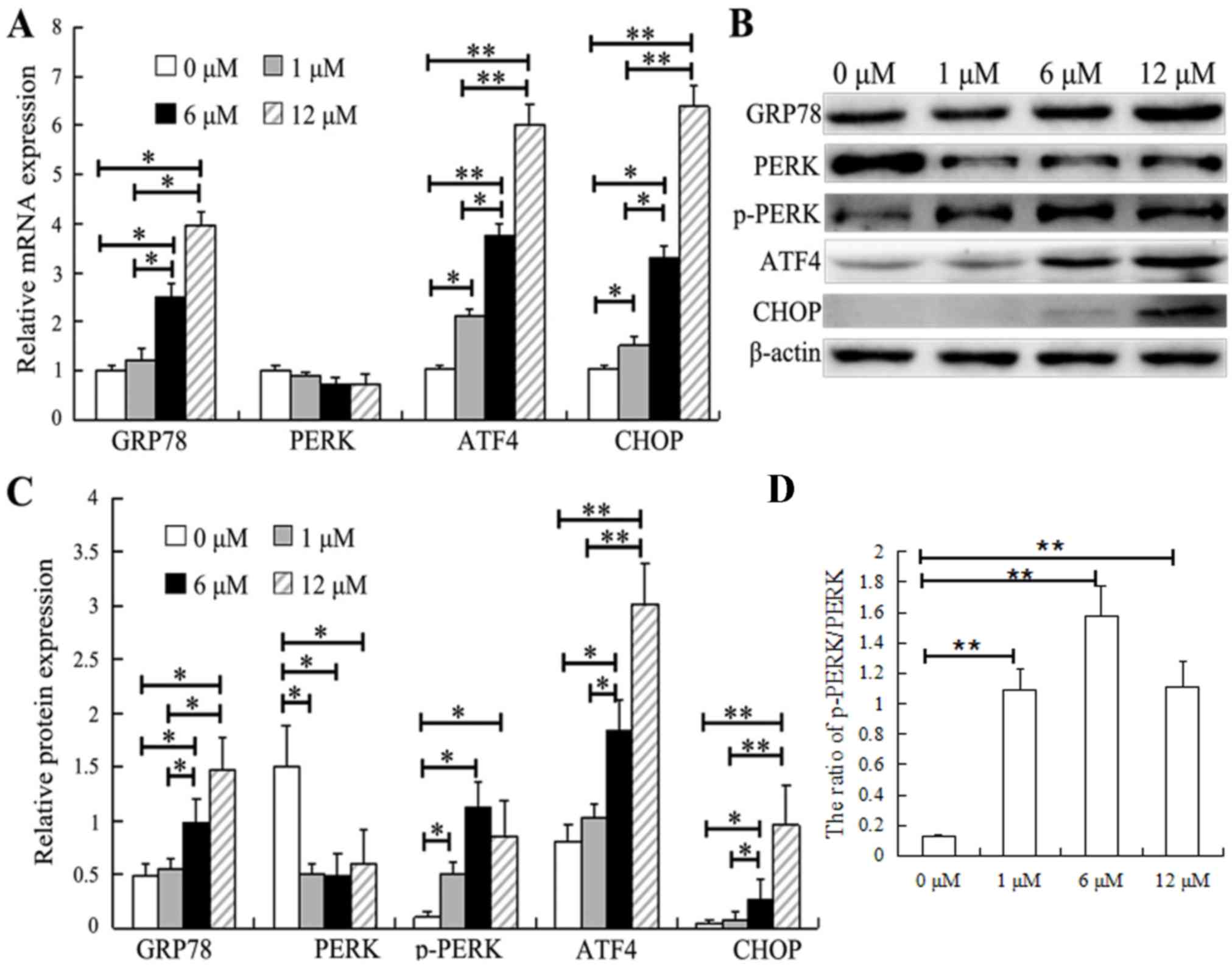
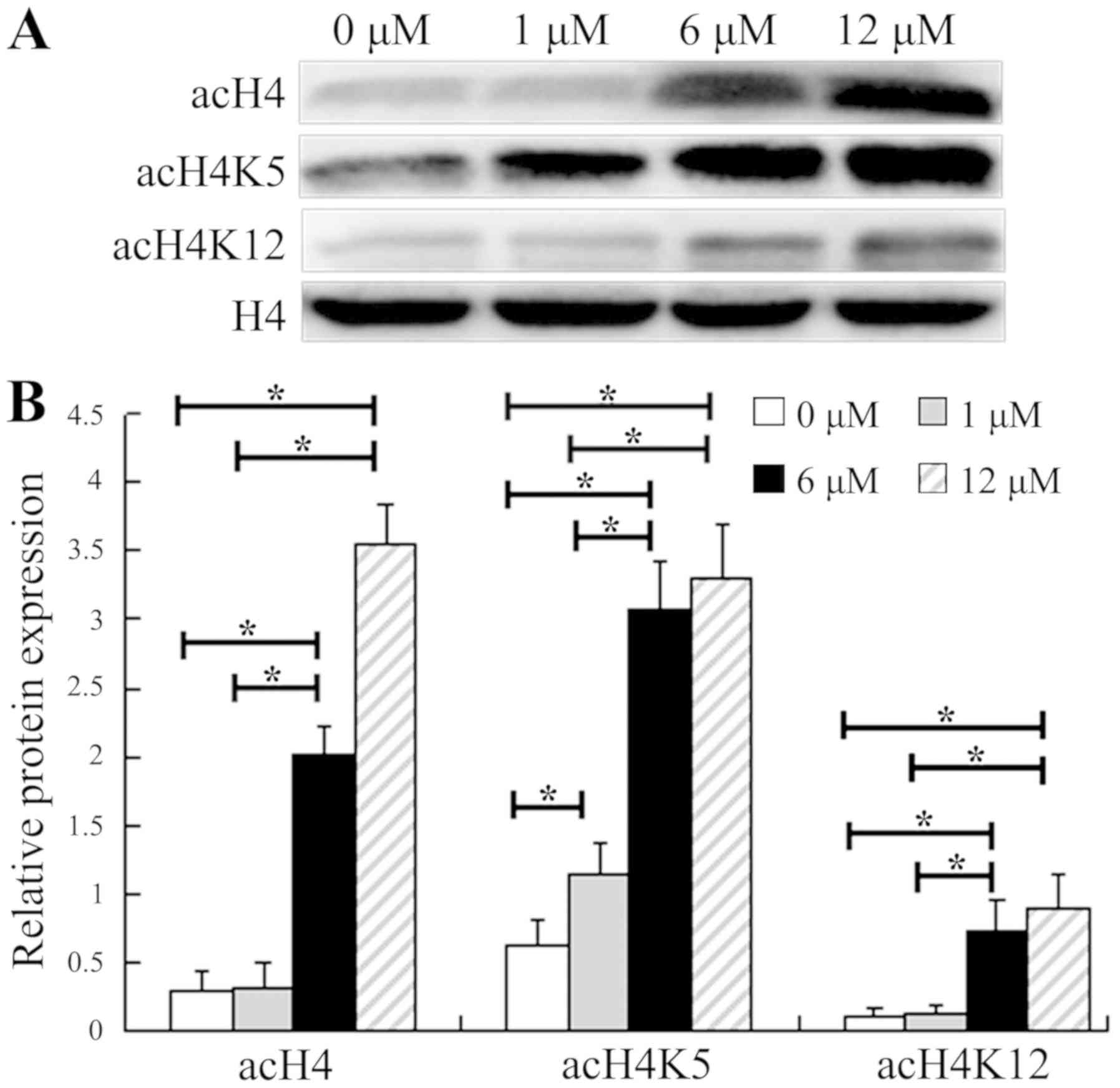
|
1
|
Buurman R, Sandbothe M, Schlegelberger B
and Skawran B: HDAC inhibition activates the apoptosome via Apaf1
upregulation in hepatocellular carcinoma. Eur J Med Res. 21:262016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen QW, Zhu XY, Li YY and Meng ZQ:
Epigenetic regulation and cancer (review). Oncol Rep. 31:523–532.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Muhammad JS, Khan MR and Ghias K: DNA
methylation as an epigenetic regulator of gallbladder cancer: An
overview. Int J Surg. 53:178–183. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shanmugam MK, Arfuso F, Arumugam S,
Chinnathambi A, Jinsong B, Warrier S, Wang LZ, Kumar AP, Ahn KS,
Sethi G and Lakshmanan M: Role of novel histone modifications in
cancer. Oncotarget. 9:11414–11426. 2017.PubMed/NCBI
|
|
5
|
Khan FS, Ali I, Afridi UK, Ishtiaq M and
Mehmood R: Epigenetic mechanisms regulating the development of
hepatocellular carcinoma and their promise for therapeutics.
Hepatol Int. 11:45–53. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang Y, Yan L, Zhang Z, Prado E, Fu L, Xu
X and Du L: Epigenetic regulation and its therapeutic potential in
pulmonary hypertension. Front Pharmacol. 9:2412018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Peng L and Zhong X: Epigenetic regulation
of drug metabolism and transport. Acta Pharm Sin B. 5:106–112.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu KY, Wang LT and Hsu SH: Modification
of epigenetic histone acetylation in hepatocellular Carcinoma.
Cancers (Basel). 10(pii): E82018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Reddy D, Khade B, Pandya R and Gupta S: A
novel method for isolation of histones from serum and its
implications in therapeutics and prognosis of solid tumours. Clin
Epigenetics. 9:302017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vahid F, Zand H, Nosrat-Mirshekarlou E,
Najafi R and Hekmatdoost A: The role dietary of bioactive compounds
on the regulation of histone acetylases and deacetylases: A review.
Gene. 562:8–15. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schneider A, Chatterjee S, Bousiges O,
Selvi BR, Swaminathan A, Cassel R, Blanc F, Kundu TK and Boutillier
AL: Acetyltransferases (HATs) as targets for neurological
therapeutics. Neurotherapeutics. 10:568–588. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhou Y, Peng J and Jiang S: Role of
histone acetyltransferases and histone deacetylases in adipocyte
differentiation and adipogenesis. Eur J Cell Biol. 93:170–177.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chrun ES, Modolo F and Daniel FI: Histone
modifications: A review about the presence of this epigenetic
phenomenon in carcinogenesis. Pathol Res Pract. 213:1329–1339.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kanno K, Kanno S, Nitta H, Uesugi N, Sugai
T, Masuda T, Wakabayashi G and Maesawa C: Overexpression of histone
deacetylase 6 contributes to accelerated migration and invasion
activity of hepatocellular carcinoma cells. Oncol Rep. 28:867–873.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu W, Xiao J, Lan J, et al: The effects
of histone acetylation on the migration and invasion of
hepatocellular carcinoma cells. J Guizhou Med Univ. 42:1365–1369.
2017.(In Chinese).
|
|
16
|
Mrakovcic M, Kleinheinz J and Fröhlich LF:
Histone deacetylase inhibitor-induced autophagy in tumor cells:
Implications for p53. Int J Mol Sci. 18(pii): E18832017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu Z, Jing S, Li Y, Gao Y, Yu S, Li Z,
Zhao Y, Piao J, Ma S and Chen X: The effects of SAHA on
radiosensitivity in pancreatic cancer cells by inducing apoptosis
and targeting RAD51. Biomed Pharmacother. 89:705–710. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lu H, Yang XF, Tian XQ, Tang SL, Li LQ,
Zhao S and Zheng HC: The in vitro and vivo anti-tumor effects and
molecular mechanisms of suberoylanilide hydroxamic acid (SAHA) and
MG132 on the aggressive phenotypes of gastric cancer cells.
Oncotarget. 7:56508–56525. 2016.PubMed/NCBI
|
|
19
|
Xue K, Gu JJ, Zhang Q, Mavis C,
Hernandez-Ilizaliturri FJ, Czuczman MS and Guo Y: Vorinostat, a
histone deacetylase (HDAC) inhibitor, promotes cell cycle arrest
and re-sensitizes rituximab- and chemo-resistant lymphoma cells to
chemotherapy agents. J Cancer Res Clin Oncol. 142:379–387. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hanke NT, Garland LL and Baker AF:
Carfilzomib combined with suberanilohydroxamic acid (SAHA)
synergistically promotes endoplasmic reticulum stress in non-small
cell lung cancer cell lines. J Cancer Res Clin Oncol. 142:549–560.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Teng Z, Kuang X, Wang J and Zhang X:
Real-time cell analysis-a new method for dynamic, quantitative
measurement of infectious viruses and antiserum neutralizing
activity. J Virol Methods. 193:364–370. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zandi K: A real-time cell analyzing assay
for identification of novel antiviral compounds against chikungunya
virus. Methods Mol Biol 1426. 255–262. 2016. View Article : Google Scholar
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lakshmaiah KC, Jacob LA, Aparna S,
Lokanatha D and Saldanha SC: Epigenetic therapy of cancer with
histone deacetylase inhibitors. J Cancer Res Ther. 10:469–478.
2014.PubMed/NCBI
|
|
25
|
Ahuja N, Sharma AR and Baylin SB:
Epigenetic therapeutics: A new weapon in the war against cancer.
Annu Rev Med. 67:73–89. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hurwitz JL, Stasik I, Kerr EM, Holohan C,
Redmond KM, McLaughlin KM, Busacca S, Barbone D, Broaddus VC, Gray
SG, et al: Vorinostat/SAHA-induced apoptosis in malignant
mesothelioma is FLIP/caspase 8-dependent and HR23B-independent. Eur
J Cancer. 48:1096–1107. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yamamoto S, Tanaka K, Sakimura R, Okada T,
Nakamura T, Li Y, Takasaki M, Nakabeppu Y and Iwamoto Y:
Suberoylanilide hydroxamic acid (SAHA) induces apoptosis or
autophagy-associated cell death in chondrosarcoma cell lines.
Anticancer Res. 28:1585–1591. 2008.PubMed/NCBI
|
|
28
|
Hrabeta J, Stiborova M, Adam V, Kizek R
and Eckschlager T: Histone deacetylase inhibitors in cancer
therapy. A review. Biomed Pap Med Fac Univ Palacky Olomouc Czech
Repub. 158:161–169. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Arhoma A, Chantry AD, Haywood-Small SL and
Cross NA: SAHA-induced TRAIL-sensitisation of multiple myeloma
cells is enhanced in 3D cell culture. Exp Cell Res. 360:226–235.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim SM, Park KC, Jeon JY, Kim BW, Kim HK,
Chang HJ, Choi SH, Park CS and Chang HS: Potential anti-cancer
effect of N-hydroxy-7-(2-naphthylthio) heptanomide (HNHA), a novel
histone deacetylase inhibitor, for the treatment of thyroid cancer.
BMC Cancer. 15:10032015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li YL, Zhang NY, Hu X, Chen JL, Rao MJ, Wu
LW, Li QY, Zhang B, Yan W and Zhang C: Evodiamine induces apoptosis
and promotes hepatocellular carcinoma cell death induced by
vorinostat via downregulating HIF-1α under hypoxia. Biochem Biophys
Res Commun. 498:481–486. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kunnimalaiyaan S, Sokolowski K, Gamblin TC
and Kunnimalaiyaan M: Suberoylanilide hydroxamic Acid, a histone
deacetylase inhibitor, alters multiple signaling pathways in
hepatocellular carcinoma cell lines. Am J Surg. 213:645–651. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Matsuo K, Gray MJ, Yang DY, Srivastava SA,
Tripathi PB, Sonoda LA, Yoo EJ, Dubeau L, Lee AS and Lin YG: The
endoplasmic reticulum stress marker, glucose-regulated protein-78
(GRP78) in visceral adipocytes predicts endometrial cancer
progression and patient survival. Gynecol Oncol. 128:552–559. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shi-Chen Ou D, Lee SB, Chu CS, Chang LH,
Chung BC and Juan LJ: Transcriptional activation of endoplasmic
reticulum chaperone GRP78 by HCMV IE1-72 protein. Cell Res.
21:642–653. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang M, Law ME, Castellano RK and Law BK:
The unfolded protein response as a target for anticancer
therapeutics. Crit Rev Oncol Hematol. 127:66–79. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yoo YS, Han HG and Jeon YJ: Unfolded
protein response of the endoplasmic reticulum in tumor progression
and immunogenicity. Oxid Med Cell Longev 2017. 29692712017.
|
|
37
|
Nakka VP, Prakash-Babu P and Vemuganti R:
Crosstalk between endoplasmic reticulum stress, oxidative stress,
and autophagy: Potential therapeutic targets for acute CNS
injuries. Mol Neurobiol. 53:532–544. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen Y, Gui D, Chen J, He D, Luo Y and
Wang N: Down-regulation of PERK-ATF4-CHOP pathway by Astragaloside
IV is associated with the inhibition of endoplasmic reticulum
stress-induced podocyte apoptosis in diabetic rats. Cell Physiol
Biochem. 33:1975–1987. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ketchum CC, Larsen CD, McNeil A,
Meyer-Ficca ML and Meyer RG: Early histone H4 acetylation during
chromatin remodeling in equine spermatogenesis. Biol Reprod.
98:115–129. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lei YU, Han B, Tian T, Zheng L, Yang T,
Liu X, Tang L, Luo X, Yang Q and Xie JR: Suberoylanilide hydroxamic
acid induces apoptosis of HepG2 cells by endoplasmic reticulum
stress apoptotic pathway. Chin J Pathophysiol. 33:2151–2156.
2017.
|
|
41
|
Chen M, Liu Q, Chen L, Zhang L and Gu E:
Remifentanil postconditioning ameliorates histone H3 acetylation
modification in H9c2 cardiomyoblasts after hypoxia/reoxygenation
via attenuating endoplasmic reticulum stress. Apoptosis.
22:662–671. 2017. View Article : Google Scholar : PubMed/NCBI
|