Introduction
Thyroid carcinoma is the most common malignancy of
the endocrine system, and its incidence rate has been increasing
worldwide over the past 10 years. The global incidence rate is 10.2
cases per 100,000 in women and 3.1 cases per 100,000 in men
(1–5). In the United States (6,7), thyroid
carcinoma is now the fifth most common cancer in women and will
replace colon cancer as the fourth leading cancer by 2030. In Korea
(8), thyroid carcinoma has rapidly
increased by 24.2% per year in women between 2000 and 2010, and the
incidence rate of 18.1 cases per 100,000 is the highest in the
world, while in China (9), thyroid
carcinoma is the third most common cancer in women, and has become
a common disease harmful to human health. This increase may partly
be explained by environmental pollution, ionizing radiation, sex
hormone levels and occupational stress (10–14).
Concurrent with the developing economy, there has been an increase
in the concentration of pollutants in the environment. This
includes air, water, soil and noise pollution as well as an
increase in contaminant garbage waste (15–18). It
is well-established that ionizing radiation is a risk factor for
thyroid carcinoma (19). Major
sources of ionizing radiation includes radioactive material from
nuclear waste and medical examinations (for example, computerized
tomography and x-rays), and there is an increased probability of
exposure to ionizing radiation. Additionally, as society and
societal roles have developed, there are now more women in the
workforce which may result in additional work-related stress and
thus changes to hormone levels (20).
Thyroid carcinoma is generally considered a
neoplastic disease, and most patients usually have a favorable
prognosis with a 10-year survival rate >90 percent when treated
by conventional surgery and adjuvant radioiodine. However, thyroid
carcinoma cells can metastasize in the early stages of the disease
to lymph nodes through the blood and lymphatic systems, which may
lead to recurrence and distant metastasis in 10–20% of patients
(21,22). Some cases have a poor response to
conventional treatment resulting in a low 10-year survival rate of
20–30% (23,24). Therefore, a better understanding of
thyroid carcinoma progression and prognosis prediction is urgently
required to improve the outcome of patients with thyroid
carcinoma.
Ryanodine receptors (RyRs) are the largest of the
ion channels. Ryanodine receptor type 2 (RyR2), a member of the RyR
family, is a homotetrameric protein complex that regulates
Ca2+ release from sarcoplasmic reticulum into the
cytosol (25). Gene transcription,
vesicle secretion, and cell proliferation are controlled by
intracellular Ca2+ levels (26), and imbalances in Ca2+
homeostasis and abnormal increases in cytosolic Ca2+
induce apoptosis (27). There is
increasing evidence to suggest that intracellular Ca2+
homeostasis is altered in cancer cells, and that these alterations
are involved in tumor angiogenesis, genetic mutations, and cellular
migration (28). Previous studies
have confirmed that RyR2 is associated with several types of
cancers, including melanoma (29),
breast cancer (30), lymphoma
(31), and prostate cancer (32). However, few studies to date have
assessed the expression and biological function of RyR2 in thyroid
carcinoma.
The present study analyzed the relationship between
RyR2 mRNA expression and thyroid carcinoma. A bioinformatics
analyses revealed that RyR2 was downregulated in thyroid carcinoma
tissues, and low expression levels of RyR2 were closely associated
with poor prognosis in thyroid carcinoma patients.
Materials and methods
Data source
Gene expression data and clinical information on
patients with thyroid carcinoma were obtained from the Genomic Data
Commons Data Portal within The Cancer Genomes Atlas (TCGA)
(https://portal.gdc.cancer.gov/) using
TCGAbiolinks R/Bioconductor package (in October 2018). First, the
RNA-seq data files were merged into a matrix file using the merge
script of the Perl language (http://www.perl.org/). Then, the gene name was
converted from the Ensembl ID to the matrix of the gene symbol
through the Ensembl database version 84 (asia.ensembl.org/index.html). These downloaded data
included a total of 506 thyroid carcinoma samples and 57 normal
thyroid samples. The data of RyR2 were extracted and analyzed. The
differentially expressed genes (DEGs) analysis with RNAseq data was
performed using R (version 3.6.0) (33) package edgeR version 3.27.6
(r-project.org/). False discovery rate <0.05,
log2 counts per million >1 and |log2 fold
change|>2 were set as inclusion criteria for the DEGs selection.
The gene expression level based on microarray data was calculated
using R package limma (version 3.40.2; bioconductor.org/packages/release/bioc/html/limma.html)
with robust multiarray average (RMA) correction.
Gene information acquisition and
clinicopathological features
Gene information for normal thyroid and thyroid
carcinoma expressing RyR2 were obtained from the downloaded data.
Cases missing relevant clinicopathological parameters (including
age, sex, race, lymphatic metastasis, extracapsular extension and
Tumor-Node-Metastasis (TNM) stage (34) and prognostic follow-up data were
eliminated. A total of 555 samples (498 tumors and 57 normal
samples) were analyzed in the present study. The median expression
level of 134 for RyR2 in thyroid carcinoma was used as the cutoff.
Low RyR2 expression in each of the 249 patients was defined as a
value below the 50th percentile. High RyR2 expression in each of
the 249 patients was defined as a value above the 50th percentile.
Significance was first evaluated using the Kruskal-Wallis test. A
Mann-Whitney test was used to evaluate the differences between any
two groups, followed Bonferroni's correction with a cut-off of
P=0.0167 was used to correct for multiple comparisons. The
χ2 and Fisher exact tests were used to evaluate the
association between clinicopathological characteristics and RyR2
expression.
Prognostic analysis
Using the median value of RyR2 expression as
mentioned above, patients with thyroid carcinoma were classified
into RyR2 high group and RyR2 low group. Kaplan-Meier analysis was
used to generate disease-free survival (DFS) curves, and log-rank
tests were performed to assess DFS differences between RyR2 high
and low expression groups. Univariate and multivariate analyses
with Cox proportional hazards regression for DFS were performed on
individual clinical risk factors with and without the RyR2
expression. Hazard ratios and 95% confidence intervals were
determined.
Functional enrichment analysis
Enrolled patients were divided into high and low
expression groups based on their RyR2 expression as described
above. In the present study, the c2.cp.kegg.v6.0.symbols.gmt data
set was downloaded from the Molecular Signatures Database from the
Gene Set Enrichment Analysis (GSEA) website (version 3.0;
software.broadinstitute.org/gsea/index.jsp).
Then, enrichment analysis was performed by the default weighted
enrichment method, and the number of random combinations was set at
1,000.
Statistical analysis
Statistical analyses were performed using SPSS v21.0
software (IBM, Corp.). The edger function was used to analyze mRNA
profiles between normal and thyroid carcinoma tissues. The
different expression of RyR2 mRNA in normal, high and low RyR2
expression groups were evaluated using the Kruskal-Wallis test,
following which the Mann-Whitney test was used to evaluate the
differences between any two groups, and the Bonferroni's correction
was performed to account for multiple corrections. Χ2
and Fisher exact tests were used to evaluate the association
between clinicopathological characteristics and RyR2 expression. In
addition, the association between RyR2 and the prognosis of
patients with thyroid carcinoma was evaluated with the Kaplan-Meier
method and log-rank test. Univariate and multivariate analyses were
performed using the Cox regression model. P<0.05 were considered
to indicate a statistically significant difference.
Results
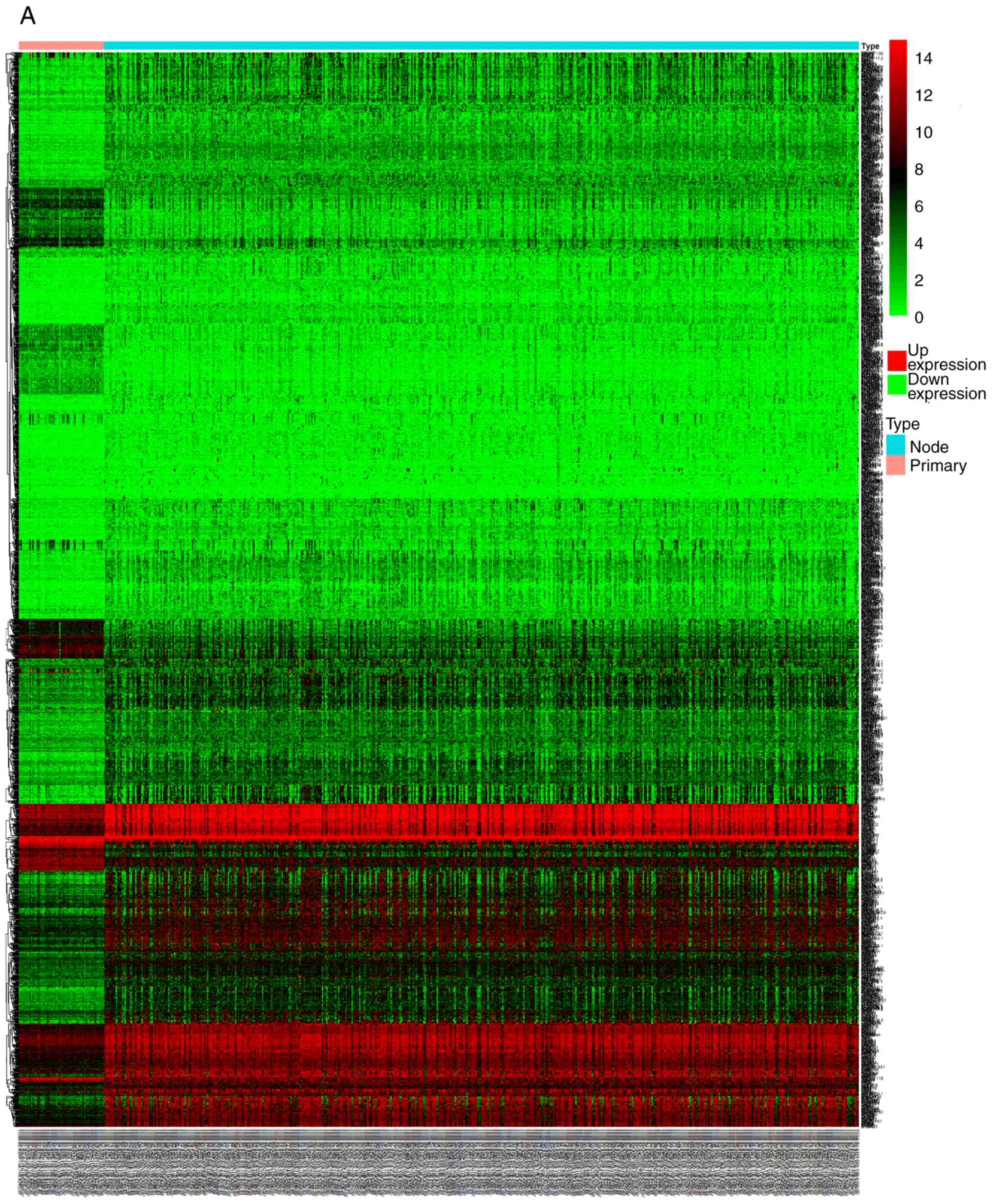
RyR2 is significantly downregulated in
thyroid carcinoma
The expression of RyR2 mRNA in normal and thyroid
carcinoma tissue was investigated using gene expression profiles
downloaded from TCGA database. The results from TCGA cohort
indicated that RyR2 expression was significantly lower in both high
RyR2 expressing and low RyR2 expressing thyroid carcinoma groups
compared with the normal thyroid group (P<0.01) (Fig. 1).
Relationship between RyR2 expression
and clinicopathological characteristics
To explore whether RyR2 expression is associated
with clinicopathological features of thyroid carcinoma, the
clinicopathological characteristics of 498 patients with thyroid
carcinoma from TCGA database were analyzed. According to the median
value of RyR2 expression, patients were divided into either high
RyR2 expression (n=249) or low RyR2 expression (n=249) groups. In
the present study, RyR2 expression was significantly associated
with lymph node metastasis (P<0.001), extracapsular extension
(P<0.001), and TNM stage (27)
(P<0.001) (Table I). However,
there was no association with other characteristics including age,
sex, or ethnic background (P>0.05).
 | Table I.Relationship between the RyR2
expression and clinicopathological characteristics in patients with
thyroid carcinoma. |
Table I.
Relationship between the RyR2
expression and clinicopathological characteristics in patients with
thyroid carcinoma.
|
| RyR2
expression |
|
|
|---|
|
|
|
|
|
|---|
| Clinicopathological
characteristics | Low, n=249 | High, n=249 | χ2
value |
P-valuea |
|---|
| Age, years |
|
| 1.168 | 0.28 |
|
<45 | 106 | 118 |
|
|
|
≥45 | 143 | 131 |
|
|
| Sex |
|
| 0.01 | 0.919 |
|
Male | 67 | 66 |
|
|
|
Female | 182 | 183 |
|
|
| Race |
|
| 2.781 | 0.249 |
|
White | 202 | 200 |
|
|
| Black
or African American | 20 | 13 |
|
|
|
Asian | 27 | 36 |
|
|
| Lymphatic
metastasis |
|
| 11.600 | P<0.001 |
| No | 107 | 145 |
|
|
|
Yes | 142 | 104 |
|
|
| Extracapsular
extension |
|
| 10.364 | P<0.001 |
| No | 135 | 170 |
|
|
|
Yes | 114 | 79 |
|
|
| TNM stage |
|
| 11.036 | P<0.001 |
| I and
II | 148 | 183 |
|
|
| III and
IV | 101 | 66 |
|
|
Association between RyR2 expression
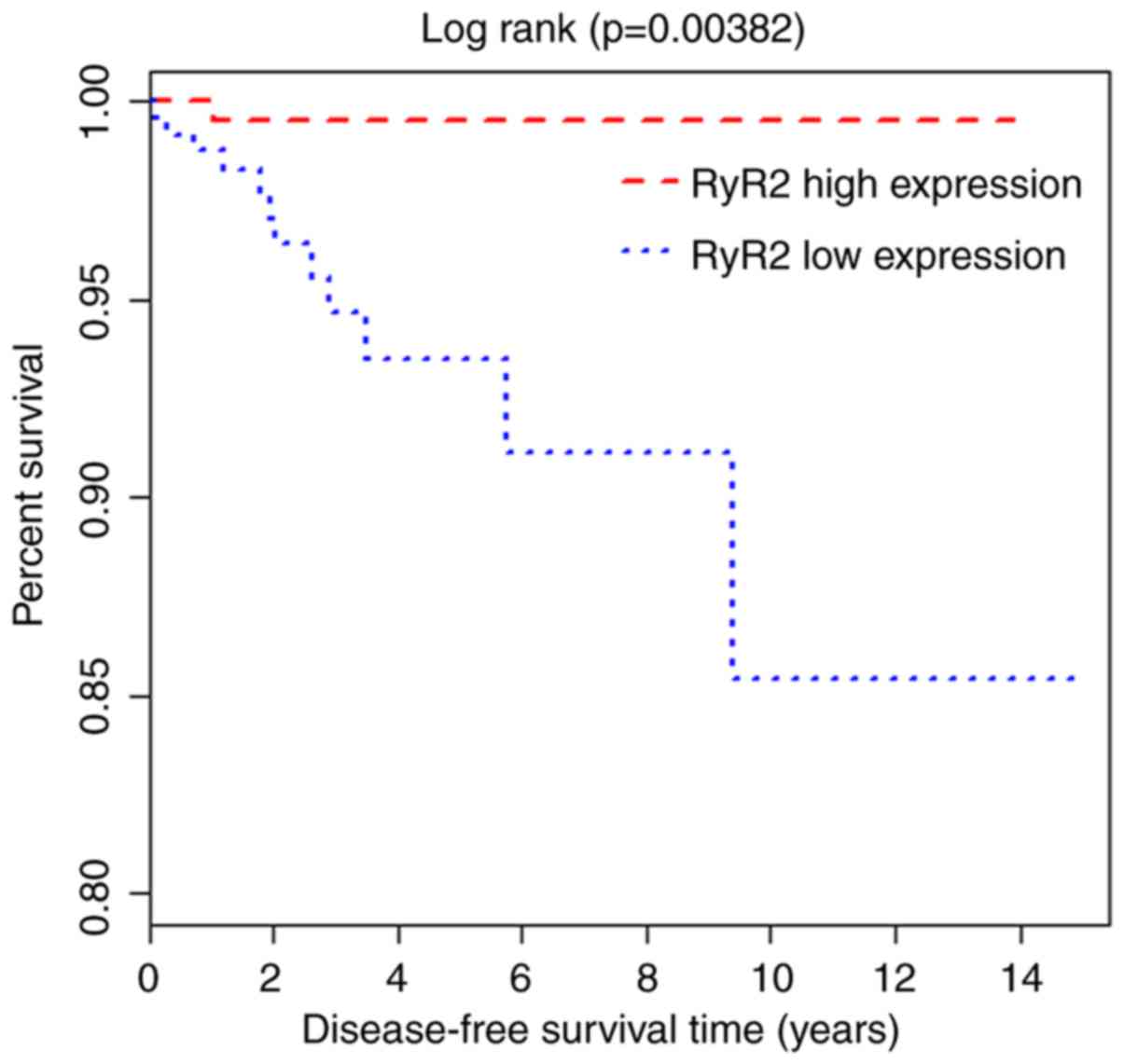
and prognosis of thyroid carcinoma
The prognostic role of RyR2 in thyroid carcinoma was
investigated using Kaplan-Meier and Cox regression analyses. The
results indicated that patients with low RyR2 mRNA levels had a
shorter disease-free survival (DFS) compared with that in patients
with high RyR2 mRNA levels (P=0.00382; Fig. 2). Furthermore, univariate analysis
showed that lymphatic metastasis, extracapsular extension, TNM
stage, and RyR2 expression were associated with DFS (P<0.05).
Multivariate analysis demonstrated that RyR2 expression was an
independent prognostic factor in thyroid carcinoma for DFS
(Table II). Moreover, RyR2
expression was negatively associated with lymphatic metastasis,
extracapsular extension, and TNM stage (Table I). The results of Tables I and II suggest that there is an association
between RyR2 and the prognosis of thyroid carcinoma patients.
 | Table II.Cox regression analysis of RyR2
expression and clinicopathological characteristics for disease-free
survival in patients with thyroid carcinoma. |
Table II.
Cox regression analysis of RyR2
expression and clinicopathological characteristics for disease-free
survival in patients with thyroid carcinoma.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Clinicopathological
characteristics | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age, years |
| <45
vs. ≥45 | 1.183
(0.437–3.325) | 0.619 | 1.351
(0.572–2.968) | 0.714 |
| Sex |
| Male
vs. female | 1.057
(0.266–2.639) | 0.894 | 1.315
(0.402–3.347) | 0.629 |
| Race |
| White
vs. Black or African American vs. Asian | 1.258
(0.714–1.893) | 0.376 | 1.421
(0.503–4.624) | 0.481 |
| Lymphatic
metastasis |
| No vs.
Yes | 2.417
(1.611–3.690) | 0.027a | 2.762
(1.783–4.176) | 0.034a |
| Extracapsular
extension |
| No vs.
Yes | 1.682
(1.012–2.656) | 0.031a | 1.538
(1.124–2.537) | 0.042a |
| TNM stage |
| I and
II vs. III and IV | 3.531
(2.219–5.647) | 0.015a | 3.726
(2.401–5.893) | 0.019a |
| RyR2
expression |
| Low vs.
higha | 5.293
(3.505–8.044) | 0.007a | 5.341
(3.547–8.128) | 0.012a |
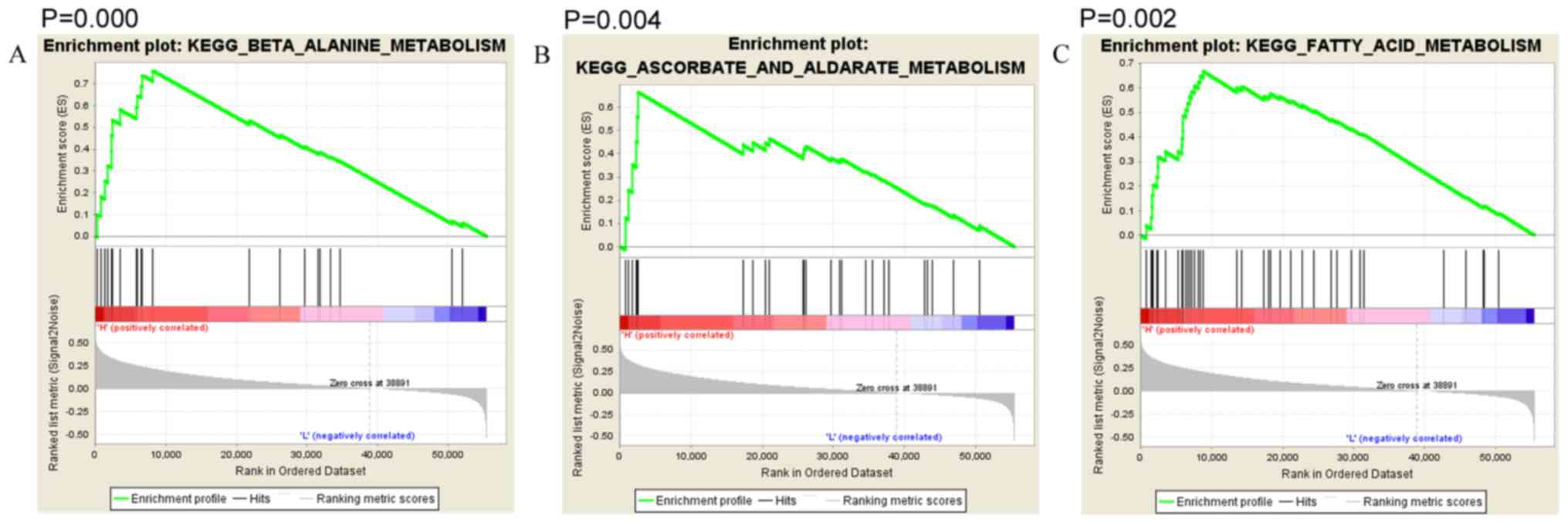
Function analysis of RyR2
Thyroid carcinoma patients were divided into high
and low expression groups according to their RyR2 expression. The
median expression level of RyR2 was used as the cutoff. To clarify
the function of RyR2 in thyroid carcinoma, Gene Set Enrichment
Analysis (GSEA) was used for enrichment. RyR2 is associated with
‘β-alanine metabolism’ (Fig. 3A;
P<0.001), ‘ascorbate and aldarate metabolism’ (Fig. 3B; P=0.004), ‘fatty acid metabolism’
(Fig. 3C; P=0.002), ‘primary bile
acid biosynthesis’ (Fig. 3D;
P=0.008), ‘glycine serine and threonine metabolism’ (Fig. 3E; P=0.008), ‘lysine degradation’
(Fig. 3F; P=0.008), ‘calcium
signaling’ (Fig. 3G; P=0.006), and
‘TGF-β signaling’ (Fig. 3H;
P=0.008).
Discussion
Thyroid carcinoma is one of the most common
malignant tumors in China. It is generally considered to be a
neoplastic disease with a good prognosis. However, thyroid
carcinoma has a high recurrence rate after surgery, and there are
individual differences in patients (35,36).
Thus, novel factors that could more effectively predict the
prognosis of thyroid carcinoma require identification. In the
present study, the expression of RyR2 in thyroid carcinoma and its
function in predicting the prognosis of patients with thyroid
carcinoma was investigated. Low RyR2 expression was associated with
poor prognosis of patients with thyroid carcinoma.
Intracellular calcium ions (Ca2+) have
important roles in fundamental cellular physiology (37). Disruption of intracellular
Ca2+ homeostasis contributes to tumor cell proliferation
and reduced apoptosis (38,39). Additionally, accumulating evidence
has demonstrated that severe and persistent endoplasmic reticulum
(ER) stress or suppression of ER stress results in tumor cell death
(40). RyR is an ER cation channel
that releases ER Ca2+ into the cytosol (41). RyRs form large protein complexes
comprising calmodulin, protein kinases, and protein phosphatases.
In previous research, it has been reported that RyR can upregulate
the activity of mitogenic pathways, including
RAS/mitogen-associated protein kinase (42), and promote T cell activation
(43). Furthermore, Abdul et
al (44) found that there was a
strong negative association between RyR levels and breast tumor
grade. However, the association between the expression of RyR2 and
clinicopathological characteristics in thyroid carcinoma remained
unclear.
According to the 8th AJCC staging system (34), minimal/minor extrathyroidal extension
(mETE) is no longer regarded as a criterion of advanced stage
cancer. Our hypothesis is that this is currently published in
different versions of mETE in 8th AJCC staging system for the
following reasons: First, capsula glanduloe thyreoideae is
imperfect, and normal thyroid tissue may contain adipose or
skeletal muscle tissue. Thus, mETE cannot be defined clearly.
Secondly, a number of studies have found that mETE is not an
independent prognostic risk factor in patients with thyroid
carcinoma. Following the publishing of the 8th AJCC staging system,
it also caused widespread controversy. Many researchers have
presented different views against the new staging system, as they
consider that mETE can increase the risk of thyroid carcinoma
recurrence (45). In the 8th AJCC
staging system, any lymph node involvement in either the central or
lateral neck defines a patient as stage group II (in the absence of
gross extrathyroidal extension or distant metastases) in patients
aged 55 years or older. While in a number of studies, the presence
of lymph node metastasis was associated with a statistically
significant decrease in survival rate (46,47).
Furthermore, lateral lymph node metastasis is considered to be an
independent prognostic risk factor in patients with thyroid
carcinoma (48,49). In the present study, RyR2 mRNA was
downregulated in thyroid carcinoma, and low expression of RyR2 was
associated with lymph node metastasis, extracapsular extension, and
high TNM staging, which suggested poor prognosis of patients with
thyroid carcinoma. Moreover, patients with low RyR2 expression had
a poorer DFS compared with that in patients with high RyR2
expression. Univariate and multivariate Cox regression analyses
showed that RyR2 was an independent prognostic factor for DFS in
thyroid carcinoma. Taken together, these results indicated that
RyR2 is involved in the protection of thyroid carcinoma. In order
to reveal the biological processes and signaling pathways of RyR2
in thyroid carcinoma, GSEA was used, which revealed that RyR2 was
enriched in ‘β-alanine metabolism’, ‘ascorbate and aldarate
metabolism’, ‘fatty acid metabolism’, ‘primary bile acid
biosynthesis’, ‘glycine serine and threonine metabolism’, and
‘lysine degradation’, these signaling pathways were closely
associated with ‘cellular metabolism’ and ‘cell activity’. RyR2 was
also enriched in ‘calcium signaling pathway’ and ‘TGF-β signaling
pathway’, these two signaling pathway themselves were closely
associated with cell signaling and tumor regression. Further
investigation is required to verify these biological processes and
signaling pathways modulated by RyR2 in thyroid carcinoma in future
studies.
Taken together, the present comprehensive analysis
demonstrated the expression and prognostic value of RyR2 in thyroid
carcinoma. We found that low expression of RyR2 is associated with
the poor prognosis of patients with thyroid carcinoma. Therefore,
RyR2 might be a potential tumor suppressor gene for thyroid
carcinoma. The exact mechanisms of RyR2 in thyroid carcinoma remain
unclear, and thus further studies investigating RyR2 and thyroid
carcinoma pathogenesis are required. A total of 555 samples (498
tumors and 57 normal samples) were analyzed in our study. A larger
cohort of tumor and normal samples is required in future studies to
improve the statistical power. In the present study, the expression
value of RyR2 in 498 patients with thyroid carcinoma were presented
as abnormal distribution, but as the median level of RyR2 was used
as the cutoff to divide patients into high and low RyR2 expression
groups, this method may be inaccurate. Therefore, more accurate
statistical methods are required in future studies. In addition,
the expression of RyR2 and its effects should be determined
separately for the different subtypes of thyroid carcinoma,
including papillary thyroid carcinoma (PTC), follicular thyroid
carcinoma (FTC), medullary thyroid carcinoma (MTC) and anaplastic
thyroid carcinoma (ATC). PTC and FTC generally present with
indolent behavior and has a favorable prognosis. MTC is an
aggressive thyroid carcinoma, deriving from parafollicular cells.
ATC is the most aggressive type of thyroid carcinoma and
responsible for more than half of all thyroid cancer deaths.
Different variants of thyroid carcinoma have distinct biological
behaviors and prognosis, thus the expression of RyR2 may differ
between different variants of thyroid carcinoma, and these will
also require further investigation.
Acknowledgements
Not applicable.
Funding
The present study was funded by The Center for
Diagnosis and Treatment of Thyroid Diseases in Sir Run Run Shaw
Hospital, School of Medicine, Zhejiang University (Zheijiang,
China).
Availability of data and materials
All the data collected and analyzed in the study are
available from the corresponding author on reasonable request.
Authors' contributions
LG and NX designed the study. NX, DZ, JC and GH
performed the data collection and analysis. All authors
participated in the writing of the manuscript. All the authors have
read and approved the final version of this manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
La Vecchia C, Malvezzi M, Bosetti C,
Garavello W, Bertuccio P, Levi F and Negri E: Thyroid cancer
mortality and incidence: A global overview. Int J Cancer.
136:2187–2195. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rahib L, Smith BD, Aizenberg R, Rosenzweig
AB, Fleshman JM and Matrisian LM: Projecting cancer incidence and
deaths to 2030: The unexpected burden of thyroid, liver, and
pancreas cancers in the United States. Cancer Res. 74:2913–2921.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pellegriti G, Frasca F, Regalbuto C,
Squatrito S and Vigneri R: Worldwide increasing incidence of
thyroid cancer: Update on epidemiology and risk factors. J Cancer
Epidemiol 2013. 9652122013.
|
|
4
|
Siegel R, Ward E, Brawley O and Jemal A:
Cancer statistics, 2011: The impact of eliminating socioeconomic
and racial disparities on premature cancer deaths. CA Cancer J
Clin. 61:212–236. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Weir HK, Thompson TD, Soman A, Møller B
and Leadbetter S: The past, present, and future of cancer incidence
in the United States: 1975 through 2020. Cancer. 121:1827–1837.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jung KW, Won YJ, Kong HJ, Oh CM, Seo HG
and Lee JS: Cancer statistics in Korea: Incidence, mortality,
survival and prevalence in 2010. Cancer Res Treat. 45:1–14. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cabanillas ME, McFadden DG and Durante C:
Thyroid cancer. Lancet. 388:2783–2795. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
McLeod DS, Cooper DS, Ladenson PW, Ain KB,
Brierley JD, Fein HG, Haugen BR, Jonklaas J, Magner J, Ross DS, et
al: Prognosis of differentiated thyroid cancer in relation to serum
thyrotropin and thyroglobulin antibody status at time of diagnosis.
Thyroid. 24:35–42. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Han MA and Kim JH: Diagnostic X-Ray
exposure and thyroid cancer risk: Systematic review and
meta-analysis. Thyroid. 28:220–228. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hong SH, Myung SK and Kim HS; Korean
Meta-Analysis (KORMA) Study Group, : Alcohol intake and risk of
thyroid cancer: A Meta-analysis of observational studies. Cancer
Res Treat. 49:534–547. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Marotta V, Russo G, Gambardella C, Grasso
M, La Sala D, Chiofalo MG, D'Anna R, Puzziello A, Docimo G, Masone
S, et al: Human exposure to bisphenol AF and diethylhexylphthalate
increases susceptibility to develop differentiated thyroid cancer
in patients with thyroid nodules. Chemosphere. 218:885–894. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kurata A: Differentiated thyroid cancer:
Why does it affect predominantly women during the reproductive
period and have higher incidence of mutual association with breast
cancer. Med Hypotheses. 122:5–7. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yan Z, Pan J, Gao F, An Z, Liu H, Huang Y
and Wang X: Seawater quality criteria derivation and ecological
risk assessment for oil pollution in China. Mar Pollut Bull.
142:25–30. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bai L, Wang J, Ma X and Lu H: Air
pollution forecasts: An overview. Int J Environ Res Public Health.
15(pii): E7802018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dehghani MH, Mosavi MF, Ale-Sheikh AA,
Heidarinejad Z and Yousefi M: Experimental data of designing an
optimal system for storage, collection and transfer of household
waste in the GIS environment: A case study of Tehran, district 22,
Iran. Data Brief. 19:1605–1613. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chae Y and An YJ: Current research trends
on plastic pollution and ecological impacts on the soil ecosystem:
A review. Environ Pollut. 240:387–395. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Albi E, Cataldi S, Lazzarini A, Codini M,
Beccari T, Ambesi-Impiombato FS and Curcio F: Radiation and thyroid
cancer. Int J Mol Sci. 18(pii): E9112017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Salem R, Haibe Y, Dagher C, Salem C,
Shamseddine A, Bitar N, Makdessi J, Khatib S, Boussen H, Benna F,
et al: Female oncologists in the Middle East and North Africa:
Progress towards gender equality. ESMO Open. 4:e0004872019.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Siraj AK, Pratheeshkumar P, Parvathareddy
SK, Bu R, Masoodi T, Iqbal K, Al-Rasheed M, Al-Dayel F, Al-Sobhi
SS, Alzahrani AS, et al: Prognostic significance of DNMT3A
alterations in Middle Eastern papillary thyroid carcinoma. Eur J
Cancer. 117:133–144. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Markovina S, Grigsby PW, Schwarz JK,
DeWees T, Moley JF, Siegel BA and Perkins SM: Treatment approach,
surveillance, and outcome of well-differentiated thyroid cancer in
childhood and adolescence. Thyroid. 24:1121–1126. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Amaral L, Bufalo NE, Peres KC, Barreto IS,
Campos A and Ward LS: ID proteins may reduce aggressiveness of
thyroid tumors. Endocr Pathol. 30:24–30. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zanotti-Fregonara P, Rubello D and Hindié
E: Bone metastases of differentiated thyroid cancer: The importance
of early diagnosis and 131I therapy on prognosis. J Nucl Med.
49:1902–1903. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ding Z, Yuan J, Liang Y, Wu J, Gong H, Ye
Y, Jiang G, Yin P, Li Y, Zhang G, et al: Ryanodine receptor type 2
plays a role in the development of cardiac fibrosis under
mechanical stretch through TGFβ-1. Int Heart J. 58:957–961. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Görlach A, Klappa P and Kietzmann T: The
endoplasmic reticulum: Folding, calcium homeostasis, signaling, and
redox control. Antioxid Redox Signal. 8:1391–1418. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shin DH, Leem DG, Shin JS, Kim JI, Kim KT,
Choi SY, Lee MH, Choi JH and Lee KT: Compound K induced apoptosis
via endoplasmic reticulum Ca2+ release through ryanodine
receptor in human lung cancer cells. J Ginseng Res. 42:165–174.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cui C, Merritt R, Fu L and Pan Z:
Targeting calcium signaling in cancer therapy. Acta Pharm Sin B.
7:3–17. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Carpi S, Polini B, Poli G, Alcantara
Barata G, Fogli S, Romanini A, Tuccinardi T, Guella G, Frontini FP,
Nieri P and Di Giuseppe G: Anticancer activity of Euplotin C,
isolated from the marine ciliate Euplotes crassus, against human
melanoma cells. Mar Drugs. 16(pii): E1662018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lu H, Chen I, Shimoda LA, Park Y, Zhang C,
Tran L, Zhang H and Semenza GL: Chemotherapy-Induced
Ca2+ release stimulates breast cancer stem cell
enrichment. Cell Rep. 18:1946–1957. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
McCarthy TV, Datar S and Mackrill JJ:
Activation of ryanodine receptor/Ca2+ release channels
downregulates CD38 in the Namalwa B lymphoma. FEBS Lett.
554:133–137. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mariot P, Prevarskaya N, Roudbaraki MM, Le
Bourhis X, Van Coppenolle F, Vanoverberghe K and Skryma R: Evidence
of functional ryanodine receptor involved in apoptosis of prostate
cancer (LNCaP) cells. Prostate. 43:205–214. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
R Core Team. R, . A language and
environment for statistical computing. R Foundation for Statistical
Computing; Vienna, Austria: 2012, ISBN 3-900051-07-0, URL.
http://www.R-project.org/
|
|
34
|
Amin MB, Greene FL, Edge SB, Compton CC,
Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR and
Winchester DP: The Eighth edition AJCC cancer staging manual:
Continuing to build a bridge from a population-based to a more
‘personalized’ approach to cancer staging. CA Cancer J Clin.
67:93–99. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
George N, Agarwal A, Kumari N, Agarwal S,
Krisnani N and Gupta SK: Molecular profiling of follicular variant
of papillary thyroid cancer reveals Low-risk noninvasive follicular
thyroid neoplasm with papillary-like nuclear features: A paradigm
shift to reduce aggressive treatment of indolent tumors. Indian J
Endocrinol Metab. 22:339–346. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yang L, Yuan Y, Sun T, Li H and Wang N:
Population-based cancer incidence analysis in Beijing, 2008–2012.
Chin J Cancer Res. 27:13–21. 2015.PubMed/NCBI
|
|
37
|
Berridge MJ, Lipp P and Bootman MD: The
versatility and universality of calcium signalling. Nat Rev Mol
Cell Biol. 1:11–21. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kondratskyi A, Yassine M, Kondratska K,
Skryma R, Slomianny C and Prevarskaya N: Calcium-permeable ion
channels in control of autophagy and cancer. Front Physiol.
4:2722013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhu H, Zhang H, Jin F, Fang M, Huang M,
Yang CS, Chen T, Fu L and Pan Z: Elevated Orai1 expression mediates
tumor-promoting intracellular Ca2+ oscillations in human esophageal
squamous cell carcinoma. Oncotarget. 5:3455–3471. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Dolai S, Pal S, Yadav RK and Adak S:
Endoplasmic reticulum stress-induced apoptosis in Leishmania
through Ca2+-dependent and caspase-independent mechanism. J Biol
Chem. 286:13638–13646. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Santulli G and Marks AR: Essential roles
of intracellular calcium release channels in muscle, brain,
metabolism, and aging. Curr Mol Pharmaco. 8:206–222. 2015.
View Article : Google Scholar
|
|
42
|
Xu C, Luo J, He L, Montell C and Perrimon
N: Oxidative stress induces stem cell proliferation via
TRPA1/RyR-mediated Ca2+ signaling in the
Drosophila midgut. Elife. 6(pii): e224412017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wolf IM, Diercks BP, Gattkowski E,
Czarniak F, Kempski J, Werner R, Schetelig D, Mittrücker HW,
Schumacher V, von Osten M, et al: Frontrunners of T cell
activation: Initial, localized Ca2+ signals mediated by NAADP and
the type 1 ryanodine receptor. Sci Signal. 8:ra1022015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Abdul M, Ramlal S and Hoosein N: Ryanodine
receptor expression correlates with tumor grade in breast cancer.
Pathol Oncol Res. 14:157–160. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Tran B, Roshan D, Abraham E, Wang L,
Garibotto N, Wykes J, Campbell P and Ebrahimi A: An analysis of the
American Joint Committee on Cancer 8th Edition T Staging system for
papillary thyroid carcinoma. J Clin Endocrinol Metab.
103:2199–2206. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wu MH, Shen WT, Gosnell J and Duh QY:
Prognostic significance of extranodal extension of regional lymph
node metastasis in papillary thyroid cancer. Head Neck.
37:1336–1343. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kim HI, Kim TH, Choe JH, Kim JH, Kim JS,
Oh YL, Hahn SY, Shin JH, Jang HW, Kim YN, et al: Restratification
of survival prognosis of N1b papillary thyroid cancer by lateral
lymph node ratio and largest lymph node size. Cancer Med.
6:2244–2251. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kim HI, Kim K, Park SY, Choe JH, Kim JH,
Kim JS, Oh YL, Hahn SY, Shin JH, Ahn HS, et al: Refining the eighth
edition AJCC TNM classification and prognostic groups for papillary
thyroid cancer with lateral nodal metastasis. Oral Oncol. 78:80–86.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kim M, Jeon MJ, Oh HS, Park S, Song DE,
Sung TY, Kim TY, Chung KW, Kim WB, Shong YK, et al: Prognostic
Implication of N1b classification in the Eighth edition of the
Tumor-Node-Metastasis staging system of differentiated thyroid
cancer. Thyroid. 28:496–503. 2018. View Article : Google Scholar : PubMed/NCBI
|