Introduction
Several lines of evidence suggest a strong
association between chronic inflammation and increased
susceptibility to neoplastic transformation and cancer development.
It has been estimated that up to 20% of all tumours arise from
conditions of persistent inflammation such as chronic infections or
autoimmune diseases. A direct link between these two conditions has
been established in particular in the gastrointestinal tract
(1,2).
The association of autoimmune thyroiditis (AIT) and
thyroid cancer was first documented in 1955 (3). Since that time the coexistence of AIT
and papillary thyroid cancer (PTC) has been well documented in the
literature. Some authors believe that coexistent thyroiditis is
associated with lower tumour stage and better prognosis of thyroid
cancer (3–8). Whether this relationship is causal or
merely fortuitous remains a point of dispute. AIT is one of the
most common autoimmune diseases, the most common inflammation of
the thyroid and the most common cause of hypothyroidism in the
industrialized world. Developed countries with sufficient iodine
intake due to iodine prophylaxis have been facing an ‘epidemic’ of
this disease. Similarly, PTC has been recognized as a cancer with
the highest increasing incidence during last three decades
(9). AIT and PTC seem to show a
stronger association than expected by simple probabilistic
explanations. Pathogenic immunobiological links between them are
possible. The results of many studies exploring this issue can be
clustered in three groups: i) AIT is induced as a response to a
pre-existing PTC, ii) PTC is induced or facilitated by a
pre-existing chronic inflammatory process, iii) common mechanisms
are responsible for both diseases (10).
The ability of thyroid cancer to produce an immune
response is caused by the RET/PTC oncogene induction of a
pro-inflammatory transcriptional program. Oncogenes responsible for
cell neoplastic transformation elicit an inflammatory
pro-tumourigenic microenvironment. Pro-inflammatory molecules, such
as cytokines and chemokines, produced by immune infiltrates,
contribute to the regulation of cellular processes for cancer onset
and progression, for tumour cell proliferation, angiogenesis and
metastases (1,2,6).
Alternatively, chronic inflammation may enhance
carcinogenesis by promoting genomic instability (11). Molecular studies showed that
thyrocytes treated with IL-1beta and TNF-alpha were able to induce
cyclooxygenase-2 (COX-2) and secrete IL-6 (10). Elevated COX-2 expression is known to
be associated with the carcinogenesis of various types of
neoplasms, by both inhibiting apoptosis and promoting angiogenesis.
COX-2 expression was found to be in thyroid cancers and thyroid
epithelium from AITs, but not in normal thyroid. This finding may
provide a basis for a relationship between carcinogenesis and
autoimmunity (12). Molecular
studies have identified activation of the RET/PTC
rearrangement-induced MAPK signalling pathway as the driving force
in the development of PTC in the context of AIT (1).
If AIT could be recognized as a precursor or a risk
condition for PTC, or at least for a subset of PTC, this would have
an obvious high clinical importance, given that AIT is a very
common disease in many countries.
Insulin-like growth factor-I (IGF-1) might also play
an important role in development of thyroid carcinoma due to its
mitogenic and anti-apoptotic properties (13). Complement component C3 (C3) has been
found overexpressed in numerous cancer tissues, such as oesophageal
cancer, gastric cancer, and lung cancer as well as in PTC tissue
when compared to normal tissue (14).
Epidemiological data report a strong female
predisposition for thyroid cancer. Female predominance of thyroid
cancer in the childbearing period suggests that oestrogen and
progesterone may play vital roles in the pathogenesis of thyroid
neoplasms (15).
Therefore, the aim of this study was to investigate
possible associations of AIT and PTC by assessing mRNA levels
expressed from key genes. The mRNA expression pattern of nuclear
thyroid hormone receptors (TR), retinoid/rexinoid receptors (RXR),
vitamin D3 receptor, progesterone receptor, selected
co-repressors, type I iodothyronine 5′-deiodinase (DIO1),
proliferation markers (MKi67, PCNA), insulin-like growth factor 1
(IGF-1), anti- and proapoptotic genes (Bcl2, BAX, p53), complement
C3 mRNA was compared in thyroid tumour tissue of PTCs without AIT
(PTC/AIT-) and with coexisting AIT (PTC/AIT+)in order to find
whether expression of selected genes may take part in the
progression of thyroid malignancies.
Materials and methods
Clinical samples
Tumour and surrounding uninvolved thyroid tissue
were collected from 33 unselected PTC patients (six male subjects
and twenty-seven female subjects) at the St. Elisabeth Cancer
Institute in Bratislava, Slovakia, whose surgery was planned by
physicians who were not connected to this study. Collected tumour
tissues and peritumoural thyroid tissue from 14 patients with
co-existent AIT [PTC/AIT+, and N+ (7 out of 14); respectively] and
19 patients without AIT [PTC/AIT-, and N- (10 out of 19);
respectively] were immediately frozen in liquid nitrogen and stored
at −70°C. Tumours were classified and the clinical stage determined
by the tumour, node, metastasis (TNM) system (16) and the presence of AIT, based on
histological evaluation. The study was approved by the Ethics
Committee of the St. Elisabeth Cancer Institute in Bratislava,
Slovakia and unambiguously conducted according to the principles of
the Declaration of Helsinki. Consent was obtained from each patient
or subject after full explanation of the purpose and nature of all
study procedures.
Reverse transcription-semiquantitative
polymerase chain reaction
Total RNA was isolated using TRI Reagent®
(Molecular Research Center, Inc, Cincinnati, USA) according to the
manufacturer's instructions. The concentration of RNA was
determined by spectrophotometry at 260 nm and the purity assessed
from the ratio of absorbance, A260/A280 nm, using a NanoDrop 2000
spectrophotometer (Thermo Scientific, Germany). Reverse
transcription (RT) was performed with 2 µg of total DNAse I-treated
(Thermo Scientific, Germany) RNA and the Ready-to-Go You-Prime
First-Strand Beads (Amersham Pharmacia Biotech, Inc., USA)
according to the manufacturer's protocol.
Semiquantitative real-time PCR was performed in
duplicates in a total volume of 20 µl using SensiFAST™ SYBR Hi-Rox
Kit (Bioline, Great Britain) and 0.25 µM of each primer and RT
product: 10 ng (RPS18) and 30 ng (for the other genes).
Amplification and detection were performed with an ABI Prisma
7900HT detection system (Applied Biosystems, USA) under the
following conditions: 95°C for 2 min, 40 cycles of denaturation
(95°C, 5 sec) and annealing (30 sec), and the final melting curve
analysis. The data are expressed using the 2−ΔΔCq method
as the relative level of each mRNA normalized to that of the
housekeeping gene RPS18. The oligonucleotide of the primers
employed in this study along with the corresponding annealing times
are summarized in Table I (17). These conditions were proven to be in
the log phase for each amplified sequence. Triton tumour tissue and
LNCaP prostatic cancer cell line were used as a positive control
(18). A negative control without
cDNA template was run with every assay batch in order to assess
overall specificity.
 | Table I.Primers for semiquantitative PCR. |
Table I.
Primers for semiquantitative PCR.
| Gene | Sequence (5′-3′) | Annealing temp
(°C) |
|---|
| RARα | F:
ACCCCCTCTACCCCGCATCTACAAG and R: CATGCCCACTTCAAAGCACTTCTGC | 60 |
| RARβ | F:
ATTCCAGTGCTGACCATCGAGTCC and R: CCTGTTTCTGTGTCATCCATTTCC | 62 |
| RARγ | F:
TACCACTATGGGGTCAGC and R: CCGGTCATTTCGCACAGCT | 60 |
| RXRα | F:
CTTTTGTTTCCGTTGCTGTTTA and R: CTGAGGTCTTTGCTGATGACAC | 60 |
| RXRβ | F:
TACAGGGCAGAACCAAGAACA and R: ATGAGGCAAGATGAGAAGGAAG | 60 |
| RXRγ | F:
AGAAAGACAGAGGAGCCGAGA and R: CAGAGAAGTGGGGAATACGC | 60 |
| RPS18 | F:
TCTAGTGATCCCTGAAAAGTTCC and R: CGTGGATTCTGCATAATGGTG | 60 |
| TRα | F:
AGGAGAACAGTGCCAGGTCA and R: TCTTGAAGCGGCACAGCTGG | 60 |
| TRβ | F:
AACTACAGGTATAAGGCTGATTCAC and R: ATGCTTCTCTGCGTATATGCC | 60 |
| VDR | F:
GACTTTGACCGGAACGTGCGG and R: CATCATGCCGATGTCCACACA | 60 |
| PR | F:
TCTATTCATTATGCCTTACCATGTG and R: AACCAATTGCCTTGATGAGC | 60 |
| SMRT | F:
TGTGGTTCATAAGCCATCTGC and R: AATCTTCCCCTCCTCCC | 60 |
| N-CoR | F:
AGCATTCCATCCCTACGGG and R: TGGACCCCTTCACCAAAG | 60 |
| IGF-1 | F:
TGACTCCACTTCCTCTAACTCCA and R: AAACCTCTCACCTCAACCTCA | 60 |
| C3 | F:
TGCGGCTACCCTACTCTGTTGTTCG and R: GACGGCAGCCTTGACTTCCACTTCC | 60 |
| PCNA | F:
AGTGGAGAACTTGGAAATGGAA and R: GAAGAGAGTGGAGTGGCTTTTG | 60 |
| MKi6 | F:
TCAGAAAGGGAAAGGAGAAGC and R: GACACACACATTGTCCTCAGC | 60 |
| Bcl2 | F:
GACTTCGCCGAGATGTCCAG and R: CAGGTGCCGGTTCAGGTACT | 60 |
| BAX | F:
TGCTTCAGGGTTTCATCCAGGA and R: ACGGCGGCAATCATCCTCTC | 60 |
| p53 | F:
CCCCTCCTGGCCCCTGTCATCTTCT and R: GCAGCGCCTCACAACCTCCGTCAT | 60 |
| DIO1 | F:
GGACATCAGAAATCACCAGA and R: TTCCTCTGGGTTGTAGTTCC | 58 |
Statistical analysis
Data are expressed as medians (range 5–95%) of two
PCR analyses. Differences between more than two groups were
assessed by one-way analysis of variance followed by Bonferroni
post hoc test using SigmaPlot® 11.0 (Systat Software
GmbH). P<0.05 was considered to indicate a statistically
significant difference.
Results
Six male subjects and twenty-seven female subjects
were enrolled in this study. The mean age of patients at surgery
was 49.91±17.06 years (mean ± SD). Clinicopathological parameters
of the 33 cases of PTC are presented in Table II. Among 33 patients, there were 14
patients with co-existent AIT (PTC/AIT+) and 19 patients without
AIT (PTC/AIT-). The mean age of patients with AIT was 45.08±16.33
and without AIT 39.85±17.63 years (mean ± SD). Histologically
confirmed lymph node metastases were present in 16 patients.
 | Table II.Clinicopathological parameters of the
33 cases of PTC. |
Table II.
Clinicopathological parameters of the
33 cases of PTC.
| Patient | Gender | Age, years | AIT status | Histology |
|---|
| P1 | F | 20 | PTC/AIT− | T1bN0M0 |
| P2 | F | 69 | PTC/AIT− | T3N0M0 |
| P3 | M | 31 | PTC/AIT− | T1bN1aM0 |
| P4 | F | 32 | PTC/AIT− | T1bN0M0 |
| P5 | F | 67 | PTC/AIT− | T1aNxM0 |
| P6 | F | 34 | PTC/AIT− | T1aN1aM0 |
| P7 | F | 37 | PTC/AIT− | T3N1bM0 |
| P8 | M | 23 | PTC/AIT− | T3N1aM0 |
| P9 | F | 34 | PTC/AIT− | T3N1bM0 |
| P10 | F | 69 | PTC/AIT− | T3N0M0 |
| P11 | F | 39 | PTC/AIT− | T3N0M0 |
| P12 | F | 20 | PTC/AIT− | T1bN0M0 |
| P13 | F | 38 | PTC/AIT− | T1aN0M0 |
| P14 | F | 69 | PTC/AIT− | T2N0M0 |
| P15 | M | 19 | PTC/AIT− | T2N1Mx |
| P16 | F | 38 | PTC/AIT− | T1bN1aM0 |
| P17 | F | 38 | PTC/AIT− | T3N1bM0 |
| P18 | M | 38 | PTC/AIT− | T3N1aM0 mikro |
| P19 | M | 62 | PTC/AIT− | T1aN1bM0 |
| P20 | F | 20 | PTC/AIT− | T1bN0M0 |
| P21 | F | 36 | PTC/AIT+ | T3N0M0 |
| P22 | F | 39 | PTC/AIT+ | T1bN1aM0 |
| P23 | F | 33 | PTC/AIT+ | T1bN0M0 |
| P24 | F | 79 | PTC/AIT+ | T1bN0M0 |
| P25 | F | 47 | PTC/AIT+ | T3N1M0 |
| P26 | M | 44 | PTC/AIT+ | T3N1bM0 |
| P27 | F | 43 | PTC/AIT+ | T3aN1bM0 |
| P28 | F | 26 | PTC/AIT+ | T1aN1aM0 |
| P29 | F | 70 | PTC/AIT+ | T1bN0M0 |
| P30 | F | 34 | PTC/AIT+ | T1aN1aM0 |
| P31 | F | 66 | PTC/AIT+ | T3N1M0 |
| P32 | F | 36 | PTC/AIT+ | T3N0M0 |
| P33 | F | 33 | PTC/AIT+ | T1bN0M0 |
We investigated the mRNA expression patterns of
selected nuclear receptors and other molecular targets in the PTC
tissue and peritumoural tissue of patients without AIT (PTC/AIT-,
and N-; respectively) and compared them to those with coexisting
AIT (PTC/AIT+, and N+; respectively).
Significantly decreased expression levels of both
TRalpha and TRbeta mRNA in PTC/AIT+ tumour tissue was seen when
compared to tumour tissue of PTC/AIT- patients (Fig. 1A and B). A similar decrease of TRbeta
mRNA (but not TRalpha mRNA) was also detected in non-tumour thyroid
tissues of PTC/AIT+ patients in comparison to non-tumour tissues of
PTC/AIT- patients.
We found significantly decreased levels of RARalpha
mRNA in thyroid tumour tissue (P<0.05) of patients with AIT
(group PTC/AIT+) compared to the tumour tissue of patients without
AIT (group PTC/AIT-; Fig. 2A). A
similar effect of AIT was found on the significantly decreased
expression of RARgamma mRNA in thyroid tumour tissue of PTC/AIT+
patients (Fig. 2B).
There was no significantly changed expression of
RARbeta mRNA in the thyroid tumour tissue between patients without
or with AIT; however, there were significantly (P<0.05) reduced
RARbeta mRNA levels in non-tumour thyroid tissue of the PTC/AIT+
subgroup when compared to non-tumour tissue of PTC/AIT- patients
and there were significantly higher levels of RXRgamma mRNA in
thyroid tumour tissue compared to non-tumour thyroid tissue of both
PTC/AIT+ and PTC/AIT- patient subgroups (data not shown). We did
not find any significant differences in expression of RXRalpha,
RXRbeta, co-repressors NCoR and SMRT mRNAs in tumour tissues of
PTC/AIT-, compared to tumour tissues of PTC/AIT+ patients (data not
shown). We found significantly increased expression of
dihydroxyvitamin D3 receptors (VDR) mRNA in tumour
tissue of PTC/AIT+ when compared to tumour tissue of PTC/AIT-
patients (Fig. 3A). On the other
hand, the levels of PR mRNA were decreased in tumour tissue of
PTC/AIT+ patients compared to expression in tumours of patients
without AIT (Fig. 3B).
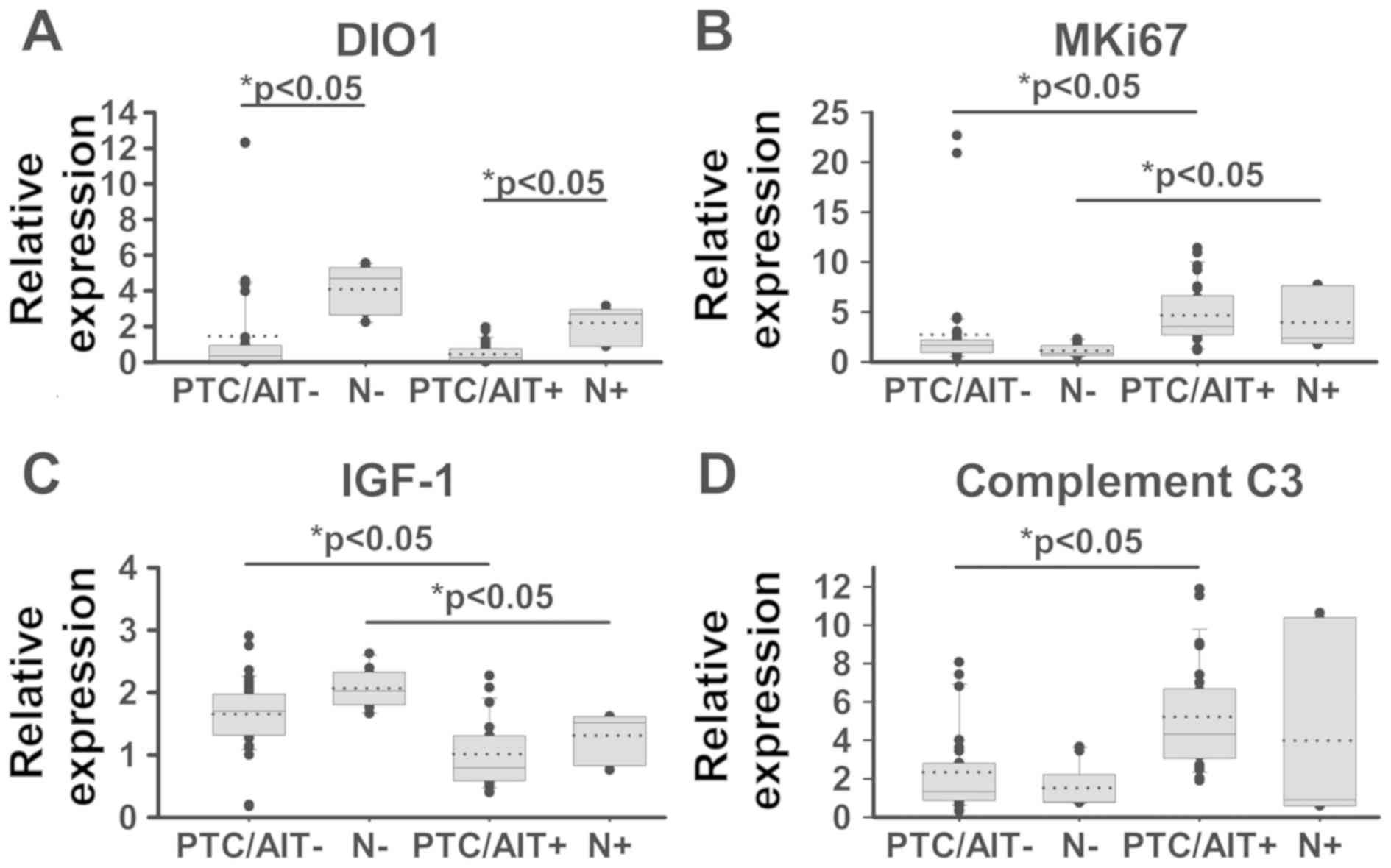
As shown in Fig. 4A,
we found that there was either absent or significantly lower
expression of type I iodothyronine 5′-deiodinase (DIO1) in tumour
tissue compared to non-tumour thyroid tissue in both of patients
and there were not any significant differences between PTC/AIT- and
PTC/AIT+ patients.
We sought to investigate the question whether the
process of AIT might take part in the proliferation and apoptosis
in both tumour and non-tumour thyroid tissue. To study
proliferation, two well-known markers, MKi67 and PCNA were
evaluated, and significantly increased expression level of MKi67
mRNA was found in tumour tissue of PTC/AIT+ when compared to
PTC/AIT- (Fig. 4B); however, the
expression of PCNA marker remained unchanged (data not shown). We
found that tumours from the PTC/AIT+ patients showed significantly
decreased levels of IGF-1 mRNA compared to expression in tumours
from the PTC/AIT- patients. A similar pattern was demonstrated in
corresponding non-tumour thyroid tissue (Fig. 4C). No differences were found in the
expression of anti-apoptotic gene Bcl2 and pro-apoptotic genes BAX
and p53 (data not shown). Significantly increased levels of
complement C3 mRNA in tumour tissue of PTC/AIT+ patients were
detected compared to the tumour tissue of PTC/AIT- patients
(Fig. 4D).
Discussion
Papillary thyroid cancer (PTC) is the most frequent
thyroid cancer histotype, accounting for more than 80% of all
thyroid malignancies (19).
Previously, a meta-analysis of the literature on Hashimoto's
thyroiditis and PTC risk was conducted in order to investigate the
question of whether AIT may predispose patients to the development
of PTC (20). Consequently, we
sought to investigate selected molecular targets in PTC comparing
those cases without AIT to those with coexisting AIT.
It has been demonstrated that large amounts of
IL-1beta are present in AIT. IL-1beta is a proinflammatory cytokine
and mediates induction of Fas on thyroid follicular cells, which
result in apoptotic tissue damage (21). IL-1beta can activate MAP kinases and
the NF-κB pathway. IL-1beta was found to modulate the retinoid
signal transduction pathway, and IL-1beta gene is a direct,
downstream target gene of retinoic acid thus it may indirectly
regulate cell growth (22).
Moreover, IL-1beta can block the insulin and insulin-like growth
factor pathways by inhibiting the receptor kinase activity.
Furthermore, IGF-1 suppresses apoptosis and induces thyroid
proliferation via protein-tyrosine kinase-dependent signalling
pathway (23). Moreover, IGF-1 and
oestrogen can act synergistically through the IGF-1 signalling
cascade (24). Previous findings
indicate that cross-talk between retinoic acids, thyroid hormone
and oestrogens pathways acting through nuclear receptors plays
important roles in the regulation of many processes in various
tissues and it has been suggested that cancer progression may be
associated with alteration in metabolism and/or signalling pathways
of these components (25).
Nuclear receptors (NRs)-ligand-inducible
transcription factors, members of the nuclear receptor superfamily
have been at the forefront of cancer research, where they are known
to act as critical regulators of cancer diseases and are also
playing a crucial role as biomarkers for tumour subclassification
and predominantly targets for hormone therapy (26). Molecular endocrinology approaches for
studying nuclear receptor superfamily members clearly demonstrate
the importance of transcription factors inducible by biologically
active molecules or hormones in the biology and possible clinical
treatment of thyroid cancer. Since no relevant data on nuclear
receptors expression showing associations of autoimmune thyroiditis
and PTC do exist, our data thus represent the first insight into
the processes, where the starting points exist at the nuclear
receptors level.
Thyroid hormone receptors (TRalpha, TRbeta) are
ligand-inducible transcription factors that mediate a variety of
the genomic actions of 3,5,3′-triiodothyronine. Loss of normal
functions of TRs by deletion, reduced expression, or by mutations
could contribute to cancer development, progression and metastasis
(27). Our data showing a marked
diminution of TRalpha and TRbeta mRNA expression in PTC tumours in
comparison with non-tumour thyroid tissues and additional decreases
in expression of both TR in PTC with coexisting AIT supports the
hypothesis suggesting that the loss or marked decrease of TR may
contribute to cancer development and progression.
Several case studies have noted the development of
AIT in patients following or within the last few weeks of
isotretinoin treatment (28).
Moreover, retinoid receptor (RAR) and retinoid X receptors (RXR)
subtypes are differentially expressed in thyroid cancer and in
thyroid carcinoma cell lines when compared to non-tumour thyroid
tissue and cells (29,30). A comparison of thyroid tumours and
case-matched normal thyroid tissue confirmed different tumour
expression of RARalpha, RARgamma, and missing or highly significant
decreases of RXRgamma expression in intact thyroid tissue (17,31).
Here, our data demonstrates a similarly marked diminution of
RARalpha and RARgamma mRNA expression in PTC with coexisting AIT
when compared to PTC without AIT. Thus, data on differences in RAR
or RXR subtype expression may have a valuable impact for the
differential diagnosis of thyroid neoplasms (30).
Recent evidence has shown that vitamin D deficiency
could be associated with autoimmune thyroid diseases such as
Hashimoto's thyroiditis and Graves' disease, and there is impaired
vitamin D signalling in thyroid tumours (32). The VDR was found to be expressed in
both normal and malignant thyroid tissue. Moreover, VDR and CYP27B1
expressions were both increased in papillary thyroid cancer when
compared to normal thyroid tissue (reviewed in 26). Our data
clearly demonstrates significant enhancement of the VDR mRNA
expression in PTC with coexisting AIT as compared to PTC without
AIT. The present study shows that malignant cells in PTC express
progesterone receptor (PR), thus opening the door for further
investigations to determine whether those patients could benefit
from hormonal therapy. Oestrogen receptors seem to have a role in
the metastatic process of PTC, as they are expressed more in the
metastatic tumours than in the primary tumours (15).
Type I, iodo-L-thyronine 5′-deiodinase (DIO1) is a
selenoezyme responsible for monodeiodination of L-thyroxine to
biologically active 3,5,3′-triiodothyronine, the cognate TR ligand.
DIO1 activity was found to be decreased in PTC, and it is
conceivable that understanding how deiodinase(s) dysregulation in
thyroid tumour cells affects thyroid hormone signalling would
relate to tumour progression could lead to new antineoplastic
approaches (33). In spite of the
fact that our data corresponded to the referenced findings, no
significant difference has been found in the mRNA expression of
DIO1 between PTC- and PTC+ patients. The Ki-67 protein (also known
as MKi67) is a cellular marker that is strongly associated with
cell proliferation. Our data has clearly shown significantly
increased MKi67 mRNA expression in PTC with coexisting AIT compared
to PTC without AIT, suggesting increased cell proliferation in the
PTC patients with AIT. Insulin-like growth factor-I (IGF-I) is a
protein with mitogenic and anti-apoptotic properties (23), and high circulating concentrations
have been shown to be associated with an increased risk of
developing cancer at several sites, such as breast, prostate, and
colorectal cancer (34). Recently,
it has been suggested that IGF-I concentrations may be positively
associated with risk of differentiated thyroid carcinoma (13). C3 is a central protein of the
complement system, playing a crucial role in the activation of the
complement system. C3 has been found to be overexpressed in
numerous cancer tissues as well as in PTC tissue when compared to
corresponding non-neoplastic tissues (14,35). Our
data clearly demonstrated significant enhancement of C3 mRNA
expression in PTC with coexisting AIT compared to PTC without
AIT.
In conclusion, the expression of investigated
molecular targets by mRNA level clearly demonstrated significant
differences in PTC with coexisting AIT compared to PTC without AIT.
Based on these novel findings, we suggest that AIT is a
predisposing factor to the development of PTC. To better understand
mechanisms underlying this association, further studies at the
molecular level are needed. Consideration of the possibility that
AIT may be a predisposing cause of PTC means that it may be
necessary to study the causes of the high prevalence of AIT and to
investigate whether reduction of exogenous factors that contribute
to the development of thyroid autoimmunity may be of value.
Acknowledgements
The authors would like to thank Professor Kenneth B.
Ain (Thyroid Oncology Program, University of Kentucky Medical
Center, USA) for editing the language of the manuscript.
Funding
The present study was supported by grants from the
APVV grant agency (grant nos. APVV-15-0372 and APVV-0160-11) and
the VEGA grant (grant no. 2/0171/17).
Availability of data and materials
All data generated or analysed during this study are
included in this published article.
Authors' contributions
DM, JP, MG and JB conceived and designed the
experiments. DM and LT conducted the PCR experiments and analysed
the data. KK and KM performed histological analyses. DM, JP and JB
reviewed the final results and drafted the paper.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the St. Elisabeth Cancer Institute in Bratislava,
Slovakia and was conducted according to the principles of the
Declaration of Helsinki. Written informed consent was obtained from
each patient/subject.
Patient consent for publication
Consent for the publication of the clinical and
pathological data was obtained from all patients who were involved
in the present study.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
AIT
|
autoimmune thyroiditis
|
|
COX-2
|
cyclooxy-genase-2
|
|
DIO1
|
type I iodothyronine 5′-deiodinase
|
|
IGF-1
|
insulin-like growth factor-I
|
|
PR
|
progesterone receptor
|
|
PTC
|
papillary thyroid carcinoma
|
|
RAR
|
retinoid receptor
|
|
RXR
|
rexinoid receptor
|
|
TR
|
thyroid hormone receptor
|
|
VDR
|
dihydroxyvitamin D3
receptor
|
References
|
1
|
Bozec A, Lassalle S, Hofman V, Ilie M,
Santini J and Hofman P: The thyroid gland: A crossroad in
inflammation-induced carcinoma? An ongoing debate with new
therapeutic potential. Curr Med Chem. 17:3449–3461. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Muzza M, Degl'Innocenti D, Colombo C,
Perrino M, Ravasi E, Rossi S, Cirello V, Beck-Peccoz P, Borrello MG
and Fugazzola L: The tight relationship between papillary thyroid
cancer, autoimmunity and inflammation: Clinical and molecular
studies. Clin Endocrinol (Oxf). 72:702–708. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Babli S, Payne RJ, Mitmaker E and Rivera
J: Effects of chronic lymphocytic thyroiditis on the
clinicopathological features of papillary thyroid cancer. Eur
Thyroid J. 7:95–101. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ott RA, Calandra DB, McCall A, Shah KH,
Lawrence AM and Paloyan E: The incidence of thyroid carcinoma in
patients with Hashimoto's thyroiditis and solitary cold nodules.
Surgery. 98:1202–1206. 1985.PubMed/NCBI
|
|
5
|
Schäffer A, Palitzsch KD, Seiffarth C,
Hohne HM, Riedhammer FJ, Hofstädter F, Schölmerich J and Rüschoff
J: Coexistent thyroiditis is associated with lower tumour stage in
thyroid carcinoma. Eur J Clin Invest. 28:838–844. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Loh KCH, Greenspan FS, Dong F, Miller TR
and Yeo PPB: Influence of lymphocytic thyroiditis on the prognostic
outcome of patients with papillary thyroid carcinoma. J Clin
Endocrinol Metab. 84:458–463. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shih ML, Lee JA, Hsieh CB, Yu JC, Liu HD,
Kebebew E, Clark OH and Duh QY: Thyroidectomy for Hashimoto's
thyroiditis: Complications and associated cancers. Thyroid.
18:729–734. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fiore E, Rago T, Latrofa F, Provenzale MA,
Piaggi P, Delitala A, Scutari M, Basolo F, Di Coscio G, Grasso L,
et al: Hashimoto's thyroiditis is associated with papillary thyroid
carcinoma: Role of TSH and of treatment with L-thyroxine. Endocr
Relat Cancer. 18:429–437. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vaccarella S, Franceschi S, Bray F, Wild
CP, Plummer M and Dal Maso L: Worldwide thyroid-cancer epidemic?
The increasing impact of overdiagnosis. N Engl J Med. 7:614–617.
2016. View Article : Google Scholar
|
|
10
|
Antonaci A, Consorti F, Mardente S and
Giovannone G: Clinical and biological relationship between chronic
lymphocytic thyroiditis and papillary thyroid carcinoma. Oncol Res.
17:495–503. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Prescott SM and Fitzpatrick FA:
Cyclooxygenase-2 and carcinogenesis. Biochim Biophys Acta.
1470:M69–M78. 2000.PubMed/NCBI
|
|
12
|
Cornetta A, Russell JP, Cunnane M, Keane
WM and Rothstein JL: Cyclooxygenase-2 expression in human thyroid
carcinoma and Hashimoto's thyroiditis. Laryngoscope. 112:238–242.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schmidt JA, Allen NE, Almquist M,
Franceschi S, Rinaldi S, Tipper SJ, Tsilidis KK, Weiderpass E,
Overvad K, Tjønneland A, et al: Insulin-like growth factor-i and
risk of differentiated thyroid carcinoma in the European
prospective investigation into cancer and nutrition. Cancer
Epidemiol Biomarkers Prev. 23:976–985. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yu J, Mai W, Ciu Y and Kong L: Key genes
and pathways predicted in papillary thyroid carcinoma based on
bioinformatics analysis. J Endocrinol Invest. 39:1285–1293. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Eldien MMS, Abdou AG, Rageh T, Abdelrazek
E and Elkholy E: Immunohistochemical expression of ER-α and PR in
papillary thyroid carcinoma. Ecancermedicalscience. 11:7482017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Brierley JD, Gospodarowicz MK and
Wittekind C: TNM classification of malignant tumours8th. Oxford:
Wiley Blackwell. ISBN 9781119263579. 2017
|
|
17
|
Brtko J, Sejnová D, Ondková S and Macejová
D: Malignant Triton tumour exhibits a complete expression pattern
of nuclear retinoid and rexinoid receptor subtypes. Gen Physiol
Biophys. 28:425–427. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Macejová D, Galbavý S, Podoba J, Bialešová
L and Brtko J: mRNA expression pattern of retinoic acid and
retinoid X nuclear receptor subtypes in human thyroid papillary
carcinoma. Oncol Rep. 30:2371–2378. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Franceschi S, Boyle P, Maisonneuve P, La
Vecchia C, Burt AD, Kerr DJ and MacFarlane GJ: The epidemiology of
thyroid carcinoma. Crit Rev Oncog. 4:25–52. 1993.PubMed/NCBI
|
|
20
|
Lai X, Xia Y, Zhang B, Li J and Jiang Y: A
meta-analysis of Hashimoto's thyroiditis and papillary thyroid
carcinoma risk. Oncotarget. 8:62414–62424. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Paolieri F, Salmaso C, Battifora M,
Montagna P, Pesce G, Bagnasco M, Richiusa P, Galluzzo A and
Giordano C: Possible pathogenetic relevance of interleukin-1 beta
in ‘destructive’ organ-specific autoimmune disease (Hashimoto's
thyroiditis). Ann N Y Acad Sci. 876:221–228. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu L and Gudas LJ: Retinoic acid induces
expression of the interleukin-1beta gene in cultured normal human
mammary epithelial cells and in human breast carcinoma lines. J
Cell Physiol. 193:244–252. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ciampolillo A, De Tullio C and Giorgino F:
The IGF-I/IGF-I receptor pathway: Implications in the
pathophysiology of thyroid cancer. Curr Med Chem. 12:2881–2891.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Surmacz E and Bartucci M: Role of estrogen
receptor alpha in modulating IGF-I receptor signaling and function
in breast cancer. J Exp Clin Cancer Res. 23:385–394.
2004.PubMed/NCBI
|
|
25
|
Garcia-Solis P and Aceves C: 5′Deiodinase
in two breast cancer cell lines: Effect of triiodothyronine,
isoproterenol and retinoids. Mol Cell Endocrinol. 201:25–31. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dhiman VK, Bolt MJ and White KP: Nuclear
receptors in cancer-uncovering new and evolving roles through
genomic analysis. Nat Rev Genet. 19:160–174. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim WG and Cheng SY: Thyroid hormone
receptors and cancer. Biochim Biophys Acta. 1830:3928–3936. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nugroho J and Schweiger B: Isotretinoin as
a possible environmental trigger to autoimmunity in genetically
susceptible patients. Case Rep Pediatr. 2017:42076562017.PubMed/NCBI
|
|
29
|
Schmutzler C, Brtko J, Winzer R, Jakobs
TC, Meissner-Weigl J, Simon D, Goretzki PE and Köhrle J: Functional
retinoid and thyroid hormone receptors in human thyroid-carcinoma
cell lines and tissues. Int J Cancer. 76:368–376. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hoftijzer HC, Liu YY, Morreau H, van Wezel
T, Pereira AM, Corssmit EP, Romijn JA and Smit JW: Retinoic acid
receptor and retinoid X receptor subtype expression for the
differential diagnosis of thyroid neoplasms. Eur J Endocrinol.
160:631–638. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Haugen BR, Larson LL, Pugazhenthi U, Hays
WR, Klopper JP, Kramer CA and Sharma V: Retinoic acid and retinoid
X receptors are differentially expressed in thyroid cancer and
thyroid carcinoma cell lines and predict response to treatment with
retinoids. J Clin Endocrinol Metab. 89:272–280. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim D: The role of vitamin D in thyroid
diseases. Int J Mol Sci. 18(pii): E19492017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Casula S and Bianco AC: Thyroid hormone
deiodinases and cancer. Front Endocrinol (Lausanne). 3:742012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen W, Wang S, Tian T, Bai J, Hu Z, Xu Y,
Dong J, Chen F, Wang X and Shen H: Phenotypes and genotypes of
insulin-like growth factor 1, IGF-binding protein-3 and cancer
risk: Evidence from 96 studies. Eur J Hum Genet. 17:1668–1675.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lin K, He S, He L, Chen J, Cheng X, Zhang
G and Zhu B: Complement component 3 is a prognostic factor of
non-small cell lung cancer. Mol Med Rep. 10:811–817. 2014.
View Article : Google Scholar : PubMed/NCBI
|