Malignant tumors have become the second-leading
cause of mortality worldwide, and it has been predicted that the
number of newly diagnosed malignancies will increase to 23.6
million by 2030 (1). The transfer of
tumor cells to distant metastases is the most common cause of
cancer-associated mortality (2). The
bone is a major target for cancer metastasis, second only to the
lungs and liver; it has unique anatomical and physiological
pathological conditions, which facilitate cancer metastasis,
especially from solid tumors (3),
including those of the prostate, breasts, kidneys and lungs
(4). Firstly, tumor cells themselves
have the ability to migrate from the primary lesion to distant
skeletal tissue; they can also accelerate tumor growth, infiltrate
the surrounding tissues and cause distance metastases, which are
all associated with tumor heterogeneity. Secondly, the anatomical
characteristics of the skeletal system are unique. For example, the
red bone marrow allows tumor cells to enter the bloodstream and
remain within the bone marrow tissue (5). Thirdly, affected bones and metastatic
tumor cells cause various types of biological response, including
adhesion molecules produced by tumor cells that bind to trabecular
bone and stromal cells. Finally, tumor cells further produce
angiogenic factors and bone resorption factors (6). In addition, hematological tumors might
directly or indirectly affect the metabolism of bone. However,
these cancer metastases remain incurable, and the 5-year survival
rate of patients with these types of metastasis is significantly
reduced (7). This results in a
series of complications, including severe bone pain, pathological
fractures, hypercalcemia and spinal cord compression, which
severely affects the quality of life and the life expectancy of
patients. Highly specific interactions between disseminating cancer
cells and the bone microenvironment determine the metastatic
process (4,8). The equilibrium between the activity of
bone resorbing osteoclasts and bone-forming osteoblasts is
interrupted by bone metastasis. Due to the abundant blood supply
and special growth microenvironment in bone tissue (9), such permissive environments
(pre-metastatic niche formation) are in favor of metastatic
development (10–12). Cells can communicate with each other
by secreting extracellular vesicles (EVs). These EVs display a
diverse range of sizes. The present review specifically highlights
the role of tumor-derived exosomes (40–100 nm diameter) in bone
metastasis.
Exosomes are small disc-shaped vesicles, with a
diameter of 40–100 nm, that contain mRNAs, miRNAs (13), lipids (14,15) and
proteins (14). Exosomes are
secreted by a wide variety of normal and malignant cells (16), formed by the endosomal network and
released from the cell via the fusion of multi-vesicular bodies
with the plasma membrane (17,18).
Exosomes are characterized by specific markers, including CD9,
CD63, CD81, Alix and TSG101 (13,14). The
role of exosomes in intercellular communication, via the transfer
of proteins (19), bioactive lipids
and miRNAs (20), has been confirmed
in numerous studies (21). Exosomes
are found in almost all body fluids, including the serum (22), saliva (23), breast milk (24), cerebrospinal fluid (25), urine (14) and semen (26). Furthermore, exosomes are largely
found in the tumor microenvironment. Tumor-derived exosomes have
received considerable attention for their role in cancer
progression and metastasis, and previous studies reported that they
serve a pivotal role in cancer growth, development and metastasis
(27–32). A recent study revealed that the
amount and contents of the exosomes secreted by tumor cells are
much larger compared with that of normal cells (33). The variable exosome contents
therefore influence their behavior and strongly modify the entire
microenvironment (34). Exosomes
significantly contribute to the communication between cells and the
subsequent reprogramming of the tumor microenvironment (35). Exosomes primarily promote tumor
metastasis as follows: i) Exosomes secreted by tumor cells directly
open the way for tumor invasion and metastasis (36); ii) tumor cells with high metastatic
potential can promote the invasion and metastasis of tumor cells
themselves, or other relatively low metastatic potential tumor
cells through exosomes (30); iii)
the cross-talk between mesenchymal cells and tumor cells via
exosomes ultimately promotes tumor metastasis (37); and iv) tumor-derived exosomes
regulate mesenchymal cells in the distant metastasis
microenvironment to promote distant colonization and proliferation
(10). Numerous specific tumor
cells, including those of the prostate, lungs and breasts, are more
prone to bone metastasis and have substantial crosstalk with bone
cells in the bone microenvironment (38). Whether tumor-derived exosomes are
involved in interactions between tumor and bone cells, and the
underlying mechanisms of such communication, remain unclear.
Long non-coding RNAs and miRNAs constitute a class
of small (19 to 25-nucleotide) RNAs that serve crucial gene
regulatory roles in humans (39).
Extensive research has revealed that miRNAs have regulatory roles
in a wide range of pathological and physiological processes
(13,40). For example, Van Balkom et al
(41) reported that human
microvascular endothelial cell (EC)-derived exosomes, containing
miR-214, promoted EC migration and angiogenesis. Furthermore,
knockout of miR-214 in ECs resulted in loss of the ability of
EC-derived exosomes to promote migration and angiogenesis. Cui
et al (42) reported that
mouse embryonic osteogenic precursor cells secreted a variety of
Wnt/β-catenin signaling pathways that activate bone marrow
mesenchymal stem cells (BMSCs) and osteogenesis-associated miRNAs
during osteogenic differentiation. Exosomes upregulated the
expression of the osteogenesis-associated genes and promoted the
formation of mineralized nodules. These findings suggest that these
miRNAs can be transferred to effector cells through exosomes to
exert their gene regulatory functions, by enriching certain miRNAs
in the source cells. Kumar and Reddy (20) reported that exosomes secreted by
cells in disease states contain mainly disease-specific or
deregulated miRNAs, and that they can be used as diagnostic
molecules. A previous study also demonstrated that exosomes from
the plasma of patients with various types of cancer present with
distinct miRNA signatures (43).
However, these characteristics do not correspond to those from the
parent tumor cell (44), which
suggests that exosomes selectively release miRNA from tumor cells.
Growing evidence indicates that some exosomes isolated from cancer
patients have distinct miRNA profiles, including those of lung
cancer (45) and breast carcinoma
(46), which suggests that these
miRNAs might be considered as specific diagnostic markers for
patients with cancer.
Over 100 years ago, Stephen Paget proposed the ‘seed
and soil’ doctrine, suggesting that tumor metastasis was not random
and emphasizing the interaction between tumor cells and target
tissues, proposing that cancer cells were like ‘seeds’ and that the
bone microenvironment was like the ‘soil’ (47). The environment provides the necessary
nutritional support for cancer cells, which have an affinity with
the bone microenvironment (48).
Tumor invasion into the bone is associated with the recruitment of
osteoclasts and osteoblasts, resulting in the release of growth
factors that accumulate in the bone matrix. This phenomenon
eventually induces positive feedback for further tumor growth, and
can be considered as a ‘vicious circle’ of bone metastasis
(49,50). Simultaneously, bone marrow also
serves as a repository for dormant tumor cells that are resistant
to chemotherapy, and these cells can then be transferred to the
bone or other organs (51,52).
Normal bone homeostasis depends on osteoblastic bone
formation and osteoclastic bone resorption (53). Bone metastasis is a complex cascade
of processes (54). Firstly, tumor
cells have a tendency to travel into the bone through specific
migration and invasion processes. Secondly, these tumor cells gain
bone-like properties and reach the bone marrow. Finally, tumor
cells interact with osteoclasts and osteoblasts. This interaction
determines whether subsequent bone metastases become osteolytic or
osteogenic. Clinically, 65 to 70% patients with bone metastases
exhibit osteolytic metastasis. Previous studies reported that
tumor-derived microvesicles, known as exosomes, facilitate the
initial communication between the primary tumor and the metastatic
site (43,55). Cancer cell metastasis to bone tissue
results in osteolytic destruction. This phenomenon is not only
caused by the direct effect of cancer cells on bone cells, but also
through the secretion of cytokines that interact with the bone
microenvironment, which results in osteoclast activation and
subsequent bone destruction (41).
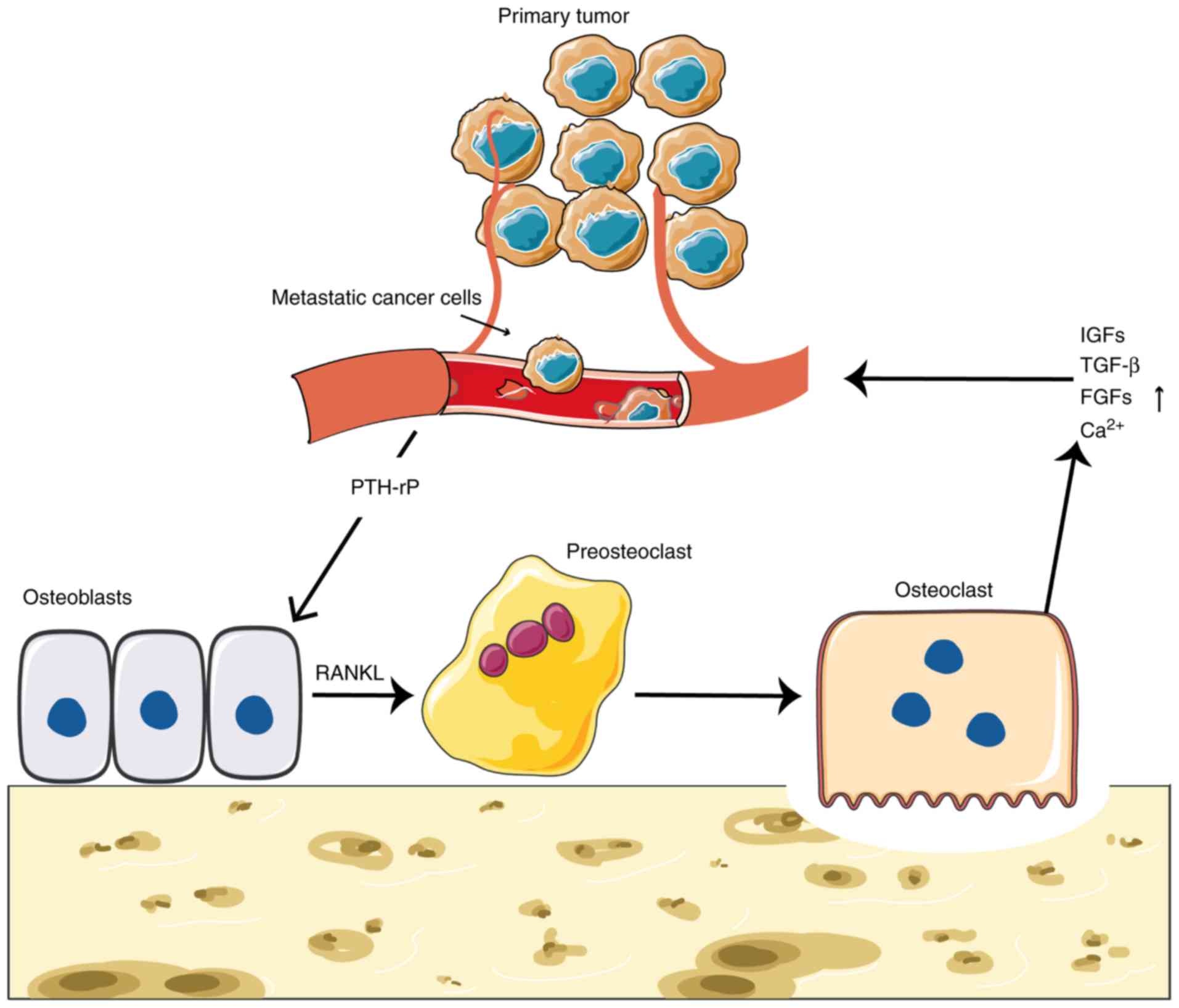
This vicious cycle between cancer cells and the bone
microenvironment results in tumor cell proliferation and continuous
bone mass destruction. Receptor activator for nuclear factor-κB
ligand (RANKL), which is a member of the tumor necrosis factor
family, is expressed and released by osteoblasts and BMSCs.
Parathyroid hormone-related protein (PTH-rP) secreted by cancer
cells directly stimulates osteoblasts to secrete RANKL (42). RANKL and macrophage
colony-stimulating factors signaling molecules, which are necessary
for the differentiation of osteoclast precursors into osteoclasts.
Once bone metastasis occurs, cancer cells can secrete cytokines
that enhance RANKL expression, which inhibits osteoblast function
and expression of other tumor-associated cells, including
fibroblasts, immune cells and osteoprotegerin (OPG). As a result,
RANKL enhances osteoclast activity, which leads to bone
destruction, and causes increases in the levels of insulin-like
growth factors (IGFs), transforming growth factor-β (TGF-β),
fibroblast growth factors (FGFs) and other cytokines released in
the bone matrix that further stimulate tumor growth (56). In addition, osteolytic lesions
increased extracellular calcium (Ca2+) concentration,
which stimulates PTH-rP secretion, resulting in increased
osteoclast activity and the formation of the aforementioned vicious
cycle (57).
Osteoblastic metastases are characterized by
increased pathological osteogenesis. These new bones do not have
the function of normal bone and destroy the normal structure of
healthy bone. Vascular endothelial growth factor (VEGF),
platelet-derived growth factor (PDGF) and endothelin-1 (ET-1) are
secreted by tumor cells themselves, which can stimulate osteoblast
proliferation (58–60). ET-1 serves an important role in the
formation of bone metastases in prostate cancer. This protein has a
dual role in stimulating proliferation and activation of prostate
cancer cells and osteoblasts (61).
In addition, ET-1 stimulates not only the growth of prostate cancer
cells but also the response to growth factors, including IGF and
PDGF (Fig. 1) (62).
The morbidity and mortality rates of bone metastatic
carcinoma are persistently high, which severely affects the
survival time and quality of life of patients with bone metastases
(63). Targeted therapy for bone
metastasis aims to reduce or delay the occurrence of
bone-associated events, including pathological fracture and bone
pain, therefore improving the quality of life of patients and
extending their survival time. Advances in the therapeutic options
for the treatment of bone disease include the use of
bisphosphonates (64), denosumab
(65) or RANKL-antibodies that
target osteoclastogenesis, which have significantly reduced the
complications of bone metastasis and offered good clinical effects
(66–68). However, the overall survival time of
patients with bone metastases has not significantly improved with
these treatments (69). Inhibiting
the occurrence of bone metastases, in particular in patients with
extremely severe bone pain, therefore remains a major challenge for
clinicians.
The ‘pre-metastatic niche’ is a supportive
microenvironment providing nutritional supplies for tumor cells
before they metastasize from the primary organ to the distal organ
(70). It has been demonstrated that
BMSCs are crucial for the generation of an appropriate
microenvironment for the primary tumor, and for the development of
metastasis (71), through the
process of pre-metastatic niche formation (72,73).
Previous studies have demonstrated that the serum of patients with
cancer contains high exosome levels, which are positively
correlated with the malignant behavior of the cancer (74–77).
Tumor cells affect surrounding cells through direct contact,
paracrine secretion, self-secretion, and by direct cross-talk via
exosomes. This newly discovered cell interaction via exosomes
serves an important role in tumor metastasis and invasion (78). Although some secreted factors recruit
BMSCs to both the primary tumor and the pre-metastatic niche
(79–81), studies about the role of exosomes in
bone metabolism and the bone microenvironment have been limited
until recently. Thanks to the emergence of fluorescence
exosome-labeling technology and the determination of specific
markers of exosomes, the research on the association between
exosomes and endothelial cells, angiogenesis and metastatic
promotion has made significant advances (70). Methods for identification and
characterization of exosomes include the observation of morphology
by transmission electron microscopy, the measurement of diameter by
dynamic light scattering, and the analysis of characteristic
surface marker proteins by flow cytometry or western blot analysis.
Furthermore, fluorescent dye kits for general cell or exosome
membrane labeling, such as green fluorescent dyes PKH67 (14) or red fluorescent dyes PKH26 (82) (both Sigma-Aldrich; Merck KGaA) can be
used to label exosomes in vitro.
1,1-dioctadecyl-3,3,3′3′-tetramethylindotricarbocyanine-iodide
(83) fluorescent dye can be used to
label exosomes in vivo. A previous study reported that the
systemic delivery of fluorescent exosomes from metastatic B16F10
melanoma cells localizes to the lungs and other tissues, including
the bone (84). Tumor-derived
exosomes are important mediators of tumorigenesis that are able to
educate stem cells for neoplastic transformation and tumor
metastasis (50). Exosomes from
highly metastatic melanomas have been reported to increase the
in vivo metastatic behavior of primary tumors via
permanently ‘educating’ bone marrow progenitors, via MET receptor
tyrosine kinase upregulation in bone marrow cells. Notably, this
reprogramming effect of exosomes on BMSCs is enduring, which may
explain how tumors that have been dormant for decades suddenly
develop metastatic disease (84).
This research may help to clarify the ‘seed and soil’ hypothesis,
and to determine the mechanism of organ-specific transfer theory
(47). In the ‘seed and oil’ theory,
exosomes may be the real seeds of cancer. Hoshino et al
(85) revealed that tumor exosome
integrins can establish a pre-metastasis microenvironment by
organ-specific colonization, and that they can therefore determine
organ-specific cancer metastasis. In this study, ~10 different
tumors were analyzed and the levels of ~1,000 proteins in the
exosomes were determined, looking for key proteins that could be a
special ‘zip code’. Exosomes can be considered as ‘signal vessels’,
and integrins, which are closely associated with cancer in the lung
and liver metastasis, are present at the surface of exosomes. This
special ‘labeling’ of exosomes may allow them to enter specific
organs and continuously accumulate to further promote metastasis.
This study also demonstrated that, when treated with lung
metastatic tumor-derived exosomes (such as those from breast cancer
cells), metastatic tumor cells that are prone to metastasize to the
bone are no longer transferred to this region, but are instead
redirected to the lungs. This finding suggests that the metastatic
characteristics of tumor cells are not autonomous and are
influenced by external factors. In addition, exosomes that target
different organs have distinctive cell adhesion receptor proteins
and cell-surface integrins. Exosomes have a tendency to enter
organs with large numbers of ligands corresponding to their surface
integrins. In conclusion, exosomes appear to serve a crucial role
in the establishment of the pre-metastatic niche. These findings
provide some directions for the identification of novel anticancer
targets in the later stage, and for the development of novel
anticancer therapies.
Recently, there has been growing interest in the
cell-cell communication roles of exosomes in cancer. Tumor-derived
exosomes serve a crucial role in cancer survival, apoptosis,
invasion, angiogenesis and resistance to chemotherapy; they are
also involved in the establishment of the metastatic niche
(86). Numerous specific tumor
cells, including those of the prostate, lungs and breasts, are
prone to bone metastasis and have substantial cross-talk with bone
cells in the bone microenvironment (Fig.
2).
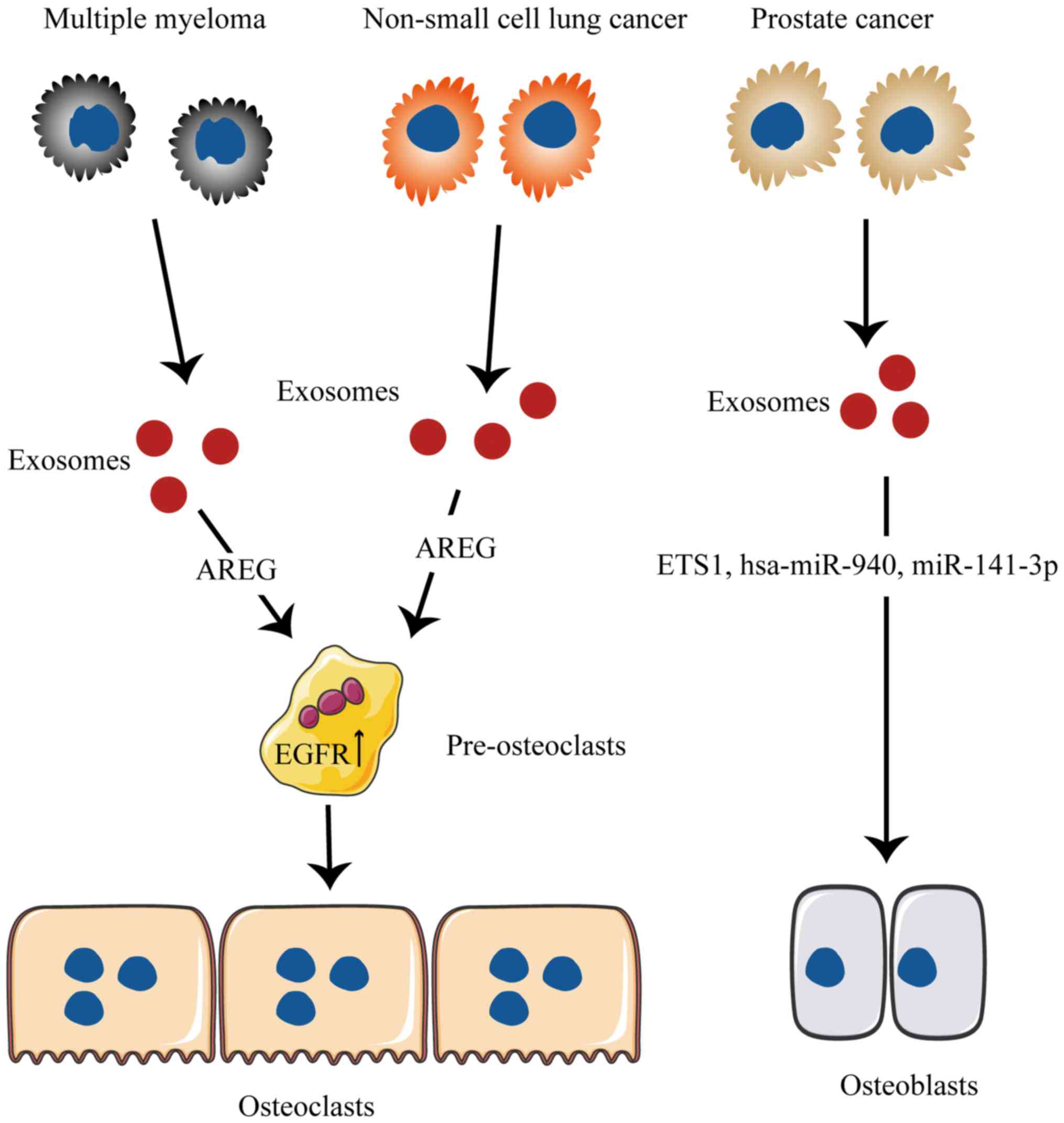
NSCLC cells release factors that alter bone
remodeling and increase osteoclast activity through a shift in the
normal balance of RANKL and OPG (103). In a study by Taverna et al
(104), it was demonstrated that
NSCLC-derived exosomes that contain AREG could induce EGFR pathway
activation in pre-osteoclasts through activation of EGFR
phosphorylation, which subsequently causes the increased expression
of RANKL. This phenomenon induces the increase of MMP9 and TRAP
expression, which triggers a vicious cycle in osteolytic bone
metastasis. A previous study by Valencia et al (105) demonstrated that miR-192 elicits
pleiotropic functions that cooperatively attenuate osseous
metastasis. This alters the cargo of cancer cell-derived exosomes
via the overexpression of a single anti-angiogenic miRNA (miR-192),
and represses the tumor-induced angiogenesis, which leads to a
reduction in the number of bone metastatic lesions in mice.
Targeting one or more miRNAs may therefore represent a potentially
beneficial strategy to block the metastatic process. However,
changing the miRNA-cargo content in exosomes could represent a
novel mechanism that may have a large positive impact on bone
metastases.
The ability of malignant blood cells to transform
the blood microenvironment niche remains currently unknown. Kumar
et al (106) recently
revealed a novel mode of niche transformation. The study
demonstrated that acute leukemia cells are able to change the
hematopoietic microenvironment, and can transform a normal niche
into a malignant niche through the transfer of exosomes [via
dickkopf WNT signaling pathway inhibitor 1 (DKK1) gene], which
provides a suitable environment for their proliferation.
Furthermore, the grafting or injection of AML-derived exosomes can
increase mesenchymal progenitor cells and block osteogenesis and
bone formation in the body. In addition, they can accelerate AML
cell proliferation. Conversely, AML-derived exosomes can be
destroyed by the targeted inhibition of Rab27a, which can
significantly delay the development of leukemia (106). Since DKK1 is a normal hematopoietic
and osteogenic inhibitory factor, subsequent studies demonstrated
that AML-derived exosomes stimulate DKK1 to cause osteocyte loss
(106). Targeting exosomes may
therefore represent a novel strategy in cancer therapy. Effective
inhibition of the hematopoietic microenvironment may be an
important new direction to control the growth of malignant blood
cells.
Together, these studies demonstrated that
tumor-derived exosomes serve a role in the cross-talk between
osteoclasts and tumor cells, which highlights the importance of
exosome cargo in cancer regulation. Furthermore, these findings
significantly enhance our understanding of intercellular
communication in bone metastasis by demonstrating that tumor cells
release biologically active exosomes that are responsible inside
the metastatic niche for the recruitment, migration and
differentiation of osteoclast precursors through re-expression of
miRNA inhibition. In addition, these studies highlight the role of
cargo contained in tumor-derived exosomes in osteoclast
differentiation, which may allow the development of novel
therapeutic strategies to inhibit the fatal attraction between
cancer and bone. The roles of the different tumor-derived exosomes
in bone constituting development and progression are summarized in
Table I. Breast cancer can also
easily translocate into the bones (107); however, the role of breast cancer
cell-derived exosomes in bone metastasis has not yet been
reported.
Bone microenvironment facilitates tumor-induced bone
destruction, and reduction of bone mass and strength. Tumor cells
have developed numerous mechanisms to counteract the effects of
chemotherapy drugs and prevent their elimination from the organism.
One of these mechanisms involves highly specific exosomes. Exosomes
serve a crucial role in the regulation of the local
microenvironment surrounding the tumor and in cell-cell
communication. However, the components of cell-derived exosomes
require further investigation. The underlying mechanisms of
tumor-derived exosomes in bone metastasis are not yet fully
understood.
Although there is debate among experts, in general,
tumor-derived exosomes have more deleterious effects than
beneficial effects as tumor-secreted vesicles. The exosomes can
stimulate tumor growth and development, and promote the process of
bone metastasis. The role of exosomes in cancer progression and
metastasis has drawn increasing attention, with studies essentially
focused on their potential role as biomarkers and targets. Emerging
evidence on exosome functions in bone metastasis may allow the
discovery of novel ways to treat bone metastases. Overall, the
results of therapies focusing on tumor-derived exosomes are
discouraging. These exosomes usually carry numerous tumor activator
molecules, which subsequently stimulate tumor growth and
metastasis, induce host immunosuppression. Thus, the inhibition of
exosome secretion may play a role in anticancer therapy.
Tumor-derived exosomes therefore lead to cancer treatment failure,
and the elimination of these exosomes seems to be applicable to the
tumor and its metastatic treatment.
Tumor metastasis mechanisms are very complex and
involve factors such as tumor cells, osteoblasts, osteoclasts and
the bone microenvironment. Positive outcomes of anticancer
treatment are therefore difficult to obtain. Further research on
bone metastasis mechanisms will thus establish new experimental
models, which could lead to more investigation on
metastasis-associated factors, including exosomes and metastasis
mechanisms. However, certain issues remain and need to be solved in
the future, including the functions of components tumor-derived
exosomes, including, RNA and DNA, in determining bone-specific
metastasis, whether inhibiting the secretion of tumor-derived
exosomes can prevent tumor metastasis, in particular bone
metastasis, and other functional damage to the body, and how to
translate future findings into clinical applications. Addressing
these questions will help highlight the underlying mechanisms of
bone metastasis. Future therapeutic strategies may involve the
combination of several drugs that could block multiple targets or
pathways at the same time, in order to improve quality of life,
increase survival time and provide a greater therapeutic benefit
for patients with bone metastases.
Not applicable.
This study was supported by the Fundamental Research
Funds for the Central Universities of Central South University
(grant no. 2018zzts918).
Not applicable.
FXZL and JJL drafted the manuscript. FX, XL, JYZ,
and FW were responsible for the collection of the relevant
literature. LQY designed the outline and revised the manuscript.
All authors have read and approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Bray F, Jemal A, Grey N, Ferlay J and
Forman D: Global cancer transitions according to the Human
Development Index (2008–2030): A population-based study. Lancet
Oncol. 13:790–801. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gupta GP and Massagué J: Cancer
metastasis: Building a framework. Cell. 127:679–695. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mundy GR: Metastasis to bone: Causes,
consequences and therapeutic opportunities. Nat Rev Cancer.
2:584–593. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Weilbaecher KN, Guise TA and McCauley LK:
Cancer to bone: A fatal attraction. Nat Rev Cancer. 11:411–425.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lalle M, De Rosa L, Marzetti L and
Montuoro A: Detection of breast cancer cells in the bone marrow or
peripheral blood: Methods and prognostic significance. Tumori.
86:183–190. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Van Der Pluijm G, Sijmons B, Vloedgraven
H, Deckers M, Papapoulos S and Löwik C: Monitoring metastatic
behavior of human tumor cells in mice with species-specific
polymerase chain reaction: Elevated expression of angiogenesis and
bone resorption stimulators by breast cancer in bone metastases. J
Bone Miner Res. 16:1077–1091. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Van Driel M and Van Leeuwen JP: Cancer and
bone: A complex complex. Arch Biochem Biophys. 561:159–166. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lynch ME and Fischbach C: Biomechanical
forces in the skeleton and their relevance to bone metastasis:
Biology and engineering considerations. Adv Drug Deliv Rev.
79-80:119–134. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Costa-Silva B, Aiello NM, Ocean AJ, Singh
S, Zhang H, Thakur BK, Becker A, Hoshino A, Mark MT, Molina H, et
al: Pancreatic cancer exosomes initiate pre-metastatic niche
formation in the liver. Nat Cell Biol. 17:816–826. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sceneay J, Parker BS, Smyth MJ and Möller
A: Hypoxia-driven immunosuppression contributes to the
pre-metastatic niche. Oncoimmunology. 2:e223552013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sceneay J, Smyth MJ and Möller A: The
pre-metastatic niche: Finding common ground. Cancer Metastasis Rev.
32:449–464. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hu Y, Rao SS, Wang ZX, Cao J, Tan YJ, Luo
J, Li HM, Zhang WS, Chen CY and Xie H: Exosomes from human
umbilical cord blood accelerate cutaneous wound healing through
miR-21-3p-mediated promotion of angiogenesis and fibroblast
function. Theranostics. 8:169–184. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen CY, Rao SS, Ren L, Hu XK, Tan YJ, Hu
Y, Luo J, Liu YW, Yin H, Huang J, et al: Exosomal DMBT1 from human
urine-derived stem cells facilitates diabetic wound repair by
promoting angiogenesis. Theranostics. 8:1607–1623. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Record M, Silvente-Poirot S, Poirot M and
Wakelam MJO: Extracellular vesicles: Lipids as key components of
their biogenesis and functions. J Lipid Res. 59:1316–1324. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Skog J, Würdinger T, van Rijn S, Meijer
DH, Gainche L, Sena-Esteves M, Curry WT Jr, Carter BS, Krichevsky
AM and Breakefield XO: Glioblastoma microvesicles transport RNA and
proteins that promote tumour growth and provide diagnostic
biomarkers. Nat Cell Biol. 10:1470–1476. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sun Y and Liu J: Potential of cancer cell
derived exosomes in clinical application: A review of recent
research advances. Clin Ther. 36:863–872. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yáñezmó M, Siljander PR, Andreu Z, Zavec
AB, Borràs FE, Buzas EI, Buzas K, Casal E, Cappello F, Carvalho J,
et al: Biological properties of extracellular vesicles and their
physiological functions. J Extracell Vesicles. 4:270662015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zeelenberg IS, Ostrowski M, Krumeich S,
Bobrie A, Jancic C, Boissonnas A, Delcayre A, Le Pecq JB,
Combadière B, Amigorena S and Théry C: Targeting tumor antigens to
secreted membrane vesicles in vivo induces efficient antitumor
immune responses. Cancer Res. 68:1228–1235. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kumar S and Reddy PH: Are circulating
microRNAs peripheral biomarkers for Alzheimer's disease? Biochim
Biophys Acta. 1862:1617–1627. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Valadi H, Ekström K, Bossios A, Sjöstrand
M, Lee JJ and Lötvall JO: Exosome-mediated transfer of mRNAs and
microRNAs is a novel mechanism of genetic exchange between cells.
Nat Cell Biol. 9:654–659. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li JJ, Wang B, Kodali MC, Chao C, Kim E,
Patters BJ, Lan L, Kumar S, Wang X, Yue J and Liao FF: In vivo
evidence for the contribution of peripheral circulating
inflammatory exosomes to neuroinflammation. J Neuroinflammation.
15:82018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lässer C, Alikhani VS, Ekström K, Eldh M,
Paredes PT, Bossios A, Sjöstrand M, Gabrielsson S, Lötvall J and
Valadi H: Human saliva, plasma and breast milk exosomes contain
RNA: Uptake by macrophages. J Transl Med. 9:92011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Admyre C, Johansson SM, Qazi KR, Filén JJ,
Lahesmaa R, Norman M, Neve EP, Scheynius A and Gabrielsson S:
Exosomes with immune modulatory features are present in human
breast milk. J Immunol. 179:1969–1978. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Street JM, Barran PE, Mackay CL, Weidt S,
Balmforth C, Walsh TS, Chalmers RT, Webb DJ and Dear JW:
Identification and proteomic profiling of exosomes in human
cerebrospinal fluid. J Transl Med. 10:52012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Vojtech L, Woo S, Hughes S, Levy C,
Ballweber L, Sauteraud RP, Strobl J, Westerberg K, Gottardo R,
Tewari M and Hladik F: Exosomes in human semen carry a distinctive
repertoire of small non-coding RNAs with potential regulatory
functions. Nucleic Acids Res. 42:7290–7304. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hong BS, Cho JH, Kim H, Choi EJ, Rho S,
Kim J, Kim JH, Choi DS, Kim YK, Hwang D and Gho YS: Colorectal
cancer cell-derived microvesicles are enriched in cell
cycle-related mRNAs that promote proliferation of endothelial
cells. BMC Genomics. 10:5562009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kumar B, Garcia M, Murakami JL and Chen
CC: Exosome-mediated microenvironment dysregulation in leukemia.
Biochim Biophys Acta. 1863:464–470. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Suetsugu A, Honma K, Saji S, Moriwaki H,
Ochiya T and Hoffman RM: Imaging exosome transfer from breast
cancer cells to stroma at metastatic sites in orthotopic nude-mouse
models. Adv Drug Deliv Rev. 65:383–390. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kogure T, Lin WL, Yan IK, Braconi C and
Patel T: Intercellular nanovesicle-mediated microRNA transfer: A
mechanism of environmental modulation of hepatocellular cancer cell
growth. Hepatology. 54:1237–1248. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yu S, Cao H, Shen B and Feng J:
Tumor-derived exosomes in cancer progression and treatment failure.
Oncotarget. 6:37151–37168. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Luga V and Wrana JL: Tumor-stroma
interaction: Revealing fibroblast-secreted exosomes as potent
regulators of Wnt-planar cell polarity signaling in cancer
metastasis. Cancer Res. 73:6843–6847. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shao Y, Shen Y, Chen T, Xu F, Chen X and
Zheng S: The functions and clinical applications of tumor-derived
exosomes. Oncotarget. 7:60736–60751. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Corrado C, Raimondo S, Chiesi A, Ciccia F,
De Leo G and Alessandro R: Exosomes as intercellular signaling
organelles involved in health and disease: Basic science and
clinical applications. Int J Mol Sci. 14:5338–5366. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kowal J, Tkach M and Théry C: Biogenesis
and secretion of exosomes. Curr Opin Cell Biol. 29:116–125. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ekström EJ, Bergenfelz C, von Bülow V,
Serifler F, Carlemalm E, Jönsson G, Andersson T and Leandersson K:
WNT5A induces release of exosomes containing pro-angiogenic and
immunosuppressive factors from malignant melanoma cells. Mol
Cancer. 13:882014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Man YG, Stojadinovic A, Mason J, Avital I,
Bilchik A, Bruecher B, Protic M, Nissan A, Izadjoo M, Zhang X and
Jewett A: Tumor-infiltrating immune cells promoting tumor invasion
and metastasis: Existing theories. J Cancer. 4:84–95. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Xue M, Zhuo Y and Shan B: MicroRNAs, long
noncoding RNAs, and their functions in human disease. Methods Mol
Biol. 1617:1–25. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Thomou T, Mori MA, Dreyfuss JM, Konishi M,
Sakaguchi M, Wolfrum C, Rao TN, Winnay JN, Garcia-Martin R,
Grinspoon SK, et al: Adipose-derived circulating miRNAs regulate
gene expression in other tissues. Nature. 542:450–455. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Luga V, Zhang L, Viloria-Petit AM,
Ogunjimi AA, Inanlou MR, Chiu E, Buchanan M, Hosein AN, Basik M and
Wrana JL: Exosomes mediate stromal mobilization of autocrine
Wnt-PCP signaling in breast cancer cell migration. Cell.
151:1542–1556. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
van Balkom BW, de Jong OG, Smits M,
Brummelman J, den Ouden K, de Bree PM, van Eijndhoven MA, Pegtel
DM, Stoorvogel W, Würdinger T and Verhaar MC: Endothelial
cellsrequire miR-214 to secrete exosomes that suppress senescence
and induce angiogenesis in human and mouse endothelial cells.
Blood. 121:3997–4006. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cui Y, Luan J, Li H, Zhou X and Han J:
Exosomes derived from mineralizing osteoblasts promote ST2 cell
osteogenic differentiation by alteration of microRNA expression.
FEBS Lett. 590:185–192. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Henderson MC and Azorsa DO: The genomic
and proteomic content of cancer cellderived exosomes. Front Oncol.
2:382012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Nolte-'t Hoen EN, Buermans HP, Waasdorp M,
Stoorvogel W, Wauben MH and 't Hoen PA: Deep sequencing of RNA from
immune cell-derived vesicles uncovers the selective incorporation
of small non-coding RNA biotypes with potential regulatory
functions. Nucleic Acids Res. 40:9272–9285. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Cazzoli R, Buttitta F, Di Nicola M,
Malatesta S, Marchetti A, Rom WN and Pass HI: microRNAs derived
from circulating exosomes as noninvasive biomarkers for screening
and diagnosing lung cancer. J Thorac Oncol. 8:1156–1162. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Corcoran C, Friel AM, Duffy MJ, Crown J
and O'Driscoll L: Intracellular and extracellular microRNAs in
breast cancer. Clin Chem. 57:18–32. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Paget S: The distribution of secondary
growths in cancer of the breast. 1889 Cancer Metastasis Rev.
8:98–101. 1889.
|
|
48
|
Brennan MF and Ekman L: Metabolic
consequences of nutritional support of the cancer patient. Cancer.
54 (11 Suppl):S2627–S2634. 1984. View Article : Google Scholar
|
|
49
|
Guise TA, Mohammad KS, Clines G, Stebbins
EG, Wong DH, Higgins LS, Vessella R, Corey E, Padalecki S, Suva L
and Chirgwin JM: Basic mechanisms responsible for osteolytic and
osteoblastic bone metastases. Clin Cancer Res. 12:6213s–6216s.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kingsley LA, Fournier PG, Chirgwin JM and
Guise TA: Molecular biology of bone metastasis. Mol Cancer Ther.
6:2609–2617. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Pantel K, Müller V, Auer M, Nusser N,
Harbeck N and Braun S: Detection and clinical implications of early
systemic tumor cell dissemination in breast cancer. Clin Cancer
Res. 9:6326–6334. 2003.PubMed/NCBI
|
|
52
|
Aft R, Naughton M, Trinkaus K, Watson M,
Ylagan L, Chavez-Macgregor M, Zhai J, Kuo S, Shannon W, Diemer K,
et al: Effect of zoledronic acid on disseminated tumour cells in
women with locally advanced breast cancer: An open label,
randomised, phase 2 trial. Lancet Oncol. 11:421–428. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Chen H, Senda T and Kubo KY: The osteocyte
plays multiple roles in bone remodeling and mineral homeostasis.
Med Mol Morphol. 48:61–68. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Nguyen DX, Bos PD and Massagué J:
Metastasis: From dissemination to organ-specific colonization. Nat
Rev Cancer. 9:274–284. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Saleem SN and Abdel-Mageed AB:
Tumor-derived exosomes in oncogenic reprogramming and cancer
progression. Cell Mol Life Sci. 72:1–10. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Clézardin P: The role of
RANK/RANKL/osteoprotegerin (OPG) triad in cancer-induced bone
diseases: Physiopathology and clinical implications. Bull Cancer.
98:837–846. 2011.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Sanders JL, Chattopadhyay N, Kifor O,
Yamaguchi T, Butters RR and Brown EM: Extracellular calcium-sensing
receptor expression and its potential role in regulating
parathyroid hormone-related peptide secretion in human breast
cancer cell lines. Endocrinology. 141:4357–4364. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Siveen KS, Prabhu K, Krishnankutty R,
Kuttikrishnan S, Tsakou M, Alali FQ, Dermime S, Mohammad RM and
Uddin S: Vascular endothelial growth factor (VEGF) signaling in
tumour vascularization: Potential and challenges. Curr Vasc
Pharmacol. 15:339–351. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Heldin CH, Lennartsson J and Westermark B:
Involvement of platelet-derived growth factor ligands and receptors
in tumorigenesis. J Intern Med. 283:16–44. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Chirgwin JM, Mohammad KS and Guise TA:
Tumor-bone cellular interactions in skeletal metastases. J
Musculoskeletal Neuronal Interact. 4:308–318. 2004.
|
|
61
|
Chang AC, Chen PC, Lin YF, Su CM, Liu JF,
Lin TH, Chuang SM and Tang CH: Osteoblast-secreted WISP-1 promotes
adherence of prostate cancer cells to bone via the VCAM-1/integrin
α4β1 system. Cancer Lett. 426:47–56. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
D'Oronzo S, Brown J and Coleman R: The
role of biomarkers in the management of bone-homing malignancies. J
Bone Oncol. 9:1–9. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Berruti A, Libè R, Laganà M, Ettaieb H,
Sukkari MA, Bertherat J, Feelders RA, Grisanti S, Cartry J,
Mazziotti G, et al: Morbidity and mortality of bone metastases in
advanced adrenocortical carcinoma: A multicenter retrospective
study. Eur J Endocrinol. 180:311–320. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Liao EY, Zhang ZL, Xia WB, Lin H, Cheng Q,
Wang L, Hao YQ, Chen DC, Tang H, Peng YD, et al: Clinical
characteristics associated with bone mineral density improvement
after 1-year alendronate/vitamin d3 or calcitriol treatment:
Exploratory results from a phase 3, randomized, controlled trial on
postmenopausal osteoporotic women in China. Medicine (Baltimore).
97:e116942018. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Stopeck AT, Lipton A, Body JJ, Steger GG,
Tonkin K, de Boer RH, Lichinitser M, Fujiwara Y, Yardley DA,
Viniegra M, et al: Denosumab compared with zoledronic acid for the
treatment of bone metastases in patients with advanced breast
cancer: A randomized, double-blind study. J Clin Oncol.
28:5132–5139. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Body JJ, Lipton A, Gralow J, Steger GG,
Gao G, Yeh H and Fizazi K: Effects of denosumab in patients with
bone metastases with and without previous bisphosphonate exposure.
J Bone Miner Res. 25:440–446. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Fizazi K, Lipton A, Mariette X, Body JJ,
Rahim Y, Gralow JR, Gao G, Wu L, Sohn W and Jun S: Randomized phase
II trial of denosumab in patients with bone metastases from
prostate cancer, breast cancer, or other neoplasms after
intravenous bisphosphonates. J Clin Oncol. 27:1564–1571. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Hirbe A, Morgan EA, Uluçkan O and
Weilbaecher K: Skeletal complications of breast cancer therapies.
Clin Cancer Res. 12:6309s–6314s. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Fizazi K, Carducci M, Smith M, Damião R,
Brown J, Karsh L, Milecki P, Shore N, Rader M, Wang H, et al:
Denosumab versus zoledronic acid for treatment of bone metastases
in men with castration-resistant prostate cancer: A randomised,
double-blind study. Lancet. 377:813–822. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Zeng Z, Li Y, Pan Y, Lan X, Song F, Sun J,
Zhou K, Liu X, Ren X, Wang F, et al: Cancer-derived exosomal
miR-25-3p promotes pre-metastatic niche formation by inducing
vascular permeability and angiogenesis. Nat Commun. 9:53952018.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Sethi N and Kang Y: Unravelling the
complexity of metastasis-molecular understanding and targeted
therapies. Nat Rev Cancer. 11:735–748. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Kaplan RN, Riba RD, Zacharoulis S, Bramley
AH, Vincent L, Costa C, MacDonald DD, Jin DK, Shido K, Kerns SA, et
al: VEGFR1-positive haematopoietic bone marrow progenitors initiate
the pre-metastatic niche. Nature. 438:820–827. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Psaila B and Lyden D: The metastatic
niche: Adapting the foreign soil. Nat Rev Cancer. 9:285–293. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
O'Brien K, Rani S, Corcoran C, Wallace R,
Hughes L, Friel AM, McDonnell S, Crown J, Radomski MW and
O'Driscoll L: Exosomes from triple-negative breast cancer cells can
transfer phenotypic traits representing their cells of origin to
secondary cells. Eur J Cancer. 49:1845–1859. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Kahlert C and Kalluri R: Exosomes in tumor
microenvironment influence cancer progression and metastasis. J Mol
Med (Berl). 91:431–437. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Logozzi M, De Milito A, Lugini L, Borghi
M, Calabrò L, Spada M, Perdicchio M, Marino ML, Federici C, Iessi
E, et al: High levels of exosomes expressing CD63 and caveolin-1 in
plasma of melanoma patients. PLoS One. 4:e52192009. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Tavoosidana G, Ronquist G, Darmanis S, Yan
J, Carlsson L, Wu D, Conze T, Ek P, Semjonow A, Eltze E, et al:
Multiple recognition assay reveals prostasomes as promising plasma
biomarkers for prostate cancer. Proc Natl Acad Sci USA.
108:8809–8814. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Steinbichler TB, Dudás J, Riechelmann H
and Skvortsovab II: The role of exosomes in cancer metastasis.
Semin Cancer Biol. 44:170–181. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Gao D, Nolan D, McDonnell K, Vahdat L,
Benezra R, Altorki N and Mittal V: Bone marrow-derived endothelial
progenitor cells contribute to the angiogenic switch in tumor
growth and metastatic progression. Biochim Biophys Acta.
1796:33–40. 2009.PubMed/NCBI
|
|
80
|
Erler JT, Bennewith KL, Cox TR, Lang G,
Bird D, Koong A, Le QT and Giaccia AJ: Hypoxia-induced lysyl
oxidase is a critical mediator of bone marrow cell recruitment to
form the premetastatic niche. Cancer Cell. 15:35–44. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Hiratsuka S, Watanabe A, Aburatani H and
Maru Y: Tumour-mediated upregulation of chemoattractants and
recruitment of myeloid cells predetermines lung metastasis. Nat
Cell Biol. 8:1369–1375. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Han C, Zhou J, Liu B, Liang C, Pan X,
Zhang Y, Zhang Y, Wang Y, Shao L, Zhu B, et al: Delivery of miR-675
by stem cell-derived exosomes encapsulated in silk fibroin hydrogel
prevents aging-induced vascular dysfunction in mouse hindlimb.
Mater Sci Eng C Mater Biol Appl. 99:322–332. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Wiklander OP, Nordin JZ, O'Loughlin A,
Gustafsson Y, Corso G, Mäger I, Vader P, Lee Y, Sork H, Seow Y, et
al: Extracellular vesicle in vivo biodistribution is determined by
cell source, route of administration and targeting. J Extracell
Vesicles. 4:263162015. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Peinado H, Alečković M, Lavotshkin S,
Matei I, Costa-Silva B, Moreno-Bueno G, Hergueta-Redondo M,
Williams C, García-Santos G, Ghajar C, et al: Melanoma exosomes
educate bone marrow progenitor cells toward a pro-metastatic
phenotype through MET. Nat Med. 18:883–891. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Hoshino A, Costa-Silva B, Shen TL,
Rodrigues G, Hashimoto A, Tesic Mark M, Molina H, Kohsaka S, Di
Giannatale A, Ceder S, et al: Tumour exosome integrins determine
organotropic metastasis. Nature. 527:329–335. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Peinado H, Lavotshkin S and Lyden D: The
secreted factors responsible for pre-metastatic niche formation:
Old sayings and new thoughts. Semin Cancer Biol. 21:139–146. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Hameed A, Brady JJ, Dowling P, Clynes M
and O'Gorman P: Bone disease in multiple myeloma: Pathophysiology
and management. Cancer Growth Metastasis. 7:33–42. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Abdi J, Chen G and Chang H: Drug
resistance in multiple myeloma: Latest findings and new concepts on
molecular mechanisms. Oncotarget. 4:2186–2207. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Rossi M, Pitari MR, Amodio N, Di Martino
MT, Conforti F, Leone E, Botta C, Paolino FM, Del Giudice T,
Iuliano E, et al: miR-29b negatively regulates human osteoclastic
cell differentiation and function: Implications for the treatment
of multiple myeloma-related bone disease. J Cell Physiol.
228:1506–1515. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Heider U, Fleissner C, Zavrski I, Kaiser
M, Hecht M, Jakob C and Sezer O: Bone markers in multiple myeloma.
Eur J Cancer. 42:1544–1553. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Raimondi L, De Luca A, Amodio N, Manno M,
Raccosta S, Taverna S, Bellavia D, Naselli F, Fontana S, Schillaci
O, et al: Involvement of multiple myeloma cell-derived exosomes in
osteoclast differentiation. Oncotarget. 6:13772–13789. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Garimella R, Washington L, Isaacson J,
Vallejo J, Spence M, Tawfik O, Rowe P, Brotto M and Perez R:
Extracellular membrane vesicles derived from 143B osteosarcoma
cells contain pro-osteoclastogenic cargo: A novel communication
mechanism in osteosarcoma bone microenvironment. Transl Oncol.
7:331–340. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Raimondo S, Saieva L, Vicario E, Pucci M,
Toscani D, Manno M, Raccosta S, Giuliani N and Alessandro R:
Multiple myeloma-derived exosomes are enriched of amphiregulin
(AREG) and activate the epidermal growth factor pathway in the bone
microenvironment leading to osteoclastogenesis. J Hematol Oncol.
12:22019. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Deng X, He G, Liu J, Luo F, Peng X, Tang
S, Gao Z, Lin Q, Keller JM, Yang T and Keller ET: Recent advances
in bone-targeted therapies of metastatic prostate cancer. Cancer
Treat Rev. 40:730–738. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Karlsson T, Lundholm M, Widmark A and
Persson E: Tumor cell-derived exosomes from the prostate cancer
cell line TRAMP-C1 impair osteoclast formation and differentiation.
PLoS One. 11:e01662842016. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Inder KL, Ruelcke JE, Petelin L, Moon H,
Choi E, Rae J, Blumenthal A, Hutmacher D, Saunders NA, Stow JL, et
al: Cavin-1/PTRF alters prostate cancer cell-derived extracellular
vesicle content and internalization to attenuate extracellular
vesicle-mediated osteoclastogenesis and osteoblast proliferation. J
Extracell Vesicles. 3:2014. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Shiozawa Y, Pedersen EA, Havens AM, Jung
Y, Mishra A, Joseph J, Kim JK, Patel LR, Ying C, Ziegler AM, et al:
Human prostate cancer metastases target the hematopoietic stem cell
niche to establish footholds in mouse bone marrow. J Clin Invest.
121:1298–1312. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Morrissey C, Lai JS, Brown LG, Wang YC,
Roudier MP, Coleman IM, Gulati R, Vakar-Lopez F, True LD, Corey E,
et al: The expression of osteoclastogenesis-associated factors and
osteoblast response to osteolytic prostate cancer cells. Prostate.
70:412–424. 2010.PubMed/NCBI
|
|
99
|
Itoh T, Ito Y, Ohtsuki Y, Ando M,
Tsukamasa Y, Yamada N, Naoe T and Akao Y: Microvesicles released
from hormone-refractory prostate cancer cells facilitate mouse
pre-osteoblast differentiation. J Mol Histol. 43:509–515. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Ye Y, Li SL, Ma YY, Diao YJ, Yang L, Su
MQ, Li Z, Ji Y, Wang J, Lei L, et al: Exosomal miR-141-3p regulates
osteoblast activity to promote the osteoblastic metastasis of
prostate cancer. Oncotarget. 8:94834–94849. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Hashimoto K, Ochi H, Sunamura S, Kosaka N,
Mabuchi Y, Fukuda T, Yao K, Kanda H, Ae K, Okawa A, et al:
Cancer-secreted hsa-miR-940 induces an osteoblastic phenotype in
the bone metastatic microenvironment via targeting ARHGAP1 and
FAM134A. Proc Natl Acad Sci USA. 115:2204–2209. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Morhayim J, van de Peppel J, Demmers JA,
Kocer G, Nigg AL, van Driel M, Chiba H and van Leeuwen JP:
Proteomic signatures of extracellular vesicles secreted by
nonmineralizing and mineralizing human osteoblasts and stimulation
of tumor cell growth. FASEB J. 29:274–285. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Peng X, Guo W, Ren T, Lou Z, Lu X, Zhang
S, Lu Q and Sun Y: Differential expression of the RANKL/RANK/OPG
system is associated with bone metastasis in human non-small cell
lung cancer. PLoS One. 8:e583612013. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Taverna S, Pucci M, Giallombardo M, Di
Bella MA, Santarpia M, Reclusa P, Gil-Bazo I, Rolfo C and
Alessandro R: Amphiregulin contained in NSCLC-exosomes induces
osteoclast differentiation through the activation of EGFR pathway.
Sci Rep. 7:31702017. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Valencia K, Luis-Ravelo D, Bovy N, Antón
I, Martínez-Canarias S, Zandueta C, Ormazábal C, Struman I, Tabruyn
S, Rebmann V, et al: miRNA cargo within exosome like vesicle
transfer influences metastatic bone colonization. Mol Oncol.
8:689–703. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Kumar B, Garcia M, Weng L, Jung X,
Murakami JL, Hu X, McDonald T, Lin A, Kumar AR, DiGiusto DL, et al:
Acute myeloid leukemia transforms the bone marrow niche into a
leukemia-permissive microenvironment through exosome secretion.
Leukemia. 32:575–587. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Guise TA: Breast cancer bone metastases:
It's all about the neighborhood. Cell. 154:957–959. 2013.
View Article : Google Scholar : PubMed/NCBI
|