Introduction
The tumor microenvironment is closely related to
cancer initiation, progression, and invasion (1). The tumor microenvironment consists of
extracellular matrix (ECM), stromal cells (such as fibroblasts,
myofibroblasts, neuroendocrine cells, adipose cells, immune and
inflammatory cells, blood, and lymphatic vascular networks) and
immune cells (including T and B lymphocytes, natural killer cells,
and tumor-associated macrophages), however, the precise function of
each constituent remains unknown (2). Tumor initiation proceeds with a complex
series of biological changes whereby normal cells acquire
uncontrolled cell growth and resistance to cell death. In
conditions of hypoxia, oxidative stress, and acidosis, the tumor
microenvironment alters cellular metabolism and leads to the
subsequent evolution of malignancies. The overexpression of
oncogenes during tumor growth and progression by stromal stimuli
can affect the aggressiveness of cancer. Cancer-associated
fibroblasts (CAFs) were shown to promote tumor growth and the
invasion of low-invasive cancer cells in xenografted mice (3). Therefore, a complex tissue
microenvironment is necessary for tumor progression and metastasis
(4,5). Research on the tumor microenvironment
is important to identify therapeutic targets and for the diagnosis
of cancer.
Cancer cells that have acquired the ability to
invade infiltrate nearby healthy tissues and spread beyond the
tissue layer. Reports that many kinds of cytokines and chemokines
were involved in every stage of tumorigenesis have suggested that
reciprocal cross-talk between cancer and adjacent stroma is
important for cancer progression (6). The role of platelet-derived growth
factor (PDGF), fibroblast activation protein (FAP), fibroblast
growth factor receptor (FGFR), vitamin D receptor (VDR),
transforming growth factor-β (TGF-β), tumor necrosis factor-α
(TNF-α), interleukin-10 (IL-10), interleukin-12 (IL-12), epidermal
growth factor (EGF), and vascular endothelial growth factor (VEGF)
have been suggested as critical tumor microenvironment factors for
tumor progression (1). In addition,
matrix metalloprotease-2 (MMP-2) from cancer-associated fibroblasts
has been reported to induce epithelial invasion and dis-cohesion of
keratinocytes into collagen (7).
Therefore, analysis of cross-talk between cancer and CAF is
important in understanding the mechanism of cancer invasion.
In this study, we observed the role of CAFs in
cancer invasion as tumor microenvironment and elucidated the
related mechanism. Our results demonstrated that CAFs were critical
inducers of OSCC invasion and that their invasive capacity was
IL-1R-dependent. CAF stimulation results in an increase in
protease, such as ADAM 9 and Kallikrein 11. The results of this
study may be useful in helping to identify the role of CAFs in
cancer invasion and progression and to generate therapeutic
strategies for treating cancers.
Materials and methods
Cell culture
HSC-2 oral squamous cell carcinoma (OSCC) cells was
grown in DMEM/F12 (3:1 ratio) medium supplemented with 10% FBS,
1×10−10 M cholera toxin, 0.4 mg/ml hydrocortisone, 5
µg/ml insulin, 5 µg/ml apo-transferrin, and 2×10−11 M
triiodothronine (T3) in a humidified atmosphere of 5%
CO2 at 37°C. Normal gingival fibroblasts (NF) and
cancer-associated fibroblasts (CAF) were maintained in complete
medium as for HSC-2 cells. Early passage (below passage 10) of the
fibroblasts were subjected to analysis.
Reagents
All reagents used in cell culture were purchased
from Gibco BRL Co. (Rockville, MD, USA). Cholera toxin,
hydrocortisone, insulin, apo-transferrin, T3, and dimethyl
sulfoxide (DMSO) were obtained from Sigma Chemical Co. (St. Louis,
MO, USA). Recombinant human interleukin 1 beta (IL-1β) was
purchased from R&D Systems (Minneapolis, MN, USA). IL-1R
antagonist was purchased from Cayman (Cayman Chemical, Ann Arbor,
MI, USA). GM6001 was purchased from Calbiochem (La Jolla, CA, USA).
Oregon Green 488 gelatin were purchased from Molecular Probes
(Carlsbad, CA, USA).
Invasion assay
8 µm pore sized polycarbonate nucleopore filter
inserts in a 24-well Transwell chamber (Corning Costar, Cambridge,
MA, USA) were coated with Matrigel (30 mg/well; Becton Dickinson,
Lincoln Park, NJ, USA) for 4 h. Cells (5×104 cells) were
added into the upper chamber, and complete medium was added to the
bottom chamber and keep for 48 h at 37°C incubator. Invaded cells
on the lower surface of the Transwell membrane was fixed with
absolute ethanol and noninvasive cells were removed with a cotton
swab. Then invaded cells were stained with hematoxylin. Cells from
five fields were counted under a microscope. The neutralizing
effect of anti-ADAM 9 antibody and anti-Kallikrein 11 antibody on
the invasion activity of CM-CAF was determined by incubating the
cells with CM-CAF after CM-CAF were treated for 1 h with 1 mg/ml
antibody to ADAM 9 (Abcam, Cambridge, MA, USA) and anti-Kallikrein
11 (R&D Systems, Saint Louis, MO, USA).
Immunostaining
The xenograft tumor tissue was used to observe the
expression of IL-1β. Tumor xenograft was established by implatation
of OSCC cells in athymic nude mouse in our previous study (8). Briefly, OSCC cells were injected into
mouse tongue and growth of tumor xenografts was observed for 5
weeks. Tissues were fixed in formalin and processed for paraffin
embedding. Deparaffinized tissue were then rehydrated and conducted
antigen retrieval via autoclave treatment of the sections in 0.01 M
citrate buffer (pH 6.0). After blocking with 10% normal goat serum,
the sections were incubated with a primary antibody at a 1:100
dilution in background reducing diluent (Dako, Carpinteria, CA,
USA). The sections were rinsed with PBS and incubated with
biotinylated anti-mouse/anti-rabbit IgG (H + L) (1:100 dilution in
background reducing diluent), followed exposure to horseradish
peroxidase streptavidin (1:200 dilution in background reducing
diluent). Staining was performed by incubating with
3,3′-diaminobenzidine (DAB) buffer. The sections were
counterstained with hematoxylin, followed by dehydration and
mounting.
Enzyme-linked immunosorbent assay
(ELISA)
The cultured medium from NF and CAF were used as
conditioned medium (CM). Media were then centrifuged, and the IL-1β
level was quantified with the Human IL-1β Quantikine ELISA kit
according to the manufacturer's protocols (R&D System Inc.,
Minneapolis, MN, USA).
ECM degradation
Oregon Green 488 gelatin-coated coverslips were
prepared as described previously (9). Cells (3×103 cells) were
plated on coverslips in 12-well plates and cultured for 16 h. Cells
were fixed with 4% paraformaldehyde followed by permeabilization
with 0.5% Triton X-100/PBS and stained for nuclei with DAPI. Areas
of matrix degradation was identified by a loss fluorescence using
an EVOS FL monochrome microscope (ThermoFisher Scientific, Waltham,
MA, USA).
Protease array
Cells were cultured in 1% medium with or without
IL-1β and harvested after 24 h. Harvested medium was centrifuged in
5,000 rpm for 10 min and used as conditioned media (CM) for
protease array. The protein concentration of the CM was normalized
by dilution with serum-free media. Then CM was incubated for 24 h
with the Proteome Profiler Human Protease Array membrane (R&D
Systems, Saint Louis, MO, USA). The relative expression levels of
the proteases were determined according to the manufacturer's
protocol and signal intensities were compared using ImageJ software
program.
Statistical analysis
The statistical analysis was conducted using InStat™
statistical software (GraphPad Software, San Diego, CA, USA). The
statistical significance of differences between groups was analyzed
using a one-way ANOVA with a Tukey's post-hoc test. P-values
of <0.05 were considered significant.
Results
IL-1β increases cancer cell
invasion
To investigate the effect of IL-1β in cancer
invasion, a Matrigel-coated Transwell invasion assay was performed
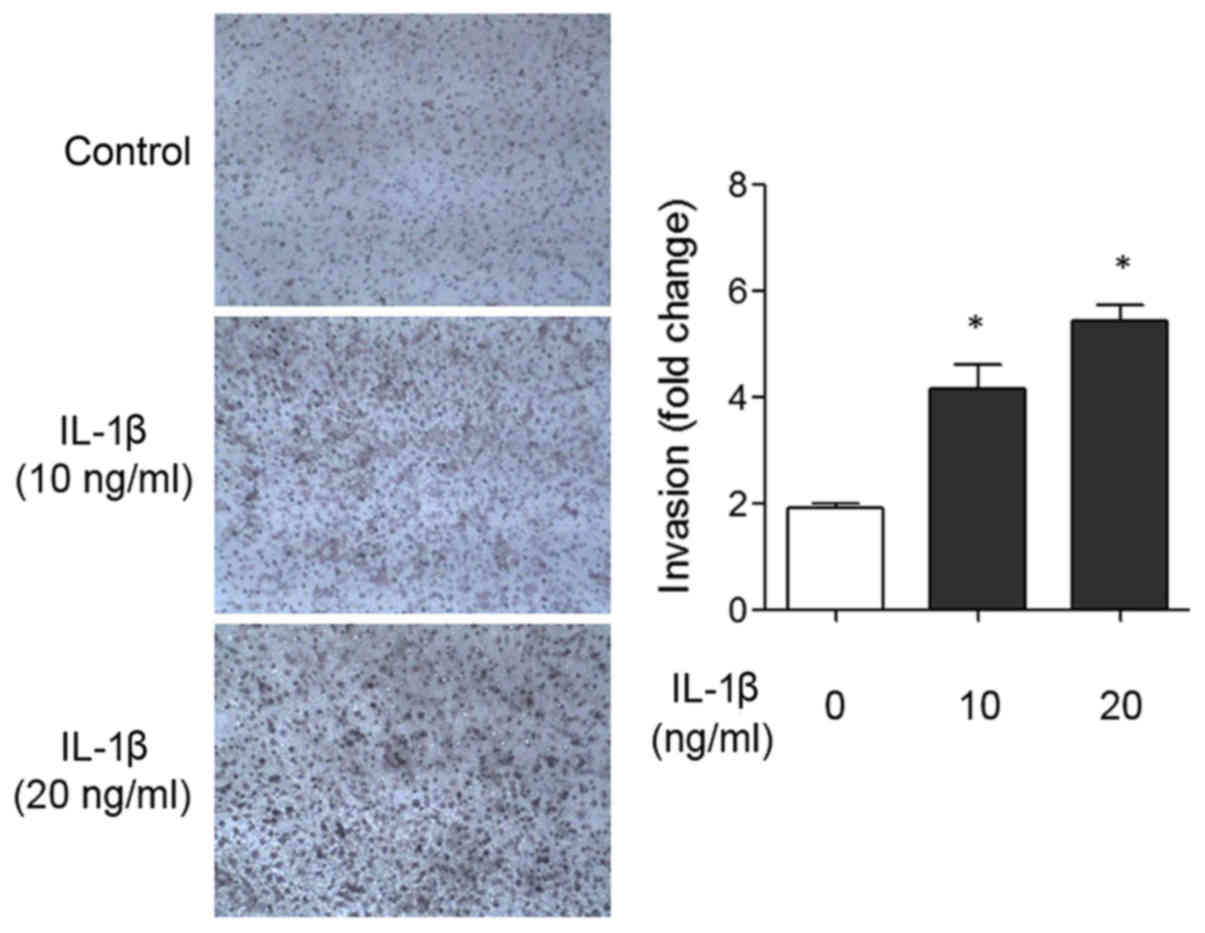
with 20 ng/ml IL-1β for 48 hours. As shown in Fig. 1, recombinant IL-1β treatment incresed
invasion 2.75-fold compared to the control. To investigate the
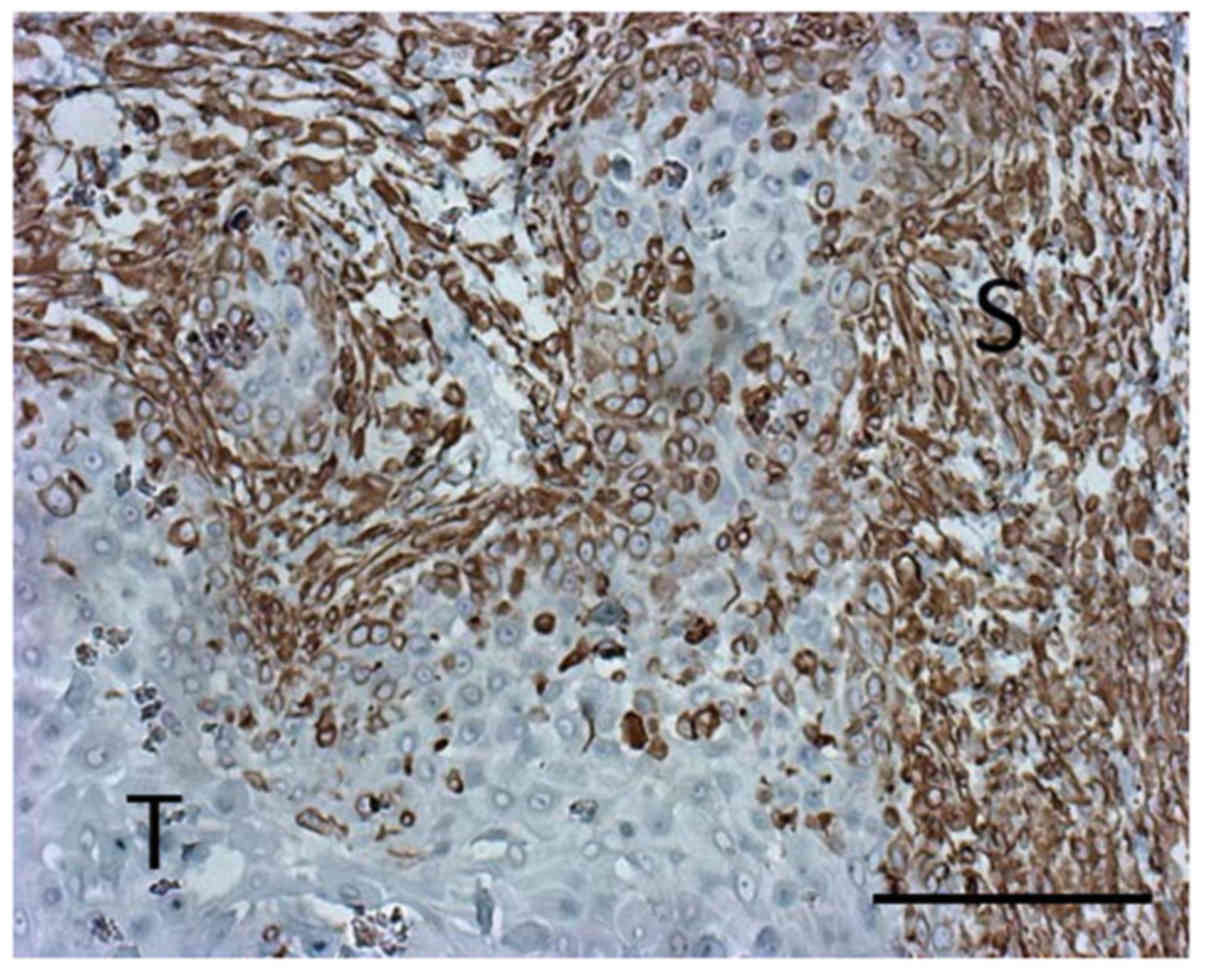
expression of IL-1β in the tumor, we used a xenofrafted tongue
tumor specimen established in our previous experiments (8). IL-1β expression was located in the
cytoplasm of the cancer cells and cancer-associate fibroblasts.
Relatively high IL-1β expression was detected in the regional
stroma (Fig. 2).
IL-1β from CAFs increases matrix
degradation
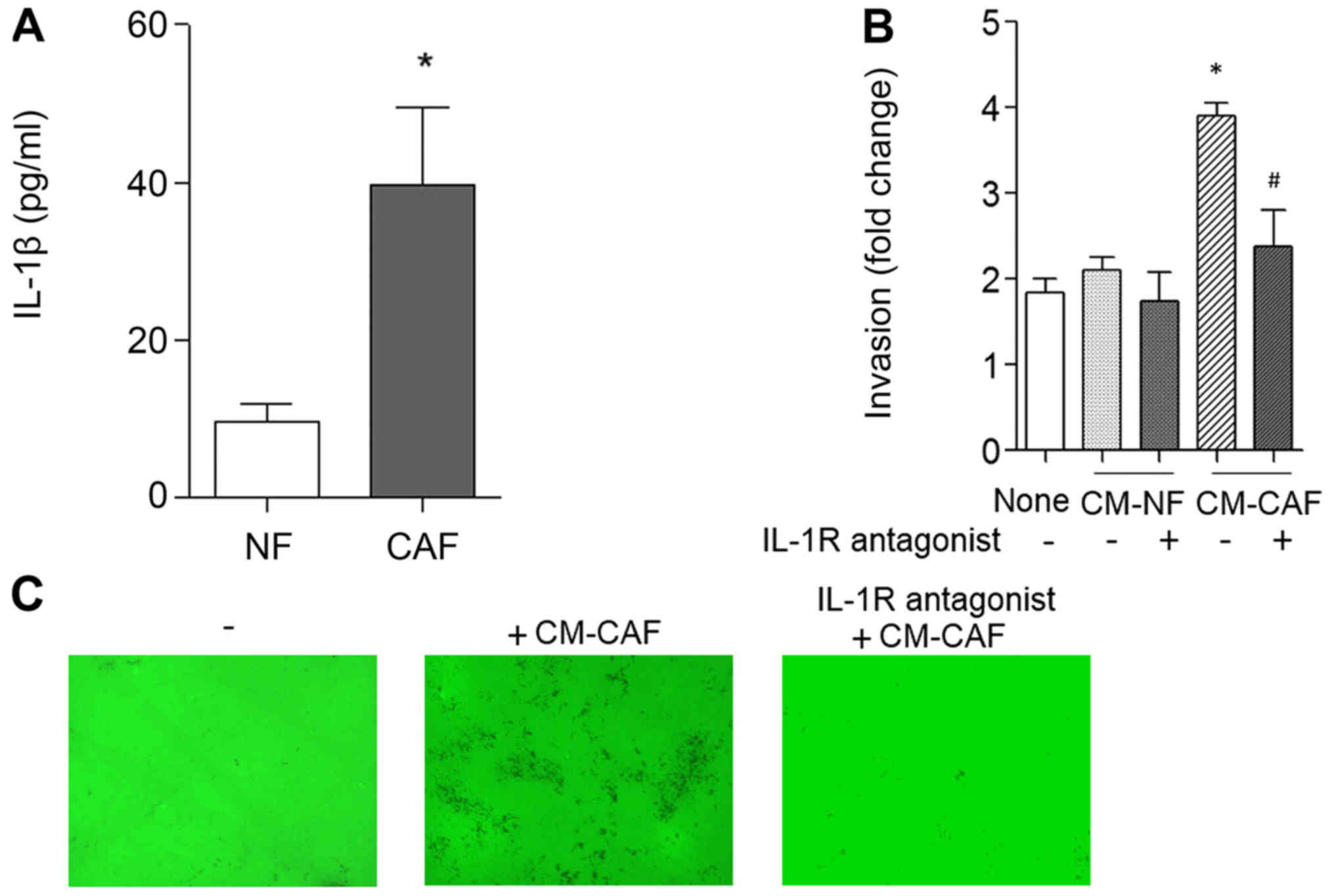
The level of IL-1β protein was measured in normal
gingival fibroblasts (NFs) and cancer-associated fibroblast (CAFs)
using a Human IL-1β Quantikine ELISA kit. As shown in Fig. 3A, IL-1β expression was 3.8-fold
higher in CAF than in NF. Conditioned medium (CM) from NF and CAF
were used for Transwell invasion assay, instead of complete medium.
Unlike NF, CAF increased cancer inasion by 2.1-fold (Fig. 3B). The increased invasion activity by
the CM of CAF (CM-CAF) was abolished by an IL-1R antagonist.
Subsequently, we observed whether the invasion activity of cancer
cells increased by CM-CAF increased matrix degradation. Cancer
cells were cultured for 16 h on FITC-gelatin-coated coverslips.
CM-CAF stimulation increased the degradation activity of
FITC-gelatin matrix compared to the control without CM-CAF
(Fig. 3C). The increased matrix
degradation by CM-CAF stimulation was abolished by IL-1R antagonist
treatment. These results indicate that CM-CAF increased proteolytic
cancer invasion in an IL-1R-dependent manner.
IL-1β increases the protease
release
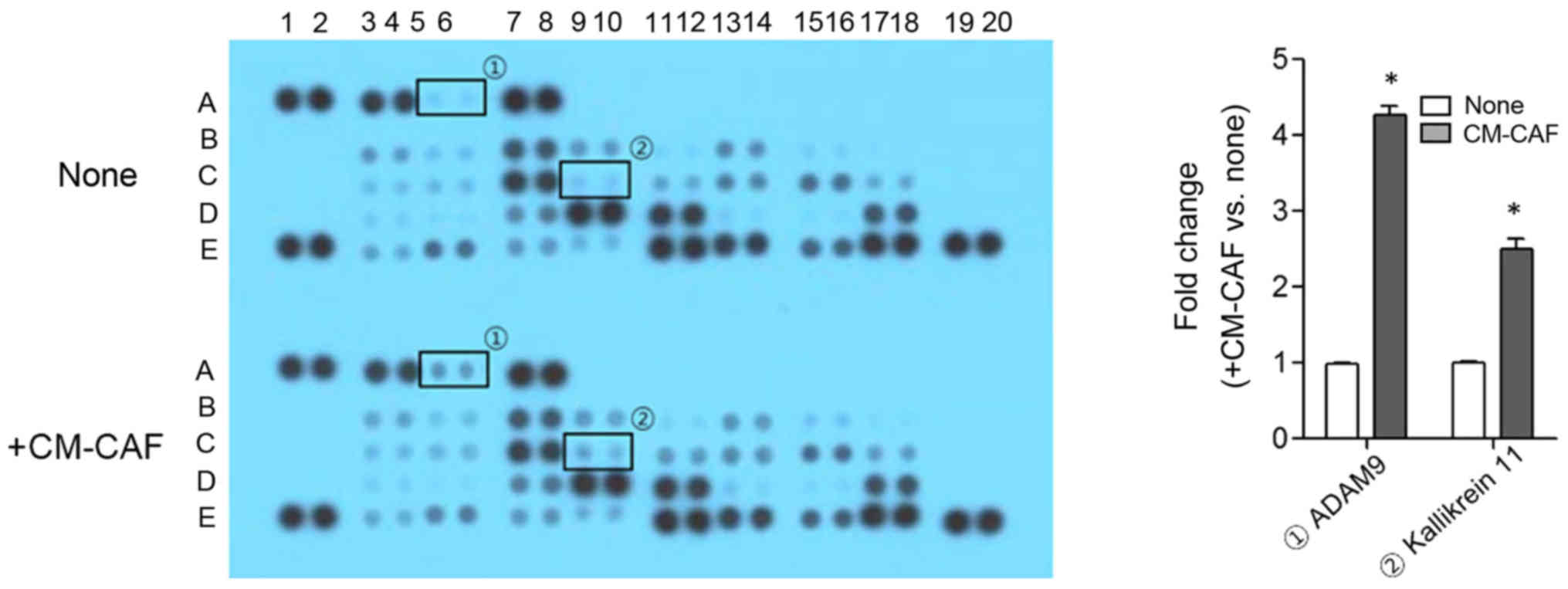
In order to explore the proteases that increased
cancer invasion by CM-CAF, the cells were stimulated with CM-CAF
and culture medium was analyzed using the Proteome Profiler Human
Protease Array. Compared to control, the secretion of ADAM 9 and
Kallikrein 11 was significantly increased by CM-CAF treatment. ADAM
9 and Kallikrein 11 were increased 4.21- and 2.48-fold,
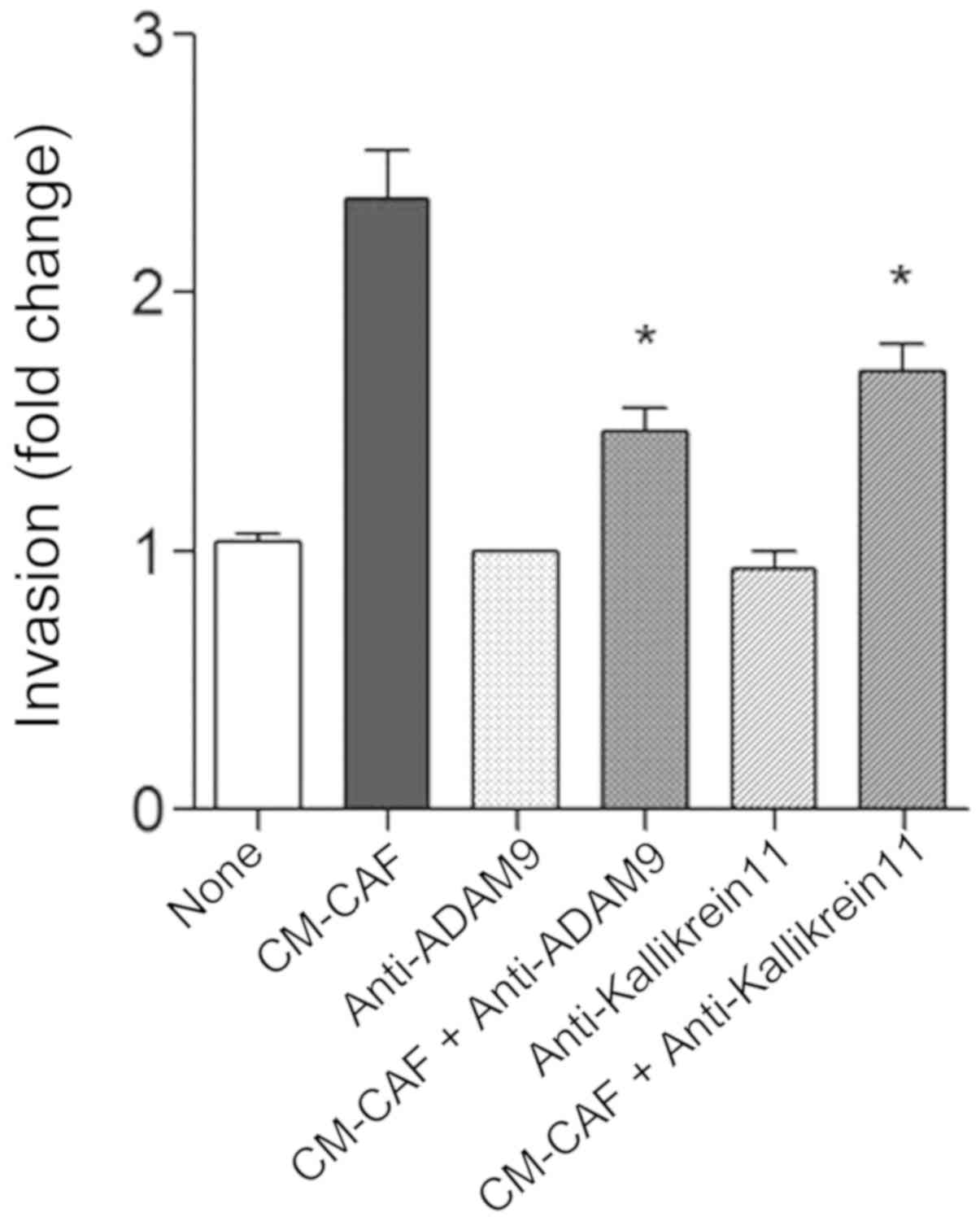
respectively (P<.01) (Fig. 4).
The increased in Transwell invasion by CM-CAF was partially
abrogated by the neutralization of ADAM 9 or Kallikrein 11
(Fig. 5).
Discussion
Protease secretion by cancer cells is a pivotal
process in cancer invasion and metastasis. Invasive cancer cells
form a protrusive cellular structure, termed invadopodia, for
effective cancer invasion, leading to the focal secretion of
proteases into the nearby extracellular matrix (ECM) (10,11).
Growth factors, such as colony stimulating factor-1 (CSF-1), TGF-β,
VEGF, PDGF, EGF, heparin-binding EGF (HB-EGF), hepatocyte growth
factor/scatter factor (HGF), and stromal cell-derived factor 1α
(SDF1α) stimulate invadopodia formation (10). MMPs, ADAM (a disintegrin and
metalloproteinase), sheddases, cysteine cathepsin proteases, and
serine proteases are localized at invadopodia (12,13).
TGF-β and EGF are synthesized in active proforms that are processed
by MMPs and another protease into active and soluble ligands,
suggesting a possible role for protease in invadopodia formation
(10,14).
In the present study, IL-1β concentration was
measured in normal fibroblast (NF) and cancer-associated fibroblast
(CAF), and higher IL-1β levels were observed in the CAF compared to
the NF. Recombinant IL-1β significantly increased cancer cell
invasion on Matrigel-coated Transwell chamber. Further, we
investigated whether stromal fibroblasts could control cancer
invasion. Conditioned media (CM) from NF and CAF were prepared and
used as cultured media in Transwell chamber for invasion assay.
Unlike CM from NF, CM from CAF (CM-CAF) increased cancer cell
invasion by increasing protease release. The invasive capacity
provided by CM-CAF was suppressed by an IL-1 receptor (IL-1R)
antagonist. These results indicate that the level of IL-1β in
CM-CAF was high enough to stimulate cancer invasion activity and
that the increased invasion activity of the cancer cells resulting
from CM-CAF treatment was sustained by an IL-1R-dependent pathway.
IL-1β is a pro-inflammatory cytokine and its expression in primary
tumors has been identified as a potential biomarker in cancer
patients at increased risk for developing bone metastasis (15). IL-1 has been suggested as a critical
molecule for tumor invasiveness and angiogenesis (16). Local tumors and lung metastases of
B16 melanoma cells were abolished in IL-1 knockout (KO) mice. IL-1β
secreted by OSCC cells reciprocally stimulated secretion of
transforming growth factor beta 1 (TGF-β1) from stromal
fibroblasts, thereby leading to cancer invasion by increasing
podoplanin (PDPN) expression, one of the components of invadopodia
(17). These results indicate that
cancer invasion is regulated by reciprocal cross-talk between
cancer and the regional microenvironment. Thus, the influence of
the tumor microenvironment is important in elucidating the
mechanism of cancer invasion. According to a recent report,
cancer-associated fibroblasts also formed invadopodia, thereby
promoting invasion activity of pancreatic cancer cells (18).
The stroma consists of the basement membrane,
fibroblasts, extracellular matrix, immune cells, and vascular
system and plays a structural and connective role in tissues.
Normal stroma maintains homeostasis by inhibiting inflammation and
neoplasia through immune systems functions. However, in the tumor
environment, it plays a role in promoting cancer growth and
malignant tumors (19).
Cancer-associated fibroblasts (CAFs) are abundant cells within the
tumor microenvironment and promote tumorigenic features by
initiating remodeling of the extracellular matrix (ECM) and
secreting cytokines. CAFs are continuously activated and lack the
ablility to revert into a normal phenotype or undergo apoptosis,
resulting in a constant number of CAFs. (19). Consequently, the reciprocal
cross-talk between stromal tissue and tumor plays a pivotal role in
cancer growth and progression (20).
We previously demonstrated that CAFs promoted tumor growth in
athymic nude mice (3). Tumor growth
was significantly increased in xenograft mixtures of CAF and cancer
cell compared to xenografts of cancer cell only in a 5-weeks
experiment. This result indicates that the existence of molecular
cross-talk between cancer cells and surrounding stroma is important
for enhancing tumor growth. Thus, targeting of CAFs is important
for understanding cancer invasion and finding keys to suppress
cancer progression. However, there is little research on the role
of IL-1β in CAF-mediated stimulation of cancer invasion. In this
study, we demonstrated that CM-CAF contained significant amounts of
IL-1β and induced the secretion of proteases from OSCC cells.
In the present study, CM-CAF was shown to stimulate
the enhanced secretion of ADAM 9 (MDC9, meltrin β) and Kallikrein
11 (KLK11, TLSP, PRSS20) from OSCC cells. The increased Transwell
invasion by CM-CAF was abrogated by neutralization of ADAM 9 or
Kallikrein 11. ADAM 9 is known as metalloprotease disintegrin
cysteine-rich protein 9 or meltrin β. Overexpression of ADAM 9 has
been identified in a variety of cancer types, including breast
cancer, renal cancer, prostate cancer, skin cancer, uterine
cervical cancer, hepatocellular carcinoma, non-small cell lung
cancer, colon cancer, gastric cancer, esophageal cancer, and head
and neck squamous cell carcinoma (HNSCC) (21). ADAM 9 is induced by oxidative stress,
such as intracellular reactive oxygen species (ROS) and/or hydrogen
peroxide, thereby supporting prostate cancer cell survival and
progression (22). Enhanced ADAM 9
expression has been reported in oral squamous cell carcinoma (OSCC)
compared to normal oral tissue. Moreover, a high degree of ADAM 9
expression has been observed in well-differentiated OSCC (21). Kallikrein 11 (KLK11) is a secreted
type of serine protease, highly expressed in many tissues including
brain, skin, salivary gland, stomach, prostate, and intestine
(23). KLK11 expression holds
prognostic significance in prostate cancer (24). In vivo studies have
demonstrated that overexpression of KLK11 led to tumor progression
and metastasis of prostate cancer. Overexpression of KLK11 in ER(+)
breast cancer cells led to breast cancer progression by increasing
the bioavailability of IGFs via degradation of insulin-like growth
factor (IGF) binding protein 3 (IGFBP-3) (25). Previously, KLK11 has been proposed as
a diagnostic biomarker of prostate and ovarian carcinoma (26). IL-1β increased the expression of an
ADAM 9 in hepatocellular carcinoma (HCC) cell to escape from the
host immune surveillance (27). The
relation between IL-1β and KLK11 has not been reported.
Neutralization of IL-1 receptor (IL-R) will be a useful tool in
elucidating the mechanisms involved in IL-1β-induced ADAM 9 or
KLK11 production. The role of ADAM 9 and KLK11 induced by CM-CAF in
OSCC cell invasion also require further investigations.
Identification of the cellular mechanism of protease controlled by
stromal factor IL-1β would be of immense significance.
In conclusion, CAFs were observed to increase cancer
invasion in an IL-1R-dependent manner. The release of ADAM 9 and
Kallikrein 11 from OSCC cells was also stimulated by CAFs. A study
on the molecular mechanism of CAF-induced proteases secretion from
cancer cells is needed to further investigate cancer invasion.
Targeting the cancer microenvironment is important for cancer
control.
Acknowledgements
Not applicable.
Funding
The current research was supported by Eulji
University in 2019 and Basic Science Research Program through the
National Research Foundation of Korea (NRF) funded by the Ministry
of Education, Science and Technology (grant no.
2018R1D1A1B07042035).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
XZ and YSH performed the experiments, analyzed and
interpreted data and wrote the manuscript.
Ethics approval and consent to
participate
Approval was received from the Animal Ethics
Committee of Eulji University (permit no. EUIACUC17-18).
Patients consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
OSCC
|
oral squamous cell carcinoma
|
|
CM
|
conditioned media
|
|
CAF
|
cancer-associated fibroblast
|
|
ELISA
|
enzyme-linked immunosorbent assay
|
References
|
1
|
Wang M, Zhao J, Zhang L, Wei F, Lian Y, Wu
Y, Gong Z, Zhang S, Zhou J, Cao K, et al: Role of tumor
microenvironment in tumorigenesis. J Cancer. 8:761–773. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen F, Zhuang X, Lin L, Yu P, Wang Y, Shi
Y, Hu G and Sun Y: New horizons in tumor microenvironment biology:
Challenges and opportunities. BMC Med. 13:452015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hwang YS, Park KK and Chung WY: Stromal
transforming growth factor-beta 1 is crucial for reinforcing the
invasive potential of low invasive cancer. Arch Oral Biol.
59:687–694. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yuan Y, Jiang YC, Sun CK and Chen QM: Role
of the tumor microenvironment in tumor progression and the clinical
applications (review). Oncol Rep. 35:2499–2515. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zi F, He J, He D, Li Y, Yang L and Cai Z:
Fibroblast activation protein α in tumor microenvironment: Recent
progression and implications (review). Mol Med Rep. 11:3203–3211.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Raman D, Baugher PJ, Thu YM and Richmond
A: Role of chemokines in tumor growth. Cancer Lett. 256:137–165.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hassona Y, Cirillo N, Heesom K, Parkinson
EK and Prime SS: Senescent cancer-associated fibroblasts secrete
active MMP-2 that promotes keratinocyte dis-cohesion and invasion.
Br J Cancer. 111:1230–1237. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang X, Cho IH, Park JH, Lee MK and Hwang
YS: Fascin is involved in cancer cell invasion and is regulated by
stromal factors. Oncol Rep. 41:465–474. 2019.PubMed/NCBI
|
|
9
|
Hwang YS, Lee J, Zhang X and Lindholm PF:
Lysophosphatidic acid activates the RhoA and NF-κB through Akt/IκBα
signaling and promotes prostate cancer invasion and progression by
enhancing functional invadopodia formation. Tumour Biol.
37:6775–6785. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hoshino D, Branch KM and Weaver AM:
Signaling inputs to invadopodia and podosomes. J Cell Sci.
126:2979–2989. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee D, Yu EJ, Ham IH and Hur H:
Clinicopathological implication of insulin-like growth factor-II
mRNA-binding protein 3 (IMP3) expression in gastric cancer.
Anticancer Res. 37:135–142. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Murphy DA and Courtneidge SA: The ‘ins’
and ‘outs’ of podosomes and invadopodia: Characteristics, formation
and function. Nat Rev Mol Cell Biol. 12:413–426. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Klemke RL: Trespassing cancer cells:
‘Fingerprinting’ invasive protrusions reveals metastatic culprits.
Curr Opin Cell Biol. 24:662–669. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kessenbrock K, Plaks V and Werb Z: Matrix
metalloproteinases: Regulators of the tumor microenvironment. Cell.
141:52–67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tulotta C and Ottewell P: The role of
IL-1B in breast cancer bone metastasis. Endocr Relat Cancer.
25:R421–R434. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Voronov E, Shouval DS, Krelin Y, Cagnano
E, Benharroch D, Iwakura Y, Dinarello CA and Apte RN: IL-1 is
required for tumor invasiveness and angiogenesis. Proc Natl Acad
Sci USA. 100:2645–2650. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hwang YS, Xianglan Z, Park KK and Chung
WY: Functional invadopodia formation through stabilization of the
PDPN transcript by IMP-3 and cancer-stromal crosstalk for PDPN
expression. Carcinogenesis. 33:2135–2146. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Goicoechea SM, García-Mata R, Staub J,
Valdivia A, Sharek L, McCulloch CG, Hwang RF, Urrutia R, Yeh JJ,
Kim HJ and Otey CA: Palladin promotes invasion of pancreatic cancer
cells by enhancing invadopodia formation in cancer-associated
fibroblasts. Oncogene. 33:1265–1273. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
De Veirman K, Rao L, De Bruyne E, Menu E,
Van Valckenborgh E, Van Riet I, Frassanito MA, Di Marzo L, Vacca A
and Vanderkerken K: Cancer associated fibroblasts and tumor growth:
Focus on multiple myeloma. Cancers (Basel). 6:1363–1381. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang FT, Sun W, Zhang JT and Fan YZ:
Cancer-associated fibroblast regulation of tumor neo-angiogenesis
as a therapeutic target in cancer. Oncol Lett. 17:3055–3065.
2019.PubMed/NCBI
|
|
21
|
Tanasubsinn P, Aung WPP, Pata S, Laopajon
W, Makeudom A, Sastraruji T, Kasinrerk W and Krisanaprakornkit S:
Overexpression of ADAM9 in oral squamous cell carcinoma. Oncol
Lett. 15:495–502. 2018.PubMed/NCBI
|
|
22
|
Sung SY, Kubo H, Shigemura K, Arnold RS,
Logani S, Wang R, Konaka H, Nakagawa M, Mousses S, Amin M, et al:
Oxidative stress induces ADAM9 protein expression in human prostate
cancer cells. Cancer Res. 66:9519–9526. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yousef GM, Scorilas A and Diamandis EP:
Genomic organization, mapping, tissue expression and hormonal
regulation of trypsin-like serine protease (TLSP PRSS20), a new
member of the human kallikrein gene family. Genomics. 63:88–96.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Stavropoulou P, Gregorakis AK, Plebani M
and Scorilas A: Expression analysis and prognostic significance of
human kallikrein 11 in prostate cancer. Clin Chim Acta.
357:190–195. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sano A, Sangai T, Maeda H, Nakamura M,
Hasebe T and Ochiai A: Kallikrein 11 expressed in human breast
cancer cells releases insulin-like growth factor through
degradation of IGFBP-3. Int J Oncol. 30:1493–1498. 2007.PubMed/NCBI
|
|
26
|
Diamandis EP, Okui A, Mitsui S, Luo LY,
Soosaipillai A, Grass L, Nakamura T, Howarth DJ and Yamaguchi N:
Human kallikrein 11: A new biomarker of prostate and ovarian
carcinoma. Cancer Res. 62:295–300. 2002.PubMed/NCBI
|
|
27
|
Kohga K, Tatsumi T, Tsunematsu H, Aono S,
Shimizu S, Kodama T, Hikita H, Yamamoto M, Oze T, Aketa H, et al:
Interleukin-1β enhances the production of soluble MICA in human
hepatocellular carcinoma. Cancer Immunol Immunother. 61:1425–1432.
2012. View Article : Google Scholar : PubMed/NCBI
|