Introduction
The inflamed colonic mucosa of patients with
ulcerative colitis (UC), an inflammatory disease affecting the
colorectal region, is suggested to be a site for the development of
precancerous lesions that can ultimately progress to colon cancer
(1). As colorectal dysplasia and
cancer is an important complication of UC, surveillance endoscopy
is recommended for the evaluation of inflammation and cancer in
patients who have had UC for more than eight years (2). Total colectomy is considered for
patients diagnosed with high-grade colorectal dysplasia or cancer
during surveillance. It is recommended that the frequency of
surveillance be increased in cases of low-grade dysplasia. However,
due to the underlying chronic inflammation in the colonic mucosa of
patients with UC, histological assessments of UC lesions for
carcinomatous changes are often challenging (3). Therefore, diagnostic markers that can
identify colorectal dysplasia and cancer in the presence of
long-standing colorectal inflammation in UC are sorely needed.
Recently, the expression levels of p53, Ki-67, and
chromogranin A have been reported to be useful for the diagnosis of
colorectal dysplasia and cancer in UC patients (4–6).
Although mismatch repair deficiency (MMRD) is reportedly associated
with carcinogenic processes in the colon, the significance of MMRD
in colitic cancer remains controversial (7,8).
Additionally, p53 expression has been used primarily as an early
diagnostic marker for colorectal dysplasia and cancer. However,
false positive results due to chronic inflammation and false
negative results despite p53 mutations are critical issues
in the diagnostic evaluation of patients with UC. Therefore,
development of highly sensitive and specific diagnostic markers is
needed for improved surveillance in this patient population.
Stathmin 1 (STMN1) is a major cytosolic
phosphoprotein that regulates microtubule dynamics by promoting
microtubule destabilization (9).
Intriguingly, increased STMN1 expression has been observed in
numerous cancers including colorectal cancer, and cancer patients
with increased STMN1 expression were reported to exhibit aggressive
tumor phenotypes and poor prognosis (10). Therefore, STMN1 is considered
potentially useful not only as a cancer biomarker but also as a
novel target for cancer treatment. However, few studies have
investigated the relationship between STMN1 expression levels and
clinical features in colorectal dysplasia and cancer in UC
patients.
The purpose of the present study was to determine
the clinical significance of STMN1 in colorectal dysplasia and
cancer due to UC. We performed immunohistochemical analysis on 31
clinical colorectal samples from eight patients with colorectal
dysplasia and/or cancer. Our results demonstrate the presence of a
relationship between STMN1 expression and several
clinicopathological features, including MMRD status, rate of Ki-67
positivity, differentiation level, TNM grade, and UC duration.
Materials and methods
Patients and samples
Eight patients (six males and two females) with UC
who underwent surgical resection in Gunma University Hospital,
Saitama Red Cross Hospital, and Gunma Prefectural Cancer Center
between 1999 and 2014 were included in this retrospective study.
The median age of the patients was 59 years (range 37–76 years).
One patient had only dysplasia. Some patients had two or more
tumors, and all dysplastic and cancerous lesions were evaluated.
All dysplastic lesion samples were only obtained from patients with
high-grade dysplasia; patients with low-grade dysplasia were not
included in the study. This study conformed to the tenets of the
Declaration of Helsinki and was approved by the Institutional
Review Board for Clinical Research at the Gunma University Hospital
(Maebashi, Gunma, Japan). Patient consent was obtained via the
opt-out method. Table I summarizes
patient information. For accurate pathological diagnosis of
dysplastic lesions in UC patients, all dysplasia and cancer tumor
sections were evaluated by a specialized pathologist, Dr. Yao T
(Department of Human Pathology, Juntendo University Graduate School
of Medicine).
 | Table I.Clinical characteristics of the
patients with UC in the present study. |
Table I.
Clinical characteristics of the
patients with UC in the present study.
| Characteristics | Patient number
(n=8) |
|---|
| Age (years) |
|
| Median
(range) | 59 (37–76) |
|
<70/≥70 | 6/2 |
| Sex |
|
|
Male/Female | 6/2 |
| Number of sample |
|
|
Normal/Dysplasia/Cancer | 8/12/11 |
| Differentiation |
|
|
Poor/Moderate/Well | 2/5/4 |
| Stage |
|
| I/II | 2/1 |
|
IIIA/IIIB | 0/2 |
|
IIIC/IV | 1/1 |
| Duration of disease
(years) |
|
| Median
(range) | 15.3 (4–27) |
Immunohistochemical staining
Paraffin-embedded blocks of all surgical resection
specimens obtained from UC patients were cut into sections 4 µm in
thickness and mounted on glass slides. Sections were deparaffinized
using xylene and dehydrated in alcohol. Endogenous peroxidase was
inhibited using 0.3% H2O2/methanol for 30 min
at room temperature. Then, the sections were soaked in heated water
supplemented with 0.5% Immunosaver (Nishin EM, Tokyo, Japan) at
98°C for 45 min. Nonspecific antigens were blocked by serum-free
Protein Block (DAKO, Glostrup, Denmark) at room temperature for 30
min. Next, the sections were incubated with primary antibodies
against STMN1 (mouse monoclonal, 1:200; Santa Cruz Biotechnology,
Santa Cruz, CA, USA), p53 (mouse monoclonal anti-human [DO-7],
1:100; DAKO), Ki67 (mouse monoclonal anti-human [MIB-1], 1:300;
DAKO), MLH1 (mouse monoclonal anti-human [ES05]; DAKO), MSH2 (mouse
monoclonal anti-human [FE11]; DAKO), MSH6 (rabbit monoclonal
anti-human [EP49]; DAKO), and PMS2 (rabbit monoclonal anti-human
[EP51]; DAKO) at 4°C for 24 h. After washing with
phosphate-buffered saline, the sections were incubated in Histofine
Simple Stain™ MAX PO (MULTI) solution (Nichirei, Tokyo, Japan) for
45 min to visualize primary antibodies. The chromogen
3,3′-diaminobenzidine tetrahydrochloride was applied as a 0.02%
solution, which contained 0.005% H2O2 in 50
mM ammonium acetate-citrate acid buffer (pH 6.0). Finally, nuclear
counterstaining was performed using Mayer's hematoxylin solution.
Negative controls for immunohistochemical staining involved
replacing primary antibodies with phosphate-buffered saline in 0.1%
bovine serum albumin and confirming a lack of staining.
Assessment of STMN1, p53, Ki-67, and
mismatch repair protein expression
We evaluated cytoplasmic staining for STMN1 in
noncancerous tissues as well as dysplastic and cancerous tissues
from patients with UC. Cytoplasmic STMN1 was scored as follows: 0,
no staining; 1+, 1–10% staining; 2+, 11–50% staining; and 3+,
51–100% staining. The optimal cutoff point was defined as follows:
Grades 0 and 1 were considered negative, and grades 2 and 3 were
considered positive. p53-positive cells were defined as those with
a brown-stained nucleus, regardless of staining intensity. The
following four staining patterns were identified: Positive cells in
most of the lesion (diffuse); positive cells aggregated in a focal
area of the lesion (nested); small numbers of isolated positive
cells scattered throughout the lesion (scattered); and negative.
Positive p53 protein expression was defined as either a diffuse or
nested pattern, whereas negative p53 protein expression was defined
as a scattered pattern throughout the lesion or negative staining,
as described previously (6). The
Ki-67 labeling index was scored as a percentage of positively
stained cells. MMRD was defined as the complete absence of the
expression of at least one MMR protein (MLH1, MSH2, MSH6, or
PMS2).
Statistical analysis
The JMP software package (SAS Institute Inc., Cary,
NC, USA) was used to perform all statistical analyses. Chi-square
tests were used to analyze associations between STMN1 and p53
expression levels. Wilcoxon's test was used to analyze associations
between STMN1 expression and the rate of Ki-67 positivity. All
differences were considered statistically significant at
P<0.05.
Results
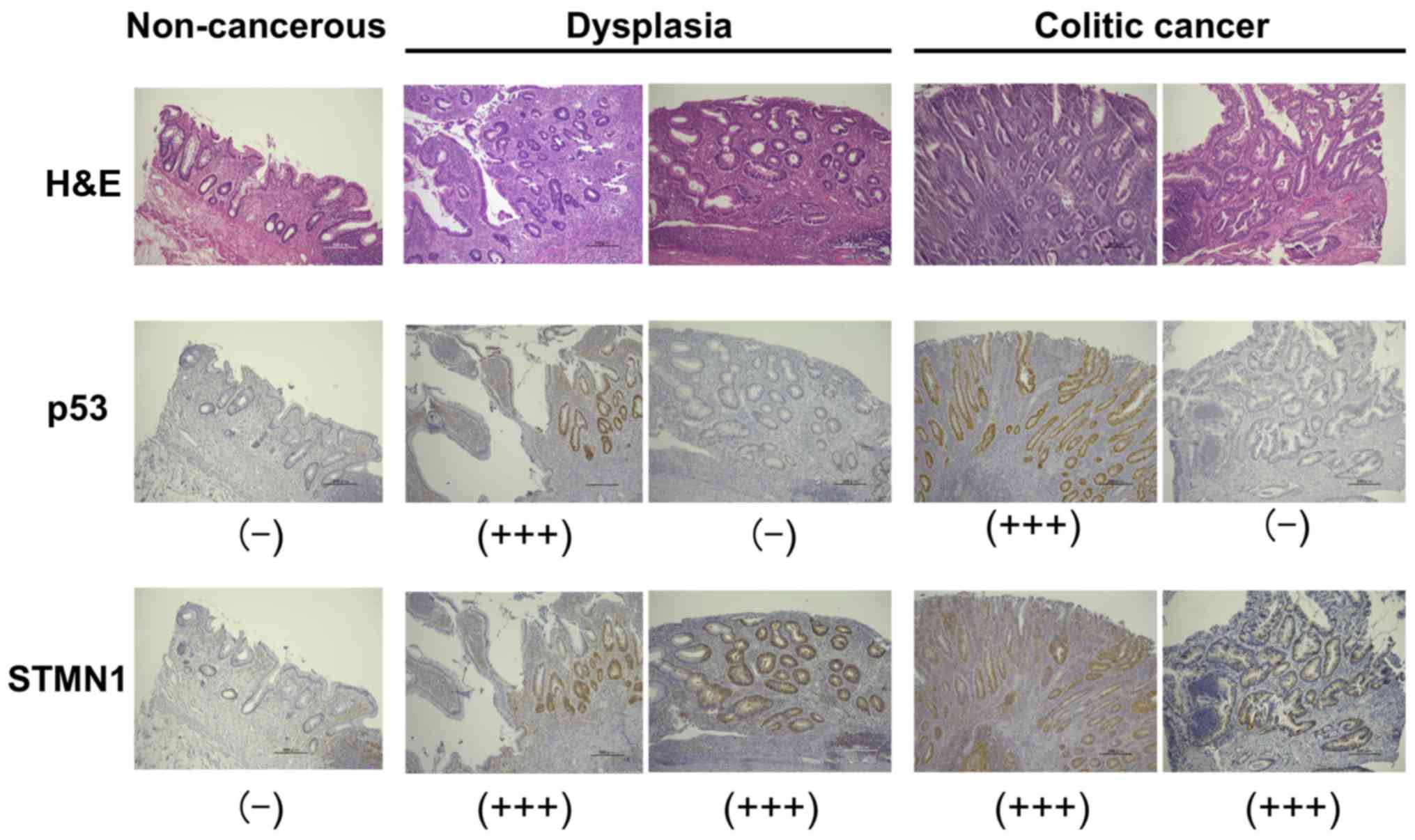
Immunohistochemical analysis of STMN1
expression in colorectal tissue specimens of patients with UC
As STMN1 was expressed in the cytoplasm of
colorectal tissue sections obtained from patients with UCs,
cytoplasmic STMN1 expression was evaluated in sections of
noncancerous tissues as well as dysplastic and cancerous lesions
from eight patients with UC. STMN1 was highly expressed in the
cytoplasm of dysplastic as well as cancerous tissue sections,
whereas cytoplasmic STMN1 staining could not be detected in
noncancerous tissue sections (Fig.
1). In contrast, p53 expression, which was identified
previously as a clinical diagnostic marker for colorectal dysplasia
and cancer, was absent in the dysplastic and cancerous sections of
some UC patients in the present study. Importantly, these
p53-negative sections were positive for STMN1, based on
immunohistochemical analysis (Fig.
1).
Significance of the expression levels
of STMN1, p53, Ki-67, and MMR proteins in the colonic mucosa of
patients with UC
Next, we analyzed the relationships among STMN1 and
p53 expression levels, Ki-67 labeling index, MMRD, level of
differentiation, TNM stage, and duration of disease in 31 colitic
mucosa samples from eight patients with UC. Table II summarizes clinicopathological
characteristics and results of immunohistochemical analysis of
these samples. In the study cohort, STMN1 was highly expressed in
dysplastic as well as cancerous specimens, whereas noncancerous
tissues were not positive for STMN1 based on immunohistochemical
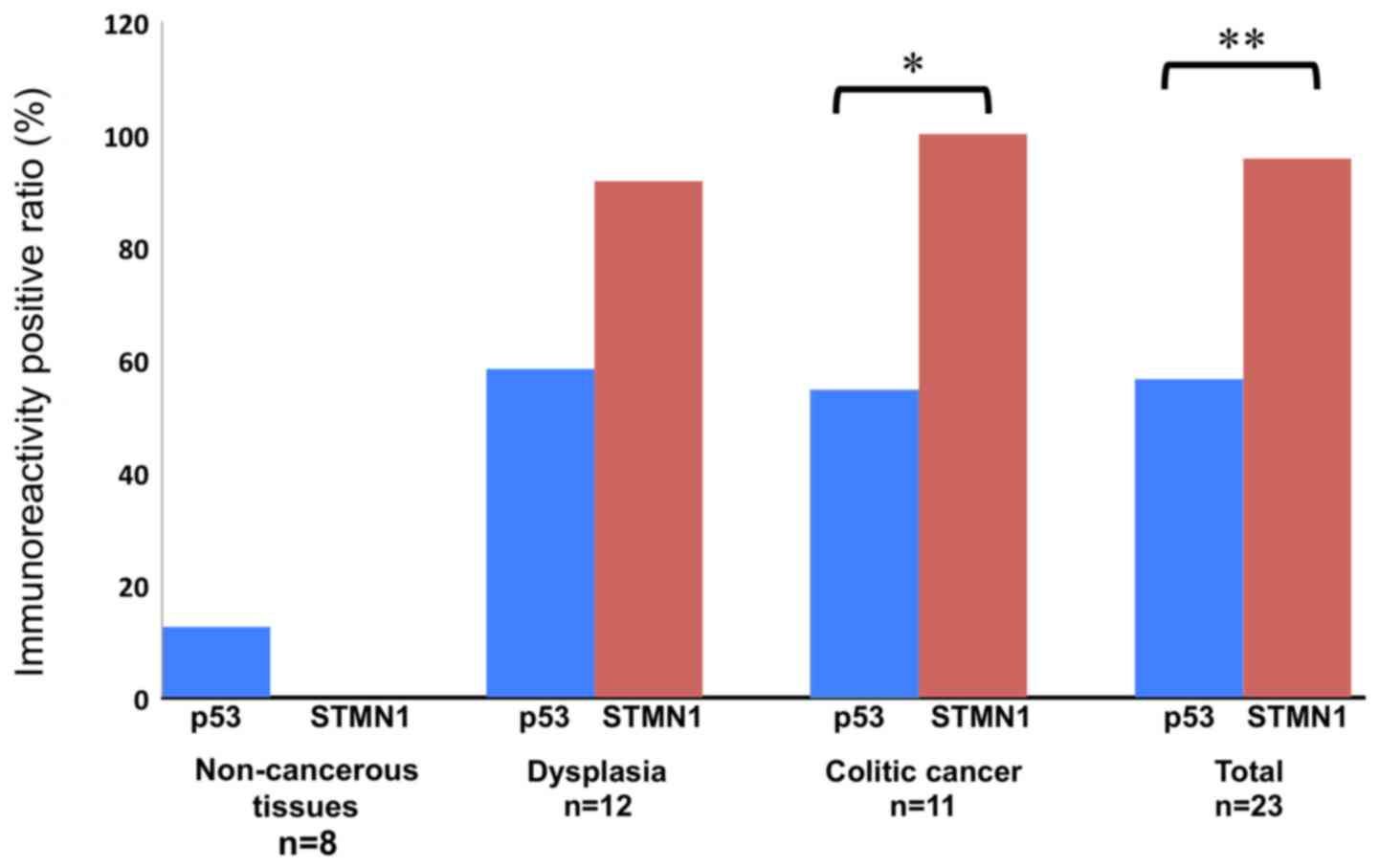
staining (Fig. 2). The rate of STMN1
positivity in 12 dysplastic and 11 colitic cancer tissue sections
was 91.7 and 100%, respectively (Fig.
2; Table II). The rate of p53
positivity in the dysplastic and cancerous tissue sections were
58.3 and 63.6%, respectively. Conversely, the rate of p53
positivity in the noncancerous tissue sections was 12.5%. The rate
of positive STNM1 staining in the cancerous tissue sections was
higher than that of p53 (Fig. 2;
P=0.001), and that the rate of positive STNM1 staining in the
dysplastic and cancerous tissue sections was higher than that of
p53 (95.7% vs 60.9%; Fig. 2;
P=0.003). The positive predictive, negative predictive, and AUC
values of STMN1 in the dysplastic and cancerous tissue sections
were defined as 100, 83.3%, and 0.97826, respectively. These values
were higher than those of p53, which were 93, 47.4%, and 0.73261
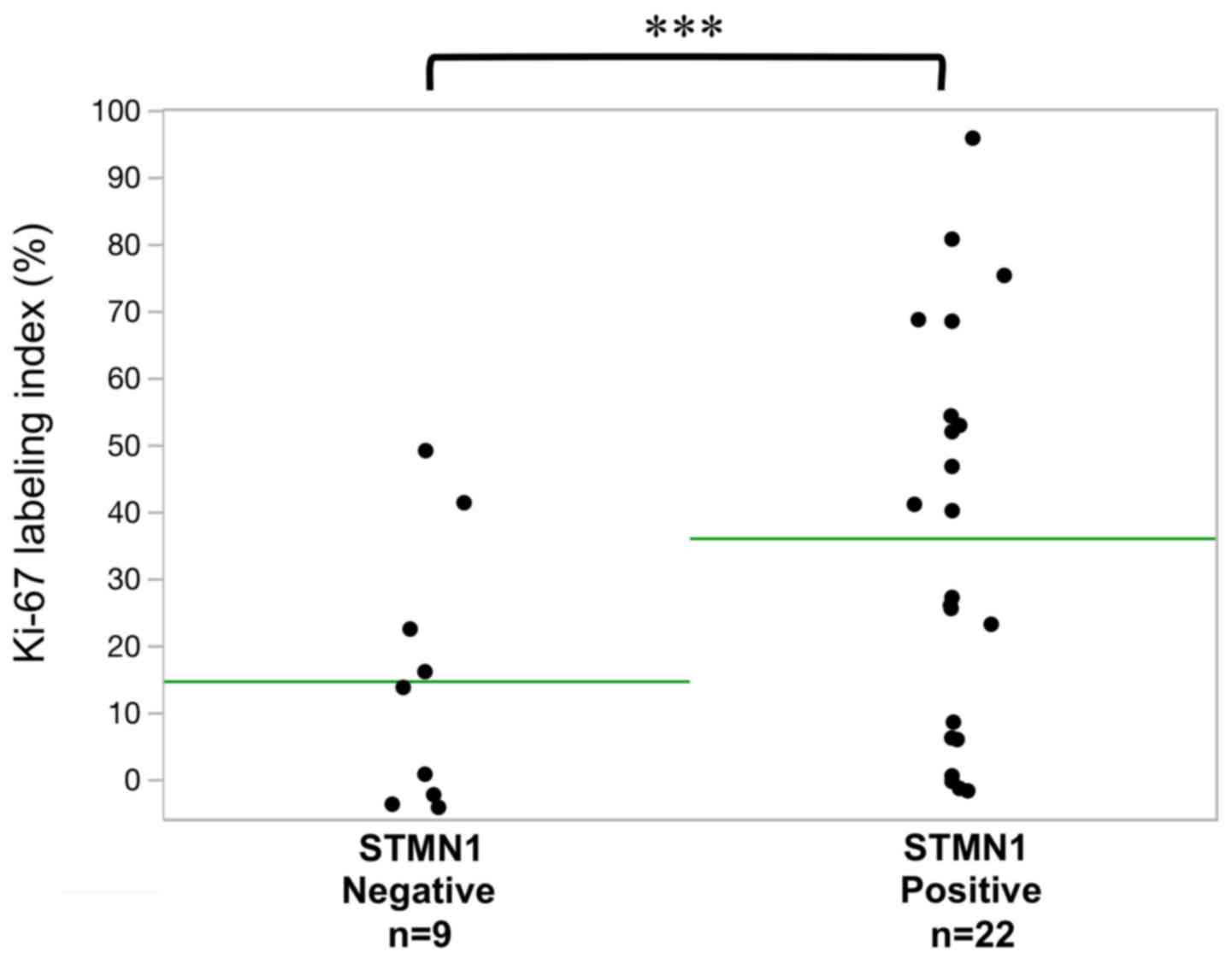
respectively. Furthermore, the median Ki-67 labeling index in
sections with high STMN1 expression (33.5%, 22/31) was higher than
that in sections with low STMN1 expression (16.6%, 9/31; Fig. 3; P=0.04). Only one colitic cancer
sample was identified as mismatch repair-deficient in this study
(Table II).
 | Table II.Clinicopathological characteristics in
ulcerative colitis patients with high-grade dysplasia and colitic
cancer. |
Table II.
Clinicopathological characteristics in
ulcerative colitis patients with high-grade dysplasia and colitic
cancer.
| Pathological
diagnosis | Location | p53 | STMN1 | MMRD | Ki-67 positive rate
(%) | Differentiation | T factor | N factor | M factor | Stage |
|---|
| Case 1 |
|
|
|
|
|
|
|
|
|
|
|
Normal | Ce | + | − | − | 3.0 | − | − | 1a | Negative | IIIB |
| Dys | Ce | + | + | − | 10.2 |
|
|
|
|
|
| Dys | Ce | − | + | − | 3.8 |
|
|
|
|
|
| Dys | Ce | − | + | − | 3.5 |
|
|
|
|
|
| Dys | A | + | − | − | 18.6 |
|
|
|
|
|
| Dys | S | + | + | − | 5.4 |
|
|
|
|
|
| Dys | S | + | + | − | 4.7 |
|
|
|
|
|
| Ca | Ce | − | + | − | 28.0 | Moderate | 3 |
|
|
|
| Ca | A | + | + | − | 10.0 | Moderate | 4a |
|
|
|
| Ca | S | + | + | − | 44.6 | Moderate | 2 |
|
|
|
| Case 2 |
|
|
|
|
|
|
|
|
|
|
|
Normal | D | − | − | − | 24.0 | − | − | 2a | Negative | IIIC |
|
Dys | D | − | + | − | 27.0 | − | − |
|
|
|
| Ca | A | + | + | + | 24.6 | Poor | 4a |
|
|
|
| Ca | D | + | + | − | 63.0 | Moderate | 1 |
|
|
|
| Case 3 |
|
|
|
|
|
|
|
|
|
|
|
Normal | T | − | − | − | 46.6 | − | − | 1a | Negative | IIIB |
|
Dys | T | + | + | − | 39.0 |
|
|
|
|
|
|
Dys | D | + | + | − | 49.0 |
|
|
|
|
|
| Ca | T | + | + | − | 68.8 | Moderate | 3 |
|
|
|
| Case 4 |
|
|
|
|
|
|
|
|
|
|
|
Normal | S | − | − | − | 1.8 | − | − | 2a | M1b | IVB |
|
Dys | S | − | + | − | 51.0 |
|
|
|
|
|
| Ca | S | − | + | − | 63.2 | Well | 4a |
|
|
|
| Case 5 |
|
|
|
|
|
|
|
|
|
|
|
Normal | S | − | − | − | 16.6 | − | − | 0 | Negative | I |
|
Dys | S | − | + | − | 73.4 |
|
|
|
|
|
| Ca | S | + | + | − | 39.8 | Well | 2 |
|
|
|
| Case 6 |
|
|
|
|
|
|
|
|
|
|
|
Normal | S | − | − | − | 5.6 | − | − | 0 | Negative | IIC |
| Ca | S | − | + | − | 26.6 | Poor | 4b |
|
|
|
| Case 7 |
|
|
|
|
|
|
|
|
|
|
|
Normal | T | − | − | − | 40.0 | − | − | − | − | − |
|
Dys | RS | + | + | − | 86.2 |
|
|
|
|
|
| Case 8 |
|
|
|
|
|
|
|
|
|
|
|
Normal | Rb | − | − | − | 1.4 | − | − | 0 | Negative | I |
| Ca | D | − | + | − | 49.8 | Well | 1 |
|
|
|
| Ca | Rb | − | + | − | 12.2 | Well | 2 |
|
|
|
Discussion
In the present study, we observed that 95.7% of
sections of dysplastic and cancerous tissues from all patients with
UC were positive for STMN1 staining. In contrast, p53, which is the
currently used diagnostic marker for colorectal dysplasia and
cancer, was expressed in only 60.9% of the sections of dysplastic
and cancerous lesions. Moreover, STMN1 expression observed in the
colonic mucosa as well as the dysplastic and cancerous lesions of
eight patients with UC in the current study was associated with a
high rate of Ki-67 positivity, a marker of proliferation.
Our data were consistent with previous studies
reporting the rate of p53 positivity as 45.0–77.8% in dysplastic
lesions and 57.0–90.9% in colitic cancer among patients with UC
(4–6,11).
Additionally, in the current study, STMN1 expression was detected
in 95.7% of sections of dysplasia and cancerous lesions; further,
STMN1 expression was not detected in any section of noncancerous
lesions. These observations suggest that STMN1 expression might be
more accurate and useful than the current diagnostic marker, p53,
for the diagnosis of dysplasia and cancer in patients with UC.
Colitic cancer secondary to chronic inflammation due
to UC presents a clinically significant problem (12), and total colonoscopy is often used
for the surveillance and diagnosis of colitic cancer in affected
patients. However, less invasive and low-cost clinical modalities
are needed to avoid invasive colonoscopy procedures, which are
associated with high medical costs relative to laboratory tests in
general. Elevated STMN1 levels in serum and urine samples from
patients with bladder cancer support its utility as a cancer
biomarker (13). Future studies are
necessary to assess whether elevated STMN1 expression in liquid
samples from patients with UC can be utilized as a new marker to
predict the presence of dysplasia and colitic cancer.
p53 staining patterns are generally classified as
diffuse, sporadic, scattered, or nested. Because the region
observable in an endoscopic biopsy is limited, assessments of
biopsy samples from patients with UC based on p53 staining might
overlook colitic cancer and dysplasia using this classification. In
the current study, we observed that STMN1 stained uniformly within
entire cancerous lesions, further providing support for STMN1
staining of biopsy samples as a useful marker of dysplasia and
colitic cancer in UC.
Previously, it was reported that 67% of colitic
cancer samples showed high-level MSI (microsatellite instability)
phenotypes as MMR protein deficiency (7). However, 9.1% of colitic cancer samples
were reported to show MMR deficiency (8). The MMR frequency in colitic cancer was
different in each report and the association of MMR with colitic
cancer is controversial. In the present study, we evaluated for the
first time the association between expression of STMN1 and MMR
deficiency; however, we detected MMR deficiency in only one sample
and we were unable to perform sufficient statistical analysis using
our limited data. In the future, multi-centric large cohort
analyses will be required to clarify the relationship between STMN1
and MMR deficiency, although sample collection will present
difficulties as colitic cancer is rare.
Zhang et al (10) reported previously that the rate of
STMN1 positivity was 62.9% in colon cancer samples from non-UC
patients. However, this rate in the present study was 100%. In
contrast to colon cancers in non-UC patients, colitic cancer has
been reported to be in accordance with the dysplasia-carcinoma
sequence hypothesis, which is associated with a higher p53 mutation
rate in dysplasia and colitic cancer in UC patients than that in
non-UC patients (14).
Interestingly, mutant p53 was reported to induce STMN1 expression
(15). Therefore, it was suggested
that the difference in p53 mutation rate between colitic cancer and
sporadic colon cancer influences the high rate of STMN1 positivity
in colitic cancers.
This study had several limitations. First, the
limited number of subjects in this study may have contributed to
less detection power in this study. Second, we did not compare the
expression significance of STMN1 in colon cancer from UC patients
with that from non-UC patients using the same immunohistochemical
method. Third, we did not implement any functional studies on the
relationship between colon cancer-related genes, including STMN1
and colon cancer, using cell lines derived from colitic cancer
patients.
Elevated STMN1 expression was observed in dysplastic
lesions from patients with UC. Our data suggest that STMN1
expression in the colonic mucosa of patients with UC might be
useful as an early diagnostic marker of dysplasia and colitic
cancer.
Acknowledgements
The authors would like to thank Ms. Yukie Saito, Ms.
Tomoko Yano, Ms. Yuka Matsui, Ms. Sayaka Okada, and Ms. Kayoko
Takahashi (Department of General Surgical Science, Gunma
University, Graduate School of Medicine) for their assistance.
Funding
The present study was supported by JSS Young
Researcher Award from Japan Surgical Society, Gunma University
Clinical Biobank, and Grants-in-Aid for Scientific Research from
the Japan Society for the Promotion of Science (JSPS) (grant nos.
JP 26461969, JP15K10129, JP15K10085, JP26350557 and 17K19893).
Availability of data and materials
The datasets used or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
The present study was designed and organized by HK,
KS, TYo and KO. Clinical samples and data were collected by HOg,
RY, YM, TT, HT, RK, RT, CK, JN, HOj and KO. Immunostaining was
evaluated by TF, TYa and KO. HK, TYo, and KO contributed to data
analysis, interpretation and drafting manuscript. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the institutional
guidelines of Gunma University Graduate School of Medicine
(Maebashi, Japan). Written informed concept was obtained by the
patients who agreed with the future research using resected samples
prior to surgery. Therefore, the opt-out method for the STMN1
project was performed using archived samples.
Patient consent for publication
Patient agreement was obtained via the opt-out
method.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Morson BC: Cancer in ulcerative colitis.
Gut. 7:425–426. 1966. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Van Assche G, Dignass A, Bokemeyer B,
Danese S, Gionchetti P, Moser G, Beaugerie L, Gomollón F, Häuser W,
Herrlinger K, et al: Second European evidence-based consensus on
the diagnosis and management of ulcerative colitis part 3: Special
situations. J Crohns Colitis. 7:1–33. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Eaden J, Abrams K, McKay H, Denley H and
Mayberry J: Inter-observer variation between general and specialist
gastrointestinal pathologists when grading dysplasia in ulcerative
colitis. J Pathol. 194:152–157. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kobayashi K, Tomita H, Shimizu M, Tanaka
T, Suzui N, Miyazaki T and Hara A: p53 Expression as a diagnostic
biomarker in ulcerative colitis-associated cancer. Int J Mol Sci.
18:E12842017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wong NA, Mayer NJ, MacKell S, Gilmour HM
and Harrison DJ: Immunohistochemical assessment of Ki67 and p53
expression assists the diagnosis and grading of ulcerative
colitis-related dysplasia. Histopathology. 37:108–114. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shigaki K, Mitomi H, Fujimori T, Ichikawa
K, Tomita S, Imura J, Fujii S, Itabashi M, Kameoka S, Sahara R and
Takenoshita S: Immunohistochemical analysis of chromogranin A and
p53 expressions in ulcerative colitis-associated neoplasia:
Neuroendocrine differentiation as an early event in the
colitis-neoplasia sequence. Hum Pathol. 44:2393–2399. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tahara T, Inoue N, Hisamatsu T, Kashiwagi
K, Takaishi H, Kanai T, Watanabe M, Ishii H and Hibi T: Clinical
significance of microsatellite instability in the inflamed mucosa
for the prediction of colonic neoplasms in patients with ulcerative
colitis. J Gastroenterol Hepatol. 20:710–715. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu X, Goldblum JR, Zhao Z, Landau M,
Heald B, Pai R and Lin J: Distinct clinicohistologic features of
inflammatory bowel disease-associated colorectal adenocarcinoma: In
comparison with sporadic microsatellite-stable and Lynch
syndrome-related colorectal adenocarcinoma. Am J Surg Pathol.
36:1228–1233. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Baldassarre G, Belletti B, Nicoloso MS,
Schiappacassi M, Vecchione A, Spessotto P, Morrione A, Canzonieri V
and Colombatti A: p27(Kip1)-stathmin interaction influences sarcoma
cell migration and invasion. Cancer Cell. 7:51–63. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang HQ, Guo X, Guo SQ, Wang Q, Chen XQ,
Li XN and Guo LS: STMN1 in colon cancer: Expression and prognosis
in Chinese patients. Eur Rev Med Pharmacol Sci. 20:2038–2044.
2016.PubMed/NCBI
|
|
11
|
Bruwer M, Schmid KW, Senninger N and
Schurmann G: Immunohistochemical expression of P53 and oncogenes in
ulcerative colitis-associated colorectal carcinoma. World J Surg.
26:390–396. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Watanabe T, Konishi T, Kishimoto J, Kotake
K, Muto T and Sugihara K; Japanese Society for Cancer of the Colon
and Rectum, : Ulcerative colitis-associated colorectal cancer shows
a poorer survival than sporadic colorectal cancer: A nationwide
Japanese study. Inflamm Bowel Dis. 17:802–808. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bhagirath D, Abrol N, Khan R, Sharma M,
Seth A and Sharma A: Expression of CD147, BIGH3 and Stathmin and
their potential role as diagnostic marker in patients with
urothelial carcinoma of the bladder. Clin Chim Acta. 413:1641–1646.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ullman TA and Itzkowitz SH: Intestinal
inflammation and cancer. Gastroenterology. 140:1807–1816. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Carney BK and Cassimeris L:
Stathmin/oncoprotein 18, a microtubule regulatory protein, is
required for survival of both normal and cancer cell lines lacking
the tumor suppressor, p53. Cancer Biol Ther. 9:699–709. 2010.
View Article : Google Scholar : PubMed/NCBI
|