Hepatocellular carcinoma (HCC) remains one of the
leading causes of cancer-associated mortality worldwide, and
involves numerous interlinked factors such as inflammation,
hypoxia, immunity and processes from liver injury, liver cirrhosis
to hepatocarcinogenesis (1). The
well-established etiological factors include chronic hepatitis B or
C viral (HBV/HCV) infection, excessive alcohol intake,
non-alcoholic steatohepatitis and exposure to aflatoxin B (2–5). In
Asia, the prevalence of HBV-infected HCC is 50–80% from 2016
(6,7). HBV/HCV infection can lead to apoptosis
and immune cell infiltration, and can cause the induction of
damaging inflammatory responses and repeated chronic tissue injury
and repair, which results in the progression of HCC (8).
Currently, the most effective treatments for HCC are
hepatic resection or liver transplantation (9). However, less than 20% of patients with
HCC can be treated surgically. Furthermore, the 5-year recurrence
rates of HCC after surgical resection were 57–75% due to the
limited efficacy of chemotherapy, radiation and target therapy
(10). Consequently, there is an
essential requirement for the development of improved therapeutic
strategies that can be used to treat HCC. The investigation of the
signaling pathways driving hepatocarcinogenesis could be helpful to
identify novel targets for HCC treatment. Hedgehog (Hh) signaling
pathway contributes to the progression of a variety of human cancer
types, including HCC, breast cancer and basal cell carcinoma
(11–14). The Hh pathway serves a crucial role
in carcinogenesis, invasiveness, recurrence and cancer stem cell
maintenance in HCC (12). In the
current review, recent studies of investigating the Shh signaling
pathway and its association with HCC, were assessed.
Hh molecules are important soluble factors that
regulate cell proliferation and differentiation during embryonic
development, adult tissue homeostasis and carcinogenesis (15,16).
Molecules that are associated with Hh signaling were first
identified in Drosophila, and later found to be highly
conserved in higher organisms, including mammals (17). In human, three types of Hh ligands
have been associated with this pathway, Sonic hedgehog (Shh),
Indian hedgehog and Desert hedgehog. In the liver, Hh ligands
generated by injured hepatocytes, activated hepatic stellate cells,
progenitor cells, Kupffer cells, natural killer T cells and
endothelial cells (18). Each Hh
ligand exhibits a different spatial and temporal expression
patterns (19). The roles of these
ligands in cellular and developmental function has been previously
reviewed (20). In the current
review, the role of the Shh signaling pathway in HCC was evaluated.
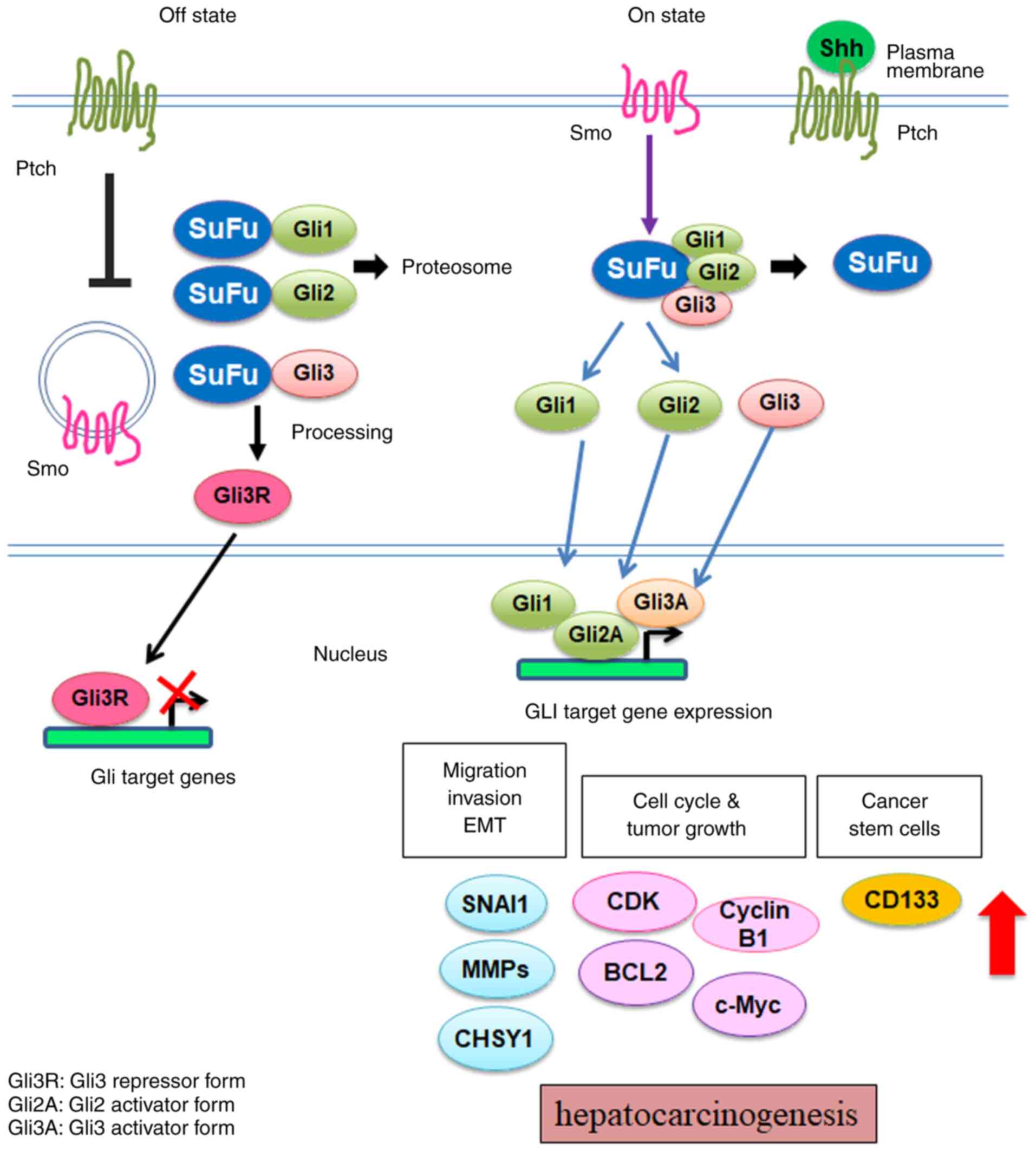
The Shh signaling pathway is activated when Shh ligands bind to
their receptors, protein patched homolog (Ptch) 1 or 2 (21,22). In
the absence of Hh ligands, Ptch continuously inhibits the activity
of G-protein-coupled receptor like receptor, SMO protein, in the
Off stage (Fig. 1, left) (23). Suppressor of fused homolog (Sufu) is
an important negative regulator of the Hh signaling pathway
(24). Sufu sequesters
glioma-associated oncogene homolog (Gli) transcription factors in
the cytosol prior to pathway activation (25). The binding of Hh ligands to Ptch
weakens the inhibition of SMO and promotes its translocation to the
plasma membrane for complete activation. The activation of SMO
results in the dissociation of the Gli-Sufu complex, and leads to
the translocation of Gli protein to the nucleus (Fig. 1, right). There are three Gli family
transcription factors: glioma-associated oncogene homolog 1
(GLI-1), GLI-2 and GLI-3 (26). Gli-1 and Gli-2 are transactivators
and Gli-3 is a repressor. In the nucleus, the Gli proteins bind to
the Gli-binding consensus sequence and upregulate the expression of
target genes, including SNAIL, c-MYC, BCL-2 and
Prominin-1 (CD133) (27).
The activation or overexpression of Hh molecules,
including PTCH-1, GLI and SMO have been frequently indicated in HCC
tissues (28–30). Che et al (28) analyzed 46 HCC tissues using reverse
transcription-quantitative PCR (RT-qPCR) and identified numerous Hh
signaling molecules that were expressed in ≥50% of tumors.
Specifically, GLI-1 expression was associated with
disease-free and overall survival in patients with HCC. Therefore,
GLI-1 expression may be a prognostic predictor of HCC.
Another study used RT-qPCR to analyze 50 patients with HCC after
surgical resection, and it was demonstrated that high PTCH-1
and GLI-1 expression increased the risk of post-section
recurrence and was associated with poor overall survival (29). The results of this study indicated
that higher PTCH-1 expression was associated with the early
recurrence of HCC and may serve as a poor prognostic marker for the
disease. Another study measured GLI-2 levels, using
immunohistochemistry in 68 patients with HCC. The results revealed
that the patients with increased GLI-2 expression exhibited
earlier HCC recurrence, and presented with both shorter
disease-free and overall survival times (31). In summary, these studies demonstrated
that the activation of the Shh signaling pathway in patients with
HCC was inversely associated with disease prognosis.
A number of studies have demonstrated that an
association exists between the activation of the Shh signaling
pathway and poor prognosis in patients with HCC. Therefore, it is
important to understand how the pathway is activated. Adult healthy
hepatocytes barely express Hh ligands, however, live epithelial
cells begin to generate Hh ligands during injury or severe stress.
The overexpression of Shh ligands and the concomitant expression in
HCC tissues can activate the Shh signaling pathway (28,32,33). In
addition, viral infection and chronic inflammation play a critical
role in the activation of the Shh signaling pathway. HBV DNA
integrates into hepatocyte chromosomes. The expression of the
HBV gene product HBx protein can increase SHH, PTCH-1
and GLI-2 expression, and is able to stabilize GLI-1 and
GLI-2 proteins (34,35). Hh signaling pathway activity has been
indicated to be necessary for HBx transformation, as demonstrated
by a study that showed that the administration of a SMO inhibitor
reduced HCC growth in HBx transgenic mice (35). HBV/HCV infection has also been
indicated to increase Hh ligands in hepatocytes and expand the
Hh-responsive cells, which promote liver fibrosis and
hepatocarcinogenesis (36). Chronic
infection with HBV or HCV leads to continuous hepatocyte apoptosis,
leukocyte infiltration and stimulates the Hh signaling pathway
(3,35,37). A
novel mouse model has revealed that chronic or acute liver injury
can induce the activation of the Hh pathway. In this aforementioned
study, primary hepatocytes upregulated SMO expression during
Fas-induced liver injury and this also increased Fas-induced
apoptosis (38). Activation of the
Hh pathway initiates downstream gene expression of cancer stem cell
marker CD133 and cytokine IL-6, which serves an important role in
the liver acute phase response and in HCC development (39–41).
PTCH-1 has been revealed to regulate cell cycle progression and
induce tumorigenicity (42).
Inhibition of the Hh pathway can reduce PTCH-1-dependent tumor
progression (43). In addition to
viral infection, mutation of the Hh signaling pathway molecules can
also activate the Hh pathway (14).
In a number of patients with HCC, SMO mutation at the C-terminal
lysine (K575M) was revealed to alter the binding between PTCH and
SMO, alleviating SMO from PTCH inhibition and subsequently
activating downstream signaling (44).
Accumulating evidence has demonstrated that an
activated Shh pathway is associated with hepatocarcinogenesis
(11,12,32). The
Shh pathway is activated in tumors and differentiates cancerous
cells from the non-cancerous cells (45). In a transgenic mouse model, Shh
expression in the liver was indicated to, not only induce liver
fibrosis, but also enhance hepatocarcinogenesis (46). Activated Shh signaling increases
cyclin B1 and cyclin-dependent kinase 1 (CDK1) protein expression,
which facilitates the G2/M transition to promote cell
proliferation and enhance hepatocarcinogenesis (47). In addition, the overexpression of
SMO and an increased ratio of SMO to PTCH mRNA
expression is associated with tumor sizes in human HCC, and
SMO-mediated c-Myc overexpression serves a crucial role in HCC
development (32). SMO is an
important regulator of adult liver repair due to its role in the
promotion of epithelial-mesenchymal transition (EMT), and serves a
key role in the early stages of HCC development (48). Lastly, GLI transcription factors are
also involved in HCC formation. GLI-2 serves a dominant role over
GLI-1 or GLI-3 in promoting HCC cell proliferation and survival
(49). This is consistent with the
observation that enhanced GLI-2 expression is associated with early
recurrence and shorter survival in patients with HCC (31). Therefore, it can be suggested that
the Shh signaling pathway is essential for the development and
progression of HCC. Shh pathway may promote HCC growth via
upregulating genes that promote cell cycle (CYCLINS, CDK and
c-MYC) and cell survival (BCL2).
In addition to inducing HCC growth, evidence has
also demonstrated that an activated Shh signaling pathway is
associated with HCC capsular/vascular invasion (28,31). The
Shh signaling pathway mediates HCC invasion and metastasis by
upregulating matrix metalloproteinase-9 (MMP-9) (50). Bromodomain 4, which is a
transcriptional and epigenetic regulator, enhances HCC cell
migration and invasion through Shh signaling pathway-mediated MMP-2
and MMP-9 activation (51). The
upregulation of GLI-1 expression in HCC tissues is associated with
clinicopathological characteristics (52). Therefore, GLI-1 may participate in
HCC progression and metastasis via the induction of EMT. Twist is a
key transcriptional factor for EMT, which promotes the invasion and
metastasis of tumor cells (53,54).
Increased Gli-1 and Twist expression has been observed in HCC
tissues, indicating the possible involvement of the Shh pathway in
EMT (55). The Shh pathway promotes
HCC migration and invasion through activation of FAK and AKT, and
the subsequent upregulation of MMP-2 and MMP-9 expression (56). Based on the available evidence, it
can be concluded that the Shh pathway can regulate invasion,
migration and EMT, at least in part, via regulating MMP
expression.
Transforming growth factor (TGF)-β serves a key role
in the induction of EMT and its expression has been revealed to be
elevated in 40% of human HCC tissues (65,66). The
activation of the Shh signaling pathway is frequently detected in
HCC; therefore, the Shh signaling pathway may interact with TGF-β
signaling to enhance EMT in HCC (28). Using computational algorithms and
confirmation of its presence in HCC cell lines, Steinway et
al (67) demonstrated that TGF-β
induced the activation of Wnt and Shh signaling to regulate EMT in
HCC. Furthermore, GLI-2 has been identified as a direct target of
TGF-β/SMAD signaling pathway in a variety of cell types, including
fibroblasts, breast cancer cells and pancreatic carcinoma (68). GLI-2 expression is associated
with the expression of active forms of SMADs in HCC tissues
(67). Therefore, it can be
suggested that TGF-β may activate the Shh signaling pathway via
upregulation of GLI-2. TGF-β is significantly elevated with the
active form of SMO in mouse keratinocytes, suggesting that the Hh
pathway can increase TGF-β expression (69). Therefore, the expression of Shh and
TGF-β signaling together, may amplify each other and participate in
the invasion and metastasis of HCC.
In other varieties of primary and tumor cells,
active Hh signaling has been demonstrated to induce EMT via WNT,
EGF/FGF, Notch and TGF-β signaling cascades (70). The Wnt/β-catenin pathway is a
well-known promoter of HCC development, and it is possible that
this pathway cross-talks with the Shh signaling pathway to regulate
HCC progression (71). A recent
study demonstrated how the cross-talk between the Hh and Wnt
pathway can contribute to HCC formation in a diethylnitrosamine
(DEN) -administrated obese mouse model (72). In chronic fibrosis, upregulated Gli
could target Myc to drive TGF-β2 expression for Wnt5a secretion
(72). Receptors for Wnt5a are
highly expressed in mouse HCC and a number of poorly differentiated
human cell lines (72). Furthermore,
elevated Wnt5a expression has also been detected in poorly
differentiated human HCC cells, suggesting that both Hh and Wnt
ligands are able to function in an autocrine-positive feedback
manner to maintain tumors (72). In
the DEN-induced HCC model, Wnt signaling and Hh pathway activity
increased during HCC development (73). An increased Gli-1 and Gli-2
expression is associated with an increase in Wnt pathway
inhibitor-secreted frizzled related protein 1 (sFRP1) in HCC
(73). It has also been found the
upregulation of sFRP1 and Gli is present in patients with
intermediate and advanced HCC (73).
Accumulated evidence has demonstrated that the Shh
pathway is associated with the growth, invasiveness, recurrence and
cancer stem cell maintenance of HCC (12,29,30).
Therefore, inhibition of the Shh signaling pathway may be used as a
potential HCC therapeutic strategy. The inhibition of Hh signaling
may inhibit liver fibrosis and decrease cancer progenitors
(74). Hh antagonist GDC-0449 and
cyclopamine have been revealed to bind to SMO and inhibit the Shh
signaling pathway (75,76). In a HBx transgenic mouse model, the
inhibition of the Hh signaling pathway by GDC-0449 delayed
hepatocarcinogenesis (35). The
results of previous studies showed that the SMO inhibitors
cyclopamine and GDC-0449 reduced HCC growth and immune infiltration
in vivo, and tumor size and Gli-1 mRNA expression were
significantly decreased (77,78).
Thus, GDC-0449 treatment is effective in reducing HCC tumor sizes
and the degree of cell infiltration (immune cell recruitment) in
mouse models.
Gli may also be another therapeutic target. The
inhibition of GLI-1 suppresses cell growth/cell cycle progression,
induces apoptosis as well as autophagy via an Erk1/2
activity-dependent mechanism in human chondrosarcoma (79). Wang et al (80)revealed that the GLI inhibitor GANT61
inhibited tumor formation and decreased tumor sizes in a Huh7
×enograft model. The autophagy inhibitor, 3-MA, partially blocked
this effect. Results of the aforementioned study suggested that
inhibition of the Hh pathway may induce autophagy through the
upregulation of Bcl2-interacting protein 3, which displaces Bcl2
from Beclin-1 to induce apoptosis. Therefore, autophagy status is a
crucial factor to determine the therapeutic responses to
Hh-targeted therapies. Other inhibitors that target the Hh
signaling pathway, including XL-139, IPI-926 and LDE-225, were
tested in clinical trials (81).
Melittin, a bee venom, inhibits HCC cell
proliferation by downregulating Methyl-CpG binding protein 2
(MeCP2) via the Shh signaling pathway (82). Melittin treatment may increase Ptch
expression due to its induction of the demethylation of Ptch1
promoter, which is associated with the downregulation of MeCP2
(82). Furthermore, the
downregulation of Shh and Gli-1 has also been revealed in Melittin
treatment (82). In addition, the
downregulation of Gli-1 alone was not sufficient to inhibit HCC
cell proliferation, and the downregulation of Gli-2 decreased both
Gli-1 and other target gene expression. These data suggest that
Gli-2 may regulate the expression of numerous downstream genes
(49). The siRNA-mediated silencing
of GLI-2 significantly reduced HCC cell proliferation,
therefore, GLI-2 may be a novel target that can be used in the
regulation of HCC growth (49).
It has been suggested that activated Hh signaling
may protect human HCC cells from radiotherapy, and that cyclopamine
is a potential radiosensitizer (83,84). Shh
ligand has been demonstrated to exhibit a protective effect on
clonogenic cell survival upon irradiation treatment in HCC cells
(84). The combination of
irradiation and cyclopamine may be a more effective way to inhibit
HCC cell proliferation than using either modality alone. The
suppression of cell proliferation by cyclopamine may be attributed
to an increase in apoptosis. Radiation upregulates Shh expression
in a dose-dependent manner and increases Gli-1 expression in the
nucleus (84). Irradiation with
cyclopamine treatment could inhibit Gli-1 and increase the
breakdown of DNA. In a previous study, when compared with
radiotherapy alone, cyclopamine with radiotherapy reduced tumor
sizes more effectively. Therefore, radiotherapy with a Shh
inhibitor may increase the radio-sensitivity of HCC cells (84). Furthermore, 5-FU treatment may
downregulate the expression of Shh signaling and inhibit motility
in hedgehog-activated HCC cell lines (85). These results suggest that 5-FU-based
chemotherapy with a Shh signaling pathway inhibitor could be a
promising treatment option for HCC.
HCC is the most common liver malignancy worldwide.
Despite advancements in diagnostic methods such as ultrasound,
multi-detector computed tomography, magnetic resonance imaging and
biomarkers and surgical techniques, the recurrence rates after
surgical resection (57–75%) or liver transplantation (15–20%)
remain high, and early recurrence decreases the survival rates of
patients with HCC (9,10,86).
Predicting early recurrence in patients after resection remains a
challenge. For patients with a high chance of recurrence,
postoperative adjuvant therapies are required. The Shh signaling
pathway is highly activated in patients with HCC, affecting
hepatocarcinogenesis, HCC progression, cancer stem cell
maintenance, invasion and HCC recurrence (Table I). Inhibiting the Shh pathway could
therefore be an effective target therapy for HCC treatment.
Not applicable.
The current review was supported by grants from The
Far Eastern Memorial Hospital (grant nos., FEMH-2017-D-010,
FEMH-2018-C-002 and FEMH-2019-C-002), The Far Eastern Memorial
Hospital-National Yang-Ming University Joint Research Program
(grant nos., 105FN05, 105DN24, 106DN03) and The Ministry of Science
and Technology, Taiwan (grant no., MOST
106-2314-B-418-001-MY3).
Not applicable.
All authors prepared literatures, revised and
approved the final manuscript. KSJ, CML, YGT and CFC performed the
literature search, wrote/edited the manuscript and prepared the
figure and the table. CJJ, WJJ, ISS and SYL contributed to
literature review and the conception of the study.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA: Cancer J Clin. 68:7–30. 2018.PubMed/NCBI
|
|
2
|
Tsai WL and Chung RT: Viral
hepatocarcinogenesis. Oncogene. 29:2309–2324. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Arzumanyan A, Reis HM and Feitelson MA:
Pathogenic mechanisms in HBV- and HCV-associated hepatocellular
carcinoma. Nature reviews. Cancer. 13:123–135. 2013.PubMed/NCBI
|
|
4
|
Stickel F: Alcoholic cirrhosis and
hepatocellular carcinoma. Adv Exp Med Biol. 815:113–130. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kew MC: Aflatoxins as a cause of
hepatocellular carcinoma. J Gastrointestin Liver Dis. 22:305–310.
2013.PubMed/NCBI
|
|
6
|
Di Bisceglie AM: Hepatitis B and
hepatocellular carcinoma. Hepatology. 49:S56–S60. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhu RX, Seto WK, Lai CL and Yuen MF:
Epidemiology of Hepatocellular carcinoma in the asia-pacific
region. Gut Liver. 10:332–339. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kanda T, Goto T, Hirotsu Y, Moriyama M and
Omata M: Molecular mechanisms driving progression of liver
cirrhosis towards hepatocellular carcinoma in chronic hepatitis B
and C infections: A review. Int J Mol Sci. 20:13582019. View Article : Google Scholar
|
|
9
|
Daher S, Massarwa M, Benson AA and Khoury
T: Current and future treatment of hepatocellular carcinoma: An
updated C omprehensive review. J Clin Transl Hepatol. 6:69–78.
2018.PubMed/NCBI
|
|
10
|
Shindoh J, Hasegawa K, Inoue Y, Ishizawa
T, Nagata R, Aoki T, Sakamoto Y, Sugawara Y, Makuuchi M and Kokudo
N: Risk factors of post-operative recurrence and adequate surgical
approach to improve long-term outcomes of hepatocellular carcinoma.
HPB (Oxford). 15:31–39. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huang S, He J, Zhang X, Bian Y, Yang L,
Xie G, Zhang K, Tang W, Stelter AA, Wang Q, et al: Activation of
the hedgehog pathway in human hepatocellular carcinomas.
Carcinogenesis. 27:1334–1340. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Della Corte CM, Viscardi G, Papaccio F,
Esposito G, Martini G, Ciardiello D, Martinelli E, Ciardiello F and
Morgillo F: Implication of the Hedgehog pathway in hepatocellular
carcinoma. World J Gastroenterol. 23:4330–4340. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Riobo-Del Galdo NA, Lara Montero A and
Wertheimer EV: Role of Hedgehog signaling in breast cancer:
Pathogenesis and therapeutics. Cells. 8(pii): E3752019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pak E and Segal RA: Hedgehog signal
transduction: Key players, oncogenic drivers and cancer therapy.
Dev Cell. 38:333–344. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jia Y, Wang Y and Xie J: The Hedgehog
pathway: Role in cell differentiation, polarity and proliferation.
Arch Toxicol. 89:179–191. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Skoda AM, Simovic D, Karin V, Kardum V,
Vranic S and Serman L: The role of the Hedgehog signaling pathway
in cancer: A comprehensive review. Bosn J Basic Med Sci. 18:8–20.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bürglin TR: The Hedgehog protein family.
Genome Biol. 9:2412008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Machado MV and Diehl AM: Hedgehog
signalling in liver pathophysiology. J Hepatol. 68:550–562. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Finco I, LaPensee CR, Krill KT and Hammer
GD: Hedgehog signaling and steroidogenesis. Annu Rev Physiol.
77:105–129. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Varjosalo M and Taipale J: Hedgehog:
Functions and mechanisms. Genes Dev. 22:2454–2472. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Stone DM, Hynes M, Armanini M, Swanson TA,
Gu Q, Johnson RL, Scott MP, Pennica D, Goddard A, Phillips H, et
al: The tumour-suppressor gene patched encodes a candidate receptor
for Sonic hedgehog. Nature. 384:129–134. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hanna A and Shevde LA: Hedgehog signaling:
Modulation of cancer properies and tumor mircroenvironment. Mol
Cancer. 15:242016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ramsbottom SA and Pownall ME: Regulation
of Hedgehog signalling inside and outside the cell. J Dev Biol.
4:232016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pham A, Therond P, Alves G, Tournier FB,
Busson D, Lamour-Isnard C, Bouchon BL, Préat T and Tricoire H: The
Suppressor of fused gene encodes a novel PEST protein involved in
Drosophila segment polarity establishment. Genetics. 140:587–598.
1995.PubMed/NCBI
|
|
25
|
Zhang Z, Shen L, Law K, Zhang Z, Liu X,
Hua H, Li S, Huang H, Yue S, Hui CC and Cheng SY: Suppressor of
fused chaperones gli proteins to generate transcriptional responses
to sonic Hedgehog signaling. Mol Cell Biol. 37:e00421–00416. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Niewiadomski P, Niedziółka SM, Markiewicz
Ł, Uśpieński T, Baran B and Chojnowska K: Gli proteins: Regulation
in development and cancer. Cells. 8:1472019. View Article : Google Scholar
|
|
27
|
Katoh Y and Katoh M: Hedgehog target
genes: Mechanisms of carcinogenesis induced by aberrant hedgehog
signaling activation. Curr Mol Med. 9:873–886. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Che L, Yuan YH, Jia J and Ren J:
Activation of sonic hedgehog signaling pathway is an independent
potential prognosis predictor in human hepatocellular carcinoma
patients. Chin J Cancer Res. 24:323–331. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jeng KS, Sheen IS, Jeng WJ, Lin CC, Lin
CK, Su JC, Yu MC and Fang HY: High expression of patched homolog-1
messenger RNA and glioma-associated oncogene-1 messenger RNA of
sonic hedgehog signaling pathway indicates a risk of postresection
recurrence of hepatocellular carcinoma. Ann Surg Oncol. 20:464–473.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dugum M, Hanouneh I, McIntyre T, Pai R,
Aucejo F, Eghtesad B and Zein N: Sonic hedgehog signaling in
hepatocellular carcinoma: A pilot study. Mol Clin Oncol. 4:369–374.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang D, Cao L, Li Y, Lu H, Yang X and Xue
P: Expression of glioma-associated oncogene 2 (Gli 2) is correlated
with poor prognosis in patients with hepatocellular carcinoma
undergoing hepatectomy. World J Surg Oncol. 11:252013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sicklick JK, Li YX, Jayaraman A, Kannangai
R, Qi Y, Vivekanandan P, Ludlow JW, Owzar K, Chen W, Torbenson MS
and Diehl AM: Dysregulation of the Hedgehog pathway in human
hepatocarcinogenesis. Carcinogenesis. 27:748–757. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cheng WT, Xu K, Tian DY, Zhang ZG, Liu LJ
and Chen Y: Role of Hedgehog signaling pathway in proliferation and
invasiveness of hepatocellular carcinoma cells. Int J Oncol.
34:829–836. 2009.PubMed/NCBI
|
|
34
|
Tarocchi M, Polvani S, Marroncini G and
Galli A: Molecular mechanism of hepatitis B virus-induced
hepatocarcinogenesis. World J Gastroenterol. 20:11630–11640. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Arzumanyan A, Sambandam V, Clayton MM,
Choi SS, Xie G, Diehl AM, Yu DY and Feitelson MA: Hedgehog
signaling blockade delays hepatocarcinogenesis induced by hepatitis
B virus X protein. Cancer Res. 72:5912–5920. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pereira Tde A, Witek RP, Syn WK, Choi SS,
Bradrick S, Karaca GF, Agboola KM, Jung Y, Omenetti A, Moylan CA,
et al: Viral factors induce Hedgehog pathway activation in humans
with viral hepatitis, cirrhosis and hepatocellular carcinoma. Lab
Invest. 90:1690–1703. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Choi SS, Bradrick S, Qiang G, Mostafavi A,
Chaturvedi G, Weinman SA, Diehl AM and Jhaveri R: Up-regulation of
Hedgehog pathway is associated with cellular permissiveness for
hepatitis C virus replication. Hepatology. 54:1580–1590. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang Y, Chen W, Han C, Zhang J, Song K,
Kwon H, Dash S, Yao L and Wu T: Adult hepatocytes are
hedgehog-responsive cells in the setting of liver injury: Evidence
for smoothened-mediated activation of NF-kappaB/epidermal growth
factor receptor/Akt in hepatocytes that counteract fas-induced
apoptosis. Am J Pathol. 188:2605–2616. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
He G and Karin M: NF-κB and STAT3-key
players in liver inflammation and cancer. Cell Res. 21:159–168.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mills LD, Zhang Y, Marler RJ,
Herreros-Villanueva M, Zhang L, Almada LL, Couch F, Wetmore C,
Pasca di Magliano M and Fernandez-Zapico ME: Loss of the
transcription factor GLI1 identifies a signaling network in the
tumor microenvironment mediating KRAS oncogene-induced
transformation. J Biol Chem. 288:11786–11794. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sarangi A, Valadez JG, Rush S, Abel TW,
Thompson RC and Cooper MK: Targeted inhibition of the Hedgehog
pathway in established malignant glioma xenografts enhances
survival. Oncogene. 28:3468–3476. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Adolphe C, Hetherington R, Ellis T and
Wainwright B: Patched1 functions as a gatekeeper by promoting cell
cycle progression. Cancer Res. 66:2081–2088. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hasanovic A and Mus-Veteau I: Targeting
the multidrug transporter ptch1 potentiates chemotherapy
efficiency. Cells. 7(pii): E1072018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ding X, Yang Y, Han B, Du C, Xu N, Huang
H, Cai T, Zhang A, Han ZG, Zhou W and Chen L: Transcriptomic
characterization of hepatocellular carcinoma with CTNNB1 mutation.
PLoS One. 9:e953072014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Efroni S, Meerzaman D, Schaefer CF,
Greenblum S, Soo-Lyu M, Hu Y, Cultraro C, Meshorer E and Buetow KH:
Systems analysis utilising pathway interactions identifies sonic
hedgehog pathway as a primary biomarker and oncogenic target in
hepatocellular carcinoma. IET Syst Biol. 7:243–251. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chung SI, Moon H, Ju HL, Cho KJ, Kim DY,
Han KH, Eun JW, Nam SW, Ribback S, Dombrowski F, et al: Hepatic
expression of Sonic Hedgehog induces liver fibrosis and promotes
hepatocarcinogenesis in a transgenic mouse model. J Hepatol.
64:618–627. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Cai H, Li H, Li J, Li X, Li Y, Shi Y and
Wang D: Sonic hedgehog signaling pathway mediates development of
hepatocellular carcinoma. Tumour Biol. Oct 15–2016.(Epub ahead of
print). View Article : Google Scholar
|
|
48
|
Michelotti GA, Xie G, Swiderska M, Choi
SS, Karaca G, Krüger L, Premont R, Yang L, Syn WK, Metzger D and
Diehl AM: Smoothened is a master regulator of adult liver repair. J
Clin Invest. 123:2380–2394. 2013.PubMed/NCBI
|
|
49
|
Kim Y, Yoon JW, Xiao X, Dean NM, Monia BP
and Marcusson EG: Selective down-regulation of glioma-associated
oncogene 2 inhibits the proliferation of hepatocellular carcinoma
cells. Cancer Res. 67:3583–3593. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Lu JT, Zhao WD, He W and Wei W: Hedgehog
signaling pathway mediates invasion and metastasis of
hepatocellular carcinoma via ERK pathway. Acta Pharmacol Sin.
33:691–700. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wang YH, Sui XM, Sui YN, Zhu QW, Yan K,
Wang LS, Wang F and Zhou JH: BRD4 induces cell migration and
invasion in HCC cells through MMP-2 and MMP-9 activation mediated
by the Sonic hedgehog signaling pathway. Oncol Lett. 10:2227–2232.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Xu Q, Liu X, Zheng X, Yao Y, Wang M and
Liu Q: The transcriptional activity of Gli1 is negatively regulated
by AMPK through Hedgehog partial agonism in hepatocellular
carcinoma. Int J Mol Med. 34:733–741. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Matsuo N, Shiraha H, Fujikawa T, Takaoka
N, Ueda N, Tanaka S, Nishina S, Nakanishi Y, Uemura M, Takaki A, et
al: Twist expression promotes migration and invasion in
hepatocellular carcinoma. BMC Cancer. 9:2402009. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Meng J, Chen S, Han JX, Qian B, Wang XR,
Zhong WL, Qin Y, Zhang H, Gao WF, Lei YY, et al: Twist1 regulates
vimentin through Cul2 circular RNA to promote EMT in hepatocellular
carcinoma. Cancer Res. 78:4150–4162. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Chun HW and Hong R: Significance of the
hedgehog pathway-associated proteins Gli-1 and Gli-2 and the
epithelial-mesenchymal transition-associated proteins Twist and
E-cadherin in hepatocellular carcinoma. Oncol Lett. 12:1753–1762.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Chen JS, Huang XH, Wang Q, Huang JQ, Zhang
LJ, Chen XL, Lei J and Cheng ZX: Sonic hedgehog signaling pathway
induces cell migration and invasion through focal adhesion
kinase/AKT signaling-mediated activation of matrix
metalloproteinase (MMP)-2 and MMP-9 in liver cancer.
Carcinogenesis. 34:10–19. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Zeller C, Hinzmann B, Seitz S, Prokoph H,
Burkhard-Goettges E, Fischer J, Jandrig B, Schwarz LE, Rosenthal A
and Scherneck S: SASH1: A candidate tumor suppressor gene on
chromosome 6q24.3 is downregulated in breast cancer. Oncogene.
22:2972–2983. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
He P, Zhang HX, Sun CY, Chen CY and Jiang
HQ: Overexpression of SASH1 inhibits the proliferation, invasion
and EMT in hepatocarcinoma cells. Oncol Res. 24:25–32. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Sun C, Zhang Z, He P, Zhou Y and Xie X:
Involvement of PI3K/Akt pathway in the inhibition of
hepatocarcinoma cell invasion and metastasis induced by SASH1
through downregulating Shh-Gli1 signaling. Int J Biochem Cell Biol.
89:95–100. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Kitagawa H, Uyama T and Sugahara K:
Molecular cloning and expression of a human chondroitin synthase. J
Biol Chem. 276:38721–38726. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Tian J, Ling L, Shboul M, Lee H, O'Connor
B, Merriman B, Nelson SF, Cool S, Ababneh OH, Al-Hadidy A, et al:
Loss of CHSY1, a secreted FRINGE enzyme, causes syndromic
brachydactyly in humans via increased NOTCH signaling. Am J Hum
Genet. 87:768–778. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Liu CH, Lan CT, Chou JF, Tseng TJ and Liao
WC: CHSY1 promotes aggressive phenotypes of hepatocellular
carcinoma cells via activation of the hedgehog signaling pathway.
Cancer Lett. 403:280–288. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Merchant AA and Matsui W: Targeting
Hedgehog-a cancer stem cell pathway. Clin Cancer Res. 16:3130–3140.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Jeng KS, Sheen IS, Jeng WJ, Yu MC, Hsiau
HI, Chang FY and Tsai HH: Activation of the sonic hedgehog
signaling pathway occurs in the CD133 positive cells of mouse liver
cancer Hepa 1–6 cells. Onco Targets Ther. 6:1047–1055. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Abou-Shady M, Baer HU, Friess H, Berberat
P, Zimmermann A, Graber H, Gold LI, Korc M and Büchler MW:
Transforming growth factor betas and their signaling receptors in
human hepatocellular carcinoma. Am J Surg. 177:209–215. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Fabregat I and Caballero-Diaz D:
Transforming growth factor-beta-induced cell plasticity in liver
fibrosis and Hepatocarcinogenesis. Front Oncol. 8:3572018.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Steinway SN, Zañudo JG, Ding W, Rountree
CB, Feith DJ, Loughran TP Jr and Albert R: Network modeling of
TGFbeta signaling in hepatocellular carcinoma
epithelial-to-mesenchymal transition reveals joint sonic hedgehog
and Wnt pathway activation. Cancer Res. 74:5963–5977. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Javelaud D, Alexaki VI, Dennler S,
Mohammad KS, Guise TA and Mauviel A: TGF-β/SMAD/GLI2 signaling axis
in cancer progression and metastasis. Cancer Res. 71:5606–5610.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Fan Q, He M, Sheng T, Zhang X, Sinha M,
Luxon B, Zhao X and Xie J: Requirement of TGFbeta signaling for
SMO-mediated carcinogenesis. J Biol Chem. 285:36570–36576. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Katoh Y and Katoh M: Hedgehog signaling,
epithelial-to-mesenchymal transition and miRNA (Review). Int J Mol
Med. 22:271–275. 2008.PubMed/NCBI
|
|
71
|
Giakoustidis A, Giakoustidis D, Mudan S,
Sklavos A and Williams R: Molecular signalling in hepatocellular
carcinoma: Role of and crosstalk among WNT/ss-catenin, Sonic
Hedgehog, Notch and Dickkopf-1. Can J Gastroenterol Hepatol.
29:209–217. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Chong YC, Lim TE, Fu Y, Shin EM,
Tergaonkar V and Han W: Indian Hedgehog links obesity to
development of hepatocellular carcinoma. Oncogene. 38:2206–2222.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Tripathy A, Thakurela S, Sahu MK,
Uthanasingh K, Behera M, Ajay AK and Kumari R: The molecular
connection of histopathological heterogeneity in hepatocellular
carcinoma: A role of Wnt and Hedgehog signaling pathways. PLoS One.
13:e02081942018. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Salaritabar A, Berindan-Neagoe I, Darvish
B, Hadjiakhoondi F, Manayi A, Devi KP, Barreca D, Orhan IE, Süntar
I, Farooqi AA, et al: Targeting Hedgehog signaling pathway: Paving
the road for cancer therapy. Pharmacol Res. 141:466–480. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Dijkgraaf GJ, Alicke B, Weinmann L,
Januario T, West K, Modrusan Z, Burdick D, Goldsmith R, Robarge K,
Sutherlin D, et al: Small molecule inhibition of GDC-0449
refractory smoothened mutants and downstream mechanisms of drug
resistance. Cancer Res. 71:435–444. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Chen JK, Taipale J, Cooper MK and Beachy
PA: Inhibition of Hedgehog signaling by direct binding of
cyclopamine to Smoothened. Genes Dev. 16:2743–2748. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Jeng KS, Sheen IS, Jeng WJ, Yu MC, Tsai
HH, Chang FY and Su JC: Blockade of the sonic hedgehog pathway
effectively inhibits the growth of hepatoma in mice: An in
vivo study. Oncol Lett. 4:1158–1162. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Jeng KS, Jeng CJ, Jeng WJ, Sheen IS, Chang
CF, Hsiau HI, Hung ZH, Yu MC and Chang FY: Sonic hedgehog pathway
inhibitor mitigates mouse hepatocellular carcinoma. Am J Surg.
210:554–560. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Sun Y, Guo W, Ren T, Liang W, Zhou W, Lu
Q, Jiao G and Yan T: Gli1 inhibition suppressed cell growth and
cell cycle progression and induced apoptosis as well as autophagy
depending on ERK1/2 activity in human chondrosarcoma cells. Cell
Death Dis. 5:e9792014. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Wang Y, Han C, Lu L, Magliato S and Wu T:
Hedgehog signaling pathway regulates autophagy in human
hepatocellular carcinoma cells. Hepatology. 58:995–1010. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Lin TL and Matsui W: Hedgehog pathway as a
drug target: Smoothened inhibitors in development. Onco Targets
Ther. 5:47–58. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Wu X, Zhao B, Cheng Y, Yang Y, Huang C,
Meng X, Wu B, Zhang L, Lv X and Li J: Melittin induces PTCH1
expression by down-regulating MeCP2 in human hepatocellular
carcinoma SMMC-7721 cells. Toxicol Appl Pharmacol. 288:74–83. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Chen YJ, Lin CP, Hsu ML, Shieh HR, Chao NK
and Chao KS: Sonic hedgehog signaling protects human hepatocellular
carcinoma cells against ionizing radiation in an autocrine manner.
Int J Radiat Oncol Biol Phys. 80:851–859. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Tsai CL, Hsu FM, Tzen KY, Liu WL, Cheng AL
and Cheng JC: Sonic Hedgehog inhibition as a strategy to augment
radiosensitivity of hepatocellular carcinoma. J Gastroenterol
Hepatol. 30:1317–1324. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Wang Q, Huang S, Yang L, Zhao L, Yin Y,
Liu Z, Chen Z and Zhang H: Down-regulation of Sonic hedgehog
signaling pathway activity is involved in 5-fluorouracil-induced
apoptosis and motility inhibition in Hep3B cells. Acta Biochim
Biophys Sin (Shanghai). 40:819–829. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Filgueira NA: Hepatocellular carcinoma
recurrence after liver transplantation: Risk factors, screening and
clinical presentation. World J Hepatol. 11:261–272. 2019.
View Article : Google Scholar : PubMed/NCBI
|