Introduction
Worldwide, breast cancer is the most common cancer
and the first leading cause of cancer mortalities in women
(1). Anthracycline agents are
effective and widely used chemotherapeutic drugs for a number of
solid tumors including breast cancer (2). However, the related cardiotoxicity is
the leading cause of discontinuation of treatment and may even
result in cardiovascular-associated death (3). Furthermore, the cardiotoxicity is
usually progressive and irreversible once exhibited, and the
treatments available are less effective (4). Therefore, it is important to identify
anthracycline-induced cardiotoxicity (AIC) as early as possible in
order to adjust or cease treatment prior to irreversible damage of
cardiac tissues.
At present, the primarily used methods to detect
cardiotoxicity include electrocardiograms and echocardiography,
which are widely available and inexpensive. However, the
irreversible cardiac cellular damage usually has occurred before
detection with electrocardiogram and echocardiography in the
majority of cases (5). Previous
studies focus on searching for selective biomarkers of
cardiotoxicity. So far, several biomarkers such as cardiac
troponins (6) and plasma natriuretic
peptides (7) were found to have
potential to contribute to monitoring chemotherapy-induced
cardiotoxicity.
Brain natriuretic peptide (BNP), a neurohormone
released by the left ventricular cardiomyocytes in response to
cardiac wall stress, is widely used to diagnose and monitor
congestive heart failure (CHF) (7).
High concentrations of BNP have been described not only in patients
with acute myocardial infarction or advanced CHF but also in
patients with asymptomatic or minimally symptomatic LV dysfunction
(8). Previously, multiple studies
demonstrated that serum BNP may be an independent biomarker in
detection of cardiotoxicity, however, relatively little known is
about the predictive applicability of BNP in anthracycline-treated
patients (9–13). Therefore, the aim of the present
study was to assess the potential role of BNP detection in the
early prediction of anthracycline-induced cardiotoxicity in
patients with breast cancer.
Materials and methods
Population
Between November 2013 and August 2016, patients
admitted to the Department of Breast Diseases of The First Hospital
of Jiaxing (Jiaxing, China) with breast cancer were prospectively
recruited. The patients underwent breast cancer surgery combined
with postoperative chemotherapy. Following the surgery, tumor
tissues were collected and used for immunohistochemistry (IHC) and
fluorescence in situ hybridization (FISH) at the Department
of Pathology of The First Hospital of Jiaxing. The inclusion
criteria were: i) Histologically confirmed primary breast cancer;
and ii) indications to suggest treatment with anthracycline-based
chemotherapy. The exclusion criteria were: i) Breast cancer in
situ or at stage IV; ii) prior exposure to anthracyclines; iii)
a history of congenital heart disease, coronary heart disease,
heart valve disease or a left ventricular ejection fraction (LVEF)
<50%; iv) loss to follow-up; v) required treatment with an
additional course of chemotherapy or other therapeutic regimen, due
to the recurrence or metastasis during the follow-up.
In total, 149 cases, all female, with a median age
of 51 (range, 28–69) years were included. Among the 149 evaluable
patients, 129 cases were confirmed as invasive ductal carcinoma and
there were 48 cases of stage I, 73 cases of stage II and 28 cases
of stage III cancer according to the 7th Edition American Joint
Committee on Cancer staging manual (14). According to the IHC or FISH results
of breast cancer tissues, tumors were classified as estrogen
receptor-positive (ER+) if >1% of the cells were
stained positively. For human epidermal growth factor receptor-2
(HER2), the staining was scored as previously described (15); tumors were classified as
HER2+ if the results of IHC were scored 3+, or if the
results of IHC were scored 2+ but confirmed positive by the further
FISH detection. Otherwise, tumors were classified as ER-negative
(ER−) or HER2-negtive (HER2−). Among the 149
patients, 77 cases were ER+ but HER2−, 21
cases were ER+ and HER2+, 29 cases were
ER− and HER2− and 22 cases were
ER− but HER2+. The details on the patients
and the tumor characteristics are summarized in Table I. The design of the present study was
approved by The Ethical Committee of The First Hospital of Jiaxing.
All the patients signed written informed consent form to
participate in the present study.
 | Table I.The association of cardiotoxicity
with clinicopathological characteristics. |
Table I.
The association of cardiotoxicity
with clinicopathological characteristics.
|
| Total (n=149) | Cardiotoxicity
group (n=52) | Non-cardiotoxicity
group (n=97) |
|
|---|
|
|
|
|
|
|
|---|
| Variable | n | % | n | % | n | % | P-value |
|---|
| Age, years |
|
|
|
|
|
| 0.991 |
|
≤50 | 66 | 44 | 23 | 44 | 43 | 44 |
|
|
>50 | 83 | 56 | 29 | 56 | 54 | 56 |
|
| Menopause
status |
|
|
|
|
|
| 0.496 |
|
Premenopausal | 83 | 56 | 27 | 52 | 56 | 58 |
|
|
Menopause | 66 | 44 | 25 | 48 | 41 | 42 |
|
| BSA |
|
|
|
|
|
| 0.777 |
| ≤1.7
m2 | 75 | 50 | 27 | 52 | 48 | 49 |
|
| >1.7
m2 | 74 | 50 | 25 | 48 | 49 | 51 |
|
| BMI |
|
|
|
|
|
| 0.991 |
|
≤23.9 | 83 | 56 | 29 | 56 | 54 | 56 |
|
|
>23.9 | 66 | 44 | 23 | 44 | 43 | 44 |
|
| Hypertension |
|
|
|
|
|
| 0.247 |
|
Yes | 37 | 25 | 10 | 19 | 27 | 28 |
|
| No | 112 | 75 | 42 | 81 | 70 | 72 |
|
| Tumor
histology |
|
|
|
|
|
| 0.308 |
|
Invasive ductal | 129 | 87 | 43 | 83 | 86 | 89 |
|
|
Others | 20 | 13 | 9 | 17 | 11 | 11 |
|
| TNM stage |
|
|
|
|
|
| 0.657 |
| I | 48 | 32 | 19 | 37 | 29 | 30 |
|
|
IIA | 26 | 17 | 9 | 17 | 17 | 18 |
|
|
IIB | 47 | 32 | 14 | 27 | 33 | 34 |
|
|
IIIA | 20 | 13 | 6 | 12 | 14 | 14 |
|
|
IIIB | 1 | 0 | 1 | 2 | 0 | 0 |
|
|
IIIC | 7 | 5 | 3 | 6 | 4 | 4 |
|
| ER/HER2
statusa |
|
|
|
|
|
| 0.303 |
|
ER+
HER2− | 77 | 52 | 26 | 50 | 51 | 53 |
|
|
ER+
HER2+ | 21 | 14 | 11 | 21 | 10 | 10 |
|
|
ER−
HER2− | 29 | 19 | 8 | 15 | 21 | 22 |
|
|
ER−
HER2+ | 22 | 15 | 7 | 13 | 15 | 15 |
|
| Chemotherapeutic
regimen |
|
|
|
|
|
| 0.536 |
|
EC/AC | 44 | 30 | 17 | 33 | 27 | 28 |
|
|
EC-T/AC-T | 105 | 70 | 35 | 67 | 70 | 72 |
|
| Cumulative dose of
adriamycin |
|
|
|
|
|
| 0.151 |
| ≤175
mg/m2 | 75 | 50 | 22 | 42 | 53 | 55 |
|
| >175
mg/m2 | 74 | 50 | 30 | 58 | 44 | 45 |
|
| Herceptin
therapy |
|
|
|
|
|
| 0.973 |
|
Yes | 26 | 38 | 9 | 17 | 17 | 18 |
|
| No | 123 | 62 | 43 | 83 | 80 | 82 |
|
| Tamoxifen
therapy |
|
|
|
|
|
| 0.871 |
|
Yes | 56 | 38 | 20 | 38 | 36 | 37 |
|
| No | 93 | 62 | 32 | 62 | 61 | 63 |
|
Chemotherapy treatment
All patients received anthracycline-based
chemotherapy. The chemotherapeutic regimen was dependent on the
disease status and risk factors, including epirubicin (E)
cyclophosphamide (C), EC-docetaxel (T), pirarubicin (A) C and AC-T.
The EC regimen was: 90 mg/m2 E and 600 mg/m2
C, both used on the first day and administered every 21 days for a
total of four courses. The EC-T regimen was similar to the EC
regimen, where E and C were used at the same dose during the first
four course, after which, T was given at 90 mg/m2 for
four courses (every 21 days). The AC regimen was: 50
mg/m2 A and 600 mg/m2 C were both
administered every 21 days for four courses in total. The AC-T
regimen was similar to the AC treatment regimen, where A and C were
used at the same dose in the first four course, after which, T was
given at 90 mg/m2 for four courses (every 21 days).
All the patients underwent baseline plasma BNP and
echocardiography examination prior to initiation of chemotherapy.
For BNP detection, all the pre-chemotherapy samples were obtained
at least 48 h prior to initiation of therapy from fasting resting
venous blood; whereas the post-chemotherapy samples were collected
24 h after each course of therapy (16). The blood samples collected were
placed in EDTA anticoagulation tubes and the serum was obtained by
centrifuging for 15 min at 4°C at 1,800 × g for detection of BNP
levels. The plasma BNP levels were determined by measuring the
chemiluminescence in a micro-particle immunoassay. Briefly, the
plasma BNP was isolated using an Abbott's B-type natriuretic
peptide assay kit (cat. no. 71008M902; Abbott Pharmaceutical Co.
Ltd.) and quantified using Architect i1000 automatic immunoassay
analyzer. Echocardiography, which measures both LVEF and left
ventricular end-systolic dimension (LVDs), was performed during
each course of chemotherapy.
Follow-up
After every 3 to 6 months of initiation
chemotherapy, the patients underwent a necessary cardiac
evaluation, including a detailed physical examination of their
symptoms and signs and echocardiography. During this period, the
moment when cardiotoxicity was determined to meet the evaluation
criteria set as the end point of observation and the
cardiotoxicity-free survival time were recorded. The median
follow-up duration was 1.5 (0.1 to 3.8) years.
Cardiotoxicity evaluation
criteria
In the present study, variations in LVEF and LVDs
were used for cardiotoxicity evaluation (17–19).
LVEF variation was measured by calculating maximum drop in LVEF
(ΔLVEF), calculated using baseline LVEF-monitored minimum LVEF.
Maximum rise in LVDs (ΔLVDs) was used to calculate variations in
LVDs and it was calculated as the monitored maximum LVDs-baseline
LVDs. If any one of these four criteria including symptoms and
signs of cardiac insufficiency, LVEF <55%, ΔLVEF ≥10% and ΔLVDs
≥7 mm was observed in the participants, they were moved to the
cardiotoxicity group, otherwise, the patients were placed in the
non-cardiotoxicity group.
Receiver operating characteristic
curves plotting
To evaluate the ability of serum BNP levels to
predict anthracycline-induced cardiotoxicity early, a receiver
operating characteristic (ROC) curve was plotted. The statistical
analysis of the ROC curve was performed using SPSS 17.0. The area
under the curve (AUC) was determined using the Wilcoxon
Mann-Whitney method. The optimal BNP threshold, diagnostic
sensitivity and specificity of BNP in predicting
anthracycline-induced cardiotoxicity were determined by the maximum
of Youden index, which was calculated as Youden index=sensitivity
(Se) + specificity (Sp) −1. The prevalence (p) of cardiotoxicity
was calculated as p=number of patients with cardiotoxicity/total
number of patients. The positive predictive value (PPV) was
calculated as PPV=p × Se/[(p × Se + (1-p) × (1-Sp)]. The negative
predictive value (NPV) was calculated as NPV=(1-p) × Sp/[(1-p) × Sp
+ p × (1-Se)].
Statistical analysis
Statistical analyses were performed using SPSS 17.0
(SPSS, Inc.). Measurements were presented as the mean ± standard
deviation and counts were expressed in absolute value. For
assessing the differences between BNP levels before and after each
chemotherapy, a paired samples t-test was used, whereas an
independent samples t-test was used for the differences in BNP
between the cardiotoxicity group and non-cardiotoxicity group.
Counting data were compared using a χ2 test. Repeated
measures analysis of variance (ANOVA) method was used to assess the
variation of the levels of BNP during each course of chemotherapy.
Kaplan-Meier method was used for calculating the survival rates and
a log-rank test was used for comparing survival rates and to infer
the cardiotoxicity-associated factors, a Cox proportional hazards
regression model was used. The optimal diagnostic threshold was
determined using ROC and the AUC was determined using the Wilcoxon
Mann-Whitney method. P<0.05 was considered to indicate a
statistically significant difference. All experiments and
statistical analyses were performed twice to ensure accuracy.
Results
Cardiotoxicity and patient
characteristics
In the present study, a total of 52 patients who met
one of the cardiotoxicity criteria were classified into the
cardiotoxicity group and the remaining 97 patients were classified
into the non-cardiotoxicity group. Table
I summarizes the characteristics of the patients. There was no
significant difference in the various clinical and pathological
features between the two groups. Chemotherapy regimens and drug
dose of these patients are also listed in Table I. Body surface area (BSA) was
calculated as=0.73× height (in m) + 0.0127× weight (in kg) −0.2106.
The median BSA value in the study was 1.7 (range 1.4–2.0)
m2. Body mass index (BMI) was calculated as=weight (in
kg)/height (in m)2. The cumulative dose of adriamycin
refers to the dose administrated per m2 BSA. In the
present study, the median value of the cumulative dose of
adriamycin was 175 (152–216) mg/m2. The dose of
epirubicin administered was determined using a 1:2 adriamycin to
epirubicin ratio (3) and that of
pirarubicin was determined using a 1:1.08 adriamycin to pirarubicin
ratio (20). For the group with a
cumulative dose of adriamycin ≤175 mg/m2, the incidence
of cardiotoxicity was reduced compared with the group with a
cumulative dose >175 mg/m2. However, the difference
was not statistically significant, possibly because the cumulative
doses of adriamycin amongst all patients were relatively low in the
present study. The incidence of cardiotoxicity in patients who
received EC-T/AC-T chemotherapy was slightly higher compared with
patients who received EC/AC therapy, indicating that docetaxel may
contribute to heart tissue damage, although the difference was not
significant, as the temporary-induced damage may be not be
permanent (Table I).
Effect of chemotherapy on the levels
of BNP
The levels of BNP were measured before and after
each treatment in the present study, and the variation was
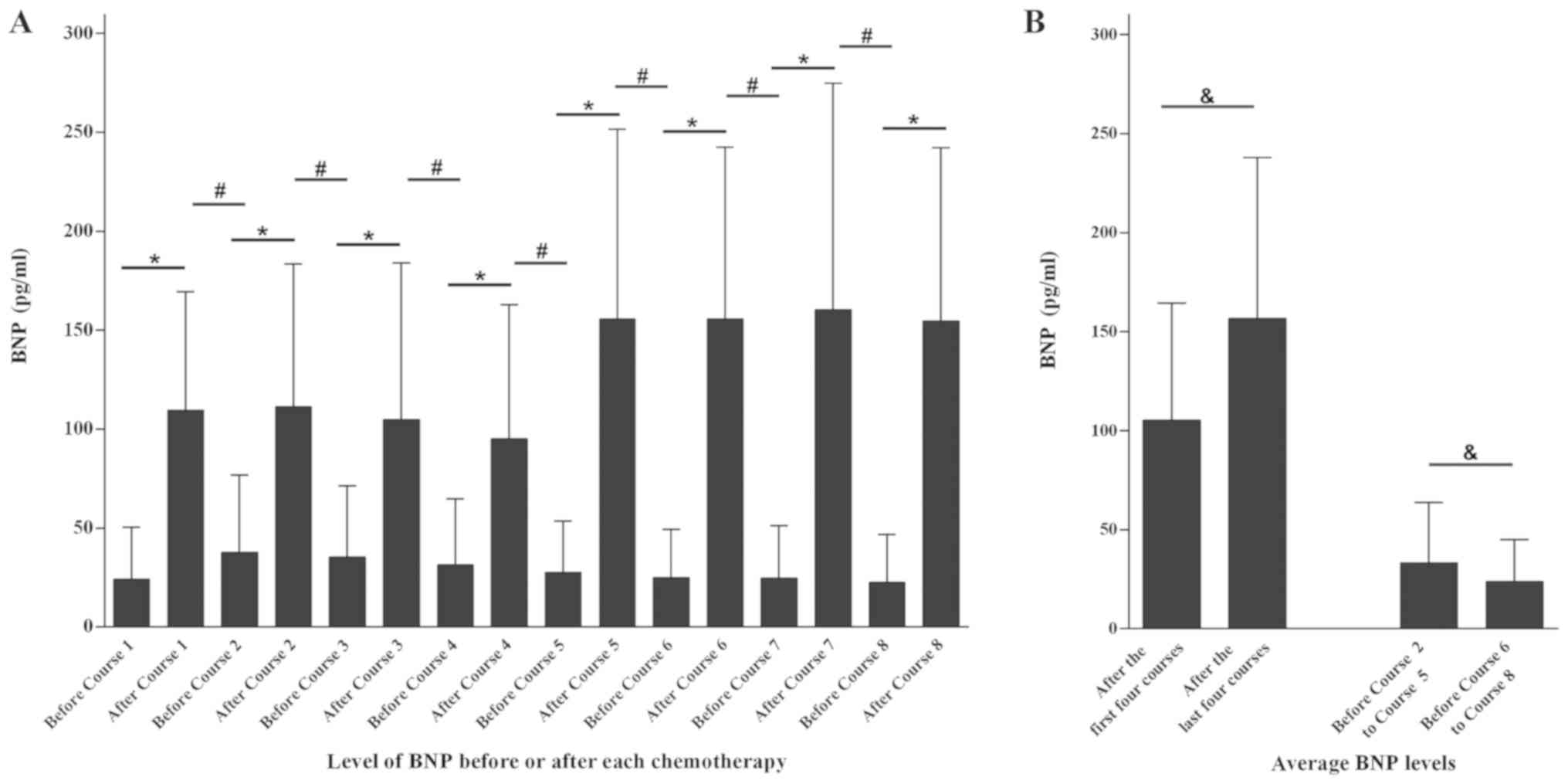
evaluated by a paired t-test (Fig.
1A). The serum BNP concentration after each therapy was
significantly increased compared with before (P<0.001),
suggesting that a single dose of chemotherapy could lead to
elevated levels of BNP. Furthermore, the paired t-test also
indicated that levels of BNP prior to the next dose of chemotherapy
were significantly decreased compared with the measurement taken
after the previous dose (P<0.001), suggesting that the increase
in BNP was transient and following clearance of chemotherapeutic
agents in vivo, the levels of BNP gradually decreased
(Fig. 1A). For patients who
underwent EC-T/AC-T chemotherapy, the average levels of BNP in each
patient after the first four and the last four course were compared
(Fig. 1B). The results showed that
the average value after the first four courses was significantly
decreased compared with last four courses (P<0.001), indicating
the ability of anthracycline to increase serum BNP levels was lower
compared with docetaxel. To compare the lowest possible
measurements of BNP throughout the recovery period after treatment
with anthracyclines and docetaxel, the average serum BNP levels of
the second to the fifth course and the sixth to eighth course prior
to each treatment were calculated. The average level of BNP in the
former set off measurements was significantly higher compared with
the latter set of measurements (P<0.001), suggesting a slower
clearance of BNP levels following treatment with anthracyclines
compared with docetaxel chemotherapy (Fig. 1B).
BNP and cardiotoxicity
In the cardiotoxicity group and non-cardiotoxicity
group, an independent t-test was performed to compare the serum BNP
levels before and after each dose of chemotherapy. Prior to the
second dose (Course 2) of chemotherapy, the serum levels of BNP
were significantly increased in the cardiotoxicity group compared
with the non-cardiotoxicity group (P=0.043; Table II), suggesting that the initial dose
of anthracyclines resulted in a slower decrease of BNP levels in
the cardiotoxicity group. The underlying reason might be that once
myocardial damage has been established, BNP would last longer due
to sustained release. However, following the fourth dose of
chemotherapy, the BNP levels were significantly increased in the
cardiotoxicity group compared with the non-cardiotoxicity group
(P=0.012; Table II), suggesting
that at this dose the administered anthracyclines had resulted in
the maximal amount of damage to cardiac tissue. These results
showed that the elevation of BNP levels induced by anthracyclines
was associated with cardiotoxicity. Furthermore, there was no
significant difference in the levels of BNP found between the
cardiotoxicity group and non-cardiotoxicity group during docetaxel
chemotherapy (Table II).
 | Table II.The relationship between BNP levels
and cardiotoxicity. |
Table II.
The relationship between BNP levels
and cardiotoxicity.
|
| Cardiotoxicity
group | Non-cardiotoxicity
group |
|
|---|
|
|
|
|
|
|---|
| Chemotherapy | BNP levels, mean ±
SD, pg/ml | n | BNP levels, mean ±
SD, pg/ml | n | P-value |
|---|
| Before course
1 | 27.2±31.5 | 52 | 22.5±23.3 | 97 | 0.306 |
| After course 1 | 119.0±65.3 | 52 | 104.6±56.5 | 97 | 0.164 |
| Before course
2 | 47.8±47.7 | 52 | 32.6±32.3 | 97 | 0.043a |
| After course 2 | 121.6±78.1 | 52 | 105.7±68.6 | 97 | 0.199 |
| Before course
3 | 42.2±44.2 | 52 | 31.7±30.4 | 97 | 0.091 |
| After course 3 | 118.3±85.2 | 52 | 97.6±75.0 | 97 | 0.127 |
| Before course
4 | 39.4±44.6 | 52 | 27.3±24.5 | 97 | 0.072 |
| After course 4 | 114.2±65.2 | 52 | 85.1±66.8 | 97 | 0.012a |
| Before course
5 | 29.3±30.0 | 35 | 26.9±23.7 | 70 | 0.654 |
| After course 5 | 161.2±123.0 | 35 | 152.8±80.1 | 70 | 0.674 |
| Before course
6 | 24.9±23.1 | 35 | 25.2±25.1 | 70 | 0.954 |
| After course 6 | 140.2±62.4 | 35 | 163.6±95.9 | 70 | 0.136 |
| Before course
7 | 27.6±31.2 | 35 | 23.4±24.0 | 70 | 0.456 |
| After course 7 | 154.9±104.9 | 35 | 163.2±119.3 | 70 | 0.727 |
| Before course
8 | 25.1±26.1 | 35 | 21.4±23.3 | 70 | 0.458 |
| After course 8 | 146.8±76.5 | 35 | 158.6±92.8 | 70 | 0.519 |
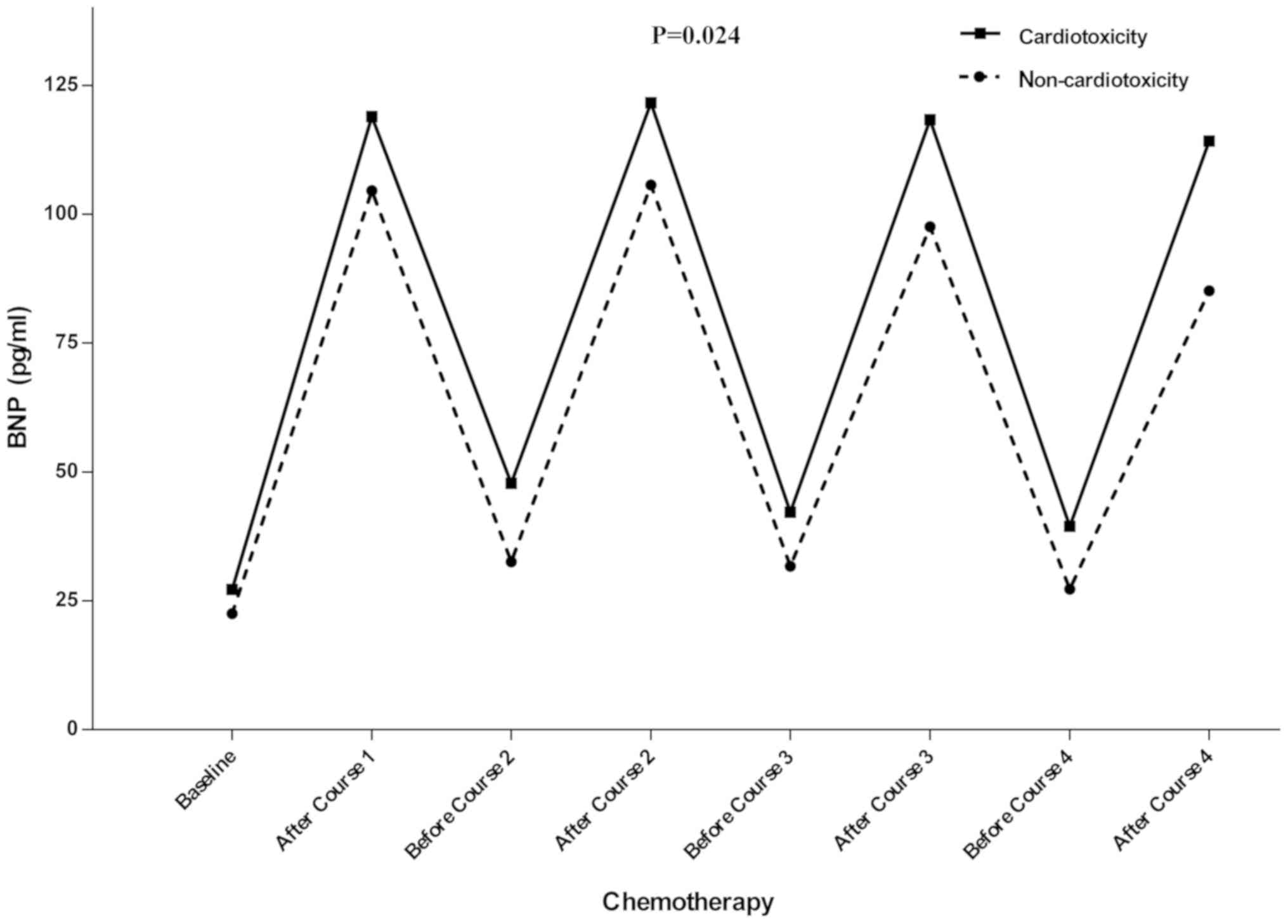
ANOVA was used to assess the variation of the levels
of BNP during the chemotherapy. The results above demonstrated that
BNP levels were associated with anthracyclines rather than
docetaxel, BNP levels from the first and fourth dose of
chemotherapy were included in this comparison. A significant
difference in the levels of BNP was determined between the two
groups, with the levels of BNP consistently being higher in the
cardiotoxicity group compared with the non-cardiotoxicity group in
each treatment with anthracycline (P=0.024). Therefore, the
increase in the levels of BNP was associated with
anthracyclines-induced cardiotoxicity (Fig. 2).
Cox proportional hazards regression
model
To plot the survival curves, the factors which may
have influenced the disease-free time, including serum BNP levels
after the fourth course of chemotherapy, cumulative dose of
adriamycin, hypertension, age, height and BSA were analyzed. These
factors were included in a multivariate analysis of Cox
proportional hazards regression model and the specific effect of
the factor and therefore its involvement in the regression model
was termed as ‘entry’. Serum BNP levels following the fourth course
of chemotherapy was an independent predictor for cardiotoxicity
(P=0.047); whereas the other factors were not (Table III).
 | Table III.Multivariate analysis of factors that
may have influenced the disease-free time. |
Table III.
Multivariate analysis of factors that
may have influenced the disease-free time.
| Covariate | Comparison | Hazard ratio | 95% CI | P-value |
|---|
| Age | Continuous | 1.010 | 0.971–1.052 | 0.612 |
| Height | Continuous | 2.677 |
0.001–5,937.427 | 0.802 |
| BSA | Continuous | 1.219 | 0.066–22.354 | 0.894 |
| BNP levels after
course 4 | Continuous | 1.003 | 1.000–1.007 | 0.047a |
| Hypertension | No vs. Yes | 1.598 | 0.747–3.417 | 0.227 |
| Cumulative dose of
adriamycin | Continuous | 1.007 | 0.996–1.018 | 0.212 |
Ability of serum BNP levels in
predicting cardiotoxicity early
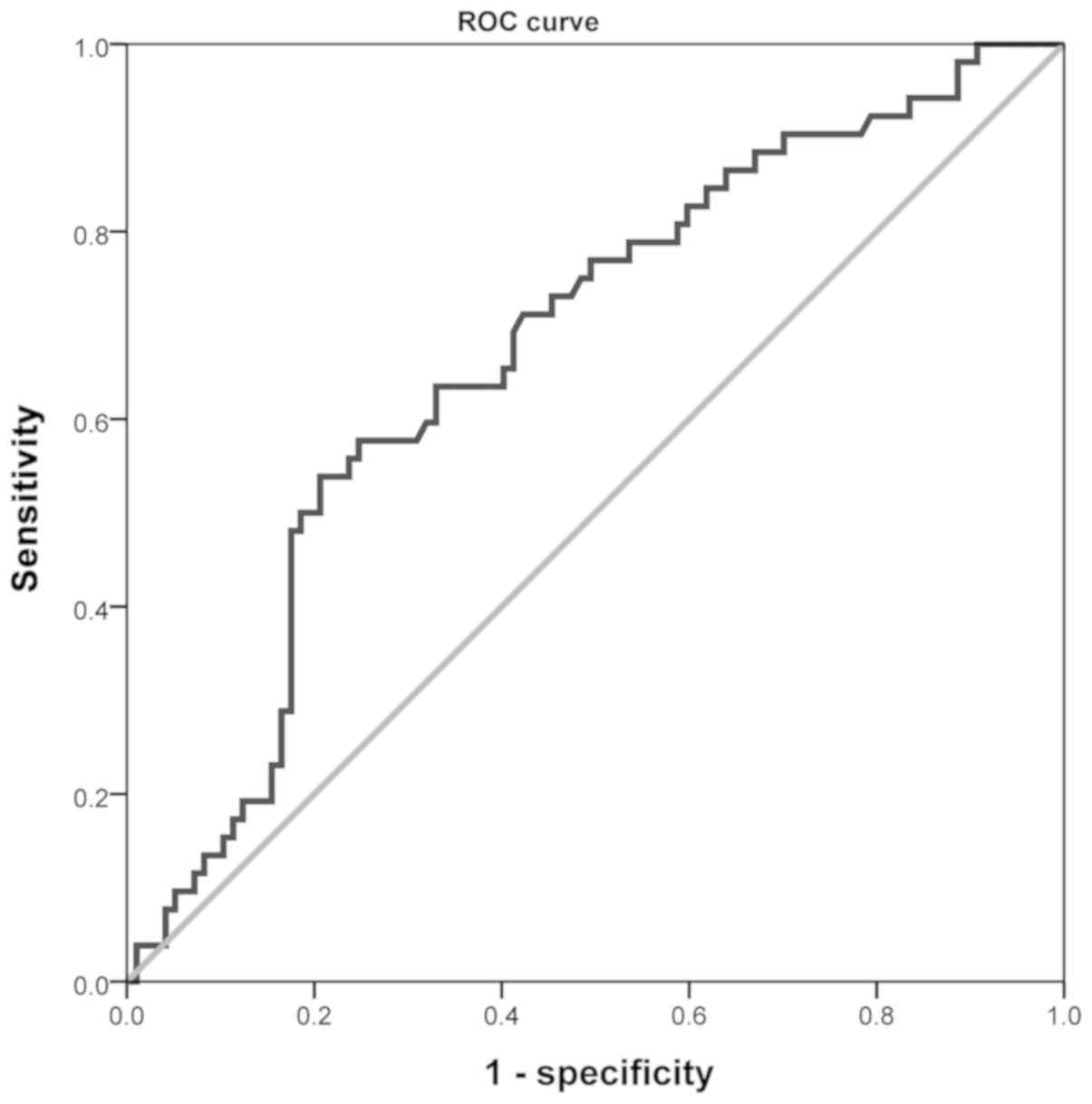
The ROC curve of BNP levels after the fourth course
of chemotherapy against cardiotoxicity is shown in Fig. 3. The statistical analysis of the ROC
curve was performed using SPSS 17.0. The area under the cure (AUC)
was 0.673 [95% confidence interval (CI), 0.584–0.763], suggesting
that the BNP level after the fourth dose of chemotherapy had a role
in diagnosing cardiotoxicity. ROC curve analysis demonstrated that
the optimal BNP threshold was 107.9 pg/ml at the maximum Youden
index of 0.332. The prevalence of cardiotoxicity was 34.9%. The
diagnostic sensitivity of anthracycline-induced cardiotoxicity was
0.538, the specificity was 0.794, the positive predictive value
(PPV) was 0.583 and the negative predictive value (NPV) was
0.762.
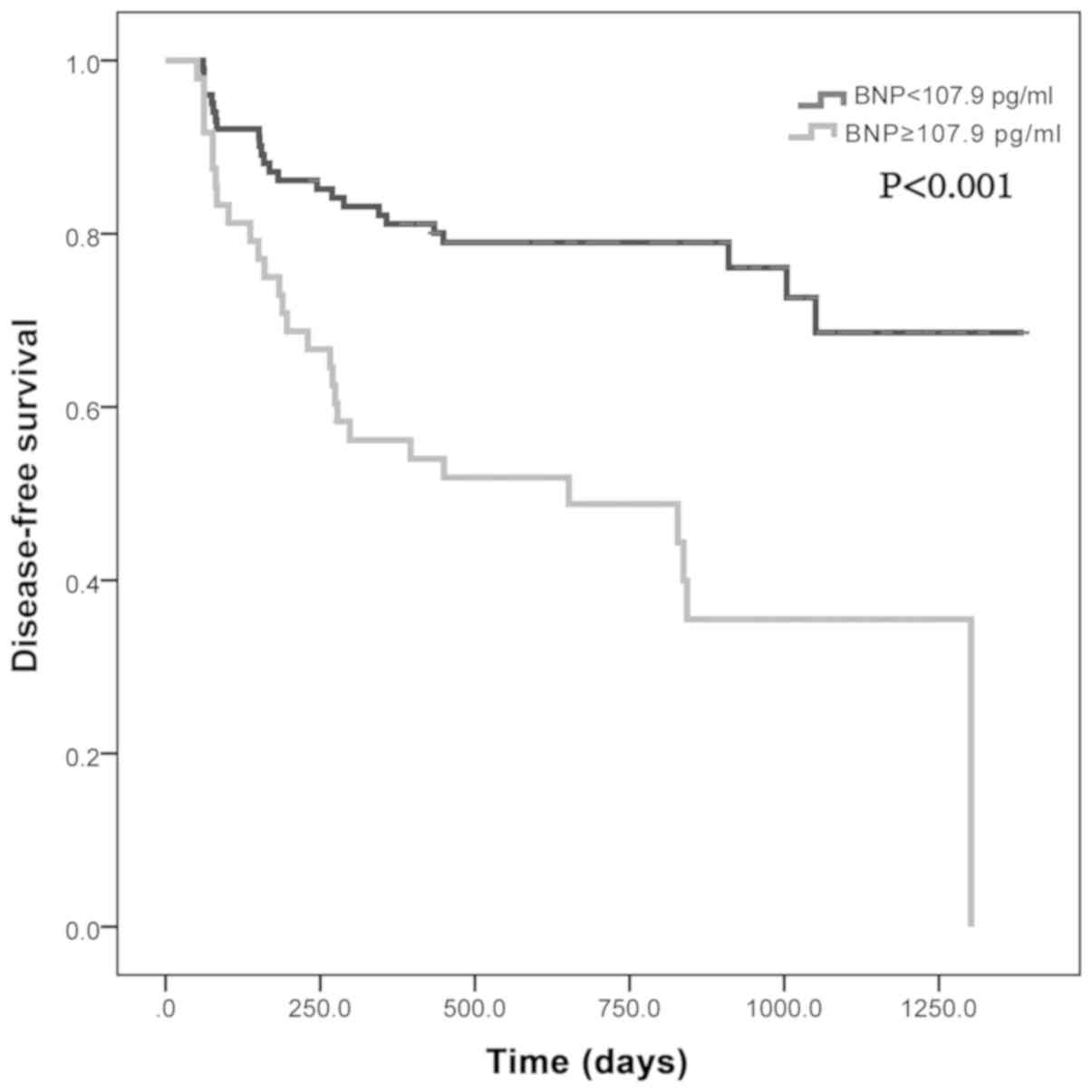
Based on this BNP threshold, patients who were
followed up were divided into two groups, the low BNP level group
(BNP <107.9 pg/ml) and the high BNP level group (BNP ≥107.9
pg/ml). According to their cardiotoxicity-free survival time, a
Log-rank test was performed (Fig. 4)
and it was shown that the cardiotoxicity-free survival rate of the
high BNP level group was consistently lower compared with the low
BNP level group (P<0.001). Therefore, it was suggested that
there was significant difference in the cardiotoxicity-free
survival rate of cardiotoxicity-free between the low BNP level
group and high BNP level group between the low BNP level group and
the high BNP level group. When patients received the final dose of
anthracycline during chemotherapy 9 weeks after the initial
chemotherapy course, the detected BNP levels after the fourth dose
of chemotherapy could predict the cardiotoxicity for a median time
period of 1.5 years, suggesting that the serum BNP levels after the
final dose of anthracycline during chemotherapy may contribute to
the detecting cardiotoxicity early.
Discussion
Anthracyclines are commonly used to treat patients
with breast cancer. However, the acute, chronic and delayed
cardiotoxicity may occur during the anthracycline therapy and may
possibly occur immediately after the initiation of chemotherapy
(21). Furthermore, aside from
clinical dilated cardiomyopathy and heart failure, ventricular
dysfunction with no symptoms was more commonly observed in the
patients receiving anthracycline based chemotherapy and the damage
was often irreversible when diagnosed (22). Therefore, to prevent irreversible
cardiotoxicity, breast cancer patients who received anthracycline,
should be monitored during treatment and early diagnosis of AIC is
necessary. A number of biomarkers, such as cardiac troponin T
(6), fluorouracil (23) and circulating microRNAs (24) have been reported to have a predictive
role in identifying AIC. Serum BNP elevation during chemotherapy
has also been well investigated (13). The present study focused on
monitoring the dynamic variation in serum BNP levels during
anthracycline chemotherapy.
In the present study, 52 of the 149 patients who
received anthracycline therapy exhibited cardiovascular events
during the observation period. The follow-up duration was to 0.1 to
3.8 years, with acute, chronic and some delayed cardiotoxicity
events observed. Since ECG detection is not highly specific,
echocardiography and clinical symptoms of patients were used to aid
in diagnosis of cardiotoxicity in the present study. Previous
studies have shown a direct association between the occurrence of
heart failure and the cumulative anthracycline dose (25,26). It
has been reported that when the cumulative dose of adriamycin is
400 mg/m2, the incidence of heart failure is 3%
increasing to 7.5% at 550 mg/m2 and to 18.0% at 700
mg/m2. However, as there was a lower cumulative dose of
adriamycin in the present study, there were fewer symptomatic
events of cardiotoxicity observed. Therefore, subclinical events of
cardiotoxicity like LVDs, which indicates a decrease in cardiac
systolic function, were used as one of the characteristics of
cardiotoxicity (27–30). In the present study, 50% of the
patients received a cumulative dose of ≤175 mg/m2
anthracycline, whereas the remaining 50% of patients received a
cumulative dose of >175 mg/m2. No significant changes
in the ejection fraction and measurements of cardiac systolic
function were observed between the two groups during the follow-up
period.
In the present study, patients with breast cancer
who were positive for ER or HER2 may have additionally received
tamoxifen and Herceptin treatment. Previous studies have suggested
that tamoxifen has a protective effect on cardiac function
(31), whereas Herceptin treatment
may be cardiotoxic (32). However,
there was no such evidence of either tamoxifen-mediated cardiac
protection or Herceptin-mediated cardiotoxicity in the study, in
agreement with other previous studies (33,34). It
may be the case that compared with the chemotherapy, both the
protective effects of tamoxifen and the cardiotoxic effects of
Herceptin were too weak to discern in these patients (35). In the follow-up period, if a case was
determined to require an additional course of chemotherapy due to
recurrence or metastasis, they were removed from the present
study.
The difference observed in the incidence of
cardiotoxicity between the respective EC/AC treatments regimens
compared with the EC-T/AC-T treatment regimens were not
significant, suggesting that the chemotherapy programs containing
docetaxel did not result in additional cardiotoxicity. Increased
concentrations of BNP were observed following docetaxel
chemotherapy and lower BNP concentrations following docetaxel
chemotherapy recovery. Together, the data suggest that
cardiotoxicity induced by docetaxel was self-limiting and could be
reversed if withdrawn (36). The
reversible nature of docetaxel-induced chemotherapy may explain why
there was a correlation between BNP after the fourth dose of
chemotherapy (subsequent to completion of treatment with
anthracyclines) and cardiotoxicity, instead of a correlation
between serum BNP levels following the eighth dose of chemotherapy
(subsequent to completion of treatment with docetaxel) and
cardiotoxicity. These data suggest that increase in the serum
levels of BNP was induced primarily by the anthracycline treatment
rather than docetaxel treatment during chemotherapy. Furthermore,
repeated measures ANOVA analysis suggested that the serum BNP
levels from the first to the fourth dose of chemotherapy in the
cardiotoxicity group were higher compared with the
non-cardiotoxicity group.
To evaluate whether BNP elevation was an effective
indicator in diagnosing cardiotoxicity, the ROC curve of serum BNP
levels following the final dose of anthracycline was plotted and
the AUC was 0.673 (95% CI, 0.584–0.763). It was also concluded that
the optimal BNP threshold was 107.9 pg/ml, the diagnostic
sensitivity of cardiotoxicity was 0.538, the specificity was 0.794,
the Youden index was 0.332, the positive predictive value was 0.583
and the negative predictive value was 0.762. The results showed
that elevation of serum BNP levels in cardiotoxicity diagnosis was
not very effective, with considerable misdiagnosis or missed
diagnoses. However, it should be noted that based on the BNP
diagnostic threshold, the Log-rank test showed that the
cardiotoxicity-free survival rate of the high BNP level group was
consistently lower than that of the low BNP level group, indicating
that serum BNP levels following the fourth dose of chemotherapy (9
weeks after the initial dose) predicted the cardiotoxicity with a
median length of 1.5 years, suggesting that elevated serum BNP
levels may assist in the detection of potential myocardial damage
prior to the occurrence of clinical symptoms and possibly prior to
detection with traditional echocardiography analysis. The baseline
serum BNP levels of the patients did not predict cardiotoxicity, in
agreement with previous studies (37–40),
although this may have been the result of the exclusion of patients
with a previous history of heart diseases.
The present study had several limitations. The
research was conducted and patients recruited at a single center
and the sample size was small. After from hypertension, other risk
factors of heart diseases such as diabetes and hyperlipidemia were
not considered. Based on the standard treatment of breast cancer,
the cumulative dose of anthracycline the patients received in the
study was not very high, potentially resulting in the lower
incidence of cardiotoxicity. In addition, blood used for serum BNP
detection in the present study was collected only once prior to or
after each therapy. As a number of previous studies (40–43)
suggested that chemotherapy could cause three types of BNP
variation, including no BNP increase, transient increase before
decrease and sustained increase. The incidence of cardiotoxicity
was associated with these types of BNP variation, as the incidence
of cardiotoxicity in the sustained BNP increase group was higher
compared with the other two groups, and subsequent impairment of LV
function only occurred in patients with persistent BNP elevation
(41). Therefore, a single change in
serum BNP levels may not be considered convincing, instead multiple
measurements of BNP should be performed during the therapy.
In conclusion, BNP can be elevated in patients who
have received anthracyclines, measuring the serum level of BNP 24 h
after final dose of anthracyclines during chemotherapy contributes
to the early detection of cardiotoxicity. As obtaining blood
samples is convenient, inexpensive and allows for more continuous
monitoring and management of patients with breast cancer, serum BNP
levels should be considered as a suitable method for diagnosing and
predicting anthracycline-induced cardiotoxicity.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
XL, YYZ, CPC and CH conceived and designed the
experiments. XL, LX and DX performed the experiments. YYZ, CPC and
DX analyzed and interpreted the data, XL and YYZ wrote the
manuscript. MT and OH designed and performed the experiments. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by The Ethical
Committee of The First Hospital of Jiaxing (Jiaxing, China). All
patients signed written informed consent form to participate in the
present study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Meinardi MT, van Veldhuisen DJ, Gietema
JA, Dolsma WV, Boomsma F, van den Berg MP, Volkers C, Haaksma J, de
Vries EG, Sleijfer DT and van der Graaf WT: Prospective evaluation
of early cardiac damage induced by epirubicin- containing adjuvant
chemotherapy and locoregional radiotherapy in breast cancer
patients. J Clin Oncol. 19:2746–2753. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lipshultz SE, Lipsitz SR, Sallan SE,
Dalton VM, Mone SM, Gelber RD and Colan SD: Chronic progressive
cardiac dysfunction years after doxorubicin therapy for childhood
acute lymphoblastic leukemia. J Clin Oncol. 23:2629–2636. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Keefe DL: Anthracycline-induced
cardiomyopathy. Semin Oncol. 28 (Suppl 12):S2–S7. 2001. View Article : Google Scholar
|
|
5
|
Armenian SH, Gelehrter SK, Vase T,
Venkatramani R, Landier W, Wilson KD, Herrera C, Reichman L,
Menteer JD, Mascarenhas L, et al: Screening for cardiac dysfunction
in anthracycline-exposed childhood cancer survivors. Clin Cancer
Res. 20:6314–6323. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kilickap S, Barista I, Akgul E, Aytemir K,
Aksoyek S, Aksoy S, Celik I, Kes S and Tekuzman G: cTnT can be a
useful marker for early detection of anthracycline cardiotoxicity.
Ann Oncol. 16:798–804. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Clerico A, Fontana M, Zyw L, Passino C and
Emdin M: Comparison of the diagnostic accuracy of brain natriuretic
peptide (BNP) and the N-terminal part of the propeptide of BNP
immunoassays in chronic and acute heart failure: A systematic
review. Clin Chem. 53:813–822. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ledwidge M, Gallagher J, Conlon C, Tallon
E, O'Connell E, Dawkins I, Watson C, O'Hanlon R, Bermingham M,
Patle A, et al: Natriuretic peptide-based screening and
collaborative care for heart failure: The STOP-HF randomized trial.
JAMA. 310:66–74. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang P, Zhang S, Zhang XB, Li WJ, Hao XM
and Zhang J: Protective effect of dexrazoxane on cardiotoxicity in
breast cancer patients who received anthracycline-containing
chemotherapy. Zhonghua Zhong Liu Za Zhi. 35:135–139. 2013.(In
Chinese). PubMed/NCBI
|
|
10
|
Lipshultz SE, Miller TL, Scully RE,
Lipsitz SR, Rifai N, Silverman LB, Colan SD, Neuberg DS, Dahlberg
SE, Henkel JM, et al: Changes in cardiac biomarkers during
doxorubicin treatment of pediatric patients with high-risk acute
lymphoblastic leukemia: Associations with long-term
echocardiographic outcomes. J Clin Oncol. 30:1042–1049. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
De Iuliis F, Salerno G, Taglieri L, De
Biase L, Lanza R, Cardelli P and Scarpa S: Serum biomarkers
evaluation to predict chemotherapy-induced cardiotoxicity in breast
cancer patients. Tumour Biol. 37:3379–3387. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lenihan DJ, Stevens PL, Massey M, Plana
JC, Araujo DM, Fanale MA, Fayad LE, Fisch MJ and Yeh ET: The
utility of point-of-care biomarkers to detect cardiotoxicity during
anthracycline chemotherapy: A feasibility study. J Card Fail.
22:433–438. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zidan A, Sherief LM, El-sheikh A, Saleh
SH, Shahbah DA, Kamal NM, Sherbiny HS and Ahmad H: NT-proBNP as
early marker of subclinical late cardiotoxicity after doxorubicin
therapy and mediastinal irradiation in childhood cancer survivors.
Dis Markers. 2015:5132192015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sinn HP, Helmchen B and Wittekind CH: TNM
classification of breast cancer: Changes and comments on the 7th
edition. Pathologe. 31:361–366. 2010.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wolff AC, Hammond MEH, Allison KH, Harvey
BE, Mangu PB, Bartlett JMS, Bilous M, Ellis IO, Fitzgibbons P,
Hanna W, et al: Human epidermal growth factor receptor 2 testing in
breast cancer: American society of clinical oncology/college of
American pathologists clinical practice guideline focused update.
Arch Pathol Lab Med. 142:1364–1382. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pichon MF, Cvitkovic F, Hacene K, Delaunay
J, Lokiec F, Collignon MA and Pecking AP: Drug-induced
cardiotoxicity studied by longitudinal B-type natriuretic peptide
assays and radionuclide ventriculography. In Vivo. 19:567–576.
2005.PubMed/NCBI
|
|
17
|
Potter E and Marwick TH: Assessment of
left ventricular function by echocardiography: The case for
routinely adding global longitudinal strain to ejection fraction.
JACC Cardiovasc Imaging. 11:260–274. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chang SA, Lim BK, Lee YJ, Hong MK, Choi JO
and Jeon ES: A novel angiotensin type I receptor antagonist,
fimasartan, prevents doxorubicin-induced cardiotoxicity in rats. J
Korean Med Sci. 30:559–568. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nitta D, Kinugawa K, Imamura T, Kato NP
and Komuro I: High dose β-blocker therapy triggers additional
reverse remodeling in patients with idiopathic non-ischemic
cardiomyopathy. Int Heart J. 57:717–724. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shimomura Y, Baba R, Watanabe A, Horikoshi
Y, Asami K, Hyakuna N, Iwai A, Matsushita T, Yamaji K, Hori T, et
al: Japanese childhood cancer and leukemia study group (JCCLSG):
Assessment of late cardiotoxicity of pirarubicin (THP) in children
with acute lymphoblastic leukemia. Pediatr Blood Cancer.
57:461–466. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bird BR and Swain SM: Cardiac toxicity in
breast cancer survivors: Review of potential cardiac problems. Clin
Cancer Res. 14:14–24. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Visscher H, Ross CJ, Rassekh SR, Barhdadi
A, Dubé MP, Al-Saloos H, Sandor GS, Caron HN, van Dalen EC, Kremer
LC, et al: Canadian pharmacogenomics network for drug safety
consortium: Pharmacogenomic prediction of anthracycline-induced
cardiotoxicity in children. J Clin Oncol. 30:1422–1428. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cao L, Zhu W, Wagar EA and Meng QH:
Biomarkers for monitoring chemotherapy-induced cardiotoxicity. Crit
Rev Clin Lab Sci. 54:87–101. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Leger KJ, Leonard D, Nielson D, de Lemos
JA, Mammen PP and Winick NJ: Circulating microRNAs: Potential
markers of cardiotoxicity in children and young adults treated with
anthracycline chemotherapy. J Am Heart Assoc. 6:e0046532017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Malik A, Jeyaraj PA, Calton R, Uppal B,
Negi P, Shankar A, Patil J and Mahajan MK: Are biomarkers
predictive of anthracycline-induced cardiac dysfunction? Asian Pac
J Cancer Prev. 17:2301–2305. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Swain SM, Whaley FS and Ewer MS:
Congestive heart failure in patients treated with doxorubicin: A
retrospective analysis of three trials. Cancer. 97:2869–2879. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nachom P and Ratanasit N: Incidence and
predictors of long-term adverse outcomes in patients with rheumatic
mitral stenosis in sinus rhythm. J Med Assoc Thai. 99:374–380.
2016.PubMed/NCBI
|
|
28
|
Singletary GE, Morris NA, Lynne O'Sullivan
M, Gordon SG and Oyama MA: Prospective evaluation of NT-proBNP
assay to detect occult dilated cardiomyopathy and predict survival
in Doberman Pinschers. J Vet Intern Med. 26:1330–1336. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Addetia K, Michel C, Holcroft CA, Sheppard
R and Rudski LG: Early improvement in serial echocardiographic
studies in heart failure patients predicts long term survival-a
pilot study. J Card Fail. 21:470–478. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Inoue T, Kawai M, Nakane T, Nojiri A,
Minai K, Komukai K, Ogawa T, Hongo K, Matsushima M and Yoshimura M:
Influence of low-grade inflammation on plasma B-type natriuretic
peptide levels. Intern Med. 49:2659–2668. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Silva FB, Romero WG, Carvalho AL, Borgo
MV, Amorim MH, Gouvea SA and Abreu GR: Hormone therapy with
tamoxifen reduces plasma levels of NT-B-type natriuretic peptide
but does not change ventricular ejection fraction after
chemotherapy in women with breast cancer. Braz J Med Biol Res.
48:154–160. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sawaya H, Sebag IA, Plana JC, Januzzi JL,
Ky B, Tan TC, Cohen V, Banchs J, Carver JR, Wiegers SE, et al:
Assessment of echocardiography and biomarkers for the extended
prediction of cardiotoxicity in patients treated with
anthracyclines, taxanes, and trastuzumab. Circ Cardiovasc Imaging.
5:596–603. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ponde N, Bradbury I, Lambertini M, Ewer M,
Campbell C, Ameels H, Zardavas D, Di Cosimo S, Baselga J, Huober J,
et al: Cardiac biomarkers for early detection and prediction of
trastuzumab and/or lapatinib-induced cardiotoxicity in patients
with HER2-positive early-stage breast cancer: A NeoALTTO sub-study
(BIG 1–06). Breast Cancer Res Treat. 168:631–638. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Matos E, Jug B, Blagus R and Zakotnik B: A
Prospective cohort study on cardiotoxicity of adjuvant trastuzumab
therapy in breast cancer patients. Arq Bras Cardiol. 107:40–47.
2016.(In English, Portuguese). PubMed/NCBI
|
|
35
|
Criscitiello C and Curigliano G: HER2
signaling pathway and trastuzumab cardiotoxicity. Future Oncol.
9:179–181. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Baldini E, Prochilo T, Salvadori B,
Bolognesi A, Aldrighetti D, Venturini M, Rosso R, Carnino F, Gallo
L, Giannessi P, et al: Multicenter randomized phase III trial of
epirubicin plus paclitaxel vs epirubicin followed by paclitaxel in
metastatic breast cancer patients: Focus on cardiac safety. Br J
Cancer. 91:45–49. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Vogelsang TW, Jensen RJ, Hesse B and Kjaer
A: BNP cannot replace gated equilibrium radionuclide
ventriculography in monitoring of anthracycline-induced
cardiotoxity. Int J Cardiol. 124:193–197. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kittiwarawut A, Vorasettakarnkij Y,
Tanasanvimon S, Manasnayakorn S and Sriuranpong V: Serum NT-proBNP
in the early detection of doxorubicin-induced cardiac dysfunction.
Asia Pac J Clin Oncol. 9:155–161. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Feola M, Garrone O, Occelli M, Francini A,
Biggi A, Visconti G, Albrile F, Bobbio M and Merlano M:
Cardiotoxicity after anthracycline chemotherapy in breast
carcinoma: Effects on left ventricular ejection fraction, troponin
I and brain natriuretic peptide. Int J Cardiol. 148:194–198. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Skovgaard D, Hasbak P and Kjaer A: BNP
predicts chemotherapy-related cardiotoxicity and death: Comparison
with gated equilibrium radionuclide ventriculography. PLoS One.
9:e967362014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Romano S, Fratini S, Ricevuto E,
Procaccini V, Stifano G, Mancini M, Di Mauro M, Ficorella C and
Penco M: Serial measurements of NT-proBNP are predictive of
not-high-dose anthracycline cardiotoxicity in breast cancer
patients. Br J Cancer. 105:1663–1668. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhang C, Shi D and Yang P: BNP as a
potential biomarker for cardiac damage of breast cancer after
radiotherapy: A meta-analysis. Medicine (Baltimore). 98:e165072019.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Michel L, Rassaf T and Totzeck M:
Biomarkers for the detection of apparent and subclinical cancer
therapy-related cardiotoxicity. J Thorac Dis. 10 (Suppl
35):S4282–S4295. 2018. View Article : Google Scholar : PubMed/NCBI
|