Introduction
Anterior gradient protein 3 (AGR3) is a homologue of
the pro-oncogenic AGR2. AGR3 and AGR2 share a 71% sequence identity
and lie adjacent to one another at chromosomal position 7p21
(1). Functionally, they belong to
the protein disulfide isomerases (PDIs) family, which act as
endoplasmic reticulum (ER)-resident molecular foldases involved in
the maintenance of cellular homeostasis. AGR2 is part of the
protein folding machinery within the ER, it is regulated by the ER
stress response (2,3) and it is known to be involved in mucin
production in intestinal, pulmonary and pancreatic tissues
(4–6). Similarly, AGR3 is also an ER resident
protein, which is required for the regulation of ciliary beat
frequency and mucociliary clearance in the airway epithelium
(7). Deregulation of AGR2 and AGR3
proteins expression has been associated with several clinical
entities, including cancer. We and others have shown similar
expression patterns of AGR2 and AGR3 in non-pathological tissue
samples as well as carcinomatous ones, including that of breast,
liver and ovary (8–11), which suggests their cognate
physiological function and role in pathology. Numerous studies have
linked AGR2 with pro-tumoral characteristics that promote cancer
aggressiveness and pertain to poor prognoses (12–15).
Although AGR2 and AGR3 harbor an ER retention signal sequence (KTEL
and QSEL, respectively), they were present in extracellular media
such as gastrointestinal mucus, blood or urine (16–19).
Intriguingly, we and others have recently demonstrated an emerging
role of AGR2 in the maintenance of the tumor microenvironment
[including properties of cancer migration (20), invasion (21), chemoresistance (22) and epithelial-to-mesenchymal
transition (EMT) (23)], which
clearly indicates that extracellular AGR2 (eAGR2) protein assumes a
gain-of-function role. AGR3 is a less characterized homologue of
AGR2, with similar but apparently not identical function in the
regulation of tumor-associated processes (11). So far, AGR3 was shown to interact
with dystroglycan-1 (DAG-1) and metastasis-associated C4.4A protein
(1), indicating its potential as a
driver of metastasis. Moreover, AGR3 mediates cisplatin resistance
in xenograft models of ovarian cancer (8). These findings collectively make AGR3 a
potential promising target for anti-tumor therapy. Elevated AGR3
expression levels were reported in some cancer types, including
breast (19,24,25),
liver (9), prostate (26) and ovary (8,27).
However, the precise role of AGR3 in tumorigenesis has not been
investigated so far. AGR3 was identified among a set of breast
cancer-associated membrane proteins (24) and later its presence was reported in
sera from breast cancer patients (19). Therefore, in the present work we aim
to investigate whether extracellular AGR3 (eAGR3) could have a
gain-of-function within the extracellular space to promote cancer
aggressiveness.
Materials and methods
Cell lines and culture reagents
Human breast carcinoma cell lines MCF-7, T-47D,
BT-474, SK-BR-3 and BT-549 were purchased from American Type
Culture Collection (ATCC). All cells were maintained in high
glucose Dulbecco's Modified Eagle's medium (DMEM), supplemented
with 10% fetal bovine serum (FBS) and 300 µg/ml L-glutamine at 37°C
in humidified atmosphere with 5% CO2. Dasatinib was
obtained from Selleckchem, 7-amino-actinomycin D (7-AAD) and
Annexin V-PE were from BD Biosciences. To inhibit EsR signaling, 10
nM 17β-estradiol, 1 µM tamoxifen and 0.5 nM fulvetrant (all from
Sigma-Aldrich) were used for 24 h.
Purification of recombinant AGR3
protein
The sequence coding for mature AGR3 (NP_789783,
amino acids 22–166) was cloned into a vector containing N-terminal
His6-GST tag, cleavable by tobacco etch virus (TEV)
protease. Recombinant fusion protein His6-GST-AGR3 was
produced in BL21-CodonPlus (DE3)-RIPL cells (Agilent) according to
a protocol described previously (28) with some minor modifications. In order
to exclude the potential impact of bacterial endotoxins on cellular
signaling (29), purified
glutathione S-transferase (His6-GST) protein served as a
control in all the experiments. Briefly, cells were lysed in buffer
containing 50 mM HEPES pH 7.4, 150 mM NaCl, 1 mM PMSF, 1 mg/ml
lysozyme. His6-GST-AGR3 fusion protein was captured on a GSTrap
glutathione-agarose column (GE Healthcare), eluted with 20 mM
glutathione and subjected to TEV protease cleavage. To remove
His6-GST and His6-TEV, proteins were applied to HisTrap column (GE
Healthcare). The purity and appropriate size of AGR3 protein was
analyzed by Coomassie blue staining of 10% SDS-PAGE gels (data not
shown). For all experiments, purified recombinant AGR3 protein was
diluted in DMEM + 10% FBS to a final concentration of 5 ng/ml or 50
ng/ml followed by media filtration.
Collection of conditioned media and
western blot analysis
For detection of secreted AGR3, conditioned media
were collected after 48 h of cell culture in serum-free DMEM,
centrifuged at 13,000 rpm for 10 min, followed by overnight
precipitated with cold acetone (at 80% final concentration). For
cellular proteins detection, cells were lysed in lysis buffer (50
mM TrisHCl, pH 7.4, 150 mM NaCl, 5 mM EDTA, 50 mM NaF, 1 mM
Na3CO4, 1% Nonidet P40) containing protease
inhibitor cocktail and phosphatase inhibitor cocktail 2 (both
Sigma-Aldrich). For detection of tyrosine-phosphorylated proteins,
the aforementioned lysis buffer was supplemented with 100 µM sodium
orthovanadate (Sigma). The primary antibodies used were as follows:
Mouse monoclonal anti-tubulin (Sigma), goat monoclonal anti-actin
(Santa Cruz Biotechnology), mouse monoclonal anti-GAPDH (Santa Cruz
Biotechnology), in-house mouse monoclonal anti-AGR3 (8,25),
Py-Plus™-HRP mouse anti-phosphotyrosine (Invitrogen), rabbit
monoclonal anti-c-Src and anti-phospho-c-Src (both Cell Signaling
Technology), mouse monoclonal anti-Gapdh (clone 6C5) (Millipore),
mouse monoclonal anti-VCP (p97) (BD Biosciences) and rabbit
monoclonal anti-EsRalpha antibody (Abcam).
Cell detachment assay
Confluent cells were seeded in the quadruplet on
24-well plates and were incubated with 5 ng/ml and 50 ng/ml eAGR3
protein for 24 h. After, cells were washed with 0.5% EGTA and
trypsin was used to detach cells from plate surface (for MCF-7,
0.0025% trypsin for 3 min; for T-47D, 0.125% trypsin for 3 min).
The remaining cells were washed with PBS, fixed with 4%
paraformaldehyde in PBS and stained with 5 mg/ml crystal violet.
Number of attached cells was quantified by absorbance measurement
on the microplate reader (Tecan Group Ltd.) at 595 nm.
Wound healing assay
Confluent cells were scraped using a sterile
micropipette tip to create an in vitro wound and
subsequently incubated in serum-free DMEM with or without eAGR3 as
indicated. For SRC family kinases inhibition, cells were
additionally treated with 1 µM dasatinib or equivalent
concentration of DMSO as control. Alternatively, cells were
transfected with wild-type or dominant negative (K298R) Src
constructs (30) to further document
the impact of Src signaling on eAGR3-induced migration. Transient
transfections of MCF-7 cells seeded in 12-well plates (at a density
of 4×105 cells/well) were performed using Fugene 6
(Promega) according to the manufacturer's recommendations using a
1:3 ratio of DNA/Fugene 6 in Opti-MEM (Invitrogen Life
Technologies). Time-lapse acquisition of the wound closure was
analyzed with Nikon eclipse Ti-E system at 10× magnification. The
pictures were captured in three randomly chosen fields within the
wounded region every 20 min for 24 h. The migration rate was
assessed using TScratch software (31) (CSE Lab, ETH) by quantification of the
cell-free area 16 h post-scratching.
Flow cytometry
Cells were treated with tyrosine kinase inhibitor
dasatinib ranging from 10 to 0.01 µM for 24 h. Following treatment,
cells were washed with PBS 2% FBS and analyzed by flow cytometry
using a FACS-Canto II flow cytometer (BD Biosciences). The
population of interest was gated according to its FSC/SSC criteria.
The dead cell population was determined using 7-amino-actinomycin D
(7AAD) and annexin V-PE staining (both BD Biosciences). Data were
analyzed with the FACS-Diva (BD Biosciences).
Statistical analyses
Graphs and statistical analyses were done using
GraphPad Prism 7.0 software. According to the experiments, either a
student t-test was applied using a two-tailed distribution of two
conditions of unequal or equal variances on groups of data obtained
in experiments, or an ANOVA following a Tukey's multiple
comparisons test was used. P<0.05 was considered to indicate a
statistically significant difference.
Results
AGR3 is secreted from breast cancer
cells
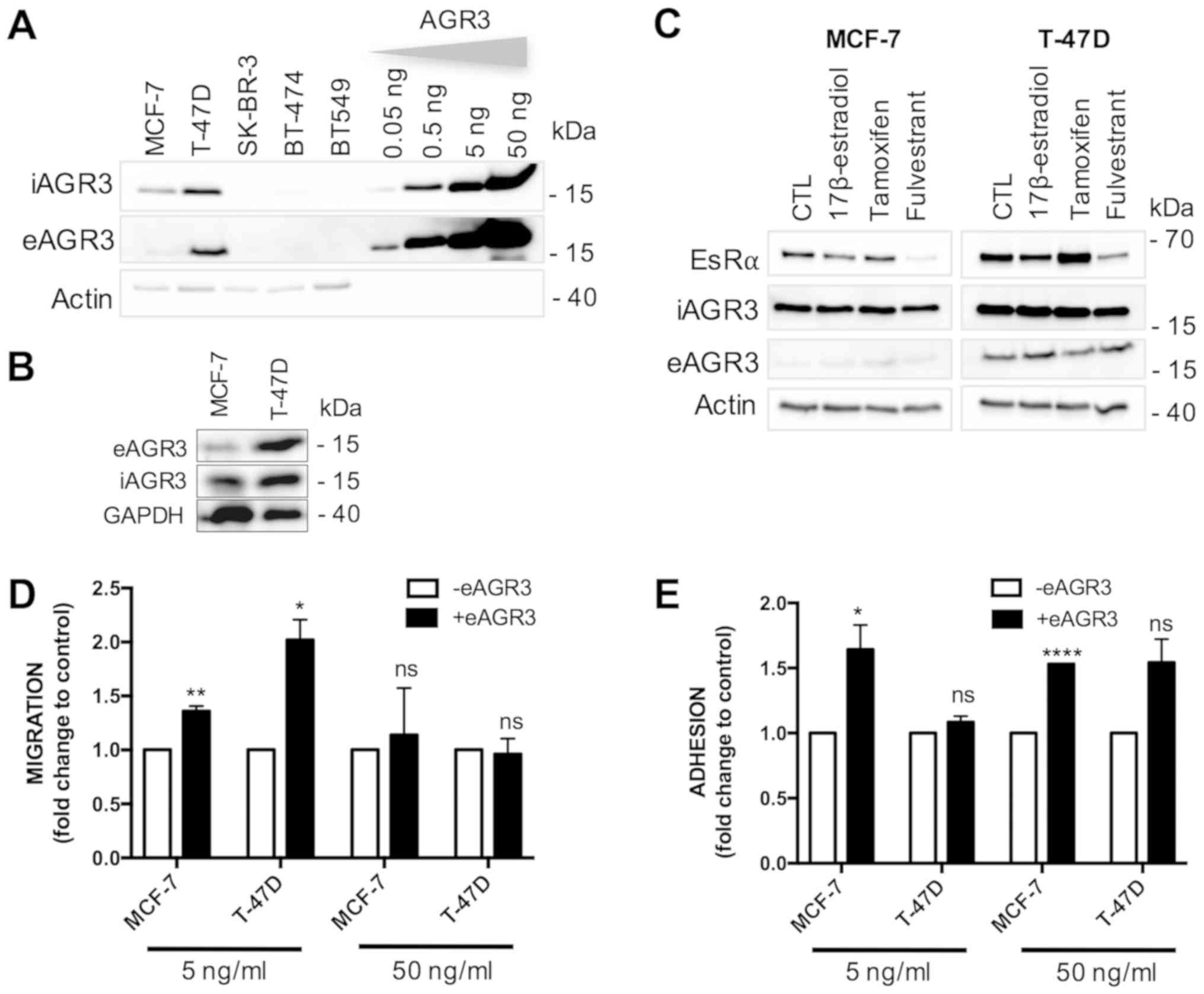
To test whether AGR3 protein could be found both
intracellularly (iAGR3) and extracellularly (eAGR3), we first
monitored AGR3 expression levels in cell extracts and in the
conditioned media of a set of distinct breast cancer cell lines
including MCF-7, T-47D, BT-474, SK-BR-3 and BT-549. We found that
iAGR3 protein is expressed only in MCF-7 and T-47D cells (Fig. 1A) and is secreted (eAGR3) in the
extracellular milieu by these two cell lines (Fig. 1A-B). We also evaluated the relative
concentration of eAGR3 using Western blotting. This was done by
comparing the intensity of the immunoreactive bands obtained from
purified recombinant AGR3 protein ranging from 50 to 0.05 ng/ml
(herein referred to as eAGR3) to that of eAGR3 acetone-precipitated
from MCF-7 and T-47D conditioned culture media. We found that in
vitro breast cancer cells secrete eAGR3 in nanomolar quantities
(Fig. 1B). Therefore, to investigate
the biological consequences of eAGR3 secretion, we further used
recombinant AGR3 protein at the concentration of 5 and 50 ng/ml. As
demonstrated here, AGR3 is only expressed in estrogen receptor
(EsR) positive breast cancer cell lines, which is in line with
previous works showing the positive correlation between AGR3 and
EsR status in breast tumor tissues (1,19,25). As
such, we investigated the effect of EsR signaling attenuation on
AGR3 expression using the EsR ligands, namely 17β-estradiol,
tamoxifen and fulvestrant. As a result, we found that none of the
used drugs affected intra- or eAGR3 expression in both cell lines
tested, indicating that the EsR pathway does not control AGR3
secretion (Fig. 1C).
eAGR3 regulates tumor migration and
adhesion
Given that the only report pertaining to AGR3
function in cancer links AGR3 with metastasis (1), we investigated whether eAGR3 could
regulate cancer cell migration and/or adhesion, one of the key
steps required for tumor cell metastasis. Firstly, we studied the
effect of eAGR3 on cell migration using a wound healing assay and
found a significant increase in migratory properties of both MCF-7
and T-47D upon eAGR3 exposure (5 ng/ml; Fig. 1D). Next, we determined the effect of
eAGR3 on cell adhesion using a cell detachment assay. To this end,
MCF-7 and T-47D cells were compared for their ability to bind to
plastic substratum upon treatment with eAGR3. Both MCF-7 and T-47D
cells exposed to low concentrations of trypsin were more prone to
stay attached to the substratum when cultivated in the presence of
eAGR3 (Fig. 1E). These results
demonstrate that eAGR3 plays an extracellular role in regulating,
tumor-associated processes, cell-adhesion and migration in breast
cancer cells.
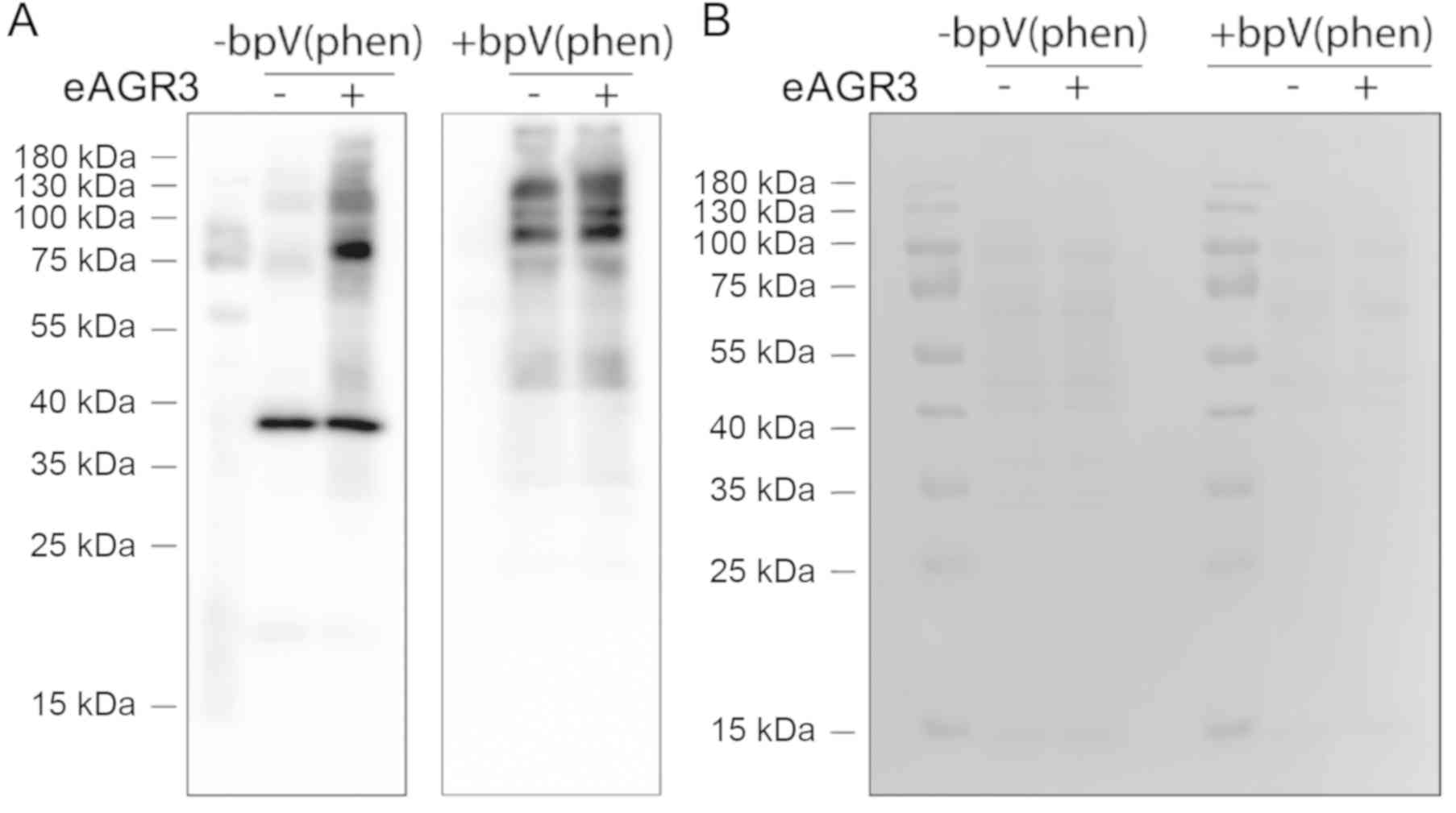
eAGR3 supports cell migration by
inducing the phosphorylation of tyrosine kinases
To investigate the intracellular signaling
mechanism(s) responsible for eAGR3-dependent migration, we then
analyzed protein tyrosine phosphorylation patterns in control MCF-7
cells and cells treated with 5 ng/ml eAGR3 using Western blot with
anti-phosphotyrosine antibodies. To enhance the detection of
tyrosine-phosphorylated proteins, cells were also treated with 15
µM bpV(phen), an inhibitor of protein phosphotyrosine phosphatases.
As shown in Fig. 2, we detected an
increase in tyrosine phosphorylation corresponding to proteins with
molecular weight of about 65, 80 and 100 kDa. This observation
suggested that eAGR3 might induce the tyrosine phosphorylation of a
major protein of a molecular weight compatible with the Src family
kinases, key actors in cell migration (32). Thus, this result indicated that eAGR3
might possibly act through Src signaling to control cell migration.
This hypothesis was further supported by a study showing that AGR2,
a pro-tumorigenic homologue of AGR3, impacts on Src signaling,
since AGR2 silencing in breast cancer cells resulted in decreased
c-Src phosphorylation with no impact on total c-Src levels
(33).
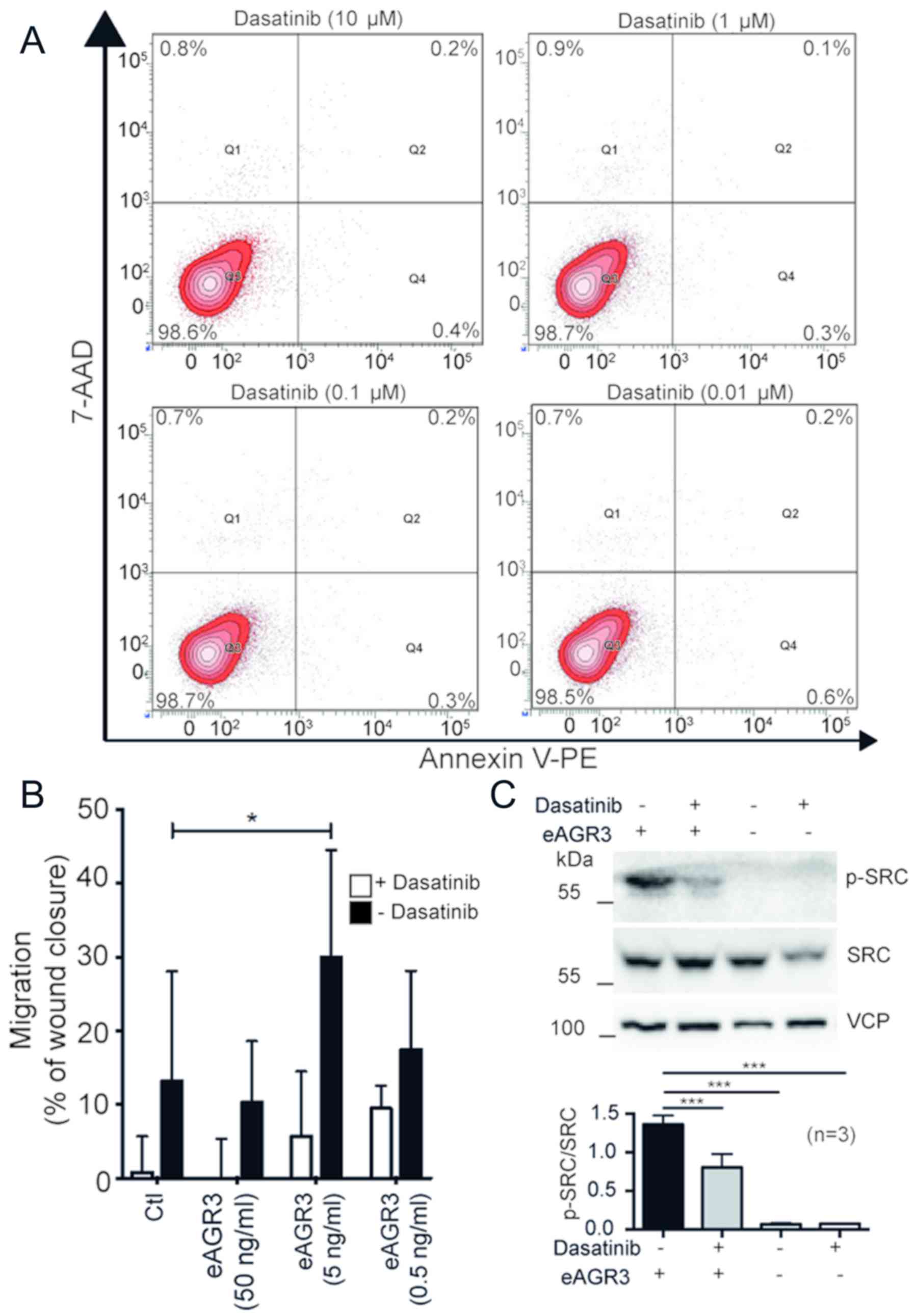
eAGR3 acts through the c-Src signaling
pathways
Therefore, to confirm our observation, dasatininb, a
tyrosine kinase inhibitor targeting Src kinases, was used and cell
migration upon treatment with eAGR3 was analyzed using wound
healing assays. Before the analysis, the toxicity of gradient
dasatinib concentrations randing from 10 to 0.01 µM was measured by
flow cytometry using Annexin V and 7-amino-actinomycin D (7-AAD)
double staining, which confirmed that dasatinib alone does not
affect cell survival at any tested concentrations (Fig. 3A). We then investigated the migration
rate of T-47D cells stimulated or not with eAGR3 (from 0.5 to 50
ng/ml) and treated or not with 1 µM dasatinib. Cell migration was
significantly downregulated upon dasatinib treatment in both
control and eAGR3-stimulated cells (Fig.
3B). Western blot analysis also revealed the induction of Src
phosphorylation in MCF-7 cells following exposure to eAGR3, which
was consistently blocked in dasatinib-treated cells (Fig. 3C). This result confirmed our initial
hypothesis that eAGR3 could signal through Src to promote cell
migration.
To further demonstrate that eAGR3 exerts its
pro-migratory functions on breast cancer cells by activating c-Src
signaling, we investigated the effect of eAGR3 on MCF-7 cells
overexpressing wild-type (WT) or dominant negative mutant of c-Src
protein, namely K298R (Fig. 4A)
(34). We found that control (Mock,
empty vector) and c-Src WT-overexpressing cells migrated
significantly faster towards in vitro wound upon treatment
with eAGR3 compared to their non-stimulated counterparts (Fig. 4B-C, E). Strikingly, cells transfected
with plasmid coding for kinase dead K298R mutant did not respond to
eAGR3 and consequently completely lost the migratory advantage of
eAGR3 stimulation (Fig. 4D-E). Taken
together, these results demonstrate the involvement of c-Src on
breast cancer cell migration and support our previous observations
that the kinase activity of c-Src is necessary for eAGR3-mediated
tumorigenic properties.
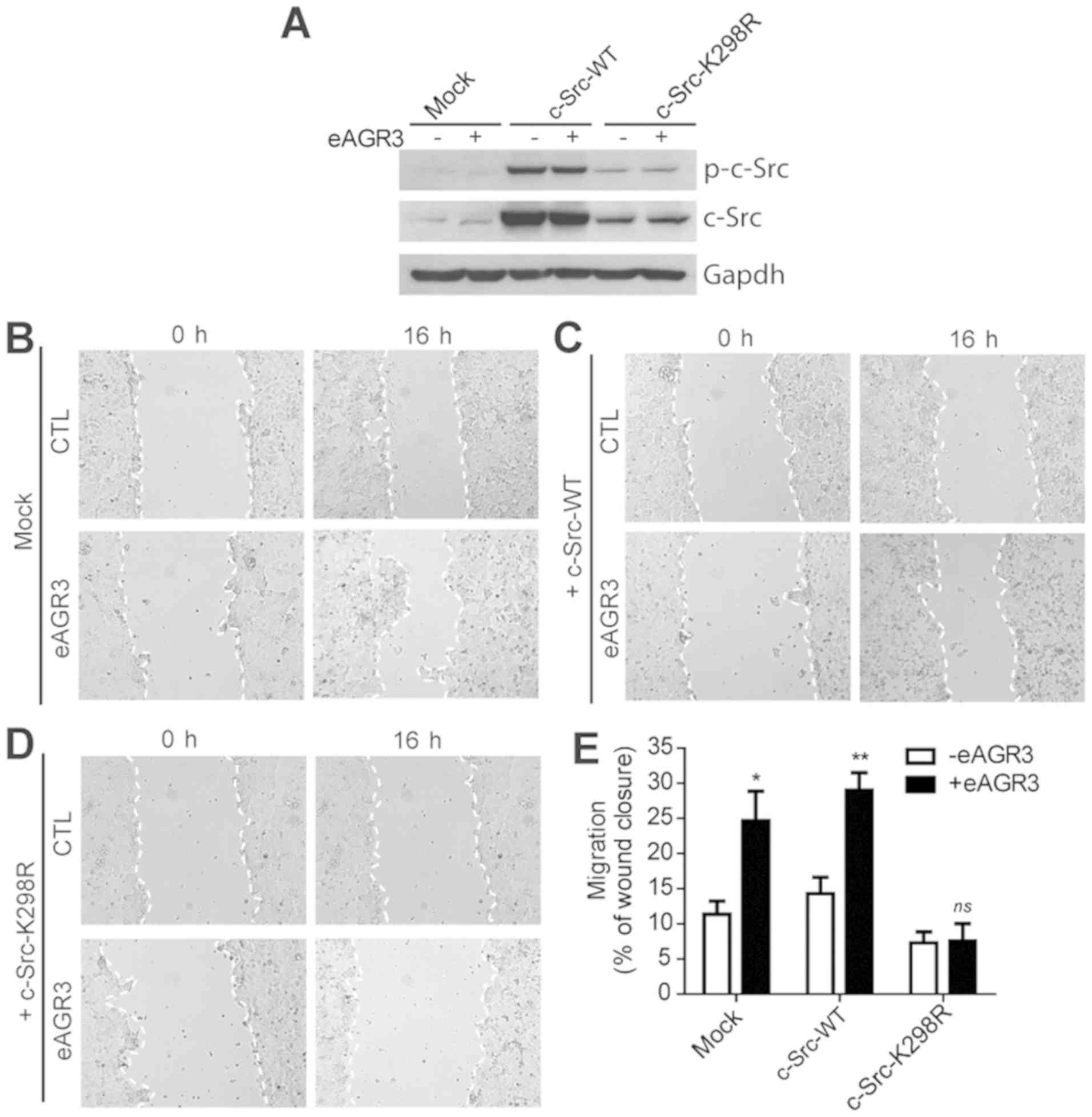 | Figure 4.eAGR3 induces cell migration via the
activation of c-Src. (A) Western blot analysis showing the level of
expression of c-Src-WT, c-Src-K298R, p-c-Src-WT, p-c-Src-K298R
following transfection of MCF-7 as compared to control cells (Mock,
empty vector). Cells were stimulated (+) or not (−) with eAGR3 (5
ng/ml). GAPDH was used as a loading control. (B-D) Wound healing
assay showing migration capacities of MCF-7 cells transfected with
empty vector (mock) (B), c-Src-wt (C) or c-Src-K298R (D), in
absence or presence of eAGR3 (5 ng/ml) for 16 h. (E) Migration rate
was quantified by measuring the size of in vitro wound 16 h
post scraping. Data are representative of three independent
experiments, *P≤0.05 and **P≤0.01 vs. -eAGR3. WT, wild-type; eAGR3,
extracellular anterior gradient protein 3; CTL, control. |
Discussion
AGR2 and AGR3 proteins are highly expressed in
breast tumors, where their expression levels correlate with
estrogen receptor (EsR) positivity and predict patient outcome
(1,25,35,36).
Accordingly, we found that AGR3 was mainly expressed in the
EsR-positive cell lines (MCF-7 and T-47D), while it was absent in
EsR-negative (SK-BR-3 or BT-549) cells, suggesting its
EsR-dependent regulation. However, the selective inhibition of EsRα
did not affect intra- or eAGR3 levels, indicating a more complex
regulation of AGR3 expression in breast cancer, which is not solely
dependent on EsR signaling. AGR2 is an emerging drug target in
breast cancer as it promotes growth, survival and dissemination of
the metastatic cells as well as resistance to treatment by
modulating tumor-associated signaling pathways and overcoming ER
stress (14). In addition, AGR2 has
been shown to be secreted by various cancer cell lines as well as
in tumors tissues (17,18,37,38).
Follow-up studies indicated that AGR2 can also act ‘from the
outside’, as eAGR2, either through binding to membrane receptors or
through incorporating within the cytoplasm of target cells
(21,22). Despite high similarity between the
two homologues-AGR2 and AGR3, there is no reported evidence of
eAGR3 extracellular activity. Recent findings have demonstrated a
significantly elevated level of eAGR3 in sera from breast cancer
patients compared to healthy controls, which is of a diagnostic and
prognostic importance (19).
However, the biological consequences of eAGR3 secretion remain
elusive.
In the current study, we showed for the first time
that eAGR3 exerts pro-tumoral functions in breast cancer by
impacting on the adhesive and migratory properties of breast cancer
cells. These findings further support the hypothesis that AGR3 may
be involved in metastatic processes either through a direct
interaction with metastasis-related proteins (such as C4.4A protein
and DAG-1 (1)) and/or by the
induction of pro-metastatic signaling pathways. In line with this,
we previously demonstrated that the AGR3 gene may be
co-expressed with genes coding for claudin 3 and hepatocyte cell
adhesion molecule (HEPACAM) (11),
both reported to regulate the metastatic potential of cancer cells
(39,40). Herein we uncover that eAGR3 acts
through the activation of c-Src signaling pathway and that
eAGR3-dependent migration can be inhibited by dasatinib and by
dominant negative Src. In addition to EsR and progesterone receptor
signaling (25), this is the first
pro-oncogenic pathway linked to the AGR3 protein. However, since
dasatinib did not completely abolish the AGR3-mediated increase in
cell migration, it is plausible that collateral signaling pathways
are involved in this process. Secreted AGR2 has been recently
demonstrated to promote colorectal cancer migration and metastasis
through non-canonical Wnt signaling, involving
Ca2+/Calmodulin-dependent protein kinase II (CaMKII) and
JNK pathways (20). However, whether
this is true for extracellular eAGR3 warrants further
investigation. Interestingly, the effect of eAGR3 does not seem to
be dose-dependent. This might be explained by the fact that high
abundance of AGR3 protein might promote formations of homodimers,
which, as we recently demonstrated for AGR2, are not secreted and
therefore lack extracellular activity (41).
The mechanism of action of extracellular AGR
proteins is in general poorly characterized and so far, it is not
clear how their signal is transduced within the cells. In line with
the existence of a cell surface receptor mediating extracellular
AGR proteins signaling both AGR2 and AGR3 are human orthologues of
the Xenopus laevis secreted XAG-2 protein (1). XAG-2 participates in frog embryogenesis
and amphibian limb regeneration by interacting with the Prod-1
receptor of Ly6 superfamily (42).
Although there is no human homologue of Prod-1, AGR2 was shown to
interact with another Ly6 receptor family member, C4.4A, which is a
GPI-anchored glycoprotein (22).
Interestingly, blocking the binding of AGR2 to C4.4A reduced
pancreatic tumor growth and metastasis in mice and improved mouse
survival. This suggests that secreted AGR2, at least partially,
exerts its pro-tumorigenic functions in a receptor-dependent
manner. Given a scarce number of reports describing the role of
AGR3 in cancer and its high homology with AGR2, we speculate that
eAGR3 may act similarly to eAGR2 and therefore could directly bind
to surface receptors thereby triggering the activation of the Src
pathway. Our hypothesis is also reinforced by the reported link
between AGR2 expression and Src phosphorylation in breast cancer
(33).
So far, the role of AGR3 in breast cancer biology
has been ambiguous, since we and others have reported the opposite
effect of elevated AGR3 expression on clinical outcomes (19,25).
However, the present work points towards rather pro-oncogenic
properties of eAGR3 such as migration and adhesion. This is of
particular importance, since AGR2 and AGR3 are co-expressed in
breast cancer tissues (25), have
been shown to be secreted in breast cancer (19) and as reported here, eAGR3 plays an
extracellular function as a signaling molecule in the tumor
microenvironment, as we previously described for eAGR2 (23). Therefore, our work highlights the
importance of AGR3 function and its prognostic significance in
breast cancer.
Acknowledgements
The authors would like to thank Mrs Katerina Kanova
from Masaryk Memorial Cancer Institute, RECAMO, 656 53 Brno, Czech
Republic, for technical assistance.
Funding
JO was supported by Ministry of Health, Czech
Republic (MMCI; grant. no. 00209805), DS was supported by an
Associazione Italiana per la Ricerca sul Cancro (grant. no.
AIRC2019) fellowship for Abroad', MD was supported by the Czech
Science Foundation (grant no. MEYS-NPS I-LO1413), RH by The Grant
Agency of the Czech Republic (grant. no. P206/12/G151); LS by The
Grant Agency of the Czech Republic (grant. no. 13-00956S;
19-02014S), and SP by the 7th Framework Program (a part of the EU
Marie Curie Initial Training Networks Biomedical engineering for
cancer and brain disease diagnosis and therapy development:
EngCaBra. Project no. PITN-GA-2010-264417. FD was supported by
grants from ‘Ligue contre le Cancer’ (Comité Charente; grant. no.
LC2018) and by the Fondation ARC pour la recherche sur le cancer
(grant. no. PJA 20181207750). This work was also funded by grants
from the Institut National du Cancer (grant. no.
PLBIO2012-PLBIO2017) to EC.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JO, LS, MD, TA, FI, DS and DF carried out the
experiments and related analyses. RH and SP provided the reagents,
designed the experiments and critically revised the manuscript. JO,
EC, FD and DF designed the study and wrote the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The author EC is a founder of Cell Stress Discovery
ltd.
Glossary
Abbreviations
Abbreviations:
|
AGR2
|
Anterior gradient 2
|
|
AGR3
|
Anterior gradient 3
|
|
DAG-1
|
dystroglycan-1
|
|
DMEM
|
Dulbecco's Modified Eagle's medium
|
|
eAGR2
|
extracellular anterior gradient
protein 2
|
|
eAGR3
|
extracellular anterior gradient
protein 3
|
|
ER
|
endoplasmic reticulum
|
|
EsR
|
estrogen receptor
|
|
PDI
|
protein disulfide isomerase
|
|
TEV
|
tobacco etch virus
|
References
|
1
|
Fletcher GC, Patel S, Tyson K, Adam PJ,
Schenker M, Loader JA, Daviet L, Legrain P, Parekh R, Harris AL and
Terrett JA: hAG-2 and hAG-3, human homologues of genes involved in
differentiation, are associated with oestrogen receptor-positive
breast tumours and interact with metastasis gene C4.4a and
dystroglycan. Br J Cancer. 88:579–585. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Higa A, Mulot A, Delom F, Bouchecareilh M,
Nguyên DT, Boismenu D, Wise MJ and Chevet E: Role of pro-oncogenic
protein disulfide isomerase (PDI) family member anterior gradient 2
(AGR2) in the control of endoplasmic reticulum homeostasis. J Biol
Chem. 286:44855–44868. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brychtova V, Mohtar A, Vojtesek B and Hupp
TR: Mechanisms of anterior gradient-2 regulation and function in
cancer. Semin Cancer Biol. 33:16–24. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Park SW, Zhen G, Verhaeghe C, Nakagami Y,
Nguyenvu LT, Barczak AJ, Killeen N and Erle DJ: The protein
disulfide isomerase AGR2 is essential for production of intestinal
mucus. Proc Natl Acad Sci USA. 106:6950–6955. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schroeder BW, Verhaeghe C, Park SW,
Nguyenvu LT, Huang X, Zhen G and Erle DJ: AGR2 is induced in asthma
and promotes allergen-induced mucin overproduction. Am J Respir
Cell Mol Biol. 47:178–185. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Norris AM, Gore A, Balboni A, Young A,
Longnecker DS and Korc M: AGR2 is a SMAD4-suppressible gene that
modulates MUC1 levels and promotes the initiation and progression
of pancreatic intraepithelial neoplasia. Oncogene. 32:3867–3876.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bonser LR, Schroeder BW, Ostrin LA,
Baumlin N, Olson JL, Salathe M and Erle DJ: The endoplasmic
reticulum resident protein AGR3. Required for regulation of ciliary
beat frequency in the airway. Am J Respir Cell Mol Biol.
53:536–543. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gray TA, MacLaine NJ, Michie CO,
Bouchalova P, Murray E, Howie J, Hrstka R, Maslon MM, Nenutil R,
Vojtesek B, et al: Anterior Gradient-3: A novel biomarker for
ovarian cancer that mediates cisplatin resistance in xenograft
models. J Immunol Methods. 378:20–32. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brychtova V, Zampachova V, Hrstka R,
Fabian P, Novak J, Hermanova M and Vojtesek B: Differential
expression of anterior gradient protein 3 in intrahepatic
cholangiocarcinoma and hepatocellular carcinoma. Exp Mol Pathol.
96:375–381. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vivekanandan P, Micchelli ST and Torbenson
M: Anterior gradient-2 is overexpressed by fibrolamellar
carcinomas. Hum Pathol. 40:293–299. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Obacz J, Takacova M, Brychtova V, Dobes P,
Pastorekova S, Vojtesek B and Hrstka R: The role of AGR2 and AGR3
in cancer: Similar but not identical. Eur J Cell Biol. 94:139–147.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Brychtova V, Vojtesek B and Hrstka R:
Anterior gradient 2: A novel player in tumor cell biology. Cancer
Lett. 304:1–7. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chevet E, Fessart D, Delom F, Mulot A,
Vojtesek B, Hrstka R, Murray E, Gray T and Hupp T: Emerging roles
for the pro-oncogenic anterior gradient-2 in cancer development.
Oncogene. 32:2499–2509. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Salmans ML, Zhao F and Andersen B: The
estrogen-regulated anterior gradient 2 (AGR2) protein in breast
cancer: A potential drug target and biomarker. Breast Cancer Res.
15:2042013. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kamal A, Valentijn A, Barraclough R,
Rudland P, Rahmatalla N, Martin-Hirsch P, Stringfellow H, Decruze
SB and Hapangama DK: High AGR2 protein is a feature of low grade
endometrial cancer cells. Oncotarget. 9:31459–31472. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bergstrom JH, Berg KA, Rodriguez-Pineiro
AM, Stecher B, Johansson ME and Hansson GC: AGR2, an endoplasmic
reticulum protein, is secreted into the gastrointestinal mucus.
PLoS One. 9:e1041862014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Edgell TA, Barraclough DL, Rajic A, Dhulia
J, Lewis KJ, Armes JE, Barraclough R, Rudland PS, Rice GE and
Autelitano DJ: Increased plasma concentrations of anterior gradient
2 protein are positively associated with ovarian cancer. Clin Sci
(Lond). 118:717–725. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kani K, Malihi PD, Jiang Y, Wang H, Wang
Y, Ruderman DL, Agus DB, Mallick P and Gross ME: Anterior gradient
2 (AGR2): Blood-based biomarker elevated in metastatic prostate
cancer associated with the neuroendocrine phenotype. Prostate.
73:306–315. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Garczyk S, von Stillfried S, Antonopoulos
W, Hartmann A, Schrauder MG, Fasching PA, Anzeneder T, Tannapfel A,
Ergönenc Y, Knüchel R, et al: AGR3 in breast cancer: Prognostic
impact and suitable serum-based biomarker for early cancer
detection. PLoS One. 10:e01221062015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tian S, Hu J, Tao K, Wang J, Chu Y, Li J,
Liu Z, Ding X, Xu L, Li Q, et al: Secreted AGR2 promotes invasion
of colorectal cancer cells via Wnt11-mediated non-canonical Wnt
signaling. Exp Cell Res. 364:198–207. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tsuji T, Satoyoshi R, Aiba N, Kubo T,
Yanagihara K, Maeda D, Goto A, Ishikawa K, Yashiro M and Tanaka M:
Agr2 mediates paracrine effects on stromal fibroblasts that promote
invasion by gastric signet-ring carcinoma cells. Cancer Res.
75:356–366. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Arumugam T, Deng D, Bover L, Wang H,
Logsdon CD and Ramachandran V: New blocking antibodies against
novel AGR2-C4.4A pathway reduce growth and metastasis of pancreatic
tumors and increase survival in mice. Mol Cancer Ther. 14:941–951.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fessart D, Domblides C, Avril T, Eriksson
LA, Begueret H, Pineau R, Malrieux C, Dugot-Senant N, Lucchesi C,
Chevet E and Delom F: Secretion of protein disulphide isomerase
AGR2 confers tumorigenic properties. Elife. 5(pii): e138872016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Adam PJ, Boyd R, Tyson KL, Fletcher GC,
Stamps A, Hudson L, Poyser HR, Redpath N, Griffiths M, Steers G, et
al: Comprehensive proteomic analysis of breast cancer cell
membranes reveals unique proteins with potential roles in clinical
cancer. J Biol Chem. 278:6482–6489. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Obacz J, Brychtova V, Podhorec J, Fabian
P, Dobes P, Vojtesek B and Hrstka R: Anterior gradient protein 3 is
associated with less aggressive tumors and better outcome of breast
cancer patients. Onco Targets Ther. 8:1523–1532. 2015.PubMed/NCBI
|
|
26
|
Bu H, Schweiger MR, Manke T, Wunderlich A,
Timmermann B, Kerick M, Pasqualini L, Shehu E, Fuchsberger C, Cato
AC and Klocker H: Anterior gradient 2 and 3-two prototype
androgen-responsive genes transcriptionally upregulated by
androgens and by oestrogens in prostate cancer cells. FEBS J.
280:1249–1266. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
King ER, Tung CS, Tsang YT, Zu Z, Lok GT,
Deavers MT, Malpica A, Wolf JK, Lu KH, Birrer MJ, et al: The
anterior gradient homolog 3 (AGR3) gene is associated with
differentiation and survival in ovarian cancer. Am J Surg Pathol.
35:904–912. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Trcka F, Durech M, Man P, Hernychova L,
Muller P and Vojtesek B: The assembly and intermolecular properties
of the Hsp70-Tomm34-Hsp90 molecular chaperone complex. J Biol Chem.
289:9887–9901. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Raetz CR, Ulevitch RJ, Wright SD, Sibley
CH, Ding A and Nathan CF: Gram-negative endotoxin: An extraordinary
lipid with profound effects on eukaryotic signal transduction.
FASEB J. 5:2652–2660. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fessart D, Simaan M and Laporte SA: c-Src
regulates clathrin adapter protein 2 interaction with beta-arrestin
and the angiotensin II type 1 receptor during clathrin-mediated
internalization. Mol Endocrinol. 19:491–503. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Geback T, Schulz MM, Koumoutsakos P and
Detmar M: TScratch: A novel and simple software tool for automated
analysis of monolayer wound healing assays. Biotechniques.
46:265–274. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang S and Yu D: Targeting Src family
kinases in anti-cancer therapies: Turning promise into triumph.
Trends Pharmacol Sci. 33:122–128. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Vanderlaag KE, Hudak S, Bald L,
Fayadat-Dilman L, Sathe M, Grein J and Janatpour MJ: Anterior
gradient-2 plays a critical role in breast cancer cell growth and
survival by modulating cyclin D1, estrogen receptor-alpha and
survivin. Breast Cancer Res. 12:R322010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen Y, Long H, Wu Z, Jiang X and Ma L:
EGF transregulates opioid receptors through EGFR-mediated GRK2
phosphorylation and activation. Mol Biol Cell. 19:2973–2983. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hrstka R, Nenutil R, Fourtouna A, Maslon
MM, Naughton C, Langdon S, Murray E, Larionov A, Petrakova K,
Muller P, et al: The pro-metastatic protein anterior gradient-2
predicts poor prognosis in tamoxifen-treated breast cancers.
Oncogene. 29:4838–4847. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fritzsche FR, Dahl E, Pahl S, Burkhardt M,
Luo J, Mayordomo E, Gansukh T, Dankof A, Knuechel R, Denkert C, et
al: Prognostic relevance of AGR2 expression in breast cancer. Clin
Cancer Res. 12:1728–1734. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bu H, Bormann S, Schafer G, Horninger W,
Massoner P, Neeb A, Lakshmanan VK, Maddalo D, Nestl A, Sültmann H,
et al: The anterior gradient 2 (AGR2) gene is overexpressed in
prostate cancer and may be useful as a urine sediment marker for
prostate cancer detection. Prostate. 71:575–587. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ramachandran V, Arumugam T, Wang H and
Logsdon CD: Anterior gradient 2 is expressed and secreted during
the development of pancreatic cancer and promotes cancer cell
survival. Cancer Res. 68:7811–7818. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
de Souza WF, Fortunato-Miranda N, Robbs
BK, de Araujo WM, de-Freitas-Junior JC, Bastos LG, Viola JP and
Morgado-Díaz JA: Claudin-3 overexpression increases the malignant
potential of colorectal cancer cells: Roles of ERK1/2 and PI3K-Akt
as modulators of EGFR signaling. PLoS One. 8:e749942013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Shao H, Gu Y, Ding J, Lu P, Ruan T and Lu
W: HEPACAM inhibited the growth and migration of cancer cells in
the progression of non-small cell lung cancer. Tumour Biol.
37:2621–2627. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Maurel M, Obacz J, Avril T, Ding YP,
Papadodima O, Treton X, Daniel F, Pilalis E, Hörberg J, Hou W, et
al: Control of anterior GRadient 2 (AGR2) dimerization links
endoplasmic reticulum proteostasis to inflammation. EMBO Mol Med.
11(pii): e101202019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Aberger F, Weidinger G, Grunz H and
Richter K: Anterior specification of embryonic ectoderm: The role
of the Xenopus cement gland-specific gene XAG-2. Mech Dev.
72:115–130. 1998. View Article : Google Scholar : PubMed/NCBI
|