Introduction
Colorectal cancer (CRC) is the third most common
type of cancer, accounting for 771,000 cases of mortality annually
worldwide (1). Metastasis is the
major cause of mortality in patients with CRC; however, effective
control strategies are limited. In the past few decades,
dysfunctional Wnt signaling has been demonstrated to be the major
contributor to CRC tumorigenesis and metastasis (2). Over 90% of CRC cases carry mutations in
genes encoding proteins involved in the Wnt/β-catenin pathway
(2). Therefore, understanding the
effects of Wnt/β-catenin signaling on the development and
metastasis of CRC may be useful in identifying potential
therapeutic targets (3).
In intestinal epithelial cells, excessive Wnt
protein expression continuously activates Wnt/β-catenin signaling
and drives epithelial-mesenchymal transition (EMT) (4), which is considered to be one of the
major determinants of metastasis (5). Conversely, inhibition of Wnt secretion
reverses EMT of CRC cells (4).
Sorting nexin 3 (SNX3) has been demonstrated to regulate the
secretion of Drosophila Wingless (Wg) which is the homolog
of human Wnt, via retromer-dependent Wntless recycling (6,7).
Therefore it has been hypothesized that human SNX3 may affect EMT
or the metastases of CRC cells. In the present study, it was
demonstrated that SNX3 inhibited β-catenin signaling in CRC cells
and reversed the EMT process, thereby reducing the metastasis of
CRC cells in vitro and in vivo.
Materials and methods
Cell culture
Human CRC cell lines (HCT116, HT29, SW480, SW620,
SW1116, HCT8, RKO, Colo205, LOVO, Colo320DM and NCI-H716) and a
human normal colon epithelial cell line, NCM460, were obtained from
the Type Culture Collection of the Chinese Academy of Medical
Sciences. All cells were maintained in RPMI-1640 medium
(Invitrogen; Thermo Fisher Scientific Inc.), supplemented with 10%
(v/v) fetal bovine serum (HyClone; GE Healthcare Life Sciences) in
a humidified incubator at 37°C and 5% CO2.
Virus infection, small interfering
(si)RNA and small hairpin (sh)RNA transfection
SNX3 expression lentivirus and a negative control
lentivirus were purchased from GeneCopoeia, Inc. HCT116 cells were
seeded into 24-well plates at a density of 4×105
cells/well. The HCT116 cells were infected by removing the old
culture medium and replacing it with 0.5 ml diluted viral
supernatant and incubated at the 37°C overnight. The clones with
stable SNX3 expression were selected using 2 µg/ml puromycin for 2
weeks.
For the siRNA-SNX3 experiments, cells were seeded in
6-well plates at a density of 1×105 cells/well and
medium was replaced with serum-free medium once confluence reached
~80%. Subsequently, 6 µl siRNA-SNX3 or negative control siRNA (NC
siRNA) (Shanghai GenePharma Co., Ltd.) was mixed with 94 µl
Opti-Minimum Essential Medium (Gibco; Thermo Fisher Scientific,
Inc.) and 12 µl HiPerfect transfection reagent (Qiagen China Co.,
Ltd.). The oligonucleotide sequences were as follows: siRNA-SNX3-01
sense strand, 5′-GCGUCAGCUUCCUUUUAGATT-3′ and antisense strand,
5′-UCUAAAAGGAAGCUGACGCTT-3′; siRNA-SNX3-02 sense strand,
5′-CCAGCAACUUCCUCGAGAUTT-3′ and antisense strand,
5′-AUCUCGAGGAAGUUGCUGGTT-3′; and NC siRNA sense strand,
5′-UUCUCCGAACGUGUCACGUTT-3′ and antisense strand,
5′-ACGUGACACGUUCGGAGAATT-3′. The mixture was gently agitated and
subsequently incubated for 5–10 min at room temperature, added to
the cells, and the cells were incubated for 48 h at 37°C.
HT29 cells were seeded in 6-well plates at
2×105 cells/well. The following day, 1 µg pLKO.1 puro
plasmid (Addgene, Inc.) encoding either human SNX3 shRNA or NC
shRNA were mixed with 3.75 µl HiPerfect transfection reagent
(Qiagen China Co., Ltd.) and 150 µl RPMI-1640 medium. The mixture
was gently mixed and subsequently incubated for 10–15 min at room
temperature, after which it was added to the cells. After 48 h,
transfected cells were selected using 2 µg/ml puromycin for 2
weeks. The medium was replaced once every three days. For the
si-β-catenin experiments, the shSNX3-HT29 and shNC-HT29 cells were
transfected with the siRNA-β-catenin or NC siRNA for 48 h.
β-catenin siRNA sense strand, 5′-GCUGCUUUAUUCUCCCAUUTT-3′ and
antisense strand, 5′-AAUGGGAGAAUAAAGCAGCTT-3′ were synthesized by
Shanghai GenePharma Ltd.
Western blot analysis
Cells were lysed in RIPA buffer (Beyotime Institute
of Biotechnology) supplemented with Protease Inhibitor Cocktail
(Roche Diagnostics). Equal quantities (20 µg) of total protein were
separated by SDS-PAGE using a 10% gel and subsequently transferred
onto polyvinylidene fluoride membranes. The membranes were blocked
with 5% skimmed milk and immunoblotted with the primary antibodies
against β-catenin (1:1,000), E-cadherin (1:1,000), vimentin
(1:1,000) cat. nos. 8480, 3195 and 5741 respectively; Cell
Signaling Technology, Inc.), SNX3 (1:1,000; cat. no. ab56078;
Abcam) and GAPDH (1:2,000; cat. no. 60004-1-Ig; ProteinTech Group,
Inc.) at 4°C overnight; antibodies were diluted in 5% BSA (Beijing
Solarbio Science & Technology Co., Ltd.) in Tris-buffered
saline. Following primary antibody incubation, membranes were
probed with anti-mouse immunoglobulin G (IgG) or anti-rabbit IgG
secondary antibodies (1:10,000; cat. no. SA00001-01 or SA00001-02,
respectively; ProteinTech Group, Inc.) at the room temperature for
2 h. The signal was visualized using Immobilon Western
Chemiluminescent HRP Substrate (EMD Millipore) according to the
manufacturer's protocol. The detection of GAPDH was used as loading
control and for densitometric analysis. The intensity of the bands
was semi-quantified using ImageJ version 1.46r (National Institutes
of Health).
Immunocytochemistry
Cells were fixed in 4% paraformaldehyde at room
temperature for 30 min and blocked with goat serum (cat. no. SL038;
Solarbio Life Sciences, Inc.) for 1 h at 37°C. Subsequently, cells
were incubated with E-cadherin and vimentin antibodies (1:100;
ProteinTech Group, Inc.) at 4°C overnight. The cells were washed
with PBS and incubated with Alexa Fluor® 594-conjugated
secondary antibodies (1:200; cat. no. A32740; Invitrogen; Thermo
Fisher Scientific, Inc.) at 1:1,000 for 1 h at 37°C. DAPI (Beijing
Solarbio Science & Technology Co., Ltd.) was used to stain the
cell nuclei for 5 min at the room temperature. Images were captured
using a fluorescence microscope (Carl Zeiss AG, Oberkochen,
Germany).
Invasion and migration assays
Cell migration and invasion assays were performed
using a 24-well migration chamber (Corning, Inc.) with or without
Matrigel™, respectively. For the cell migration assays, cells at a
density of 5×105 in 200 µl serum-free medium were seeded
onto Transwell inserts. The bottom chamber was filled with 600 µl
medium containing 20% FBS. For the invasion assays, Transwell
inserts were coated with 25 µg Matrigel™. After incubation for 48
h, the inserts were fixed with neat methanol at room temperature
for 20 min and stained with 2% crystal violet for 30 sec at the
room temperature. The number of cells which had invaded through the
membrane per field was counted and imaged under a light microscope
(magnification, ×200; Carl Zeiss AG).
Wound healing assay
The HT29 and HCT116 cells were seeded in 6-well
plates at a density of 1×106 cells/well. A scratch was
made in the center of the well using a sterile 100-µl pipette tip
once the confluence reached ~95%. The cells were washed three times
with PBS and the medium was replaced with fresh serum-free medium.
Images were captured on an inverted light microscope
(magnification, ×100; Carl Zeiss AG) at 0 and 24 h. Results were
expressed as the migration index; the distance migrated by HT29
relative to the distance migrated by HCT116 (8).
In vivo metastasis
A total of 10 female nude mice (aged 4–5 weeks and
weighing 16–22 g), purchased from Chengdu Dashuo Experimental
Animal Co., Ltd, were maintained at 37°C and 50% humidity under a
12-h light/dark cycle in an animal environmental control chamber
with free access to food and water under specific pathogen-free
conditions. HCT116 cells stably expressing SNX3 or vector control,
were harvested from cell culture plates, washed with PBS, and
resuspended at a concentration of 1×107 cells/ml in PBS.
A total of 10 mice were equally divided into two groups. Nude mice
were injected with 1×106 cells in 100 µl PBS in to the
tail vein. After 6 weeks, these mice were sacrificed, and body
weight was examined. All animal experiments were approved by the
Animal Experimental Ethics Committee of The Third People's Hospital
of Chengdu, and all procedures performed on animals were in
accordance with the ethical standards of The Third People's
Hospital of Chengdu.
Hematoxylin and eosin staining
Upon culling of the mice, the lungs were dissected
and fixed with 4% paraformaldehyde for 30 min at room temperature,
followed by embedding in paraffin. The tissues were sliced in to 5
µm thick sections, and were stained with hematoxylin and eosin
staining for 30 sec at room temperature. The metastatic foci in the
lungs were imaged and counted under a light microscope
(magnification, ×50 and ×100, respectively).
Statistical analysis
SPSS 20.0 (IBM Corp.) and GraphPad Prism 5.0
(GraphPad Software, Inc.) were used for data analysis. Quantitative
data are presented as the mean ± standard error of the mean of
three independent experiments. Comparisons between groups were
analyzed using the unpaired two-tailed Student's t-test, or a
one-way analysis of variance where appropriate with a Student's
Newman-Keuls test for post-hoc analysis. P<0.05 was considered
to indicate a statistically significant difference.
Results
SNX3 expression is downregulated in
human CRC cell lines
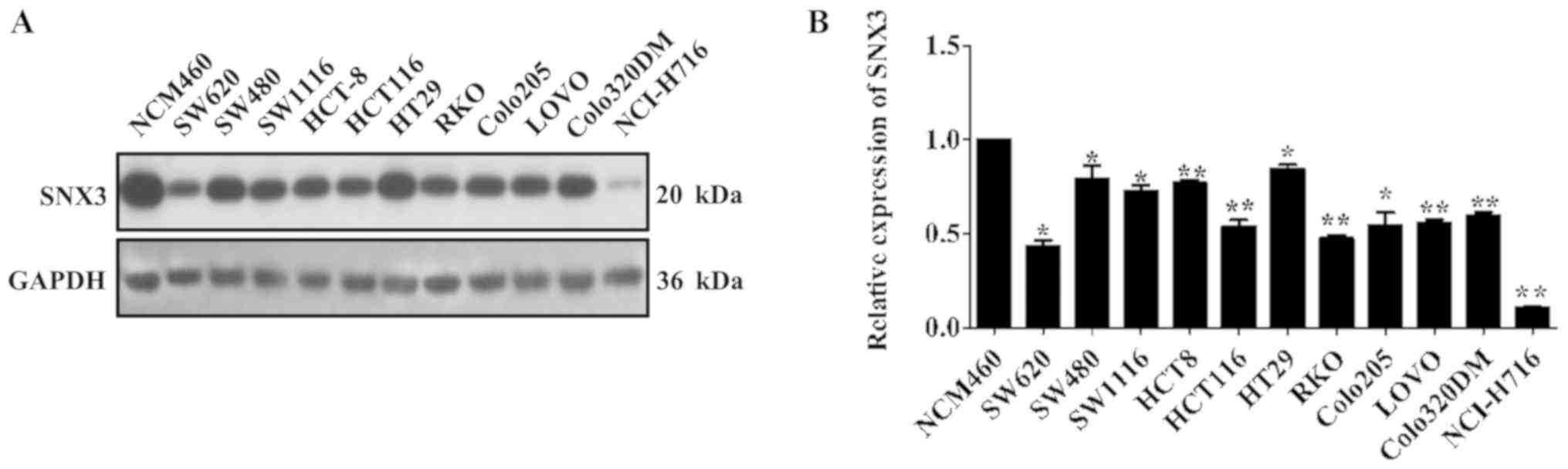
It has previously been reported that different CRC
cell lines exhibit variable expression of Wnt protein (9). Considering the ability of SNX3 to
mediate the secretion of Wg, which is the Drosophila homolog
of Wnt (7), the expression levels of
SNX3 were examined in 11 CRC cell lines and a human normal colon
epithelial cell line. The results demonstrated that SNX3 expression
was significantly decreased in the CRC cell lines compared with in
the normal colon epithelial cell line NCM460 (Fig. 1A and B). These findings suggested
that SNX3 expression may be downregulated in human CRC cell
lines.
Expression of SNX3 is inversely
associated with the migratory and invasive ability of CRC
cells
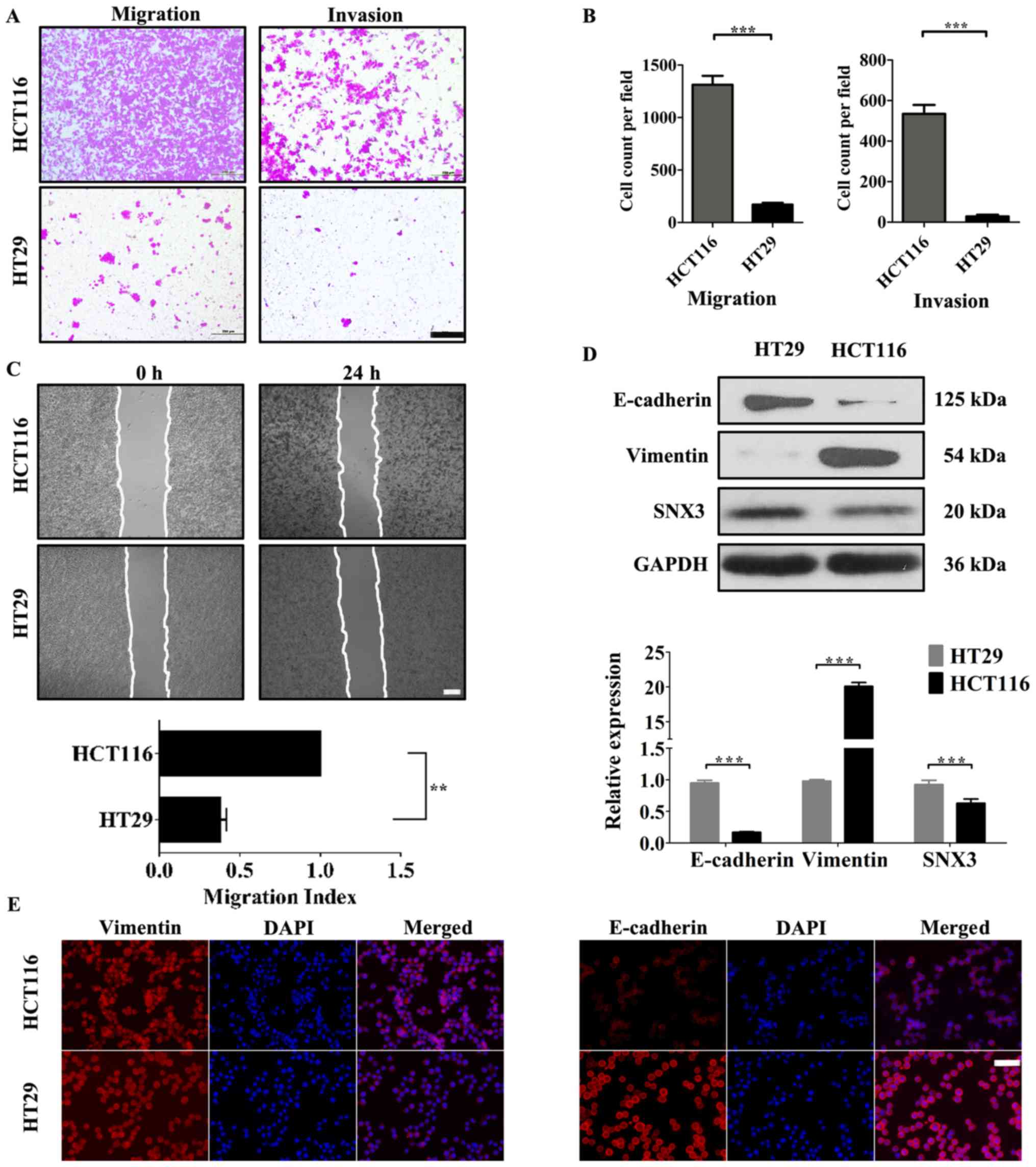
To understand the role of SNX3 in CRC cells, the
association between SNX3 expression and the migratory and invasive
ability of CRC cells was evaluated. HCT116 and HT29 cells were used
to investigate the effects of SNX3 on the migration and invasion of
human CRC cells. HCT116 cells exhibited significantly increased
migratory and invasive activity compared with HT29 cells (Fig. 2A-C). In addition, compared with HT29,
the protein expression levels of vimentin were significantly
upregulated, and E-cadherin was significantly downregulated in
HCT116 (Fig. 2D and E). Furthermore,
the expression levels of SNX3 were higher in HT29 cells compared
with in HCT116 cells (Fig. 2D).
These data indicated an inverse association between the expression
of SNX3 and the invasive capacity of CRC cells.
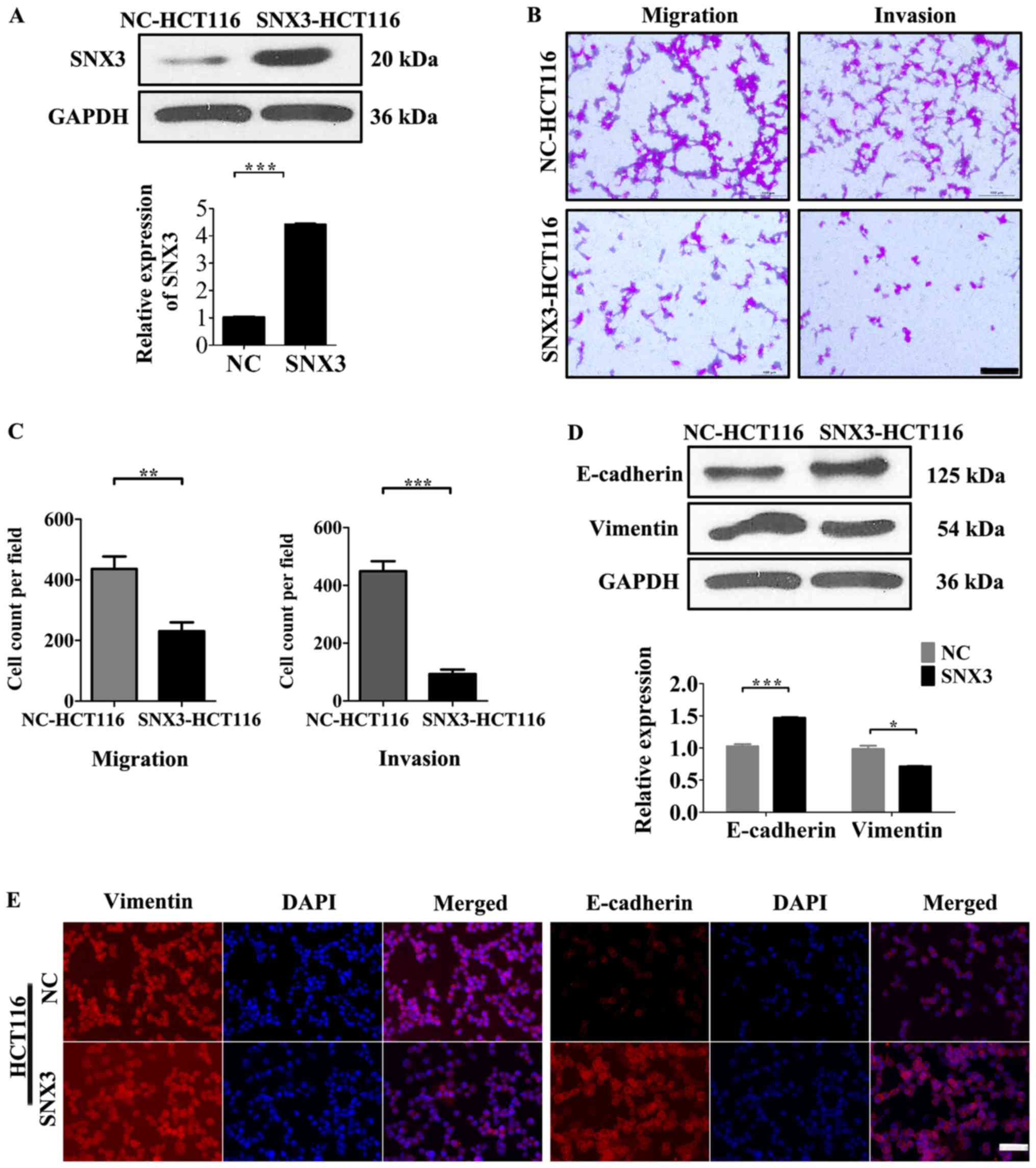
SNX3 overexpression suppresses HCT116
migration and invasion
To determine whether SNX3 overexpression affects
cellular processes that are involved in tumor metastasis, the
effects of SNX3 overexpression on the migration and invasion of
tumor cells was measured. Since HCT116 cells exhibited low SNX3
expression levels, a SNX3 lentivirus was transfected into these
cells (Fig. 3A). Overexpression of
SNX3 led to a decrease in the motility and invasiveness of HCT116
cells in vitro (Fig. 3B and
C).
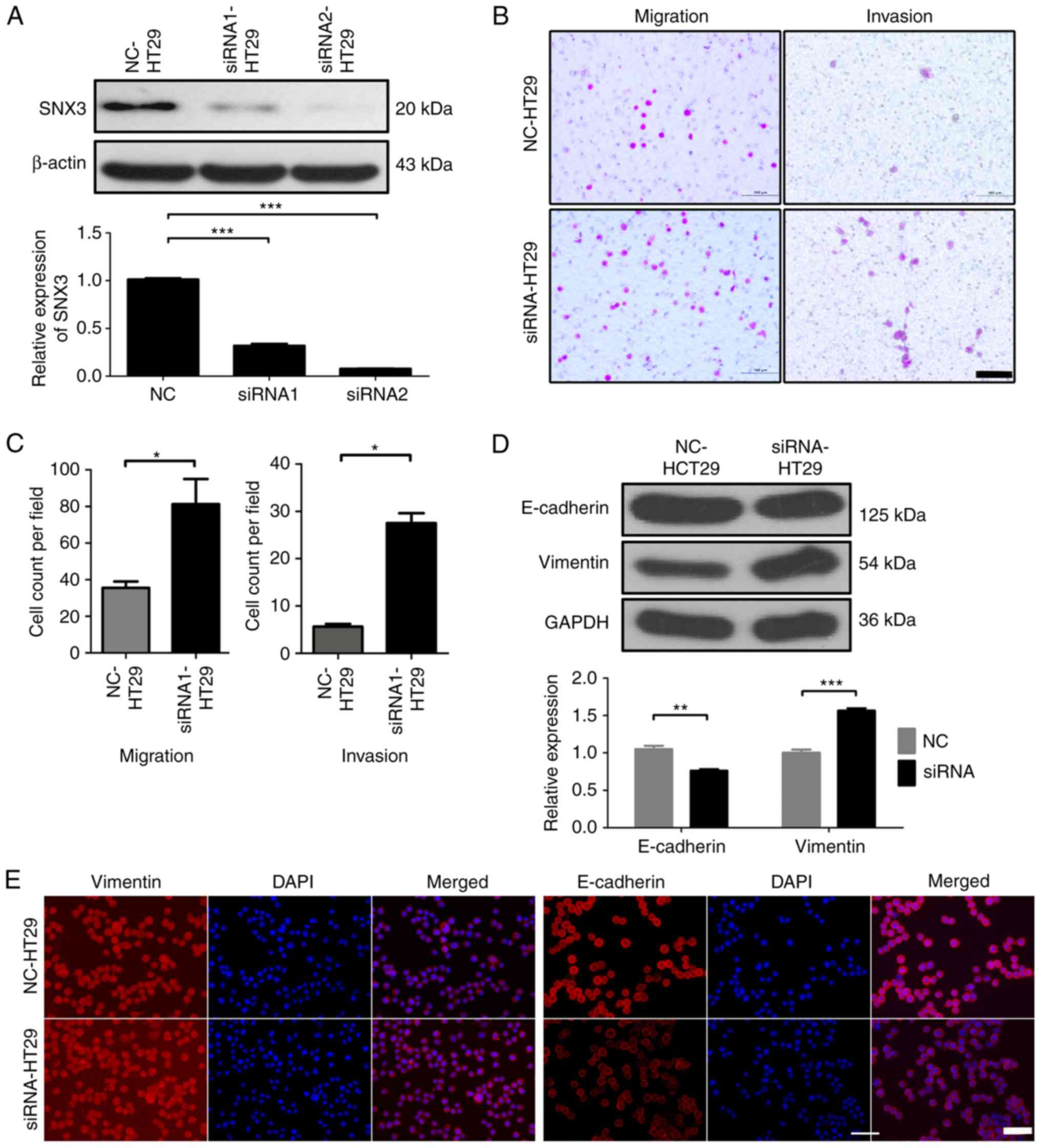
Silencing SNX3 promotes HT29 migration
and invasion
To further evaluate the function of SNX3 on the
migration and invasion of CRC cells, siRNAs were designed to
knockdown SNX3 expression in HT29 cells. siRNA1 and siRNA2 were
demonstrated to effectively knock down expression (Fig. 4A). siRNA2 was used in all subsequent
experiments. Transwell assays demonstrated that SNX3 knockdown
significantly increased the migratory and invasive ability of HT29
cells (Fig. 4B and C).
SNX3 expression decreases expression
of EMT-associated proteins
Since EMT is largely attributable to the migratory
and invasive capacity of CRC cells (10), the expression levels of EMT markers
were measured. To identify whether SNX3 inhibits EMT of HCT116
cells, the expression levels of EMT-associated proteins, E-cadherin
and vimentin, which are considered markers of epithelial cells and
mesenchymal cells, respectively, were determined. Overexpression of
SNX3 increased the expression levels of E-cadherin and decreased
the expression levels of vimentin (Fig.
3D and E). Conversely, the protein expression levels of
E-cadherin were decreased, whereas vimentin expression was
increased in HT29 cells following SNX3 knockdown (Fig. 4D and E). These findings suggested
that SNX3 may be able to reverse EMT.
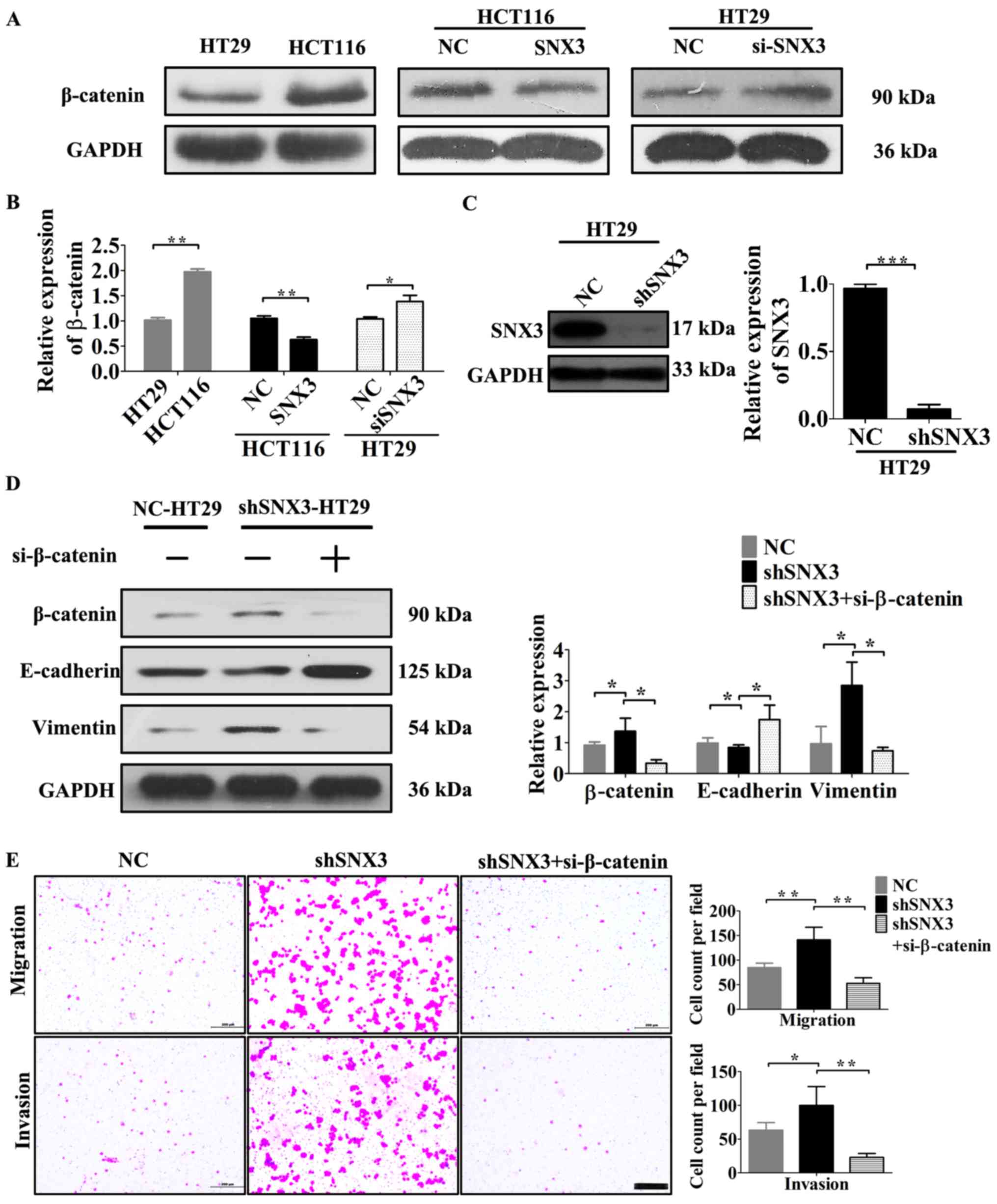
SNX3 may reverse EMT of CRC cells by
regulating β-catenin
β-catenin signaling contributes to CRC pathogenesis
and EMT (2). Based on assessment of
the expression levels of β-catenin and SNX3 in HCT116 and HT29
cells, β-catenin may be negatively associated with SNX3 expression
(Figs. 2D and 5A). Notably, SNX3 overexpression decreased
the expression levels of β-catenin in HCT116 cells (Fig. 5A and B), whereas SNX3 knockdown in
HT29 cells increased β-catenin expression (Fig. 5A-B). To examine the association
between EMT and β-catenin, the EMT status and invasive ability of
β-catenin-knockdown on HT29 cells transfected with SNX3 shRNA were
examined. The results demonstrated that β-catenin knockdown in
shRNA-SNX3-transfected HT29 cells significantly abrogated the EMT,
migration and invasion induced by SNX3 shRNA (Fig. 5D and E). Therefore, the results
suggested that SNX3 may reverse EMT in CRC cells in a β-catenin
dependent manner.
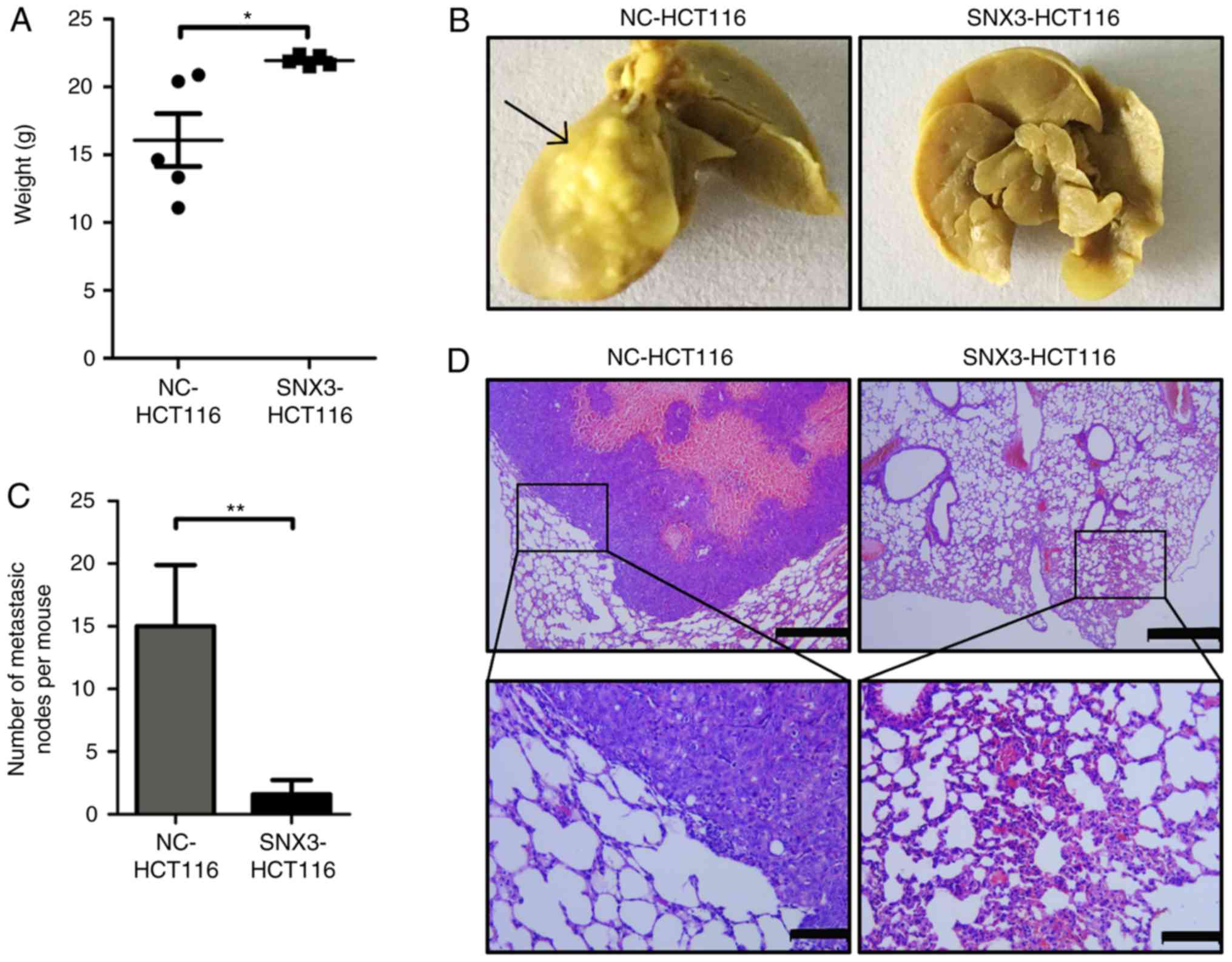
SNX3 inhibits the metastasis of CRC
cells to the lung in vivo
To further investigate the role of SNX3 in lung
metastasis of CRC cells in vivo, HCT116 cells were infected
with SNX3 overexpression lentivirus or negative control to
establish stable CRC cell lines. The effect of SNX3 on lung
metastasis was assessed in immunodeficient female nude mice using
tail vein injection. The degree of weight loss may be inversely
associated with the severity of the lung metastasis. After 8 weeks,
the weight of mice and the metastatic tumor nodules in the lungs
were measured. The results demonstrated that the weight of mice was
significantly decreased in mice injected with the negative control
cells compared with those injected with SNX3 overexpression cells
(Fig. 6A). Additionally, a decreased
number of total metastatic foci in the SNX3-overexpression group
was observed (Fig. 6B and C).
Hematoxylin and eosin staining was used to confirm the presence of
metastatic tumor nodules in the lungs (Fig. 6D). These findings suggested that SNX3
may promote metastasis of CRC cells to the lungs.
Discussion
The most common cause of cancer-associated mortality
is metastasis and EMT is the most common process by which
tumorigenic cells become metastatic (11). Therefore, inhibiting this
transformation may prove beneficial in cancer prevention.
Wnt/β-catenin signaling is considered to be the primary pathway
that regulates EMT and the expression of EMT-associated molecules,
including E-cadherin, vimentin and Snail (12,13).
Therefore, suppression of the Wnt/β-catenin signaling pathway and
reversal of EMT in cancer cells may be a potential approach to
cancer therapy (3). In the present
study, the results suggested that SNX3 may reverse EMT, and inhibit
the migratory and invasive potential of CRC cells. In addition, it
was demonstrated that SNX3 was differentially expressed in CRC
cells with different metastatic capacities. When SNX3 was
overexpressed in HCT116 cells, the typically high migratory and
invasive potential of the cells was suppressed, and the expression
of EMT-associated proteins were decreased. the mesenchymal
phenotype reverted back to that of epithelial cells. Conversely,
HT29 cells exhibited an increase in migratory and invasive capacity
following knockdown of SNX3. SNX3 is a transporter for the
secretion of Wnt proteins; therefore, it was hypothesized that the
actions of SNX3 may be associated with β-catenin signaling.
β-catenin was negatively associated with SNX3 expression, and
knockdown of β-catenin expression reduced migration and invasion.
Furthermore, SNX3 overexpression prevented lung metastasis of
injected HCT116 cells in vivo.
Sorting nexins are part of a large, evolutionarily
ancient family of proteins in mammals. These proteins regulate the
trafficking of various proteins among intracellular membranes,
including endocytosis, protein degradation and recycling (14). SNX3 is the only sorting nexin that
mediates secretion of Wnt protein and retrieval of its receptor,
Wntless (6,7). Although SNX3 is important for Wnt
signaling and epidermal growth factor receptor trafficking
(15), information on its role in
cancer prevention is limited. The present study demonstrated that
SNX3 may reverse the EMT in CRC cells by suppressing β-catenin,
thus inhibiting metastasis. These findings provide novel insights
into the functions of SNX3, particularly in regulating the EMT and
cancer metastasis.
At least 19 Wnt members have been identified to
date. Various Wnt proteins initiate canonical or noncanonical
β-catenin signals for distinctive biological purposes (16,17). For
example, in CRC, the secretion of Wnt3a, a common canonical Wnt,
acts as an oncogene that activates β-catenin (5); whereas, a noncanonical Wnt, Wnt5a,
serves as a tumor suppressor that impairs invasion, migration and
metastasis of CRC cells (18).
Therefore, two possible mechanisms by which SNX3 inhibits β-catenin
are hypothesized. Firstly, SNX3 may enhance non-canonical Wnt
signaling. Notably, Wnt5a enhances β-catenin degradation,
downregulates its expression in CRC cells (19), and is associated with a good
prognosis for patients with CRC (18). Secondly, SNX3 may affect the
stability of Wnt receptors, through a similar mechanism to that of
SNX27, which enhances the endocytosis of Fzd7 and promotes the
degradation of Fzd7, thus repressing the signals transduced by
β-catenin (20). In the present
study, examining the secretion of Wnt3 or Wnt5a by HT29 cells
overexpressing SNX3 was attempted; however, in the supernatant of
cultured CRC cells, the quantity of secretory Wnt was too low to be
effectively detected (data not shown). Future studies need to
further investigate the effects of SNX3 on CRC.
Acknowledgements
Not applicable.
Funding
This study was supported by grants from The National
Natural Science Foundation of China (grant no. 81270465 to YG), the
Natural Science Foundation of Sichuan Province (grant nos.
2015FZ0072 to YG and 2014JY0017 to CZ) and the Science and
Technology Foundation of Chengdu City (grant nos.
2014-HM01-00217-SF to CZ and 2015-HM01-00139-SF to ZZ).
Availability of data and material
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
BRP, CZ and YBG were responsible for the study
design, original article drafting and editing, data acquisition and
data analysis. BRP, TTZ, WY, YJL, ZZ, YT, YNC, JWZ and YLL
performed the experiments. BRP performed the tumor xenograft
experiments. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
All animal experiments were approved by the Animal
Experimental Ethics Committee of the Third People's Hospital of
Chengdu, and all procedures performed on animals were in accordance
with the ethical standards of the Third People's Hospital of
Chengdu.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
GBD 2013 Mortality and Causes of Death
Collaborators: Global, regional, and national age-sex specific
all-cause and cause-specific mortality for 240 causes of death,
1990–2013, . A systematic analysis for the Global Burden of Disease
Study 2013. Lancet. 385:117–171. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
White BD, Chien AJ and Dawson DW:
Dysregulation of Wnt/β-catenin signaling in gastrointestinal
cancers. Gastroenterology. 142:219–232. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Masuda M, Sawa M and Yamada T: Therapeutic
targets in the Wnt signaling pathway: Feasibility of targeting TNIK
in colorectal cancer. Pharmacol Ther. 156:1–9. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schwab RHM, Amin N, Flanagan DJ, Johanson
TM, Phesse TJ and Vincan E: Wnt is necessary for mesenchymal to
epithelial transition in colorectal cancer cells. Dev Dyn.
247:521–530. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Voloshanenko O, Erdmann G, Dubash TD,
Augustin I, Metzig M, Moffa G, Hundsrucker C, Kerr G, Sandmann T,
Anchang B, et al: Wnt secretion is required to maintain high levels
of Wnt activity in colon cancer cells. Nat Commun. 4:26102013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Harterink M, Port F, Lorenowicz MJ,
McGough IJ, Silhankova M, Betist MC, van Weering JRT, van Heesbeen
RGHP, Middelkoop TC, Basler K, et al: A SNX3-dependent retromer
pathway mediates retrograde transport of the Wnt sorting receptor
Wntless and is required for Wnt secretion. Nat Cell Biol.
13:914–923. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang P, Wu Y, Belenkaya TY and Lin X:
SNX3 controls Wingless/Wnt secretion through regulating
retromer-dependent recycling of Wntless. Cell Res. 21:1677–1690.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Thompson CC, Ashcroft FJ, Patel S, Saraga
G, Vimalachandran D, Prime W, Campbell F, Dodson A, Jenkins RE,
Lemoine NR, et al: Pancreatic cancer cells overexpress gelsolin
family-capping proteins, which contribute to their cell motility.
Gut. 56:95–106. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Holloway KR, Calhoun TN, Saxena M, Metoyer
CF, Kandler EF, Rivera CA and Pruitt K: SIRT1 regulates Dishevelled
proteins and promotes transient and constitutive Wnt signaling.
Proc Natl Acad Sci USA. 107:9216–9221. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhu QC, Gao RY, Wu W and Qin HL:
Epithelial-mesenchymal transition and its role in the pathogenesis
of colorectal cancer. Asian Pac J Cancer Prev. 14:2689–2698. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huber AH and Weis WI: The structure of the
beta-catenin/E-cadherin complex and the molecular basis of diverse
ligand recognition by beta-catenin. Cell. 105:391–402. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gilles C, Polette M, Mestdagt M,
Nawrocki-Raby B, Ruggeri P, Birembaut P and Foidart JM:
Transactivation of vimentin by beta-catenin in human breast cancer
cells. Cancer Res. 63:2658–2664. 2003.PubMed/NCBI
|
|
14
|
Cullen PJ and Korswagen HC: Sorting nexins
provide diversity for retromer-dependent trafficking events. Nat
Cell Biol. 14:29–37. 2012. View
Article : Google Scholar
|
|
15
|
Chiow KH, Tan Y, Chua RY, Huang D, Ng ML,
Torta F, Wenk MR and Wong SH: SNX3-dependent regulation of
epidermal growth factor receptor (EGFR) trafficking and degradation
by aspirin in epidermoid carcinoma (A-431) cells. Cell Mol life
Sci. 69:1505–1521. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee JM, Kim IS, Kim H, Lee JS, Kim K, Yim
HY, Jeong J, Kim JH, Kim JY, Lee H, et al: RORalpha attenuates
Wnt/beta-catenin signaling by PKCalpha-dependent phosphorylation in
colon cancer. Mol Cell. 37:183–195. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kikuchi A, Yamamoto H and Sato A:
Selective activation mechanisms of Wnt signaling pathways. Trends
Cell Biol. 19:119–129. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dejmek J, Dejmek A, Säfholm A, Sjölander A
and Andersson T: Wnt-5a protein expression in primary dukes B colon
cancers identifies a subgroup of patients with good prognosis.
Cancer Res. 65:9142–9146. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ying J, Li H, Yu J, Ng KM, Poon FF, Wong
SC, Chan AT, Sung JJ and Tao Q: WNT5A exhibits tumor-suppressive
activity through antagonizing the Wnt/beta-catenin signaling, and
is frequently methylated in colorectal cancer. Clin Cancer Res.
14:55–61. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sun L, Hu X, Chen W, He W, Zhang Z and
Wang T: Sorting nexin 27 interacts with Fzd7 and mediates Wnt
signalling. Biosci Rep. 36:e002962016. View Article : Google Scholar : PubMed/NCBI
|