Introduction: MicroRNAs (miRNAs/miRS) and
miR-146a
miRNAs, of 18–22 nucleotides in length have been
demonstrated to post-transcriptionally downregulate target mRNA
expression by binding to the 3′-untranslated region (UTR) of target
mRNA sequences (1,2). Each miRNA can regulate multiple target
genes, and several miRNAs can also regulate the same gene (1,2). Thus,
several miRNAs can cooperatively control the expression of a single
target gene with high precision. In addition, miRNAs are typically
highly conserved, which reflects the importance of their various
functions (3,4). miRNAs play key roles in diverse
biological processes, including cell development and
differentiation, signaling pathways, cell proliferation, apoptosis
and metabolism (5). Moreover,
dysregulation of miRNAs results in a number of diseases, such as
viral infections, genetic disorders and several types of cancer,
such as breast, pancreatic and gastric cancer (6–9).
miR-146a and miR-146b are two members of the miR-146 family. These
two miRNAs are expressed in chromosomes 5 and 10, respectively, and
exhibit a high level of structural similarity, differing in mature
sequence by only two nucleotides at the 3′ end (10). Such small differences in structure
indicate that these two miRNAs have similar biological functions
(10). For example, only mature
forms of miR-146a were found when expression of these miRNAs was
stimulated by lipopolysaccharide (LPS) in human monocytes,
suggesting different post-transcriptional processing mechanisms for
these miRNAs (11).
Studies that aimed to determine whether miRNAs play
an important role in the innate immune response to microbial
infection initially revealed that miR-146a is nuclear factor κB
(NF-κB)-dependent (12).
Importantly, miR-146a regulates the innate immune response by
binding to the 3′-UTR of the TNF receptor-associated factor 6
(TRAF6) and interleukin IL-1 receptor associated kinase 1 (IRAK1)
genes, which encode two key adapter molecules downstream of
Toll-like receptors (TLRs) and cytokine receptors (Table I) (12). Another study provided evidence that
in addition to acting as a modulator of chronic physiological and
pathological responses, miR-146a also regulated acute inflammatory
responses in human lung alveolar epithelial cells (13). Perry et al (13) demonstrated that exposure of human
lung alveolar epithelial cells to interleukin-1β (IL-1β) resulted
in a rapid time- and concentration-dependent increase in
miRNA-146a; to a lesser extent, an increase in miRNA-146b
expression was only observed at high IL-1β concentrations. This
analysis showed that increased miR-146a expression negatively
regulated the release of the proinflammatory chemokines IL-8 and
CCL-5 by targeting the 3′UTRs of their respective mRNAs (Table I) (13). In addition to the two known miR-146a
targets, TRAF6 and IRAK1, Hou et al (14) demonstrated that IL-1
receptor-associated kinase 2 (IRAK2) is another target of miR-146a.
And in several types of cancer, such as breast, pancreatic and
gastric cancer, miR-146a was found to suppress cancer cell
proliferation, invasion and metastasis by repressing the expression
of epidermal growth factor receptor (EGFR) through a direct
mechanism that involves targeting the 3′-UTR of EGFR mRNA (Table I) (7–9).
Furthermore, vesicular stomatitis virus infection resulted in the
upregulation of miR-146a expression in mouse macrophages through
the TLR-myeloid differentiation factor Myd88-independent TLR, but
in a retinoic acid-inducible gene I (RIG-I)-NF-κB-dependent manner
by targeting TRAF6, IRAK1 and IRAK2 (Table I) (14).
 | Table I.Targets of microRNA-146a. |
Table I.
Targets of microRNA-146a.
| Target gene | Type of cell | Functions | (Refs.) |
|---|
| EGFR | Breast, pancreatic
and gastric cancer cells | Cell proliferation,
invasion and metastasis | (7–9) |
| TRAF6, IRAK1 | LPS-stimulated
monocytes, Th17 cells, human stellate cells | Innate immunity
response, T cell-mediated autoimmunity, anti-fibrotic effect | (12,43,59) |
| IL-8, CCL-5 | Lung epithelial
alveolar | Acute inflammatory
responses | (13) |
| IRAK2 | VSV-infected
macrophages | Innate immunity
response | (14) |
| IL-6 | Pulmonary
macrophages | Paraquat
poison | (15) |
| KDM2B | HPV16-positive
keratinocytes | Cell proliferation,
migration | (16) |
| COX-2, FLAP | Lung adenocarcinoma
cells | Cell proliferation,
migration | (17,18) |
| FADD | T lymphocytes | Anti-apoptotic
effect | (34) |
| Stat1 | Treg cells, Tfh
cells, NK/T cells | Immune homeostasis,
limiting the number of Tfh cells, NK/T cell function | (39,54,55) |
| Itch | Th2 cells | Th1/Th17
skewing | (41) |
| ICOS | Tfh cells | Limiting the number
of GC B cells | (44) |
| Numb | MZ B cells | MZ B cell
differentiation | (49) |
| CFH | HBV-infected
hepatocytes | Hepatitis | (51) |
| HNF1α | Hepatocytes | Hepatitis, hepatic
fibrosis | (57) |
| BRCA1 | HUVECS | Microvascular
invasion | (62) |
| HAb18G | Hepatocytes | Cell migration,
metastasis | (63) |
Based on experiments using pulmonary macrophages,
peripheral blood mononuclear cells and serum from patients with
lung injury caused by Paraquat poisoning, a dual-luciferase
reporter assay demonstrated that the IL-6 mRNA is a direct target
of miR-146a. Accordingly, it was suggested that increased
expression of IL-6 in patients with lung injury caused by Paraquat
poisoning was associated with decreased expression of miR-146a
(Table I) (15). Furthermore, studies performed using
human papillomavirus (HPV)16 E6/E7-positive keratinocytes
identified the histone demethylase KDM2B as a new direct target of
miR-146a, and two putative binding sites for miR-146a were
identified in its 3′-UTR sequence (Table
I) (16). These results revealed
that the transcriptional repressor c-MYC mediated the
downregulation of miR-146a through the binding sites in the
miR-146a promoter, resulting in KDM2B overexpression in
HPV-mediated tumorigenesis. Thus, miR-146a-5p may have therapeutic
potential to significantly inhibit the proliferation and migration
of keratinocytes and cervical cancer cells (16). Two novel targets of miR-146a,
cyclooxygenase-2 (COX-2) (17) and
5-lipoxygenase-activating protein (FLAP) (Table I) (18), the functions of which are associated
with arachidonic acid metabolism, were also reported. Arachidonic
acid can be converted to prostaglandins (PGs) or leukotrienes (LTs)
by the enzymatic activities of COX-1, COX-2 or 5-lipoxygenase
(5-LO), respectively. FLAP functions with 5-LO to convert
arachidonic acid to the intermediate leukotriene B4 (LTB4), one of
the most potent LTs. FLAP and LTB4 were found to be upregulated in
lung cancer due to a hypermethylated miR-146a promoter, and high
LTB4 expression supported a favorable microenvironment for tumor
growth and metastasis, leading to low overall survival time in
patients with lung adenocarcinoma (18). Similarly, decreased miR-146a
expression contributed to the upregulation of COX-2 in lung cancer
cells (17). Thus, in lung cancer
cells, miR-146a acts as an endogenous dual inhibitor of arachidonic
acid metabolism by regulating both PG and LT production via direct
targeting of the COX-2 and FLAP 3′-UTRs (17,18).
In addition to miRNA, next-generation sequencing
revealed that long non-coding RNAs (lncRNAs), a class of regulatory
RNAs >200 nucleotides without protein-coding function, play
important roles in regulating pre-miRNA splicing, mRNA degradation
and epigenetic modification (19).
Emerging studies have shown that lncRNAs function as endogenous
sponges to regulate miR-146a expression by competitively binding to
miR-146a (20–23). For instance, lncRNA HCG18 functions
as a competitive endogenous RNA (ceRNA) to upregulate TRAF6
expression by sponging miR-146a in intervertebral disc degeneration
(20). One study showed that the
lncRNA MALAT1 promoted the pro-inflammatory NF-κB pathway by
targeting miR-146a in LPS-induced acute kidney injury (21). Similarly, the lncRNA NIFK-AS1
suppressed M2 macrophage polarization in endometrial cancer by
binding to miR-146a (22), and the
lncRNA CHRF downregulated miR-146a in osteoarthritis (23). lncRNAs also inhibited miR-146a
expression by inducing methylation of the CpG island in its
promoter, such as lncRNA PVT1 (24).
However, no lncRNA was found to regulate the expression of miR-146a
in hepatocellular carcinoma (HCC).
Role of miR-146a in the toll-like receptor 4
(TLR4) signaling pathway
As the first barrier of the body against infectious
diseases, TLRs serve as the eyes of natural immunity by monitoring
and identifying various pathogen-associated molecular patterns. To
date, 10 members of the human TLR family have been found in mammals
and humans: TLRs −1, −2, −4, −5, −6 and −10 are expressed on the
surface of cells, whereas TLRs −3, −7, −8 and −9 are found
intracellularly. There are 12 members of the TLR family (TLR1 to
TLR9, and TLR11 to TLR13) in rats (25). Among the TLRs, TLR4 signaling plays
an important role in initiating the innate immune response. LPS,
the principal component of the outer membrane of Gram-negative
bacteria, is a strong stimulator of monocytes and macrophages,
involved in innate immunity, and induces the production of a
variety of inflammatory mediators, including tumor necrosis
factor-α (TNF-α), both in vitro and in vivo. However,
TLR4 does not bind to LPS directly; the adaptor protein myeloid
differentiation factor 2 (MD-2) binds directly to and recognizes
the lipophilic component of LPS (lipid A) (26). The process of TLR4 activation is
accomplished through a series of steps in which LPS is bound by
different LPS-binding proteins and transferred to the MD-2/TLR4
complex. The LPS-binding protein joins with the LPS monomer from
the LPS and delivers it to cluster of differentiation-14 (CD14)
proteins, which form the final complex comprising of LPS and
MD-2/TLR4 (27). TLR4 identifies
ligands in two ways, namely, via the TLR4/MyD88/NF-kB and
TLR4/TRIF/IRF3 pathways. In the former pathway, the combination of
TLR4 and its ligand forms the MD-2/TLR4 complex that subsequently
binds to the Toll/IL-1R homology (TIR) domain structure of MyD88 to
activate it. The activated TLR4/MyD88 complexes further stimulate
IL-1R-associated kinase 4 (IRAK4). MyD88 recruits IRAK4, and the
MyD88-IRAK4 complex recruits the IRAK4 substrate IRAK2 or related
IRAK1 (26–28). Based on the crystal structure, the
MyD88-IRAK4-IRAK2 complex occurs at a stoichiometry of 6:4:4
(29). Phosphorylated IRAK1 and
IRAK2 bind to TRAF6 leading to the activation of inhibitor of
nuclear factor κB kinase (IKK). Subsequently, the inhibitor of κB
(IκB) is phosphorylated by IKK, which triggers ubiquitination and
proteolysis and removes IκB from NF-κB. As a result, NF-κB enters
the nucleus and triggers subsequent inflammatory reactions by
promoting gene transcription of immune-responsive genes and release
of cytokines, such as IL-1β, TNF-α, IL-6 and miR-146a (26–29). A
scheme describing how miR-146a functions in the TLR4 signaling
pathway is provided in Fig. 1.
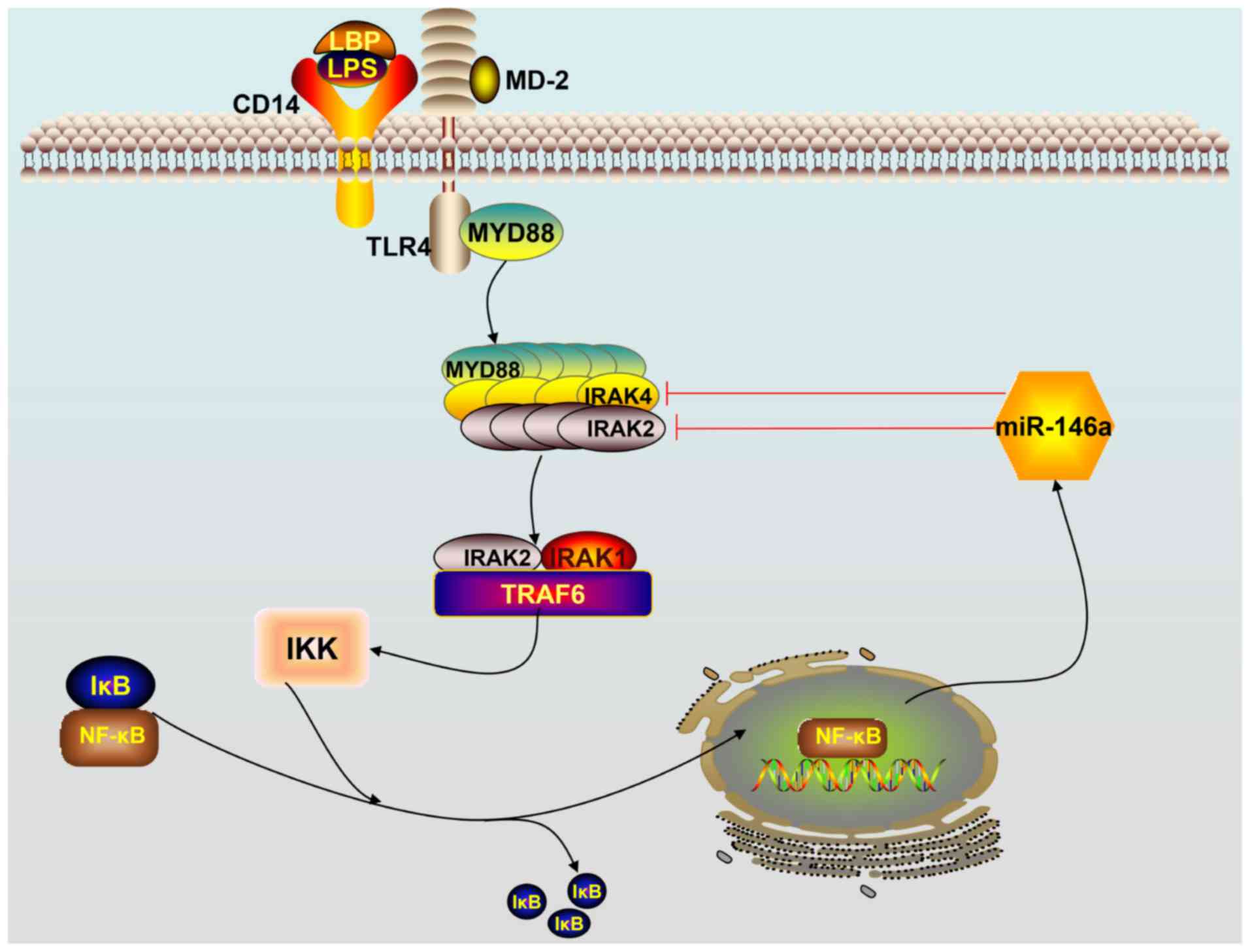 | Figure 1.Schematic overview of the TLR4
signaling pathway. Activation of the TLR4 receptor by
lipopolysaccharides triggers downstream NF-κB signaling. NF-κB
enters the nucleus, leading to the expression of miR-146a. Mature
miR-146a downregulates IRAK1 and TRAF6 levels by targeting their
mRNAs and subsequently terminates the TLR4 signaling pathway
cascade. TLR, Toll-like receptor; miR, microRNA; TRAF6, TNF
receptor-associated factor 6; IRAK1, interleukin IL-1
receptor-associated kinase 1; NF-κB, nuclear factor κB; LPS,
lipopolysaccharides; LBP, lipopolysaccharide binding protein; IKK,
inhibitor of nuclear factor-κB kinase; IκB, inhibitor of κB; CD14,
cluster of differentiation-14 protein; MD-2, myeloid
differentiation factor 2. |
On the other hand, overexpression or inappropriate
expression of TLR4 has been implicated in several immune-mediated
and inflammatory diseases. A variety of extracellular and
intracellular negative feedback pathways are involved in
maintaining balanced cellular homeostasis following the activation
of TIR receptors. miR-146a was shown to terminate the TLR4
signaling cascade to control the activation of mammalian innate
immune responses (12). As early as
2006, miR-146a, miR-132 and miR-155 were reported to be upregulated
in human monocytes in response to LPS treatment (12). In one study, 200 miRNAs were analyzed
after exposing THP-1 cells of the human acute monocytic leukemia
cell line to LPS production, and expression of miR-146a/b, miR-132
and miR-155 was enhanced (12).
Further investigation revealed that transcriptional induction of
miR-146a by LPS, TNF-α and IL-1β was dependent on NF-κB. Notably,
miR-146 was found to play a significant role in controlling TLR and
cytokine signaling through a negative feedback regulation loop
involving the downregulation of IRAK1 and TRAF6 levels, each a key
component involved in amplifying the responses of TLR4 signaling
(12).
In addition to functions in THP-1 monocytes,
miR-146a was also reported to be upregulated in lymphocytes and
lung alveolar epithelial cells through the activation of
TLR-mediated NF-κB signaling (13,30).
Moreover, it is well established that soluble decoy TLRs (sTLRs)
are effective in blocking TLR signaling (31). For example, LPS-induced NF-κB
activation and TNF production were inhibited by recombinant sTLR4
expressed by macrophages in vitro. In addition, recent
investigation in cells of mammals and fish demonstrated that IRF3
negatively regulates the TLR-mediated NF-κB signaling pathway by
targeting TRIF for ubiquitination and degradation (32). In brief, hosts initiate robust
activation of the innate immune system to guard against pathogens,
including LPS, and mobilize a variety of extracellular and
intracellular negative feedback pathways to avoid an overstimulated
inflammatory state.
Role of miR-146a in the adaptive immune
response
In addition to its expression in monocytes,
macrophages that are involved in innate immune responses, miR-146a
was also expressed in T cells and B cells, which are associated
with adaptive immune responses. During the adaptive immune
response, the binding of T cell receptors (TCRs) to an antigen
results in the activation of three main transcription factors,
activator protein 1 (AP-1), NF-κB and NFAT, which are involved in
the secretion of early cytokines, particularly IL-2 (33). After the body has defeated the
invading organisms, activated T lymphocytes must be removed in a
timely manner to terminate the immune response; this is
accomplished by killing the cells that may have developed
self-recognition and that are capable of producing reactions to
autoantigens (33). miR-146a has a
crucial role in accumulating or maintaining the memory T cell pool
(34). Evidence that miR-146a is
present at a low level in naïve human T cells and is abundantly
expressed in human memory T cells suggests that miR-146a is
upregulated during T cell differentiation (34). miR-146a expression is also induced
following T cell activation, upon T cell receptor stimulation. A
study found that miR-146a impairs AP-1 activity, halts the IL-2
signal to modulate T lymphocyte differentiation and functions as an
antiapoptotic factor to protect T lymphocytes from Fas-mediated
apoptosis by targeting the Fas-associated via death domain mRNA
(Table I) (34). As well as acting as an antiapoptotic
factor, miR-146a functions as a T cell-autonomous factor that
controls T-cell-mediated autoimmune responses by targeting TRAF6
and IRAK1 (35). Similar to innate
immunity, adaptive immunity (particularly T-cell responses) must be
accurately modulated to effectively protect the body and prevent
inflammatory diseases, including autoimmune diseases (33–35).
Thus, miR-146a is an indispensable modulator in adaptive
immunity.
CD4+ T cells play a pivotal role in
orchestrating the reactions of the innate and adaptive immune
systems (36). Following activation,
CD4+ T cells can differentiate into distinct subsets of
effector T helper (Th) cells, including Th1, Th2, Th9, Th17, Th22,
regulatory T cells (Tregs) and T follicular helper cells (Tfh),
which have distinct functions in the immune system and regulate
appropriate cellular and humoral immune responses to various
pathogens (37). Th1 cells produce
IL-2, interferon-γ (IFN-γ) and TNF-β, which protect the host
against intracellular pathogens; Th2 cells produce IL-4, IL-5, IL-6
and IL-13, and stimulate B cells to proliferate and produce IgE,
which is associated with the humoral immune response and allergic
inflammation (37). Th17 cells
produce the IL-17 family proinflammatory cytokines, which are
involved in eliminating certain fungi and extracellular pathogens
(38). Thus, tightly regulated
differentiation of Th cells is vital for providing an effective
immune response and avoiding unwanted autoimmunity. Indeed,
miR-146a has emerged as a critical modulator of effector T helper
cell differentiation. In Tregs, miR-146a suppresses Th1 responses
and controls Treg-maintained immune homeostasis by downregulating
the expression of Stat1, a key transcription factor for Th1
effector cell differentiation (Table
I) (39). Luo et al
(40) revealed that epithelial
cell-derived miR-146a induced the expression of IL-10 in monocytes
and the inducible IL-10+ monocytes are capable of
suppressing the activities of CD4+ effector T cells and
skewing Th2 polarization to avoid allergic reactions in a mouse
model of allergic rhinitis. Moreover, miR-146a also prevented
aggressive Th1/17 skewing in Th2 cells by targeting Itch, which
regulates the production of cytokines (Table I) (41). Interestingly, it was recently
reported that miR-146a may be associated with the imbalance of
Th1/Th2 differentiation upon decreased IL-4 and increased IFN-γ
levels, leading to a Th1-driven immune response following acute
exposure to airborne particulate matter PM2.5 (42). However, further experiments are
needed to verify this hypothesis. In Th17 cells, miR-146a blocked
the autocrine IL-6- and IL-21-induced Th17 differentiation pathways
in autoreactive CD4+ T cells by targeting TRAF6 and
IRAK1 to control T-cell-mediated autoimmunity, such as autoimmune
encephalomyelitis (Table I)
(43).
miR-146a also serves as a post-transcriptional brake
to prevent the accumulation of Tfh cells and germinal center (GC)
B-cells, which is crucial for optimal GC B-cell selection (44,45).
Moreover, miR-146a-mediated repression of STAT1 contributed to
limiting the number of Tfh cells and miR-146a-mediated suppression
of inducible T cell costimulator (ICOS) on Tfh cells, and the
corresponding decreased expression of ICOSL on GC B cells
contributed to limiting the number of GC B cells (Table I) (44). A recent study highlighted the crucial
role of miR-146b, another member of the miR-146 family, in
regulating the GC reaction orchestrated by B cells and Tfh cells
(46). Similar to previous reports,
it was found that the loss of miR-146a in B cells alone is
sufficient to cause enhanced GC responses and spontaneous
autoimmunity (44,45). However, the inconsistency was that
specific deletion of miR-146a in T cells did not alter the number
of Tfh cells, although specific deletion of both miR-146a and
miR-146b in T cells increased Tfh cell numbers and elevated GC
responses (46). Thus, further
studies are needed to explain the intricate molecular mechanism of
the miR-146 family in Tfh cells. Although miR-146a was identified
as a critical negative regulator of immune reactions, miR-146a
overexpression led to enlargement of the spleen and lymph node,
inflammatory infiltration in the liver and lung, increased levels
of T cells in peripheral blood and imbalanced homeostasis, and
abnormal development of T cells (47,48).
Therefore, superabundant or insufficient miR-146 expression is
harmful to the maintenance of immune homeostasis. Regardless, the
use of miR-146 for the treatment of diseases is challenged by the
determination of the optimum dosage.
Despite a good understanding of the role of miR-146a
in myeloid and T-cell subsets, its role in B cells is not well
understood. Recently, King et al (49) observed an increase in preceding
transitional B-cell stages, intact splenic retention and decreased
marginal zone (MZ) B cells in miR-146a-deficient mice. Thus, it was
speculated that MZ B-cell differentiation was disrupted due to
decreased Notch2 signaling and increased Numb expression, with the
latter serving not only as a negative regulator of the Notch2
pathway, but also as a direct target of miR-146a (Table I) (49). Overall, miR-146a-mediated regulation
of the Notch2 pathway is required for the development of MZ B
cells.
Role of miR-146a in HCC
HCC, the most common primary liver cancer, is the
third leading cause of cancer-associated death in the world,
according to statistics reported by the World Health Organization
(WHO) in 2006 (50). The etiology of
HCC is a complex, multistep and multifactor process involving a
number of factors, such as chronic infection with hepatitis B virus
(HBV) or hepatitis C virus (HCV), liver cirrhosis, habitual alcohol
abuse and aflatoxin B1 exposure (50). Recently, miR-146a was reported to
participate in a variety of pathogenic pathways associated with
hepatocarcinogenesis. HBV infects hepatocytes, but does not
directly cause cytopathic changes; indeed, immune responses of the
host are the main cause of hepatic injury. Li et al
(51) found that the protein HBV X
(HBx) promoted the expression of miR-146a through the NF-κB
signaling pathway in HBV-expressing hepatocytes, which subsequently
expressed increased levels of miR-146a, leading to inflammation of
the liver via downregulation of the target complement factor H
(CFH), an important negative regulator of the complement
alternative pathway (Table I;
Fig. 2). In the alternative pathway,
CFH accelerates the transfer of Bb from C3b by competing with B or
Bb for binding to C3b, through the inhibition of C3 convertase
(C3bBb) formation. A decreased level of CFH in hepatocytes was
associated with excessive activation of the complement alternative
pathway and was a direct cause of liver inflammation (51). Hence, elevated HBx-induced expression
of miR-146a led to more severe inhibition of CFH in hepatocytes,
resulting in accumulation of C3bBb and enhancement of the
alternative pathway-mediated cytotoxicity, and the subsequent
outcomes of hepatitis, liver fibrosis and HCC (51). Similarly, the level of miR-146a was
consistently increased in HCV-infected hepatocyte-like cells and
liver tissue from HCV-infected patients (52). Further investigation revealed that
persistent HCV infection induced upregulation of miR-146a through
the NF-κB signaling pathway and that increased expression of
miR-146a was involved in key metabolic pathways in liver cells,
including the proteasome, fatty acid metabolism and anaerobic
energetic metabolism pathways (52).
Moreover, increased expression of miR-146a may weaken the immune
responses of hepatocytes and help HCV-infected cells escape immune
surveillance (52). In HBV or HCV
infections, dysregulation of miR-146a promotes liver inflammation
and disease progression, in contrast to the usual role of miR-146a
in suppressing cancer growth and inhibiting inflammation (16–18,51,52). The
finding that miR-371, miR-373 and miR-543 dramatically decreased
caspase-8 (Casp-8) gene expression in HCC, by binding to the Casp-8
mRNA, supports the pro-necrotic and pro-inflammatory functions of
miRNAs (53). Furthermore, Visalli
et al (53) identified that
miR-371, miR-373 and miR-543 were overexpressed in HCC, resulting
in a significantly decreased level of Casp-8, a marked increase in
programmed cell necrosis and an intensive inflammatory process.
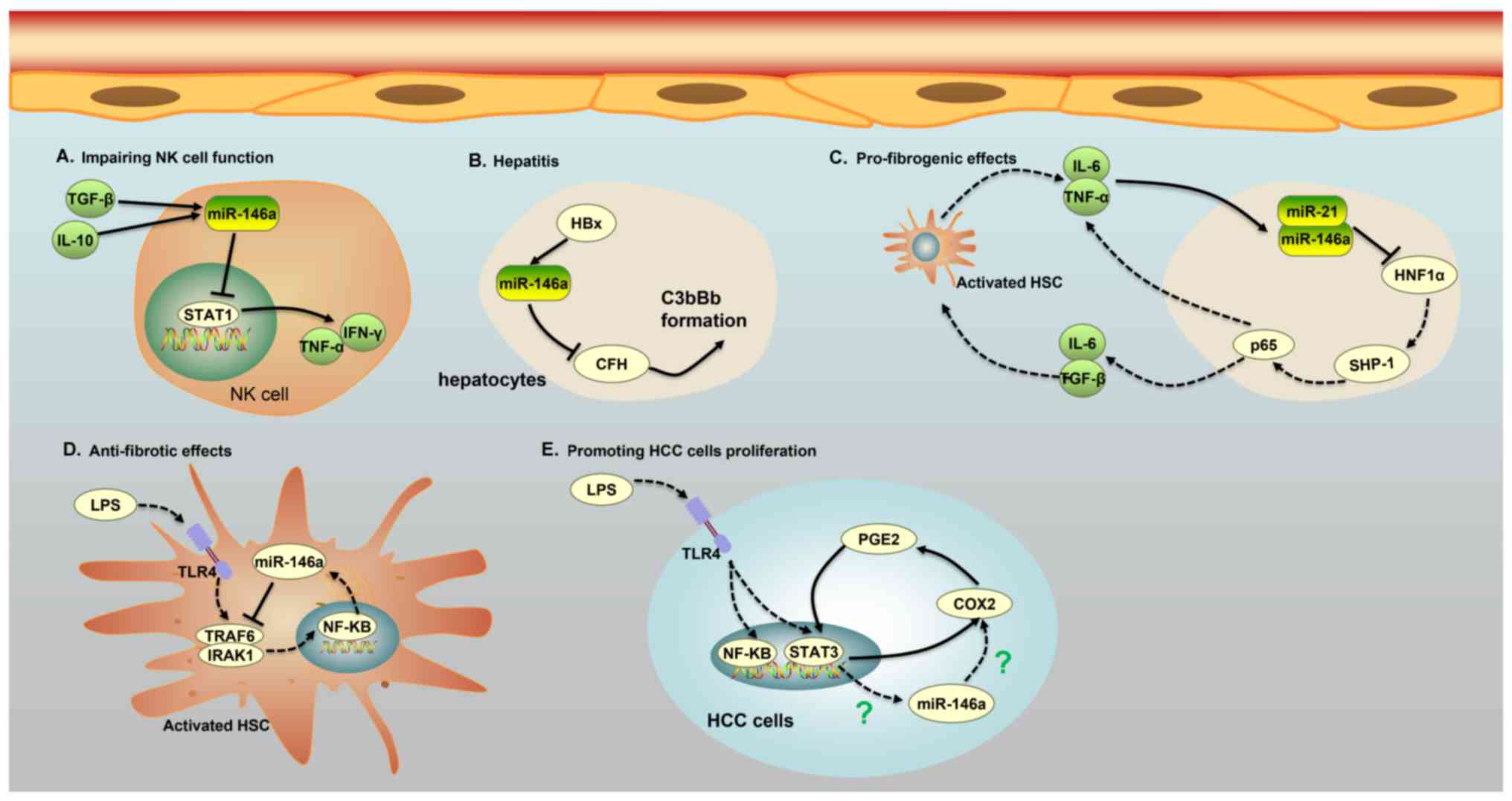 | Figure 2.Five major roles of miR-146a in
different cells of patients with HCC. (A) miR-146a expression in NK
cells of patients with chronic hepatitis B and HCC is induced by
IL-10 and TGF-β, and increased expression of miR-146a weakens the
function of NK cells and the secretion of TNF-α and interferon-γ by
targeting STAT1. (B) The protein HBx promotes the expression of
miR-146a, leading to liver inflammation via the downregulation of
CFH, an important negative regulator of the complement alternative
pathway in HBV-expressing hepatocytes. The low expression of CFH
causes a cascade of C3bBb formation, which is associated with liver
inflammation. (C) An miR-146a-mediated feedback circuit modulates
the crosstalk between HSCs and hepatocytes leading to hepatic
fibrosis. Inhibition of HNF1α in hepatocytes by miR-21 and miR-146a
leads to an increase in IL-6 and TGF-β production, which causes the
activation of HSCs. On the other hand, activated HSCs secrete IL-6
and TNFα, which further suppress the expression of HNF1α and SH2
domain-containing phosphatase-1 in hepatocytes. (D) miR-146a can
impede the phosphorylation of NF-κB and suppress the secretion of
pro-inflammatory cytokines by binding to IRAK1 and TRAF6 in
LPS-induced HSC activation. (E) A COX-2/PGE2/STAT3 positive
feedback loop exists in HCC cells, which may involve miR-146a. HCC,
hepatocellular carcinoma; IL, interleukin; TGF-β, transforming
growth factor-β; NK, natural killer; miR, microRNA; TNF, tumor
necrosis factor; CFH, target complement factor H; HSC, human
stellate cells; HNF, hepatocyte nuclear factor; HBV, hepatitis B
virus; HBx, HBV x; LPS, lipopolysaccharide; C3bBb, C3 convertase;
NF-κB, nuclear factor κB; COX-2, cyclooxygenase-2; PGE2,
prostaglandin E2; SHP-1, SH2 domain-containing phosphatase-1;
HNF1α, hepatocyte nuclear factor 1α; STAT3, signal transducer and
activator of transcription 3. |
Indeed, the level of miR-146a expression was
increased not only in HBV/HCV-infected hepatocytes and liver tissue
from HBV/HCV-infected patients, but also in natural killer (NK)
cells and T cells from chronic hepatitis B (CHB) and HCC patients
(54,55). miR-146a expression in NK cells of
patients with CHB and HCC was induced by IL-10 and TGF-β, and
increased expression of miR-146a weakened the function of NK cells
and the secretion of TNF-α and IFN-γ by targeting STAT1 (Table I; Fig.
2) (54). Additionally, miR-146a
levels were significantly increased in CD4+ and
CD8+ T cells of patients infected with HBV, possibly due
to inflammatory cytokines and viral factors, although the details
of the underlying mechanisms remain unclear (55). The overexpression of miR-146a in CHB
patients impaired T-cell function by targeting STAT1 (55). As a transcription factor, STAT1 plays
an important role in antiviral responses by inducing the expression
of antiviral IFN-stimulated genes and epitope-specific CTLs. Thus,
the upregulation of miR-146a observed in patients with CHB may lead
to decreased expression of NK cell-mediated antiviral cytokines and
antiviral IFN-stimulated genes, as well as impairment of T/B cell
function, promotion of T-cell functional deficiency and immune
tolerance to HBV infection (54,55).
Liver fibrosis and cirrhosis are well known to be
the main causes of HCC (56).
miR-146a was found to be involved in the progression of
hepatocellular damage and hepatic fibrogenesis through an
inflammatory feedback circuit regulating the crosstalk between
hepatocytes and human stellate cells (HSCs) (57). The activation of HSCs into
fibroblast-like cells in the injured liver mediated a gradual
accumulation of excessive extracellular matrix proteins, leading to
hepatic fibrosis (58). In rat
models of liver fibrosis, inflammatory cytokines such as IL-6 and
TNF-α were shown to increase the expression of miR-146a and miR-21,
which in turn sustained the repression of hepatocyte nuclear factor
1α (HNF1α), a liver-enriched transcription factor that markedly
alleviated hepatic fibrosis in hepatocytes. Moreover, HNF1α
suppression in hepatocytes induced phosphorylation of signal
transducer and activator of transcription 3 (STAT3) and p65 by
regulating the transcriptional expression of SH2 domain-containing
phosphatase-1 (SHP-1), which contributed to IL-6, TNF-α and TGFβ1
production, thus enhancing hepatocellular inflammation, HSC
activation and hepatic fibrosis progression (Table I; Fig.
2) (57). Importantly, another
study found that HNF1α regulated the transcriptional expression of
SHP-1 by binding to the SHP-1 promoter in hepatocytes, which was
associated with activation of the NF-κB and JAK/STAT pathways,
including the phosphorylation of STAT3 and p65 (57). This discovery highlights the central
role of miR-146a in liver cell homeostasis and signaling; thus,
impairment of miR-146a regulation and signaling may participate in
hepatocellular injury and liver cirrhosis. Nonetheless, it was not
determined whether HNF1α binds to the SHP-1 promoter directly or
whether other adaptor proteins are required. In addition, it
remains to be determined whether other factors are involved in this
inflammatory feedback circuit consisting of HNF1α, SHP-1, STAT3,
p65, miR-21 and miR-146a. Interestingly, another study revealed the
anti-proinflammatory and anti-fibrotic effects of miR-146a on liver
injury via the inhibition of LPS/TLR4 signaling (59). TLR4 signaling occurs in activated
HSCs, and TLR4 signaling cascades were capable of promoting the
secretion of inflammatory cytokines, chemokines and adhesion
molecules, which contributed to the progression of liver injury and
fibrogenesis (59). Nevertheless,
miR-146a downregulated the expression levels of TLR4, IRAK1 and
TRAF6, and impeded the phosphorylation of NF-κB to suppress the
secretion of pro-inflammatory cytokines and cell proliferation, and
to promote apoptosis (Table I;
Fig. 2) (59). As most miRNAs exert multiple
biological properties due to their multiple modes of action, it was
not unexpected that miR-146a had controversial functions in the
progression of liver injury and fibrogenesis (1). A single miRNA can target multiple mRNA
transcripts, which may be controlled by multiple miRNAs. Thus, it
may be reasonable for miR-146a to exhibit different expression
characteristics and function in some specific inflammatory settings
by downregulating various target genes.
In addition to being expressed in HSCs, TLR4 were
found to be functionally expressed in HCC cells upon LPS
stimulation, and by activating TLR4 signaling, LPS was able to
induce proliferation and clone formation in HCC cells (60). Moreover, positive associations were
found among the expression of TLR4, COX-2 and STAT3 in the liver
tumor tissues of patients with HCC, and further investigation
demonstrated the existence of a COX-2/PGE2/STAT3 positive feedback
loop in HCC cells (Fig. 2) (60). Regardless, it was not determined
whether miR-146a was involved in the COX-2/PGE2/STAT3 positive
feedback loop in HCC cells by LPS stimulation, although miR-146a
was demonstrated to be an endogenous dual inhibitor of arachidonic
acid metabolism in lung cancer cells by regulating the production
of both PG and LT via direct targeting of the COX-2 and FLAP 3′
UTRs (17,18). Importantly, another study confirmed
that miR-146a expression was regulated by aberrantly activated
STAT3 in HCC cells (61). Based on
this evidence, it was speculated that miR-146a may be involved in
the COX-2/PGE2/STAT3 positive feedback loop in HCC cells through
LPS stimulation. Further analysis is needed to verify this
speculation and provide a deeper understanding.
miR-146a was also shown to promote the angiogenic
activity of endothelial cells in patients with HCC (62). Angiogenesis, a hallmark of cancer, is
important in the multistep development of cancer, and endothelial
cells (ECs) play a crucial role in the proliferation, migration and
arrangement of vascular cavities. By targeting BRCA1, miR-146a
upregulated the expression of platelet-derived growth factor
receptor α in human umbilical vein endothelial cells, leading to
microvascular invasion in patients with HCC (Table I) (62). In contrast to the aforementioned
results, Zhang et al (63)
demonstrated that miR-146a expression was notably decreased in
hepatoma cells and hepatoma tissues due to the methylation of the
miR-146a promoter. Furthermore, the recovery of miR-146a was able
to prominently inhibit cancer migration, invasion and metastasis by
downregulating vascular endothelial growth factor through dual
pathways in HCC. miR-146a inhibits nuclear accumulation of
β-catenin by upregulating the expression of adenomatosis polyposis
coli (APC), a tumor suppressor, whereas miR-146a downregulates
NF-κB p65 by targeting HAb18G expression (Table I) (63). As a previous study showed that
miR-146a directly downregulated the expression of UHRF1, which
modulated tumor suppressor gene silencing via DNA methylation
(64), it was speculated that the
suppression of miR-146a may facilitate self-methylation by
upregulating UHRF1 in HCC. However, this possibility was not fully
explored and verified, and the molecular mechanism by which
miR-146a resulted in upregulation of APC expression requires
further investigation.
In addition to the environmental risk factors,
genetic factors also have an important function in hepatocellular
carcinogenesis. Accumulating studies have provided evidence for a
complex link between dysregulated miR-146 expression and HCC
development (65–68). A G>C polymorphism (rs2910164) in
the stem region opposite the mature miR-146a sequence is caused by
a G:U to C:U mismatch in the stem region of the miR-146a precursor.
Xu et al (65) demonstrated
that male individuals carrying the GG genotype of the miR-146a gene
had a 2-fold greater susceptibility to HCC than those with the CC
genotype; individuals carrying the miR-146a GG genotype also had
increased production of mature miR-146a. Jazdzewski et al
(69) reported the expression of
pre-miR-146a or mature miR-146a from the C allele to be
approximately 2-fold lower than that from the G allele, with the GC
genotype of miR-146a conferring greater susceptibility to an
increased risk for papillary thyroid carcinoma compared with the GG
or CC genotype. Overall, different etiological factors for
papillary thyroid carcinoma and HCC may account for this
discrepancy. Furthermore, a large number of meta-analyses based on
several cases showed that the miR-146a rs2910164 polymorphism
contributed to increased susceptibility to the development of HCC
(66–68). Thus, the genetic variants of miR-146a
may be part of a spectrum of genes involved in the etiology of
HCC.
Orthotopic liver transplantation is considered as
the best choice for patients with end-stage liver disease, whereas
acute rejection (AR) of transplants represents the most difficult
problem to solve (70). Moreover, it
was reported that plasma miRNAs, such as miR-122, miR-192 and
miR-146a, can be used as potential diagnostic biomarkers for acute
rejection following liver transplantation; as plasma miR-122 and
miR-192 indicate liver injury and miR-146a may reflect cellular
rejection (71). The association
between miR-146a and AR is not surprising as AR is mainly mediated
through T-cell-dependent immune pathways, and miR-146a plays a
significant role in the control of T-lymphocyte maturation and
activation. Surprisingly, increased expression of miR-146a may be
associated with multidrug resistance in drug-resistant HCC
(72).
Conclusions and perspectives
Cumulative evidence has demonstrated that miR-146a
serves as a key modulator of innate and adaptive immune responses.
However, the mechanism by which miR-146a directly or indirectly
affects T-cell differentiation remains controversial, and further
studies are required to address this. A number of studies have
demonstrated the utility of microRNAs as cancer-associated
biomarkers, supported by the finding that some microRNAs displayed
altered expression profiles in cancer compared with those in normal
tissues. In several types of cancer, such as breast, pancreatic and
gastric cancer, miR-146a was found to suppress cancer cell
proliferation, invasion and metastasis by repressing the expression
of EGFR through a direct mechanism that involves targeting the
3′-UTR of EGFR mRNA. However, an increasing number of studies have
demonstrated that miR-146a not only acts as a tumor suppressor, but
also functions in hepatocellular oncogenesis, and that genetic
variants of miR-146a may be part of a spectrum of genes involved in
the etiology of HCC. Several questions remain about the role of
miR-146a in oncogenesis. Considering the various roles of miR-146a
and its association with HCC, further investigations are required
in order to understand the molecular mechanisms of
miR-146a-mediated liver disease pathogenesis and HCC development.
Finally, the potential of miR-146a as a diagnostic biomarker of
liver injury, hepatic fibrosis or HCC requires further
investigation.
Acknowledgements
Not applicable.
Funding
This study was supported by the Natural Science
Foundation of China (grant nos. 81571572, 81201488 and
30801088).
Availability of data and materials
Not applicable.
Authors' contributions
HW and XL were major contributors to writing the
manuscript. LW and JL produced the tables and figures. HW, XL, LW,
JL, XW, TL, YX and WW checked and revised the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lagos-Quintana M, Rauhut R, Lendeckel W
and Tuschl T: Identification of novel genes coding for small
expressed RNAs. Science. 294:853–858. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lau NC, Lim LP, Weinstein EG and Bartel
DP: An abundant class of tiny RNAs with probable regulatory roles
in Caenorhabditis elegans. Science. 294:858–862. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang H, Qu Y, Duan J, Deng T, Liu R,
Zhang L, Bai M, Li J, Zhou L, Ning T, et al: Integrated analysis of
the miRNA, gene and pathway regulatory network in gastric cancer.
Oncol Rep. 35:1135–1146. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shi XB, Tepper CG and deVere White RW:
Cancerous miRNAs and their regulation. Cell Cycle. 7:1529–1538.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hurst DR, Edmonds MD, Scott GK, Benz CC,
Vaidya KS and Welch DR: Breast cancer metastasis suppressor 1
up-regulates miR-146, which suppresses breast cancer metastasis.
Cancer Res. 69:1279–1283. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li Y, Vandenboom TG, Wang Z, Kong D, Ali
S, Philip PA and Sarkar FH: miR-146a suppresses invasion of
pancreatic cancer cells. Cancer Res. 70:1486–1495. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kogo R, Mimori K, Tanaka F, Komune S and
Mori M: Clinical significance of miR-146a in gastric cancer cases.
Clin Cancer Res. 17:4277–4284. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Labbaye C and Testa U: The emerging role
of MIR-146A in the control of hematopoiesis, immune function and
cancer. J Hematol Oncol. 5:132012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Thomson JM, Newman M, Parker JS,
Morin-Kensicki EM, Wright T and Hammond SM: Extensive
post-transcriptional regulation of microRNAs and its implications
for cancer. Genes Dev. 20:2202–2207. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Taganov KD, Boldin MP, Chang KJ and
Baltimore D: NF-kappaB-dependent induction of microRNA miR-146, an
inhibitor targeted to signaling proteins of innate immune
responses. Proc Natl Acad Sci USA. 103:12481–12486. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Perry MM, Moschos SA, Williams AE,
Shepherd NJ, Larner-Svensson HM and Lindsay MA: Rapid changes in
microRNA-146a expression negatively regulate the IL-1beta-induced
inflammatory response in human lung alveolar epithelial cells. J
Immunol. 180:5689–5698. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hou J, Wang P, Lin L, Liu X, Ma F, An H,
Wang Z and Cao X: MicroRNA-146a feedback inhibits RIG-I-dependent
type I IFN production in macrophages by targeting TRAF6, IRAK1 and
IRAK2. J Immunol. 183:2150–2158. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu W and Li Y: Lung injury caused by
paraquat poisoning results in increased interleukin-6 and decreased
microRNA-146a levels. Exp Ther Med. 16:406–412. 2018.PubMed/NCBI
|
|
16
|
Peta E, Sinigaglia A, Masi G, Di Camillo
B, Grassi A, Trevisan M, Messa L, Loregian A, Manfrin E, Brunelli
M, et al: HPV16 E6 and E7 upregulate the histone lysine demethylase
KDM2B through the c-MYC/miR-146a-5p axys. Oncogene. 37:1654–1668.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cornett AL and Lutz CS: Regulation of
COX-2 expression by miR-146a in lung cancer cells. RNA.
20:1419–1430. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Iacona JR, Monteleone NJ and Lutz CS:
miR-146a suppresses 5-lipoxygenase activating protein (FLAP)
expression and Leukotriene B4 production in lung cancer cells.
Oncotarget. 9:26751–26769. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Batista PJ and Chang HY: Long noncoding
RNAs: Cellular address codes in development and disease. Cell.
152:1298–1307. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xi Y, Jiang T, Wang W, Yu J, Wang Y, Wu X
and He Y: Long non-coding HCG18 promotes intervertebral disc
degeneration by sponging miR-146a-5p and regulating TRAF6
expression. Sci Rep. 7:132342017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ding Y, Guo F, Zhu T, Li J, Gu D, Jiang W,
Lu Y and Zhou D: Mechanism of long non-coding RNA MALAT1 in
lipopolysaccharide-induced acute kidney injury is mediated by the
miR-146a/NF-κB signaling pathway. Int J Mol Med. 41:446–454.
2018.PubMed/NCBI
|
|
22
|
Zhou YX, Zhao W, Mao LW, Wang YL, Xia LQ,
Cao M, Shen J and Chen J: Long non-coding RNA NIFK-AS1 inhibits M2
polarization of macrophages in endometrial cancer through targeting
miR-146a. Int J Biochem Cell Biol. 104:25–33. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yu C, Shi D, Li Z, Wan G and Shi X: Long
noncoding RNA CHRF exacerbates IL-6-induced inflammatory damages by
downregulating microRNA-146a in ATDC5 cells. J Cell Physiol.
234:21851–21859. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu HT, Fang L, Cheng YX and Sun Q: LncRNA
PVT1 regulates prostate cancer cell growth by inducing the
methylation of miR-146a. Cancer Med. 5:3512–3519. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kawai T and Akira S: The role of
pattern-recognition receptors in innate immunity: Update on
Toll-like receptors. Nat Immunol. 11:373–384. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shimazu R, Akashi S, Ogata H, Nagai Y,
Fukudome K, Miyake K and Kimoto M: MD-2, a molecule that confers
lipopolysaccharide responsiveness on Toll-like receptor 4. J Exp
Med. 189:1777–1782. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Meng J, Lien E and Golenbock DT:
MD-2-mediated ionic interactions between lipid A and TLR4 are
essential for receptor activation. J Biol Chem. 285:8695–8702.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Guven-Maiorov E, Keskin O, Gursoy A,
VanWaes C, Chen Z, Tsai CJ and Nussinov R: The architecture of the
TIR domain signalosome in the toll-like receptor-4 signaling
pathway. Sci Rep. 5:131282015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lin SC, Lo YC and Wu H: Helical assembly
in the MyD88-IRAK4-IRAK2 complex in TLR/IL-1R signalling. Nature.
465:885–890. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cameron JE, Yin Q, Fewell C, Lacey M,
McBride J, Wang X, Lin Z, Schaefer BC and Flemington EK:
Epstein-Barr virus latent membrane protein 1 induces cellular
MicroRNA miR-146a, a modulator of lymphocyte signaling pathways. J
Virol. 82:1946–1958. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Iwami KI, Matsuguchi T, Masuda A, Kikuchi
T, Musikacharoen T and Yoshikai Y: Cutting edge: Naturally
occurring soluble form of mouse Toll-like receptor 4 inhibits
lipopolysaccharide signaling. J Immunol. 165:6682–6686. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhao X, Huo R, Yan X and Xu T: IRF3
negatively regulates toll-like receptor-mediated NF-κB signaling by
targeting TRIF for degradation in teleost fish. Front Immunol.
9:8672018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Schorle H, Holtschke T, Hünig T, Schimpl A
and Horak I: Development and function of T cells in mice rendered
interleukin-2 deficient by gene targeting. Nature. 352:621–624.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Curtale G, Citarella F, Carissimi C,
Goldoni M, Carucci N, Fulci V, Franceschini D, Meloni F, Barnaba V
and Macino G: An emerging player in the adaptive immune response:
microRNA-146a is a modulator of IL-2 expression and
activation-induced cell death in T lymphocytes. Blood. 115:265–273.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yang L, Boldin MP, Yu Y, Liu CS, Ea CK,
Ramakrishnan P, Taganov KD, Zhao JL and Baltimore D: miR-146a
controls the resolution of T cell responses in mice. J Exp Med.
209:1655–1670. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhou L, Chong MM and Littman DR:
Plasticity of CD4+ T cell lineage differentiation.
Immunity. 30:646–655. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Murphy KM and Stockinger B: Effector T
cell plasticity: Flexibility in the face of changing circumstances.
Nat Immunol. 11:674–680. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Korn T, Bettelli E, Oukka M and Kuchroo
VK: IL-17 and Th17 cells. Annu Rev Immunol. 27:485–517. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lu LF, Boldin MP, Chaudhry A, Lin LL,
Taganov KD, Hanada T, Yoshimura A, Baltimore D and Rudensky AY:
Function of miR-146a in controlling Treg cell-mediated regulation
of Th1 responses. Cell. 142:914–929. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Luo X, Han M, Liu J, Wang Y, Luo X, Zheng
J, Wang S, Liu Z, Liu D, Yang PC and Li H: Epithelial cell-derived
micro RNA-146a generates interleukin-10-producing monocytes to
inhibit nasal allergy. Sci Rep. 5:159372015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Okoye IS, Czieso S, Ktistaki E, Roderick
K, Coomes SM, Pelly VS, Kannan Y, Perez-Lloret J, Zhao JL,
Baltimore D, et al: Transcriptomics identified a critical role for
Th2 cell-intrinsic miR-155 in mediating allergy and antihelminth
immunity. Proc Natl Acad Sci USA. 111:E3081–E3090. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hou T, Liao J, Zhang C, Sun C, Li X and
Wang G: Elevated expression of miR-146, miR-139 and miR-340
involved in regulating Th1/Th2 balance with acute exposure of fine
particulate matter in mice. Int Immunopharmacol. 54:68–77. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Li B, Wang X, Choi IY, Wang YC, Liu S,
Pham AT, Moon H, Smith DJ, Rao DS, Boldin MP and Yang L: miR-146a
modulates autoreactive Th17 cell differentiation and regulates
organ-specific autoimmunity. J Clin Invest. 127:3702–3716. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Pratama A, Srivastava M, Williams NJ, Papa
I, Lee SK, Dinh XT, Hutloff A, Jordan MA, Zhao JL, Casellas R, et
al: MicroRNA-146a regulates ICOS-ICOSL signalling to limit
accumulation of T follicular helper cells and germinal centres. Nat
Commun. 6:64362015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Boldin MP, Taganov KD, Rao DS, Yang L,
Zhao JL, Kalwani M, Garcia-Flores Y, Luong M, Devrekanli A, Xu J,
et al: miR-146a is a significant brake on autoimmunity,
myeloproliferation and cancer in mice. J Exp Med. 208:1189–1201.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Cho S, Lee HM, Yu IS, Choi YS, Huang HY,
Hashemifar SS, Lin LL, Chen MC, Afanasiev ND, Khan AA, et al:
Differential cell-intrinsic regulations of germinal center B and T
cells by miR-146a and miR-146b. Nat Commun. 9:27572018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Guo Q, Zhang J, Li J, Zou L, Zhang J, Xie
Z, Fu X, Jiang S, Chen G, Jia Q, et al: Forced miR-146a expression
causes autoimmune lymphoproliferative syndrome in mice via
downregulation of Fas in germinal center B cells. Blood.
121:4875–4883. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Li Z, Zhang S, Wan Y, Cai M, Wang W, Zhu
Y, Li Z, Hu Y, Wang H, Chen H, et al: MicroRNA-146a overexpression
impairs the positive selection during T cell development. Front
Immunol. 8:20062018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
King JK, Ung NM, Paing MH, Contreras JR,
Alberti MO, Fernando TR, Zhang K, Pellegrini M and Rao DS:
Regulation of marginal Zone B-cell differentiation by
MicroRNA-146a. Front Immunol. 7:6702017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Mao B and Wang G: MicroRNAs involved with
hepatocellular carcinoma (Review). Oncol Rep. 34:2811–2820. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Li JF, Dai XP, Zhang W, Sun SH, Zeng Y,
Zhao GY, Kou ZH, Guo Y, Yu H, Du LY, et al: Upregulation of
microRNA-146a by hepatitis B virus X protein contributes to
hepatitis development by downregulating complement factor H. MBio.
6:e02459–14. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Bandiera S, Pernot S, El Saghire H, Durand
SC, Thumann C, Crouchet E, Ye T, Fofana I, Oudot MA, Barths J, et
al: Hepatitis C virus-induced upregulation of MicroRNA miR-146a-5p
in hepatocytes promotes viral infection and deregulates metabolic
pathways associated with liver disease pathogenesis. J Virol.
90:6387–6400. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Visalli M, Bartolotta M, Polito F, Oteri
R, Barbera A, Arrigo R, Di Giorgio RM, Navarra G and Aguennouz M:
miRNA expression profiling regulates necroptotic cell death in
hepatocellular carcinoma. Int J Oncol. 53:771–780. 2018.PubMed/NCBI
|
|
54
|
Xu D, Han Q, Hou Z, Zhang C and Zhang J:
miR-146a negatively regulates NK cell functions via STAT1
signaling. Cell Mol Immunol. 14:712–720. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Wang S, Zhang X, Ju Y, Zhao B, Yan X, Hu
J, Shi L, Yang L, Ma Z, Chen L, et al: MicroRNA-146a feedback
suppresses T cell immune function by targeting Stat1 in patients
with chronic hepatitis B. J Immunol. 191:293–301. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Fattovich G, Stroffolini T, Zagni I and
Donato F: Hepatocellular carcinoma in cirrhosis: Incidence and risk
factors. Gastroenterology. 127 (5 Suppl 1):S35–S50. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Qian H, Deng X, Huang ZW, Wei J, Ding CH,
Feng RX, Zeng X, Chen YX, Ding J, Qiu L, et al: An HNF1α-regulated
feedback circuit modulates hepatic fibrogenesis via the crosstalk
between hepatocytes and hepatic stellate cells. Cell Res.
25:930–945. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Weiskirchen R and Tacke F: Cellular and
molecular functions of hepatic stellate cells in inflammatory
responses and liver immunology. Hepatobiliary Surg Nutr. 3:344–363.
2014.PubMed/NCBI
|
|
59
|
Chen Y, Wu Z, Yuan B, Dong Y, Zhang L and
Zeng Z: MicroRNA-146a-5p attenuates irradiation-induced and
LPS-induced hepatic stellate cell activation and hepatocyte
apoptosis through inhibition of TLR4 pathway. Cell Death Dis.
9:222018. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Lin A, Wang G, Zhao H, Zhang Y, Han Q,
Zhang C, Tian Z and Zhang J: TLR4 signaling promotes a
COX-2/PGE2/STAT3 positive feedback loop in
hepatocellular carcinoma (HCC) cells. Oncoimmunology.
5:e10743762015. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Sun X, Zhang J, Hou Z, Han Q, Zhang C and
Tian Z: miR-146a is directly regulated by STAT3 in human
hepatocellular carcinoma cells and involved in anti-tumor immune
suppression. Cell Cycle. 14:243–252. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Zhu K, Pan Q, Zhang X, Kong LQ, Fan J, Dai
Z, Wang L, Yang XR, Hu J, Wan JL, et al: miR-146a enhances
angiogenic activity of endothelial cells in hepatocellular
carcinoma by promoting PDGFRA expression. Carcinogenesis.
34:2071–2079. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Zhang Z, Zhang Y, Sun XX, Ma X and Chen
ZN: microRNA-146a inhibits cancer metastasis by downregulating VEGF
through dual pathways in hepatocellular carcinoma. Mol Cancer.
14:52015. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Zhou L, Zhao X, Han Y, Lu Y, Shang Y, Liu
C, Li T, Jin Z, Fan D and Wu K: Regulation of UHRF1 by miR-146a/b
modulates gastric cancer invasion and metastasis. FASEB J.
27:4929–4239. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Xu T, Zhu Y, Wei QK, Yuan Y, Zhou F, Ge
YY, Yang JR, Su H and Zhuang SM: A functional polymorphism in the
miR-146a gene is associated with the risk for hepatocellular
carcinoma. Carcinogenesis. 29:2126–2131. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Peng Q, Li S, Lao X, Chen Z, Li R, Deng Y
and Qin X: The association of common functional polymorphisms in
mir-146a and mir-196a2 and hepatocellular carcinoma risk: Evidence
from a meta-analysis. Medicine (Baltimore). 93:e2522014. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Tian T, Wang M, Zhu W, Dai ZM, Lin S, Yang
PT, Liu XH, Liu K, Zhu YY, Zheng Y, et al: miR-146a and miR-196a-2
polymorphisms are associated with hepatitis virus-related
hepatocellular cancer risk: A meta-analysis. Aging (Albany NY).
9:381–392. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Dong S, Miao AY, Lei W and Chen QW:
miR-146a rs2910164 and hepatocellular carcinoma: A meta-analysis.
Minerva Med. 108:287–292. 2017.PubMed/NCBI
|
|
69
|
Jazdzewski K, Murray EL, Franssila K,
Jarzab B, Schoenberg DR and de la Chapelle A: Common SNP in
pre-miR-146a decreases mature miR expression and predisposes to
papillary thyroid carcinoma. Proc Natl Acad Sci USA. 105:7269–7274.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Moreno R and Berenguer M: Post-liver
transplantation medical complications. Ann Hepatol. 5:77–85. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Hu J, Wang Z, Tan CJ, Liao BY, Zhang X, Xu
M, Dai Z, Qiu SJ, Huang XW, Sun J, et al: Plasma microRNA, a
potential biomarker for acute rejection after liver
transplantation. Transplantation. 95:991–999. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Zhuo L, Liu J, Wang B, Gao M and Huang A:
Differential miRNA expression profiles in hepatocellular carcinoma
cells and drug-resistant sublines. Oncol Rep. 29:555–562. 2013.
View Article : Google Scholar : PubMed/NCBI
|
















