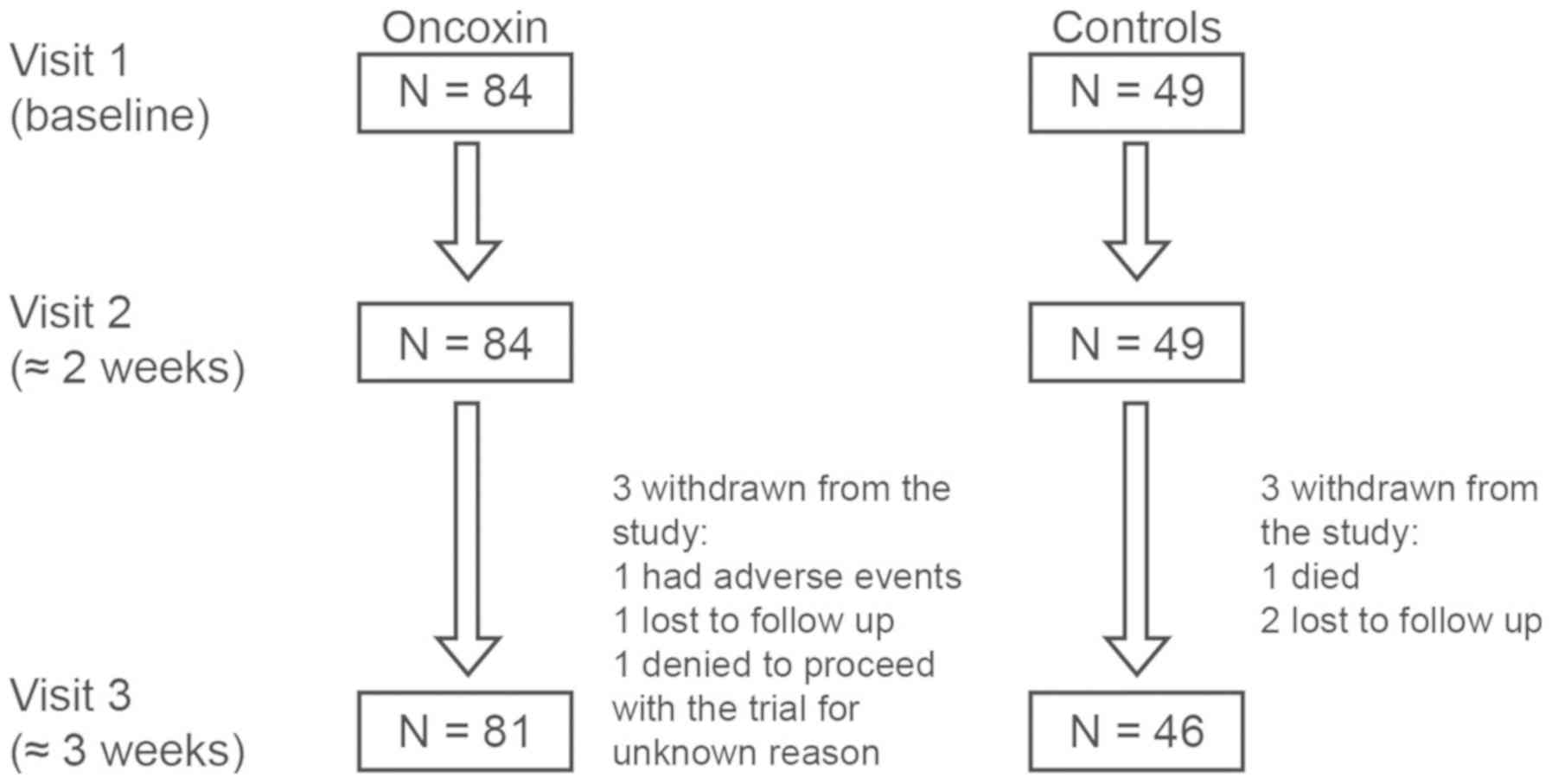
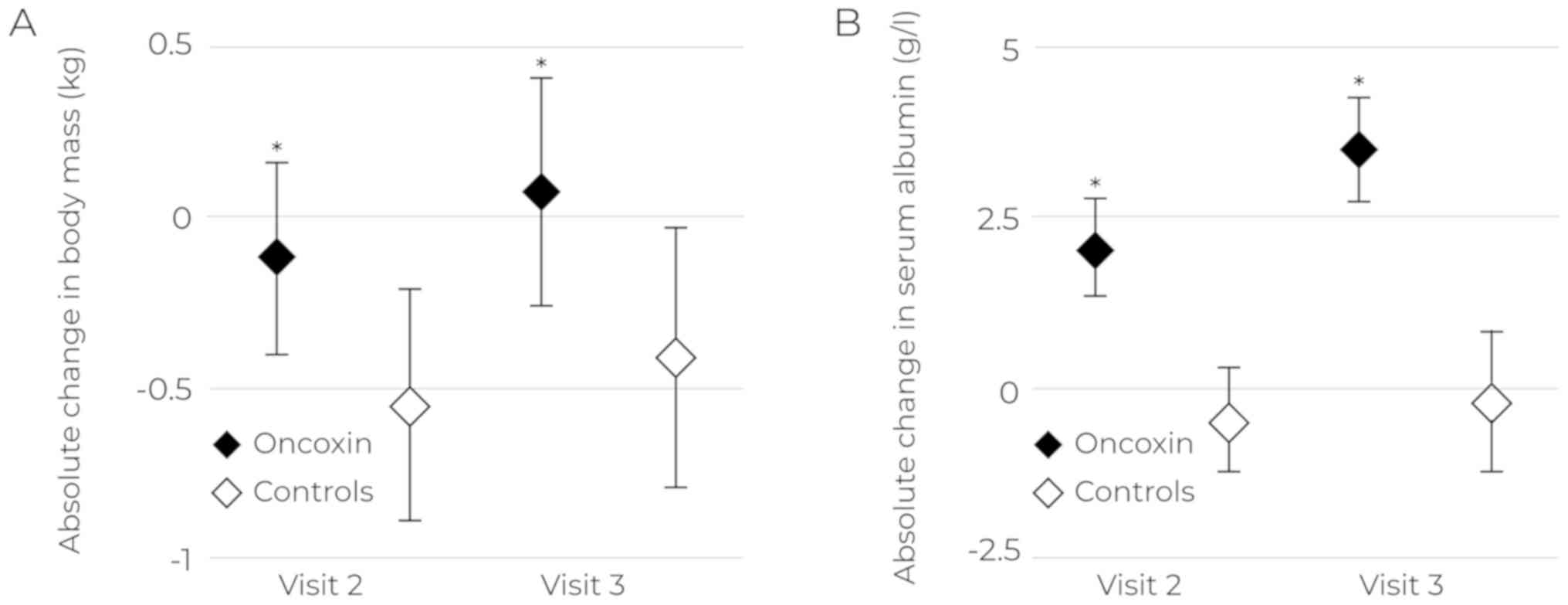
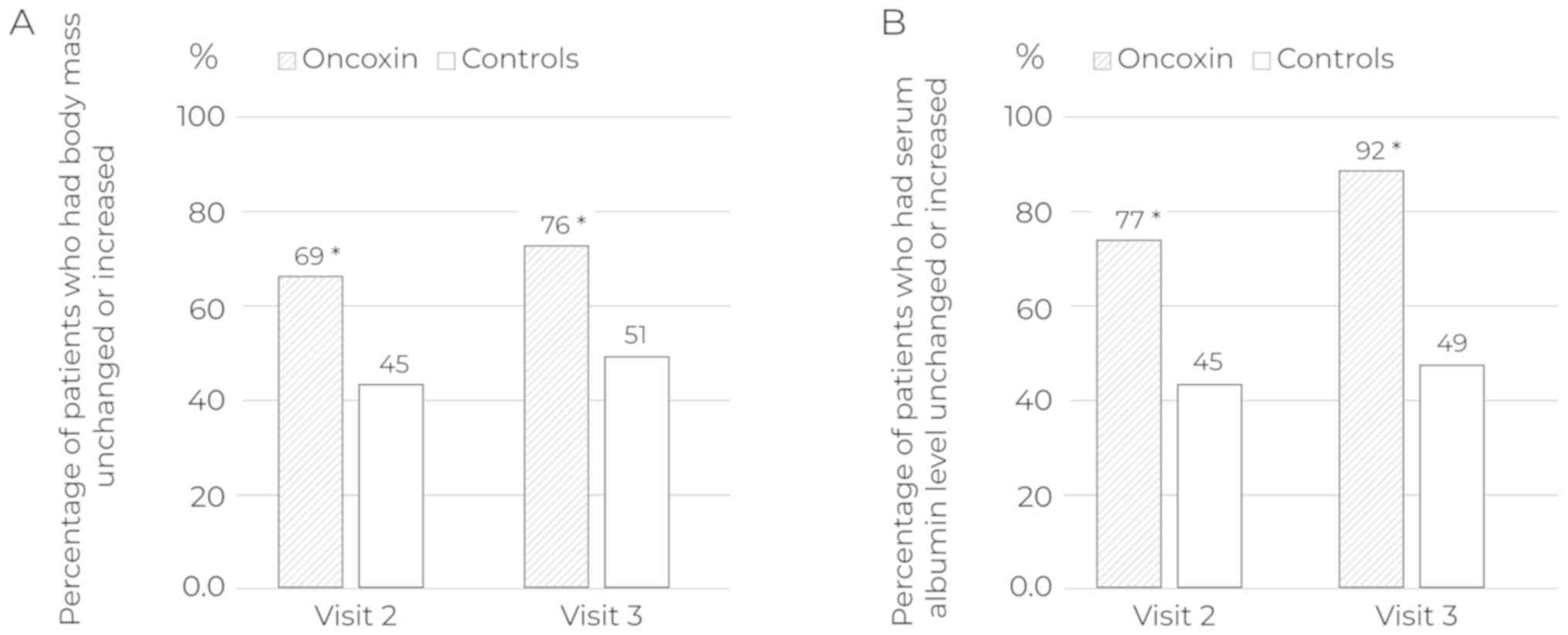
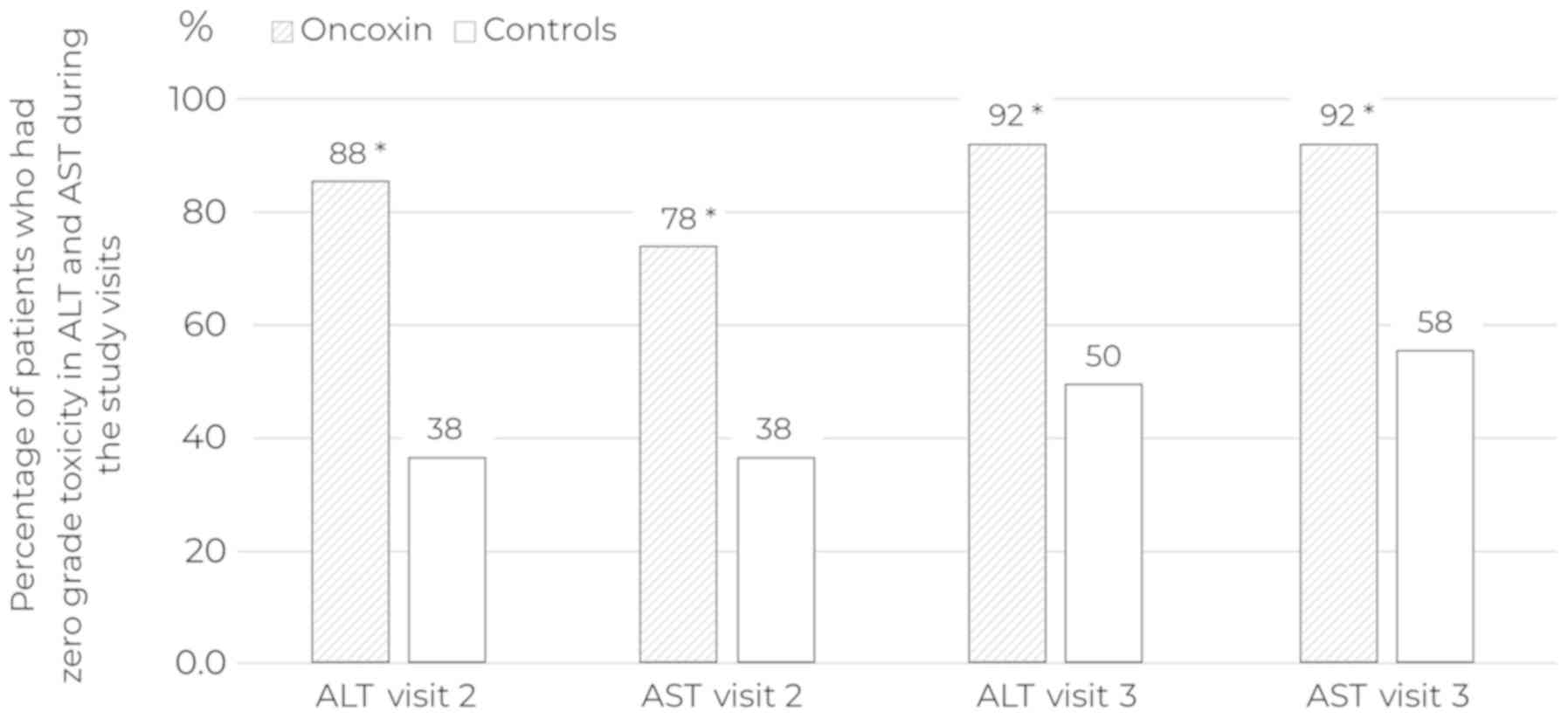
|
1
|
Prasanna T, Beith J, Kao S, Boyer M and
McNeil CM: Dose modifications in adjuvant chemotherapy for solid
organ malignancies: A systematic review of clinical trials. Asia
Pac J Clin Oncol. 14:125–133. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lakhanpal SH: Docetaxel and
cyclophosphamide as adjuvant chemotherapy for early breast cancer:
Primary prophylaxis with g-CSF is required. Breast Cancer Manage.
2:367–374. 2013. View Article : Google Scholar
|
|
3
|
Jones SE, Savin MA, Holmes FA,
O'Shaughnessy JA, Blum JL, Vukelja S, McIntyre KJ, Pippen JE,
Bordelon JH, Kirby R, et al: Phase III trial comparing doxorubicin
plus cyclophosphamide with docetaxel plus cyclophosphamide as
adjuvant therapy for operable breast cancer. J Clin Oncol.
24:5381–5387. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Haller DG, Tabernero J, Maroun J, de Braud
F, Price T, Van Cutsem E, Hill M, Gilberg F, Rittweger K and
Schmoll HJ: Capecitabine plus oxaliplatin compared with
fluorouracil and folinic acid as adjuvant therapy for stage III
colon cancer. J Clin Oncol. 29:1465–1471. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
André T, Boni C, Mounedji-Boudiaf L,
Navarro M, Tabernero J, Hickish T, Topham C, Zaninelli M, Clingan
P, Bridgewater J, et al: Oxaliplatin, fluorouracil, and leucovorin
as adjuvant treatment for colon cancer. N Engl J Med.
350:2343–2351. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Eichler M, Singer S, Janni W, Harbeck N,
Rack B, Augustin D, Wischnik A, Kiechle M, Ettl J, Scholz C, et al:
Pretreatment quality of life, performance status and their relation
to treatment discontinuation and treatment changes in high-risk
breast cancer patients receiving chemotherapy: Results from the
prospective randomized ADEBAR trial. Breast Cancer. 24:319–325.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yan Y, Mo Y and Zhang D: Magnesium
isoglycyrrhizinate prevention of chemotherapy-induced liver damage
during initial treatment of patients with gastrointestinal tumors.
Zhonghua Gan Zang Bing Za Zhi. 23:204–208. 2015.(In Chinese).
PubMed/NCBI
|
|
8
|
Zhao H, Zhu W, Xie P, Li H, Zhang X, Sun
X, Yu J and Xing L: A phase I study of concurrent chemotherapy and
thoracic radiotherapy with oral epigallocatechin-3-gallate
protection in patients with locally advanced stage III
non-small-cell lung cancer. Radiother Oncol. 110:132–136. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhao H, Xie P, Li X, Zhu W, Sun X, Sun X,
Chen X, Xing L and Yu J: A prospective phase II trial of EGCG in
treatment of acute radiation-induced esophagitis for stage III lung
cancer. Radiother Oncol. 114:351–356. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Al-Mahtab M, Akbar SM, Khan MS and Rahman
S: Increased survival of patients with end-stage hepatocellular
carcinoma due to intake of ONCOXIN(®), a dietary
supplement. Indian J Cancer. 52:443–446. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shumsky A, Bilan E, Sanz E and Petrovskiy
F: Oncoxin nutritional supplement in the management of
chemotherapy- and/or radiotherapy-associated oral mucositis. Mol
Clin Oncol. 10:463–468. 2019.PubMed/NCBI
|
|
12
|
Hui D, Shamieh O, Paiva CE, Khamash O,
Perez-Cruz PE, Kwon JH, Muckaden MA, Park M, Arthur J and Bruera E:
Minimal clinically important difference in the physical, emotional,
and total symptom distress scores of the edmonton symptom
assessment system. J Pain Symptom Manage. 51:262–269. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yagi T, Asakawa A, Ueda H, Ikeda S,
Miyawaki S and Inui A: The role of zinc in the treatment of taste
disorders. Recent Pat Food Nutr Agric. 5:44–51. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Prasad AS: Impact of the discovery of
human zinc deficiency on health. J Trace Elem Med Biol. 28:357–363.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sweeney JD, Ziegler P, Pruet C and
Spaulding MB: Hyperzincuria and hypozincemia in patients treated
with cisplatin. Cancer. 63:2093–2095. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ciubotariu D, Ghiciuc CM and Lupușoru CE:
Zinc involvement in opioid addiction and analgesia-should zinc
supplementation be recommended for opioid treated persons? Subst
Abuse Treat Prev Policy. 4:10–29. 2015.
|
|
17
|
Weinstein SJ, Stolzenberg-Solomon R,
Pietinen P, Taylor PR, Virtamo J and Albanes D: Dietary factors of
one-carbon metabolism and prostate cancer risk. Am J Clin Nutr.
84:929–935. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Buijs N, Luttikhold J, Houdijk AP and van
Leeuwen PA: The role of a disturbed arginine/NO metabolism in the
onset of cancer cachexia: A working hypothesis. Curr Med Chem.
19:5278–5286. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ham DJ, Murphy KT, Chee A, Lynch GS and
Koopman R: Glycine administration attenuates skeletal muscle
wasting in a mouse model of cancer cachexia. Clin Nutr. 33:448–458.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Grotz MR, Pape HC, van Griensven M, Stalp
M, Rohde F, Bock D and Krettek C: Glycine reduces the inflammatory
response and organ damage in a two-hit sepsis model in rats. Shock.
16:116–121. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kantor ED, Lampe JW, Navarro SL, Song X,
Milne GL and White E: Associations between glucosamine and
chondroitin supplement use and biomarkers of systemic inflammation.
J Altern Complement Med. 20:479–485. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dominiak K, McKinney J, Heilbrun LK and
Sarkar FH: Critical need for clinical trials: An example of a pilot
human intervention trial of a mixture of natural agents protecting
lymphocytes against TNF-alpha induced activation of NF-kappa B.
Pharm Res. 27:1061–1065. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Khan R, Khan AQ, Lateef A, Rehman MU,
Tahir M, Ali F, Hamiza OO and Sultana S: Glycyrrhizic acid
suppresses the development of precancerous lesions via regulating
the hyperproliferation, inflammation, angiogenesis and apoptosis in
the colon of Wistar rats. PLoS One. 8:e560202013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Prasad AS: Zinc: Mechanisms of host
defense. J Nutr. 137:1345–1349. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fukagawa NK and Galbraith RA: Advancing
age and other factors influencing the balance between amino acid
requirements and toxicity. J Nutr 134 (6 Suppl). 1569S–1574S.
2004.
|
|
26
|
Miller GW, Lock EA and Schnellmann RG:
Strychnine and glycine protect renal proximal tubules from various
nephrotoxicants and act in the late phase of necrotic cell injury.
Toxicol Appl Pharmacol. 125:192–197. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhong Z, Li X, Yamashina S, von
Frankenberg M, Enomoto N, Ikejima K, Kolinsky M, Raleigh JA and
Thurman RG: Cyclosporin a causes a hypermetabolic state and hypoxia
in the liver: Prevention by dietary glycine. J Pharmacol Exp Ther.
299:858–865. 2001.PubMed/NCBI
|
|
28
|
Mauriz JL, Matilla B, Culebras JM,
Gonzalez P and Gonzalez-Gallego J: Dietary glycine inhibits
activation of nuclear factor kappa B and prevents liver injury in
hemorrhagic shock in the rat. Free Radic Biol Med. 31:1236–1244.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Robbins D and Zhao Y: Manganese superoxide
dismutase in cancer prevention. Antioxid Redox Signal.
20:1628–1645. 2014. View Article : Google Scholar : PubMed/NCBI
|