Introduction
The treatment of cancer often requires chemotherapy
(CT). Adjuvant chemotherapy (ACT) is often prescribed after surgery
and required to eradicate residual tumour cells. The appropriate
doses and regimens of CT depend on the type of cancer, stage,
performance status of patient, and several other factors. The dose
intensity is known to be critically important to increase the
disease-free and overall survival in patients with potentially
curable tumours, such as diffuse B-cell lymphoma or germ cell
tumours. However, for ACT used in early breast cancer, colorectal
cancer, non-small cell lung cancer (NSCLC) and pancreatic tumours,
the decision to commence cytotoxic therapy is not an easy one
since, for some patients, ACT may be unnecessary and accompanied by
significant, even fatal adverse effects (1).
Despite the use of guideline recommended doses, in
real clinical practice, the rate of patients with serious
manifestations of treatment-associated toxicity can be
significantly higher compared to the data published in randomised
trials. For example, in a retrospective study by Lakhanpal
(2) (real practice) the febrile
neutropenia rate in patients with breast cancer, who had received
docetaxel/cyclophosphamide ACT, accounted for 25% of cases.
Likewise, the Jones et al (3)
clinical trial showed that only 2.4% of patients had this severe
adverse event. Poor tolerability of anticancer drugs often requires
dose decrease or treatment discontinuation. A study of oxaliplatin
in patients with colorectal cancer showed that the rate of patients
who discontinued participation early in the study rose up to 31%,
and the rate of those who required dose reduction accounted for 62%
of cases (4,5). Reduction of doses and even treatment
cessation may be caused by several undesirable effects:
Haematological toxicity, hepatic toxicity, renal toxicity, severe
mucositis, poor nutritional status, progressive weakness. Therapy
toxicity and side effects are associated with poor quality of life
(QoL) that in turn may negatively affect patients' mood, appetite,
compliance and decision-making regarding CT continuation (6).
To maintain QoL and effective CT dosages, the use of
all available supportive therapy options needs to be explored. In
this regard, the development of new approaches to maintain QoL and
comply with recommended CT regimens is an important task to
increase the survival of patients with ACT.
Recent studies showed that a number of amino acids,
micronutrients, vitamins and biologically active substances can
reduce the severity of CT's side effects, enhance appetite and may
reduce infectious complications. For example, the use of
glycyrrhizin as a supplement to FOLFOX and XELOX CT regimens was
accompanied by significant liver function improvement and fewer
cases of liver dysfunction (cases of hepatic dysfunction reduced by
more than twice compared to control group) (7). Due to its antioxidant and
anti-inflammatory properties, epigallocatechin gallate (EGCG), a
natural polyphenol, is highly effective in relieving acute
esophagitis induced by radiation therapy or CT (8,9). The
present prospective study was performed to evaluate the impact of
the multicomponent nutritional supplement Oncoxin on QoL and
tolerability of anticancer drugs in patients receiving ACT. Oncoxin
is a solution containing amino acids, vitamins, micronutrients and
biologically active substances. Previous studies showed that ONCX
was able to increase life expectancy, improve QoL and appetite in
patients with end-stage hepatocellular carcinoma (10) and effectively reduce the severity of
oral mucositis symptoms in patients receiving CT, radiation therapy
or their combination (11).
Materials and methods
Study population
The eligibility criteria for inclusion in the study
were as follows: Male and female patients who had signed an
informed consent, aged 50–70 years, with gastric cancer IIВ-IIIС,
NSCLC IIВ-IIIА; R0 surgery, ACT required, 2nd and further course of
ACT, XELOX regimen of ACT for gastric cancer and
paclitaxel+carboplatin regimen for NSCLC, body mass index (BMI)
>15, serum albumin ≥25 g/l, Eastern Cooperative Oncology Group
performance status ≤2. The exclusion criteria were as follows:
Severe concomitant diseases or conditions that may complicate or
make the patient's participation in the study impossible, or make
it difficult to explain clinical findings (including mental
disorders, severe infectious and parasitic diseases, and an
intolerability to any of the ONCX components), the patient's family
or official relations with a member of staff of the clinical site,
the patient's failure to assess his/her physical and/or emotional
condition, the patient's failure to comply with the study
requirements, the patient's refusal to participate in the study, as
well as pregnancy or breastfeeding.
The present study was approved by the Ethics
Committee of the Loginov Moscow Clinical Scientific Centre,
protocol 3/2017, April 17, 2017. All patients were enrolled between
November 2017 and March 2019. In accordance with the Declaration of
Helsinki, all patients provided written informed consent to
participate in the study and to publish the results. The study was
retrospectively registered under the study registration number
NCT03550482 at ClinicalTrials.gov resource, June 8, 2018.
Study design and treatment
The present study was multicentre, open-label,
non-randomised clinical trial in two parallel groups with a 20-day
treatment period. No follow-up period was intended. The following
visits were scheduled: Visit 1-the first day of 2nd or further
course of ACT; Visit 2–7±1 days before the next course of ACT;
Visit 3-the first day of the next course of ACT (before
administration of drugs) (21±3 days after Visit 1). The following
primary endpoint was used: The percentage of patients who had an
improvement in QoL corresponding to the minimal clinically
important difference (MCID) that any patient felt at Visits 2 in
total symptom distress score of the Edmonton Symptom Assessment
System questionnaire (SDS ESAS) (12). This MCID corresponds to 6 points of
improvement within-patient change for improvement (12). ESAS is a validated set of questions
that assesses the average intensity of 10 symptoms (pain, fatigue,
nausea, depression, anxiety, drowsiness, shortness of breath,
appetite, feelings of well-being and sleep) over the past 24 h,
each with an 11-point numerical rating scale that ranges from 0 (no
symptom) to 10 (worst intensity). Secondary endpoints included
total SDS ESAS, emotional SDS ESAS, physical SDS ESAS scores,
separate symptoms of ESAS, body mass, serum albumin level, and the
Common Toxicity Criteria (ver. 2) of the National Cancer Institute
(https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcv20_4-30-992.pdf)
using the blood and hepatic scales.
The study was conducted in 10 clinical sites in
Russia and Kazakhstan. Patients were grouped in ONCX and control
groups as 2:1. A total of 133 patients were enrolled in the study;
84 in the ONCX group and 49 in the control group.
In addition to ACT treatment (XELOX or
paclitaxel+carboplatin), patients in the ONCX group received 25 ml
of ONCX (Catalysis S.L., 28016 Madrid, Spain) twice daily for 20
days. ONCX's composition is shown in Table I. In case of nausea/vomiting after
ONCX use, patients were advised to dilute it in water, juice or
milk. Patients with BMI <20 and serum albumin levels <30 g/l
received nutritional support. Nutritional support was provided
through nutritionally complete, high energy, high protein, ready to
drink supplement enriched with n-3 fatty acids and fibre.
 | Table I.Composition of ONCX per 100 ml. |
Table I.
Composition of ONCX per 100 ml.
| Component | Mass |
|---|
| Glycine | 2,000 mg |
| Glucosamine | 2,000 mg |
| Malic acid | 1,200 mg |
| Arginine | 640 mg |
| Cysteine | 204 mg |
| Mono-ammonium
glycyrrhizinate | 200 mg |
| Ascorbic acid | 200 mg |
| Sodium
methylparaben | 120 mg |
| Zinc sulphate | 100 mg |
| Green tea
extract | 80 mg |
| Calcium
pantothenate | 25 mg |
| Pyridoxine | 12 mg |
| Manganese
sulphate | 4 mg |
| Cinnamon
extract | 3 mg |
| Folic acid | 400 µg |
| Cyanocobalamin | 2 µg |
Because of Glycyrrhizin may increase blood pressure
(BP), the information regarding possible BP increase was added to
Study protocol and Informed consent form. Thus, both investigators
and patients were informed of possible BP increase. Patients' BP
was monitored as a part of routine practice and corrected if
needed.
To decrease therapy toxicity, the following
drugs/methods were available and used if needed: filgrastim,
epoetin alfa, blood transfusions, corticosteroids, 5H3 antagonists,
ademetionine, polyunsaturated phosphatidyl choline and nutritional
supplements containing vitamins and minerals.
Statistical analysis
Statistical analysis was performed using StatSoft
Statistica 10.0 software (http://statsoft.ru/). For odds ratios' (OR) 95%
confidence interval (CI) VassarStats online service (vassarstats.net) was used.
Sample size was calculated based on the following
conditions:
1. It was expected that the proportion of patients
with MCID improvement in total SDS ESAS is 50% in ONCX group and
20% in control group (with 30-point baseline total SDS ESAS).
2. Patients' allocation as 2:1 in comparison groups
for the ONCX and control groups, respectively.
3. Alpha 0.05 and power not less than 0.9.
Based on the above conditions, data from 120
patients had to be analysed. With a dropout of 25%, 150 patients
had to be included in the study (100 ONCX/50 controls).
Baseline characteristics (quantitative and
semiquantitative) are presented as mean and (standard deviation),
unless otherwise stated; when comparisons between groups or within
a group were made, the data are presented as mean and [95% CI].
Categorical variables are expressed as absolute numbers and
percentages. The differences of quantitative and semiquantitative
variables between the ONCX and control groups were compared using
the Mann-Whitney U test and the differences within each group were
compared using the sign test. For tables 2×2, OR and OR's [95% CI]
were calculated. The differences of categorical variables were
compared using the Yates corrected Chi-square or two-sided exact
Fisher test. P<0.05 was considered to indicate a statistically
significant difference.
Results
One hundred thirty-three patients were enrolled in
the study; of which 84 received ONCX and 49 were included in the
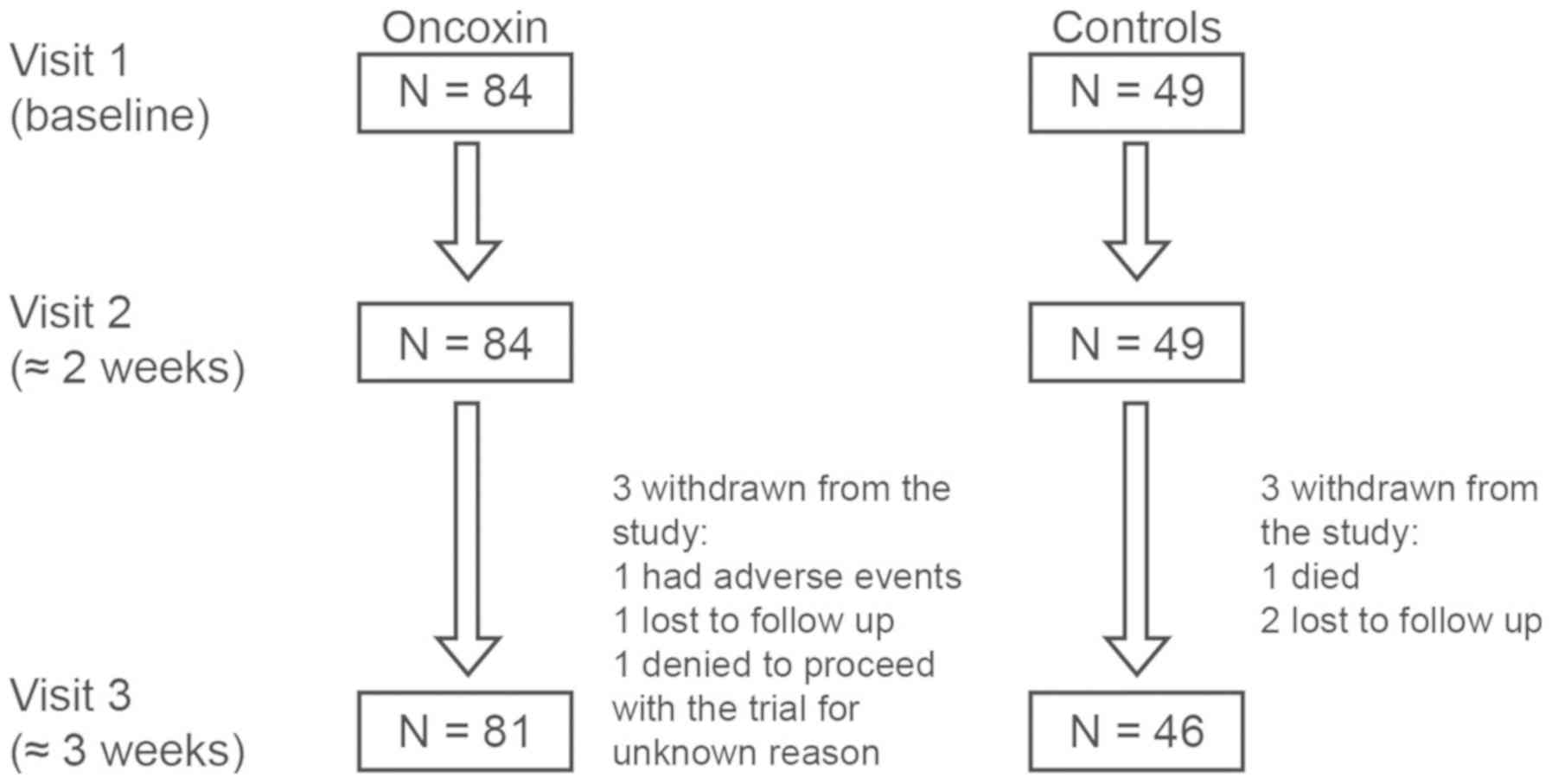
control group. Patient disposition is shown in Fig. 1. The initial clinical characteristics
of the subjects are provided in Table
II. No significant differences were found between the compared
groups at baseline.
 | Table II.Patient baseline characteristics for
each group. |
Table II.
Patient baseline characteristics for
each group.
| Characteristic | ONCX | Controls | P-value |
|---|
| Number of patients,
n | 84 | 49 |
|
| Non-small cell lung
cancer, abs (%) | 53 (63.1) | 31 (63.3) | 0.9 |
| Males, abs (%) | 50 (59.5) | 33 (67.3) | 0.5 |
| Age, years | 59.0 (6.1) | 57.2 (5.2) | 0.1 |
| Height, cm | 167.5 (6.9) | 170.1 (6.5) | 0.1 |
| Body mass, kg | 63.7 (10.7) | 67.5 (11.3) | 0.1 |
| BMI (min-max) | 22.7
(15.9–32.4) | 23.2
(17.9–31.7) | 0.2 |
| Serum albumin,
g/l | 34.5 (3.9) | 35.6 (5.0) | 0.2 |
| ESAS |
|
|
|
|
Emotional SDS ESAS | 3.1 (3.6) | 3.0 (3.5) | 0.9 |
|
Physical SDS ESAS | 16.6 (9.1) | 15.0 (7.4) | 0.2 |
| Total
SDS ESAS | 22.9 (12.5) | 21.2 (11.0) | 0.4 |
| Mean toxicity
grades |
|
|
|
|
Leukocytes | 0.03 (0.17) | 0.15 (0.37) | 0.3 |
|
Platelets | 0 | 0.05 (0.22) | 0.7 |
|
Haemoglobin | 0.78 (0.71) | 1.00 (0.76) | 0.2 |
|
Lymphocytes | 0.12 (0.44) | 0.31 (0.61) | 0.2 |
|
Alkaline phosphatase | 0.27 (0.54) | 0.18 (0.56) | 0.4 |
|
ALT | 0.09 (0.29) | 0.08 (0.27) | 0.9 |
|
AST | 0 | 0.05 (0.22) | 0.7 |
| Total
bilirubin | 0 | 0 |
|
Improvement in QoL corresponding to the MCID that
any patient felt at Visits 2 and 3 was assessed. At Visit 2, the
number of such patients in the ONCX group accounted for 52% vs. 35%
in the control group, at Visit 3–59% and 43%, respectively. At
Visit 2, the chance of ONCX-treated patients having MCID
improvement in total SDS ESAS was twice as high compared to the
control group: OR=2.07 [1.00–4.29], P=0.005. The difference was
insignificant by Visit 3: OR=1.89 [0.91–3.93].
Total SDS ESAS declined significantly in each group
by Visit 2. However, there were no significant differences noted
between the groups. By Visit 3, the QoL in patients receiving ONCX
was significantly better, including the total SDS ESAS and physical
SDS ESAS (see Table III).
 | Table III.Alterations in emotional, physical
and total SDS ESAS and separate ESAS symptoms during the present
study. |
Table III.
Alterations in emotional, physical
and total SDS ESAS and separate ESAS symptoms during the present
study.
| Time point | Variable | ONCX, mean (95%
CI) | Controls, mean (95%
CI) | P-value |
|---|
| Baseline | Emotional SDS
ESAS | 3.11
(2.32–3.90) | 2.98
(1.96–4.00) | 0.887 |
|
| Physical SDS
ESAS | 16.6
(14.7–18.6) | 15.0
(12.9–17.1) | 0.173 |
|
| Total SDS ESAS | 22.9
(20.2–25.6) | 21.2
(18.0–24.4) | 0.358 |
| Week 2 | Appetite | 1.75
(1.18–2.32) | 3.55
(2.56–4.54) | 0.002 |
|
| Well-being | 2.15
(1.62–2.69) | 3.02
(2.52–3.52) | <0.001 |
|
| Emotional SDS
ESAS | 2.49
(1.81–3.16) | 1.94
(0.90–2.98) | 0.142 |
|
| Physical SDS
ESAS | 11.3
(9.8–12.8) | 13.5
(11.4–15.5) | 0.092 |
|
| Total SDS ESAS | 16.0
(13.7–18.2) | 18.5
(15.5–21.4) | 0.112 |
| Week 3 | Appetite | 1.20
(0.79–1.61) | 2.76
(2.06–3.46) | <0.001 |
|
| Tiredness | 1.56
(1.19–1.92) | 2.85
(2.30–3.40) | <0.001 |
|
| Well-being | 1.46
(1.05–1.87) | 2.96
(2.45–3.46) | <0.001 |
|
| Emotional SDS
ESAS | 1.65
(1.15–2.16) | 1.85
(0.95–2.75) | 0.789 |
|
| Physical SDS
ESAS | 8.68
(7.49–9.87) | 11.9
(10.2–13.8) | 0.001 |
|
| Total SDS ESAS | 11.8
(10.0–13.6) | 16.8
(14.1–19.5) | <0.001 |
A few symptoms within the ESAS questionnaire
improved in ONXC patients by Visit 2. Those values included QoL
aspects such as appetite and well-being. Furthermore, at Visit 3,
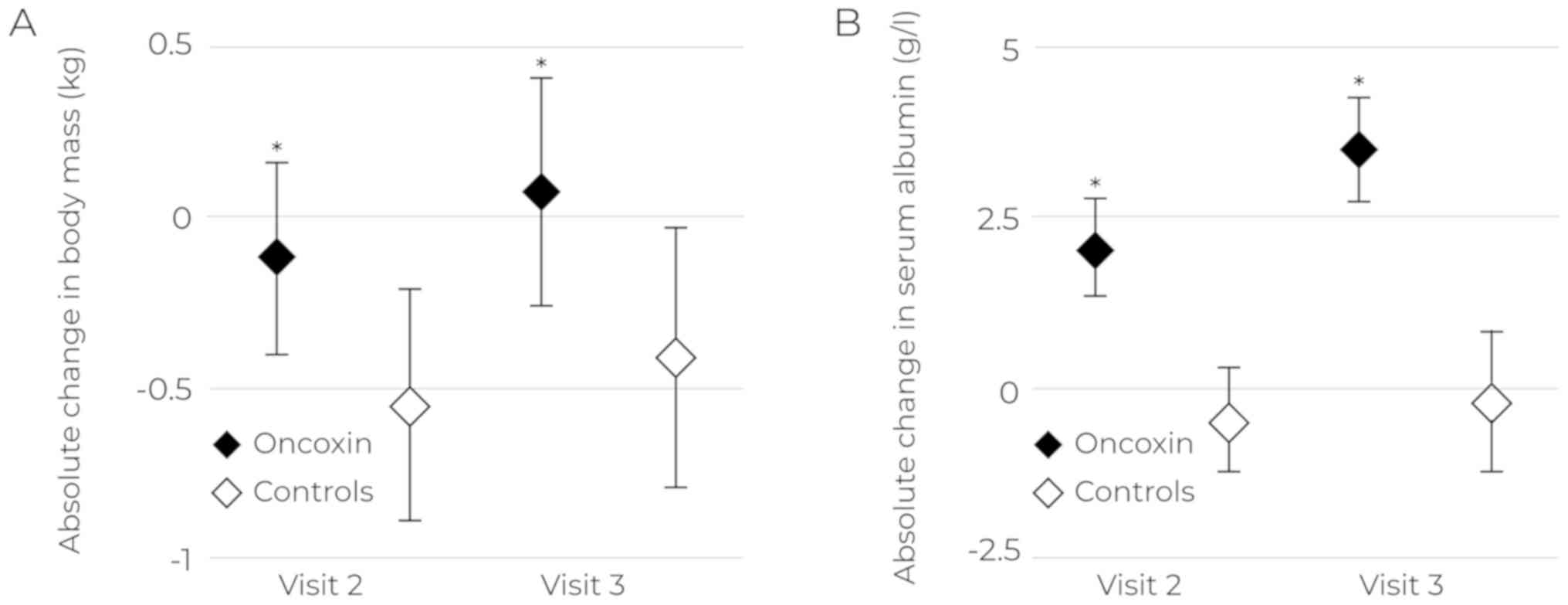
differences in the fatigue severity were detected (Table III). Changes in body weight and
serum albumin levels were insignificant by Visit 2, with no
differences found between groups or within groups. By Visit 3,
albumin levels were significantly higher in the ONCX group compared
to the control group [38.1 (37.1–39.1) g/l vs. 35.5 (33.9–37.0),
P=0.03, respectively]. Weight loss in the ONCX group was less
pronounced by Visit 2, and patients regained their body weight by
Visit 3 (Fig. 2A). Similar results
were obtained for serum albumin levels at Visits 2 and 3 (Fig. 2B).
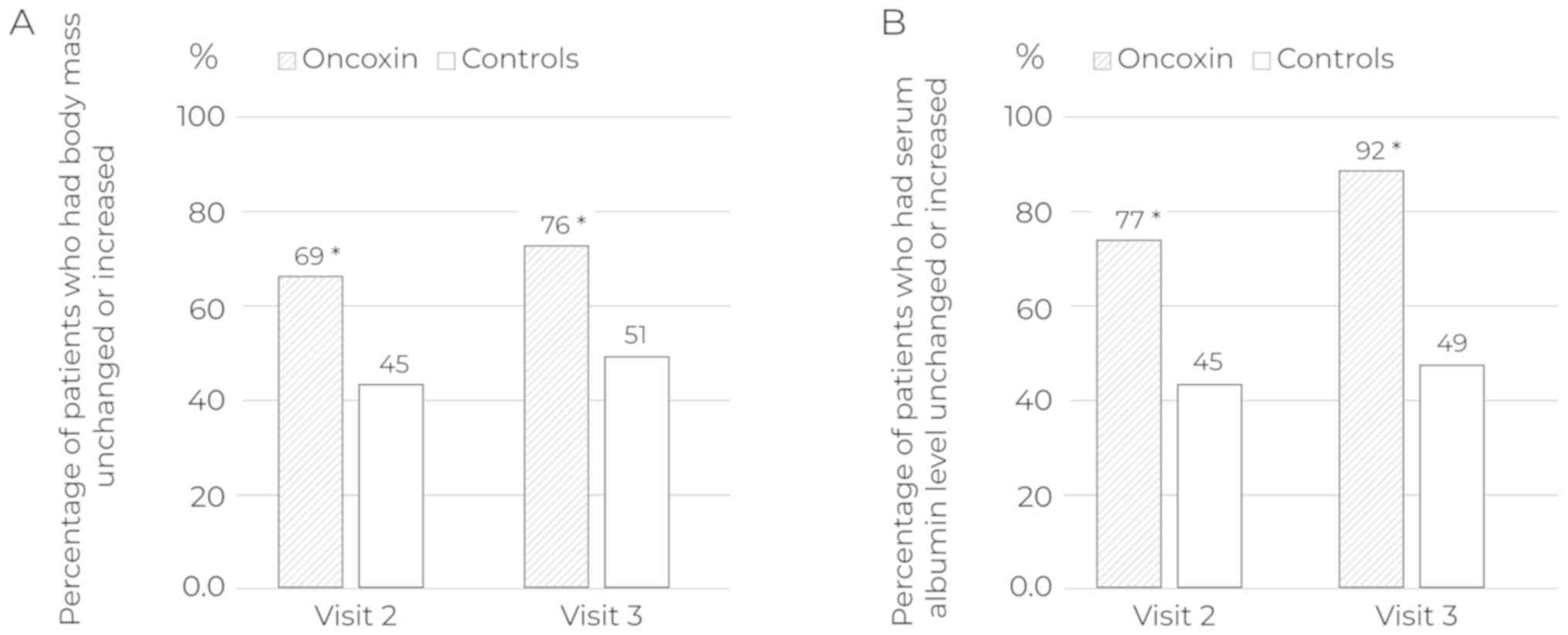
During the study, the rate of patients whose body
weight and serum albumin levels remained unchanged or increased was
significantly higher in the ONCX group (Fig. 3; Table
IV). Initially, 25% of patients among those received ONCX and
29% in the control group needed nutritional support. By the end of
the study, the proportions of such patients were 19 and 30%,
respectively. However, the difference was not significant
(P=0.19).
 | Table IV.Odds ratios of unchanged or increased
body mass and serum albumin level in the Oncoxin group compared
with in the control group. |
Table IV.
Odds ratios of unchanged or increased
body mass and serum albumin level in the Oncoxin group compared
with in the control group.
| Variable | Week of the
study | OR (95% CI) |
|---|
| Body mass | Week 2 | 2.74
(1.32–5.67) |
|
| Week 3 | 3.07
(1.45–6.52) |
| Serum albumin | Week 2 | 4.20
(1.96–8.98) |
|
| Week 3 | 11.46
(4.41–29.8) |
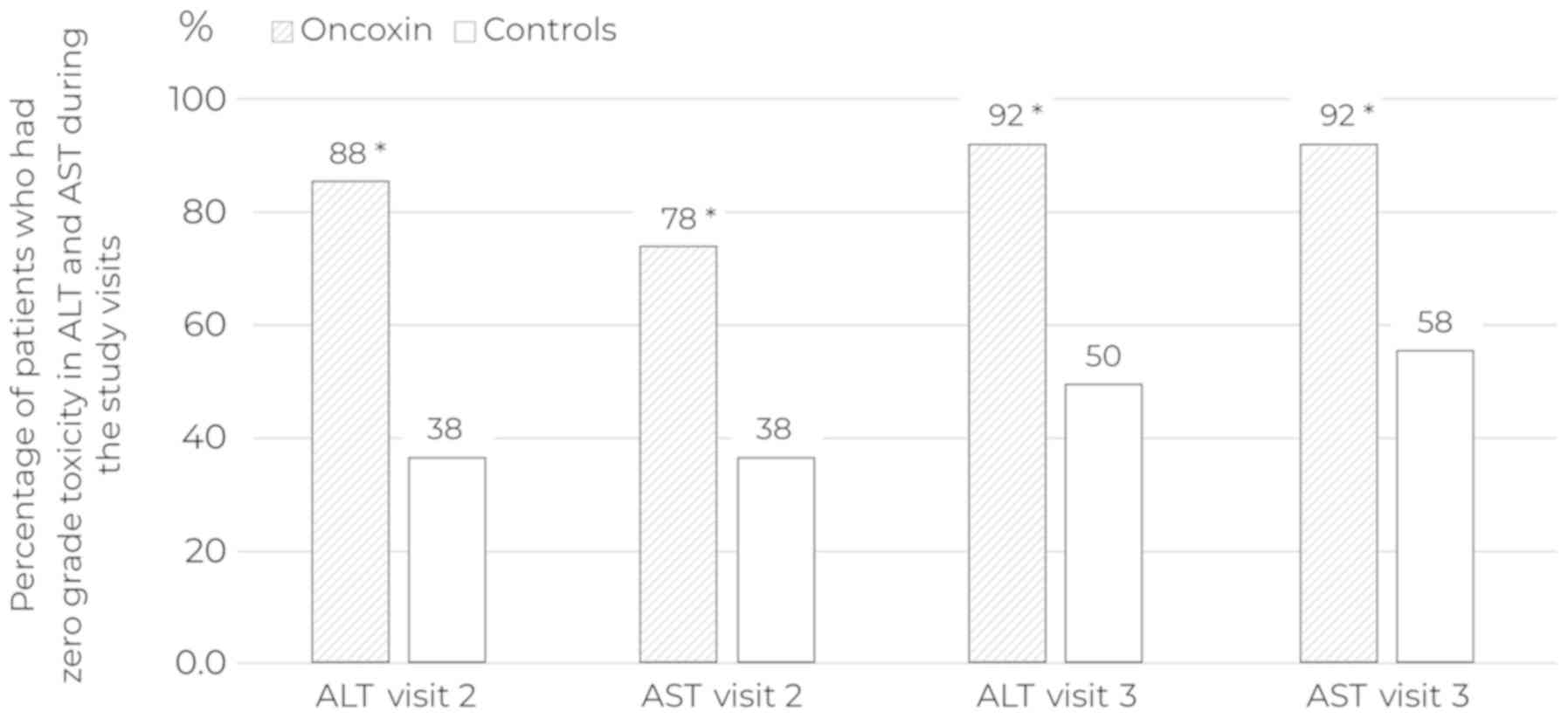
Patients distribution across toxicity grades
according to allocation group and study progress is presented in
Table V. The therapy toxicity
analysis showed that the use of ONCX reduced drops in haemoglobin
and alanine aminotransferase (ALT)/aspartate aminotransferase (AST)
elevation at Visit 2 and ALT/AST elevation at Visit 3 (Table VI). Special attention should be paid
to the differences in the rate of patients in the ONCX and control
groups, in which AST and ALT levels corresponded to a zero degree
of toxicity at Visits 2 and 3 (Fig.
4).
 | Table V.Patient distribution across toxicity
grades according to group and study progress. |
Table V.
Patient distribution across toxicity
grades according to group and study progress.
|
|
| Baseline | Week 2 | Week 3 |
|---|
|
|
|
|
|
|
|---|
| Variable | Toxicity
gradesa | ONCX, abs (%) | Control, abs
(%) | ONCX, abs (%) | Control, abs
(%) | ONCX, abs (%) | Control, abs
(%) |
|---|
| Number of patients
with assessed toxicity |
| 67 | 39 | 67 | 39 | 64 | 36 |
| Hemoglobin | 0 | 26 (39) | 11 (28) | 44 (66) | 9 (23) | 51 (80) | 21 (58) |
|
| 1 | 30 (45) | 17 (44) | 22 (33) | 26 (67) | 12 (19) | 15 (42) |
|
| 2 | 11 (16) | 11 (28) | 1 (1) | 4 (10) | 1 (1) | 0 |
| ALT | 0 | 61 (91) | 36 (92) | 59 (88) | 15 (38) | 59 (92) | 18 (50) |
|
| 1 | 6 (9) | 3 (8) | 8 (12) | 14 (36) | 5 (8) | 18 (50) |
|
| 2 | 0 | 0 | 0 | 10 (26) | 0 | 0 |
| AST | 0 | 67 (100) | 37 (95) | 52 (78) | 15 (38) | 59 (92) | 21 (58) |
|
| 1 | 0 | 2 (5) | 15 (22) | 22 (56) | 5 (8) | 15 (42) |
|
| 2 | 0 | 0 | 0 | 1 (3) | 0 | 0 |
|
| 3 | 0 | 0 | 0 | 1 (3) | 0 | 0 |
 | Table VI.Mean toxicity grades during the study
visits. |
Table VI.
Mean toxicity grades during the study
visits.
| Time point | Parameter | ONCX, mean (95%
CI) | Controls, mean (95%
CI) | P-value |
|---|
| Week 2 | Haemoglobin | 0.36
(0.23–0.48) | 0.87
(0.69–1.06) | <0.001 |
|
| ALT | 0.22
(0.12–0.33) | 0.69
(0.48–0.90) | <0.001 |
|
| AST | 0.12
(0.04–0.20) | 0.87
(0.61–1.13) | <0.001 |
| Week 3 | ALT | 0.08
(0.01–0.15) | 0.42
(0.25–0.59) | 0.005 |
|
| AST | 0.08
(0.01–0.15) | 0.50
(0.33–0.67) | <0.001 |
ONCX was well tolerated except for nausea. Seven
patients reported ONCX-related nausea immediately after swallowing
with or without vomiting; one of them refused to participate in
Visit 2 of the study and the rest of the subjects were able to
continue participation once ONCX was diluted, as they were advised
at Visit 1.
Discussion
This is the first study to show that the Oncoxin
nutritional supplement containing amino acids, vitamins,
micronutrient elements and naturally-occurring biologically-active
macromolecules is able to improve the patients' QoL, prevent the
loss of body weight and reduction of albumin, and equally as
important, reduce ACT hepatic toxicity as it is evident by the
proportions of patient with ALT and AST levels within normal
limits. An important feature of this study was that it was
conducted in the context of real clinical practice. The only
exception was the requirement to fill out the ESAS patient
questionnaire. There were no other special requirements for the
centres that participated in the study.
The cancer patients' QoL is one of the key
influencing factors for patient compliance and determines the
possibility of implementation or continuation of treatment. QoL is
what a person feels independently, without considering the
objective state, findings of instrumental/laboratory tests and
knowledge of the essence of the disease. Thus, QoL is vitally
important in assessing the therapy's effects; it shows how patients
feel about their condition, and how this attitude changes during
the disease progression or medical intervention (6).
ONCX was previously found to improve the QoL and
life expectancy, as well as appetite in patients with end-stage
hepatocellular carcinoma (10). The
authors assumed that ONCX acted as a nutrient and expanded the food
allowance, eliminating the possible shortage of its individual
components. Indeed, various ONCX components can have such effect.
Zinc has the capacity to correct taste disorders, including those
associated with cancer, as well as stimulate food consumption,
which is extremely important in the development of
anorexia-cachexia syndrome (13). Up
to 35% of elderly people in developed countries have deficiency of
this nutrient element (14), and
such deficiency is typical to lung and ovarian cancer (15), opioid use (16) and cisplatin CT (15). Vitamins can also affect the
well-being of patients, e.g. vitamin B12 (17). Some amino acids, namely, arginine and
glycine, can prevent muscle loss in cancer diseases (18,19). As
demonstrated, a significant number of ONCX components suppress the
severity of systemic inflammation, and this suppression seems to be
a key mechanism to improve the QoL when using ONCX. Suppression of
systemic inflammation has been found with glycine (20), glucosamine (21), EGCG (22), glycyrrhizin (23), zinc (24).
The ability of ONCX to reduce therapy toxicity is an
important aspect of its use demonstrated in the study. The previous
studies showed the role of several of its components in reducing
the xenobiotics toxicity. Cysteine participates in remethylation of
methionine, whose level is closely related to chemical toxicity
manifestation threshold due to amino acid involvement in a series
of antioxidant systems, including glutathione (25). Glycine is capable of reducing nephro-
and hepatotoxicity of drugs and a number of toxic compounds
(26), e.g. cyclosporine A
nephrotoxicity (27). It is assumed
that due to suppression of prostaglandin E2 release from Kupffer
cells, glycine blocks the liver damage caused by this drug
(28). The hepatoprotective
properties of glycyrrhizin are well known and may be important in
toxic liver damage caused by CT. This feature has been reported in
patients with gastric cancer. It was established that the use of
glycyrrhizin with FOLFOX and XELOX CT regimens was accompanied by a
significantly smaller number of liver function abnormalities (more
than twice as compared to the control group) (7). EGCG is another component with a
clinically proven ability to affect the antitumor therapy's
toxicity. EGCG is a basic green tea extract polyphenol that
possesses radioprotective and chemoprotective effects. Due to its
antioxidant and anti-inflammatory effects, EGCG was highly
effective in relieving acute esophagitis induced by radiotherapy or
chemoradiotherapy (8,9).
The antioxidant and anti-inflammatory properties of
ONCX components are likely to be the basis of its ability to reduce
ACT toxicity. The antioxidant activity was also found in other
components of ONCX, including zinc (14,24) and
manganese (29).
Being a real clinical setting study, it was planned
to be as simple and non-burdening as possible for investigators.
Therefore, it has several disadvantages. Except for the ESAS
questionnaire, outcome measures were limited by current clinical
practice. Another disadvantage of this study was the short
observation period of patients between successive courses of ACT,
i.e. about three weeks. It was not possible to assess the delayed
effects of ONCX and to identify any details related to the effect
of this nutritional supplement on the relative ACT dose intensity,
as well as other measures that characterise the condition of
patients during administration of anticancer agents.
The study showed that the use of the nutritional
supplement ONCX, when administered concurrently with ACT, increased
the proportions of patients with clinically meaningful improvement
of QoL by 16% as early as after 2 weeks of use and who have not
experienced a loss in body weight and a decrease in albumin levels,
by 25 and 43%, respectively. In addition, ONCX reduced the severity
of appetite disturbance and hepatic toxicity of anticancer
therapy.
In conclusion, it should be noted that it was the
first study carried out within the real clinical setting that
showed the high efficacy of ONCX in improving the ACT patients' QoL
and reducing the therapy's toxicity. Even though the obtained
results look promising, further studies of multicomponent
nutritional supplements, such as ONCX, are required to explore
opportunities to improve patients' QoL and to achieve the best ACT
efficacy with minimal toxicity.
Acknowledgements
The authors would like to thank Mr. Ivan Vasilievich
Besprozvannykh for his help with graphs/figures.
Funding
Catalysis S.L. provided Oncoxin nutritional
supplement for the purposes of the present study. No other funding
was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DRK was involved in study design and interpretation
of final results, patient selection and follow up. MVK was involved
in study design, protocol and procedure development and
interpretation of final results. VSP was involved in study design,
protocol and procedure development, interpretation of final
results, and patient selection and follow up. MD was involved in
study design and interpretation of final results. EVB was
responsible for patient selection and follow up, and involved in
study design and interpretation of final results. ZMA, RZA, ENB,
AVB, SNG, ASM, SSP, MVR and ASS were responsible for patient
selection and follow up, and involved in study design and
interpretation of final results. IAK was responsible for patient
selection and treatment and involved in study design and
interpretation of final results. ES was involved in study design,
and protocol and procedures development. FIP performed statistical
analysis and was a major contributor in writing the manuscript. All
authors have read and approved the final version of this
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Loginov Moscow Clinical Scientific Centre
(protocol 3/2017 April 17, 2017). In accordance with the
Declaration of Helsinki, all patients provided written informed
consent to participate in the present study.
Patient consent for publication
All patients provided written informed consent for
publication of the results.
Competing interests
ES is a Scientific Department Managing Director of
Catalysis S.L., the manufacturer and provider of Oncoxin for the
purposes of the present study. All other authors declare that they
have no competing interests.
Glossary
Abbreviations
Abbreviations:
|
ACT
|
adjuvant chemotherapy
|
|
ALT
|
alanine aminotransferase
|
|
AST
|
aspartate aminotransferase
|
|
BMI
|
body mass index
|
|
CI
|
confidence interval
|
|
CT
|
chemotherapy
|
|
EGCG
|
epigallocatechin gallate
|
|
ESAS
|
Edmonton Symptom Assessment System
|
|
FOLFOX
|
folinic acid (leucovorin),
fluorouracil, oxaliplatin
|
|
MCID
|
minimal clinically important
difference
|
|
NSCLC
|
non-small cell lung cancer
|
|
ONCX
|
Oncoxin
|
|
OR
|
odds ratio
|
|
QoL
|
quality of life
|
|
SDS
|
symptom distress score
|
|
XELOX
|
oxaliplatin, capecitabine
|
References
|
1
|
Prasanna T, Beith J, Kao S, Boyer M and
McNeil CM: Dose modifications in adjuvant chemotherapy for solid
organ malignancies: A systematic review of clinical trials. Asia
Pac J Clin Oncol. 14:125–133. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lakhanpal SH: Docetaxel and
cyclophosphamide as adjuvant chemotherapy for early breast cancer:
Primary prophylaxis with g-CSF is required. Breast Cancer Manage.
2:367–374. 2013. View Article : Google Scholar
|
|
3
|
Jones SE, Savin MA, Holmes FA,
O'Shaughnessy JA, Blum JL, Vukelja S, McIntyre KJ, Pippen JE,
Bordelon JH, Kirby R, et al: Phase III trial comparing doxorubicin
plus cyclophosphamide with docetaxel plus cyclophosphamide as
adjuvant therapy for operable breast cancer. J Clin Oncol.
24:5381–5387. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Haller DG, Tabernero J, Maroun J, de Braud
F, Price T, Van Cutsem E, Hill M, Gilberg F, Rittweger K and
Schmoll HJ: Capecitabine plus oxaliplatin compared with
fluorouracil and folinic acid as adjuvant therapy for stage III
colon cancer. J Clin Oncol. 29:1465–1471. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
André T, Boni C, Mounedji-Boudiaf L,
Navarro M, Tabernero J, Hickish T, Topham C, Zaninelli M, Clingan
P, Bridgewater J, et al: Oxaliplatin, fluorouracil, and leucovorin
as adjuvant treatment for colon cancer. N Engl J Med.
350:2343–2351. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Eichler M, Singer S, Janni W, Harbeck N,
Rack B, Augustin D, Wischnik A, Kiechle M, Ettl J, Scholz C, et al:
Pretreatment quality of life, performance status and their relation
to treatment discontinuation and treatment changes in high-risk
breast cancer patients receiving chemotherapy: Results from the
prospective randomized ADEBAR trial. Breast Cancer. 24:319–325.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yan Y, Mo Y and Zhang D: Magnesium
isoglycyrrhizinate prevention of chemotherapy-induced liver damage
during initial treatment of patients with gastrointestinal tumors.
Zhonghua Gan Zang Bing Za Zhi. 23:204–208. 2015.(In Chinese).
PubMed/NCBI
|
|
8
|
Zhao H, Zhu W, Xie P, Li H, Zhang X, Sun
X, Yu J and Xing L: A phase I study of concurrent chemotherapy and
thoracic radiotherapy with oral epigallocatechin-3-gallate
protection in patients with locally advanced stage III
non-small-cell lung cancer. Radiother Oncol. 110:132–136. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhao H, Xie P, Li X, Zhu W, Sun X, Sun X,
Chen X, Xing L and Yu J: A prospective phase II trial of EGCG in
treatment of acute radiation-induced esophagitis for stage III lung
cancer. Radiother Oncol. 114:351–356. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Al-Mahtab M, Akbar SM, Khan MS and Rahman
S: Increased survival of patients with end-stage hepatocellular
carcinoma due to intake of ONCOXIN(®), a dietary
supplement. Indian J Cancer. 52:443–446. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shumsky A, Bilan E, Sanz E and Petrovskiy
F: Oncoxin nutritional supplement in the management of
chemotherapy- and/or radiotherapy-associated oral mucositis. Mol
Clin Oncol. 10:463–468. 2019.PubMed/NCBI
|
|
12
|
Hui D, Shamieh O, Paiva CE, Khamash O,
Perez-Cruz PE, Kwon JH, Muckaden MA, Park M, Arthur J and Bruera E:
Minimal clinically important difference in the physical, emotional,
and total symptom distress scores of the edmonton symptom
assessment system. J Pain Symptom Manage. 51:262–269. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yagi T, Asakawa A, Ueda H, Ikeda S,
Miyawaki S and Inui A: The role of zinc in the treatment of taste
disorders. Recent Pat Food Nutr Agric. 5:44–51. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Prasad AS: Impact of the discovery of
human zinc deficiency on health. J Trace Elem Med Biol. 28:357–363.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sweeney JD, Ziegler P, Pruet C and
Spaulding MB: Hyperzincuria and hypozincemia in patients treated
with cisplatin. Cancer. 63:2093–2095. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ciubotariu D, Ghiciuc CM and Lupușoru CE:
Zinc involvement in opioid addiction and analgesia-should zinc
supplementation be recommended for opioid treated persons? Subst
Abuse Treat Prev Policy. 4:10–29. 2015.
|
|
17
|
Weinstein SJ, Stolzenberg-Solomon R,
Pietinen P, Taylor PR, Virtamo J and Albanes D: Dietary factors of
one-carbon metabolism and prostate cancer risk. Am J Clin Nutr.
84:929–935. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Buijs N, Luttikhold J, Houdijk AP and van
Leeuwen PA: The role of a disturbed arginine/NO metabolism in the
onset of cancer cachexia: A working hypothesis. Curr Med Chem.
19:5278–5286. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ham DJ, Murphy KT, Chee A, Lynch GS and
Koopman R: Glycine administration attenuates skeletal muscle
wasting in a mouse model of cancer cachexia. Clin Nutr. 33:448–458.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Grotz MR, Pape HC, van Griensven M, Stalp
M, Rohde F, Bock D and Krettek C: Glycine reduces the inflammatory
response and organ damage in a two-hit sepsis model in rats. Shock.
16:116–121. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kantor ED, Lampe JW, Navarro SL, Song X,
Milne GL and White E: Associations between glucosamine and
chondroitin supplement use and biomarkers of systemic inflammation.
J Altern Complement Med. 20:479–485. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dominiak K, McKinney J, Heilbrun LK and
Sarkar FH: Critical need for clinical trials: An example of a pilot
human intervention trial of a mixture of natural agents protecting
lymphocytes against TNF-alpha induced activation of NF-kappa B.
Pharm Res. 27:1061–1065. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Khan R, Khan AQ, Lateef A, Rehman MU,
Tahir M, Ali F, Hamiza OO and Sultana S: Glycyrrhizic acid
suppresses the development of precancerous lesions via regulating
the hyperproliferation, inflammation, angiogenesis and apoptosis in
the colon of Wistar rats. PLoS One. 8:e560202013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Prasad AS: Zinc: Mechanisms of host
defense. J Nutr. 137:1345–1349. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fukagawa NK and Galbraith RA: Advancing
age and other factors influencing the balance between amino acid
requirements and toxicity. J Nutr 134 (6 Suppl). 1569S–1574S.
2004.
|
|
26
|
Miller GW, Lock EA and Schnellmann RG:
Strychnine and glycine protect renal proximal tubules from various
nephrotoxicants and act in the late phase of necrotic cell injury.
Toxicol Appl Pharmacol. 125:192–197. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhong Z, Li X, Yamashina S, von
Frankenberg M, Enomoto N, Ikejima K, Kolinsky M, Raleigh JA and
Thurman RG: Cyclosporin a causes a hypermetabolic state and hypoxia
in the liver: Prevention by dietary glycine. J Pharmacol Exp Ther.
299:858–865. 2001.PubMed/NCBI
|
|
28
|
Mauriz JL, Matilla B, Culebras JM,
Gonzalez P and Gonzalez-Gallego J: Dietary glycine inhibits
activation of nuclear factor kappa B and prevents liver injury in
hemorrhagic shock in the rat. Free Radic Biol Med. 31:1236–1244.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Robbins D and Zhao Y: Manganese superoxide
dismutase in cancer prevention. Antioxid Redox Signal.
20:1628–1645. 2014. View Article : Google Scholar : PubMed/NCBI
|