Introduction
Autophagy is the process of transporting damaged,
denatured or aging proteins, and organelles into lysosomes for
digestion and degradation. Under normal physiological conditions,
autophagy helps cells to maintain a self-stable state; however,
during stress, autophagy prevents accumulation of toxic or
carcinogenic damaged proteins and organelles, and inhibits cell
carcinogenesis (1–4). Once a tumor is formed, autophagy can be
harmful as cells provide additional nutrients and promote tumor
growth. Therefore, the role of autophagy in the development of
tumors is two-sided (5–9). Since autophagy can regulate cancer
formation, proliferation, metastasis and energy metabolism of
tumors, antitumor drugs based on regulation of autophagy activity
have been used in clinical treatment (10–12).
Additionally, inhibition of tumors by improving autophagy activity
has become a novel concept for cancer treatment (10–14).
Depending on the way a lysosome accepts the
substance to be degraded, autophagy can be divided into
macroautophagy, microautophagy and chaperone-mediated autophagy.
Macroautophagy, the usual form of autophagy, is the most common
type and is characterized by the formation of cup-shaped bilayer
membrane structures surrounding the cytoplasmic component, followed
by the formation of autophagosomes (15–18). The
outer membrane of autophagosomes and the enzymatic fusion form a
monolayer membrane structure of autophagosomes, while the inner
membrane and contents of autophagosomes are digested (15–18). The
aforementioned process is mediated by autophagy-related genes
(ARGs). Autophagocytosis is a process in which cells use lysosomes
to degrade damaged organelles and macromolecules under regulation
of ARGs (19–26). Previous studies have identified 234
ARGs (27). These ARGs have been
identified as direct or indirect participants in the process of
autophagy; thus, analysis of a list of ARGs can provide a
comprehensive overview of the alterations of autophagy in multiple
myeloma (MM). Several studies have demonstrated that these ARGs
have significant clinical implications for various types of cancer,
including glioma, liver cancer and thyroid cancer (23,24,28).
Autophagy has an important role in the pathogenesis
of plasma cell development and MM, the incidence rate of which is
estimated to be 2–3/100,000, and which mostly affects patients
>40 years old (29–31). Generally, autophagy is considered to
be involved in pro-survival mechanisms of MM cells and to interact
with the ubiquitin-proteasome system to maintain homeostasis of MM
cells via degraded and misfolded proteins for energy recovery
(32–36). Therefore, inhibiting autophagy may
effectively induce MM cell death and can act synergistically using
proteasome inhibitors. However, exaggerated activation of autophagy
may result in excessive degradation of organelles, which can induce
autophagic cell death. Thus, activation of autophagic cell death
may represent a promising approach for treatment of MM (32–36).
Recent studies have demonstrated that autophagy mediates drug
resistance in MM cells and leads to development of clinical
complications for MM, while inhibition of autophagy may reverse the
response to drugs (37,38). However, the clinical role,
particularly the prognostic role of ARGs in MM, has yet to be
determined.
In the present study, the expression profiles of
ARGs and prognosis data of MM were integrated, and used to develop
a risk score to predict the clinical outcome of patients with MM.
Previous studies on the role of autophagy in MM tended to focus on
a single gene, on the contrary, the present study proposed a novel
predictor by integrating several effective indexes, which could
provide more effective information concerning autophagy and offer
more favorable performance in survival prediction of patients with
MM. The present study evaluated the prognostic value of ARGs in MM
clinical samples by data mining and bioinformatics analysis of gene
expression profiles. Additionally, a risk score was constructed
using the prognosis-associated ARGs, which was expected to provide
novel ideas for clinical applications in MM.
Materials and methods
Dataset and data processing
Microarray expression profiles were obtained from
the Gene Expression Omnibus (GEO) database (ncbi.nlm.nih.gov/geo/) using the accession number
GSE24080, which contained 559 samples of patients with MM (39). GSE24080 belongs to the MAQC-II
Project: Multiple myeloma (MM) dataset. All patients had complete
data for event-free survival (EFS), which included the recurrence
of MM or the onset of certain symptoms associated with MM.
Additionally, the platform was [HG-U133_Plus_2] Affymetrix Human
Genome U133 Plus 2.0 Array (GPL570), which contains 54,675 probes
(Affymetrix; Thermo Fisher Scientific, Inc.). To generate the gene
expression profile, the expression matrix and microarray platform
annotation file were downloaded. When more than one probe detected
the same gene expression value, the average value was considered as
the gene expression.
For further analysis, a total of 234 human genes and
proteins involved in autophagy were acquired from the human
autophagy database (HADb; autophagy.lu/). HADb is the first human
autophagy-dedicated database, and is a public repository that
contains annotation information associated with the up-to-date
human genes the have been reported to be involved in autophagy. By
October 2018, there were 234 ARGs included in the dataset (27).
Survival analysis and functional
characteristics
A total of 234 ARGs were selected and their
prognostic values were assessed. ARGs that were significantly
associated with the EFS of MM were identified using univariate Cox
analysis. Kaplan-Meier plots were used to further analyze the
potential of ARGs as prognostic factors in patients. In order to
determine the best cut-off value for grouping the patients to
observe significant difference in outcome, all gene expression
values from the 20 to 80th percentiles were considered. The cut-off
with the lowest log-rank P-value was selected to group the
patients. Survival analysis was conducted using the ‘survival’
package of R software (version 3.5.1; http://CRAN.R-project.org/package=survival). P<0.05
was considered to indicate a statistically significant difference
(40). To reveal molecular
functional characteristics, in addition to the autophagy of these
prognostic ARGs, functional enrichment analysis was conducted for
the Gene Ontology (GO; http://geneontology.org/) and Kyoto Encyclopedia of
Genes and Genomes (KEGG; http://www.kegg.jp/) databases using the R package
‘clusterProfiler’ (version 3.12.0) (41,42). The
protein-protein interaction (PPI) network was generated to display
the associations between the prognosis-associated ARGs, by using
the Search Tool for the Retrieval of Interacting Genes/Proteins
(STRING) database (https://string-db.org/; version 10.0).
Risk score construction
To develop a risk score using independent factors
with ARGs, least absolute shrinkage and selection operator (LASSO)
multivariate Cox regression analysis was performed. Subsequently,
for each patient, the risk score was derived by multiplying the
expression level of prognosis-associated ARGs and its corresponding
coefficient as follows: Risk
score=ΣniARGi * βi; in
detail, LASSO Cox analysis selected the eligible ARGs for the risk
score based on the expression levels of each sample and generated
the corresponding coefficients for each of them. Accordingly, a
risk score formula consisting of 16 ARGs weighted by the
coefficients from LASSO penalized regression was established, where
‘β’ is the coefficient, ‘i’ refers to each ARG and ‘n’ is the
number of the prognostic ARGs included in the calculation. The risk
score for each patient was calculated, and all patients were
divided into high- or low-risk groups based on the median level of
the risk score. The performance of the risk score was assessed
using the ‘survival receiver-operator characteristic (ROC)’ package
for R software (https://CRAN.R-project.org/package=survivalROC;
version 1.0.3), which provides an effective approach for evaluating
time-dependent ROC using censored data. To quantitatively evaluate
the prognostic value, the area under the curve (AUC) of the ROC
curves was calculated (24,43–48).
Gene set enrichment analysis
In order to explore the pathways that are affected
in the high- or low-risk group, gene set enrichment analysis (GSEA)
(http://software.broadinstitute.org/gsea/index.jsp;
version 3.0) was performed (49–52).
Using GSEA, the present study tested whether the
activated/repressed gene signatures were enriched for high-risk vs.
low-risk cases. The pre-defined hallmarks were calculated using a
normalized enrichment score (NES) and false discovery rate (FDR).
Pathways with NES>1 and FDR<0.05 were considered to be
significant.
Validation of the autophagy-associated
risk score in other independent cohorts
MM-related microarray and RNA-sequencing datasets
were screened in the GEO (https://www.ncbi.nlm.nih.gov/geo/), ArrayExpress
(https://www.ebi.ac.uk/arrayexpress/)
and SRA databases (https://www.ncbi.nlm.nih.gov/sra), and the search
strategy was as follows: ‘Myeloma AND Homo sapiens’. Subsequently,
prognosis-associated datasets were selected for further analysis.
Four independent cohorts met the inclusion criteria, including
GSE57317, GSE4581, GSE4452 and GSE4204. The expression data of the
aforementioned 16 genes [5-aminoimidazole-4-carboxamide
ribonucleotide formyltransferase/IMP cyclohydrolase (ATIC), BCL2
interacting protein 3 like (BNIP3L), calcium binding and
coiled-coil domain 2 (CALCOCO2), DnaJ heat shock protein family
(Hsp 40) member B1 (DNAJB1), DnaJ heat shock protein family (Hsp
40) member B9 (DNAJB9), eukaryotic translation initiation factor 4E
binding protein 1 (EIF4EBP1), eva-1 homolog A (EVA1A), FKBP prolyl
isomerase 1B (FKBP1B), forkhead box O1 (FOXO1), forkhead box O3
(FOXO3), GABA type A receptor-associated protein (GABARAP),
hypoxia-inducible factor 1 subunit α (HIF1A), NCK associated
protein 1 (NCKAP1), protein kinase cAMP-dependent type I regulatory
subunit α (PRKAR1A), SPT20 homolog SAGA complex component (SUPT20H)
and transmembrane 9 superfamily member 1 (TM9SF1)] were extracted
from these selected datasets. The risk score was calculated and
one-way Cox regression analysis was performed using SPSS version
19.0 (IBM Corp.) and individual hazard ratios (HRs) were
calculated. The HR and corresponding 95% confidence interval (CI)
estimates were calculated and pooled to determine the association
of risk score with clinical outcome. The random-effects model was
conducted. Finally, a meta-analysis was carried out to impute the
summarized HR combining all five datasets.
Results
Prognostic autophagy-specific gene
screening
Univariate Cox regression analysis using
autophagy-specific gene expression values for MM samples identified
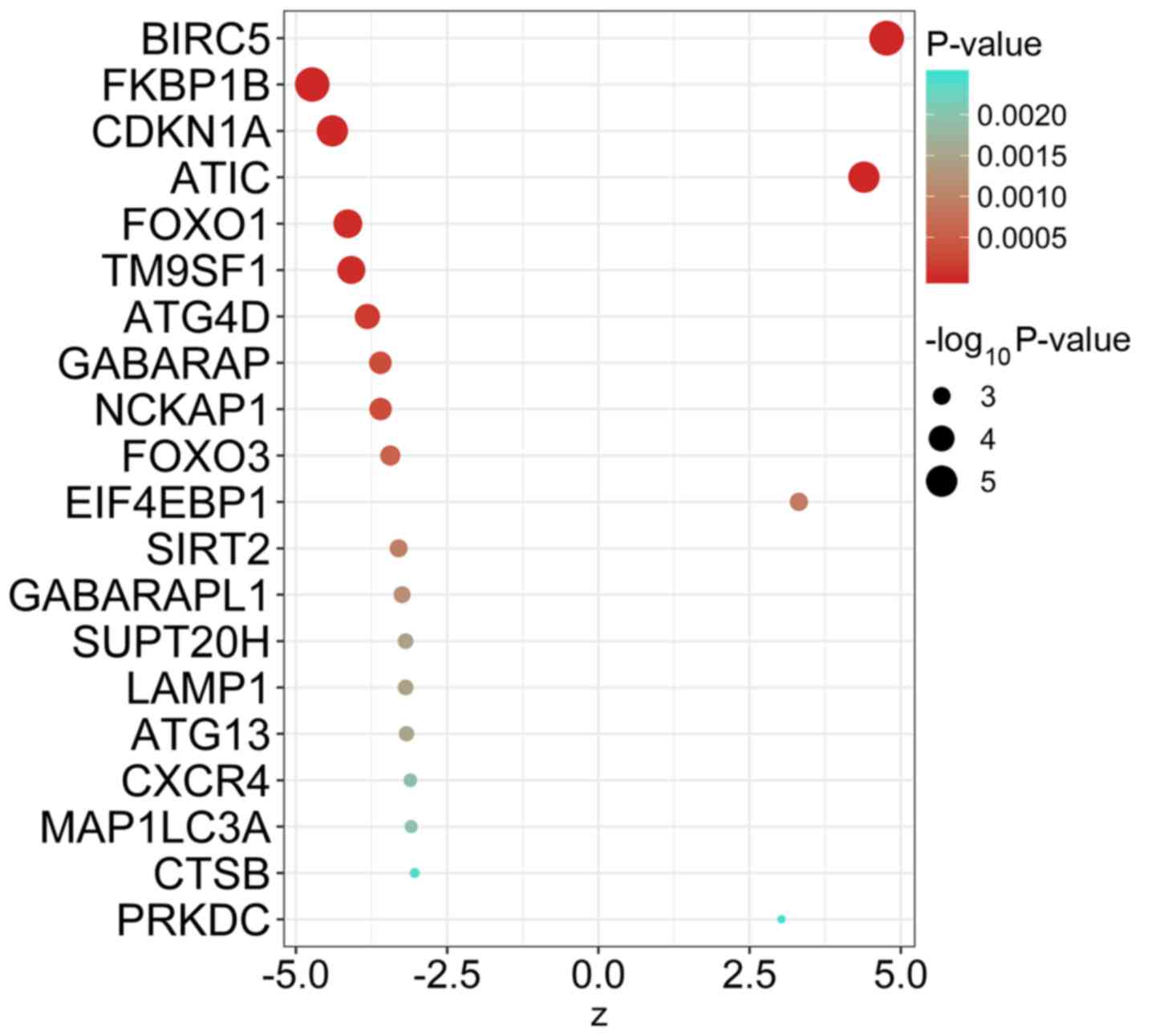
55 ARGs that were significantly associated with EFS of MM (Table I). The top 20 ARGs with significant
association with survival are displayed in Fig. 1.
 | Table I.Prognosis-associated
autophagy-related genes in multiple myeloma. |
Table I.
Prognosis-associated
autophagy-related genes in multiple myeloma.
| Gene | Hazard ratio | Z-score | P-value |
|---|
| BIRC5 | 1.370446 | 4.764858 |
1.89×10−6 |
| FKBP1B | 0.804927 | −4.731650 |
2.23×10−6 |
| CDKN1A | 0.693169 | −4.397310 |
1.10×10−5 |
| ATIC | 1.828409 | 4.392418 |
1.12×10−5 |
| FOXO1 | 0.685527 | −4.142160 |
3.44×10−5 |
| TM9SF1 | 0.607627 | −4.082530 |
4.45×10−5 |
| ATG4D | 0.651409 | −3.819260 |
1.34×10−4 |
| GABARAP | 0.572865 | −3.603840 |
3.14×10−4 |
| NCKAP1 | 0.813461 | −3.598490 |
3.20×10−4 |
| FOXO3 | 0.678902 | −3.439260 |
5.83×10−4 |
| X10IF4X10BP1 | 1.245795 | 3.315612 |
9.14×10−4 |
| SIRT2 | 0.754843 | −3.30136 |
9.62×10−4 |
| GABARAPL1 | 0.760565 | −3.245810 |
1.17×10−3 |
| SUPT20H | 0.645072 | −3.185440 |
1.45×10−3 |
| LAMP1 | 0.756851 | −3.185010 |
1.45×10−3 |
| ATG13 | 0.677256 | −3.172030 |
1.51×10−3 |
| CXCR4 | 0.840891 | −3.106610 |
1.89×10−3 |
| MAP1LC3A | 0.736496 | −3.094860 |
1.97×10−3 |
| CTSB | 0.758494 | −3.034600 |
2.41×10−3 |
| PRKDC | 1.470160 | 3.026135 |
2.48×10−3 |
| PARP1 | 1.485479 | 3.024757 |
2.49×10−3 |
| ITGA6 | 0.868587 | −2.995980 |
2.74×10−3 |
| SH3GLB1 | 0.708930 | −2.987360 |
2.81×10−3 |
| DRAM1 | 0.756615 | −2.938770 |
3.30×10−3 |
| FADD | 1.517498 | 2.908387 |
3.63×10−3 |
| ITGA3 | 0.809097 | −2.867680 |
4.14×10−3 |
| APOL1 | 0.843190 | −2.866750 |
4.15×10−3 |
| PTX10N | 1.574460 | 2.833041 |
4.61×10−3 |
| PPP1R15A | 0.842720 | −2.822870 |
4.76×10−3 |
| HSPA5 | 0.698868 | −2.774470 |
5.53×10−3 |
| VAMP7 | 1.347821 | 2.757183 |
5.83×10−3 |
| BNIP3L | 0.734860 | −2.653410 |
7.97×10−3 |
| MAPK1 | 1.497021 | 2.526023 |
1.15×10−2 |
| PINK1 | 0.738836 | −2.496230 |
1.26×10−2 |
| CALCOCO2 | 0.688453 | −2.458060 |
1.40×10−2 |
| HIF1A | 1.101043 | 2.440301 |
1.47×10−2 |
| BCL2L1 | 0.797140 | −2.431050 |
1.51×10−2 |
| DNAJB9 | 0.746170 | −2.406550 |
1.61×10−2 |
| SQSTM1 | 0.705885 | −2.383290 |
1.72×10−2 |
| ATG9A | 0.789362 | −2.358180 |
1.84×10−2 |
| PRKAR1A | 1.414585 | 2.347303 |
1.89×10−2 |
| X10VA1A | 1.174754 | 2.328178 |
1.99×10−2 |
| HSP90AB1 | 1.237892 | 2.277807 |
2.27×10−2 |
| RAB24 | 0.824202 | −2.270130 |
2.32×10−2 |
| VX10GFA | 1.251725 | 2.246293 |
2.47×10−2 |
| ATG5 | 0.659360 | −2.23166 |
2.56×10−2 |
| ATF4 | 0.752327 | −2.221500 |
2.63×10−2 |
| WDR45B | 0.676826 | −2.189410 |
2.86×10−2 |
| XBP1 | 0.778841 | −2.135780 |
3.27×10−2 |
| DNAJB1 | 0.825023 | −2.131930 |
3.30×10−2 |
| ARNT | 1.323780 | 2.109022 |
3.49×10−2 |
| NAF1 | 1.324480 | 2.089132 |
3.67×10−2 |
| MTMR14 | 0.808476 | −2.054530 |
3.99×10−2 |
| CASP8 | 1.271856 | 1.981271 |
4.76×10−2 |
| GAPDH | 1.263585 | 1.973326 |
4.85×10−2 |
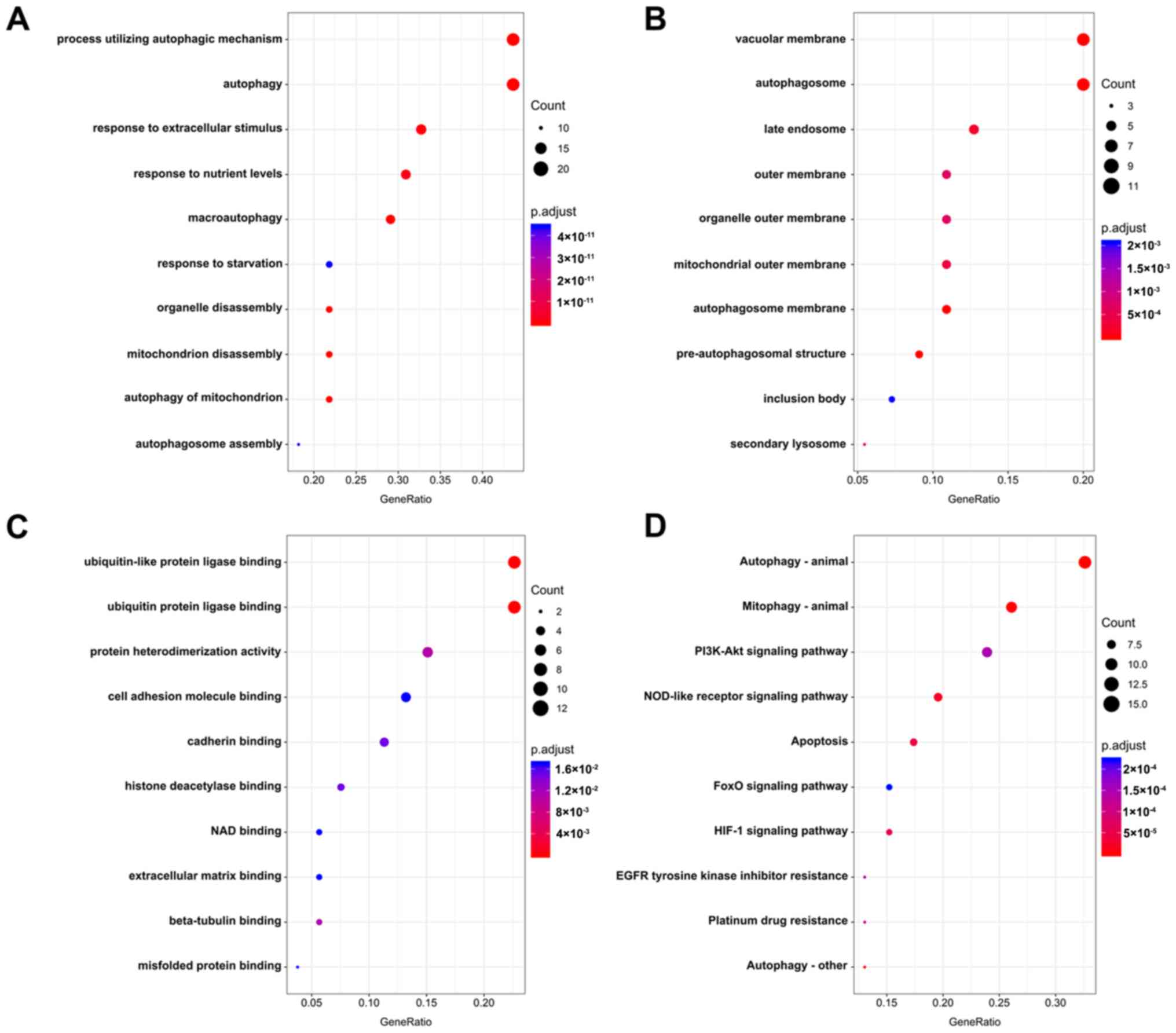
Molecular characteristics of ARGs in
MM
The association of these prognostic ARGs with the GO
terms of the biological process (BP), cellular component (CC) and
molecular function (MF) categories were analyzed. The top three
enriched BP terms were ‘process utilizing autophagic mechanism’,
‘autophagy’ and ‘response to extracellular stimulus’ (Fig. 2A). For CC, the tops three terms were
‘vacuolar membrane’, ‘autophagosome’ and ‘late endosome’ (Fig. 2B). The top three enriched MF terms
were ‘ubiquitin-like protein ligase binding’, ‘ubiquitin protein
ligase binding’ and ‘protein heterodimerization activity’ (Fig. 2C). Accordingly, genes involved in
KEGG pathways were enriched in autophagy-related pathways,
including ‘autophagy-animal’, ‘mitophagy-animal’ and ‘PI3K-Akt
signaling pathway’ (Fig. 2D;
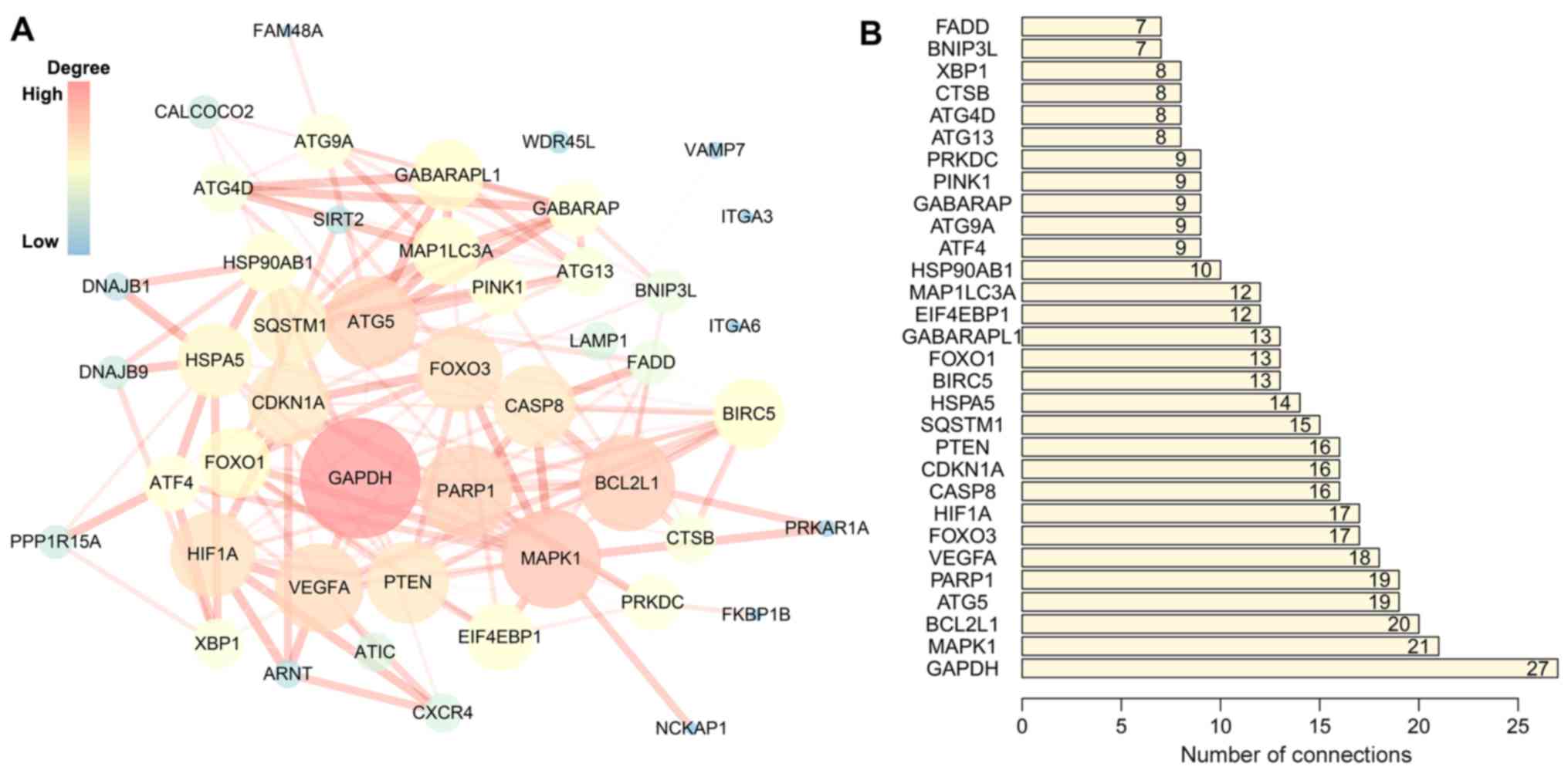
Table II). The PPI network
suggested that these genes have important interactions with each
other. GAPDH, MAPK1, BCL2L1, ATG5 and PARP1 were at the center of
the PPI network, which suggested that these genes had a broader
connection to the other genes (Fig.
3).
 | Table II.Gene functional enrichment analysis
of prognosis-associated autophagy-related genes. |
Table II.
Gene functional enrichment analysis
of prognosis-associated autophagy-related genes.
| Category | ID | Description | GeneRatio | P.adjust | Gene ID (top
10) | Count |
|---|
| Biological
process | GO:0061919 | Process utilizing
autophagic mechanism | 24/55 |
1.82×10−20 | FOXO1, TM9SF1,
ATG4D, GABARAP, SIRT2, GABARAPL1, SUPT20H, ATG13, MAP1LC3A,
SH3GLB1 | 24 |
| Biological
process | GO:0006914 | Autophagy | 24/55 |
1.82×10−20 | FOXO1, TM9SF1,
ATG4D, GABARAP, SIRT2, GABARAPL1, SUPT20H, ATG13, MAP1LC3A,
SH3GLB1 | 24 |
| Biological
process | GO:0009991 | Response to
extracellular stimulus | 18/55 |
5.30×10−13 | FKBP1B, CDKN1A,
FOXO1, GABARAP, FOXO3, EIF4EBP1, SIRT2, GABARAPL1, MAP1LC3A,
ITGA6 | 18 |
| Biological
process | GO:0031667 | Response to
nutrient levels | 17/55 |
2.68×10−12 | FKBP1B, CDKN1A,
FOXO1, GABARAP, FOXO3 EIF4EBP1, SIRT2, GABARAPL1, MAP1LC3A,
SH3GLB1 | 17 |
| Biological
process | GO:0016236 | Macro
autophagy | 16/55 |
1.38×10−13 | ATG4D, GABARAP,
GABARAPL1, ATG13, MAP1LC3A, SH3GLB1, BNIP3L, PINK1, CALCOCO2,
HIF1A |
| Biological
process | GO:0042594 | Response to
starvation | 12/55 |
4.42×10−11 | CDKN1A, FOXO1,
GABARAP, EIF4EBP1, GABARAPL1, MAP1LC3A, SH3GLB1, HSPA5, MAPK1,
ATG5 | 12 |
| Biological
process | GO:1903008 | Organelle
disassembly | 12/55 |
3.16×10−13 | ATG4D, GABARAP,
GABARAPL1, ATG13, MAP1LC3A, BNIP3L, PINK1, HIF1A, SQSTM1 ATG9A | 12 |
| Biological
process | GO:0061726 | Mitochondrion
disassembly | 12/55 |
3.36×10−14 | ATG4D, GABARAP,
GABARAPL1, ATG13, MAP1LC3A, BNIP3L, PINK1, HIF1A, SQSTM1,
ATG9A | 12 |
| Biological
process | GO:0000422 | Autophagy of
mitochondrion | 12/55 |
3.36×10−14 | ATG4D GABARAP,
GABARAPL1, ATG13, MAP1LC3A, BNIP3L, PINK1, HIF1A, SQSTM1,
ATG9A | 12 |
| Biological
process | GO:0000045 | Autophagosome
assembly | 10/55 |
4.42×10−11 | ATG4D, GABARAP,
GABARAPL1, ATG13, MAP1LC3A, SH3GLB1, PINK1, ATG9A, ATG5,
WDR45B | 10 |
| Cellular
component | GO:0005774 | Vacuolar
membrane | 11/55 |
1.65×10−06 | TM9SF1, GABARAP,
GABARAPL1, LAMP1, MAP1LC3A, SH3GLB1, DRAM1 VAMP7, CALCOCO2,
EVA1A | 11 |
| Cellular
component | GO:0005776 | Autophagosome | 11/55 |
3.63×10−13 | TM9SF1, GABARAP,
GABARAPL1, LAMP1, MAP1LC3A, SH3GLB1 CALCOCO2, SQSTM1, ATG9A,
RAB24 | 11 |
| Cellular
component | GO:0005770 | Late endosome | 7/55 |
2.65×10−04 | LAMP1 CXCR4,
MAP1LC3A, VAMP7, MAPK1, SQSTM1, ATG9A | 7 |
| Cellular
component | GO:0019867 | Outer membrane | 6/55 |
7.08×10−04 | SH3GLB1, PPP1R15A,
BNIP3L, PINK1, BCL2L1, CASP8 | 6 |
| Cellular
component | GO:0031968 | Organelle outer
membrane | 6/55 |
7.08×10−04 | SH3GLB1, PPP1R15A,
BNIP3L, PINK1, BCL2L1, CASP8 | 6 |
| Cellular
component | GO:0005741 | Mitochondrial outer
membrane | 6/55 |
4.38×10−04 | SH3GLB1, PPP1R15A,
BNIP3L, PINK1, BCL2L1, CASP8 | 6 |
| Cellular
component | GO:0000421 | Autophagosome
membrane | 6/55 |
4.42×10−08 | TM9SF1, GABARAP,
GABARAPL1, MAP1LC3A, SH3GLB1, CALCOCO2 | 6 |
| Cellular
component | GO:0000407 | Pre-autophagosomal
structure | 5/55 |
2.23×10−06 | ATG13, SQSTM1,
ATG9A, ATG5, WDR45B | 5 |
| Cellular
component | GO:0016234 | Inclusion body | 4/55 |
2.07×10−03 | PINK1, SQSTM1,
HSP90AB1, ATF4 | 4 |
| Cellular
component | GO:0005767 | Secondary
lysosome | 3/55 |
3.60×10−04 | LAMP1, MAP1LC3A,
SQSTM1 | 3 |
| Molecular
function | GO:0044389 | Ubiquitin-like
protein ligase binding | 12/53 |
1.81×10−08 | CDKN1A, FOXO1,
GABARAP, GABARAPL1, CXCR4, MAP1LC3A, HSPA5, PINK1, HIF1A,
SQSTM1 | 12 |
| Molecular
function | GO:0031625 | Ubiquitin protein
ligase binding | 12/53 |
1.81×10−08 | CDKN1A, FOXO1,
GABARAP, GABARAPL1, CXCR4, MAP1LC3A, HSPA5, PINK1, HIF1A,
SQSTM1 | 12 |
| Molecular
function | GO:0046982 | Protein
heterodimerization activity | 8/53 |
1.08×10−02 | ITGA3, BNIP3L,
HIF1A, BCL2L1, VEGFA, ATF4, XBP1, ARNT | 8 |
| Molecular
function | GO:0050839 | Cell adhesion
molecule binding | 7/53 |
1.70×10−02 | ATIC, ITGA6,
SH3GLB1, ITGA3, HSPA5, HSP90AB1, DNAJB1 | 7 |
| Molecular
function | GO:0045296 | Cadherin
binding | 6/53 |
1.49×10−02 | ATIC, ITGA6,
SH3GLB1, HSPA5, HSP90AB1, DNAJB1 | 6 |
| Molecular
function | GO:0042826 | Histone deacetylase
binding | 4/53 |
1.49×10−02 | SIRT2, PARP1,
HIF1A, HSP90AB1 | 4 |
| Molecular
function | GO:0051287 | NAD binding | 3/53 |
1.70×10−02 | SIRT2, PARP1,
GAPDH | 3 |
| Molecular
function | GO:0050840 | Extracellular
matrix binding | 3/53 |
1.70×10−02 | ITGA6, ITGA3,
VEGFA | 3 |
| Molecular
function | GO:0048487 | Beta-tubulin
binding | 3/53 |
1.12×10−02 | GABARAP, SIRT2,
GABARAPL1 | 3 |
| Molecular
function | GO:0051787 | Misfolded protein
binding | 2/53 |
1.70×10−02 | HSPA5, DNAJB9 | 2 |
| KEGG pathway | hsa04140 |
Autophagy-animal | 15/46 |
7.91×10−14 | ATG4D, GABARAP,
GABARAPL1, SUPT20H, LAMP1, ATG13, CTSB, SH3GLB1, PTEN, MAPK1 | 15 |
| KEGG pathway | hsa04137 |
Mitophagy-animal | 12/46 |
1.73×10−13 | GABARAP, FOXO3,
GABARAPL1, BNIP3L, PINK1, CALCOCO2, HIF1A, BCL2L1, SQSTM1,
ATG9A | 12 |
| KEGG pathway | hsa04151 | PI3K-Akt signaling
pathway | 11/46 |
1.48×10−04 | CDKN1A, FOXO3,
EIF4EBP1, ITGA6, ITGA3, PTEN, MAPK1, BCL2L1, HSP90AB1 VEGFA | 11 |
| KEGG pathway | hsa04621 | NOD-like receptor
signaling pathway | 9/46 |
2.77×10−05 | GABARAP, GABARAPL1,
CTSB FADD, MAPK1, BCL2L1, HSP90AB1, ATG5, CASP8 | 9 |
| KEGG pathway | hsa04210 | Apoptosis | 8/46 |
4.95×10−05 | BIRC5, CTSB, PARP1,
FADD, MAPK1, BCL2L1, ATF4, CASP8 | 8 |
| KEGG pathway | hsa04068 | FoxO signaling
pathway | 7/46 |
2.22×10−04 | CDKN1A, FOXO1,
GABARAP, FOXO3, GABARAPL1, PTEN, MAPK1 | 7 |
| KEGG pathway | hsa04066 | HIF-1 signaling
pathway | 7/46 |
6.28×10−05 | CDKN1A, EIF4EBP1,
MAPK1, HIF1A, VEGFA, ARNT, GAPDH | 7 |
| KEGG pathway | hsa01521 | EGFR tyrosine
kinase inhibitor resistance | 6/46 |
1.48×10−04 | FOXO3, EIF4EBP1,
PTEN, MAPK1, BCL2L1, VEGFA | 6 |
| KEGG pathway | hsa01524 | Platinum drug
resistance | 6/46 |
1.20×10−04 | BIRC5, CDKN1A,
FADD, MAPK1, BCL2L1, CASP8 | 6 |
| KEGG pathway | hsa04136 |
Autophagy-other | 6/46 |
1.80×10−06 | ATG4D, GABARAP,
GABARAPL1, ATG13, ATG9A, ATG5 | 6 |
Risk score identification
I order to construct novel risk assessment models
for the prognosis of patients with MM, prognostic signatures were
generated using multivariate Cox regression analysis. Finally, to
develop the prognostic signature, 16 prognosis-associated ARGs were
included (Figs. 4 and 5). Kaplan-Meier survival plots demonstrated
that all 16 genes were significantly associated with the EFS of
patients with MM. Furthermore, the risk score was calculated for
each patient by multiplying the regression coefficient and
expression value for each gene. Risk score=ATIC × 0.3374 + BNIP3L ×
(−0.2126) + CALCOCO2 × (−0.2682) + DNAJB1 × (−0.3848) + DNAJB9 ×
(−0.3443) + EIF4EBP1 × 0.1397 + EVA1A × 0.1794 + FKBP1B × (−0.1205)
+ FOXO1 × (−0.3114) + FOXO3 × (−0.2853) + GABARAP × (−0.3557) +
HIF1A × 0.0876 + NCKAP1 × (−0.1487) + PRKAR1A × 0.7314 + SUPT20H ×
(−0.3261) + TM9SF1 × (−0.1992). Using these prognostic signatures,
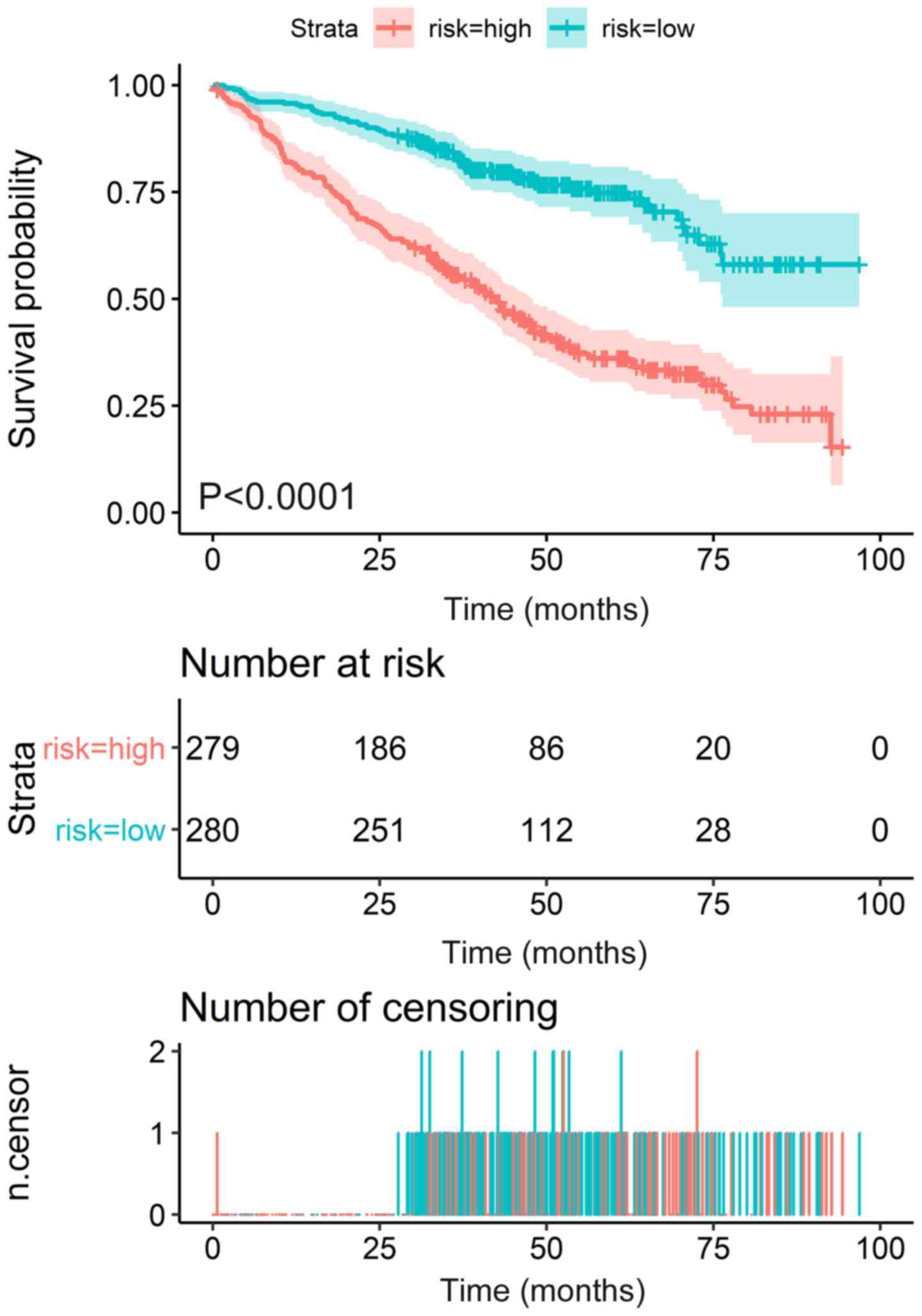
patients with MM were separated into high- and low-risk groups with
distinct clinical outcomes (Fig. 6).
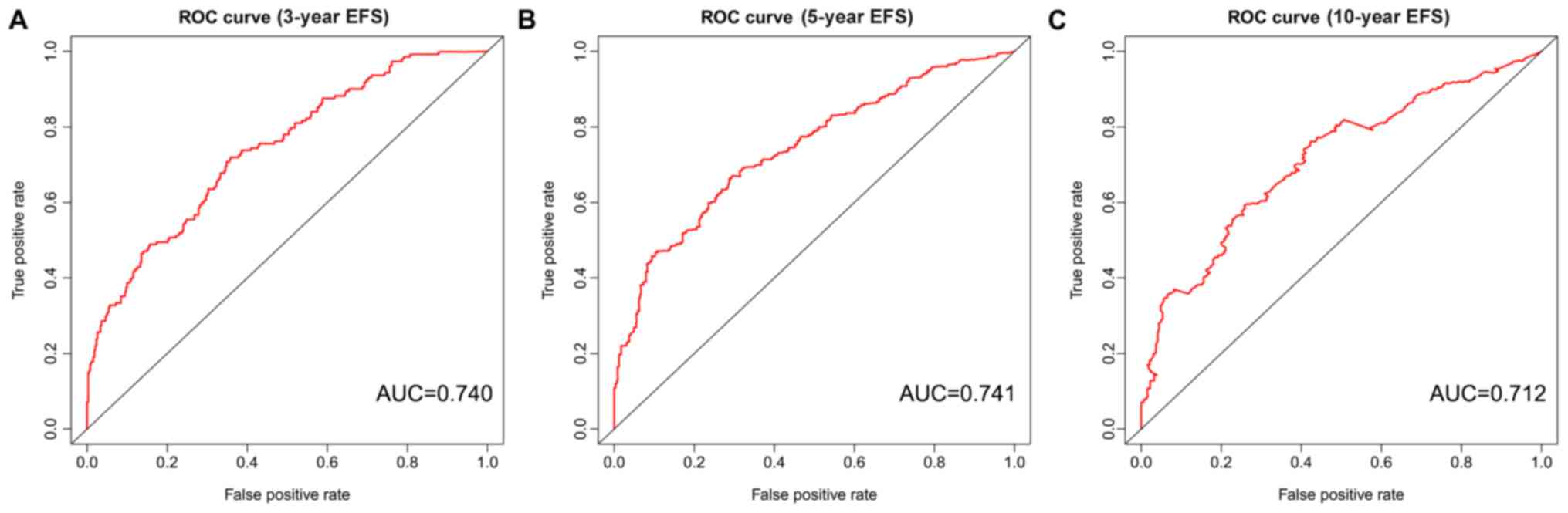
The AUC values for the ROC curve were 0.740, 0.741 and 0.712 for 3,
5 and 10 years, respectively (Fig.
7).
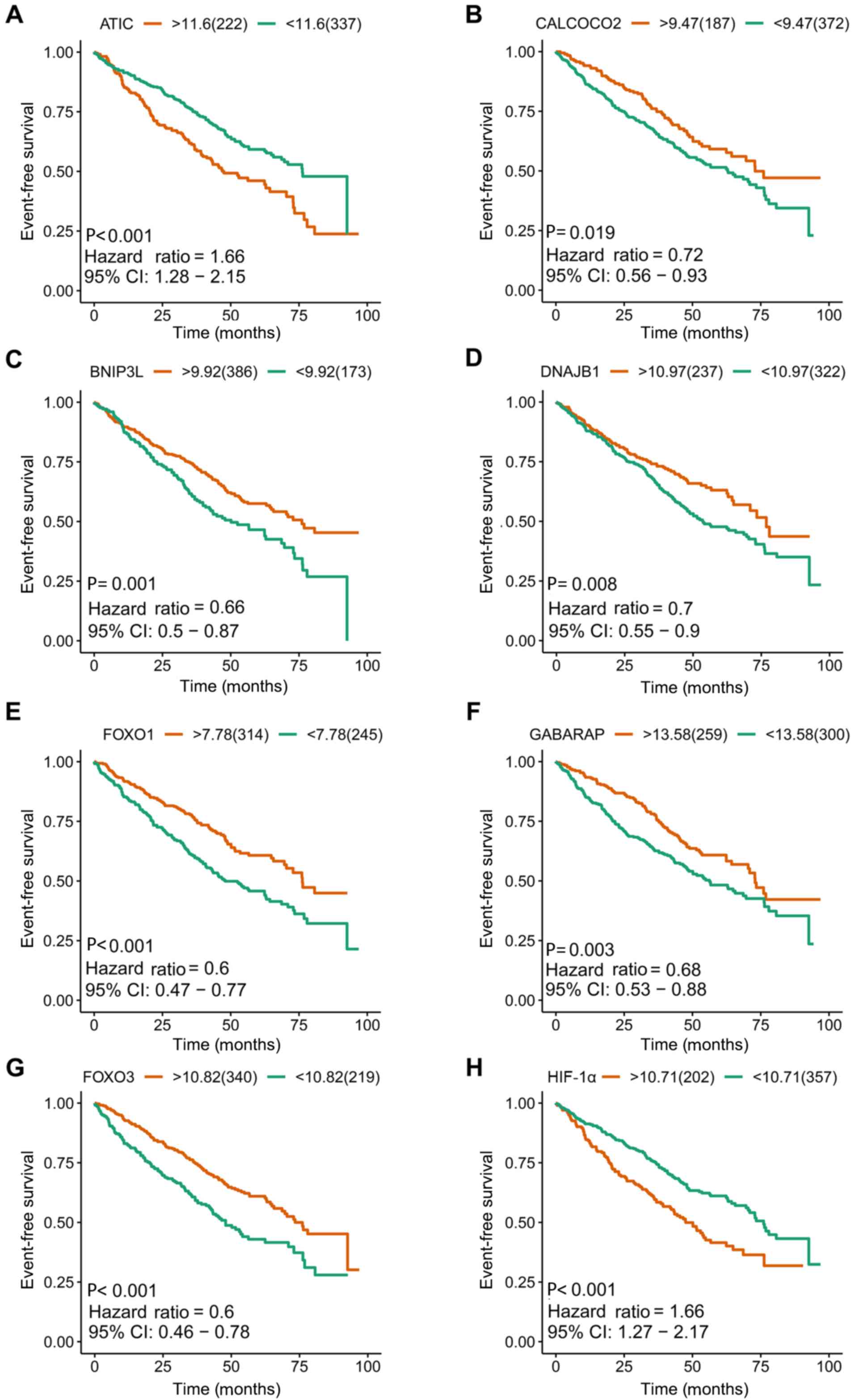 | Figure 4.Kaplan-Meier survival plots of eight
included prognostic predictors for multiple myeloma. (A) ATIC, (B)
CALCOCO2, (C) BNIP3L, (D) DNAJB1, (E) FOXO1, (F) GABARAP, (G) FOXO3
and (H) HIF-1α. ATIC, 5-aminoimidazole-4-carboxamide ribonucleotide
formyltransferase/IMP cyclohydrolase; CI, confidence interval;
CALCOCO2, calcium binding and coiled-coil domain 2; BNIP3L, BCL2
interacting protein 3 like; DNAJB1, DnaJ heat shock protein family
(Hsp 40) member B1; FOXO1, forkhead box O1; GABARAP, GABA type A
receptor-associated protein; FOXO3, forkhead box O3; HIF-1α,
hypoxia-inducible factor 1 subunit α. |
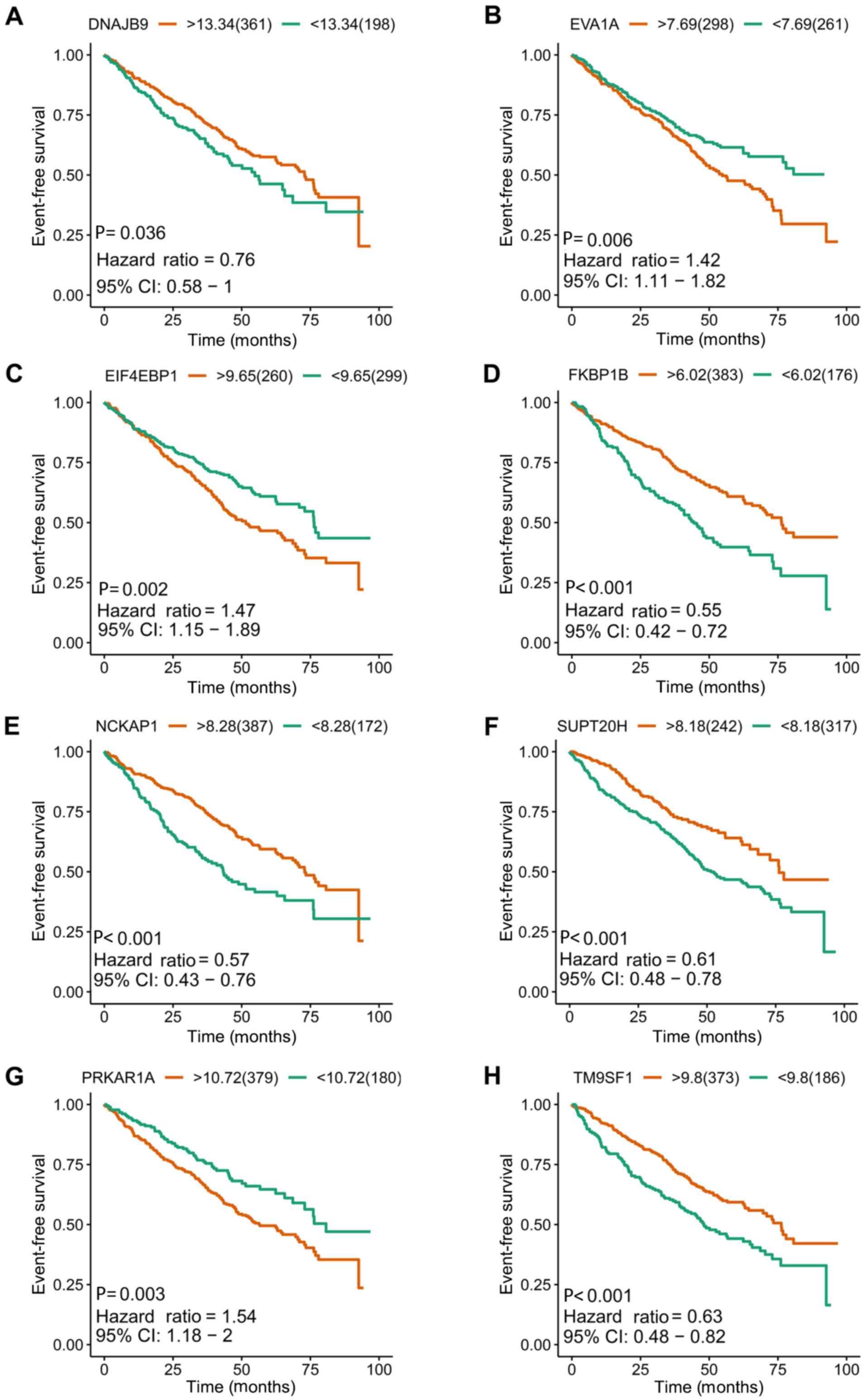 | Figure 5.Kaplan-Meier survival plots of the
other eight included prognostic predictors for multiple myeloma.
(A) DNAJB9, (B) EVA1A, (C) EIF4EBP1, (D) FKBP1B, (E) NCKAP1, (F)
SUPT20H, (G) PRKAR1A and (H) TM9SF1. DNAJB9, DnaJ heat shock
protein family (Hsp 40) member B9; CI, confidence interval;
EIF4EBP1, eukaryotic translation initiation factor 4E binding
protein 1; EVA1A, eva-1 homolog A; FKBP1B, FKBP prolyl isomerase
1B; NCKAP1, NCK associated protein 1; SUPT20H, SPT20 homolog, SAGA
complex component; PRKAR1A, protein kinase cAMP-dependent type I
regulatory subunit α; TM9SF1, transmembrane 9 superfamily member
1. |
Hallmarks of high- and low-risk
groups
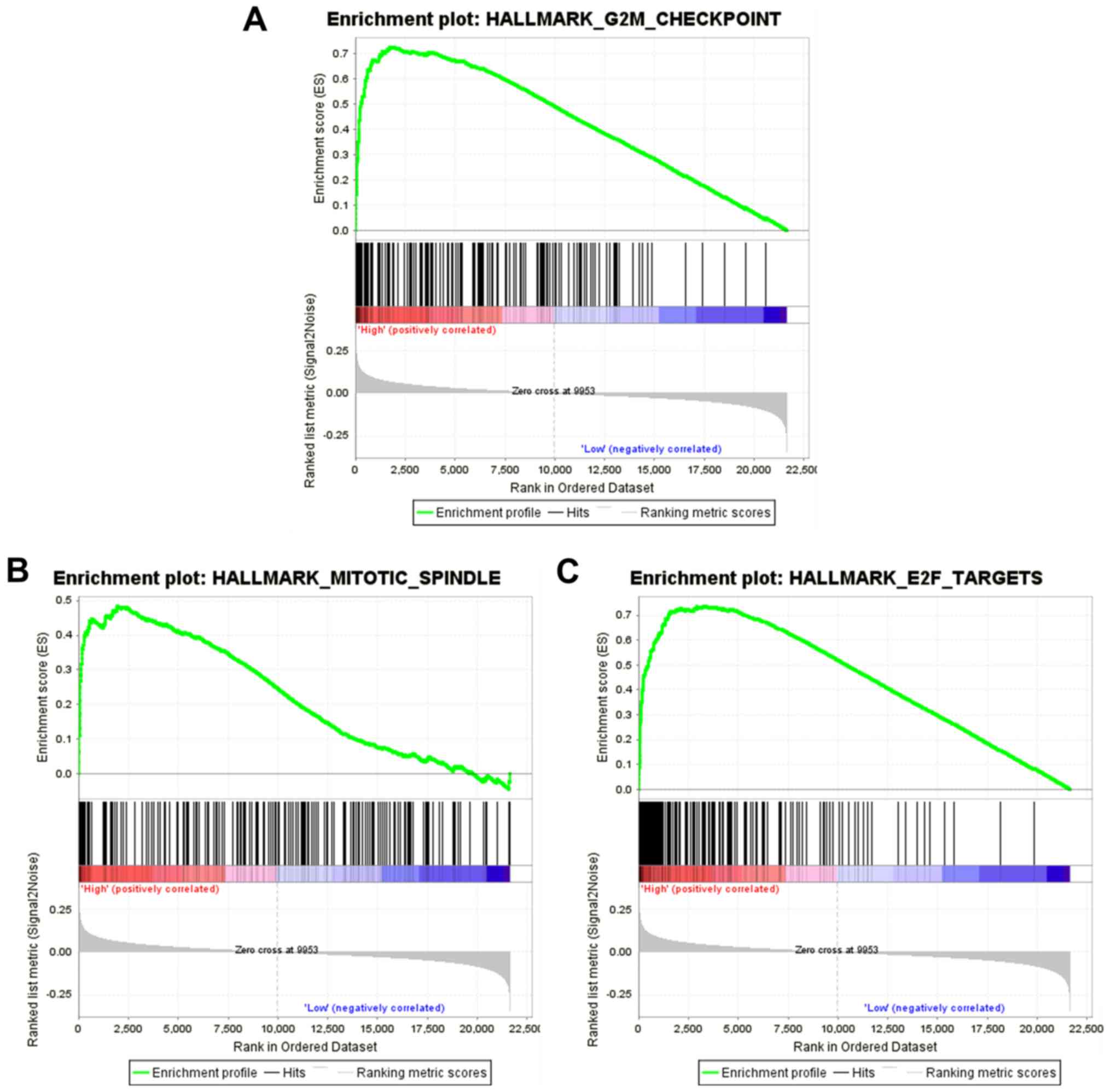
The results of the GSEA analysis indicated that five
hallmarks were significantly associated with high-risk patients,
including ‘G2M_CHECKPOINT’, ‘MITOTIC_SPINDLE’, ‘E2F_TARGETS’,
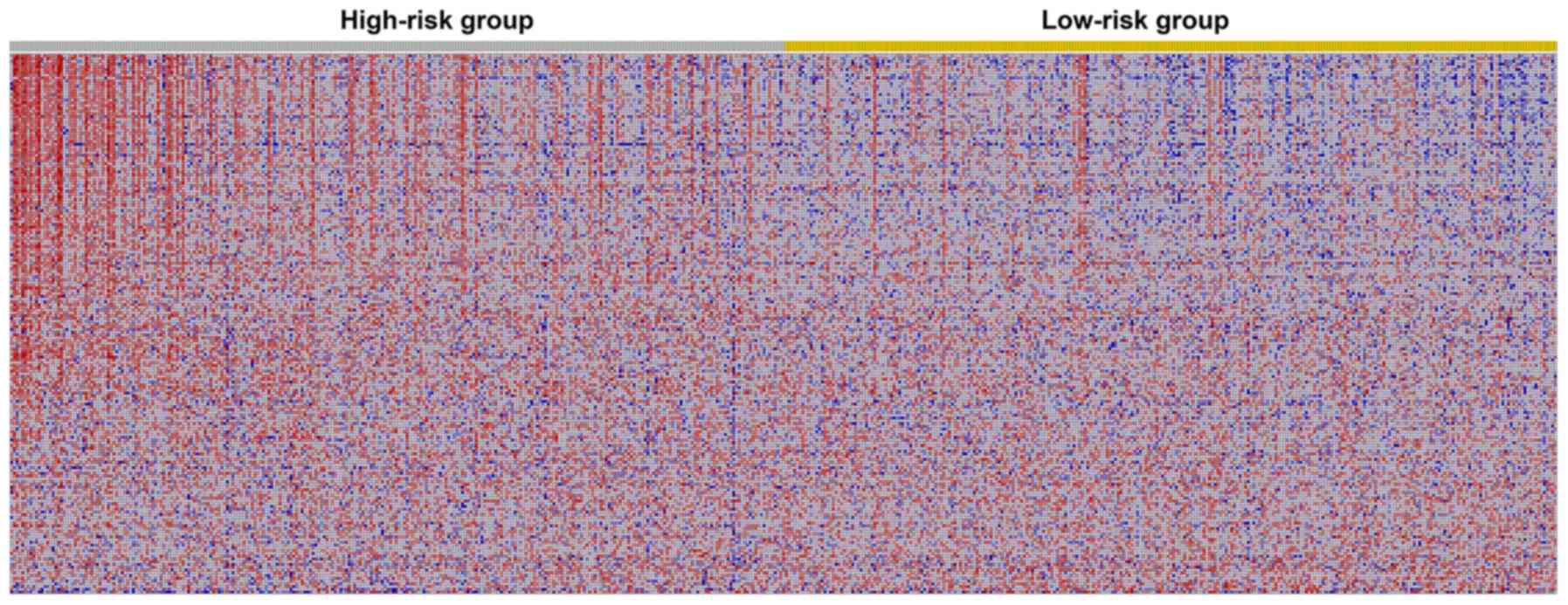
‘MYC_TARGETS_V1’ and ‘MYC_TARGETS_V2’. Fig. 8 shows the three most significant
hallmarks, and heatmaps of the G2M checkpoint revealed that genes
in the high-risk group differed greatly from genes in the low-risk
group (Fig. 9).
Validation of the autophagy-associated
risk score in other independent cohorts
In addition to the original testing cohort
(GSE24080), four independent cohorts met the inclusion criteria,
including GSE57317, GSE4581, GSE4452 and GSE4204. The expression
data of the 16 ARGs were extracted from these microarray datasets
and the prognostic signatures were calculated based on the
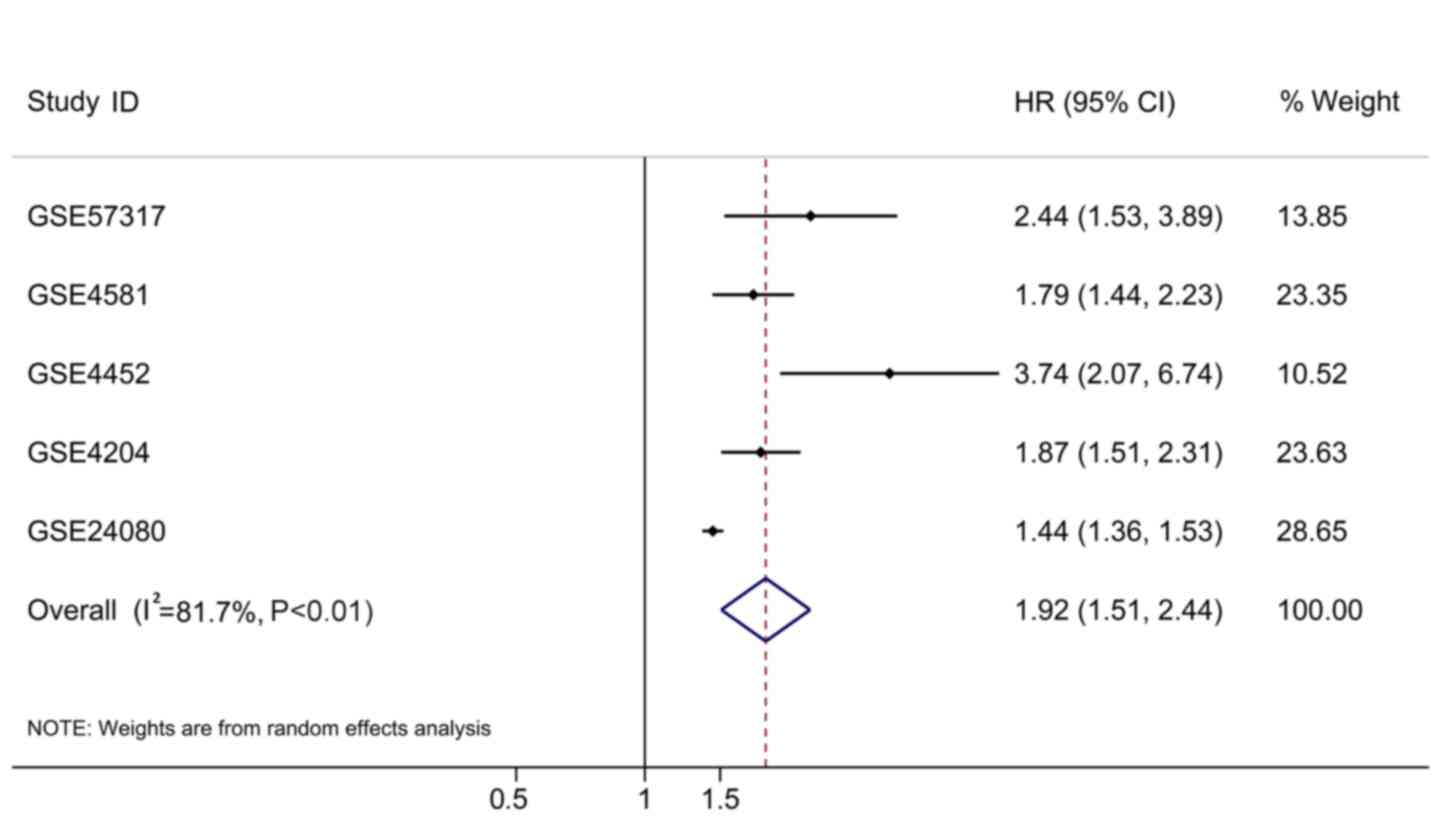
aforementioned formula. The HRs were all >1 (Table III). To have a comprehensive view
of the clinical role of the risk score based on all the rational
cases, the overall HR was 1.92 (95% CI, 1.51–2.44; P<0.01;
Table III; Fig. 10) with 1,631 cases, which supported
the findings from the testing cohort.
 | Table III.Validation of the autophagy-related
risk score in other independent cohorts. |
Table III.
Validation of the autophagy-related
risk score in other independent cohorts.
| GEO datasets | HR | LCI | UCI | P-value | Number of
samples | Platform |
|---|
| GSE57317 | 2.442 | 1.533 | 3.892 | <0.001 | 55 | GPL570 |
| GSE4581 | 1.793 | 1.438 | 2.235 | <0.001 | 414 | GPL570 |
| GSE4452 | 3.737 | 2.072 | 6.740 | <0.001 | 65 | GPL570 |
| GSE4204 | 1.867 | 1.508 | 2.312 | <0.001 | 538 | GPL570 |
| GSE24080 | 1.442 | 1.363 | 1.526 | <0.001 | 559 | GPL570 |
| Combination | 1.920 | 1.510 | 2.440 | <0.001 | 1,631 | NA |
Discussion
The role of autophagy in tumors is well known, and
the function of autophagy in the development and treatment of MM
has been previously reported (29–30).
However, the clinical significance of ARGs, particularly their
prognostic effect in MM has not been extensively studied.
Furthermore, there is no comprehensive analysis of the prognostic
significance of all ARGs. Therefore, in the present study, the
expression levels of ARGs were analyzed, and the prognostic value
of ARGs was subsequently examined. Finally, a prognostic model was
constituted using the prognostic ARGs. Furthermore, this model
could predict the prognosis of patients well and provided novel
ideas for clinical applications in MM. Given the clinical
significance of these prognostic ARGs in MM, if drugs could be used
to intervene in their expression, they may provide novel directions
for clinical MM treatment.
With the assistance of GEO GSE24080 from the MAQC-II
Project, the prognostic value of all 234 ARGs in MM was evaluated.
By performing univariate Cox analysis, 55 prognostic ARGs were
identified, which indicated that autophagy served an essential role
in the development of MM and could influence the outcome of
patients with MM. As, in addition to autophagy, the ARGs could have
multiple functions, GO and KEGG pathway analyses were also
conducted. Indeed, most pathways that were enriched were
autophagy-related pathways. Interestingly, certain other
annotations were identified, including ‘response to extracellular
stimulus’, ‘vacuolar membrane’ and ‘late endosome’. For KEGG
pathways, the ‘PI3K-Akt signaling pathway’ was identified. It has
been documented that there was a close association between PI3K-Akt
signaling and autophagy (53–55). In
general, activation of the PI3K-Akt signaling pathway may induce
autophagy in numerous types of cancer (53–58).
To assess the prognosis of patients with MM more
accurately, multivariate Cox regression analysis was used to
analyze prognostic significance of ARGs. The results narrowed the
scope of ARGs to 16. Due to the limited predictive power of
individual prognostic indicators, these ARGs were combined into a
prognostic assessment model. The prognostic model exhibited AUCs of
>0.7, suggesting that it had a moderate power to evaluate the
prognosis of patients with MM. The establishment of such a
prognostic index confirmed the role of autophagy for the
development and patient prognosis of MM; however, it also provided
novel biomarkers for clinical applications in MM. Notably, another
four independent cohorts were used to validate the results based on
the 559 patients from GSE24080, and the current ARG-based risk
score succeeded in yielding concordant prognostic values in
individual cohorts and when all cohorts were combined (1,631
cases).
To identify why these ARGs may hold any value for
the prognosis of MM, the mechanisms involved in the development of
MM were explored. However, among these 16 prognostic ARGs, 11
(ATIC, CALCOCO2, DNAJB1, DNAJB9, EVA1A, FKBP1B, GABARAP, NCKAP1,
PRKAR1A, SUPT20H and TM9SF1) have not been reported to be
associated with MM, to the best of our knowledge. Their roles in MM
are yet to be determined; however, several well-established ARGs
for MM, including HIF1A, EIF4EBP1, FOXO1, FOXO3 and BNIP3L were
identified as prognostic ARGs in the present study.
Among the five previously identified ARGs associated
with MM, HIF1A is the most widely studied (59,60).
Hypoxia, a central characteristic for cancer incidence and
progression, occurs when most types of cancer are evolving
(59–64). HIF1A is a hypoxia-inducible factor,
and constitutive expression of HIF1A in MM indicates that
suppression of HIF1A-mediated transcription could become a
favorable target for MM (65–68). For
instance, chetomin, an inhibitor of the HIF1A/p300 interaction, can
inhibit tumor cell growth of MM (69). Due to the function of HIF1A in
inducing autophagy, the suppressive effects of inhibitors of HIF1A
could exert their effect by modulating the autophagy of MM cells
(70–73). The expression levels of EIF4EBP1,
which is a target of mTOR, have been reported to be upregulated for
MM cases (74). As a master
regulator of protein synthesis control, phosphorylation of EIF4EBP1
has a close functional association with Myc and mTOR (75). In Myc-dependent tumor initiation and
maintenance of MM, the mTOR-dependent phosphorylation of EIF4EBP1
is required for tumor cell survival (75). This may explain the prognostic role
of EIF4EBP1 in MM, which, to the best of our knowledge, has not
been reported previously. FOXO1 has been reported to act as a tumor
suppressor for MM (76). The
activation of FOXO1 could subsequently inhibit the tumor growth and
induce cell autophagy and cell death (77–80).
FOXO3, another family member of the FOXO family, has been studied
in MM (81). In primary MM cells,
FOXO transcription factors are highly phosphorylated (81). The activation of FOXO3 has been
observed in response to thiadiazolidinone, a non-competitive
inhibitor of glycogen synthase kinase-3 (82). An increase in FOXO3a expression was
observed following treatment of MM cells with 4-chlorobenzoyl
berbamine, a novel berbamine derivative (83). The prognostic roles of FOXO1 and
FOXO3 have not yet been investigated, to the best of our knowledge,
and the present study demonstrated their prognostic value for MM.
As for BNIP3L, an increase in BNIP3L occurs when MM cells are
treated with panobinostat, a pan-histone deacetylase inhibitor used
to treat MM (84). However, its
prognostic value, to the best of our knowledge, has not been
documented. The activation of BNIP3L under drug treatment may
improve the understanding of its potential mechanism in the
development of MM. However, the molecular mechanism of these 16
prognostic ARGs in MM requires further exploration.
There were several limitations of the present study.
Firstly, of the 16 identified prognostic ARGs, 11 have not been
previously reported to be associated with MM. Their prognostic
value needs to be confirmed by other cohorts. Additionally, the
potential mechanism of these 11 ARGs in MM progression remains
unclear, which requires additional research. Secondly, the efficacy
of the prognostic model based on 16 ARGs identified in the present
study has to be validated using other independent samples.
Furthermore, multiple detection methods are also required for
validation of the results of the present study using clinical
samples. For instance, RNA-sequencing, microarray or reverse
transcription-quantitative PCR, are also promising starting points
for future research investigating ARGs in MM.
In conclusion, the present study focused on
autophagy, an important phenomenon for tumors, and extracted ARGs
with prognostic value for MM. Furthermore, an ARG-based MM
prognostic evaluation model, with moderate performance in
predicting the clinical outcome patients with MM, was constructed.
However, the results require validation, and the working principles
and molecular mechanisms of the ARGs in MM require additional
research.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Program of
Scientific and Technology Project (Guilin Science Research and
Technology Development; grant no. 2016012706-2), Sanming Project of
Medicine in Shenzhen (grant no. SZSM201602087), National Natural
Science Foundation of China (grant no. 81460038), Guangxi Natural
Science Foundation of China (grant no. 2017GXNSFAA198178) and
Shenzhen Futian Public Welfare Scientific Research Project (grant
nos. FTWS2017020 and FTWS2018005).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
FZ analyzed the data, generated figures and wrote
the manuscript. XW, HZ and ZHY performed bioinformatics analysis
and wrote the manuscript. ZZY made substantial contributions to the
design of the study, conducted data analysis and figure generation,
and wrote the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Boga JA, Caballero B, Potes Y,
Perez-Martinez Z, Reiter RJ, Vega-Naredo I and Coto-Montes A:
Therapeutic potential of melatonin related to its role as an
autophagy regulator: A review. J Pineal Res. 66:e125342018.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Guillaume JD, Celano SL, Martin KR and
MacKeigan JP: Determining the impact of metabolic nutrients on
autophagy. Methods Mol Biol. 1862:151–162. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liang ZG, Lin GX, Yu BB, Su F, Li L, Qu S
and Zhu XD: The role of autophagy in the radiosensitivity of the
radioresistant human nasopharyngeal carcinoma cell line CNE-2R.
Cancer Manag Res. 10:4125–4134. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhao Y, Onda K, Sugiyama K, Yuan B, Tanaka
S, Takagi N and Hirano T: Antitumor effects of arsenic disulfide on
the viability, migratory ability, apoptosis and autophagy of breast
cancer cells. Oncol Rep. 41:27–42. 2019.PubMed/NCBI
|
|
5
|
Jiao YN, Wu LN, Xue D, Liu XJ, Tian ZH,
Jiang ST, Han SY and Li PP: Marsdenia tenacissima extract
induces apoptosis and suppresses autophagy through ERK activation
in lung cancer cells. Cancer Cell Int. 18:1492018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sun T, Liu H and Ming L: Multiple roles of
autophagy in the sorafenib resistance of hepatocellular carcinoma.
Cell Physiol Biochem. 44:716–727. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wu JS, Li L, Wang SS, Pang X, Wu JB, Sheng
SR, Tang YJ, Tang YL, Zheng M and Liang XH: Autophagy is positively
associated with the accumulation of myeloid-derived suppressor
cells in 4-nitroquinoline-1-oxide-induced oral cancer. Oncol Rep.
40:3381–3391. 2018.PubMed/NCBI
|
|
8
|
Wu Y, Liu X, Qin Z, Hu L and Wang X:
Low-frequency ultrasound enhances chemotherapy sensitivity and
induces autophagy in PTX-resistant PC-3 cells via the endoplasmic
reticulum stress-mediated PI3K/Akt/mTOR signaling pathway. Onco
Targets Ther. 11:5621–5630. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhu J, Zhao B, Xiong P, Wang C, Zhang J,
Tian X and Huang Y: Curcumin induces autophagy via inhibition of
yes-associated protein (YAP) in human colon cancer cells. Med Sci
Monit. 24:7035–7042. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Duan X, Chen B, Cui Y, Zhou L, Wu C, Yang
Z, Wen Y, Miao X, Li Q, Xiong L and He J: Ready player one?
Autophagy shapes resistance to photodynamic therapy in cancers.
Apoptosis. 23:587–606. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Han Y, Fan S, Qin T, Yang J, Sun Y, Lu Y,
Mao J and Li L: Role of autophagy in breast cancer and breast
cancer stem cells (Review). Int J Oncol. 52:1057–1070.
2018.PubMed/NCBI
|
|
12
|
Ianniciello A, Rattigan KM and Helgason
GV: The Ins and outs of autophagy and metabolism in hematopoietic
and leukemic stem cells: Food for thought. Front Cell Dev Biol.
6:1202018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jacomin AC, Taillebourg E and Fauvarque
MO: Deubiquitinating enzymes related to autophagy: New therapeutic
opportunities? Cells. 7(pii): E1122018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jin S, Wei J, You L, Liu H and Qian W:
Autophagy regulation and its dual role in blood cancers: A novel
target for therapeutic development (Review). Oncol Rep.
39:2473–2481. 2018.PubMed/NCBI
|
|
15
|
Feldmann A, Bekbulat F, Huesmann H,
Ulbrich S, Tatzelt J, Behl C and Kern A: The RAB GTPase RAB18
modulates macroautophagy and proteostasis. Biochem Biophys Res
Commun. 486:738–743. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Han Q, Deng Y, Chen S, Chen R, Yang M,
Zhang Z, Sun X, Wang W, He Y, Wang F, et al: Downregulation of
ATG5-dependent macroautophagy by chaperone-mediated autophagy
promotes breast cancer cell metastasis. Sci Rep. 7:47592017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pajares M, Jimenez-Moreno N, Garcia-Yague
AJ, Escoll M, de Ceballos ML, Van Leuven F, Rábano A, Yamamoto M,
Rojo AI and Cuadrado A: Transcription factor NFE2L2/NRF2 is a
regulator of macroautophagy genes. Autophagy. 12:1902–1916. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang C, Wang H, Zhang D, Luo W, Liu R, Xu
D, Diao L, Liao L and Liu Z: Phosphorylation of ULK1 affects
autophagosome fusion and links chaperone-mediated autophagy to
macroautophagy. Nat Commun. 9:34922018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bednarczyk M, Muc-Wierzgon M, Waniczek D,
Fatyga E, Klakla K, Mazurek U and Wierzgoń J: Autophagy-related
gene expression in colorectal cancer patients. J Biol Regul Homeost
Agents. 31:923–927. 2017.PubMed/NCBI
|
|
20
|
Cao QH, Liu F, Yang ZL, Fu XH, Yang ZH,
Liu Q, Wang L, Wan XB and Fan XJ: Prognostic value of autophagy
related proteins ULK1, Beclin 1, ATG3, ATG5, ATG7, ATG9, ATG10,
ATG12, LC3B and p62/SQSTM1 in gastric cancer. Am J Transl Res.
8:3831–3847. 2016.PubMed/NCBI
|
|
21
|
Chen D, Chen J, Guo Y and Li Y:
Cinobufacini promotes apoptosis of bladder cancer cells by
influencing the expression of autophagy-related genes. Oncol Lett.
15:7104–7110. 2018.PubMed/NCBI
|
|
22
|
Li WL, Xiong LX, Shi XY, Xiao L, Qi GY and
Meng C: IKKβ/NFκBp65 activated by interleukin-13 targets the
autophagy-related genes LC3B and beclin 1 in fibroblasts
co-cultured with breast cancer cells. Exp Ther Med. 11:1259–1264.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lin P, He RQ, Dang YW, Wen DY, Ma J, He Y,
Chen G and Yang H: An autophagy-related gene expression signature
for survival prediction in multiple cohorts of hepatocellular
carcinoma patients. Oncotarget. 9:17368–17395. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lin P, He Y, Wen DY, Li XJ, Zeng JJ, Mo
WJ, Li Q, Peng JB, Wu YQ, Pan DH, et al: Comprehensive analysis of
the clinical significance and prospective molecular mechanisms of
differentially expressed autophagy-related genes in thyroid cancer.
Int J Oncol. 53:603–619. 2018.PubMed/NCBI
|
|
25
|
Ma Y, Zhang Y, Zhao Y, Wang X, Lin Y and
Ma A: Expression of autophagy-related genes in cerebrospinal fluid
of patients with tuberculous meningitis. Exp Ther Med.
15:4671–4676. 2018.PubMed/NCBI
|
|
26
|
Zheng LQ, Li SY and Li CX: Expression
profiling analysis of autophagy-related genes in perineural
invasion of cutaneous squamous cell carcinoma. Oncol Lett.
15:4837–4848. 2018.PubMed/NCBI
|
|
27
|
Moussay E, Kaoma T, Baginska J, Muller A,
Van Moer K, Nicot N, Nazarov PV, Vallar L, Chouaib S, Berchem G and
Janji B: The acquisition of resistance to TNFα in breast cancer
cells is associated with constitutive activation of autophagy as
revealed by a transcriptome analysis using a custom microarray.
Autophagy. 7:760–770. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang H, Lu X, Wang N, Wang J, Cao Y, Wang
T, Zhou X, Jiao Y, Yang L, Wang X, et al: Autophagy-related gene
expression is an independent prognostic indicator of glioma.
Oncotarget. 8:60987–61000. 2017.PubMed/NCBI
|
|
29
|
Gandolfi S, Prada CP and Richardson PG:
How I treat the young patient with multiple myeloma. Blood.
132:1114–1124. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Raje NS, Bhatta S and Terpos E: Role of
the RANK/RANKL pathway in multiple myeloma. Clin Cancer Res.
25:12–20. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhu B, Ju S, Chu H, Shen X, Zhang Y, Luo X
and Cong H: The potential function of microRNAs as biomarkers and
therapeutic targets in multiple myeloma. Oncol Lett. 15:6094–6106.
2018.PubMed/NCBI
|
|
32
|
Lu D, Yang C, Zhang Z, Cong Y and Xiao M:
Knockdown of Linc00515 inhibits multiple myeloma autophagy and
chemoresistance by upregulating miR-140-5p and downregulating
ATG14. Cell Physiol Biochem. 48:2517–2527. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ma R, Zhang Y, Wang W, Wu J, Yang Q, Xu W,
Jiang S, Han Y, Yu K and Zhang S: Inhibition of autophagy enhances
the antitumour activity of tigecycline in multiple myeloma. J Cell
Mol Med. 22:5955–5963. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mei H, Xiang Y, Mei H, Fang B, Wang Q, Cao
D, Hu Y and Guo T: Pterostilbene inhibits nutrient metabolism and
induces apoptosis through AMPK activation in multiple myeloma
cells. Int J Mol Med. 42:2676–2688. 2018.PubMed/NCBI
|
|
35
|
Su N, Wang P and Li Y: Role of
Wnt/β-catenin pathway in inducing autophagy and apoptosis in
multiple myeloma cells. Oncol Lett. 12:4623–4629. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zheng Z, Liu T, Zheng J and Hu J:
Clarifying the molecular mechanism associated with carfilzomib
resistance in human multiple myeloma using microarray gene
expression profile and genetic interaction network. Onco Targets
Ther. 10:1327–1334. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Desantis V, Saltarella I, Lamanuzzi A,
Mariggiò MA, Racanelli V, Vacca A and Frassanito MA: Autophagy: A
new mechanism of prosurvival and drug resistance in multiple
myeloma. Transl Oncol. 11:1350–1357. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yun Z, Zhichao J, Hao Y, Ou J, Ran Y, Wen
D and Qun S: Targeting autophagy in multiple myeloma. Leuk Res.
59:97–104. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shi L, Campbell G, Jones WD, Campagne F,
Wen Z, Walker SJ, Su Z, Chu TM, Goodsaid FM, Pusztai L, et al: The
MicroArray quality control (MAQC)-II study of common practices for
the development and validation of microarray-based predictive
models. Nat Biotechnol. 28:827–838. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ioannidis JPA: The proposal to lower P
value thresholds to .005. JAMA. 319:1429–1430. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ostaszewski M, Kieffer E, Danoy G,
Schneider R and Bouvry P: Clustering approaches for visual
knowledge exploration in molecular interaction networks. BMC
Bioinformatics. 19:3082018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yu G, Wang LG, Han Y and He QY:
clusterProfiler: An R package for comparing biological themes among
gene clusters. OMICS. 16:284–287. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
He RQ, Zhou XG, Yi QY, Deng CW, Gao JM,
Chen G and Wang QY: Prognostic signature of alternative splicing
events in bladder urothelial carcinoma based on spliceseq data from
317 cases. Cell Physiol Biochem. 48:1355–1368. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Liang L, Zeng JH, Qin XG, Chen JQ, Luo DZ
and Chen G: Distinguishable prognostic signatures of left- and
right-sided colon cancer: A study based on sequencing data. Cell
Physiol Biochem. 48:475–490. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lin P, He RQ, Ma FC, Liang L, He Y, Yang
H, Dang YW and Chen G: Systematic analysis of survival-associated
alternative splicing signatures in gastrointestinal
pan-adenocarcinomas. EBioMedicine. 34:46–60. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lin P, Wen DY, Li Q, He Y, Yang H and Chen
G: Genome-wide analysis of prognostic lncRNAs, miRNAs, and mRNAs
forming a competing endogenous RNA network in hepatocellular
carcinoma. Cell Physiol Biochem. 48:1953–1967. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yang H, Lin P, Wu HY, Li HY, He Y, Dang YW
and Chen G: Genomic analysis of small nucleolar RNAs identifies
distinct molecular and prognostic signature in hepatocellular
carcinoma. Oncol Rep. 40:3346–3358. 2018.PubMed/NCBI
|
|
48
|
Zhang R, Lin P, Yang X, He RQ, Wu HY, Dang
YW, Gu YY, Peng ZG, Feng ZB and Chen G: Survival associated
alternative splicing events in diffuse large B-cell lymphoma. Am J
Transl Res. 10:2636–2647. 2018.PubMed/NCBI
|
|
49
|
Lu X, Sun W, Tang Y, Zhu L, Li Y, Ou C,
Yang C, Su J, Luo C, Hu Y and Cao J: Identification of key genes in
hepatocellular carcinoma and validation of the candidate gene,
cdc25a, using gene set enrichment analysis, meta-analysis and
cross-species comparison. Mol Med Rep. 13:1172–1178. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ni Y, Song C, Jin S, Chen Z, Ni M, Han L,
Wu J and Jin Y: Gene set enrichment analysis: A genome-wide
expression profile-based strategy for discovering functional
microRNA-disease relationships. J Int Med Res. 46:596–611. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wang J, Vasaikar S, Shi Z, Greer M and
Zhang B: WebGestalt 2017: A more comprehensive, powerful, flexible
and interactive gene set enrichment analysis toolkit. Nucleic Acids
Res. 45:W130–W137. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zyla J, Marczyk M, Weiner J and Polanska
J: Ranking metrics in gene set enrichment analysis: Do they matter?
BMC Bioinformatics. 18:2562017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Han X, Zhong Z, Kou J, Zheng Y, Liu Z,
Jiang Y, Zhang Z, Gao Z, Cong L, Tian Y and Yang L: ROS Generated
by upconversion nanoparticle-mediated photodynamic therapy induces
autophagy via PI3K/AKT/mTOR signaling pathway in M1 peritoneal
macrophage. Cell Physiol Biochem. 48:1616–1627. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Li X, Huang Q, Wang M, Yan X, Song X, Ma
R, Jiang R, Zhao D and Sun L: Compound K inhibits
autophagy-mediated apoptosis through activation of the PI3K-Akt
signaling pathway thus protecting against Ischemia/reperfusion
injury. Cell Physiol Biochem. 47:2589–2601. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Liu M, Zhao G, Zhang D, An W, Lai H, Li X,
Cao S and Lin X: Active fraction of clove induces apoptosis via
PI3K/Akt/mTOR-mediated autophagy in human colorectal cancer HCT-116
cells. Int J Oncol. 53:1363–1373. 2018.PubMed/NCBI
|
|
56
|
Luo X, Ye S, Jiang Q, Gong Y, Yuan Y, Hu
X, Su X and Zhu W: Wnt inhibitory factor-1-mediated autophagy
inhibits Wnt/β-catenin signaling by downregulating dishevelled-2
expression in non-small cell lung cancer cells. Int J Oncol.
53:904–914. 2018.PubMed/NCBI
|
|
57
|
Wang J, Sun P, Chen Y, Yao H and Wang S:
Novel 2-phenyloxypyrimidine derivative induces apoptosis and
autophagy via inhibiting PI3K pathway and activating MAPK/ERK
signaling in hepatocellular carcinoma cells. Sci Rep. 8:109232018.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Yin S, Yang S, Pan X, Ma A, Ma J, Pei H,
Dong Y, Li S, Li W and Bi X: MicroRNA155 promotes ox-LDL-induced
autophagy in human umbilical vein endothelial cells by targeting
the PI3K/Akt/mTOR pathway. Mol Med Rep. 18:2798–2806.
2018.PubMed/NCBI
|
|
59
|
Daskalaki I, Gkikas I and Tavernarakis N:
Hypoxia and selective autophagy in cancer development and therapy.
Front Cell Dev Biol. 6:1042018. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Du L, Shen T, Liu B, Zhang Y, Zhao C, Jia
N, Wang Q and He Q: Shock wave therapy promotes cardiomyocyte
autophagy and survival during hypoxia. Cell Physiol Biochem.
42:673–684. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Liang Y, Chen X and Liang Z: MicroRNA-320
regulates autophagy in retinoblastoma by targeting hypoxia
inducible factor-1alpha. Exp Ther Med. 14:2367–2372. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Niu G, Zhu D, Zhang X, Wang J, Zhao Y and
Wang X: Role of hypoxia-inducible factors 1a (HIF1a) in SH-SY5Y
cell autophagy induced by oxygen-glucose deprivation. Med Sci
Monit. 24:2758–2766. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Wang H, Zhang D, Jia S, Huang S, Xiao L,
Ma L, Liu G, Gong K and Xu L: Effect of sustained hypoxia on
autophagy of genioglossus Muscle-derived stem cells. Med Sci Monit.
24:2218–2224. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Wang Z, Deng M, Liu Z and Wu S:
Hypoxia-induced miR-210 promoter demethylation enhances
proliferation, autophagy and angiogenesis of schwannoma cells.
Oncol Rep. 37:3010–3018. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Bosseler M, Marani V, Broukou A, Lequeux
A, Kaoma T, Schlesser V, François JH, Palissot V, Berchem GJ,
Aouali N and Janji B: Inhibition of HIF1a-dependent upregulation of
Phospho-l-Plastin resensitizes multiple myeloma cells to frontline
therapy. Int J Mol Sci. 19(pii): E15512018. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Coudre C, Alani J, Ritchie W, Marsaud V,
Sola B and Cahu J: HIF-1a and rapamycin act as gerosuppressant in
multiple myeloma cells upon genotoxic stress. Cell Cycle.
15:2174–2182. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Filippi I, Saltarella I, Aldinucci C,
Carraro F, Ria R, Vacca A and Naldini A: Different adaptive
responses to hypoxia in normal and multiple myeloma endothelial
cells. Cell Physiol Biochem. 46:203–212. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Muz B, Kusdono HD, Azab F, de la Puente P,
Federico C, Fiala M, Vij R, Salama NN and Azab AK: Tariquidar
sensitizes multiple myeloma cells to proteasome inhibitors via
reduction of hypoxia-induced P-gp-mediated drug resistance. Leuk
Lymphoma. 58:2916–2925. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Viziteu E, Grandmougin C, Goldschmidt H,
Seckinger A, Hose D, Klein B and Moreaux J: Chetomin, targeting
HIF-1a/p300 complex, exhibits antitumour activity in multiple
myeloma. Br J Cancer. 114:519–523. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Wang P, Long M, Zhang S, Cheng Z, Zhao X,
He F, Liu H and Ming L: Hypoxia inducible factor-1a regulates
autophagy via the p27-E2F1 signaling pathway. Mol Med Rep.
16:2107–2112. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Wang X, Wu TT, Jiang L, Rong D and Zhu YQ:
Deferoxamine-induced migration and odontoblast differentiation via
ROS-dependent autophagy in dental pulp stem cells. Cell Physiol
Biochem. 43:2535–2547. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Zhang W and Zhang J: Dexmedetomidine
preconditioning protects against lung injury induced by
ischemia-reperfusion through inhibition of autophagy. Exp Ther Med.
14:973–980. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Zhu SM, Rao T, Yang X, Ning JZ, Yu WM,
Ruan Y, Yuan R, Li CL, Jiang K, Hu W, et al: Autophagy may play an
important role in varicocele. Mol Med Rep. 16:5471–5479. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Seegmiller AC, Wang HY, Hladik C and Chen
W: Uniform expression of Notch1, suppressor of B-cell-specific gene
expression, in plasmablastic lymphoma. Arch Pathol Lab Med.
135:770–775. 2011.PubMed/NCBI
|
|
75
|
Pourdehnad M, Truitt ML, Siddiqi IN,
Ducker GS, Shokat KM and Ruggero D: Myc and mTOR converge on a
common node in protein synthesis control that confers synthetic
lethality in Myc-driven cancers. Proc Natl Acad Sci USA.
110:11988–11993. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Liu X, Zhang Y, Wang Z, Wang X, Zhu G, Han
G, Chen G, Hou C, Wang T, Shen B, et al: Metabotropic glutamate
receptor 3 is involved in B-cell-related tumor apoptosis. Int J
Oncol. 49:1469–1478. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Kinoshita S, Ri M, Kanamori T, Aoki S,
Yoshida T, Narita T, Totani H, Ito A, Kusumoto S, Ishida T, et al:
Potent antitumor effect of combination therapy with sub-optimal
doses of Akt inhibitors and pomalidomide plus dexamethasone in
multiple myeloma. Oncol Lett. 15:9450–9456. 2018.PubMed/NCBI
|
|
78
|
Kishino A, Hayashi K, Hidai C, Masuda T,
Nomura Y and Oshima T: XBP1-FoxO1 interaction regulates ER
stress-induced autophagy in auditory cells. Sci Rep. 7:44422017.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Miki Y, Tanji K, Mori F, Utsumi J, Sasaki
H, Kakita A, Takahashi H and Wakabayashi K: Autophagy mediators
(FOXO1, SESN3 and TSC2) in Lewy body disease and aging. Neurosci
Lett. 684:35–41. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Shen M, Cao Y, Jiang Y, Wei Y and Liu H:
Melatonin protects mouse granulosa cells against oxidative damage
by inhibiting FOXO1-mediated autophagy: Implication of an
antioxidation-independent mechanism. Redox Biol. 18:138–157. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
De Bruyne E, Bos TJ, Schuit F, Van
Valckenborgh E, Menu E, Thorrez L, Atadja P, Jernberg-Wiklund H and
Vanderkerken K: IGF-1 suppresses Bim expression in multiple myeloma
via epigenetic and posttranslational mechanisms. Blood.
115:2430–2440. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Zhou Y, Uddin S, Zimmerman T, Kang JA,
Ulaszek J and Wickrema A: Growth control of multiple myeloma cells
through inhibition of glycogen synthase kinase-3. Leuk Lymphoma.
49:1945–1953. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Shen JK, Du HP, Ma Q, Yang M, Wang YG and
Jin J: 4-Chlorobenzoyl berbamine, a novel berbamine derivative,
induces apoptosis in multiple myeloma cells through the IL-6 signal
transduction pathway and increases FOXO3a-Bim expression. Oncol
Rep. 30:425–432. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Liu XF, Zhou Q, Hassan R and Pastan I:
Panbinostat decreases cFLIP and enhances killing of cancer cells by
immunotoxin LMB-100 by stimulating the extrinsic apoptotic pathway.
Oncotarget. 8:87307–87316. 2017.PubMed/NCBI
|