Esophageal cancer (EC) is the sixth leading cause of
cancer-associated mortality worldwide due to its highly aggressive
nature and poor prognosis (1).
Although the incidence of esophageal adenocarcinoma and
esophagogastric junctional carcinoma has increased in the United
States and Europe, esophageal squamous cell carcinoma (ESCC) still
accounts for ~78% of EC cases (2,3). At
present, the standard therapy for ESCC includes surgery,
radiotherapy and chemotherapy (4).
Despite the use of multidisciplinary therapies, the prognosis of
patients with ESCC remains poor. The overall survival (OS) rate at
5 years is only 30–40% for ESCC cases due to primary site tumor
recurrence, metastasis development and treatment complications
(5). It is therefore crucial to
determine novel and effective treatment strategies for ESCC.
Recently, the application of next-generation
sequencing in ESCC allowed for the identification of several
processes that may contribute to carcinogenesis and disease
prognosis, including driver gene mutations, changes in molecular
and protein dynamics, dysregulation of cellular signaling pathways
and alterations of the tumor microenvironment (6–8).
Furthermore, it has been demonstrated that molecular targeted
therapy can provide effective treatment in several types of cancer,
including lung cancer and colon cancer (9,10).
However, the benefits from this type of therapy on the development
of locally advanced and metastatic ECs is lower than expected
(11–14). The successful use of
immune-checkpoint inhibitors (ICI), including monoclonal antibodies
against programmed cell death 1 (PD-1), has considerably improved
the prognosis of various types of malignancy, including melanoma
and non-small cell lung cancer (15,16).
Previous clinical trials reported promising antitumor activity of
anti-PD-1-mAb in the treatment of ESCC (17–19). In
addition, numerous phase II/III clinical trials examined whether
combining immunotherapy with radiotherapy (RT) could enhance
anti-tumor effects (20,21). However, the application of combined
therapy in cancer requires further investigation.
At present, RT remains the main treatment for ESCC.
RT results in a significant reduction in local tumor growth and
simultaneously relieves dysphagia (22,23). The
use of ionizing radiation on local tumor cells leads to direct or
indirect DNA damage and induces a series of molecular events
associated with cell death (24). It
has been suggested that the combination of RT and immunotherapy can
enhance treatment efficacy in non-small cell lung cancer and
melanoma brain metastases (25).
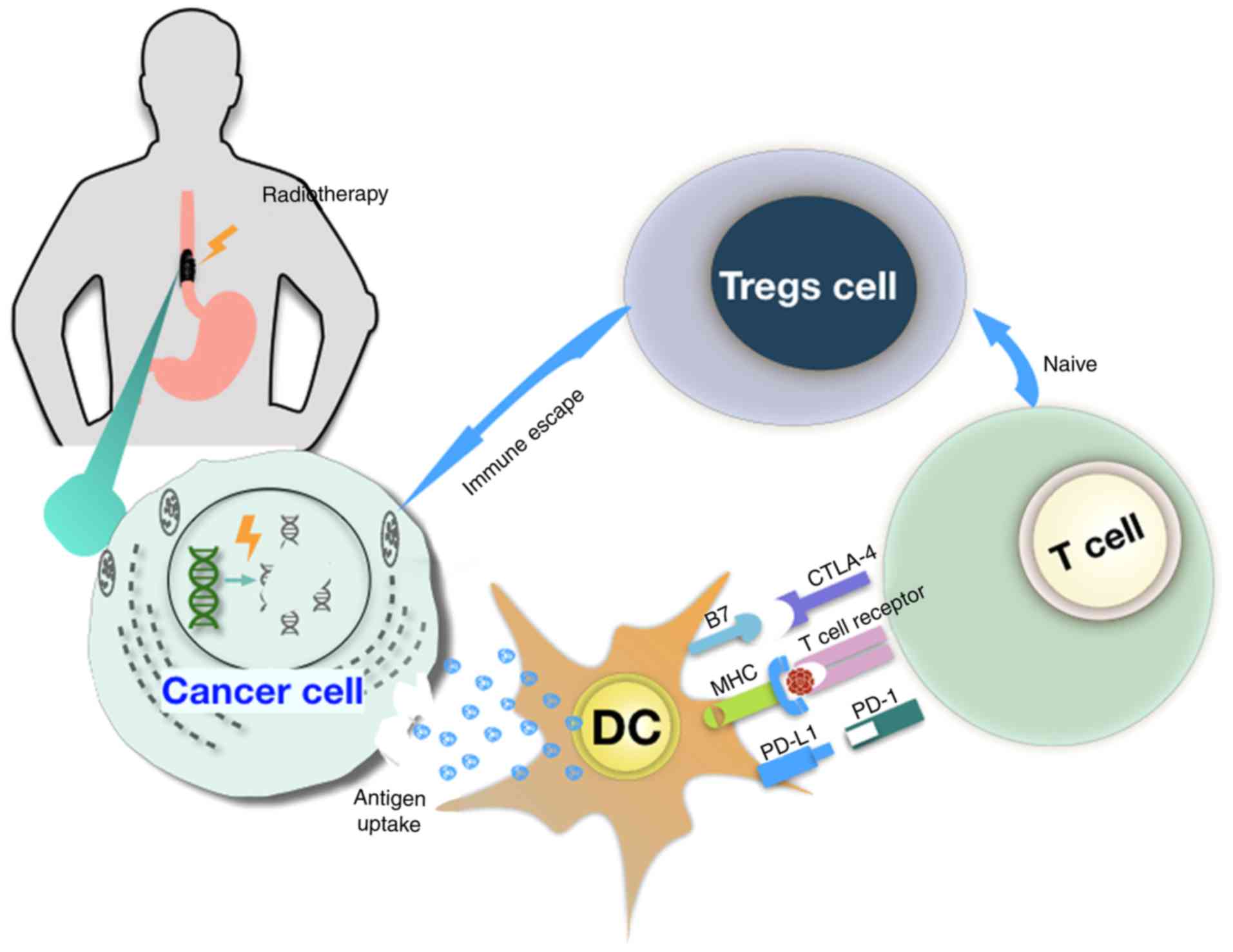
Tumor cells release tumor-associated antigens and cytokines,
including interferon-γ and tumor necrosis factor-α, which modulate
the tumor immune microenvironment and subsequently target dendritic
cells (DC) (26,27). This phenomenon increases the
expression of molecules of the major histocompatibility complex I
and causes the upregulation of programmed death ligand 1 (PD-L1) in
dendritic cells (26,27). However, RT also accelerates the
production of regulatory T cells (Tregs) in systemic and
intratumoral sites where Tregs acquire subsequently a highly
suppressive phenotype (28).
Subsequently, reduced radiation-induced tumor death can contribute
to tumor escape from the host immune surveillance, and can suppress
the antitumor immune response (29,30)
(Fig. 1).
Numerous studies have demonstrated that PD-L1 tumor
expression is associated with disease prognosis in patients with
ESCC. The majority of these studies reported that high expression
of PD-L1 is associated with poor prognosis in these patients
(36–40). Wang et al (38) recruited 180 patients with ESCC and
reported that patients with high expression of PD-L1 exhibited
worse clinical outcome compared with patients with low expression
(P=0.0010). In addition, this study by Wang et al (38) reported that the number of
CD8+ T cells is lower in ESCC tissues compared with
normal tissues (P=0.0346), and the results suggested that PD-L1
expression may be considered as a predictive factor for OS
(P=0.0114). A meta-analysis came to a similar conclusion, with the
results suggesting that PD-L1 overexpression is associated with
unfavorable outcomes and lower OS in patients with ESCC, notably in
Eastern Asian countries such as China, Japan and South Korea
[hazard ratio=1.43; 95% confidence interval (CI)=1.10–1.88]
However, a limited number of studies reported that increased PD-L1
expression is associated with improved disease-free survival and OS
(41,42). This controversy may be attributed to
numerous factors, including different methodological approaches,
different assessment criteria to define high PD-L1 expression and
heterogeneity of PD-L1 expression. These factors may result in
differing detection of infiltrating lymphocytes in tumor from the
biopsy or the postoperative pathological specimens. However,
staining cut-off values tumor proportion score (TPS) of 1 or 5% are
frequently used to define the positive rate of PD-L1 expression.
Various studies have defined the cut-off values differently.
Borghaei et al (43) and
Katsuya et al (44) defined a
positive tumor PD-L1 protein expression as an incidence of TPS ≥1%,
whereas other studies used TPS ≥5% as the threshold (45–47).
At present, anti-PD-1 agents are approved by the
Food and Drug Administration (FDA) for the treatment of melanoma
and non-squamous cell lung cancer (48). Numerous anti-PD-1 antibodies,
including pembrolizumab and nivolumab, anti-PD-L1 antibodies,
including durvalumab, have demonstrated promising antitumor
activity in advanced ESCC (Table I).
The multicohort KEYNOTE-028 study investigated the use of
pembrolizumab monotherapy for the treatment of advanced esophageal
carcinoma (49). Preliminary results
reported that 41% of patients had PD-L1 upregulation in tumors, and
among these patients, the objective response rate (ORR) was 23%
(49). Updated versions of this
study reported promising antitumor effects of pembrolizumab
monotherapy, with a response rate of 28% (5/18) for tumors
exhibiting squamous histology, and a partial response of 30% for
the samples (50,51). In addition, the median duration of
response was 15 months (range, 6 to ≥26 months), the OS was 7
months, and the median progression free survival (PFS) was 1.8
months. In a phase II study using nivolumab administration for
refractory ESCC, the median follow-up was 10.8 months (18). Another ongoing clinical trial,
KEYNOTE 181, is currently evaluating the efficacy of an anti-PD-1
mAb in disease progression of patients with advanced ESCC following
chemotherapy as first-line therapy (52). The preliminary results demonstrated
that immune-related adverse events included rash (13%), decreased
appetite (9%) and decreased lymphocyte count (9%). No
treatment-associated mortality was reported (52). The KEYNOTE-180 study evaluated the
efficacy of pembrolizumab in patients with metastatic EC, including
ESCC (53). A total of 121 patients
were enrolled in this trial, including 63 patients (52%) with ESCC
and 58 patients (48%) with ESCS and PD-L1 overexpression. The
results revealed that the median PFS was 2 months (95% CI, 1.9–2.1)
and the ORR was 14% (95% CI, 5–17%) in patients with high PD-L1
expression. The comparison of the two trials revealed that the
number of adverse events reported in the KEYNOTE-180 study was
higher than in the KEYNOTE-028 study. In the latter trial,
treatment-related grade 3–5 adverse events were observed in 15
patients (12%). Among these patients, 5 patients (4%) discontinued
treatment and 1 patient died due to treatment-associated
pneumonitis.
A previous similar study (ONO-4538-07) reported that
nivolumab therapy had promising activity in patients with ESCC who
were refractory or intolerant to chemotherapy (54). The results, based on a 2 year
follow-up, reported that the ORR was 17.2% (95% CI, 9.9–28.2) and
that 3 patients had a complete response (54). The toxicity observed with nivolumab
was higher than perbrolizumab, and the majority of the patients
exhibited grade 3–4 adverse events following nivolumab treatment. A
total of 7 patients (10.8%) discontinued the treatment due to
drug-associated adverse events. A recent study, CHECKMATE-032
(ClinicalTrials.gov Identifier code,
NCT01928394), demonstrated that the combination of nivolumab and
ipilimumab significantly improved the median OS and ORR of patients
with advanced EC (55). However,
this combined treatment resulted in higher toxicity compared with
PD1 or PD-L1 blockade as monotherapy, with adverse effects
including diarrhea and increased levels of alanine aminotransferase
and aspartate transaminase in the serum (55).
A recent ambitious trial investigated pembrolizumab
as an alternative treatment for the second-line strategy reported
in the KEYNOTE-181 study (52). A
phase III clinical trial (KEYNOTE-590/MK-3475-590) will assess the
efficacy of two different groups
[pembrolizumab+cisplatin+5-fluorouracil (5-FU) vs.
placebo+cisplatin+5-FU] in patients with esophageal neoplasms. The
two treatments will be compared with pembrolizumab, which is used
as the first-line treatment for locally advanced or metastatic EC.
Early results of PFS and OS of patients are expected in 2021.
Furthermore, preliminary results from clinical trials demonstrated
that other anti-PD-L1 mAbs, including avelumab, durvalumab
(56) and atezolizumab, have some
potential antitumor activity in patients with previously-treated
advanced gastric/gastroesophageal junction/esophageal (G/GEJ/E)
cancers (57–59). In addition, avelumab treatment
resulted in a similar ORR (15%) compared with findings from Taieb
et al (60).
The association between RT efficacy and the
immunomodulatory effects on metastatic carcinoma cells was
initially described by Mole in 1953 as the ‘abscopal’ effect
(61). In this phenomenon, local
tumor irradiation can cause metastasis regression in sites distant
from the irradiated area, this rare abscopal effect has only been
reported for a few metastatic solid tumors following radiotherapy
treatment (62,63). Previous studies reported that the
combination of immunotherapy with RT has additional efficacy in
solid tumors (25,64–66).
Retrospective studies, including 23 case reports of lymphoma and
solid malignancy, revealed that the combination of RT and
immunotherapy can enhance treatment efficacy (67,68).
Furthermore, clinical studies reported an abscopal effect from
primary tumor cell irradiation in metastatic carcinoma (69–71).
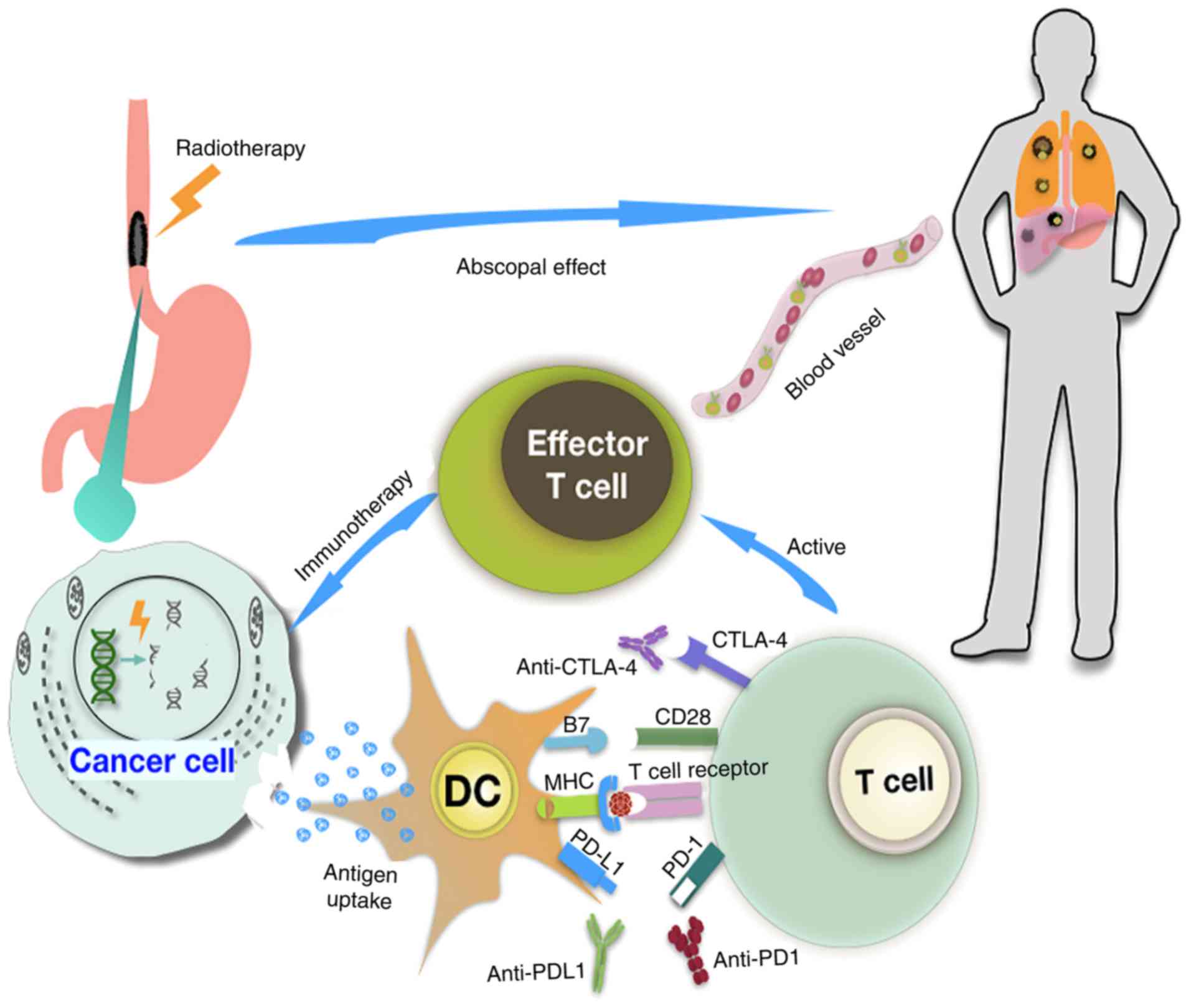
Tumor-associated antigens are released from tumor cells following
exposure to radiation, and are taken up by APCs, which cause
priming and activation of cytotoxic T cells. Radiation can
therefore stimulate antitumor immunity (72). The combination of radiation and ICI
can thus have a direct cytotoxic effect on tumor cells and further
activate effector T cells, enhancing the immune surveillance of
tumor cells. This process also promotes the recruitment and
infiltration of immune cells in the tumor, and stimulates the
recognition and killing of tumor cells by the immune system
(Fig. 2) (27).
The combination of irradiation and anti-PD-L1
treatment synergistically promotes antitumor activity in
vitro (73,74). In mouse models, RT induces
upregulation of PD-L1 expression in DCs and promotes antigen
cross-presentation in tumor-draining lymph nodes (75,76).
Pre-clinical results demonstrated that irradiated effector T cells
induce a decrease in the number of PD-L1-pexpressing tumor cells,
which suggests that combinating RT with anti-PD-L1 mAbs may enhance
the antitumor effects of RT as monotherapy (77). Accumulating clinical evidence has
demonstrated that 2–3 courses of combination therapy (checkpoint
inhibitors with RT) has promising potential and is a well-tolerated
treatment in patients with various types of locally advanced or
metastatic malignancy, including non-small cell lung cancer
(NSCLC), melanoma and renal cell carcinoma (78,79). A
previous study reported that administration of nivolumab in 26
patients with metastatic brain melanoma (BM) during or after RT
resulted in an increased 1-year OS rate of 55% and a median OS of
11.8 months (80). A previous study
investigated 75 patients with BM who were treated concurrently or
at different time points with RT and pembrolizumab, nivolumab or
ipilimumab (81). The results
demonstrated that concurrent treatment improved the volume
reduction of the lesion compared with non-concurrent treatment
after 3 and/or 6 months treatment. In addition, the median
percentage of the lesion volume was reduced to a greater extent
following anti-PD-L1 treatment compared with anti-cytotoxic
T-lymphocyte-associated protein 4 (CTLA-4) treatment (81). A retrospective study reviewed
consecutive patients with metastatic NSCLC and melanoma. Among the
59 patients who received radiation and anti-PD-1 therapy, 25
patients continued to receive PD-1 inhibition treatment for a
median of 238 additional days (82).
The majority of studies have reported that the high
expression of PD-L1 in EC is associated with poor treatment outcome
(87). Patients with high PD-L1
expression tend to respond well to anti-PD1/PD-L1 mAbs and exhibit
a significant increase in OS rate (88). These results suggest that PD-L1
expression may be used as a predictive biomarker for suitablility
of anti-PD1/PD-L1 treatment. Recently, PD-L1 status has been used
to evaluate the number of circulating tumor cells (CTCs) in breast
cancer (89). Furthermore, previous
studies have demonstrated that PD-L1 is associated with the number
of CTCs present in advanced NSCLC, and that the combination of
PD-L1 status and CTC number can be used as a potential noninvasive
biopsy to evaluate disease progression (90–92). A
previous study revealed that the abundance of CTCs with high
expression of PD-L1 at baseline could be used as a predictor of
immunotherapy response in advanced solid tumors, including EC
(93). It is therefore crucial to
develop a highly sensitive, accessible and reliable assay for the
evaluation of PD-L1 expression, and for the detection of PD-L1
status in CTCs (94). PD-L1
expression is a potential biomarker for determining the feasibility
of immunotherapy. Further investigation is required to confirm the
correlation of PD-L1 expression in CTCs.
In 2016, the FDA approved the use of checkpoint
inhibitors, including pembrolizumab and nivolumab, in the treatment
of recurrent or metastatic head and neck squamous cell carcinoma
(HNSCC) due to their antitumor efficacy and safety (95). Patients with HNSCC may also develop
EC, as these tumors share a common origin and clonal expansion
(96). ESCC gene expression was
similar to the classical subtype described in The Cancer Genome
Atlas studies of HNSCC, which possess similar somatic alterations
(97). Furthermore, numerous in
vitro studies reported a significant antitumor effect of
checkpoint inhibitors in esophageal cell lines (98). Additional clinical trials are
therefore required to fully examine the OS rate, tumor response and
toxicity caused by immunotherapy in ESCC. At present, ICB is used
as a salvage therapy following treatment failure, disease relapse
or metastasis in ESCC due to ineffective chemoradiation and/or
unsuccessful surgery. The combination of immunotherapy and
radiation has been reported to enhance the antitumor effect
compared to treatment with only one of the two (27,99,100).
Immunotherapy can influence the tumor microenvironment (101,102)
and the tumor-associated blood and lymphatic vasculature (103,104),
and can further improve local oxygen and nutritional conditions
(105). These elements and changes
in the surrounding stromal cells can markedly influence the
efficacy of radiation (106).
Immunotherapy can therefore be a potential sensitizing agent for
RT.
At present, the evaluation of clinical response
following CRT is determined by Response Evaluation Criteria in
Solid Tumors (RECIST) (107).
Additional criteria that have not been included in RECIST are used
for immunotherapy evaluation, and are designated as immune-Related
Response Criteria (108). When T
cells are recruited to tumors, they may increase the tumor volume
as a result of immunotherapy, a process termed ‘pseudoprogression’
(109,110). Therefore, to accurately evaluate
the tumor response to the combination of RT and immunotherapy,
additional evidence is required from in vitro functional
studies and clinical trials.
The majority of common immune-related adverse events
occur in the gastrointestinal tract, endocrine glands, skin and
liver (111). A treatment-related
patient death occurred due to pneumonia in the KEYNOTE-180 trial
(53). Furthermore, the grade 3–4
adverse events in the KEYNOTE-180 trial were significantly higher
than in the KEYNOTE-028 trial (49),
which may be due to the different inclusion criteria. The
KEYNOTE-180 trial included disease progression of patients treated
with chemotherapy and/or RT. The results demonstrated that patient
organs, including heart, lung, liver, bone marrow and
gastrointestinal tract, suffered considerable tissue damage
following immunosuppression. The patients further exhibited reduced
healing abilities, although results from blood tests and
radiographs were normal. It has been demonstrated that radiation
can lead to T lymphocyte inactivation at a dose of 2 Gy/fraction
(112). A previous study reported
that the incidence of grade 4 absolute lymphocyte count was 27% in
patients with EC treated with chemoradiation therapy (113). Ongoing clinical trials include
concurrent CRT combined with immunotherapy as neoadjuvant treatment
for ESCC. The radiation-related adverse events of concurrent CRT
including early radiation-induced esophagitis, cardiac toxicity,
radiation-associated pneumonia and whole blood cell reduction
(114). Furthermore, the extent of
adverse events can be increased if the chemotherapy is provided
concomitantly with RT. Esophageal perforation is one of these
adverse events, which is a rare and life-threatening event
(115). Additional mechanistic
studies and clinical trials are therefore required.
The delivery of the radiation optimal dose is
unclear when administered in combination with immunotherapy.
Definitive CRT is the established treatment of choice in advanced
ESCC, and the maximum dose of 60 Gy is considered feasible to limit
side effects in patients (116).
The combination of neoadjuvant CRT with immunotherapy can be used
following surgery, with a radiation dose of 44.1 Gy in 21 fractions
(117). A previous study reported a
radiation treatment at 50.4 Gy in 28 fractions (118). Concurrent RT (41.4 Gy in 23
fractions, 5 days per week) followed by surgery is also used as a
protocol in EC treatment strategies (119). Optimizing the RT parameters and
dose, clinical methodology, fraction number and duration with the
course of immunotherapy in order to maximize antitumor effects and
minimize the adverse events is therefore very challenging. The
insights highlighted in this review suggest that immunotherapy can
be applied to patients with ESCC, and that a combination of
multiple strategies may be the future direction of treatment.
Not applicable.
This review was supported by the Chongqing Health
and Family Planning Committee Science Foundation of China (grant
no. 2017ZBXM004).
Not applicable.
TZ and LSW participated in the conception and design
of the manuscript. XYL wrote the manuscript. CYL, TZ and JFH
critically reviewed the manuscript for important intellectual
content. All authors have read and approved the final version of
the manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Arnold M, Soerjomataram I, Ferlay J and
Forman D: Global incidence of esophageal cancer by histological
subtype in 2012. Gut. 64:381–387. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Edgren G, Adami HO, Weiderpass E and Nyrén
O: A global assessment of the oesophageal adenocarcinoma epidemic.
Gut. 62:1406–1414. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kitagawa Y, Uno T, Oyama T, Kato K, Kato
H, Kawakubo H, Kawamura O, Kusano M, Kuwano H, Takeuchi H, et al:
Esophageal cancer practice guidelines 2017 edited by the Japan
Esophageal Society: Part 1. Esophagus. 16:1–24. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lin DC, Wang MR and Koef HP: Genomic and
epigenomic aberrations in esophageal squamous cell carcinoma and
implications for patients. Gastroenterology. 154:374–389. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sawada G, Niida A, Uchi R, Hirata H,
Shimamura T, Suzuki Y, Shiraishi Y, Chiba K, Imoto S, Takahishi Y,
et al: Genomic landscape of esophageal squamous cell carcinoma in a
Japanese population. Gastroenterology. 150:1171–1182. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Talukdar FR, di Pietro M, Secricer M,
Moehler M, Goepgert K, Lima SSC, Pinto LFR, Hendricks D, Parker MI
and Herceg Z: Molecular landscape of esophageal cancer:
Implications for early detection and personalized therapy. Ann NY
Acad Sci. 1434:342–359. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pakkala S and Ramalingam SS: Personalized
therapy for lung cancer: Striking a moving target. JCI Insight.
3:1208582018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ducreux M, Chamseddine A, Laurent-Puig P,
Smolenschi C, Hollebecque A, Dartigues P, Samallin E, Boige V,
Malka D and Gelli M: Molecular targeted therapy of BRAF-mutant
colorectal cancer. Ther Adv Med Oncol. 11:17588359198564942019.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lam KO and Kwong DLW: Target therapy for
esophageal adenocarcinoma. Methods Mol Biol. 1756:51–65. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Thuss-Patience PC, Shah MA, Ohtsu A, Van
Cutsem E, Ajani JA, Castro H, Mansoor W, Chung HC, Bodoky G,
Shitara K, et al: Trastuzumab emtansine versus taxane use for
previously treated HER2-positive locally advanced or metastatic
gastric or gastro-esophageal junction adenocarcinoma (GATSBY): An
international randomised, open-label, adaptive, phase 2/3 study.
Lancet Oncol. 18:640–653. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cunningham D, Tebbutt NC and Davidenko I:
Phase III, randomized, double-blind, multicenter, placebo
(P)-controlled trial of rilotumumab (R) plus epirubicin, cisplatin
and capecitabine (ECX) as first-line therapy in patients (pts) with
advanced MET-positive (pos) gastric or gastresophageal junction
(G/GEJ) cancer: RILOMET-1 study. J Clin Oncol. 33 (Suppl
15):40002017. View Article : Google Scholar
|
|
14
|
Hecht JR, Bang YJ, Qin SK, Chung HC, Xu
JM, Park JO, Jeziorski K, Shparyk Y, Hoff PM, Sobrero A, et al:
Lapatinib in combination with capecitabine plus oxaliplatin in
Human Epidermal Growth Factor Receptor 2-positive advanced or
metastatic gastric, esophageal, or gastresophageal adenocarcinoma:
TRIO-013/LOGiC-A randomized phase III trial. J Clin Oncol.
34:443–451. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Karlsson AK and Saleh SN: Checkpoint
inhibitors for malignant melanoma: A systematic review and
meta-analysis. Clin Cosmet Investig Dermatol. 10:325–339. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Herzberg B, Campo MJ and Gainor JF: Immune
checkpoint inhibitors in non-small cell lung cancer. Oncologist.
22:81–88. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Raufi AG and Klempner SJ: Immunotherapy
for advanced gastric and esophageal cancer: Preclinical rationale
and ongoing clinical investigations. J Gastrointest Oncol.
6:561–569. 2015.PubMed/NCBI
|
|
18
|
Kudo T, Hamamoto Y, Kato K, Ura T, Kojima
T, Tsushima T, Hironaka S, Hara H, Satoh T, Iwasa S, et al:
Nivolumab treatment for oesophageal squamous-cell carcinoma: An
open-label, multicentre, phase 2 trial. Lancet Oncol. 18:631–639.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kojima T and Doi T: Immunotherapy for
esophageal squamous cell carcinoma. Curr Oncol Rep. 19:332017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kang J, Demaria S and Formenti S: Current
clinical trials testing the combination of immunotherapy with
radiotherapy. J Immunother Cancer. 4:512016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Weichselbaum RR, Liang H, Deng L and Fu
YX: Radiotherapy and immunotherapy: A beneficial liaison? Nat Rev
Clin Oncol. 14:365–379. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Prasad NR, Karthigeyan M, Vikram K,
Parthasarathy R and Reddy KS: Palliative radiotherapy in esophageal
cancer. Indian J Surg. 77:34–38. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Matuschek C, Bölke E, Zahra T, Knoefel WT,
Peiper M, Budach W, Erhardt A, Scherer A, Baldus SE, Gerber PA, et
al: Gattermann N, Orth K. Trimodal therapy in squamous cell
carcinoma of the esophagus. Eur J Med Res. 16:437–444. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Baskar R, Dai J, Wenlong N, Yeo R and Yeoh
KW: Biological response of cancer cells to radiation treatment.
Front Mol Biosci. 1:242014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gong J, Le TQ, Massarelli E, Hendifar AE
and Tuli R: Radiation therapy and PD-1/PD-L1 blockade: The clinical
development of an evolving anticancer combination. J Immunother
Cancer. 6:462018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wan S, Pestka S, Jubin RG, Lyu YL, Tsai YC
and Liu LF: Chemotherapeutics and radiation stimulate MHC class I
expression through elevated interferon-beta signaling in breast
cancer cells. PLoS One. 7:e325422012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang Y, Deng W, Li N, Neri S, Sharma A,
Jiang W and Lin SH: Combining immunotherapy and radiotherapy for
cancer treatment: Current challenges and future directions. Front
Pharmacol. 9:1852018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Persa E, Balogh A, Sáfrány G and Lumniczky
K: The effect of ionizing radiation on regulatory T cells in health
and disease. Cancer Lett. 368:252–261. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Facciabene A, Motz GT and Coukos G:
T-regulatory cells: Key players in tumor immune escape and
angiogenesis. Cancer Res. 72:2162–2171. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu S and Sun X, Luo J, Zhu H, Yang X, Guo
Q, Song Y and Sun X: Effects of radiation on T regulatory cells in
normal states and cancer: Mechanisms and clinical implications. Am
J Cancer Res. 5:3276–3285. 2015.PubMed/NCBI
|
|
31
|
Tang J, Shalabi A and Hubbard-Lucey VM:
Comprehensive analysis of the clinical immune-oncology landscape.
Ann Oncol. 29:84–91. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pardoll DM: The blockade of immune
checkpoints in cancer immunotherapy. Nat Rev Cancer. 12:252–264.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Riaz N, Havel JJ, Makarov V, Desrichard A,
Urba WJ, Sims JS, Hodi FS, Marin-Algarra S, Mandal R, Sharfman WH,
et al: Tumor and microenvironment evoluation during immunotherapy
with nivolumab. Cell. 171:934–949.e16. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kono K, Mimura K, Yamada R, Ujiie D,
Hayase S, Tada T, Hanayama H, Min AKT, Shibata M, Momma T, et al:
Current status of cancer immunotherapy for esophageal squamous cell
carcinoma. Esophagus. 15:1–9. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Memarnejadian A, Meilleur CE, Shaler CR,
Khazaie K, Bennink JR, Schell TD and Haeryfar SMM: PD-1 blockade
promotes epitope spreading in anticancer CD8+ T cell
responses by preventing fratricidal death of subdominant clones to
relieve immunodomination. J Immunol. 199:3348–3359. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tanaka K, Miyata H, Sugimura K, Kanemura
T, Hamada-Uematsu M, Mizote Y, Yamasaki M, Wada H, Nakajima K,
Takiguchi S, et al: Negative influence of programmed
death-1-ligands on the survival of esophageal cancer patients
treated with chemotherapy. Cancer Sci. 107:726–733. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen L, Deng H, Lu M, Xu B, Wang Q, Jiang
J and Wu C: B7-H1 expression associates with tumor invasion and
predicts patient's survival in human esophageal cancer. Int J Clin
Exp Pathol. 7:6015–6023. 2014.PubMed/NCBI
|
|
38
|
Wang X, Teng F, Kong L and Yu J: PD-L1
expression in human cancers and its association with clinical
outcomes. Onco Targets Ther. 9:5023–5039. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liu Y, Cheng Y, Xu Y, Wang Z, Du X, Li C,
Peng J, Gao L, Liang X and Ma C: Increased expression of programmed
cell death protein 1 on NK cells inhibits NK-cell-mediated
anti-tumor function and indicates poor prognosis in digestive
cancers. Oncogene. 36:6143–6153. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Leng C, Li Y, Qin J, Ma J, Liu X, Cui Y,
Sun H, Wang Z, Hua X, Yu Y, et al: Relationship between expression
of PD-L1 and PD-L2 on esophageal squamous cell carcinoma and the
antitumor effects of CD8+ T cells. Oncol Rep.
35:699–708. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hatogai K, Kitano S, Fujii S, Kojima T,
Daiko H, Nomura S, Yoshino T, Ohtsu A, Takiguchi Y, Doi T and
Ochiai A: Comprehensive immunohistochemical analysis of tumor
microenvironment immune status in esophageal squamous cell
carcinoma. Oncogarget. 7:47252–47264. 2016.
|
|
42
|
Jiang D, Song Q, Wang H, Huang J, Wang H,
Hou J, Li X, Xu Y, Sujie A, Zeng H, et al: Independent prognostic
role of PD-L1expression in patients with esophageal squamous cell
carcinoma. Oncogarget. 8:8315–8329. 2017.
|
|
43
|
Borghaei H, Paz-Ares L, Horn L, Spigel DR,
Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al:
Nivolumab versus docetaxel in advanced non-squamous non-small cell
lung cancer. N Engl J Med. 373:1627–1639. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Katsuya Y, Fujita Y, Horinouchi H, Ohe Y,
Watanabe S and Tsuta K: Immunohistochemical status of PD-L1 in
thymoma and thymic carci-noma. Lung Cancer. 88:154–159. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Fay AP, Signoretti S, Callea M, Telό GH,
McKay RR, Song J, Carvo I, Lampron ME, Kaymakcalan MD,
Poli-de-Figueiredo CE, et al: Programmed death ligand-1 expression
in adrenocortical carcinoma: An exploratory biomarker study. J
Immunother Cancer. 3:32015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Mansfield AS, Roden AC, Peikert T, Sheinin
YM, Harrington SM, Krco CJ, Dong H and Kwon ED: B7-H1 expression in
malig-nant pleural mesothelioma is associated with sarcomatoid
histology and poor prognosis. J Thorac Oncol. 9:1036–1040. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lipson EJ, Vincent JG, Loyo M, Kagohara
LT, Luber BS, Wang H, Xu H, Nayar SK, Wang TS, Sidransky D, et al:
PD-L1 expression in the Merkel cell carcinoma microenvironment:
Association with in ammation, Merkel cell polyomavirus and overall
survival. Cancer Immunol Res. 1:54–63. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Conway JR, Kofman E, Mo SS, Elmarakeby H
and Van Allen E: Genomics of response to immune checkpoint
therapies for cancer: Implications for precision medicine. Genome
Med. 10:932018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Doi T, Piha-Paul SA, Jalal SI, Mai-Dang H,
Yuan S, Koshiji M, Csiki I and Bennouna J: Pembrolizumab (MK-3475)
for patients (pts) with advanced esophageal carcinoma: Preliminary
results from KEYNOTE-028. J Clin Oncol. 33 (15 Suppl):S40102015.
View Article : Google Scholar
|
|
50
|
Doi T, Piha-Paul SA, Jalal SI, Mai-Dang H,
Saraf S, Koshiji M, Csiki I and Bennouna J: Updated results for the
advanced esophageal carcinoma cohort of the phase Ib KEYNOTE-028
study of pembrolizumab. J Clin Oncol. 34 (15 Suppl):S40462016.
View Article : Google Scholar
|
|
51
|
Stenger M: Immunotherapy in Advanced
Esophageal Carcinoma. The ASCO Post. 2017, https://www.ascopost.com/News/58296November
29–2017
|
|
52
|
Doi T, Bennouna J, Shen L, Enzinger PC,
Wang R, Csiki I, Koshiji M and Shah MA: KEYNOTE-181: Phase 3,
open-label study of second-line pembrolizumab vs single-agent
chemotherapy in patients with advanced/metastatic esophageal
adenocarcinoma. J Clin Oncol. 34 (15 Suppl):2017.
|
|
53
|
Shah MA, Kojima T, Enzinger PC, Hochhauser
D, Raimbourg J, Hollebecque A, Lordick F, Kim SB, Tajika M, Kim HT,
et al: Pembrolizumab for patients with previously treated
metastatic adenocarcinoma or squamous cell carcinoma of the
esophagus: Phase 2 KEYNOTE-180 study. J Clin Oncol. 36 (15
Suppl):S40492018. View Article : Google Scholar
|
|
54
|
Kitagawa Y, Doki Y, Kato K and Ura T: Two
year survival and safety update for esophageal squamous cell
carcinoma treated with nivolumab (ATTRACTION-01/ONO-4538-07). Anna
Oncol. 28 (Suppl 5):v209–v268. 2017.
|
|
55
|
Yuriy Y, Alexander PO, Calvo E, Joseph W,
Kim, Antonio PA, Sharma P and Johanna KP: Nivolumab ± ipilimumab in
pts with advanced (adv)/metastatic chemotherapy-refractory (CTx-R)
gastric (G), esophageal (E), or gastroesophageal junction (GEJ)
cancer: CheckMate 032 study. J Clin Oncol. 35 (Suppl 15):40142017.
View Article : Google Scholar
|
|
56
|
Greally M, Molena D, Sihag S, Wu Abraham
JC, Shah PM, Fein Carly, Capanu M, Kelsen DP, Janjigian YY, Ilson
DH, et al: Phase Ib/II trial of durvalumab and chemoradiation (CRT)
with carboplatin/paclitaxel for esophageal and gastroesophageal
junction (GEJ) adenocarcinoma. J Clin Oncol. 4:1722018. View Article : Google Scholar
|
|
57
|
Chung HC, Arkenau HT, Wyrwicz L, Oh DY,
Lee KW, Infante JR, Chin KM, Heydebreck AV, Kang YK and Safran H:
Safety, PD-L1 expression, and clinical activity of avelumab
(MSB0010718C), an anti-PD-L1 antibody, in patients with advanced
gastric or gastroesophageal junction cancer. J Clin Oncol. 34 (4
Suppl):S1672016. View Article : Google Scholar
|
|
58
|
Smyth E and Thuss-Patience PC: Immune
checkpoint inhibition in gastro-oesophageal cancer. Oncol Res
Treat. 41:272–280. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Bang Y, Golan T, Lin CC, Kang YK, Wainberg
ZA, Wasserstrom H, Jin J, Mi G, McNeely SC, Laing N, et al: Interim
safety and clinical activity in patients (pts) with locally
advanced and unresectable or metastatic gastric or gastroesophageal
junction (G/GEJ) adenocarcinoma from a multicohort phase I study of
ramucirumab (R) plus durvalumab (D). J Clin Oncol. 36 (4
Suppl):S922018. View Article : Google Scholar
|
|
60
|
Taieb J, Moehler M, Boku N, Ajani JA,
Yañez Ruiz E, Ryu MH, Guenther S, Chand V and Bang YJ: Evolution of
checkpoint inhibitors for the treatment of metastatic gastric
cancers: Current status and future perspectives. Cancer Treat Rev.
66:104–113. 2016. View Article : Google Scholar
|
|
61
|
Mole RH: Whole body
irradiation-radiobiology or medicine? Br J Radiol. 26:234–241.
1953. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Abuodeh Y, Venkat P and Kim S: Systematic
review of case reports on the abscopal effect. Curr Probl Cancer.
40:25–37. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Siva S, MacManus MP, Martin RF and Martin
OA: A Abscopal effects of radiation therapy: A clinical review for
the radiobiologist. Cancer Lett. 356:82–90. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Van Limbergen EJ, De Ruysscher DK, Olive
Pimentel V, Marcus D, Berbee M, Hoeben A, Rekers N, Theys J,
Yaromina A, Dubois LJ and Lambin P: Combining radiotherapy with
immunotherapy: The past, the present and the future. Br J Radiol.
90:201701572017. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Jing W, Gershan JA, Weber J, Tlomak D,
Mcolash L, Sabatos-Peyton C and Johnson BD: Combined immune
checkpoint protein blockade and low dose whole body irradiation as
immunotherapy for myeloma. J Immunother Cancer. 3:22015. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Salama AK, Postow MA and Salama JK:
Irradiation and immunotherapy: From concept to the clinic. Cancer.
122:1659–1671. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Reynders K, Illidge T, Siva S, Chang JY
and De Ruysscher D: The abscopal effect of local radiotherapy:
Using immunotherapy to make a rare event clinically relevant.
Cancer Treat Rev. 41:503–510. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Twyman-Saint Victor C, Rech AJ, Maity A,
Rengan R, Pauken KE, Stelekati E, Benci JL, Xu B, Dada H, Odorizzi
PM, et al: Radiation and dual checkpoint blockade activates
non-redundant immune mechanisms in cancer. Nature. 520:373–377.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Schoenhals JE, Seyedin SN, Tang C, Cortez
MA, Niknam S, Tsouka E, Chang JY, Hahn SM and Welsh JW: Preclinical
rationale and clinical considerations for radiotherapy plus
immunotherapy: Going beyond local control. Cancer J. 22:130–137.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Son CH, Fleming GF and Moroney JW:
Potential role of radiation therapy in augmenting the activity of
immunotherapy for gynecologic cancers. Cancer Manag Res. 9:553–563.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Frey B, Rückert M, Deloch L, Rühle PF,
Derer A, Fietkau R and Gaipl US: Immunomodulation by ionizing
radiation-impact for design of radio- immunotherapies and for
treatment of inflammatory diseases. Immunol Rev. 280:231–248. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Lhuillier C, Rudqvist NP, Elemento O,
Formenti SC and Demaria S: Radiation therapy and anti-tumor
immunity: Exposing immunogenic mutations to the immune system.
Genome Med. 11:402019. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Kim KJ, Kim JH, Lee SJ, Lee EJ, Shin EC
and Seong J: Radiation improves antitumor effect of immune
checkpoint inhibitor in murine hepatocellular carcinoma model.
Oncotarget. 8:41242–41255. 2017.PubMed/NCBI
|
|
74
|
Oweida A, Lennon S, Calame D, Korpela S,
Bhatia S, Sharma J, Graham C, Binder D, Serkova N, Raben D, et al:
Ionizing radiation sensitizes tumors to PD-L1 immune checkpoint
blockade in orthotopic murine head and neck squamous cell
carcinoma. Oncoimmunology. 6:e13561532017. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Aguilera T, Rafat M, Kariolis M, Eyben RV,
Graves E and Giaccia A: Tumor immunologic heterogeneity influences
response to radiation and combination immunotherapy. J Immunother
Cancer. 3:P3452015. View Article : Google Scholar
|
|
76
|
Sharabi AB, Nirschl CJ, Kochel CM, Nirschl
TR, Francica BJ, Velarde E, Deweese TL and Drake CG: Stereotactic
radiation therapy augments antigen-specific PD-1-mediated antitumor
immune responses via cross-presentation of tumor antigen. Cancer
Immunol Res. 3:345–355. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Deng L, Liang H, Burnette B, Beckett M,
Darga T, Weichselbaum RR and Fu YX: Irradiation and anti-PD-L1
treatment synergistically promote antitumor immunity in mice. J
Clin Invest. 124:687–695. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Asna N, Livoff A, Batash R, Debbi R,
Schaffer P, Rivkind T and Schaffer M: Radiation therapy and
immunotherapy-a potential combination in cancer treatment. Curr
Oncol. 25:e454–e460. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Lazzari C, Karachaliou N, Bulotta A,
Viganó M, Mirabile A, Brioschi E, Santarpia M, Gianni L, Rosell R
and Gregorc V: Combination of immunotherapy with chemotherapy and
radiotherapy in lung cancer: Is this the beginning of the end for
cancer? Ther Adv Med Oncol. 10:17588359187620942018. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Ahmed KA, Stallworth DG, Kim Y, Johnstone
PA, Harrison LB, Caudell JJ, Yu HH, Etame AB, Weber JS and Gibney
GT: Clinical outcomes of melanoma brain metastases treated with
stereotactic radiation and anti-PD-1 therapy. Ann Oncol.
27:434–441. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Qian JM, Yu JB, Kluger HM and Chiang VL:
Timing and type of immune checkpoint therapy affect the early
radiographic response of melanoma brain metastases to stereotactic
radiosurgery. Cancer. 122:3051–3058. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Pike LRG, Bang A, Ott P, Balboni T, Taylor
A, Catalano P, Rawal B, Spektor A, Krishnan M, Cagney D, et al:
Radiation and PD-1 inhibition: Favorable outcomes after
brain-directed radiation. Radiother Oncol. 124:98–103. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Chen MF, Chen PT, Chen WC, Lu MS, Lin PY
and Lee KD: The role of PD-L1 in the radiation response and
prognosis for esophageal squamous cell carcinoma related to IL-6
and T-cell immunosuppression. Oncotarget. 7:7913–7924.
2016.PubMed/NCBI
|
|
84
|
Stahl M and Budach W: Definitive
chemoradiotherapy. J Thorac Dis. 9 (Suppl 8):S792–S798. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Matzenauer M, Vrána D, Vlachová Z, Aujesky
R, Vrba R, Neoral C and Melichar B: Stereotactic radiotherapy in
the treatment of local recurrences of esophageal cancer. Oncol
Lett. 13:1807–1810. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Luke JJ, Lemons JM, Karrison TG, Pitroda
SP, Melotek JM, Zha Y, Al-Hallaq HA, Arina A, Khodarev NN, Janisch
L, et al: Safety and clinical activity of pembrolizumab and
multisite stereotactic body radiotherapy in patients with advanced
solid tumors. J Clin Oncol. 36:1611–1618. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Wu P, Wu D, Li L, Chai Y and Huang J:
PD-L1 and survival in solid tumors: A meta-analysis. PLoS One.
10:e01314032015. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Alsaab HO, Sau S, Alzhrani R, Tatiparti K,
Bhise K, Kashaw SK and Iyer AK: PD-1 and PD-L1 checkpoint signaling
inhibition for cancer immunotherapy: Mechanism, combinations, and
clinical outcome. Front Pharmacol. 8:5612017. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Mazel M, Jacot W, Pantel K, Bartkowiak K,
Topart D, Cayrefourcq L, Rossille D, Maudelonde T, Fest T and
Alix-Panabières C: Frequent expression of PD-L1 on circulating
breast cancer cells. Mol Oncol. 9:1773–1782. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Ilié M, Szafer-Glusman E, Hofman V,
Chamorey E, Lalvee S, Selva E, Leroy S, Marquette CH, Kowanetz M,
Hedge P, et al: Detection of PD-L1 in circulating tumor cells and
white blood cells from patients with advanced non-small-cell lung
cancer. Ann Oncol. 29:193–199. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Nicolazzo C, Raimondi C, Mancini M,
Caponnetto S, Gradilone A, Gandini O, Mastromartino M, Del Bene G,
Prete A, Longo F, et al: Monitoring PD-L1 positive circulating
tumor cells in non-small cell lung cancer patients treated with the
PD-1 inhibitor nivolumab. Sci Rep. 6:317262016. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Guibert N, Delaunay M, Lusque A, Boubekeur
N, Rouquette I, Clermont E, Mourlanette J, Gouin S, Dormoy I, Favre
G, et al: PD-L1 expression in circulating tumor cells of advanced
non-small cell lung cancer patients treated with nivolumab. Lung
Cancer. 120:108–112. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Yue C, Jiang Y, Li P, Wang Y, Xue J, Li N,
Li D, Wang R, Dang Y, Hu Z, et al: Dynamic change of PD-L1
expression on circulating tumor cells in advanced solid tumor
patients undergoing PD-1 blockade therapy. Oncoimmunology.
7:e14381112918. View Article : Google Scholar
|
|
94
|
Zhu X and Lang J: Soluble PD-1 and PD-L1:
Predictive and prognostic significance in cancer. Oncotarget.
8:97671–97682. 2017.PubMed/NCBI
|
|
95
|
Cohen EEW, Bell RB, Bifulco CB, Burtness
B, Gillison ML, Harrington KJ, Le QT, Lee NY, Leidner R, Lewis RL,
et al: The Society for Immunotherapy of Cancer consensus statement
on immunotherapy for the treatment of squamous cell carcinoma of
the head and neck (HNSCC). J Immunother Cancer. 7:1842019.
View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Califano J, Leong PL, Koch WM, Eisenberger
CF, Sidransky D and Westra WH: Second esophageal tumors in patients
with head and neck squamous cell carcinoma: An assessment of clonal
relationships. Clin Cancer Res. 5:1862–1867. 1999.PubMed/NCBI
|
|
97
|
The Cancer Genome Atlas Research Network,
. Integrated genomic characterization of oesophageal carcinoma.
Nature. 541:169–175. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Predina JD, Judy B, Aliperti LA,
Fridlender ZG, Blouin A, Kapoor V, Laguna B, Nakagawa H, Rustgi AK,
Aguilar L, et al: Neoadjuvant in situ gene-mediated cytotoxic
immunotherapy improves postoperative outcomes in novel syngeneic
esophageal carcinoma models. Cancer Gene Ther. 18:871–883. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Sharabi AB, Lim M, DeWeese TL and Drake
CG: Radiation and checkpoint blockade immunotherapy:
Radiosensitisation and potential mechanisms of synergy. Lancet
Oncol. 16:e498–509. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Marciscano AE, Walker JM, McGee HM, Kim
MM, Kunos CA, Monjazeb AM, Shiao SL, Tran PT and Ahmed MM:
Incorporating radiation oncology into immunotherapy: Proceedings
from the ASTRO-SITC-NCI immunotherapy workshop. J Immunother
Cancer. 6:62018. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Pitt JM, Marabelle A, Eggermont A, Soria
JC, Kroemer G and Zitvogel L: Targeting the tumor microenvironment:
Removing obstruction to anticancer immune responses and
immunotherapy. Anna Oncol. 27:1482–1492. 2016. View Article : Google Scholar
|
|
102
|
Tang H, Qiao J and Fu YX: Immunotherapy
and tumor microenvironment. Cancer Lett. 370:85–90. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Schaaf MB, Garg AD and Agostinis P:
Defining the role of the tumor vasculature in antitumor immunity
and immunotherapy. Cell Death Dis. 9:1152018. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Hendry SA, Farnsworth RH, Solomon B, Achen
MG, Stacker SA and Fox SB: The role of the tumor vasculature in the
host immune response: Implications for therapeutic strategies
targeting the tumor microenvironment. Front Immunol. 7:6212016.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Kallman RF and Dorie MJ: Tumor oxygenation
and reoxygenation during radiation therapy: Their importance in
predicting tumor response. Int J Radiat Oncol Biol Phys.
12:681–685. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Jiang W, Chan CK, Weissman IL, Kim BYS and
Hahn SM: Immune priming of the tumor microenvironment by radiation.
Trends Cancer. 2:638–645. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Wolchok JD, Hoos A, O'Day S, Weber JS,
Hamid O, Lebbé C, Maio M, Binder M, Bohnsack O, Nichol G, et al:
Guidelines for the evaluation of immune therapy activity in solid
tumors: Immune-related response criteria. Clin Cancer Res.
15:7412–7420. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Somarouthu B, Lee SI, Urban T, Sadow CA,
Harris GJ and Kambadakone A: Immune-related tumour response
assessment criteria: A comprehensive review. Br J Radiol.
91:201704572018. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Hoos A, Wolchok JD, Humphrey RW and Hodi
FS: CCR 20th anniversary commentary: Immune-related response
criteria-capturing clinical activity in immuno-oncology. Clin
Cancer Res. 21:4989–4991. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Hodi FS, Hwu WJ, Kefford R, Weber JS, Daud
A, Hamid O, Patnaik A, Ribas A, Robert C, Gangadhar TC, et al:
Evaluation of immune-related response criteria and RECIST v1.1 in
patients with advanced melanoma treated with pembrolizumab. J Clin
Oncol. 34:1510–1517. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Postow MA, Sidlow R and Hellmann MD:
Immune-related adverse events associated with immune checkpoint
blockade. N Engl J Med. 378:158–168. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Yovino S and Grossman SA: Severity,
etiology and possible consequences of treatment-related lymphopenia
in patients with newly diagnosed high-grade gliomas. CNS Oncol.
1:149–154. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Davuluri R, Jiang W, Fang P, Xu C, Komaki
R, Gomez DR, Welsh J, Cox JD, Crane CH, Hsu CC and Lin SH:
Lymphocyte nadir and esophageal cancer survival outcomes after
chemoradiation therapy. Int J Radiat Oncol Biol Phys. 99:128–135.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Yamashita H, Haga A, Takenaka R, Kiritoshi
T, Okuma K, Ohtomo K and Nakagawa K: Efficacy and feasibility of
ambulatory treatment-based monthly nedaplatin plus S-1 in
definitive or salvage concurrent chemoradiotherapy for early,
advanced, and relapsed esophageal cancer. Radiat Oncol. 11:42016.
View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Chen HY, Ma XM, Ye M, Hou YL, Xie HY and
Bai YR: Esophageal perforation during or after conformal
radiotherapy for esophageal carcinoma. J Radiat Res. 55:940–947.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Roeder F, Nicolay NH, Nguyen T,
Saleh-Ebrahimi L, Askoxylakis V, Bostel T, Zwicker F, Debus J,
Timke C and Huber PE: Intensity modulated radiotherapy (IMRT) with
concurrent chemotherapy as definitive treatment of locally advanced
esophageal cancer. Radiat Oncol. 9:1912014. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Hong MH, Kim H, Park SY, Kim DJ, Lee CG,
Cho J, Kim JH, Kim HR, Kim YH, Park SR and Cho BC: A phase II trial
of preoperative chemoradiotherapy and pembrolizumab for locally
advanced esophageal squamous cell carcinoma (ESCC). J Clin Oncol.
37 (15 Suppl):S40272019. View Article : Google Scholar
|
|
118
|
Katz M, Bauer TW, Varadhachary G,
Acquavella N, Petroni G, Bullock T, Slingluff CL and Rahma OE: A
randomized multicenter phase Ib/II study to assess the safety and
the immunological effect of chemoradiation therapy (CRT) in
combination with pembrolizumab (anti-PD1) to CRT alone in patients
with resectable or borderline resectable pancreatic cancer. J Clin
Oncol. 3 (Suppl 2):P1672015.
|
|
119
|
Van Hagen P, Hulshof MC, Van Lanschot JJ,
Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, Richel DJ,
Nieuwenhuijzen GA, Hospers GA, Bonenkamp JJ, et al: Preoperative
chemora-diotherapy for esophageal or junctional cancer. N Engl J
Med. 366:2074–2084. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
U.S. Department of Health and Human
Services, . Common Terminology Criteria for Adverse Events (CTCAE)
v5.0. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5×7.pdfNovember
27–2017
|