Introduction
Acute promyelocytic leukemia (APL) is characterized
by a balanced reciprocal translocation between chromosomes 15 and
17, causing a fusion between promyelocytic leukemia (PML) and
retinoic acid receptor α (1).
Despite recent progress in the diagnosis and treatment of APL, its
prognosis remains poor (2).
Therefore, novel prognostic markers that are associated with APL
progression would be of great clinical relevance.
Long non-coding RNAs (lncRNAs) refer to non-protein
coding transcripts of >200 nucleotides in length, which are
involved in a wide range of biological behaviors, including
epigenetic regulation, chromatin modification, transcription and
post-transcriptional processing (3–5).
Increasing evidence has confirmed the role of lncRNAs in
carcinogenesis, acting as proto-oncogenes or tumor suppressor genes
(6,7). For example, lncRNA growth
arrest-specific 5 and maternally expressed 3 act as tumor
suppressor genes, and metastasis associated lung adenocarcinoma
transcript 1 and HOX transcript antisense RNA function as oncogenes
(8–11). The newly identified lncRNA zinc
finger antisense 1 (ZFAS1) is highly expressed in mammary glands
and functions as a tumor suppressor gene in human breast cancer
(12). Moreover, ZFAS1 is
upregulated in colorectal cancer tissue and is an oncogene in
hepatocellular carcinoma (13,14). To
date, there have been several studies on the function of lncRNAs in
APL. For example, lncRNA nuclear paraspeckle assembly transcript 1
(NEAT1) is involved in myeloid differentiation, which can be
abrogated following the inhibition of NEAT1 (15). Moreover, lncRNA Pvt1 oncogene (PVT1)
was found to be overexpressed in APL, and knockdown of PVT1 led to
suppression of MYC proto-oncogene bHLH transcription factor
expression and cell viability (16).
These findings implicated the potential role of lncRNAs in the
development of APL. However, to the best of our knowledge, the role
of ZFAS1 in APL has not yet been described.
The present study aimed to characterize the role and
regulation of ZFAS1 in APL. This is, to the best of our knowledge,
the first study to demonstrate the potential function of ZFAS1 in
APL. The findings from this study may provide novel insights into
the mechanisms of APL progression.
Materials and methods
Clinical samples
A total of 59 peripheral blood samples were
collected with informed consent. This included 33 samples from
patients with APL (mean age ± standard deviation, 62.4±4.8 years)
collected at the time of diagnosis and 26 samples from healthy
donors (mean age, 47 years). The peripheral blood mononuclear cells
of APL samples were isolated using Ficoll-Hypaque gradient
centrifugation method (d=1.077 g/mol; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) (17). Regarding
the isolation of the granulocyte fraction, contaminating
erythrocytes were hemolysed with cold ammonium chloride solution
(250 mM). The ammonium chloride solution was added directly into
the peripheral blood mononuclear cell fraction and subsequent
granulocytes were collected and washed once with PBS as described
earlier (18). The granulocyte was
diluted in PBS and collected by centrifugation at 300 × g for 15
min at 4°C and subsequently, the pellets were stored at 80°C.
Patient characteristics are described in Table I. All of the procedures were
conducted according to the guidelines of the Medical Ethics
Committees of the Health Bureau of the Zhejiang Province of China,
and ethical approval was obtained from the Ethics Committee of the
Medical School of Zhejiang University (Hangzhou, China).
 | Table I.Clinical characteristics of patients
with acute promyelocytic leukemia (n=33). |
Table I.
Clinical characteristics of patients
with acute promyelocytic leukemia (n=33).
| Characteristic | n | % |
|---|
| Cytogenetics
t(15;17) | 33 | 100.0 |
| Age, years |
|
|
|
10–20 | 6 | 18.2 |
|
20–30 | 17 | 51.5 |
|
30–40 | 9 | 27.3 |
|
40–50 | 1 | 3.0 |
| Sex |
|
|
| Male | 20 | 60.6 |
|
Female | 13 | 39.4 |
| WBC count
(×109) |
|
|
|
<10 | 14 | 42.4 |
|
10–50 | 10 | 30.3 |
|
>50 | 4 | 12.1 |
|
N/A | 5 | 15.2 |
| Percentage of PB
blasts |
|
|
|
<80 | 16 | 48.5 |
|
≥80 | 9 | 27.3 |
|
N/A | 8 | 24.2 |
Cell culture and transfection
The NB4 and NB4-all-trans retinoic acid-resistant
(NB4-R-ATRA) cell lines were generous gifts provided by Dr Jianguo
Chen (Sun Yat-Sen Hospital, Shanghai, China). The HL-60 cell line
was purchased from the Cell Bank of Shanghai Institute of
Biological Sciences (www.cellbank.org.cn; Chinese Academy of Sciences,
Shanghai, China). All cells were cultured in RPMI-1640 medium
supplemented with 10% (v/v) fetal calf serum, 1% (v/v)
antibiotic-antimycotic solution and 2 mM L-glutamine (all Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) in a humidified
atmosphere at 37°C and with 5% CO2.
Chemicals and reagents
ATRA was purchased from Sigma-Aldrich; Merck KGaA,
and dissolved in EtOH at the stock concentration of 20 mM. Cells
were treated with 1 µM ATRA for 24 h. All other chemical reagents
were also obtained from Sigma-Aldrich; Merck KGaA.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total cellular RNA was purified using
TRIzol® (Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocols. The expression levels of ZFAS1 were
detected by RT-qPCR using a SYBR ExScript RT-PCR kit (Takara
Biotechnology Co., Ltd., Dalian, China), performed in an Applied
Biosystems 7500 PCR system (Thermo Fisher Scientific, Inc.). qPCR
was performed with the following cycling conditions: 40 cycles of
denaturation at 95°C for 30 sec, annealing at 59°C for 30 sec and
extension at 72°C for 15 sec. Samples were amplified in triplicate,
and data were normalized to the expression of glyceraldehyde
3-phosphate dehydrogenase (GAPDH). The primer sequences were as
follows: GAPDH forward, 5′-AGATGTTCCAATATGATTCC-3′ and reverse,
5′-TGGACTCCACGACGTACTCAG-3′ and ZFAS1 forward,
5-ACGTGCAGACATCTACAACCT-3′ and reverse, 5-TACTTCCAACACCCGCAT-3′.
The relative mRNA expressions of target genes were calculated using
the 2−∆∆Cq method (19).
RNA interference
Two small interfering (si)RNA constructs against
ZFAS1 were generated. ZFAS1-1 siRNA
(5′-CTGGCTGAACCAGTTCCACAAGGTT-3′), ZFAS1-2 siRNA
(5′-TACTTCTCCTAGTTGCAGTCAGG-3′) and the scramble siRNA
(5′-ACGTGACACGTTCGGAGAATT-3′) were purchased from Shanghai Sangon
Co., Ltd. (Shanghai, China). NB4 cells were transfected with 20 µM
siRNAs using the Neon Transfection system (Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol.
Following 24 h transfection, ZFAS1 level was determined by
RT-qPCR.
Cell proliferation assay
Cell proliferation was measured using Cell Counting
Kit-8 (CCK-8; Promega Corporation, Madison, WI, USA) according to
the manufacturer's protocol. Briefly, cells were plated at a
density of 1×104 cells/well in 96-well plates and
cultured in RPMI-1640 medium. The CCK-8 reagent (10 µl) was added
to the wells at the end of the experiment. Following incubation at
37°C for 2 h, the absorbance in each well was determined using a
microplate reader at 450 nm.
Cytosolic fraction isolation
To isolate the cytosolic fraction, cells were washed
with ice-cold PBS. The cells were lysed using the Cell Lysis and
Mitochondria Intact buffer (Beyotime Institute of Biotechnology,
Haimen, China) on ice for 5 min, and the cell suspension was
centrifuged at 500 × g for 5 min at 4°C. The supernatant was
removed and stored at −20°C as the cytosolic fraction. This was
subsequently separated by 12% SDS-PAGE and the proteins were
detected by the western blot assay.
Western blot assay
The cells were lysed using radioimmunoprecipitation
assay buffer on ice for 30 min and the protein concentration was
determined with the Bradford assay (both Beyotime Institute of
Biotechnology). The proteins (25 µg) were separated by 12% SDS-PAGE
and transferred onto polyvinylidene fluoride membranes (EMD
Millipore, Billerica, MA, USA). The membranes were blocked with 5%
skimmed milk for 1 h at room temperature, and incubated at 4°C
overnight with primary antibodies diluted at 1:1,000 against Mcl-1
(cat. no. ab32087; Abcam, Cambridge, MA, USA), caspase-9 (cat. no.
9502), cleaved caspase-9 (cat. no. 9505), caspase-3 (cat. no.
9662), cleaved caspase-3 (cat. no. 9661), caspase-8 (cat. no.
9746), Bcl-2 (cat. no. 4223), cytochrome c (cat. no. 12963),
Smac/DIABLO (cat. no. 15108), GAPDH (cat. no. 5174), PARP (cat. no.
9532), XIAP (cat. no. 2045) and BAX (cat. no. 5023) (all Cell
Signaling Technology, Inc., Danvers, MA, USA). Membranes were
subsequently incubated with the goat anti-mouse (cat. no. 31430) or
goat anti-rabbit (cat. no. 31460) horseradish peroxidase-conjugated
secondary antibodies (1:2,000; both from Thermo Fisher Scientific,
Inc.) for 1 h at room temperature. Enhanced chemiluminescence
reagent (Thermo Fisher Scientific, Inc.) was used to detect the
signal on the membrane with a Bio Image Intelligent Quantifier 1D
(Bio Image Systems, Inc., Jackson, MI, USA). Densitometric analysis
was performed with Quantity One software (version 4.6.8; Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
Flow cytometry analysis
Cells (2×105) were washed twice PBS and
then fixed with 70% ethanol at room temperature for 30 min. Next,
the cell cycle distribution was analyzed by flow cytometry
(FACSCalibur; Becton, Dickinson and Company, Franklin, Lakes, NJ,
USA) using DNA staining with propidium iodide (1 mg/ml;
Sigma-Aldrich; Merck KGaA) and Annexin V in PBS. The cells
undergoing apoptosis were Annexin V-FITC-positive and PI-negative.
Data analysis was performed using FlowJo version 8.8.7 software
(Tree Star, Inc., Ashland, OR, USA).
Statistical analysis
Differences between two groups were estimated using
Student's t-test with Prism version 5.0 (GraphPad Software, Inc.,
La Jolla, CA, USA). Differences among three groups were analyzed
using one-way analysis of variance followed by Tukey's test
post-hoc with SPSS (version 17; SPSS, Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
ZFAS1 is upregulated in patients with
APL
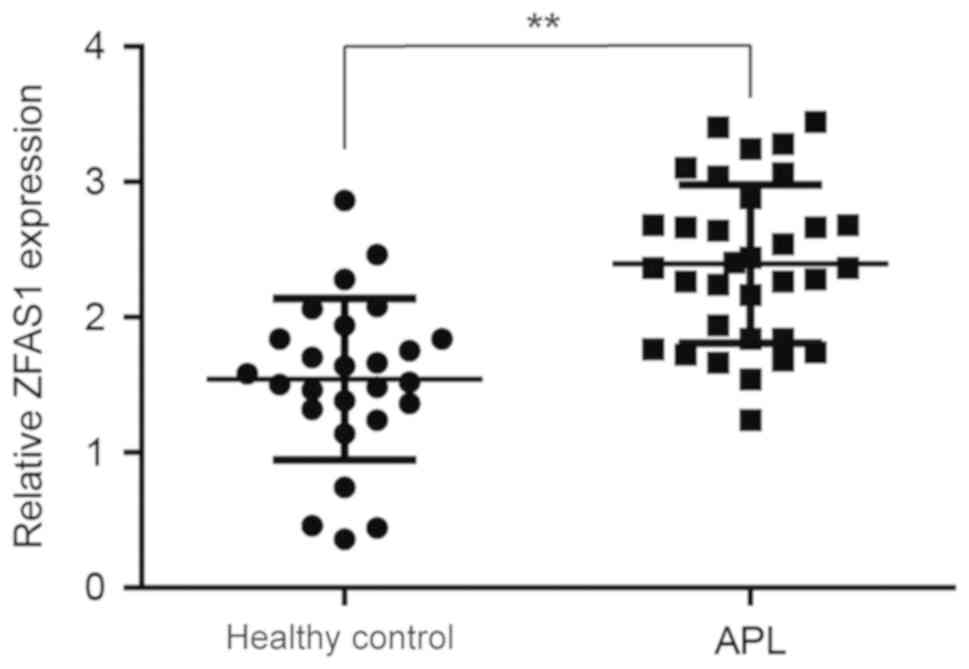
To investigate whether ZFAS1 is involved in the
development of APL, the ZFAS1 expression levels in 33 APL patient
samples (Table I) were first
compared with the levels in 26 healthy donor samples. As shown in
Fig. 1, the ZFAS1 expression level
was significantly increased in APL samples compared with that in
healthy controls. This result suggests that ZFAS1 upregulation may
have a positive association with the development of APL.
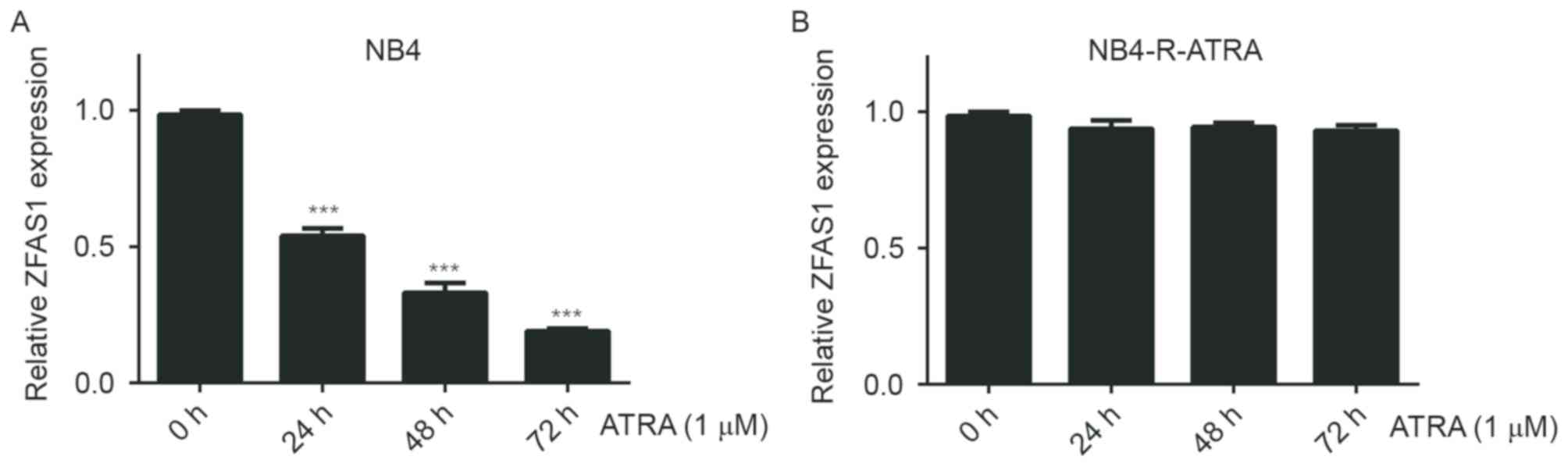
ATRA treatment results in the
downregulation of ZFAS1
NB4 cells has been widely accepted as a model of
myeloid maturation in which ATRA treatment leads to a proliferation
block at the G1 phase and terminates the differentiation
of myeloid cells (20–22). Therefore, the present study
investigated whether ATRA treatment affects ZFAS1 expression in NB4
cells. As shown in Fig. 2A, ZFAS1
expression levels in NB4 cells decreased following treatment with
ATRA (1 µM). At the same time, there was little ZFAS1
downregulation found in the ATRA-resistant cell line, therefore
excluding the possibility of non-specific stress responses to ATRA
treatment (Fig. 2B). These data
suggest that ZFAS1 may be involved in the proliferation of NB4
cells.
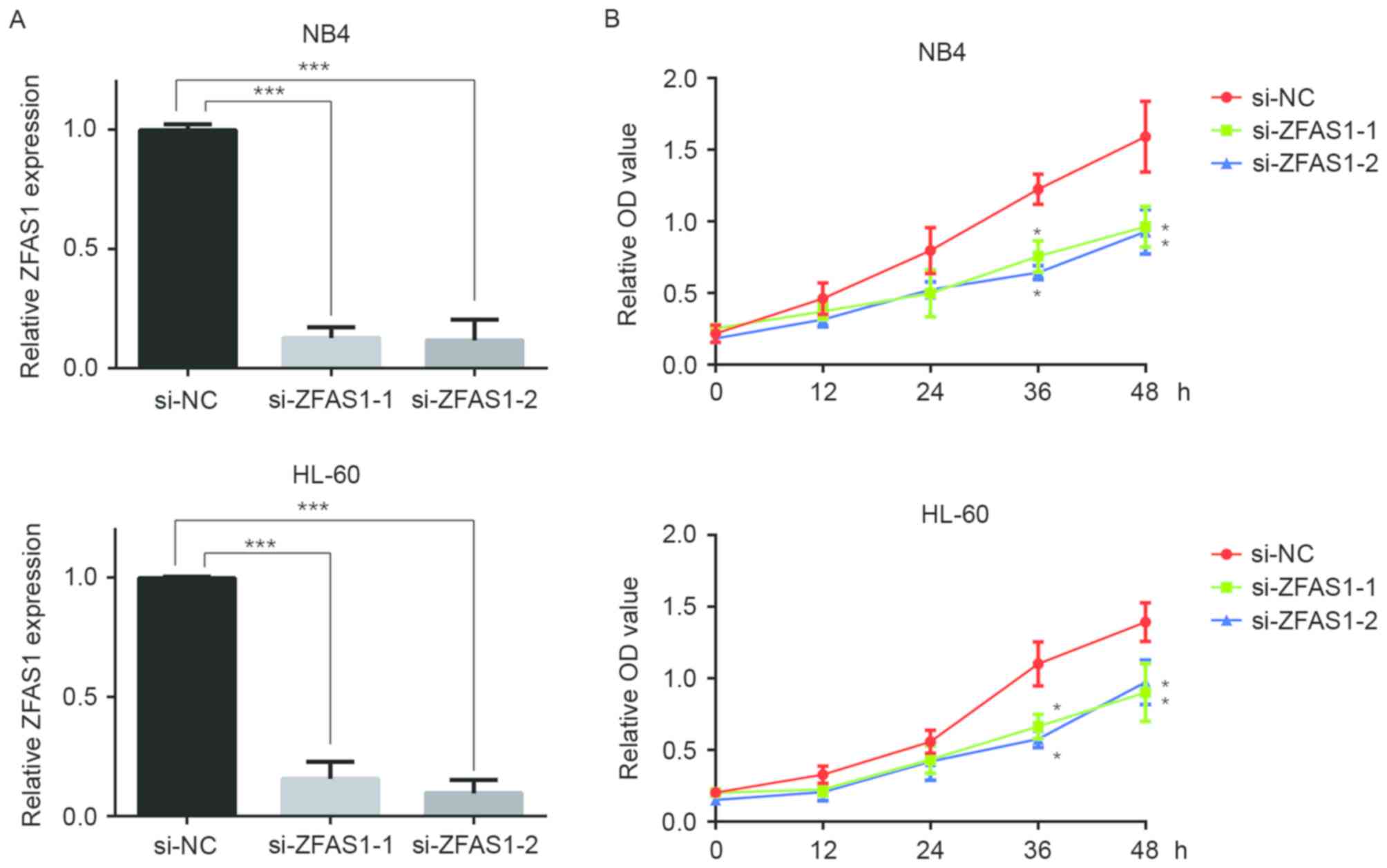
Downregulation of ZFAS1 decreases the
proliferation of APL cells
To further dissect the function of ZFAS1 in cellular
proliferation, two ZFAS1-specific siRNAs were used to repress the
expression of ZFAS1 in two APL cell lines, NB4 and HL-60 (Fig. 3A). As shown in Fig. 3B, APL cells transfected with ZFAS1
siRNAs exhibited a lower proliferation rate than the control group.
These data further confirmed that ZFAS1 is involved in the
proliferation of APL cells.
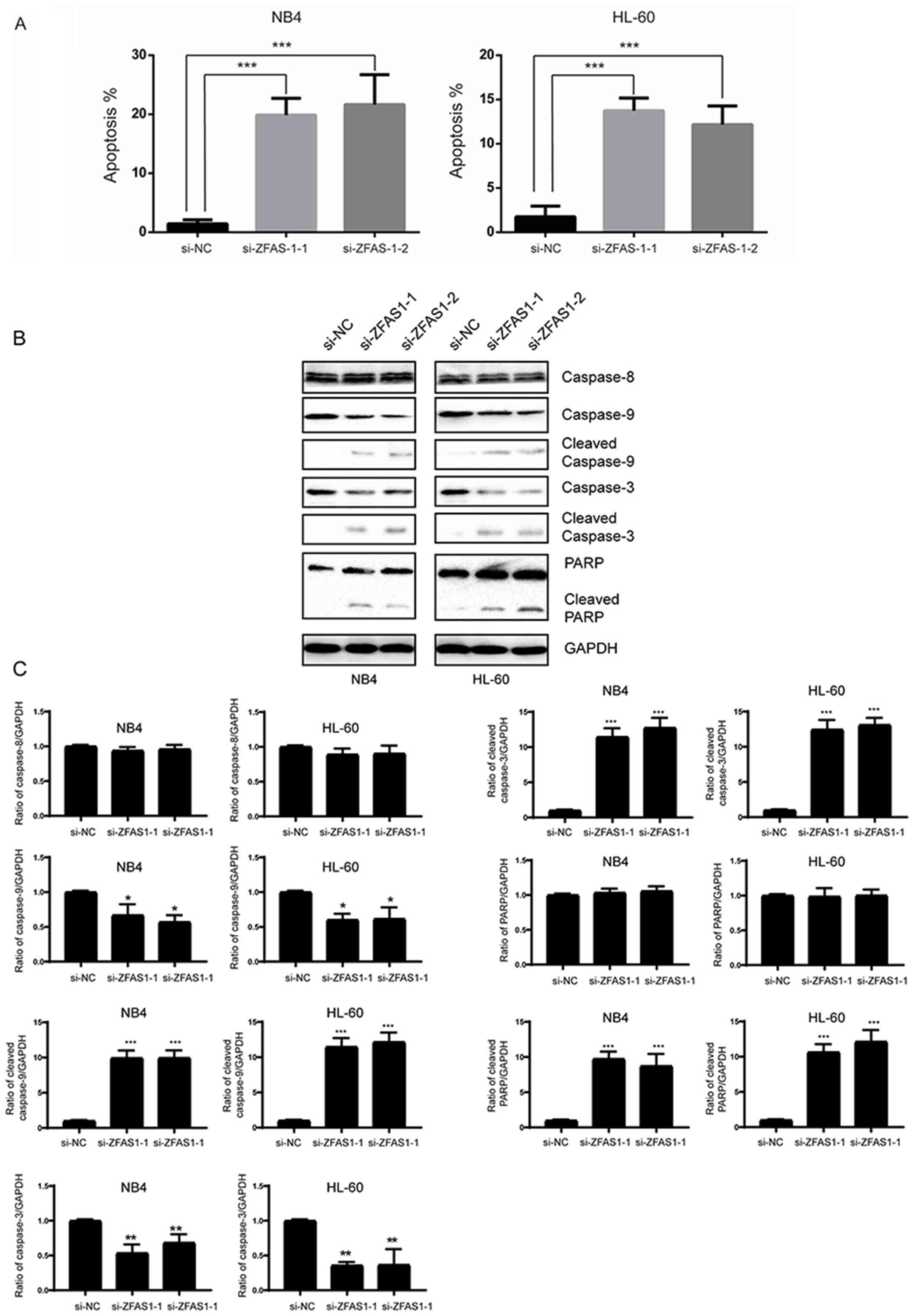
ZFAS1 regulates APL cell apoptosis
through the intrinsic apoptotic pathway
To investigate whether ZFAS1 affects the apoptosis
of APL cells, Annexin V/PI staining and flow cytometry analysis
were employed. As shown in Fig. 4A,
silencing of ZFAS1 markedly promoted the apoptosis of the APL
cells. There are two major pathways that lead to apoptosis: The
extrinsic pathway, also known as the death receptor signaling
pathway, and the intrinsic pathway, also known as the mitochondrial
pathway. Caspases are a group of cysteine proteases that are vital
regulators of apoptosis. The extrinsic and intrinsic pathways are
initiated by caspase-8 and caspase-9, respectively, leading to the
activation of caspase-3 (23). To
further investigate the potential role of ZFAS1 in APL cell
apoptosis in the present study, a western blot assay was performed
to detect caspase-3, caspase-8 and caspase-9 levels. When ZFAS1 was
silenced, cleaved caspase-9, caspase-3 and PARP were increased. At
the same time, cleaved caspase-8 was not significantly affected
(Fig. 4B and C). These data
suggested that ZFAS1 affects apoptosis via the intrinsic
pathway.
ZFAS1 affects the expression of the
Bcl-2 family proteins in APL cells
The intrinsic pathway is mainly regulated by the
Bcl-2 protein family. Therefore, levels of Bcl-2 family proteins
were investigated by western blotting. As shown in Fig. 5A, Bcl-2 and Mcl-1 expression levels
were decreased, while the expression level of XIAP was not
significantly affected following the silencing of ZFAS1.
Additionally, Bax expression and release of cytochrome c and
Smac/DIABLO were increased in the cytosolic fraction (Fig. 5B). Based on these results, ZFAS1
regulates the intrinsic pathway through the modulation of Bcl-2
family proteins.
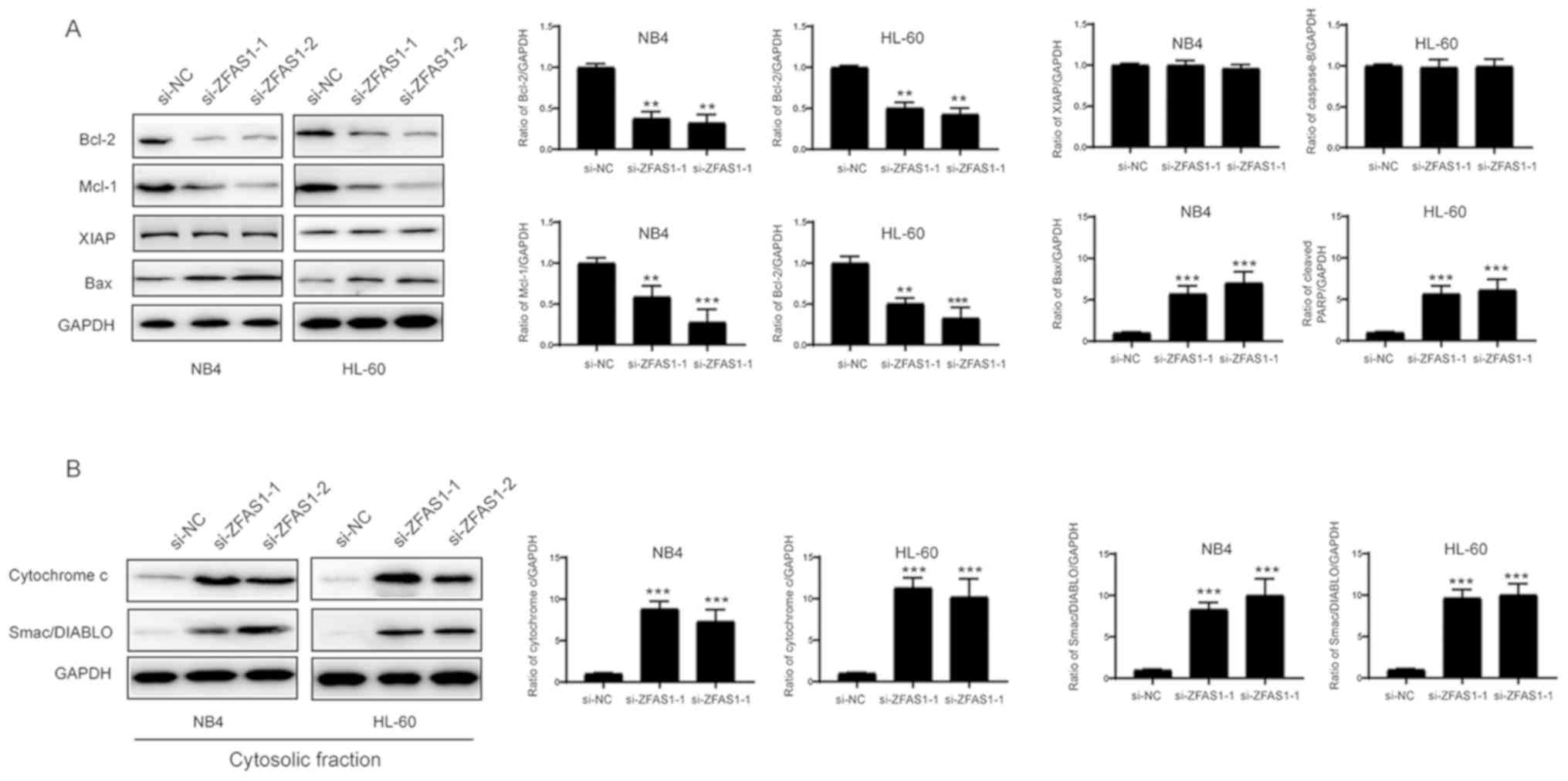 | Figure 5.ZFAS1 regulates APL cell apoptosis
through the modulation of Bcl-2 family proteins. (A) APL cells were
transfected with si-NC, si-ZFAS1-1 or si-ZFAS1-2 for 48 h. The
levels of Bcl-2, Mcl-1, XIAP and Bax were examined by western blot
analyses with the indicated antibodies. Densitometry analysis of
the western blots is presented on the right. **P<0.01,
***P<0.001 vs. si-NC. (B) APL cells were transfected with si-NC,
si-ZFAS1-1 or si-ZFAS1-2 for 48 h. Cytosolic fractions were
subjected to western blotting with the indicated antibodies and
densitometry analysis is presented on the right. ***P<0.001 vs.
si-NC. Values are presented as the mean ± standard deviation (n=3).
ZFAS1, long non-coding RNA zinc finger antisense 1; APL, acute
promyelocytic leukemia; si- small interfering RNA; NC, negative
control; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; Bcl-2,
B-cell lymphoma-2; Mcl-1, induced myeloid leukemia cell
differentiation protein Mcl-1; XIAP, E3 ubiquitin-protein ligase
XIAP; Bax, apoptosis regulator BAX; Smac/DIABLO, Diablo homolog
mitochondrial. |
Discussion
APL is a unique form of acute myeloid leukemia (AML)
caused by an arrest in granulocyte differentiation during the
promyelocytic stage (24). Although
clinical advances in AML have been achieved, its prognosis remains
quite poor. Therefore, novel approaches are required to target AML.
Increasing evidence indicates that lncRNAs are involved in a
variety of biological processes, including cell proliferation,
survival and differentiation (4,6). In
light of the potential value of lncRNAs as biomarkers and
therapeutic targets, the present study sought to investigate an
lncRNA involved in APL.
The present data showed that the expression of the
lncRNA ZFAS1 is significantly upregulated in APL patients compared
with that in healthy controls. The lncRNA ZFAS1 has previously been
found to be dysregulated in several different cancer types. For
example, ZFAS1 is overexpressed in gastric cancer and its increased
level is associated with a poor prognosis and shorter survival
times (25). ZFAS1 also promotes
metastasis and is associated with a poor prognosis in
hepatocellular carcinoma (13).
However, ZFAS1 expression is decreased in breast tumors and
functions as a tumor suppressor gene in breast cancer (12). The discrepancy with regard to the
function of ZFAS1 may be due to the different cancer type.
Currently, the role of ZFAS1 in APL remains unknown. The present
study data showed that ZFAS1 expression was significantly increased
in APL samples compared with that in healthy samples, indicating
that ZFAS1 may serve an important role in the development and
progression of APL. Moreover, silencing of ZFAS1 by RNAi lead to
the marked inhibition of APL cell proliferation. In addition, an
Annexin V/PI staining assay showed that the downregulation of ZFAS1
repressed APL cell proliferation by inducing apoptosis.
Apoptosis is a programmed cellular death mechanism
that is initiated by two pathways: The extrinsic and intrinsic
pathways. In the present study, subsequent to downregulating ZFAS1,
increased cleaved caspase-3 and caspase-9, and PARP was detected,
while caspase-8 was not affected. Therefore, ZFAS1 appears to
regulate apoptosis via the intrinsic pathway rather than the
extrinsic pathway. It is well documented that the intrinsic pathway
is mainly mediated by the Bcl-2 protein family, which controls the
stability of the mitochondrial membrane (6). Among the Bcl-2 protein family members,
Bcl-2, Mcl-2 and Bcl2-associated agonist of cell death (Bcl-xl) are
all upregulated in numerous types of tumor (26). Bax and Bcl-2 homologous
antagonist/killer (Bak) are involved in the formation of pores in
the outer mitochondrial membrane and the release of cytochrome
c and Smac/DIABLO. Together, these steps finally lead to the
activation of caspase-3 (27,28). A
number of studies have shown that lncRNAs affect the levels of
Bcl-2 family proteins (29). For
example, the knockdown of hepatocellular carcinoma upregulated
lncRNA is able to inhibit Bcl-2, thereby promoting apoptosis in
diffuse large B-cell lymphoma (30).
The lncRNA CDKN2B antisense RNA 1, which is found to be upregulated
in bladder cancer, serves as a positive regulator of Bcl-2 and a
negative regulator of Bax (31).
Similarly, silencing of ZFAS1 reduced the levels of Bcl-2, Mcl-1,
and increased the levels of Bax, cytochrome c and
Smac/DIABLO. These data confirm that ZFAS1 regulates APL cell
apoptosis through modulation of Bcl-2 protein family members and
the intrinsic pathway.
To the best of our knowledge, the present study is
the first to show that the lncRNA ZFAS1 regulates proliferation as
well as apoptosis in APL cells. Taken together, the results showed
that ZFAS1 was highly upregulated in samples from APL patients, and
affected APL cell proliferation and apoptosis in vitro. The
present study highlights the importance of the largely unexplored
population of non-protein-coding genes in understanding the
molecular basis of disease, and may provide a potential biomarker
and therapeutic target for the clinical management of APL.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LS performed the cell culture, cell transfection,
western blotting and RT-qPCR. HK repeated the experiments and
drafted the first version of manuscript. FW performed the cell
viability assays. HL performed the flow cytometry analysis. WW
performed the cell culture experiments and collected the clinical
data. GW, XY and JW performed the cell culture experiments. QF
designed the experiment and revised the final version of the
manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
the First Affiliated Hospital, School of Medicine, Zhejiang
University (Hangzhou, China). All procedures that involved human
participants were in accordance with the Ethical Standards of The
National Research Committee and with the 1964 Declaration of
Helsinki and following amendments. Written informed consent was
obtained from all study participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
He Y, Meng XM, Huang C, Wu BM, Zhang L, Lv
XW and Li J: Long noncoding RNAs: Novel insights into hepatocelluar
carcinoma. Cancer Lett. 344:20–27. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Testa U and Lo-Coco F: Prognostic factors
in acute promyelocytic leukemia: Strategies to define high-risk
patients. Ann Hematol. 95:673–680. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wilusz JE, Sunwoo H and Spector DL: Long
noncoding RNAs: Functional surprises from the RNA world. Genes Dev.
23:1494–1504. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gutschner T and Diederichs S: The
hallmarks of cancer: A long non-coding RNA point of view. RNA Biol.
9:703–719. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Carninci P, Kasukawa T, Katayama S, Gough
J, Frith MC, Maeda N, Oyama R, Ravasi T, Lenhard B, Wells C, et al:
The transcriptional landscape of the mammalian genome. Science.
309:1559–1563. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mercer TR, Dinger ME and Mattick JS: Long
non-coding RNAs: Insights into functions. Nat Rev Genet.
10:155–159. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kogo R, Shimamura T, Mimori K, Kawahara K,
Imoto S, Sudo T, Tanaka F, Shibata K, Suzuki A, Komune S, et al:
Long noncoding RNA HOTAIR regulates polycomb-dependent chromatin
modification and is associated with poor prognosis in colorectal
cancers. Cancer Res. 71:6320–6326. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mourtada-Maarabouni M, Pickard MR, Hedge
VL, Farzaneh F and Williams GT: GAS5, a non-protein-coding RNA,
controls apoptosis and is downregulated in breast cancer. Oncogene.
28:195–208. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Benetatos L, Vartholomatos G and
Hatzimichael E: MEG3 imprinted gene contribution in tumorigenesis.
Int J Cancer. 129:773–779. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gutschner T, Hämmerle M, Eissmann M, Hsu
J, Kim Y, Hung G, Revenko A, Arun G, Stentrup M, Gross M, et al:
The noncoding RNA MALAT1 is a critical regulator of the metastasis
phenotype of lung cancer cells. Cancer Res. 73:1180–1189. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Svoboda M, Slyskova J, Schneiderova M,
Makovicky P, Bielik L, Levy M, Lipska L, Hemmelova B, Kala Z,
Protivankova M, et al: HOTAIR long non-coding RNA is a negative
prognostic factor not only in primary tumors, but also in the blood
of colorectal cancer patients. Carcinogenesis. 35:1510–1515. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Askarian-Amiri ME, Crawford J, French JD,
Smart CE, Smith MA, Clark MB, Ru K, Mercer TR, Thompson ER, Lakhani
SR, et al: SNORD-host RNA Zfas1 is a regulator of mammary
development and a potential marker for breast cancer. RNA.
17:878–891. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li T, Xie J, Shen C, Cheng D, Shi Y, Wu Z,
Deng X, Chen H, Shen B, Peng C, et al: Amplification of long
noncoding RNA ZFAS1 promotes metastasis in hepatocellular
carcinoma. Cancer Res. 75:3181–3191. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Thorenoor N, Faltejskova-Vychytilova P,
Hombach S, Mlcochova J, Kretz M, Svoboda M and Slaby O: Long
non-coding RNA ZFAS1 interacts with CDK1 and is involved in
p53-dependent cell cycle control and apoptosis in colorectal
cancer. Oncotarget. 7:622–637. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zeng C, Xu Y, Xu L, Yu X, Cheng J, Yang L,
Chen S and Li Y: Inhibition of long non-coding RNA NEAT1 impairs
myeloid differentiation in acute promyelocytic leukemia cells. BMC
Cancer. 14:6932014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zeng C, Yu X, Lai J, Yang L, Chen S and Li
Y: Overexpression of the long non-coding RNA PVT1 is correlated
with leukemic cell proliferation in acute promyelocytic leukemia. J
Hematol Oncol. 8:1262015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
English D and Andersen BR: Single-step
separation of red blood cells. Granulocytes and mononuclear
leukocytes on discontinuous density gradients of Ficoll-Hypaque. J
Immunol Methods. 5:249–252. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fuss IJ, Kanof ME, Smith PD and Zola H:
Isolation of whole mononuclear cells from peripheral blood and cord
blood. Curr Protoc Immunol Chapter. 7:Unit 7.1. 2009.
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lanotte M, Martin-Thouvenin V, Najman S,
Balerini P, Valensi F and Berger R: NB4, a maturation inducible
cell line with t(15;17) marker isolated from a human acute
promyelocytic leukemia (M3). Blood. 77:1080–1086. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dimberg A and Oberg F: Retinoic
acid-induced cell cycle arrest of human myeloid cell lines. Leuk
Lymphoma. 44:1641–1650. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dimberg A, Bahram F, Karlberg I, Larsson
LG, Nilsson K and Oberg F: Retinoic acid-induced cell cycle arrest
of human myeloid cell lines is associated with sequential
down-regulation of c-Myc and cyclin E and posttranscriptional
up-regulation of p27(Kip1). Blood. 99:2199–2206. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Galluzzi L, López-Soto A, Kumar S and
Kroemer G: Caspases connect cell-death signaling to organismal
homeostasis. Immunity. 44:221–231. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Adams J and Nassiri M: Acute promyelocytic
leukemia: A review and discussion of variant translocations. Arch
Pathol Lab Med. 139:1308–1313. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nie F, Yu X, Huang M, Wang Y, Xie M, Ma H,
Wang Z, De W and Sun M: Long noncoding RNA ZFAS1 promotes gastric
cancer cells proliferation by epigenetically repressing KLF2 and
NKD2 expression. Oncotarget. 8:38227–38238. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yu R, Yu BX, Chen JF, Lv XY, Yan ZJ, Chen
Y and Ma Q: Anti-tumor effects of Atractylenolide I on bladder
cancer cells. J Exp Clin Cancer Res. 35:402016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Delbridge AR and Strasser A: The BCL-2
protein family, BH3-mimetics and cancer therapy. Cell Death Differ.
22:1071–1080. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Martinou JC and Youle RJ: Mitochondria in
apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev
Cell. 21:92–101. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gross A, McDonnell JM and Korsmeyer SJ:
BCL-2 family members and the mitochondria in apoptosis. Genes Dev.
13:1899–1911. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Peng W, Wu J and Feng J: Long noncoding
RNA HULC predicts poor clinical outcome and represents
pro-oncogenic activity in diffuse large B-cell lymphoma. Biomed
Pharmacother. 79:188–193. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhu H, Li X, Song Y, Zhang P, Xiao Y and
Xing Y: Long non-coding RNA ANRIL is up-regulated in bladder cancer
and regulates bladder cancer cell proliferation and apoptosis
through the intrinsic pathway. Biochem Biophys Res Commun.
467:223–228. 2015. View Article : Google Scholar : PubMed/NCBI
|