Introduction
Pancreatic ductal adenocarcinoma (PDAC) is a highly
complex and malignant type of cancer in humans. It is the seventh
leading cause of cancer-associated mortality, and is expected to
rise to the third due to its increasing incidence and poor
prognosis (1). Despite considerable
improvements in surgical, radiation and chemotherapeutic
treatments, 80% of patients with PDAC miss the optimal period for
effective systemic therapy due to a lack of symptoms, anesis or
disease regression at the time of diagnosis (2). Hence, the five-year overall survival
rate for PDAC remains at 3–5%. There is thus an urgent need to
identify new biomarkers to improve the understanding of the
molecular mechanisms involved in PDAC pathogenesis (3). Potential prognostic biomarkers and
novel therapeutic targets may help to improve current poor
treatment outcomes.
Microarrays have been widely used to identify more
sensitive and effective biomarkers for PDAC. Shen et al
(4) reported that ribosomal protein
genes nucleoporin 170, nucleoporin 160 and heterogeneous nuclear
ribonucleoprotein U may be useful as molecular markers for early
diagnosis of the disease. Ger et al (5) reported that the fms related tyrosine
kinase 3 and poly(rC) binding protein 3 could potentially be used
as prognostic biomarkers for pancreatic cancer. Another analysis
considered dickkopf WNT signaling pathway inhibitor 1 and high
mobility group AT-hook 2 to be hub genes that are strongly
associated with Wnt family member 3A and tumor protein p53,
respectively (6). In addition, KRAS,
TP53, CDKN2A, SMAD4, RNF43, ARID1A, TGFbR2, GNAS, RREB1 and PBRM1
were identified as driver genes in PDAC (7). Variation in the significantly expressed
genes associated with PDAC pathogenesis between different studies
may be due to small sample sizes, the use of different microarray
platforms and different statistical methods. To overcome these
limitations, integrative meta-analysis using different microarray
platforms with larger sample sizes may prove to be a powerful
bioinformatics tool, improving the accuracy and reliability of data
analysis.
In the present study, multiple Gene Expression
Omnibus (GEO) datasets containing a large number of samples were
acquired from two different microarray platforms (Affymetrix and
Agilent). The RobustRankAggreg (RRA) 1.1 package (8) in R is based on a statistical model that
allows for the evaluation of the significance of results. It can be
used to identify differentially expressed genes (DEGs) across
multiple datasets from different microarray platforms. By defining
the rank vector for each gene, based only on the datasets where it
is present, the results include DEGs that are not present in every
dataset (9). Hence, the gene
expression module was investigated to reveal the genes influencing
PDAC tumorigenesis.
Materials and methods
Selection and retrieval of microarray
datasets
A total of 8 gene expression profiles [GSE15471
(10), GSE16515 (11), GSE41368 (12), GSE62165 (13), GSE62452 (14), GSE71729 (15), GSE71989 (16) and GSE91035 (17)] from two platforms (Affymetrix and
Agilent) were retrieved from the GEO (http://www.ncbi.nlm.nih.gov/geo/) database, using the
keywords ‘pancreatic ductal adenocarcinoma’, ‘Homo sapiens’ and
‘microarray’. The selected microarray datasets met the following
inclusion criteria: i) Expression profiling by array; ii) samples
included human PDAC and corresponding adjacent or normal pancreatic
tissue; iii) n>10; and iv) the gene expression profile is
complete. A total of 8 microarray datasets were retrieved from two
different microarray platforms. The data included 452 PDAC samples
and 204 normal pancreatic tissue samples (Table I). For further validation, normalized
datasets (fragments per kilobase of transcript per million mapped
reads upper quartile) of 146 PDAC samples (with complete expression
profiles and clinical prognoses) were retrieved from The Cancer
Genome Atlas (TCGA) database (7).
 | Table I.Gene expression profile data
characteristics. |
Table I.
Gene expression profile data
characteristics.
| Author, Year | Dataset | Count | Tumor | Normal | Platform | Region | (Refs.) |
|---|
| Badea et al,
2009 | GSE15471 | 78 | 39 | 39 | GPL570 | Romania | 10 |
| Pei et al,
2009 | GSE16515 | 52 | 36 | 16 | GPL570 | USA | 11 |
| Frampton et
al, 2012 | GSE41368 | 12 | 6 | 6 | GPL6244 | Italy | 12 |
| Janky et al,
2014 | GSE62165 | 131 | 118 | 13 | GPL13667 | Belgium | 13 |
| Yang et al,
2014 | GSE62452 | 130 | 69 | 61 | GPL6244 | USA | 14 |
| Moffitt et
al, 2015 | GSE71729 | 191 | 145 | 46 | GPL20769 | USA | 15 |
| Schmittgen,
2015 | GSE71989 | 22 | 14 | 8 | GPL570 | USA | 16 |
| Schmittgen,
2016 | GSE91035 | 40 | 25 | 15 | GPL22763 | USA | 17 |
Data pre-processing and DEG
analysis
Initially, log2 conversion and quantile
normalization was performed on each individual GEO dataset. DEGs of
each dataset were then screened using the limma package (http://bioinf.wehi.edu.au/limma) with
R/Bioconductor 3.9 software (http://www.bioconductor.org/). The RRA package was
used for gene integration analysis of the DEGs in the eight
datasets. P<0.05 was considered to indicate a statistically
significant result and a fold-change (log-scaled) of mean + 2SD was
set as the threshold in the limma package. In the RRA package, an
adjusted P<0.05 was considered to indicate a statistically
significant difference.
Functional enrichment analysis
The functions of common DEGs were further analyzed
using the clusterProfiler package (https://guangchuangyu.github.io/software/clusterProfiler)
with R/Bioconductor software for functional enrichment analysis.
This included the following gene ontology (GO) categories:
Molecular function (MF), biological process (BP) and cellular
component (CC), as well as enrichment analysis of the Kyoto
Encyclopedia of Genes and Genomes (KEGG) pathways. P<0.05 was
set as the threshold value for MF, BP and CC; and P<0.01 was set
as the threshold value for KEGG analysis.
Protein-protein interaction (PPI)
network construction
In the present study, the Search Tool for the
Retrieval of Interacting Genes/Proteins (STRING; string-db.org) database was used to construct the PPI
network of common DEGs, which was then visualized using Cytoscape
(3.7.1) software (18). The
Cytoscape MCODE plug-in was used to search for clustered
sub-networks, and the default parameters were as follows: Degree
cutoff, ≥2; node score cutoff, ≥0.2; K-core, ≥2; max depth,
100.
Prediction system construction
As the GSE62452 dataset and TCGA data contain
patient survival information, they can be used as training and
validation datasets, respectively. Univariate Cox proportional
hazard analysis was applied to identify the prognosis-associated
genes in GEO datasets (training set), using survival analysis in R,
with P<0.05 set as the significance threshold. Multivariate Cox
regression analysis was then applied to further screen for factors
associated with patient survival. Subsequently, a prediction system
was constructed consisting of five signature prognostic genes
[laminin subunit γ 2 (LAMC2), laminin subunit β 3 (LAMB2), Serpin
family B member 5 (SERPINB5), amphiregulin (AREG) and secreted
frizzled related protein 4 (SFRP4)], and was used to construct a
risk score formula. Each patient's risk score was calculated and
the median risk score was regarded as the cutoff point. Patients
were divided into a high- and a low-risk group, in accordance with
their prognostic risk scores. Kaplan-Meier (KM) analysis [with
log-rank test (Mantel-Cox)] was then performed to calculate and
compare the survival time between the two groups, with P<0.05
selected to indicate a significant difference. KM curves were
constructed using the R ‘survival’ package. Finally, receiver
operating characteristic (ROC) analysis was conducted using the R
‘survivalROC’ package to identify the sensitivity and specificity
of the prediction system. The five aforementioned prognostic
signature genes were applied to TCGA dataset (validation set) to
verify whether they could effectively predict the prognosis of PDAC
(19).
Results
Identification of DEGs
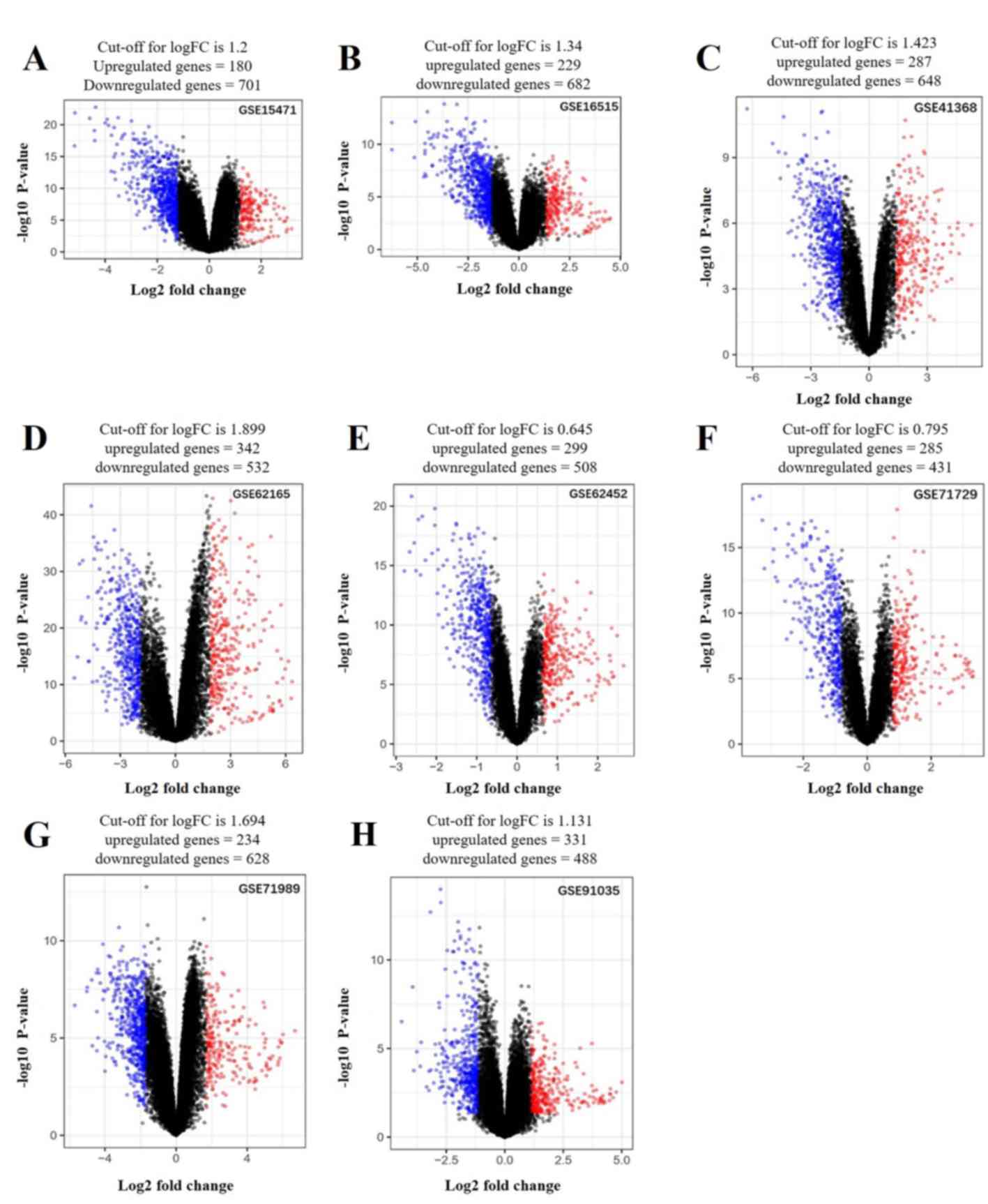
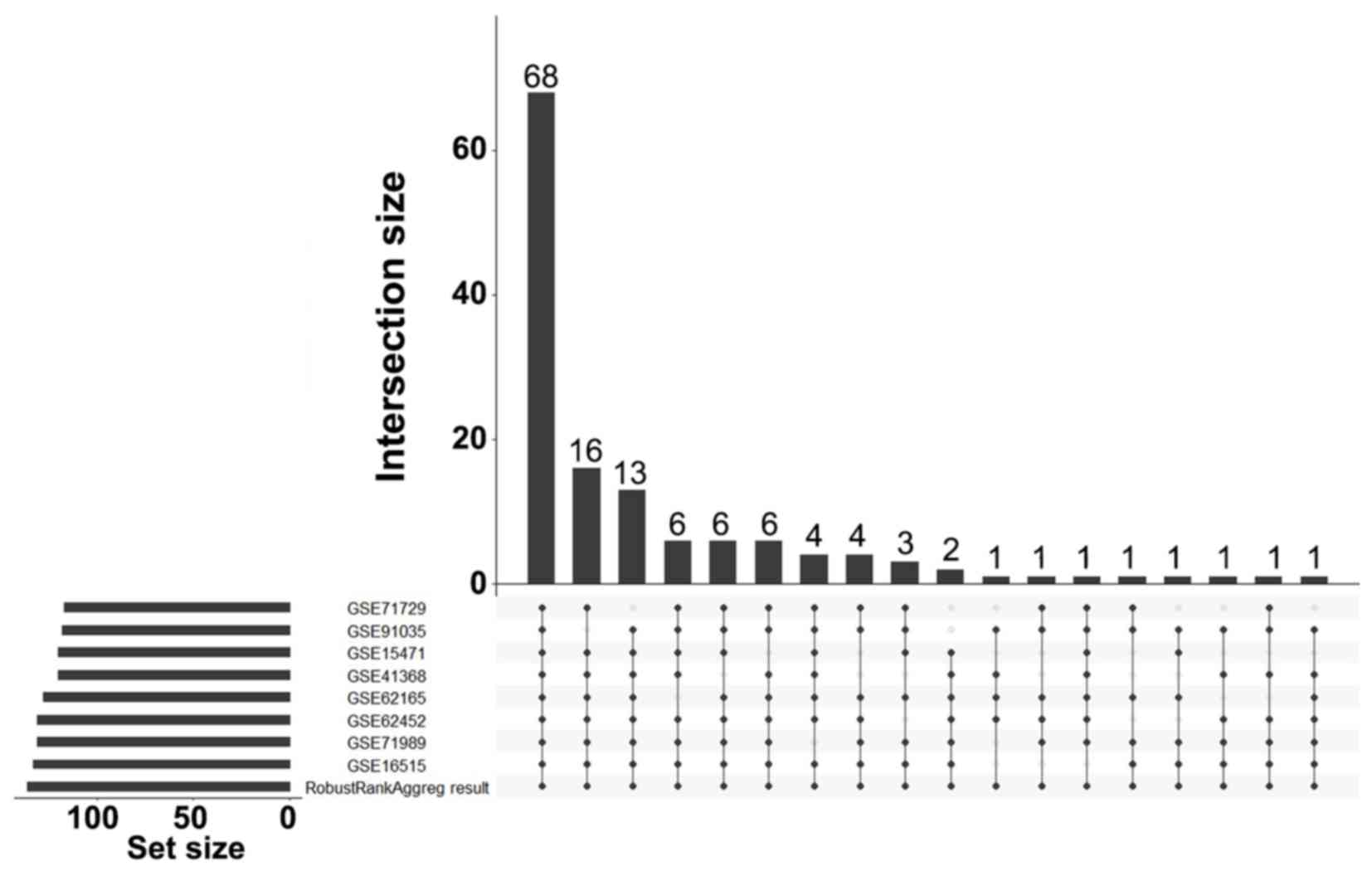
A total of 136 DEGs (67 up- and 69 downregulated
genes) were identified between PDAC tissues and normal tissues by
analyzing eight gene expression profiles retrieved from the GEO
database. Volcano plots of the gene expression profile data, and a
heat map and histogram of DEGs across the datasets are displayed in
Figs. 1–3.
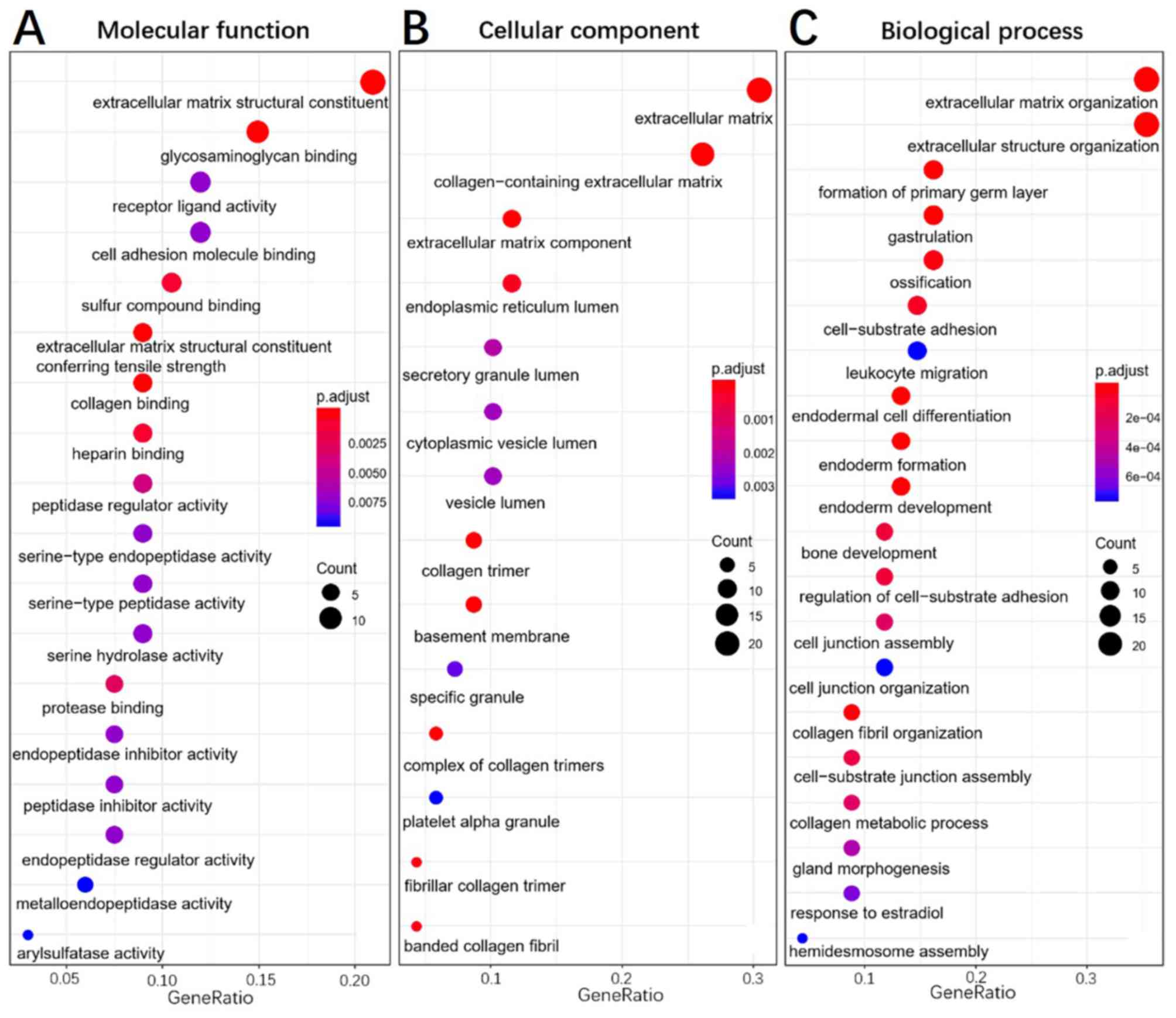
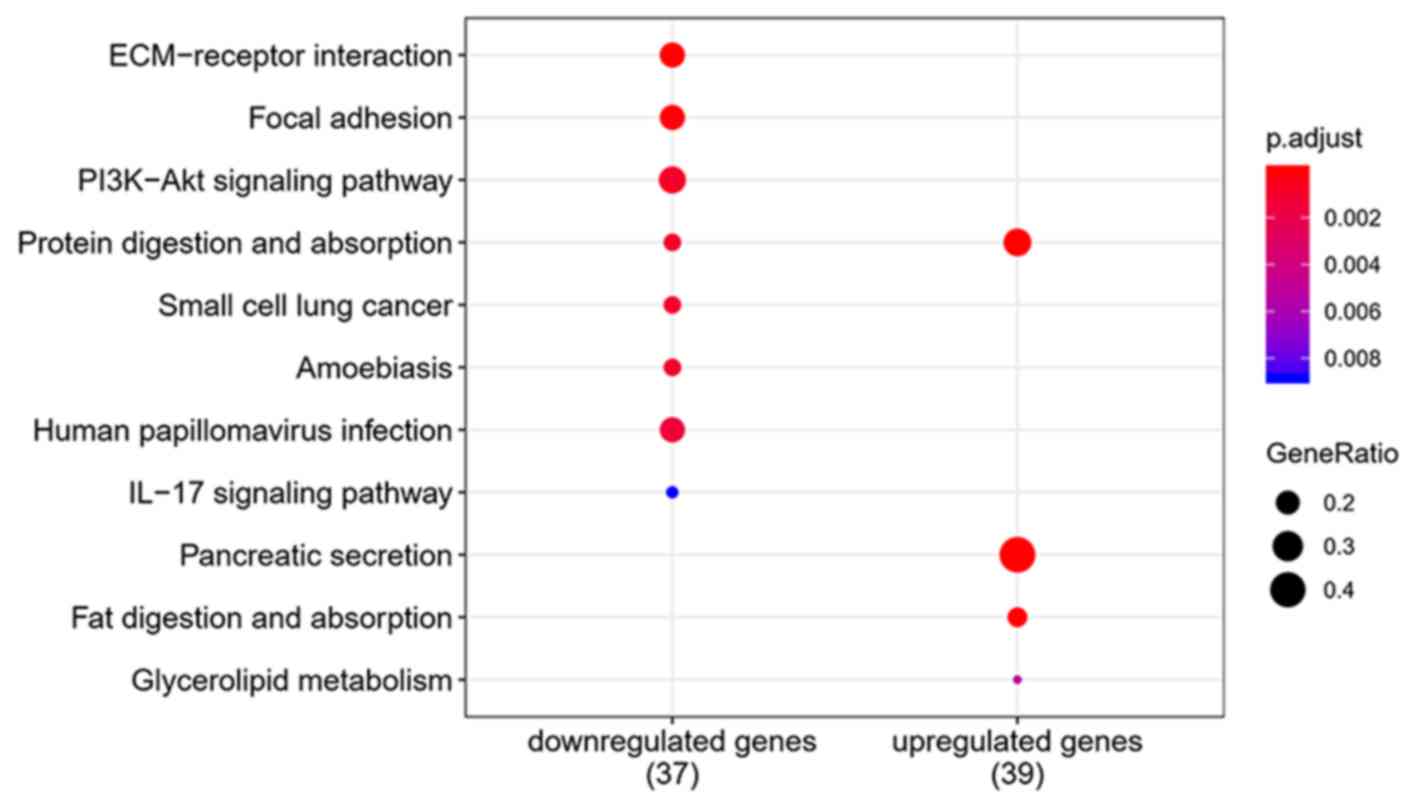
Functional enrichment analysis
To elucidate the functions of common DEGs, GO and
KEGG pathway enrichment analyses were performed. The GO results
determined that, in the MF category, the upregulated genes were
predominantly enriched in ‘extracellular matrix constituent’ and
‘glycosaminoglycan binding’, whilst the downregulated genes were
mainly enriched in ‘serine-type endopeptidase activity’,
‘serine-type peptidase activity’ and ‘serine hydrolase activity’.
In the BP category, the upregulated genes were mainly enriched in
‘ECM organization’ and ‘extracellular structure organization’,
whilst the downregulated genes were enriched in ‘antimicrobial
humoral response’ and ‘digestion’. In the CC category, the
upregulated genes were mainly enriched in ‘ECM’ and
‘collagen-containing ECM’, whilst the downregulated genes were not
enriched. ‘Pancreatic secretion’,
‘phosphoinositide-3-kinase-protein kinase B/Akt (PI3K-Akt)
signaling pathway’, ‘protein digestion and absorption’ and
‘ECM-receptor interaction’ were the most enriched pathways in the
KEGG pathway analysis. The results of the functional enrichment
analysis are exhibited in Figs.
4–6 and support the results of
previous studies (20,21).
Hub gene identification using PPI
network construction and modular analysis
A PPI network based on the DEGs was constructed
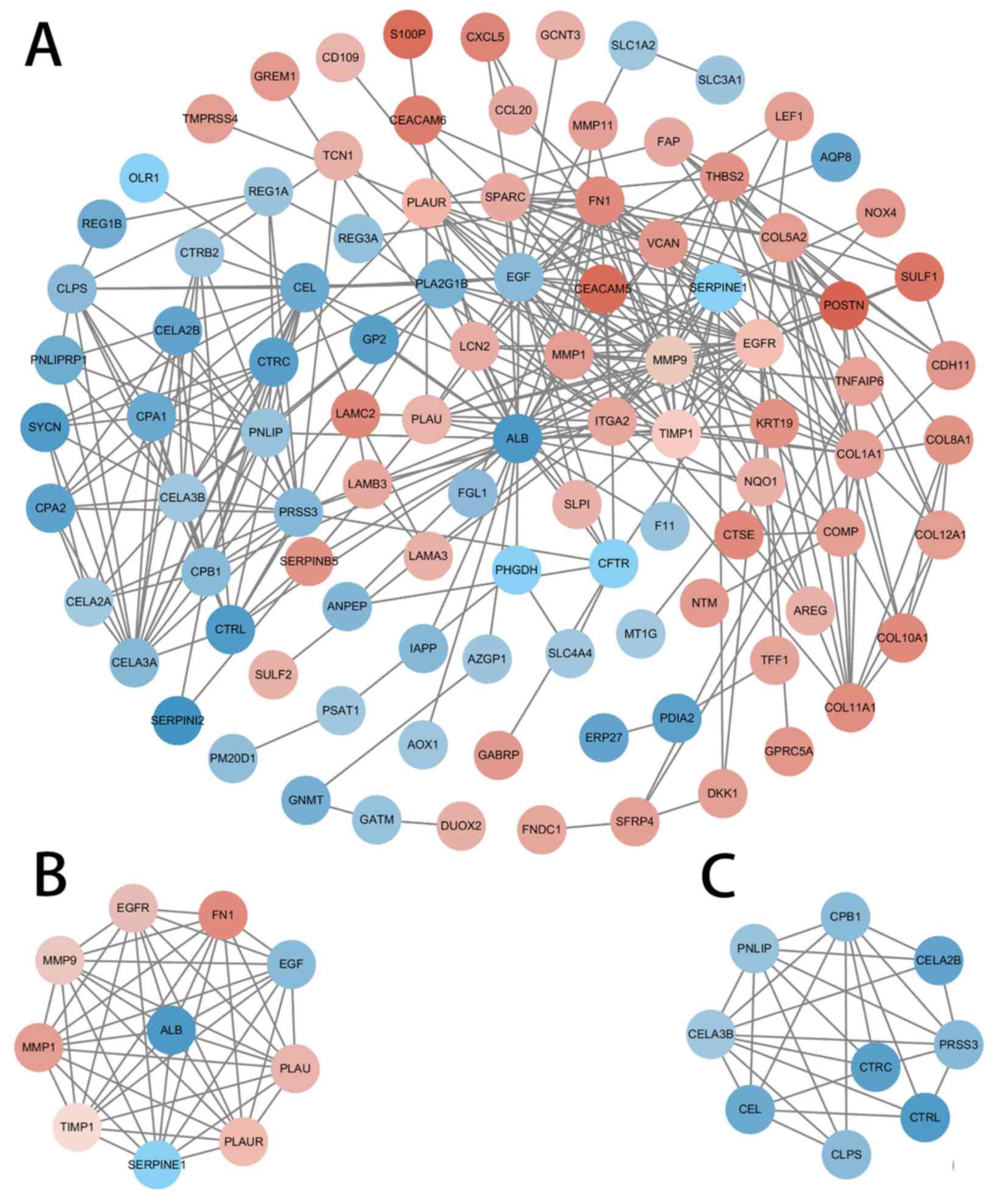
using Cytoscape software and the STRING database (Fig. 7). The ten genes with the highest
degree of connectivity [albumin (ALB), epidermal growth factor
(EGF), MMP9, epidermal growth factor receptor (EGFR), fibronectin 1
(FN1), matrix metalloproteinase (MMP) 1, plasminogen activator
inhibitor-1 (SERPINE1), tissue inhibitors of metalloproteinases
(TIMP1), plasminogen activator urokinase (PLAU) and PLAU receptor
(PLAUR)] were selected as the hub genes; two modules with MCODE
scores>5 were selected from the PPI network (Fig. 7B and C). Coincidentally, Module 1 was
composed of the 10 hub genes previously selected (Table II).
 | Table II.Hub genes with high degree of
connectivity. |
Table II.
Hub genes with high degree of
connectivity.
| Name | MCODE cluster | MCODE score | Degree |
|---|
| FN1 | Cluster 1 | 7.363636 | 20 |
| ALB | Cluster 1 | 7.363636 | 33 |
| MMP1 | Cluster 1 | 7.363636 | 16 |
| TIMP1 | Cluster 1 | 7.363636 | 14 |
| MMP9 | Cluster 1 | 7.363636 | 23 |
| SERPINE1 | Cluster 1 | 7.363636 | 13 |
| PLAUR | Cluster 1 | 7.363636 | 12 |
| PLAU | Cluster 1 | 7.363636 | 11 |
| EGF | Cluster 1 | 7.363636 | 26 |
| EGFR | Cluster 1 | 7.363636 | 23 |
Construction of the prediction
system
The prognostic prediction system, composed of five
signature genes (LAMC2, LAMB3, SERPINB5, AREG and SFRP4), was
constructed using the survival information of 66 patients in the
training set (GSE62452). The risk score formula for each patient
was calculated as shown below: Risk score=(1.0656) × LAMC2 +
(−0.5804) × LAMB3 + (−0.4488) × SERPINB5 + (0.3060) × AREG +
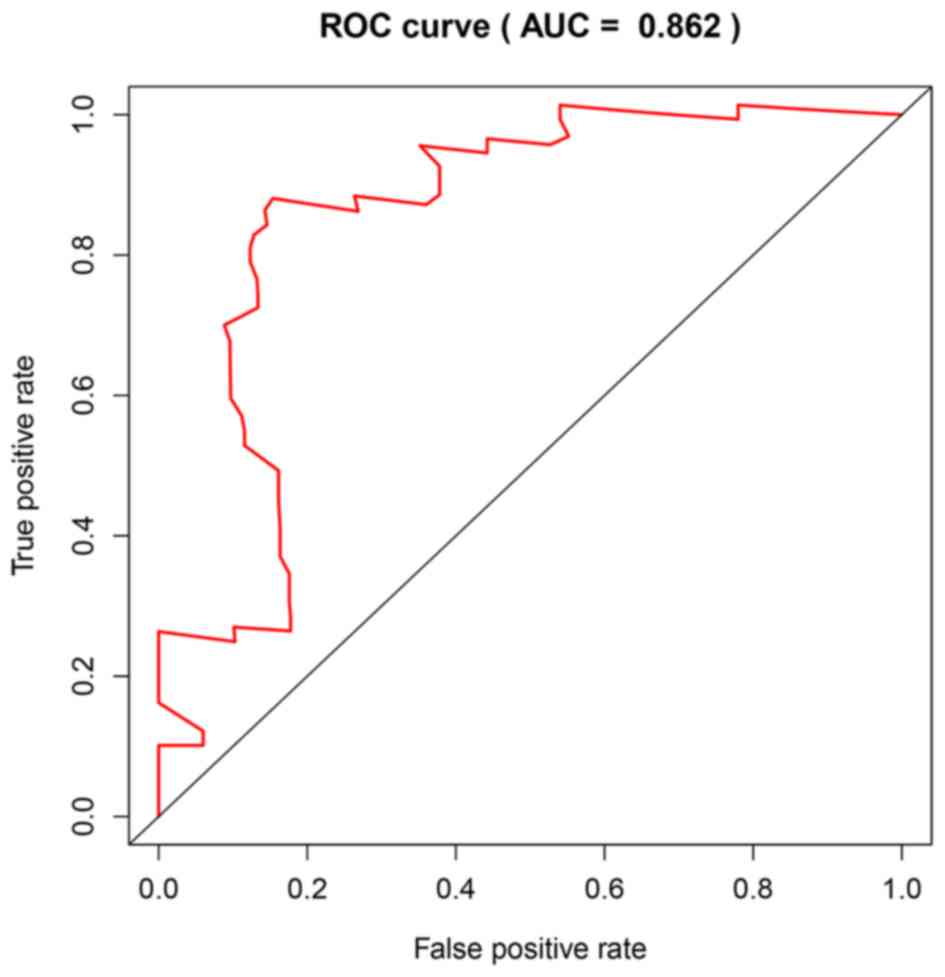
(−0.5294) × SFRP4. The area under the ROC curve was found to be
0.862, and consequently, specificity and sensitivity were both
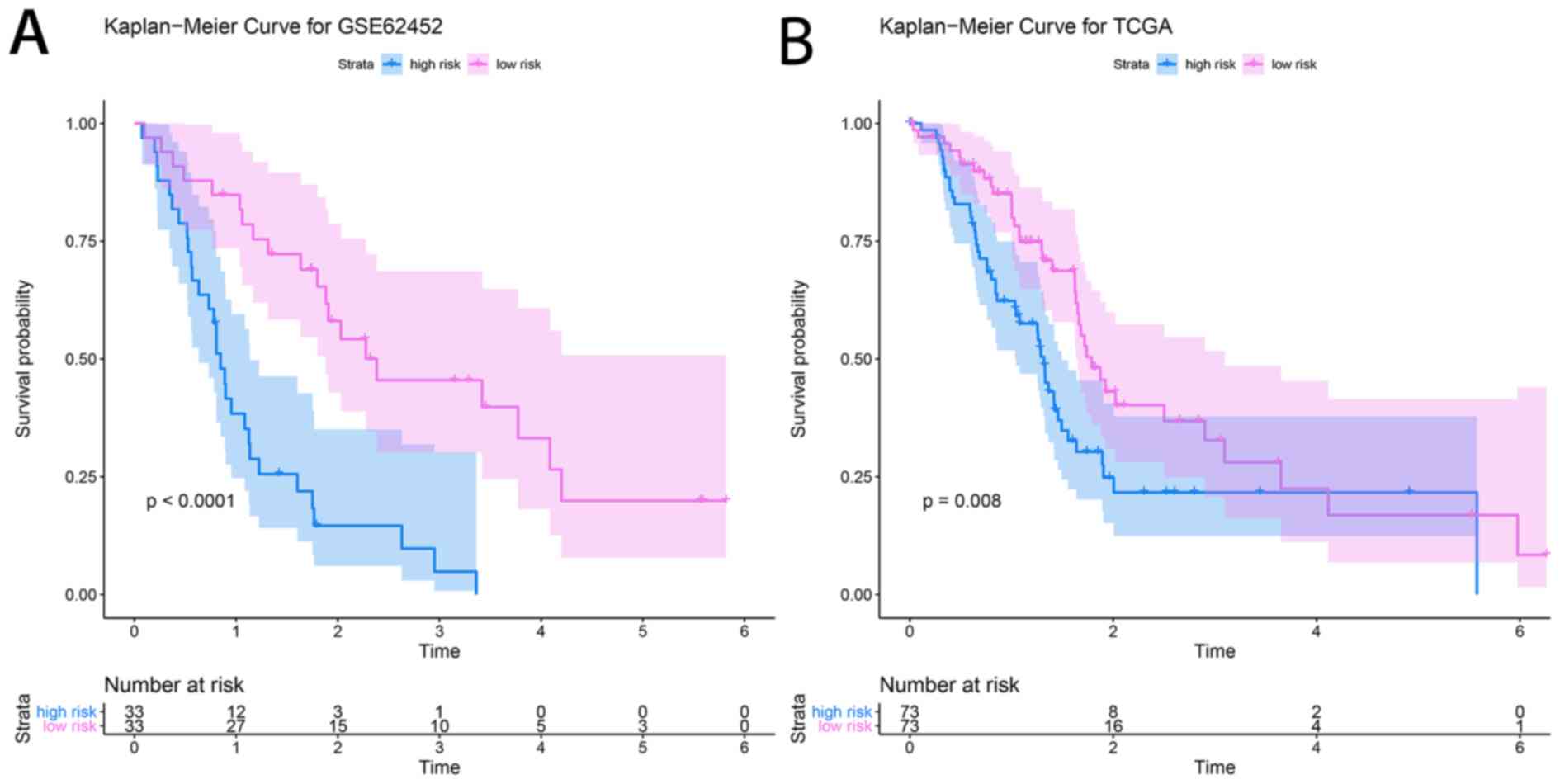
determined to be highest when the risk score was 0.960 (Fig. 8). The PDAC patients of the GSE62452
dataset were divided into a high-risk group (risk score, ≥0.960;
n=33) and a low-risk group (risk score, <0.960; n=33). The
patients in the low-risk group (45.5%; 95% CI, 30.1–68.7%) had a
significantly higher survival rate than those in the high-risk
group (48.0%; 95% CI, 33.5–68.7%; P=4×10−6; Fig. 9A).
Validation of the prediction
system
A dataset retrieved from TCGA, consisting of the
clinical prognostic information of 146 patients with PDAC, was used
as an independent validation dataset for the prognostic prediction
system. The individual risk score of each patient was calculated
using the aforementioned formula. A risk score of 0.960 was used as
the threshold and the patient samples were divided into high- and
low-risk groups (n=73 each). KM survival analysis showed that the
high-risk group (48.7%; 95% CI, 37.8–62.7%) had significantly
poorer OS scores than the low-risk group (48.1%; 95% CI,
36.0–64.4%; P=0.008; Fig. 9B).
Discussion
The pathogenesis of PDAC is extremely complex.
Whilst there have been many studies on the biological mechanisms
underpinning PDAC, the results are inconsistent and various aspects
remain unclear. This could be attributable to the three following
aspects: i) Small study sample sizes; ii) datasets retrieved from
different platforms; and iii) different statistical analysis
methods. However, using a combination of multiple datasets from
different platforms, the present study aimed to improve the
accuracy and reliability of these results. In the present study,
eight PDAC datasets (GSE15471, GSE16515, GSE41368, GSE62165,
GSE62452, GSE71729, GSE71989 and GSE91035) from two
platforms (Affymetrix and Agilent) were analyzed, and the RRA
package was used to account for the differences between the
platforms. Finally, a dataset from TCGA was used to verify the data
and to further improve reliability.
A total of 136 DEGs were identified from the GEO
datasets. These included 67 up- and 69 downregulated genes, which
were differentially expressed in PDAC samples, compared with the
normal controls.
GO enrichment analysis determined that the most
significant enrichments in MF, BP and CC were related to the
extracellular matrix (ECM), especially for the upregulated genes.
For KEGG pathways analysis, ‘pancreatic secretion’, ‘PI3K-Akt
signaling pathway’ and ‘ECM-receptor interaction’ were highly
enriched. High enrichment of ECM related genes and pathways
suggests that ECM regulation is closely associated with PDAC
progression. The ECM is a complex, three-dimensional structure
composed of both structural and non-structural proteins (22,23). It
plays a fundamental role in facilitating cell differentiation,
apoptosis, proliferation and migration (24). The ECM is also the most abundant
component in the tumor microenvironment (TME), which profoundly
influences the behavior of cancer cells (25). Furthermore, certain studies have
suggested that alterations in the ECM can induce cell
transformation and metastasis, promoting the development and
progression of tumors (26,27). In the TME of PDAC, some ECM proteins,
such as collagen, fibronectin and laminin, are significantly
upregulated (28). Each of these
proteins promotes the growth and invasion of PDAC cells (29–32).
Moreover, the results of the present study indicated LAMC2 and
LAMB2 are significantly associated with PDAC prognosis.
Based on PPI network analysis of the common DEGs in
the selected studies, the 10 most highly connected genes (ALB, EGF,
MMP9, EGFR, FN1, MMP1, SERPINE1, TIMP1, PLAU and PLAUR) were
screened and Module 1 is, coincidentally, composed of the 10 hub
genes. Furthermore, FN1 and PLAU have previously been identified as
hub genes in a similar study (10).
Multiple studies have determined that these genes are associated
with tumorigenesis and progression. For example, the FN1 gene
encodes fibronectin, which is a major constituent of the ECM within
the TME. The binding of FN1 to its receptor activates the FN1
signaling pathway in pancreatic cancer cells, and promotes tumor
cell survival, invasion, metastasis and angiogenesis (33). The expression of fibronectin in
pancreatic cancer cells is also associated with a low survival rate
(34,35). In addition, FN1 participates in the
progression of ovarian cancer by increasing the expression level of
matrix metalloproteinase MMP-9 (36). MMPs and TIMPs are important enzymes
in the process of ECM degradation. Expression of the MMP-9 protein
is upregulated during the progression of various cancer types,
including pancreatic cancer, and is directly involved in tumor cell
migration, invasion, metastasis, tumor-related inflammation and
angiogenesis (37,38). Therefore, its expression is
associated with malignant progression (39).
The MMP-1 protein is one of the most widely
expressed MMPs. It activates the G protein-coupled receptor
protease-activated receptor-1 (PAR1) and induces secretion of
bioactive proteins (most prominently interleukin-8,
growth-regulated oncogene-α and C-C motif chemokine ligand 2) to
regulate tumor migration (40).
TIMP-1 enzyme is a natural inhibitor of MMPs, but it can also
stimulate cell proliferation and prevent apoptosis, promoting
cancer progression (41). The
binding of TIMP1 to receptors on the cell surface activates the
PI3K/Akt signaling pathway via Ras, and the EGF signal then induces
TIMP1 expression (42,43). High plasma TIMP1 levels may interact
with EGFR signaling, and thereby reduce the anti-tumor effects of
EGFR inhibitors (44). The EGF and
EGFR proteins are widely recognized for their role in numerous
cancer types, including PDAC (45,46).
Upon binding to EGF, EGFR forms homologous dimers,
auto-phosphorylates and interacts with downstream factors to
activate genes involved in cell proliferation, differentiation,
survival and migration (47,48). As such, inhibitors of the EGFR
signaling pathway have received attention as potential therapeutic
agents (49). Moreover, EGF-induced
activation of EGFR increases MMP-9 expression levels through the
activation of the PI3K/Akt pathway in patients with glioblastoma
(50).
In the present study, the ALB gene showed the
highest connectivity to other genes in Module 1A, and this gene
encodes the most abundant protein found in human blood (51). Cachexia is expressed in <80% of
patients with PDAC, and plasma albumin is often reduced (52,53). In
the current study, it was speculated that besides digestive and
absorption disorders, cachexia may also be related to the low
expression of ALB. Nevertheless, investigations into the regulatory
genetic mechanism of ALB in PDAC have rarely been reported. PLAU,
PLAUR and SERPINE1 represent the main components of the system.
This system not only participates in cell signaling pathways (such
as angiogenesis, cell growth, cell adhesion and migration) to
influence cancer-related processes, but also in the disruption of
the ECM through various pathways, such as the downregulation of the
tumor suppressor gene p53, interference of Hoxa5 function,
inhibition of the p38 pathway and activation of mitogen-activated
protein kinase 1 (54–57).
An accurate and credible prognostic prediction
system may help to better evaluate patient prognosis, provide a
basis for clinical decision-making and indicate new therapeutic
targets. Of the five genes associated with prognosis in the present
study, LAMC2 and SER5 have also been reported to be
prognosis-associated genes in PDAC (6,10). The
LAMC2 and laminin subunit α 3 proteins are important components of
laminin 5. Through interaction with cell surface receptors, they
participate in a variety of biological processes, including cell
adhesion, differentiation, tumor angiogenesis and metastasis. The
upregulation of LAMC2 is considered an indicator of adverse
prognosis and the high metastatic potential of multiple cancer
types, including colorectal cancer and lung adenocarcinoma
(58). LAMC2 gene-silencing has been
shown to significantly inhibit cell migration and invasion in head
and neck squamous cell carcinoma cells (59). The LAMB3 protein regulates epithelial
mesenchymal transition-related proteins and MMP-9 via the
activation of the Akt signaling pathway, to promote the invasion
and metastasis of tumors in papillary thyroid cancer (60). The important role of LAMA3 in the
metastasis of lung adenocarcinoma has also been reported (61).
SERPINB5 is regarded as a tumor suppressor and an
important senescence-associated biomarker. Notably, it is expressed
in PDAC, but not in healthy pancreatic tissue (62). There is abundant evidence that
SERPINB5 plays an oncogenic and pro-metastatic role (63,64), and
is associated with the prognosis of patients with PDAC (62).
AREG is a ligand of EGFR and is overexpressed in
various cancer tissues. It has been demonstrated that high AREG
expression can be used as an independent prognostic indicator of
poor overall survival in patients with PDAC, and there is a
significant association between AREG/EGFR co-expression and poor
tumor differentiation (65). The
AREG protein has been clinically indicated as a prognostic and
predictive biomarker, and numerous novel strategies have been
developed to disrupt AREG-mediated oncogenic pathways (66).
SFRP4 is a Wnt-signaling antagonist. Due to its
pro-apoptotic properties, SFRP4 provides axon-guidance information
and functions as a tumor suppressor in multiple tissue types.
Highly methylated SFRP4 induces transcriptional silencing, which
activates abnormal Wnt signaling, leading to tumorigenesis and
tumor progression (67). Moreover,
SFRP4 monotherapy (or in combination with chemotherapy) can inhibit
proliferation, reduce cell survival and initiate apoptosis in
cancer stem cells in breast, prostate and ovarian cancer cell
lines. This increases cell sensitivity to chemotherapy, amplifying
the treatment effect (68). However,
the mechanism of SFRP4 in PDAC remains poorly characterized.
Certain limitations of the present study should be
noted. Firstly, the effects of certain patient and disease
characteristics (tumor grade/stage, sex, age and race) on gene
expression were not accounted for. Secondly, the GEO and TCGA data
were obtained from public databases, and consequently, evaluation
of data quality was challenging. Thirdly, variation in the accuracy
of the prediction system for different molecular subtypes of PDAC
has not been investigated due to a lack of consensus on the
clinical classification of different molecular subtypes. Fourthly,
TCGA dataset was used to validate 136 DEGs identified in the
meta-analysis of the GEO datasets, but the results are not as
similar as anticipated. This could be due to the small sample size
of normal tissues in TCGA dataset. Finally, analysis was performed
on gene expression databases; further molecular experimentation is
required to validate the results.
In conclusion, the present study confirmed certain
DEGs that were previously indicated in similar studies, and has
also identified a set of genes that were not discovered in
individual analyses. Therefore, this technique should be considered
useful for the further analysis of heterogeneous expression
datasets, improvement of DEG recognition techniques and the
discovery of new biomarkers for PDAC diagnosis or therapeutic
targeting. Furthermore, the present study identified 10 hub genes
that may be involved in the pathogenesis of PDAC using
multi-platform, multi-gene expression profile datasets and
bioinformatics meta-analysis. In addition, a reliable predictive
system, composed of five genes, for determining the prognosis of
patients with PDAC has been constructed. The results of the current
study may help to guide individualized clinical decision-making and
future molecular-targeted therapies.
Acknowledgements
Not applicable.
Funding
The present study was an independent study funded by
the following grants: Medical science and technology plan projects
of Zhejiang (grant no. 2017196257), the Youth Foundation of
Southwest Medical University (grant no. 0903-00031099) and the
Doctoral research start-up funding project of Affiliated Hospital
of Southwest Medical University (grant no. 16229).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the GEO and TCGA databases.
Authors' contributions
XT and YP conceived and designed the study. YQP and
YM drafted the manuscript. YM and YQP collected and analyzed the
data. LP critically revised the manuscript. XL, JX and LP were
responsible for interpretation of the data. The manuscript was
revised by YP XT, with final approval from YP.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hidalgo M: Pancreatic cancer. N Engl J
Med. 362:1605–1617. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shen Q, Yu M, Jia JK, Li WX, Tian YW and
Xue HZ: Possible Molecular markers for the diagnosis of pancreatic
ductal adenocarcinoma. Med Sci Monit. 24:2368–2376. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ger M, Kaupinis A, Petrulionis M,
Kurlinkus B, Cicenas J, Sileikis A, Valius M and Strupas K:
Proteomic identification of FLT3 and PCBP3 as potential prognostic
biomarkers for pancreatic cancer. Anticancer Res. 38:5759–5765.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tang Y, Zhang Z, Tang Y, Chen X and Zhou
J: Identification of potential target genes in pancreatic ductal
adenocarcinoma by bioinformatics analysis. Oncol Lett.
16:2453–2461. 2018.PubMed/NCBI
|
|
7
|
Cancer Genome Atlas Research Network:; and
Cancer Genome Atlas Research Network, . Integrated Genomic
Characterization of Pancreatic Ductal Adenocarcinoma. Cancer Cell.
32:185–203 e13. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kolde R and Laur S.; RobustRankAggreg, :
Methods for robust rank aggregation. 2013.
|
|
9
|
Kolde R, Laur S, Adler P and Vilo J:
Robust rank aggregation for gene list integration and
meta-analysis. Bioinformatics. 28:573–580. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Badea L, Herlea V, Dima SO, Dumitrascu T
and Popescu I: Combined gene expression analysis of whole-tissue
and microdissected pancreatic ductal adenocarcinoma identifies
genes specifically overexpressed in tumor epithelia.
Hepatogastroenterology. 55:2016–2027. 2008.PubMed/NCBI
|
|
11
|
Pei H, Li L, Fridley BL, Jenkins GD,
Kalari KR, Lingle W, Petersen G, Lou Z and Wang L: FKBP51 affects
cancer cell response to chemotherapy by negatively regulating Akt.
Cancer Cell. 16:259–266. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Frampton AE, Castellano L, Colombo T,
Giovannetti E, Krell J, Jacob J, Pellegrino L, Roca-Alonso L, Funel
N, Gall TM, et al: MicroRNAs cooperatively inhibit a network of
tumor suppressor genes to promote pancreatic tumor growth and
progression. Gastroenterology. 146:268–277 e18. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Janky R, Binda MM, Allemeersch J, Van den
Broeck A, Govaere O, Swinnen JV, Roskams T, Aerts S and Topal B:
Prognostic relevance of molecular subtypes and master regulators in
pancreatic ductal adenocarcinoma. BMC Cancer. 16:6322016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang S, He P, Wang J, Schetter A, Tang W,
Funamizu N, Yanaga K, Uwagawa T, Satoskar AR, Gaedcke J, et al: A
Novel MIF signaling pathway drives the malignant character of
pancreatic cancer by targeting NR3C2. Cancer Res. 76:3838–3850.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Moffitt RA, Marayati R, Flate EL, Volmar
KE, Loeza SG, Hoadley KA, Rashid NU, Williams LA, Eaton SC, Chung
AH, et al: Virtual microdissection identifies distinct tumor- and
stroma-specific subtypes of pancreatic ductal adenocarcinoma. Nat
Genet. 47:1168–1178. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jiang J, Azevedo-Pouly AC, Redis RS, Lee
EJ, Gusev Y, Allard D, Sutaria DS, Badawi M, Elgamal OA, Lerner MR,
Brackett DJ, et al: Globally increased ultraconserved noncoding RNA
expression in pancreatic adenocarcinoma. Oncotarget. 7:53165–53177.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sutaria DS, Jiang J, Azevedo-Pouly ACP,
Lee EJ, Lerner MR, Brackett DJ, Vandesompele J, Mestdagh P and
Schmittgen TD: Expression Profiling Identifies the Noncoding
Processed Transcript of HNRNPU with Proliferative Properties in
Pancreatic Ductal Adenocarcinoma. Noncoding RNA.
3:ncrna30300242017.
|
|
18
|
Kohl M, Wiese S and Warscheid B.
Cytoscape: Software for visualization and analysis of biological
networks. Methods Mol Biol. 696:291–303. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhao X, Sun S, Zeng X and Cui L:
Expression profiles analysis identifies a novel three-mRNA
signature to predict overall survival in oral squamous cell
carcinoma. Am J Cancer Res. 8:450–461. 2018.PubMed/NCBI
|
|
20
|
Zhu T, Gao YF, Chen YX, Wang ZB, Yin JY,
Mao XY, Li X, Zhang W, Zhou HH and Liu ZQ: Genome-scale analysis
identifies GJB2 and ERO1LB as prognosis markers in patients with
pancreatic cancer. Oncotarget. 8:21281–21289. 2017.PubMed/NCBI
|
|
21
|
Li H, Wang X, Fang Y, Huo Z, Lu X, Zhan X,
Deng X, Peng C and Shen B: Integrated expression profiles analysis
reveals novel predictive biomarker in pancreatic ductal
adenocarcinoma. Oncotarget. 8:52571–52583. 2017.PubMed/NCBI
|
|
22
|
Hynes RO and Naba A: Overview of the
matrisome-an inventory of extracellular matrix constituents and
functions. Cold Spring Harb Perspect Biol. 4:a0049032012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Humphrey JD, Dufresne ER and Schwartz MA:
Mechanotransduction and extracellular matrix homeostasis. Nat Rev
Mol Cell Biol. 15:802–812. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Venning FA, Wullkopf L and Erler JT:
Targeting ECM disrupts cancer progression. Front Oncol. 5:2242015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fang M, Yuan J, Peng C and Li Y: Collagen
as a double-edged sword in tumor progression. Tumour Biol.
35:2871–2882. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Miles FL and Sikes RA: Insidious changes
in stromal matrix fuel cancer progression. Mol Cancer Res.
12:297–312. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pickup MW, Mouw JK and Weaver VM: The
extracellular matrix modulates the hallmarks of cancer. EMBO Rep.
15:1243–1253. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mollenhauer J, Roether I and Kern HF:
Distribution of extracellular matrix proteins in pancreatic ductal
adenocarcinoma and its influence on tumor cell proliferation in
vitro. Pancreas. 2:14–24. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Armstrong T, Packham G, Murphy LB, Bateman
AC, Conti JA, Fine DR, Johnson CD, Benyon RC and Iredale JP: Type I
collagen promotes the malignant phenotype of pancreatic ductal
adenocarcinoma. Clin Cancer Res. 10:7427–7437. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Vaquero EC, Edderkaoui M, Nam KJ, Gukovsky
I, Pandol SJ and Gukovskaya AS: Extracellular matrix proteins
protect pancreatic cancer cells from death via mitochondrial and
nonmitochondrial pathways. Gastroenterology. 125:1188–1202. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Koenig A, Mueller C, Hasel C, Adler G and
Menke A: Collagen type I induces disruption of E-cadherin-mediated
cell-cell contacts and promotes proliferation of pancreatic
carcinoma cells. Cancer Res. 66:4662–4671. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Grzesiak JJ and Bouvet M: The alpha2beta1
integrin mediates the malignant phenotype on type I collagen in
pancreatic cancer cell lines. Br J Cancer. 94:1311–1319. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Topalovski M and Brekken RA: Matrix
control of pancreatic cancer: New insights into fibronectin
signaling. Cancer Lett. 381:252–258. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Glasner A, Levi A, Enk J, Isaacson B,
Viukov S, Orlanski S, Scope A, Neuman T, Enk CD, Hanna JH, et al:
NKp46 receptor-mediated interferon-ү production by natural killer
cells increases fibronectin 1 to alter tumor architecture and
control metastasis. Immunity. 48:107–119 e4. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hu D, Ansari D, Zhou Q, Sasor A, Said
Hilmersson K and Andersson R: Stromal fibronectin expression in
patients with resected pancreatic ductal adenocarcinoma. World J
Surg Oncol. 17:292019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shibata K, Kikkawa F, Nawa A, Thant AA,
Naruse K, Mizutani S and Hamaguchi M: Both focal adhesion kinase
and c-Ras are required for the enhanced matrix metalloproteinase 9
secretion by fibronectin in ovarian cancer cells. Cancer Res.
58:900–903. 1998.PubMed/NCBI
|
|
37
|
Himelstein BP, Canete-Soler R, Bernhard
EJ, Dilks DW and Muschel RJ: Metalloproteinases in tumor
progression: The contribution of MMP-9. Invasion Metastasis.
14:246–258. 1994-1995.
|
|
38
|
Bloomston M, Zervos EE and Rosemurgy AS
II: Matrix metalloproteinases and their role in pancreatic cancer:
A review of preclinical studies and clinical trials. Ann Surg
Oncol. 9:668–674. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Grunwald B, Vandooren J, Locatelli E,
Fiten P, Opdenakker G, Proost P, Kruger A, Lellouche JP, Israel LL,
Shenkman L and Comes Franchini M: Matrix metalloproteinase-9
(MMP-9) as an activator of nanosystems for targeted drug delivery
in pancreatic cancer. J Control Release. 239:39–48. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Agarwal A, Tressel SL, Kaimal R, Balla M,
Lam FH, Covic L and Kuliopulos A: Identification of a
metalloprotease-chemokine signaling system in the ovarian cancer
microenvironment: Implications for antiangiogenic therapy. Cancer
Res. 70:5880–5890. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Grunwald B, Schoeps B and Kruger A:
Recognizing the molecular multifunctionality and interactome of
TIMP-1. Trends Cell Biol. 29:6–19. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hirsch FR, Varella-Garcia M and Cappuzzo
F: Predictive value of EGFR and HER2 overexpression in advanced
non-small-cell lung cancer. Oncogene. 28((Suppl 1)): S32–S37. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Normanno N, De Luca A, Bianco C, Strizzi
L, Mancino M, Maiello MR, Carotenuto A, De Feo G, Caponigro F and
Salomon DS: Epidermal growth factor receptor (EGFR) signaling in
cancer. Gene. 366:2–16. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Olayioye MA, Neve RM, Lane HA and Hynes
NE: The ErbB signaling network: Receptor heterodimerization in
development and cancer. EMBO J. 19:3159–3167. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chong CR and Janne PA: The quest to
overcome resistance to EGFR-targeted therapies in cancer. Nat Med.
19:1389–1400. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Rivera F, Lopez-Tarruella S, Vega-Villegas
ME and Salcedo M: Treatment of advanced pancreatic cancer: From
gemcitabine single agent to combinations and targeted therapy.
Cancer Treat Rev. 35:335–339. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wang F, Xiao W, Sun J, Han D and Zhu Y:
MiRNA-181c inhibits EGFR-signaling-dependent MMP9 activation via
suppressing Akt phosphorylation in glioblastoma. Tumour Biol.
35:8653–8658. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Qiu Q, Yang M, Tsang BK and Gruslin A:
EGF-induced trophoblast secretion of MMP-9 and TIMP-1 involves
activation of both PI3K and MAPK signalling pathways. Reproduction.
128:355–363. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wang T, Yamashita K, Iwata K and Hayakawa
T: Both tissue inhibitors of metalloproteinases-1 (TIMP-1) and
TIMP-2 activate Ras but through different pathways. Biochem Biophys
Res Commun. 296:201–205. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Tarpgaard LS, Orum-Madsen MS, Christensen
IJ, Nordgaard C, Noer J, Guren TK, Glimelius B, Sorbye H, Ikdahl T,
Kure EH, et al: TIMP-1 is under regulation of the EGF signaling
axis and promotes an aggressive phenotype in KRAS-mutated
colorectal cancer cells: A potential novel approach to the
treatment of metastatic colorectal cancer. Oncotarget.
7:59441–59457. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Curry S, Mandelkow H, Brick P and Franks
N: Crystal structure of human serum albumin complexed with fatty
acid reveals an asymmetric distribution of binding sites. Nat
Struct Biol. 5:827–835. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
52
|
Mueller TC, Burmeister MA, Bachmann J and
Martignoni ME: Cachexia and pancreatic cancer: Are there treatment
options? World J Gastroenterol. 20:9361–9373. 2014.PubMed/NCBI
|
|
53
|
Bachmann J, Buchler MW, Friess H and
Martignoni ME: Cachexia in patients with chronic pancreatitis and
pancreatic cancer: Impact on survival and outcome. Nutr Cancer.
65:827–833. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Mengele K, Napieralski R, Magdolen V,
Reuning U, Gkazepis A, Sweep F, Brunner N, Foekens J, Harbeck N and
Schmitt M: Characteristics of the level-of-evidence-1 disease
forecast cancer biomarkers uPA and its inhibitor PAI-1. Expert Rev
Mol Diagn. 10:947–962. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Asuthkar S, Stepanova V, Lebedeva T,
Holterman AL, Estes N, Cines DB, Rao JS and Gondi CS:
Multifunctional roles of urokinase plasminogen activator (uPA) in
cancer stemness and chemoresistance of pancreatic cancer. Mol Biol
Cell. 24:2620–2632. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Xue A, Xue M, Jackson C and Smith RC:
Suppression of urokinase plasminogen activator receptor inhibits
proliferation and migration of pancreatic adenocarcinoma cells via
regulation of ERK/p38 signaling. Int J Biochem Cell Biol.
41:1731–1738. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Botla SK, Savant S, Jandaghi P, Bauer AS,
Mucke O, Moskalev EA, Neoptolemos JP, Costello E, Greenhalf W,
Scarpa A, et al: Early epigenetic downregulation of microRNA-192
expression promotes pancreatic cancer progression. Cancer Res.
76:4149–4159. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Ding J, Yang C and Yang S: LINC00511
interacts with miR-765 and modulates tongue squamous cell carcinoma
progression by targeting LAMC2. J Oral Pathol Med. 47:468–476.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Kinoshita T, Nohata N, Hanazawa T, Kikkawa
N, Yamamoto N, Yoshino H, Itesako T, Enokida H, Nakagawa M, Okamoto
Y and Seki N: Tumour-suppressive microRNA-29s inhibit cancer cell
migration and invasion by targeting laminin-integrin signalling in
head and neck squamous cell carcinoma. Br J Cancer. 109:2636–2645.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Jung SN, Lim HS, Liu L, Chang JW, Lim YC,
Rha KS and Koo BS: LAMB3 mediates metastatic tumor behavior in
papillary thyroid cancer by regulating c-MET/Akt signals. Sci Rep.
8:27182018. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Wang XM, Li J, Yan MX, Liu L, Jia DS, Geng
Q, Lin HC, He XH, Li JJ and Yao M: Integrative analyses identify
osteopontin, LAMB3 and ITGB1 as critical pro-metastatic genes for
lung cancer. PLoS One. 8:e557142013. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Ohike N, Maass N, Mundhenke C, Biallek M,
Zhang M, Jonat W, Luttges J, Morohoshi T, Kloppel G and Nagasaki K:
Clinicopathological significance and molecular regulation of maspin
expression in ductal adenocarcinoma of the pancreas. Cancer Lett.
199:193–200. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Kashima K, Ohike N, Mukai S, Sato M,
Takahashi M and Morohoshi T: Expression of the tumor suppressor
gene maspin and its significance in intraductal papillary mucinous
neoplasms of the pancreas. Hepatobiliary Pancreat Dis Int. 7:86–90.
2008.PubMed/NCBI
|
|
64
|
Cao D, Zhang Q, Wu LS, Salaria SN, Winter
JW, Hruban RH, Goggins MS, Abbruzzese JL, Maitra A and Ho L:
Prognostic significance of maspin in pancreatic ductal
adenocarcinoma: Tissue microarray analysis of 223 surgically
resected cases. Mod Pathol. 20:570–578. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Wang L, Wu H, Wang L, Lu J, Duan H, Liu X
and Liang Z: Expression of amphiregulin predicts poor outcome in
patients with pancreatic ductal adenocarcinoma. Diagn Pathol.
11:602016. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Busser B, Sancey L, Brambilla E, Coll JL
and Hurbin A: The multiple roles of amphiregulin in human cancer.
Biochim Biophys Acta. 1816:119–131. 2011.PubMed/NCBI
|
|
67
|
Pawar NM and Rao P: Secreted frizzled
related protein 4 (sFRP4) update: A brief review. Cell Signal.
45:63–70. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Deshmukh A, Kumar S, Arfuso F, Newsholme P
and Dharmarajan A: Secreted Frizzled-related protein 4 (sFRP4)
chemo-sensitizes cancer stem cells derived from human breast,
prostate, and ovary tumor cell lines. Sci Rep. 7:22562017.
View Article : Google Scholar : PubMed/NCBI
|