Introduction
Hypopharyngeal carcinoma, which originates in the
mucosal epithelia of the hypopharynx, accounts for 5% of head and
neck cancer cases worldwide (1–3). Once
diagnosed, this disease has limited treatment options and a poor
prognosis (4). Despite the
combination of surgery, radiotherapy and chemotherapy benefiting
the patients, the overall 5-year survival rate remains <20%
(5–7). Therefore, there is a constant need to
develop novel and effective therapeutic targets for hypopharyngeal
carcinoma.
The T-box transcription factor family, which
comprises TBX1, TBX2 and TBX3, serves an important role in
embryonic development. TBX3 is widely expressed in various tissues
and is associated with the pluripotency of embryonic stem cells
(8–10). Overexpression of this protein has
been demonstrated to be associated with various types of cancer,
including breast cancer (11),
gastric cancer (12), colorectal
cancer (13), bladder cancer
(14), head and neck cancer
(15) and melanoma (16). Ectopic TBX3 expression promotes the
growth and invasion of gastric cancer (12). Mechanistically, TBX3 accelerates
papillary thyroid carcinoma cell proliferation by potentiating
polycomb repressive complex 2-mediated cyclin-dependent kinase
(CDK) inhibitor 1C (p57KIP2) repression. It also drives the growth
of sarcoma by suppressing CDK inhibitor 1 (p21) (17). In addition, TBX3 is targeted by
microRNA (miR)-17–92 and miR-206, contributing to their suppressive
role in pancreatic and breast cancer stem cell viability (18,19).
These findings suggest that targeting TBX3 may be helpful in
treating patients with cancer. However, the role of this factor in
hypopharyngeal carcinoma remains largely unclear.
In the present study, TBX3 was identified as a
potential oncogene in hypopharyngeal carcinoma. Its upregulation
was observed in hypopharyngeal carcinoma samples when compared with
normal tissue samples. The silencing of TBX3 caused cell cycle
arrest at the S phase and increased apoptosis, potentially
contributing to the suppressed proliferation of TBX3-knockdown
hypopharyngeal carcinoma FaDu cells. By contrast, ectopic TBX3
expression led to an increased viability of FaDu cells. Therefore,
this transcription factor maybe a promising target for the
treatment and monitoring of hypopharyngeal carcinoma.
Materials and methods
Patient information
Samples from 30 patients (25 male and 5 female) with
hypopharyngeal carcinoma and the adjacent tissues were collected
from the Taizhou People's Hospital (Taizhou, China) between January
2010 and June 2015. The adjacent non-cancerous tissues were
obtained 2 cm away from the cancer sites. The median age of the
patients at the time of surgery was 64.63 years (range, 41–76
years). Written informed consent was obtained from all patients and
the study was approved by the Ethics Committee of the Taizhou
People's Hospital.
Immunohistochemical analysis of
clinical hypopharyngeal cancer and normal tissues
Human hypopharyngeal cancer and normal
hypopharyngeal tissue samples were fixed with 4% formalin for 24 h
at room temperature and embedded in paraffin (5 µm thick). The
tissues were then subjected to immunohistochemical analysis for
TBX3, E-cadherin and N-cadherin. Briefly, the sides were
deparaffinized in xylene and hydrated in a graded alcohol series
(100, 85 and 75%). Antigens were retrieved using citrate buffer at
95°C (pH 6), and 3% hydrogen peroxide was used for endogenous
peroxidase blocking, followed by incubation with 10% goat serum
(Abcam) at room temperature for 1 h. The slides were then incubated
with the primary antibody at 4°C overnight. The incubation with the
secondary antibodies was performed at room temperature for 30 min.
After staining with 3,3′-diaminobenzidineat room temperature for 20
min, sections were counterstained with hematoxylin at room
temperature for 5 min. Images of protein expression were captured
using a Zeiss microscope using the brightfield lens at ×100 and
×400 magnification. Immunostaining scores were analyzed using
Image-Pro Plus version 4.1 software (Media Cybernetics, Inc.). The
extent of protein expression was graded as follows: Negative, 0;
weak, 1; moderate, 2; and strong, 4. The extent of staining was
grouped according to the percentage of cells with high staining in
the cancer nest: Negative, 0; 1–25%, 1; 26–50%, 2; 51–75%, 3; and
76–100%, 4. The final score of staining was the sum of the protein
expression grade and the extent of staining score. The primary
antibodies for TBX3 (rabbit polyclonal; 1:200; cat. no. ab99302)
and N-cadherin (rabbit polyclonal; 1:600; cat. no. ab76057) were
purchased from Abcam, and the primary antibody for E-cadherin
(mouse monoclonal; 1:100; cat. no. 14472) was purchased from Cell
Signaling Technology, Inc. The horseradish peroxidase-conjugated
secondary antibodies (goat anti-rabbit IgG; cat. no. sc-2004;
1:100; and goat anti-mouse IgG; cat. no. sc-2005; 1:100) were from
Santa Cruz Biotechnology, Inc.
Cell culture
FaDu and 293T cells (American Tissue Culture
Collection) were cultured in DMEM (Hyclone; GE Healthcare Life
Sciences) supplemented with 10% fetal bovine serum (Gibco; Thermo
Fisher Scientific, Inc.) and 1% penicillin and streptomycin
solution (Corning Life Sciences) at 37°C with 5% CO2.
Cells were passaged when they had reached 80% confluence.
TBX3 knockdown in hypopharyngeal
cancer cells
pGCSIL-GFP lentivirus vectors were used for TBX3
knockdown, and pHelper1.0 and Helper2.0 served as the packaging
vectors. All the vectors and short hairpin RNA (shRNA) targeting
TBX3 (shTBX3) and non-targeting shRNA (shCtrl) lentivirus particles
were designed and purchased from Shanghai GeneChem Co., Ltd. The
sequences were as follows: shTBX3, 5′-TGCTGATGACTGTCGTTAT-3′; and
shCtrl, 5′-TTCTCCGAACGTGTCACGT-3′. Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) was used to transfect
the vectors (1 µg/ml) into 293T cells. Virus supernatants were
collected and used for the infection of FaDu cells. After 72 h of
infection, the knockdown efficiency was determined using reverse
transcription-quantitative PCR (RT-qPCR) and western blotting.
TBX3 overexpression in hypopharyngeal
cancer cells
The open reading frame sequence of the TBX3 gene
(accession number: NM_016569) was inserted into the pCDH lentivirus
vectors (purchased from Shanghai GeneChem Co., Ltd.) between the
BamHI and SacI sites. 293T cells were used for virus packaging,
using Lipofectamine® 2000. The virus supernatants with
pCDH-TBX3 (OE-TBX3) or pCDH-empty (OE-Empty) were collected and
used for infection of FaDu cells, three times (three days per
time). After 72 h of infection with the lentivirus, the efficiency
of transfection was determined by RT-qPCR and western blotting.
Western blot analysis
Total protein was extracted from the cancer cells
using RIPA buffer (Beyotime Institute of Biotechnology), and the
concentration was measured using a Pierce™ BCA protein assay kit
(Thermo Fisher Scientific, Inc.). The protein samples (40 µg per
lane) were separated via SDS-PAGE (12% gel) and transferred onto
PVDF membranes (Merck KGaA). The membranes were blocked with 5%
skimmed milk at room temperature for 1 h and then incubated with
primary antibodies overnight at 4°C. Subsequently, the membranes
were incubated with secondary antibodies at room temperature for 3
h. The primary antibodies against TBX3 (rabbit polyclonal; cat. no.
ab99302), p21 (rabbit monoclonal; cat. no. ab109520), p57KIP2
(rabbit monoclonal; cat. no. ab75974), CDK inhibitor 1B (p27;
rabbit monoclonal; cat. no. ab32034) and N-cadherin (rabbit
polyclonal; cat. no. ab76057) were purchased from Abcam (all
1:1,000). The primary antibodies against E-cadherin (mouse
monoclonal; cat. no. 14472), CDK2 (rabbit monoclonal; cat. no.
2546), CDK4 (rabbit monoclonal; cat. no. 12790), proliferating cell
nuclear antigen (PCNA; rabbit monoclonal; cat. no. 13110), cyclin E
(rabbit monoclonal; cat. no. 20808), Bcl-2 (mouse monoclonal; cat.
no. 15071), caspase 3 (rabbit polyclonal; cat. no. 9662), poly
(ADP-ribose) polymerase (PARP; rabbit monoclonal; cat. no. 9532)
and GAPDH (rabbit monoclonal; cat. no. 5174) were purchased from
Cell Signaling Technology, Inc. (all 1:1,000). Following incubation
with the secondary antibodies, the proteins were detected by
chemiluminescence (Thermo Fisher Scientific, Inc.). The secondary
antibodies (goat anti-mouse IgG; cat. no. sc-2005; and goat
anti-rabbit IgG; cat. no. sc-2004) were obtained from Santa Cruz
Biotechnology, Inc. (both 1:5,000). Signals were visualized using
Pierce™ enhanced chemiluminescence western blotting substrate
(Thermo Fisher Scientific, Inc.), and developed using Blue Devil
Auto-radiography film (Tanon Science and Technology Co., Ltd.).
Total RNA isolation and RT-qPCR
Total RNA was extracted from FaDu cells transfected
with shCtrl or shTBX3, using TRIzol® reagent
(Invitrogen; Thermo Fisher Scientific, Inc.). A total of 1 µg RNA
was subjected to RT using the One Step RT-PCR kit (Takara
Biotechnology Co., Ltd.), according to the manufacturer's protocol.
RT-qPCR was performed to determine the relative amounts of the
transcripts using SYBR master mix (Beijing Transgen Biotech Co.,
Ltd.) on an IQ5 Real Time PCR System (Bio-Rad Laboratories, Inc.).
The thermocycling conditions were as follows: Initial denaturation,
95°C for 3 min; followed by 40 cycles of denaturation at 95°C for
15 sec, annealing at 60°C for 30 sec and elongation at 72°C for 30
sec. GAPDH served as the internal normalization control. The primer
sequences are as follows: TBX3 forward, 5′-TGAACTCAACAGCCGCTCCTC-3′
and reverse, 5′-CTTCCAAGCCGCTAACCAACC-3′; and GAPDH forward,
5′-TGACTTCAACAGCGACACCCA-3′ and reverse,
5′-CACCCTGTTGCTGTAGCCAAA-3′. The 2−ΔΔCq method was used
for quantification of the relative mRNA expression levels (20).
MTT assay
shCtrl- and shTBX3-transfected FaDu cells were
seeded in 96-well plates at a density of 3×103
cells/well and cultured and viability was measured every 24 h for 5
days. The cells were washed with PBS and incubated with 0.5 mg/ml
MTT solution at 37°C for 4 h. MTT reagent was removed and DMSO was
added to each well. Subsequently, the cell viability was determined
by the measurement of the optical density at 570 nm.
Cell cycle assay
Propidium iodide (PI; Sigma-Aldrich; Merck KGaA)
staining was employed to determine cell cycle progression.
Hypopharyngeal cancer FaDu cells expressing shCtrl or shTBX3
lentivirus were seeded at a density of 5×105 cells/well
in a 6-well culture plates. The cells were harvested, washed twice
with ice-cold PBS, fixed with 70% ice-cold ethanol overnight and
rehydrated in PBS at 4°C for 30 min. Subsequently, PI staining was
performed at 37°C for 30 min and fluorescence activated cell
sorting on a flow cytometer (FACSCalibur; Becton, Dickinson and
Company) was used to determine the PI absorbance of the indicated
cells. The cell cycle phases were analyzed using ModFit software
(version 2.0; Becton, Dickinson and Company).
Apoptosis assay
The Annexin V-APC apoptosis detection kit
(eBioscience; Thermo Fisher Scientific, Inc.) was used to detect
the apoptosis of shCtrl- and shTBX3-transfected FaDu cells,
according to the manufacturer's protocol. In brief, FaDu cells
transfected with shCtrl or shTBX3 were washed with PBS three times
and re-suspended using 100 µl staining buffer. The cells were then
incubated with 5 µl Annexin V-APC for 15 min. Flow cytometry
(FACSCalibur; Becton, Dickinson and Company) was used to detect
apoptosis. FlowJo Vx 10.0 software was used to analyze the results
(FlowJo LLC).
Statistical analysis
All the data in the present study were analyzed with
SPSS software (version 22.0; IBM Corp.). All statistical data are
presented as the mean ± standard deviation of ≥3 independent
experiments. Comparisons between two groups were performed using
Student's t-test or Fisher's exact test. Spearman's correlation
test was also used. A Mann-Whitney U test was applied to analyze
the immunohistochemistry results. P<0.05 was considered to
indicate a statistically significant difference.
Results
TBX3 is highly expressed in cancer
tissues of patients with hypopharyngeal cancer
In order to assess the clinical relevance of TBX3 in
hypopharyngeal carcinoma, an immunohistochemistry assay was
performed to investigate the expression of TBX3 in hypopharyngeal
cancer tissue and normal hypopharyngeal specimens in the present
study. The association between the expression of TBX3 and
clinicopathological factors in patients with hypopharyngeal
carcinoma is demonstrated in Table
I. The results revealed that TBX3 protein levels were
significantly increased in hypopharyngeal carcinoma compared with
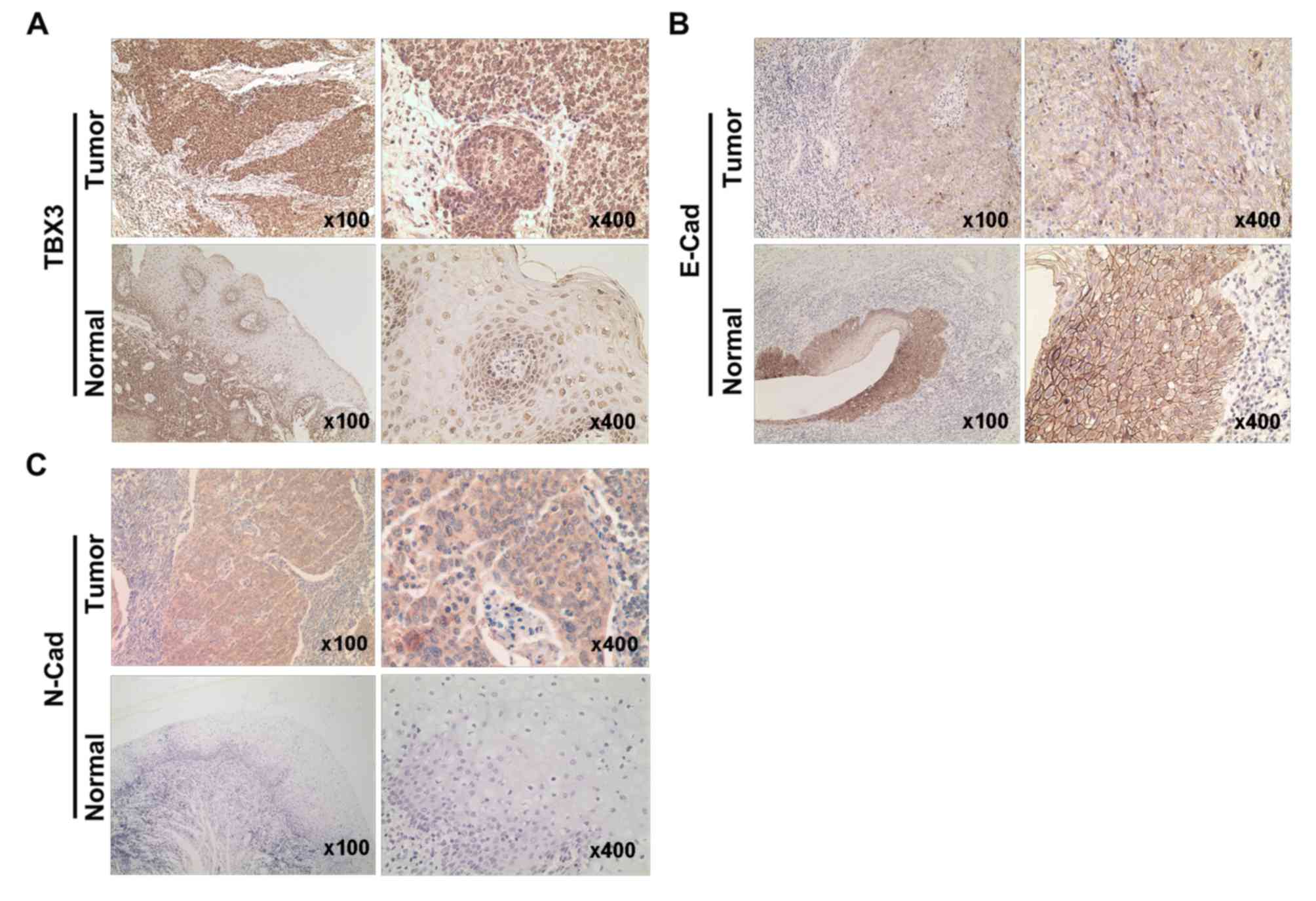
the normal hypopharyngeal tissues (Fig.
1A; Table II). Furthermore, the
expression of N-cadherin and E-cadherin was examined in
hypopharyngeal carcinoma and normal hypopharyngeal tissues. The
results demonstrated a higher expression of N-cadherin and lower
expression of E-cadherin in hypopharyngeal carcinoma tissues
compared with the normal hypopharyngeal tissues (Fig. 1B and C; Table II). Further analysis revealed a
negative correlation between TBX3 and E-cadherin
(r2=−0.389; P=0.034; Table
III). Collectively, these data indicate that TBX3 is a
potential biomarker for hypopharyngeal carcinoma and may be
essential for the progression of this disease.
 | Table I.Association between the expression of
TBX3 and clinicopathological factors in patients with
hypopharyngeal carcinoma. |
Table I.
Association between the expression of
TBX3 and clinicopathological factors in patients with
hypopharyngeal carcinoma.
|
|
| TBX3
expression |
|
|---|
|
|
|
|
|
|---|
| Clinical
factors | No. of
patients | High | Low | P-value |
|---|
| Age |
|
|
| >0.999 |
| <65
years | 16 | 12 | 4 |
|
| ≥65
years | 14 | 10 | 4 |
|
| Sex |
|
|
| >0.999 |
|
Male | 25 | 18 | 7 |
|
|
Female | 5 | 4 | 1 |
|
|
Differentiation |
|
|
|
0.210 |
|
Low | 10 | 9 | 1 |
|
|
Moderate/High | 20 | 13 | 7 |
|
| T stage |
|
|
| >0.999 |
|
1–2 | 18 | 13 | 5 |
|
|
3–4 | 12 | 9 | 3 |
|
| Lymphatic
metastasis |
|
|
|
0.391 |
| No | 8 | 7 | 1 |
|
|
Yes | 22 | 15 | 7 |
|
 | Table II.Immunohistochemical staining score of
TBX3, E-cadherin and N-cadherin. |
Table II.
Immunohistochemical staining score of
TBX3, E-cadherin and N-cadherin.
| Variable | N | TBX3 | E-cadherin | N-cadherin |
|---|
| Normal | 30 | 5 (0–7) | 7 (5–7) | 0 (0–2) |
| Cancer | 30 | 7 (5–7) | 5 (3–7) | 3 (0–6) |
| P-value |
| <0.001 | <0.001 | <0.001 |
 | Table III.Correlation between the expression of
TBX3, E-cadherin and N-cadherin. |
Table III.
Correlation between the expression of
TBX3, E-cadherin and N-cadherin.
| Variable | TBX3 | E-Cadherin |
|---|
| TBX3 |
|
|
| E-cadherin | −0.389a |
|
| N-cadherin |
0.105 | −0.010 |
TBX3 promotes hypopharyngeal carcinoma
cell proliferation
TBX3 was upregulated in hypopharyngeal carcinoma
specimens, but whether it is associated with the progression of
hypopharyngeal carcinoma remains less clear. In order to address
this question, TBX3 was knocked-down and overexpressed in
hypopharyngeal carcinoma FaDu cells, using lentivirus-based shRNA
and overexpressing strategies, respectively. Green fluorescence
data demonstrated that the infection efficiency was similar between
the shCtrl and shTBX3 (Fig. 2A).
RT-qPCR and western blotting results verified that TBX3 was
efficiently knocked-down in the FaDu cells (Fig. 2B). In addition, TBX3 was efficiently
overexpressed (Fig. 2C). The
upregulation of E-cadherin, p57KIP2 and p21 was observed, as well
as the downregulation of N-cadherin in the shTBX3 group compared
with shCtrl FaDu cells (Fig. 2D).
The inverse results were observed in the TBX3-overexpressed cells
(Fig. 2E). Subsequently, the effect
of TBX3 on hypopharyngeal carcinoma cell proliferation was
examined. The results of the MTT assay demonstrated that although
no significant inhibition was observed on days 1 and 2, TBX3
silencing notably inhibited the proliferation of FaDu cells on days
3–5 (Fig. 2F). By contrast, TBX3
ectopic expression promoted cell proliferation (Fig. 2G). Taken together, the results from
the present study demonstrate that TBX3 silencing inhibited the
proliferation of hypopharyngeal carcinoma cells, indicating that
this protein is critical for hypopharyngeal carcinoma cell
viability.
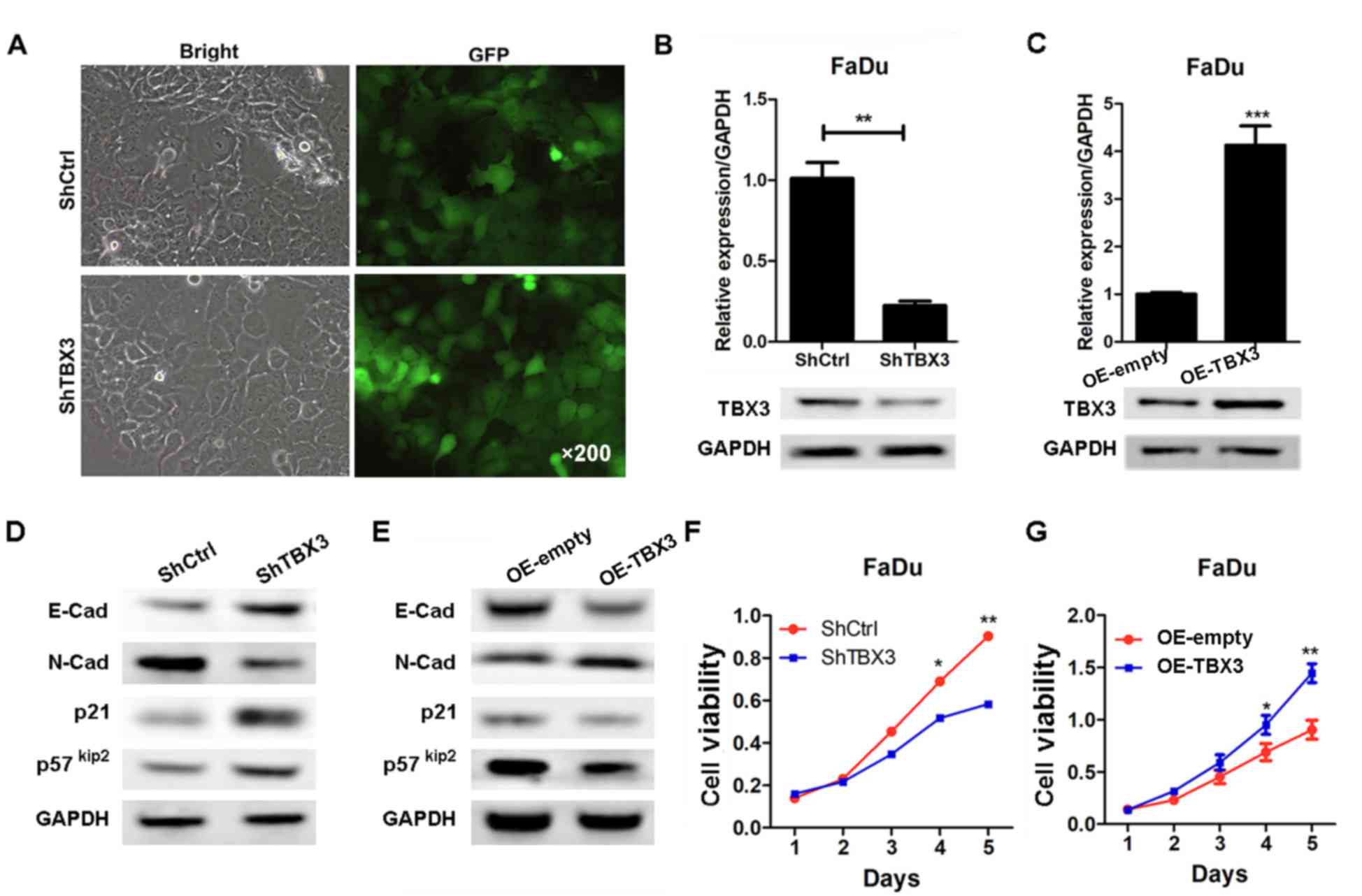 | Figure 2.TBX3 expression is efficiently
suppressed and overexpressed in FaDu cells using a
lentivirus-mediated knockdown strategy. (A) Green fluorescence
images of FaDu cells transfected with shCtrl or shTBX3 lentivirus.
(B) RT-qPCR and western blotting results of TBX3 in FaDu cells
expressing shCtrl or shTBX3 lentivirus (**P<0.01 vs. shCtrl).
(C) RT-qPCR and western blotting results of TBX3 in OE-empty or
OE-TBX3 FaDu cells (***P<0.001 vs. shCtrl). Western
blot analysis of E-cadherin, N-cadherin, p21 and p57KIP2 in the
cells described in (D) FaDu cells expressing shCtrl or shTBX3
lentivirus and (E) OE-empty or OE-TBX3 FaDu cells. An MTT assay was
performed in order to detect the proliferation of the cells
described in (F) FaDu cells expressing shCtrl or shTBX3 lentivirus
(*P<0.05, **P<0.01 vs. shCtrl) and (G) OE-empty or OE-TBX3
FaDu cells (*P<0.05, **P<0.01 vs. OE-empty). TBX3, T-box
transcription factor TBX3; shCtrl, short hairpin RNA control;
shTBX3, short hairpin RNA targeting TBX3; RT-qPCR, reverse
transcription-quantitative PCR; p21, cyclin-dependent kinase
inhibitor 1; p57KIP2, cyclin-dependent kinase inhibitor 1C. |
TBX3 silencing induces cell cycle
arrest in FaDu cells
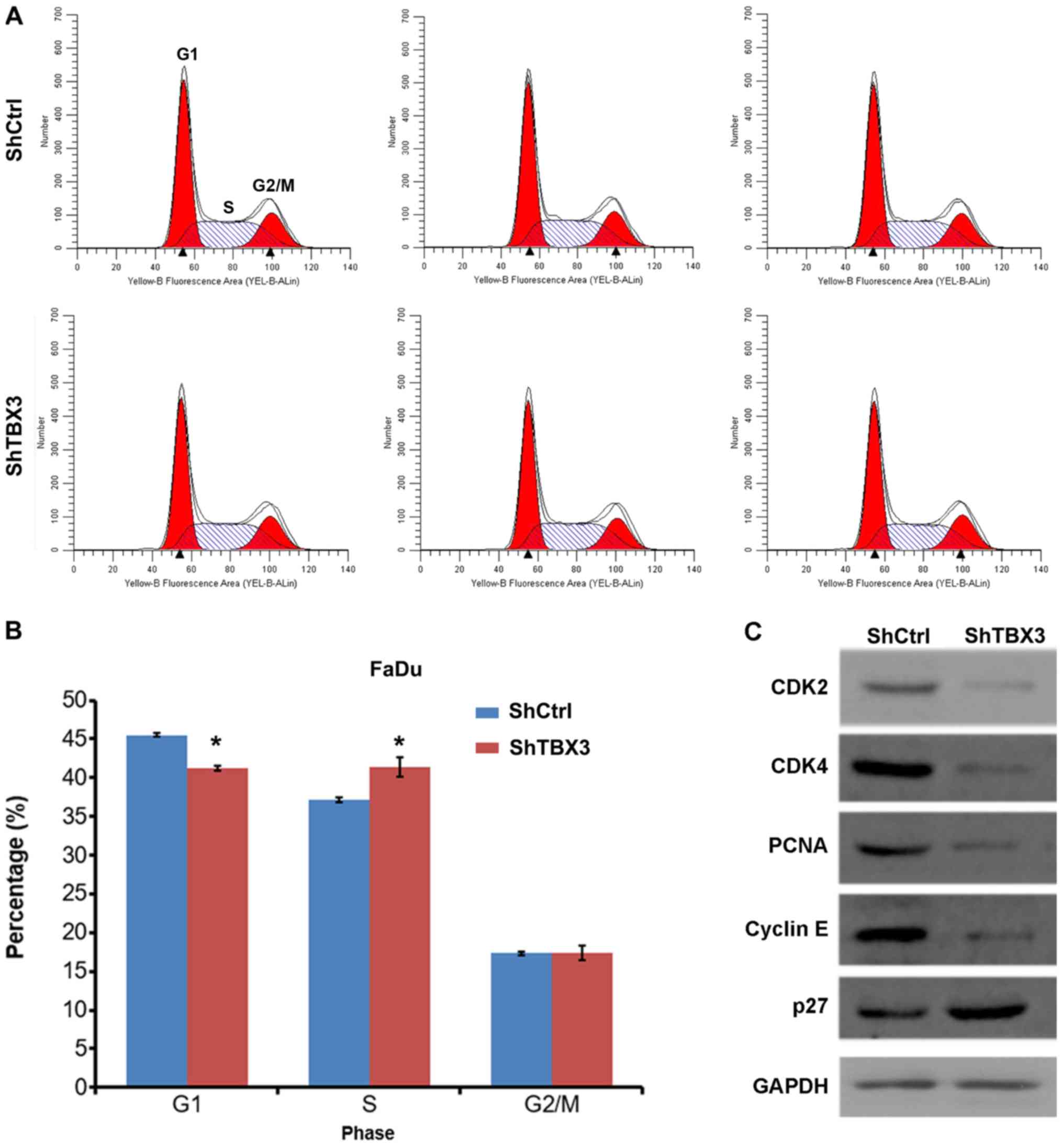
Accelerated cell cycle progression is one of the
main reasons that cancer cells proliferate at a higher rate than
normal cells (21). The cell cycle
progression in shCtrl- and shTBX3-transfected FaDu cells was
investigated in the present study. Decreased G1 and
increased S phase distribution were exhibited in shTBX3 FaDu cells
compared with shCtrl cells, whereas no significant change was
observed in the distribution of cells in the G2/M phase
(Fig. 3A and B), indicating that the
cell cycle was arrested at the S phase in the shTBX3-transfected
FaDu cells. Furthermore, the expression of cell cycle proteins was
investigated. The western blotting results revealed that CDK2,
CDK4, PCNA and cyclin E expression was decreased, whereas p27 was
upregulated in the shTBX3 FaDu cells (Fig. 3C). Taken together, the data from the
present study demonstrated that TBX3 silencing in FaDu cells
results in enhanced cell cycle arrest.
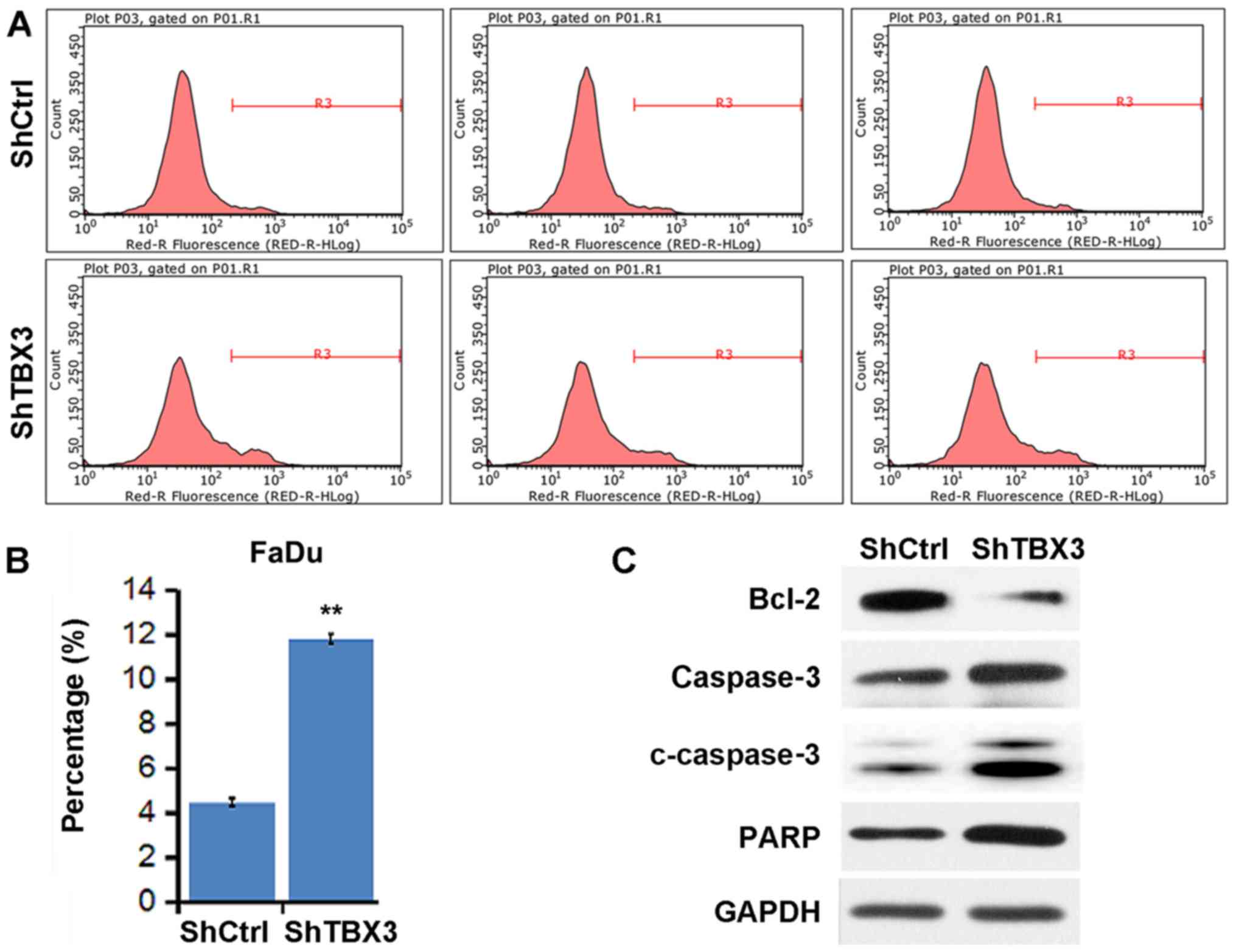
TBX3 silencing enhances the apoptosis
rate of FaDu cells
The present study then determined whether TBX3
regulates the apoptosis rate of FaDu cells. The apoptosis rate of
cells transfected with shCtrl and shTBX3 was analyzed using Annexin
V-APC staining and flow cytometry. TBX3 knockdown led to an
increase in the apoptosis rate of the FaDu cells (Fig. 4A and B). The expression of
cleaved-caspase 3, caspase 3 and PARP was increased, whereas Bcl-2
was downregulated following TBX3 knockdown (Fig. 4C). These results suggest that the
induction of apoptosis may contribute to suppressed cell viability
in shTBX3-transfected FaDu cells.
Discussion
Although hypopharyngeal carcinoma is a relatively
rare disease among the different types of head and neck cancer, it
is often diagnosed when it has already reached a malignant stage
(22). Treatment strategies remain
ineffective against this fatal disease (22). In the last decade, certain studies
have identified proto-oncogenes and tumor suppressors in
hypopharyngeal carcinoma, including c-Myc (23), PI3K-mTOR (24) and phosphate and tensin homolog (PTEN)
(25); however, the molecular
mechanisms underlying the progression of hypopharyngeal carcinoma
are currently poorly understood. Therefore, identifying novel
targets triggering hypopharyngeal carcinoma may assist the
development of effective targeted treatments.
The transcription factor TBX3, which belongs to the
T-box family, is involved in the development of multiple organs,
including the breast, lung and pancreas (26). Numerous studies have demonstrated
that TBX3 participates in the development of different types of
human cancer. For example, TBX3 serves as a downstream effector of
Wnt/β-catenin signaling in liver cancer growth (27). TBX3 is overexpressed in head and neck
squamous cell carcinoma and represses the expression of PTEN, which
is a common tumor suppressor in various types of cancer (15). Notably, TBX3 also functions as a
downstream target of AKT serine/threonine kinase 3. Upregulation of
TBX3 is observed in melanoma tissues and contributes to the growth
and invasion of melanoma (28). At
the molecular level, cell adhesion molecule E-cadherin has been
reported to be inhibited by TBX3 (29,30).
E-cadherin is also regulated by hepatocyte growth factor and serves
as a prognostic factor for hypopharyngeal carcinoma (31). However, the association between TBX3
and E-cadherin in hypopharyngeal carcinoma remains unclear. In the
present study, the involvement of TBX3 in hypopharyngeal carcinoma
was investigated. First, TBX3 was observed to be significantly
upregulated in hypopharyngeal carcinoma tissues, whereas E-cadherin
was downregulated. In addition, N-cadherin expression was enhanced
in these tissues. This suggests that TBX3 may contribute to the
progression of hypopharyngeal carcinoma. TBX3 expression was then
knocked-down in FaDu cells, which are widely used to study
hypopharyngeal carcinoma, using shRNA, leading to decreased cell
proliferation. By contrast, TBX3 ectopic expression led to enhanced
viability of the cells. Furthermore, E-cadherin and N-cadherin were
negatively and positively regulated by TBX3, respectively, which
was consistent with the results from the patient samples in the
present study. However, certain limitations remained in the present
study. As it was not possible to obtain another hypopharyngeal
carcinoma cell line, the functional experiments of TBX3 were not
investigated in other cells. Further studies should be performed
when another hypopharyngeal carcinoma cell line becomes available.
Overall, the results from the present study indicate that TBX3 is a
potential oncogene in hypopharyngeal carcinoma.
It has been demonstrated that TBX3 is regulated by
cyclin A-CDK2 and c-Myc, and is important for S-phase progression
(32). p21, a CDK inhibitor, is
transcriptionally repressed by TBX3 (17). These results suggest that TBX3 is
critical for the cell cycle process. In the present study, TBX3
silencing led to an increased number of cells at the S phase of the
cell cycle, and a decreased proportion at the G1 phase,
while the cells at the G2/M phase remained unchanged.
Increasing evidence supports the important role that CDK2, CDK4,
cyclin E and p27 serve in cell cycle progression (33–36).
AsTBX3 was demonstrated to regulate the cell cycle progression of
FaDu cells, the present study then detected whether cell
cycle-associated proteins were modulated by TBX3. In the FaDu
cells, CDK2, CDK4 and cyclin E were repressed following TBX3
knockdown. By contrast, p27 was upregulated by TBX3 silencing. In
addition, TBX3 has been reported to regulate apoptosis via various
mechanisms. This transcription factor disturbs the p53 pathway to
suppress apoptosis, leading to dysregulated cell transformation and
myogenic differentiation (37). TBX3
also serves as an anti-apoptotic protein in mesangial cells
(38). Consistent with these
results, enhanced apoptosis was observed in the TBX3-knockdown FaDu
cells in the present study. Cleaved-caspase 3, PARP and Bcl-2 are
markers of apoptosis. Dysregulation of these genes results in the
apoptosis of cancer cells (39,40). The
present study then determined whether TBX3 regulated the expression
of these proteins. The results revealed that cleaved-caspase 3 and
PARP were upregulated, whereas the survival marker Bcl-2 was
downregulated in the TBX3-silenced cells. Therefore, the results of
the present study were consistent with previous studies on TBX3 in
cell cycle and apoptosis regulation (41).
In conclusion, the present study provided evidence
to suggest that TBX3 acts as a potential oncogene in hypopharyngeal
carcinoma. Elevated TBX3 expression was observed in a cohort of
hypopharyngeal carcinoma samples. Furthermore, the knockdown of
TBX3 largely inhibited the proliferation of hypopharyngeal
carcinoma FaDu cells and led to cell cycle arrest at the S phase
and the induction of apoptosis. Therefore, targeting TBX3 may be a
promising targeted strategy for hypopharyngeal carcinoma.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
PD conceived the study, carried out the experimental
design and data interpretation, and prepared and revised the
manuscript. YH, HZ, XJ, YC and YZ performed the experiments. RH and
JX performed the statistical analysis. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Written informed consent was obtained from all
participating patients, and the study was approved by the Ethics
Committee of Taizhou People's Hospital (Taizhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
TBX3
|
T-box transcription factor TBX3
|
|
shRNA
|
short hairpin RNA
|
|
RT-qPCR
|
reverse transcription-quantitative
PCR
|
|
PI
|
propidium iodide
|
|
p57KIP2
|
cyclin-dependent kinase inhibitor
1C
|
|
PTEN
|
phosphate and tensin homolog
|
|
CDK
|
cyclin-dependent kinase
|
References
|
1
|
Chung CH, Parker JS, Karaca G, Wu J,
Funkhouser WK, Moore D, Butterfoss D, Xiang D, Zanation A, Yin X,
et al: Molecular classification of head and neck squamous cell
carcinomas using patterns of gene expression. Cancer Cell.
5:489–500. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zvrko E, Mikic A and Vuckovic L:
Clinicopathologic significance of CD105-assessed microvessel
density in glottic laryngeal squamous cell carcinoma. Auris Nasus
Larynx. 37:77–83. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gooi Z, Richmon J, Agrawal N, Blair E,
Portugal L, Vokes E, Seiwert T, de Souza J, Saloura V, Haraf D, et
al: AHNS Series- Do you know your guidelines? Principles of
treatment for nasopharyngeal cancer: A review of the National
Comprehensive Cancer Network guidelines. Head Neck. 39:201–205.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kotwall C, Sako K, Razack MS, Rao U,
Bakamjian V and Shedd DP: Metastatic patterns in squamous cell
cancer of the head and neck. Am J Surg. 154:439–442. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Milisavljevic D, Stankovic M, Zivic M,
Popovic M and Radovanović Z: Factors affecting results of treatment
of Hypopharyngeal Carcinoma. Hippokratia. 13:154–160.
2009.PubMed/NCBI
|
|
6
|
Chu PY, Wang LW and Chang SY: Surgical
treatment of squamous cell carcinoma of the hypopharynx: Analysis
of treatment results, failure patterns, and prognostic factors. J
Laryngol Otol. 118:443–449. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chu PY and Chang SY: Reconstruction of the
hypopharynx after surgical treatment of squamous cell carcinoma. J
Chin Med Assoc. 72:351–355. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim J, Chu J, Shen X, Wang J and Orkin SH:
An extended transcriptional network for pluripotency of embryonic
stem cells. Cell. 132:1049–1061. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Weidgang CE, Russell R, Tata PR, Kühl SJ,
Illing A, Müller M, Lin Q, Brunner C, Boeckers TM, Bauer K, et al:
TBX3 directs cell-fate decision toward mesendoderm. Stem Cell
Reports. 1:248–265. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Russell R, Ilg M, Lin Q, Wu G, Lechel A,
Bergmann W, Eiseler T, Linta L, Kumar PP, Klingenstein M, et al: A
dynamic role of TBX3 in the pluripotency circuitry. Stem Cell
Reports. 5:1155–1170. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fan W, Huang X, Chen C, Gray J and Huang
T: TBX3 and its isoform TBX3+2a are functionally distinctive in
inhibition of senescence and are overexpressed in a subset of
breast cancer cell lines. Cancer Res. 64:5132–5139. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Miao ZF, Liu XY, Xu HM, Wang ZN, Zhao TT,
Song YX, Xing YN, Huang JY, Zhang JY, Xu H and Xu YY: Tbx3
overexpression in human gastric cancer is correlated with advanced
tumor stage and nodal status and promotes cancer cell growth and
invasion. Virchows Arch. 469:505–513. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shan ZZ, Yan XB, Yan LL, Tian Y, Meng QC,
Qiu WW, Zhang Z and Jin ZM: Overexpression of Tbx3 is correlated
with Epithelial-Mesenchymal Transition phenotype and predicts poor
prognosis of colorectal cancer. Am J Cancer Res. 5:344–353.
2014.PubMed/NCBI
|
|
14
|
Beukers W, Kandimalla R, Masius RG,
Vermeij M, Kranse R, van Leenders GJ and Zwarthoff EC:
Stratification based on methylation of TBX2 and TBX3 into three
molecular grades predicts progression in patients with pTa-bladder
cancer. Mod Pathol. 28:515–522. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Burgucu D, Guney K, Sahinturk D, Ozbudak
IH, Ozel D, Ozbilim G and Yavuzer U: Tbx3 represses PTEN and is
over-expressed in head and neck squamous cell carcinoma. BMC
Cancer. 12:4812012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Peres J, Davis E, Mowla S, Bennett DC, Li
JA, Wansleben S and Prince S: The highly homologous T-box
transcription factors, TBX2 and TBX3, have distinct roles in the
oncogenic process. Genes Cancer. 1:272–282. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Willmer T, Hare S, Peres J and Prince S:
The T-box transcription factor TBX3 drives proliferation by direct
repression of the p21(WAF1) cyclin-dependent kinase inhibitor. Cell
Div. 11:62016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cioffi M, Trabulo SM, Sanchez-Ripoll Y,
Miranda-Lorenzo I, Lonardo E, Dorado J, Reis Vieira C, Ramirez JC,
Hidalgo M, Aicher A, et al: The miR-17–92 cluster counteracts
quiescence and chemoresistance in a distinct subpopulation of
pancreatic cancer stem cells. Gut. 64:1936–1948. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Amir S, Simion C, Umeh-Garcia M, Krig S,
Moss T, Carraway KL III and Sweeney C: Regulation of the T-box
transcription factor Tbx3 by the tumor suppressor microRNA-206 in
breast cancer. Br J Cancer. 114:1125–1134. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pfaffl MW: A new mathematical model for
relative quantification in real-time RT-PCR. Nucleic Acids Res.
29:e452001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jang JY, Kim EH, Cho J, Jung JH, Oh D, Ahn
YC, Son YI and Jeong HS: Comparison of oncological and functional
outcomes between initial surgical versus non-surgical treatments
for hypopharyngeal cancer. Ann Surg Oncol. 23:2054–2061. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kleszcz R, Paluszczak J, Krajka-Kuźniak V
and Baer-Dubowska W: The inhibition of c-MYC transcription factor
modulates the expression of glycolytic and glutaminolytic enzymes
in FaDu hypopharyngeal carcinoma cells. Adv Clin Exp Med.
27:735–742. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hsu CM, Lin PM, Tsai YT, Tsai MS, Tseng
CH, Lin SF and Yang MY: NVP-BEZ235, a dual PI3K-mTOR inhibitor,
suppresses the growth of FaDu hypopharyngeal squamous cell
carcinoma and has a synergistic effect with Cisplatin. Cell Death
Discov. 4:572018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu J, Lei DP, Jin T, Zhao XN, Li G and
Pan XL: Altered expression of miR-21 and PTEN in human laryngeal
and hypopharyngeal squamous cell carcinomas. Asian Pac J Cancer
Prev. 12:2653–2657. 2011.PubMed/NCBI
|
|
26
|
Washkowitz AJ, Gavrilov S, Begum S and
Papaioannou VE: Diverse functional networks of Tbx3 in development
and disease. Wiley Interdiscip Rev Syst Biol Med. 4:273–283. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Renard CA, Labalette C, Armengol C, Cougot
D, Wei Y, Cairo S, Pineau P, Neuveut C, de Reyniès A, Dejean A, et
al: Tbx3 is a downstream target of the Wnt/beta-catenin pathway and
a critical mediator of beta-catenin survival functions in liver
cancer. Cancer Res. 67:901–910. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Peres J, Mowla S and Prince S: The T-box
transcription factor, TBX3, is a key substrate of AKT3 in
melanomagenesis. Oncotarget. 6:1821–1833. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Feng X, Yao W, Zhang Z, Yuan F, Liang L,
Zhou J, Liu S and Song J: T-box transcription factor Tbx3
contributes to human hepatocellular carcinoma cell migration and
invasion by repressing E-cadherin expression. Oncol Res.
26:959–966. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Du HF, Ou LP, Yang X, Song XD, Fan YR, Tan
B, Luo CL and Wu XH: A new PKCα/β/TBX3/E-cadherin pathway is
involved in PLCε-regulated invasion and migration in human bladder
cancer cells. Cell Signal. 26:580–593. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kim CH, Kim J, Kahng H and Choi EC: Change
of E-cadherin by hepatocyte growth factor and effects on the
prognosis of hypopharyngeal carcinoma. Ann Surg Oncol.
14:1565–1574. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Willmer T, Peres J, Mowla S, Abrahams A
and Prince S: The T-Box factor TBX3 is important in S-phase and is
regulated by c-Myc and cyclin A-CDK2. Cell Cycle. 14:3173–3183.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
García-Reyes B, Kretz AL, Ruff JP, von
Karstedt S, Hillenbrand A, Knippschild U, Henne-Bruns D and Lemke
J: The emerging role of cyclin-dependent kinases (CDKs) in
pancreatic ductal adenocarcinoma. Int J Mol Sci. 19:2018.
View Article : Google Scholar
|
|
34
|
Xiao W, Jiang Y, Men Q, Yuan L, Huang Z,
Liu T, Li W and Liu X: Tetrandrine induces G1/S cell cycle arrest
through the ROS/Akt pathway in EOMA cells and inhibits angiogenesis
in vivo. Int J Oncol. 46:360–368. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Swaffer MP, Jones AW, Flynn HR, Snijders
AP and Nurse P: CDK substrate phosphorylation and ordering the cell
cycle. Cell. 167:1750.e16–1761.e16. 2016. View Article : Google Scholar
|
|
36
|
Galea CA, Nourse A, Wang Y, Sivakolundu
SG, Heller WT and Kriwacki RW: Role of intrinsic flexibility in
signal transduction mediated by the cell cycle regulator, p27 Kip1.
J Mol Biol. 376:827–838. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Carlson H, Ota S, Song Y, Chen Y and
Hurlin PJ: Tbx3 impinges on the p53 pathway to suppress apoptosis,
facilitate cell transformation and block myogenic differentiation.
Oncogene. 21:3827–3835. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wensing LA and Campos AH: TBX3, a
downstream target of TGF-β1, inhibits mesangial cell apoptosis. Exp
Cell Res. 328:340–350. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Warren CFA, Wong-Brown MW and Bowden NA:
BCL-2 family isoforms in apoptosis and cancer. Cell Death Dis.
10:1772019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Brown JM and Attardi LD: The role of
apoptosis in cancer development and treatment response. Nat Rev
Cancer. 5:231–237. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li X, Ruan X, Zhang P, Yu Y, Gao M, Yuan
S, Zhao Z, Yang J and Zhao L: TBX3 promotes proliferation of
papillary thyroid carcinoma cells through facilitating
PRC2-mediated p57KIP2 repression. Oncogene.
37:2773–2792. 2018. View Article : Google Scholar : PubMed/NCBI
|