Introduction
In 1993, Lee et al (1) discovered the first microRNA (miRNA),
lin-4, which by repressing the lin-14 gene is essential for
controlling the timing of Caenorhabditis elegans larval
development. In 2000, the miRNAlet-7 was discovered to repress
lin-41 to promote a later developmental transition in C.
elegans (2). Since then, a
number of evolutionarily conserved miRNAs have been identified,
from plants and fungi to humans, and have been shown to play
various roles in biological and pathophysiological processes. To
date, thousands of studies on miRNAs using well-developed methods
which are now performed routinely (3). Mature miRNAs are short, single-stranded
RNA molecules,~22 nucleotides in length, processed from
well-characterized precursors through a highly accurate pathway
involving a fold-back hairpin structure (4). The majority of miRNA genes are located
in intergenic regions; however, a small portion are located in
intron and exon sequences. miRNAs function via their seed sequence
(5′-end 2–8 nucleotide sequence), which is completely complementary
or partially complementary to the 3′untranslated region (3′UTR), or
even the coding sequence and 5′UTR, of the target gene. A
ribonucleoprotein complex, named the RNA-induced silencing complex
(RISC), is involved in regulating diverse biological processes,
with argonaute (AGO) being the catalytic component (5). Gene silencing occurs either through RNA
cleavage promotion or translational inhibition. In addition, some
miRNAs do not inhibit target gene expression, but rather bind to
the 5′UTR of ribosomal protein mRNA and promote ribosomal protein
synthesis (6).
The discovery of long non-coding RNAs (lncRNAs)
occurred earlier than that of miRNAs: The first lncRNA, H19, was
discovered by Brannan et al (7) in 1990. However, defining lncRNAs based
simply on the size and the absence of protein-coding capability is
insufficient. Thus far, miRNAs which greatly expand the functional
genome from a large-scale regulatory network are well understood,
while the lncRNA counterpart of the transcriptome has been
relatively neglected. Nonetheless, the evolution and functions of
lncRNAs have recently piqued interest among researchers due to the
availability of sensitive detection techniques. lncRNAs are most
commonly defined as non-protein-coding RNA molecules (>200
nucleotides) transcribed by RNA polymerase that may or may not be
polyadenylated, and can be present within the nucleus or cytoplasm
(8). lncRNAs share a similar
conserved structure with mRNAs (9,10) and
are considered sense, antisense, bidirectional, intronicor
intergenic mRNAs, according to their location in the gene sequence.
Some lncRNAs tend to be transcribed away from the 5′ or the 3′ends
of the gene and are concentrated near promoters. The initial exons
and introns of these genes suggest that the transcription of these
lncRNAs comprises a potential regulatory aspect (11). ncRNAs also participate in a wide
variety of biological processes (12,13),
such as post-transcriptional regulation (14). In general, weak conservation of
lncRNAs exists due to evolution, and due to selective pressure,
several local highly conserved sequences are often distributed in
fragile chromosome sites. A number of studies on lncRNAs have
focused on their regulation of protein-coding genes, and little is
known about interactions between RNA classes. In addition, recent
reports (15) suggest that lncRNAs
may interact with other RNA classes, including miRNAs. Thus,
non-coding RNAs (ncRNAs) are not mere evolutionary relics; rather,
they provide a ‘Rosetta Stone’, facilitating the interpretation of
much of the genomic repertoire of non-coding transcripts.
Interactions between lncRNAs and miRNAs
With the development of gene networks, and
differential expression and pathway analyses, lncRNAs are emerging
as important regulators implicated in various biological processes
(16). However, our understanding of
the impact of miRNA-lncRNA regulatory networks remains limited.
‘Sponge effect’ of lncRNAs on
miRNAs
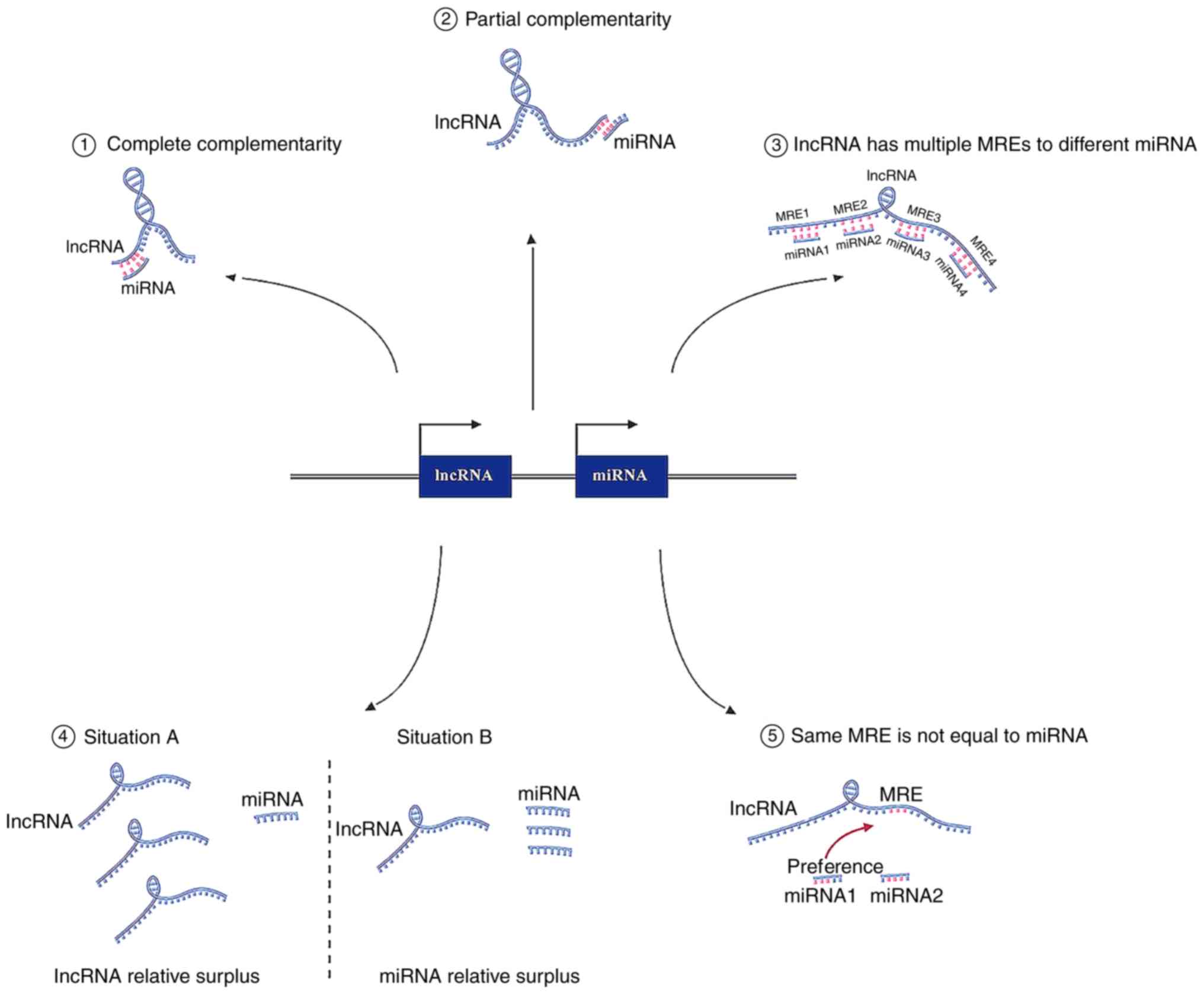
Competing endogenous RNAs (ceRNAs) and microRNA
response elements (MREs), two important components involved in the
‘sponge effect’, can act in almost all interaction mechanisms as
lncRNAs and miRNAs (Fig. 1). ceRNAs
were first proposed by Salmena et al (17), who hypothesized molecular regulation
patterns, such as an lncRNA that competes with a miRNA to release
the inhibition of other genes; this lncRNA was called a ceRNA.
Lewis et al (18) described
more fully the concept of an MRE in 2004; an MRE (miRNA response
element) is a seed region that comprises nucleotides 2–8 of the
5′portion of the miRNA and is particularly crucial for mRNA
recognition and silencing or interaction with ncRNAs. Moreover,
MREs and ceRNAs play an irreplaceable role in the ‘sponge effect’
of lncRNAs and miRNAs. The characteristics of this ‘sponge effect’
can be observed via the following aspects.
At present, there are two modes used to describe the
‘sponge effect’ of lncRNAs and miRNAs, namely complete
complementary mode and partial complementary mode. miRNAs that bind
to target gene sequences are partially complementary, and this
process is mediated by MREs that harbor conserved target sites. In
2009, Seitz (19) proposed that
miRNA-binding sites identified via bioinformatics can titrate
miRNAs and thereby impair their activity. Such ceRNAs regulate MREs
on their targets, and thus play an important role in
post-transcriptional regulation. When the sponging effect of an
lncRNA and a miRNA occurs, it is usually complete complementation.
However, when an miRNA negatively controls an lncRNA, the mature
lncRNA usually has a hat and a poly5-A tail, that is, a 5′UTR and a
3′UTR. Therefore, miRNAs can also have partial complementation with
an lncRNA, similar to an mRNA (11).
In general, an lncRNA has multiple MREs, and the
more it has, the more the lncRNAs and miRNAs communicate with each
other. This has an important effect indifferent physiological and
pathological conditions. For instance, lncRNA-BGL3 functions as a
ceRNA for miR-17, miR-93, miR-20a, miR-20b, miR-106a and miR-106b
to prevent repression of the mRNA for phosphatase and tensin
homolog (20). lncRNAs that share
multiple MREs will crosstalk effectively, which is also of great
significance in a variety of biochemical processes (21).
Moreover, the same MREs on a ceRNA are not equal.
For miRNA, all lncRNAs that do not contain an MRE will have a
sponging effect with the corresponding miRNA, exhibiting a
preference when several miRNAs are present at the same time
(22). For instance, the lncRNA
BC032469 contains elements complementary to the miR-1207-5p and
miR-1266 seed regions; however, BC032469 functions as a ceRNA by
impairing only miR-1207-5p-dependent target gene downregulation
(23). It is proposed that the
primary targets of a certain miRNA are preferentially affected,
whereas the remainder are less affected Moreover, previous studies
(24,25) have suggested that MREs in lncRNAs
show a positional preference for the AGO binding sites in
mid-regions and at the 3′ends of the lncRNAs (11). These sites harbor a possible pattern
of regulatory elements across transcripts.
In addition, the overall influence of the sponging
effect depends on the specific spatial-temporal distribution. For
example, during embryonic development, an miRNA has been proven to
be an important post-transcriptional regulator that can promote the
rapid clearance of core transcription factors (TFs) during human
embryonic stem cell (hESC) differentiation (26). Long intergenic non-coding RNA,
regulator of reprogramming (Linc-RoR) can serve as the endogenous
‘sponge’ for differentiation-related miRNAs. In hESC self-renewal,
Linc-RoR suppresses miRNAs at a certain stage when highly expressed
or under treatment with various agents. However, in hESCs with
strong differentiation ability, the relevant miRNA is highly
transcribed, and Linc-RoR levels decrease. Linc-RoR is important
for suppressing miRNA expression in the early stage of hESC
differentiation, which may facilitate further hESC differentiation
(26). Moreover, these results may
provide an insight into miRNA-lncRNA interactions occurring in
multiple stem cells.
In summary, the sponging effect is the basis of the
molecular mechanism of the network involved in various biochemical
processes mediated by miRNAs, lncRNAs and related molecules.
Main mechanisms of regulation between
lncRNAs and miRNAs
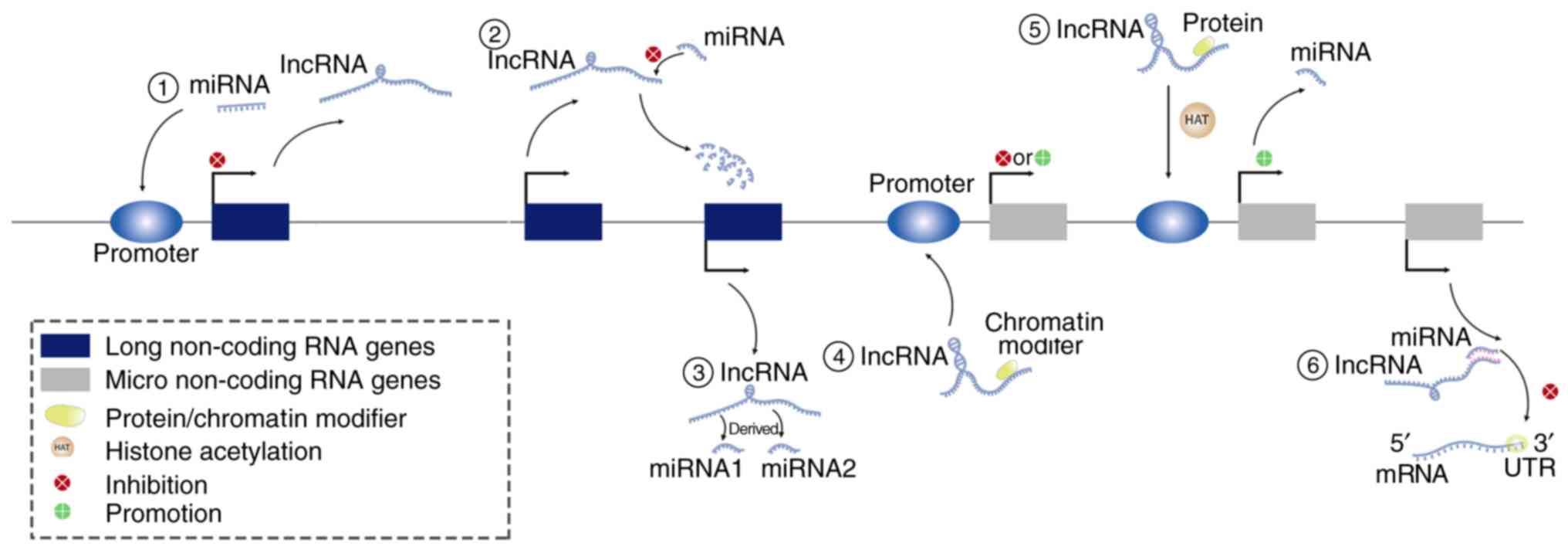
There are two aspects of regulatory factors and
regulatory targets: Οne is the regulation of lncRNAs by miRNAs, and
the other is the regulation of miRNAs by lncRNAs (Fig. 2). Regarding the former, miRNAs can
indirectly regulate the expression of lncRNAs. lncRNAs and miRNAs
interact to form the transcriptome of regulatory networks, an
interaction that is sometimes similar to an enhancer function,
influencing the expression of flanking genes (27). An interesting example of the
interaction between an miRNA and an lncRNA is the DLK1-MEG3
imprinted domain, which includes the tumor suppressor factor MEG3
lncRNA, and an miRNA, such as miR-29, which is involved in a number
of cancer types (28). miR-29
negatively regulates DNA methylase and indirectly regulates the
expression of MEG3 in liver cancer. In addition, miRNAs degrade
lncRNAs in an AGO-dependent manner. Within the RISC, miRNA binds to
the target lncRNA 3′UTR, leading to full mRNA degradation or
blockade of the ribosomal machinery, both of which result in gene
silencing (29). lncRNAs also
regulate miRNAs in further ways. The most common involves
lncRNA-mediated inhibition of miRNA expression via the sponging
effect. lncRNAs can be used as precursors of miRNAs to directly
affect miRNA regulation; some differences between the two sequences
may exist (30). Guo et al
(31) examined H19 and the product
of miR-675 sequencing analysis, and found that the H19 main base is
G, with miR-675 having a G or C. These two types of mature miRNA
sequences are reversed inmiR-675-3p/5p, and this structure ensures
the stability of the pre-miRNA stem-loop structure. Differences in
nucleotide composition tend to indicate that different lengths are
required for functioning. Although miR-675 and H19 belong to the
ncRNA family, they exhibit different conservation and nucleotide
mutation frequencies. Additionally, lncRNAs bind competitively with
miRNA targets (some mRNAs), and lncRNAs compete with the 3′UTR of
the target mRNA, which indirectly inhibits the negative regulation
of the target mRNA by the miRNA (18,31). For
example, lncRNA FEZF1-AS1 can competitively inhibit miRNA-30a,
leading to Nanog silencing in breast cancer (32). lncRNAs also bind to several proteins
in complex to regulate miRNA expression, such as H19, which can act
on PCAF/hnRNPU/Pol RNA II and enhance the histone acetylation of
the region upstream of miR-200 (33), and lncRNAs can affect the expression
of miRNAs via other chromatin modifications (34).
miRNAs and lncRNAs constitute a
negative feedback loop
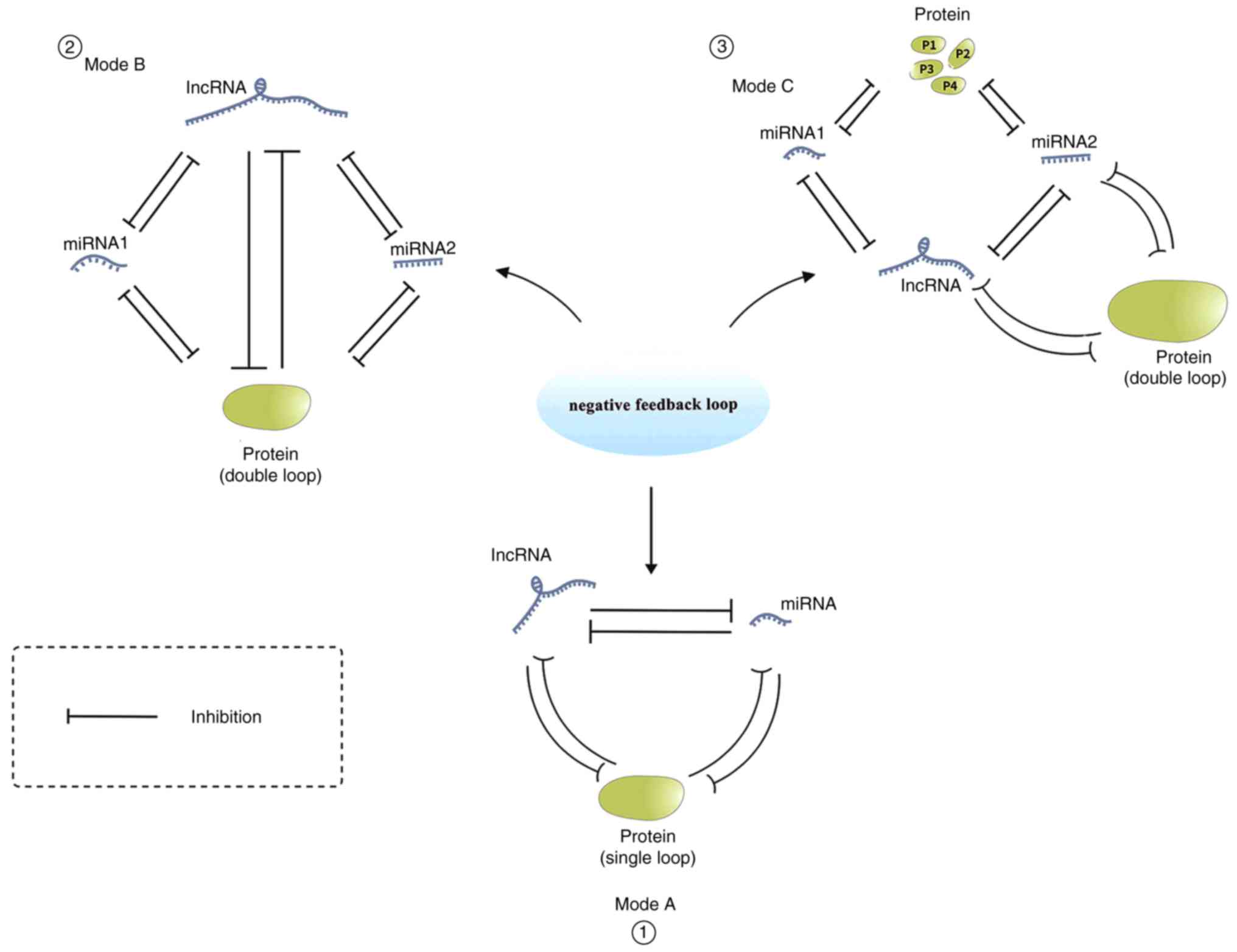
Another important mechanism of interaction between
miRNAs and lncRNAs is that they can function together to form a
negative feedback regulation pathway. A relatively simple example
is that miRNA-200a and histone deacetylase4 (HDAC4) can form a
negative feedback regulation loop in hepatocellular carcinoma; that
is, HDAC4 overexpression can inhibit miR-200a (33). Other studies have confirmed that
HDAC4 overexpression also inhibits H19, indicating that H19,
miR-200A and HDAC4 together constitute a negative feedback
regulation loop (33,35). Another example involves the tumor
formation process, whereby the enhancer of zeste homolog 2 (EZH2)
gene participates in polycomb complex 2 core subunit inhibition,
with epigenetic modification playing a crucial role. EZH2 has been
confirmed to interact with a variety of miRNAs and has been
accepted as a negative regulator of miRNAs (36). Some miRNAs can bind directly to the
3′UTR of EZH2, and EZH2 regulates miRNA expression at two
transcriptional levels. By interacting with EZH2, miRNAs can affect
H3K27 methylation and regulate cellular processes. Thus, miRNAs and
EZH2 constitute an important regulatory and feedback pathway in
which EZH2 is a stable factor. In addition, miR-26a-2 forms a
negative feedback loop with miR-101 and EZH2 and is under the
negative regulation of MYC and HIF-1a/1b (36,37).
EZH2 inhibits cell cycle regulatory factors and the tumor
suppressor gene Rap1GAP, and participates in the
epithelial-mesenchymal transition process via molecules such as
E-cadherin (36). Moreover, EZH2 and
lncRNAs are closely related, affecting both each other and the
expression of target genes. Therefore, miRNA-101, miR-26a, EZH2 and
HOTAIR lncRNA also comprise a negative feedback loop (38). Furthermore, lncRNAs and related
molecules can form other negative feedback loops. In addition,
MIR100HG, miR-100 and miR-125b overexpression has been observed in
cetuximab-resistant colorectal cancer, head and neck squamous cell
cancer cell lines, as well as in tumors from patients with
colorectal cancer whose disease progressed on cetuximab, and
miR-100 and miR-125b have been observed to coordinately repress
five Wnt/β-catenin negative regulators (mitochondrial genome
maintenance exonuclease 1, Dbf4-dependent kinase 3, ring finger
protein 4, cell division cycle 27 and nuclear factor, erythroid 2
like 2). These results describe a double-negative feedback loop
between MIR100HG and the TF GATA binding protein 6 (GATA6): GATA6
represses MIR100HG; however, this repression is relieved through
targeting of GATA6 by miR-125b, which results in increased Wnt
signaling, and Wnt inhibition in cetuximab-resistant cells restores
cetuximab responsiveness (39).
Thus, the lncRNA MIR100HG, miR-100, miR-125b and GATA6 form a
double-negative feedback loop. These examples also indicate that
lncRNAs and miRNAs may be involved in the diversity of the negative
feedback loop (Fig. 3).
Methods of research into lncRNAs and
miRNAs
Databases for studying interactions
between miRNAs and lncRNAs
In recent years, a number of lncRNA/miRNA-related
databases have been established by researchers in combination with
bioinformatics technology (Table I)
(40–50). The establishment of these databases
not only provides comprehensive information on various types of
lncRNAs, but also a very important platform for studying the
regulatory relationship between lncRNAs and miRNAs. Three
representative databases (44,46,50) are
discussed below. The DIANA-LncBase database (http://www.microrna.gr/LncBase) is a tool developed by
the DIANA Laboratory for researchers to explore potential
interactions between miRNAs and lncRNAs. The DIANA-LncBase database
offers detailed information for each miRNA-lncRNA pair, such as
graphical plots of the genomic location of the transcript, a
representation of binding sites, lncRNA tissue expression, and MRE
conservation and prediction scores. The CHIP database (http://rna.sysu.edu.cn/chipbase/) is an open
database developed by Zhongshan University (Guangzhou, China). The
CHIP database is mainly used for comprehensive annotation of
ncRNAs, including TF binding sites and motifs, and for decoding the
transcriptional regulatory networks of lncRNAs, miRNAs, other
ncRNAs and protein-coding genes based on chromatin
immunoprecipiation-sequencing (seq) data. By integrating
experimental and predicted ncRNA-disease associations from manual
literature curation and other resources under one common framework,
the MNDR v2.0 database (http://www.rna-society.org/mndr/index.html) was
developed by Harbin Medical University (Harbin, China) for ncRNAs
and related diseases.
 | Table I.Related databases for the study of
interactions between miRNAs and lncRNAs. |
Table I.
Related databases for the study of
interactions between miRNAs and lncRNAs.
| Author, year | Database | Website | Applicable
ncRNA | (Refs.) |
|---|
| Erdmann et
al, 2000 | Non-codingRNA
database | http://biobases.ibch.poznan.pl/ncRNA | lncRNA/miRNA | (40) |
| Mituyama et
al, 2009 | fRNAdb | http://www.ncRNA.org/frnadb | lncRNA/miRNA | (41) |
| Dinger et
al, 2009 | NERD | http://jsm-research.imb.uq.edu.au/NRED | lncRNA | (42) |
| Amaral et
al, 2011 | lncRNAdb | http://www.lncrnadb.org/ | lncRNA | (43) |
| Yang et al,
2013 | ChipBase v2.0 | http://rna.sysu.edu.cn/chipbase/ | lncRNA/miRNA | (44) |
| Volders et
al, 2013 | LNCipedia | http://www.lncipedia.org | lncRNA | (45) |
| Paraskevopoulou
et al, 2013 | DIANA-LncBase | http://www.microrna.gr/LncBase | lncRNA/miRNA | (46) |
| Cook et al,
2011 | RBPDB | http://rbpdb.ccbr.utoronto.ca/ | lncRNA/miRNA | (47) |
| Li et al,
2014 |
lncRNABase(starBase) v2.0 | http://starbase.sysu.edu.cn/ | lncRNA | (48) |
| Hsu et al,
2006 | miRNAMap | http://mirnamap.mbc.nctu.edu.tw | miRNA | (49) |
| Cui et al,
2018 | MNDR v2.0 | http://www.rna-society.org/mndr/index.html | lncRNA/miRNA | (50) |
Research technology for lncRNAs and
miRNAs
As lncRNAs/miRNAs have various functions through
numerous types of mechanisms, the establishment and application of
molecular biology research methods has a very important role in
investigating lncRNA/miRNA functions (Table II). At present, there are several
well-developed research methods for qualitative and quantitative
analysis of ncRNAs. Microarray, RNA-seq, northern blotting, reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) and
fluorescence in situ hybridization (FISH) have been used for
such analyses of ncRNAs (51).
Indeed, Ørom et al (52)
observed the expression of 3,019 types of lncRNAs in a variety of
human cell lines through microarray detection spectrum analysis.
Using RNA-seq technology from induced pluripotent stem cells of
human neurons, Lin et al (53) found that a number of lncRNAs are
involved in the development of nervous system diseases. Northern
blotting (54) and RT-qPCR (55) assays have been employed to examine
the expression of ncRNAs, and to verify the authenticity of the
results of microarray experiments. In addition to FISH technology,
combined knockdown and localization of ncRNAs (c-KLAN) can be used
to analyze the localization of non-coding RNAs in cells or tissues
(56). Subcellular fractionation
(57), which is a process used to
separate cellular components while preserving the individual
functions of each component, is has also been applied to the study
of ncRNA localization. The main technologies currently being used
in research into ncRNA function, in terms of silencing ncRNA gene
expression, include RNA interference (RNAi; small interfering or
short hairpin RNA) (48), locked
nucleic acids (58) and clustered
regularly interspaced short palindromic repeats (CRISPR) (59). As another approach to the study of
ncRNA functions, the target ncRNA gene can be overexpressed by a
plasmid or lentiviral vector, followed by the observation of
changes in cell phenotype. Among these techniques, RNAi has been
widely employed to examine a specific lncRNA (60), and RNA binding protein
immunoprecipitation (RIP) has been widely used for examining the
mechanism of ncRNA action. RIP is also used to screen proteins
related to lncRNA binding. With the development of molecular
biology technologies, researchers have combined RIP with
microarrays to develop a new technology, RIP-Chip (61), and the combination of RIP and RNA-seq
has resulted in another technology, RIP-seq (62). With regards to bioinformatics
research methods for ncRNAs, numerous new approaches have been
reported, such as catRAPID [fast predictions of RNA and protein
interactions and domains; (63)],
which is being used in the rapid prediction of RNA and protein
interactions (thepredictive function can provide guidance for
finding the lncRNA target). Capture long-read sequencing (CLS), a
new technology for accelerating lncRNA annotation developed by the
GENCODE alliance, combines targeted RNA capture with the third
generation of long read sequencing. The full-length transcriptional
model produced by CLS is superior to that of the existing
short-reading technology, revealing the genomic features of
lncRNAs, including promoter and gene structure, and protein coding
potential (64). Chromatin
conformation capture (3C technology) is another technology that
implements quantitative or semi-quantitative methods to assess
changes in the interactions between the three-dimensional
structures of genomic regions (65).
In recent years, 4C-seq (66),
5C-seq (67) and Hi-C (68) have been derived from next-generation
sequencing. With the increase in the number of lncRNAs involved in
chromatin conformation interactions, this technology is also used
to study lncRNA-mediated chromatin interactions (65–68). The
results of ncRNA research technology have recently aided in the
development of the new technique of frozen electron microscopy,
which analyzes the structure of nucleic acids (including
ncRNAs/proteins and their complexes), providing a more thorough
understanding of their functions (69). Moreover, the rapid amplification of
cDNA ends technique is used to study the function of ncRNAs for
loss-of-function studies, as well as the cellular localization of
lncRNAs, starting from a known cDNA fragment and extending through
the ends to obtain the complete 3′and 5′ends and, subsequently, the
full-length lncRNA sequence (70).
The development of new technologies in functional areas is helpful
for discovering the biological functions of lncRNAs, their
molecular mechanisms, and the pathological mechanisms involved in
the development of tumors.
 | Table II.Related technology for the study of
interactions between miRNAs and lncRNAs. |
Table II.
Related technology for the study of
interactions between miRNAs and lncRNAs.
| Author, year | Technology | Function | Significance | Applicable
ncRNA | (Refs.) |
|---|
| Yan et al,
2012 | Microarray | Qualitativeand
quantitative research on ncRNAs | Detection spectrum
analysis of lncRNAsin a variety of cell lines | lncRNA/miRNA | (51) |
| Yan et al,
2012 | RNA-seq | Qualitative and
quantitative research on ncRNAs | Analysis of the
sequence and expression level of target ncRNAs in cells | lncRNA/miRNA | (51) |
| Yan et al,
2012 | Northern blotting;
RT-qPCR | Quantitative study
of target ncRNA | Analysis of the
expression level of ncRNAs in cells or tissues | lncRNA/miRNA | (51) |
| Yan et al,
2012 | FISH | Quantitative study
on the location of target ncRNA | Analysis of the
location and expression level of ncRNAs in cells or tissues | lncRNA/miRNA | (51) |
| Chakraborty et
al, 2012; Alberts et al, 2002 | c-KLAN; subcellular
fractionation | Study on the
location of target ncRNA | Clarification on
the site of action of ncRNAs | lncRNA/miRNA | (56,57) |
| Keene et al,
2006 | RIP-Chip | Study on the
composition of complex compound with ncRNA | Provides guidance
on the changes in the expression levels of target RNA in cancer or
other diseases. | lncRNA/miRNA | (61) |
| Bellucci et
al, 2011 | catRAPID | Predictions of RNA
and fast interactions and domains | Provides positive
guidance for finding ncRNA targets, and it can be used to detect
the interaction between RNA and DNA/protein | lncRNA/miRNA | (63) |
| Lagarde et
al, 2017 | CLS | Accelerating lncRNA
annotations | To articulate the
genome features of lncRNAs, including promoter and gene structure,
and protein coding potential | lncRNA | (64) |
| Olivarius et
al, 2009 | RACE | Rapidly amplifying
the 5′ and 3′ ends of cDNA from low abundance transcripts based on
PCR | To obtain the
full-length lncRNA sequence for research | lncRNA | (65) |
| Yan et al,
2012; Koshkin et al, 1998; Zhang et al, 2014 |
RNAi/CRISPR/LNA | Interfering with
the expression of ncRNA in varying degrees | Study of the
function of ncRNAs by cell phenotype | lncRNA/miRNA | (51,58,59) |
| Tanizawa et
al, 2012; Splinter et al, 2012; Dostieand Dekker, 2007;
Belton et al, 2012 | 3C/4C/5C/Hi-c
technology | Study on the
changes in the interaction between the three dimensional structural
changes between genomic regions | Used for
lncRNA-mediated chromatin interaction | lncRNA | (65–68) |
lncRNAs and miRNAs in cancer
Overall, miRNAs and lncRNAs have important roles in
the diagnosis and treatment of cancer. Recent studies have
indicated that specific lncRNAs and miRNAs related to cancer can be
detected in blood and serum samples (71,72). For
example, lncRNA GHET1 is overexpressed in various cancers, which
can predict unfavorable survival and clinical parameters in
patients (73). Recent literature
has shown that miR-20a is overexpressed in colon cancer and acts as
a diagnostic and prognostic biomarker (74). lncRNAs and miRNAs can also be
combined for the diagnosis of cancer. Permuth et al
(75) reported on the use of eight
lncRNAs (C00472, MEG3, PANDA, PVT1, UCA1, ANRIL, GLIS3-AS1, and
ADARB2-AS1) for the diagnosis of pancreatic ductal carcinoma
(PDAC), and Li et al (76)
found that miRNA1209 in the serum of patients with PDAC has a
higher diagnostic accuracy than CA-199. In recent years, the
mechanisms by which lncRNAs and miRNAs mediate cancer progression
have been gradually studied (Table
III) (77–86), and the main mechanisms involved are
epigenetic regulation, transcriptional and post-transcriptional
regulation, and ceRNAs. The knowledge on these mechanisms can be
useful in the treatment and diagnosis of tumors. In China, ncRNAs
have been used for cancer diagnosis in the form of diagnostic kits.
Qadir et al (87) attempted
to summarize the emerging field of ncRNAs and their role in
different diseases, including cancer, their modes of action, and
their potential in target identification and therapeutic drug
development. In addition, some gene editing techniques have been
used for cancer treatment, such as CRISPR. Although, at present,
there is no systematic evaluation of the clinical prognosis of
ncRNA-mediated tumors, more clinical diagnoses and treatments of
tumors could be explored, according to the interaction mechanisms
of different ncRNAs in the processes of tumorigenesis and
development. It is worth noting that in recent years, some other
types of ncRNA have been involved in the development of diseases
including cancer, such as circular RNAs(circRNAs) and
PIWI-interacting RNAs (piRNAs). circRNA is a novel form of RNA
which is distinct from the traditional linear RNA. circRNA has a
strong cyclic structure, species conservation and tissue
specificity, and it is not readily cleaved by nucleases (88,89).
piRNAs, ncRNAs of 25–33 nt in length, function by interacting with
the PIWI protein (90,91). Thus, various ncRNAs involved in the
regulation of the occurrence and development of cancer still need
further study.
 | Table III.lncRNAs and miRNAs associated with
molecular mechanisms in human cancer. |
Table III.
lncRNAs and miRNAs associated with
molecular mechanisms in human cancer.
|
|
| Expression |
|
|
|
|
|---|
| Author, year | ncRNAs | Upregulated | Downregulated | Mechanism | Outcomes | Cancer | (Refs.) |
|---|
| Yang et al,
2019 | lncRNA00173,
miRNA182-5p | miRNA182-5pl | lncRNA00173 | Sponge effect | Facilitate cell
proliferation, migration and apoptosis | Non-small cell lung
cancer | (77) |
| Li et al,
2019 | lncRNA XIST,
miR-497-5p | miR-191 | lncRNA XIST | Sponge effect | Facilitate cell
proliferation, migration | Hepatocellular
carcinoma | (78) |
| Zhang et al,
2019 | miR-191 | miR-191 | – | Regulation of
signaling pathway (axis) | Facilitate cell
proliferation | Hepatocellular
carcinoma | (79) |
| Hu et al,
2016 | miRNA 21, lncRNA
GAS5 | miRNA 21 | lncRNA GAS5 | Transcriptional
regulation | Promote cell
migration and invasion | Hepatocellular
carcinoma | (80) |
| Wu et al,
2015 | lncRNA
uc002yug.2 | lncRNA
uc002yug.2 | – |
Post-transcriptional regulation | Promote cell
proliferation, migration and invasion | Esophageal
cancer | (81) |
| Chen et al,
2014; Kang et al, 2015 | lncRNA ANRIL | – | lncRNA ANRIL | Transcriptional
regulation | Promote cell
proliferation and anchorage-dependent growth; inhibits
apoptosis | Esophageal
cancer | (82,83) |
| Zhang et al,
2017 | lncRNA CCAT1 | lncRNA CCAT1 | – | Epigenetic
modification | Promote cell
growth, migration, tumour occurrence | Esophageal
cancer | (84) |
| Mazzu et al,
2019 | miRNA 193b | miRNA 193b | – | Epigenetic
modification | Facilitate prostate
cancer progression | Prostate
cancer | (85) |
| Chen et al,
2019 | miRNA 31-5p, miRNA
223-3p | miRNA 31-5p, miRNA
223-3p | – |
Post-transcriptional regulation | Promote the
occurrence of colon cancer | Colitis-associated
cancer | (86) |
Conclusion
In conclusion, the mechanisms by which miRNAs and
lncRNAs mediate the occurrence and development of cancer still need
further exploration in order to provide broader prospects for
cancer treatment. Currently, the miRNA-lncRNA interaction
mechanisms are an important part of research and treatment for most
cancer types. However, there are numerous challenges to be
addressed, such as the safety of miRNAs for the treatment of
tumors. Nonetheless, with additional research on ncRNAs in the
field of cancer, it is considered likely that the specific
mechanism of ncRNA-mediated tumorigenesis and development will be
found, offering accurate entry points for the treatment of tumors.
Therefore, exploring miRNA and lncRNA interactions could provide
new breakthroughs for the clinical treatment of tumors.
Acknowledgements
The authors would like thank Professor Shiming Yang
of the Army Military Medical University and Professor Rui Xie of
Zunyi Medical University for their guidance.
Funding
The study was supported by the Science and
Technology Commission Foundation of Sichuan Province (grant no.
2016JY0101), the Science and Technology Commission Foundation of
Sichuan Province (grant no. 2017JQ0052), the Luzhou city-Luzhou
medical college [grant no. 2015-LZCYD-S01(9/15)], and the Southwest
medical university Funding (grant no. 2017-R-63).
Availability of data and materials
Not applicable.
Authors' contributions
BS and CL wrote the manuscript draft. BS, CL, HL and
LZ contributed to the preparation of the manuscript. ML, SL and GL
revised the manuscript. BS, CL, ML conceived the design of the
figures. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ncRNA
|
non-coding RNA
|
|
lncRNA
|
long non-coding RNA
|
|
miRNA
|
microRNA
|
|
AGO
|
argonaute
|
|
RISC
|
RNA-induced silencing complex
|
|
3′UTR
|
3′untranslated region
|
|
5′UTR
|
5′untranslated region
|
|
ceRNA
|
competing endogenous RNA
|
|
MRE
|
microRNA response element
|
|
HAT
|
histone acetylation
|
References
|
1
|
Lee RC, Feinbaum RL and Ambros V: The C.
elegans heterochronic gene lin-4 encodes small RNAs with antisense
complementarity to lin-14. Cell. 75:843–854. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Reinhart BJ, Slack FJ, Basson M,
Pasquinelli AE, Bettinger JC, Rougvie AE, Horvitz HR and Ruvkun G:
The 21-nucleotide let-7 RNA regulates developmental timing in
Caenorhabditis elegans. Nature. 403:901–906. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Remsburg C, Konrad K, Sampilo NF and Song
JL: Analysis of microRNA functions. Methods Cell Biol. 151:323–334.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Suzuki H, Gabrielson E, Chen W, Anbazhagan
R, van England M, Herman JG, Baylin SB and Weijenberg MP: A genomic
screen for genes upregulated by demethylation and histone
deacetylase inhibition in human colorectal cancer. Nat Genet.
31:141–149. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vishnoi A and Rani S: MiRNA biogenesis and
regulation of diseases: An overview. Methods Mol Biol. 1509:1–10.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lintner NG and Cate JHD: Regulating the
ribosome: A spotlight on RNA dark matter. Mol Cell. 54:1–2. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brannan CI, Dees EC, Ingram RS and
Tilghman SM: The product of the H19 gene may function as an RNA.
Mol Cell Biol. 10:28–36. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Spizzo R, Almeida MI, Colombatti A and
Calin GA: Long non-coding RNAs and cancer: A new frontier of
translational research? Oncogene. 31:4577–4587. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Arriaga-Canon C, Fonseca-Guzmán Y,
Valdes-Quezada C, Arzate-Mejía R, Guerrero G and Recillas-Targa F:
A long non-coding RNA promotes full activation of adult gene
expression in the chicken α-globin domain. Epigenetics. 9:173–181.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bergmann JH and Spector DL: Long
non-coding RNAs: Modulators of nuclear structure and function. Curr
Opin Cell Boil. 26:10–18. 2014. View Article : Google Scholar
|
|
11
|
Majoros WH and Ohler U: Spatial
preferences of microRNA targets in 3′untranslated regions. BMC
Genomics. 8:1522007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cabili MN, Trapnell C, Goff L, Koziol M,
Tazon-Vega B, Regev A and Rinn JL: Integrative annotation of human
large intergenic noncoding RNAs reveals global properties and
specific subclasses. Genes Dev. 25:1915–1927. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Du Z, Fei T, Verhaak RG, Su Z, Zhang Y,
Brown M, Chen Y and Liu XS: Integrative genomic analyses reveal
clinically relevant long noncoding RNAs in human cancer. Nat Struct
Mol Biol. 20:908–913. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou Q, Yu Q, Gong Y, Liu Z, Xu H, Wang Y
and Shi Y: Construction of a lncRNA-miRNA-mRNA network to determine
the regulatory roles of lncRNAs in psoriasis. Exp Ther Med.
18:4011–4021. 2019.PubMed/NCBI
|
|
15
|
Dong Z, Zhang A, Liu S, Lu F, Guo Y, Zhang
G, Xu F, Shi Y, Shen S, Liang J and Guo W: Aberrant
methylation-mediated silencing of lncRNA MEG3 functions as aceRNA
in esophageal cancer. Mol Cancer Res. 15:800–810. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang KC and Chang HY: Molecular mechanisms
of long noncoding RNAs. Mol Cell. 43:904–914. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Salmena L, Poliseno L, Tay Y, Kats L and
Pandolfi PP: A ceRNA hypothesis: The Rosetta Stone of a hidden RNA
language? Cell. 146:353–358. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lewis BP, Burge CB and Bartel DP:
Conserved seed pairing, often flanked by adenosines, indicates that
thousands of human genes are microRNA targets. Cell. 120:15–20.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Seitz H: Redefining microRNA targets. Curr
Biol. 19:870–873. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Guo G, Kang Q, Zhu X, Chen Q, Wang X, Chen
Y, Ouyang J, Zhang L, Tan H, Chen R, et al: A long noncoding RNA
critically regulates Bcr-Abl-mediated cellular transformation by
acting as a competitive endogenous RNA. Oncogene. 34:1768–1779.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ergun S and Oztuzcu S: Oncocers:
CeRNA-mediated cross-talk by sponging miRNAs in oncogenic pathways.
Tumour Biol. 36:3129–3136. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jalali S, Bhartiya D, Lalwani MK,
Sivasubbu S and Scaria V: Systematic transcriptome wide analysis of
lncRNA-miRNA interactions. PLoS One. 8:e538232013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lü MH, Tang B, Zeng S, Hu CJ, Xie R, Wu
YY, Wang SM, He FT and Yang SM: Long noncoding RNA BC032469, a
novel competing endogenous RNA, upregulates hTERT expression by
sponging miR-1207-5p and promotes proliferation in gastric cancer.
Oncogene. 35:3524–3534. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Karreth FA and Pandolfi PP: ceRNA
cross-talk in cancer: When ce-bling rivalries go awry. Cancer
Discov. 3:1113–1121. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Guo LL, Song CH, Wang P, Dai LP, Zhang JY
and Wang KJ: Competing endogenous RNA networks and gastric cancer.
World J Gastroenterol. 21:11680–11687. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang Y, Xu Z, Jiang J, Xu C, Kang J, Xiao
L, Wu M, Xiong J, Guo X and Liu H: Endogenous miRNA sponge
lincRNA-RoR regulates Oct4, Nanog, and Sox2 in human embryonic stem
cell self-renewal. Dev Cell. 25:69–80. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ørom UA and Shiekhattar R: Long non-coding
RNAs and enhancers. Curr Opin Genet Dev. 21:194–198. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Keniry A, Oxley D, Monnier P, Kyba M,
Dandolo L, Smits G and Reik W: The H19 lincRNA is a developmental
reservoir of miR-675 that suppresses growth and Igf1r. Nat Cell
Biol. 14:659–665. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Da Sacco L and Masotti A: Recent insights
and novel bioinformatics tools to understand the role of microRNAs
binding to 5′untranslated region. Int J Mol Sci. 14:480–495. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
López-Urrutia E, Bustamante Montes LP,
Ladrón de Guevara Cervantes D, Pérez-Plasencia C and Campos-Parra
AD: Crosstalk between long non-coding RNAs, Micro-RNAs and mRNAs:
Deciphering molecular mechanisms of master regulators in cancer.
Front Oncol. 9:6692019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Guo L, Zhao Y, Yang S, Zhang H, Wu Q and
Chen F: An integrated evolutionary analysis of miRNA-lncRNA in
mammals. Mol Biol Rep. 41:201–207. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang Z, Sun L, Zhang Y, Lu G, Li Y and
Wei Z: Long non-coding RNA FEZF1-AS1 promotes breast cancer
stemness and tumorigenesis via targeting miR-30a/Nanog axis. J Cell
Physiol. 233:8630–8638. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang L, Yang F, Yuan JH, Yuan SX, Zhou
WP, Huo XS, Xu D, Bi HS, Wang F and Sun SH: Epigenetic activation
of the MiR-200 family contributes to H19-mediated metastasis
suppression in hepatocellular carcinoma. Carcinogenesis.
34:577–586. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sun M and Kraus W: From discovery to
function: The expanding roles of long non-coding RNAs in physiology
and disease. Endocr Rev. 7:er000099992015.(Epub ahead of print).
View Article : Google Scholar
|
|
35
|
Yuan JH, Yang F, Chen BF, Lu Z, Huo XS,
Zhou WP, Wang F and Sun SH: The histone deacetylase
4/SP1/microrna-200a regulatory network contributes to aberrant
histone acetylation in hepatocellular carcinoma. Hepatology.
54:2025–2035. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cao P, Deng Z, Wan M, Huang W, Cramer SD,
Xu J, Lei M and Sui G: MicroRNA-101 negatively regulates Ezh2 and
its expression is modulated by androgen receptor and
HIF-1alpha/HIF-1beta. Mol Cancer. 9:1082010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sander S, Bullinger L, Klapproth K,
Fiedler K, Kestler HA, Barth TF, Möller P, Stilgenbauer S, Pollack
JR and Wirth T: MYC stimulates EZH2 expression by repression of its
negative regulator miR-26a. Blood. 112:4202–4212. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Benetatos L, Voulgaris E, Vartholomatos G
and Hatzimichael E: Non-coding RNAs and EZH2 interactions in
cancer: Long and short tales from the transcriptome. Int J Cancer.
133:267–274. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lu Y, Zhao X, Liu Q, Li C, Graves-Deal R,
Cao Z, Singh B, Franklin JL, Wang J, Hu H, et al: lncRNA
MIR100HG-derived miR-100 and miR-125b mediate cetuximab resistance
via Wnt/β-catenin signaling. Nat Med. 23:1331–1341. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Erdmann VA, Szymanski M, Hochberg A, Groot
N and Barciszewski J: Non-coding, mRNA-like RNAs database Y2K.
Nucleic Acids Res. 28:197–200. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mituyama T, Yamada K, Hattori E, Okida H,
Ono Y, Terai G, Yoshizawa A, Komori T and Asai K: The Functional
RNA Database 3.0: Databases to support mining and annotation of
functional RNAs. Nucleic Acids Res. 37:D89–D92. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Dinger ME, Pang KC, Mercer TR, Crowe ML,
Grimmond SM and Mattick JS: NRED: A database of long noncoding RNA
expression. Nucleic Acids Res. 37:D122–D126. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Amaral PP, Clark MB, Gascoigne DK, Dinger
ME and Mattick JS: lncRNAdb: A reference database for long
noncoding RNAs. Nucleic Acids Res. 39:D146–D151. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yang JH, Li JH, Jiang S, Zhou H and Qu LH:
ChIPBase: A database for decoding the transcriptional regulation of
long non-coding RNA and microRNA genes from ChIP-Seq data. Nucleic
Acids Res. 41:D177–D187. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Volders PJ, Helsens K, Wang X, Menten B,
Martens L, Gevaert K, Vandesompele J and Mestdagh P: LNCipedia: A
database for annotated human lncRNA transcript sequences and
structures. Nucleic Acids Res. 41:D246–D251. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Paraskevopoulou MD, Georgakilas G,
Kostoulas N, Reczko M, Maragkakis M, Dalamagas TM and Hatzigeorgiou
AG: DIANA-LncBase: Experimentally verified and computationally
predicted microRNA targets on long non-coding RNAs. Nucleic Acids
Res. 41:D239–D245. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Cook KB, Kazan H, Zuberi K, Morris Q and
Hughes TR: RBPDB: A database of RNA-binding specificities. Nucleic
Acids Res. 39:D301–D308. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Li JH, Liu S, Zhou H, Qu LH and Yang JH:
starBase v2.0: Decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA
interaction networks from large-scale CLIP-Seq data. Nucleic Acids
Res. 42:D92–D97. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Hsu PW, Huang HD, Hsu SD, Lin LZ, Tsou AP,
Tseng CP, Stadler PF, Washietl S and Hofacker IL: miRNAMap: Genomic
maps of microRNA genes and their target genes in mammalian genomes.
Nucleic Acids Res. 34:D135–D139. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Cui T, Zhang L, Huang Y, Yi Y, Tan P, Zhao
Y, Hu Y, Xu L, Li E and Wang D: MNDR v2.0: An updated resource of
ncRNA-disease associations in mammals. Nucleic Acids Res.
46:D371–D374. 2018.PubMed/NCBI
|
|
51
|
Yan B, Wang ZH and Guo JT: The research
strategies for probing the function of long noncoding RNAs.
Genomics. 99:76–80. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ørom UA, Derrien T, Beringer M, Gumireddy
K, Gardini A, Bussotti G, Lai F, Zytnicki M, Notredame C, Huang Q,
et al: Long noncoding RNAs with enhancer-like function in human
cells. Cell. 143:46–58. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Lin M, Pedrosa E, Shah A, Hrabovsky A,
Maqbool S, Zheng D and Lachman HM: RNA-Seq of human neurons derived
from iPS cells reveals candidate long non-coding RNAs involved in
neurogenesis and neuropsychiatric disorders. PLoS One.
6:e233562011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Alwine JC, Kemp DJ and Stark GR: Method
for detection of specific RNAs in agarose gels by transfer to
diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc
Natl Acad Sci USA. 74:5350–5354. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Heid CA, Stevens J, Livak KJ and Williams
PM: Real time quantitative PCR. Genome Res. 6:986–994. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Chakraborty D, Kappei D, Theis M, Nitzsche
A, Ding L, Paszkowski-Rogacz M, Surendranath V, Berger N, Schulz H,
Saar K, et al: Combined RNAi and localization for functionally
dissecting long noncoding RNAs. Nat Methods. 9:360–362. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Alberts B, Johnson A and Lewis J:
Fractionation of Cells. Molecular Biology of the Cell. 4th. New
York: Garland Science; 2002
|
|
58
|
Koshkin AA, Singh SK, Nielsen P, Rajwanshi
VK, Kumar R, Meldgaard M, Olsen CE and Wengel J: LNA (Locked
Nucleic Acids): Synthesis of the adenine, cytosine, guanine,
5-methylcytosine, thymine and uracil bicyclonucleoside monomers,
oligomerisation, and unprecedented nucleic acid recognition.
Tetrahedron. 54:3607–3630. 1998. View Article : Google Scholar
|
|
59
|
Zhang F, Wen Y and Guo X: CRISPR/Cas9 for
genome editing: Progress, implications and challenges. Hum Mol
Genet. 23:R40–R46. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Fire A, Xu S, Montgomery MK, Kostas SA,
Driver SE and Mello CC: Potent and specific genetic interference by
double-stranded RNA in Caenorhabditis elegans. Nature. 391:806–811.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Keene JD, Komisarow JM and Friedersdorf
MB: RIP-Chip: The isolation and identification of mRNAs, microRNAs
and protein components of ribonucleoprotein complexes from cell
extracts. Nat Protoc. 1:302–307. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Zhao J, Ohsumi TK, Kung JT, Ogawa Y, Grau
DJ, Sarma K, Song JJ, Kingston RE, Borowsky M and Lee JT:
Genome-wide identification of polycomb-associated RNAs by RIP-seq.
Mol Cell. 40:939–953. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Bellucci M, Agostini F, Masin M and
Tartaglia GG: Predicting protein associations with long noncoding
RNAs. Nat Methods. 8:444–445. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Lagarde J, Uszczynska-Ratajczak B,
Carbonell S, Pérez-Lluch S, Abad A, Davis C, Gingeras TR, Frankish
A, Harrow J, Guigo R and Johnson R: High-throughput annotation of
full-length long noncoding RNAs with capture long-read sequencing.
Nat Genet. 49:1731–1740. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Tanizawa H and Noma K: Unravelling global
genome organization by 3C-seq. Semin Cell Dev Biol. 23:213–221.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Splinter E, de Wit E, van de Werken HJ,
Klous P and de Laat W: Determining long-range chromatin
interactions for selected genomic sites using 4C-seq technology:
From fixation to computation. Methods. 58:221–230. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Dostie J and Dekker J: Mapping networks of
physical interactions between genomic elements using 5C technology.
Nat Protoc. 2:988–1002. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Belton JM, McCord RP, Gibcus JH, Naumova
N, Zhan Y and Dekker J: Hi-C: A comprehensive technique to capture
the conformation of genomes. Methods. 58:268–276. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Severs NJ: Freeze-fracture electron
microscopy. Nat Protoc. 2:547–576. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Olivarius S, Plessy C and Carninci P:
High-throughput verification of transcriptional starting sites by
Deep-RACE. BioTechniques. 46:130–132. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Cossu AM, Mosca L, Zappavigna S, Misso G,
Bocchetti M, De Micco F, Quagliuolo L, Porcelli M, Caraglia M and
Boccellino M: Long non-coding RNAs as important biomarkers in
laryngeal cancer and other head and neck tumours. Int J Mol Sci.
20:E34442019. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Nappi L and Nichols C: MicroRNAs as
biomarkers for germ cell tumors. Urol Clin North Am. 46:449–457.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Jiang YF, Zhang HY, Ke J, Shen H, Ou HB
and Liu Y: Overexpression of LncRNA GHET1 predicts an unfavourable
survival and clinical parameters of patients in various cancers. J
Cell Mol Med. 23:4891–4899. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Moody L, Dvoretskiy S, An R, Mantha S and
Pan YX: The efficacy of miR-20a as a diagnostic and prognostic
biomarker for colorectal cancer: A systematic review and
meta-analysis. Cancers (Basel). 11:E11112019. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Permuth JB, Chen DT, Yoder SJ, Li J, Smith
AT, Choi JW, Kim J, Balagurunathan Y, Jiang K, Coppola D, et al:
Linc-ing Circulating Long Non-coding RNAs to the Diagnosis and
Malignant Prediction of Intraductal Papillary Mucinous Neoplasms of
the Pancreas. Sci Rep. 7:104842017. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Li A, Yu J, Kim H, Wolfgang CL, Canto MI,
Hruban RH and Goggins M: Serum miR-1290 as a marker of pancreatic
cancer-response. Clin Cancer Res. 19:5252–5253. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Yang Q, Tang Y, Tang C, Cong H, Wang X,
Shen X and Ju S: Diminished LINC00173 expression induced miR-182-5p
accumulation promotes cell proliferation, migration and apoptosis
inhibition via AGER/NF-κB pathway in non-small-cell lung cancer. Am
J Transl Res. 11:4248–4262. 2019.PubMed/NCBI
|
|
78
|
Li XY, Zhou LY, Luo H, Zhu Q, Zuo L, Liu
GY, Feng C, Zhao JY, Zhang YY and Li X: The long noncoding RNA
MIR210HG promotes tumor metastasis by acting as a ceRNA of
miR-1226-3p to regulate mucin-1c expression in invasive breast
cancer. Aging (Albany NY). 11:5646–5665. 2019.PubMed/NCBI
|
|
79
|
Zhang Y, Zhu Z, Huang S, Zhao Q, Huang C,
Tang Y, Sun C, Zhang Z, Wang L, Chen H, et al: lncRNA XIST
regulates proliferation and migration of hepatocellular carcinoma
cells by acting as miR-497-5p molecular sponge and targeting PDCD4.
Cancer Cell Int. 19:1982019. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Hu L, Ye H, Huang G, Luo F, Liu Y, Liu Y,
Yang X, Shen J, Liu Q and Zhang J: Long noncoding RNA GAS5
suppresses the migration and invasion of hepatocellular carcinoma
cells via miR-21. Tumour Biol. 37:2691–2702. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Wu H, Zheng J, Deng J, Zhang L, Li N, Li
W, Li F, Lu J and Zhou Y: LincRNAuc002yug.2 involves in alternative
splicing of RUNX1 and serves as a predictor for esophageal cancer
and prognosis. Oncogene. 34:4723–4734. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Chen D, Zhang Z, Mao C, Zhou Y, Yu L, Yin
Y, Wu S, Mou X and Zhu Y: ANRIL inhibits p15(INK4b) through the
TGFβ1 signaling pathway in human esophageal squamous cell
carcinoma. Cell Immunol. 289:91–96. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Kang M, Sang Y, Gu H, Zheng L, Wang L, Liu
C, Shi Y, Shao A, Ding G, Chen G, et al: Long noncoding RNAs POLR2E
rs3787016 C/T and HULC rs7763881 A/C polymorphisms are associated
with decreased risk of esophageal cancer. Tumour Biol.
36:6401–6408. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Zhang E, Han L, Yin D, He X, Hong L, Si X,
Qiu M, Xu T, De W, Xu L, et al: H3K27 acetylation activated-long
non-coding RNA CCAT1 affects cell proliferation and migration by
regulating SPRY4 and HOXB13 expression in esophageal squamous cell
carcinoma. Nucleic Acids Res. 45:3086–3101. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Mazzu YZ, Yoshikawa Y, Nandakumar S,
Chakraborty G, Armenia J, Jehane LE, Lee GM and Kantoff PW:
Methylation-associated miR-193b silencing activates master drivers
of aggressive prostate cancer. Mol Oncol. 13:1944–1958. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Chen G, Feng Y, Li X, Jiang Z, Bei B,
Zhang L, Han Y, Li Y and Li N: Post-transcriptional gene regulation
in colitis associated cancer. Front Genet. 10:5852019. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Qadir MI, Bukhat S, Rasul S, Manzoor H and
Manzoor M: RNA therapeutics: Identification of novel targets
leading to drug discovery. J Cell Biochem. 2019:(Epub ahead of
print).
|
|
88
|
Meng X, Li X, Zhang P, Wang J, Zhou Y and
Chen M: Circular RNA: An emerging key player in RNA world. Brief
Bioinform. 18:547–557. 2017.PubMed/NCBI
|
|
89
|
Qu S, Yang X, Li X, Wang J, Gao Y, Shang
R, Sun W, Dou K and Li H: Circular RNA: A new star of noncoding
RNAs. Cancer Lett. 365:141–148. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Sato K and Siomi MC: Piwi-interacting
RNAs: Biological functions and biogenesis. Essays Biochem.
54:39–52. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Busch J, Ralla B, Jung M, Wotschofsky Z,
Trujillo-Arribas E, Schwabe P, Kilic E, Fendler A and Jung K:
Piwi-interacting RNAs as novel prognostic markers in clear cell
renal cell carcinomas. J Exp Clin Cancer Res. 34:612015. View Article : Google Scholar : PubMed/NCBI
|