Introduction
Transitional cell carcinoma (TCC) of the bladder is
the most common malignancy in the urinary tract. American Cancer
Society estimates about 80,470 new cases and about 17,670 deaths
from bladder cancer in 2019 in United States, with an almost three
times higher incidence in male patients (1). Bladder cancer incidence peaks between
ages 50 and 70 years and the leading risk factors for bladder
cancer development are tobacco smoking and occupational exposure to
various chemical substances (mainly benzene and aryl amines)
(2,3).
When diagnosed for the first time, usually by
transurethral bladder resection, approximately 75% of tumors are
identified as non-muscle invasive bladder cancer (NMIBC) and are
confined to the bladder mucosa and submucosa (4). The remaining are muscle invasive
bladder cancers which have a much worse prognosis and require more
aggressive treatment with very little chance of bladder
preservation. But NMIBC is also a serious disease, well known for
its recurrence and progression rates, which are not easy to
predict. Furthermore, it is estimated that up to 20% of NMIBC will
progress to muscle invasive disease (5,6). The
most important risk factors for recurrence and progression are
number of tumors (single vs. two or more tumors), tumor grade (low
vs. high), tumor size (< vs. ≥3 cm), presence of CIS and prior
recurrence rate (≤ vs. >1 recurrence/year) (4). It has also been shown that the response
to intravesical treatment with bacillus Calmette-Guerin (BCG) is an
important prognostic factor for NMIBC (7).
Bone morphogenetic proteins (BMPs), named so because
they were first isolated from bone, are members of the transforming
growth factor beta family (TGF-β). More than 20 members of BMP have
been isolated to date. BMPs have a critical function during normal
mammalian development and cellular differentiation (8–10).
Recent studies have also shown that they may play an important role
in the regulation of malignant cells, including prostate (11) and bladder cancer (12,13). We
have investigated BMP-6 and −7 in human clear cell renal carcinoma
and we have found a significantly higher BMP-6 mRNA expression in
malignant than in healthy tissue. Nevertheless, BMP-6 mRNA and
protein expression have not shown a significant correlation with
disease presentation, disease progression and patients'
characteristics (14). BMP-7
expression was down-regulated in our clear cell renal carcinoma
samples (15). Our research has been
limited by a relatively small number of samples and a relatively
short follow-up period.
BMPs' expression and a possible role in urological
cancers are still not clear. Presumably, it is different for
distinct members of BMPs and diverse urological cancers as well as
for their histology, grade, stage and tissue heterogeneity. Thus,
in order to proceed with our research of BMPs in urological cancer,
in this study we aimed to examine their (BMP-2, −4, −6, and −7
proteins) expression in NMIBC and their possible role in the
progression and recurrence of the disease.
Huge efforts have been invested in finding novel
pathways associated with the evolution of different malignancies,
which may lead to an improvement in patients' prognosis. It is
necessary to identify molecular markers that may predict the
outcome or potentially serve as therapeutic targets for NMIBC.
Preliminary data have shown that BMPs are important molecular
markers for urothelial carcinomas (16–19).
Materials and methods
This study is based on tissue samples from 71
patients with NMIBC treated with transurethral resection (TUR) at
our institution from 2007–2010. Written informed consent was
obtained in accordance with the local Ethics Code. The study was
conducted in accordance with the Helsinki Declaration, and was
approved by the Ethics Committee of the University Hospital Center
Zagreb. Patient data, as well as data on previous surgery for
bladder cancer, including the time to recurrence, were obtained.
Number of tumors in the bladder, size of tumors in cm, and
pathohistological findings were collected. We also stained 10
samples of macroscopically and histologically healthy bladder
tissue obtained during brain death, heart-beating donor organ
explantation. The pathological stage was assigned according to the
American Joint Committee on Cancer criteria using the 2004TNM
staging system.
Immunohistochemistry
Formalin-fixed, paraffin-embedded tissues (3–4 µm)
were deparaffinized in xylene and then rehydrated through graded
alcohol. Endogenous peroxidase activity was blocked with 0.3%
hydrogen peroxide for 10 min. The sections were blocked with 20%
normal rabbit serum for 30 min. prior to 1 h of incubation with
primary antibody (mouse monoclonal BMP-2, −4, −6 antibodies,
(Abcam, UK) and antihuman BMP-7 monoclonal antibody [(R&D
Systems, USA)]. The slides were washed twice in Tris-buffered
saline and incubated with biotinylated rabbit-antimyocyte antibody
(DAKO, Glostrup, Denmark) diluted 1:500 in blocking serum. The
detection of antibody reaction was carried out with a standard
streptavidin-biotin complex (Dako, Glostrup, Denmark). Negative
control sections were processed in an identical manner after
omitting the primary antibody: They showed no staining.
Immunohistochemical staining for BMPs was evaluated
semiquantitatively and the specimens were scored according to the
distribution of positive cells. Immunostaining results were graded
according to the following protocol: 3+, more than 50% of cells
positive; 2+, 10–50% of cells positive; 1+, up to 10% of cells
positive; and 0, if cells demonstrated no positive staining. The
cellular localization and pattern of immunoreactivity were examined
in a blinded fashion independently by two investigators.
The patients were followed-up every 3 months for 2
years, then every 6 months for 2 years and annually thereafter. The
routine check-up included office visits, serum electrolytes, urine
cytology, cystoscopy and ultrasound. Bladder and upper urinary
tracts, using multislice computed tomography were imaged at the
discretion of the treating physician. The patients who were
followed elsewhere were evaluated by correspondence or a phone
interview. Time to recurrence was calculated as the time interval
from surgical intervention to the first evidence of recurrence or
until the last follow-up if the patient did not have
recurrence.
Statistical analysis
Statistical analysis was performed using the
STATISTICA software package version 6.1, SN AGA304B211928E61,
StatSoft Inc.; USA.
Generalized one-way ANOVA as test in Generalized
Linear/Nonlinear Model for Poisson distribution was performed from
pairwise/multiple group comparison (Comparison between BMP
categories in numerical parameters). Descriptive statistics and
t-tests for single samples were also performed. Comparison between
BMP categories in qualitative parameters were assessed using
cross-tabulation tables and Pearson chi-square test. All
statistical analyses were 2-tailed, with P<0.05 considered to
indicate a statistically significant difference..
Results
The study included 49 men and 22 women. Mean age at
the time of TUR was 65.5 years (from 39–84). Out of 71 patients, 24
(34%) had previous TUR for bladder cancer. Based on
pathohistological findings, 38 patients (54%) had low-grade and 33
(46%) high-grade bladder cancer. Twenty-eight patients (39%) had Ta
and 43 (61%) T1 stage cancer. The patients were followed-up from 5
to 67 months (mean 33). Thirty-four patients (48%) had at least one
recurrence, mean 1.6 (from 1 to 4) (Table I). Out of 34 patients with
recurrence, 11 (32%) had a change in the grade status (three
progressed to a higher grade and eight showed downgrading to a
lower grade). Regarding the stage, five patients progressed from Ta
to T1 cancer, and six patients demonstrated a downstaging from T1
to Ta cancer. Expression of BMPs was as follows: BMP-2 in normal
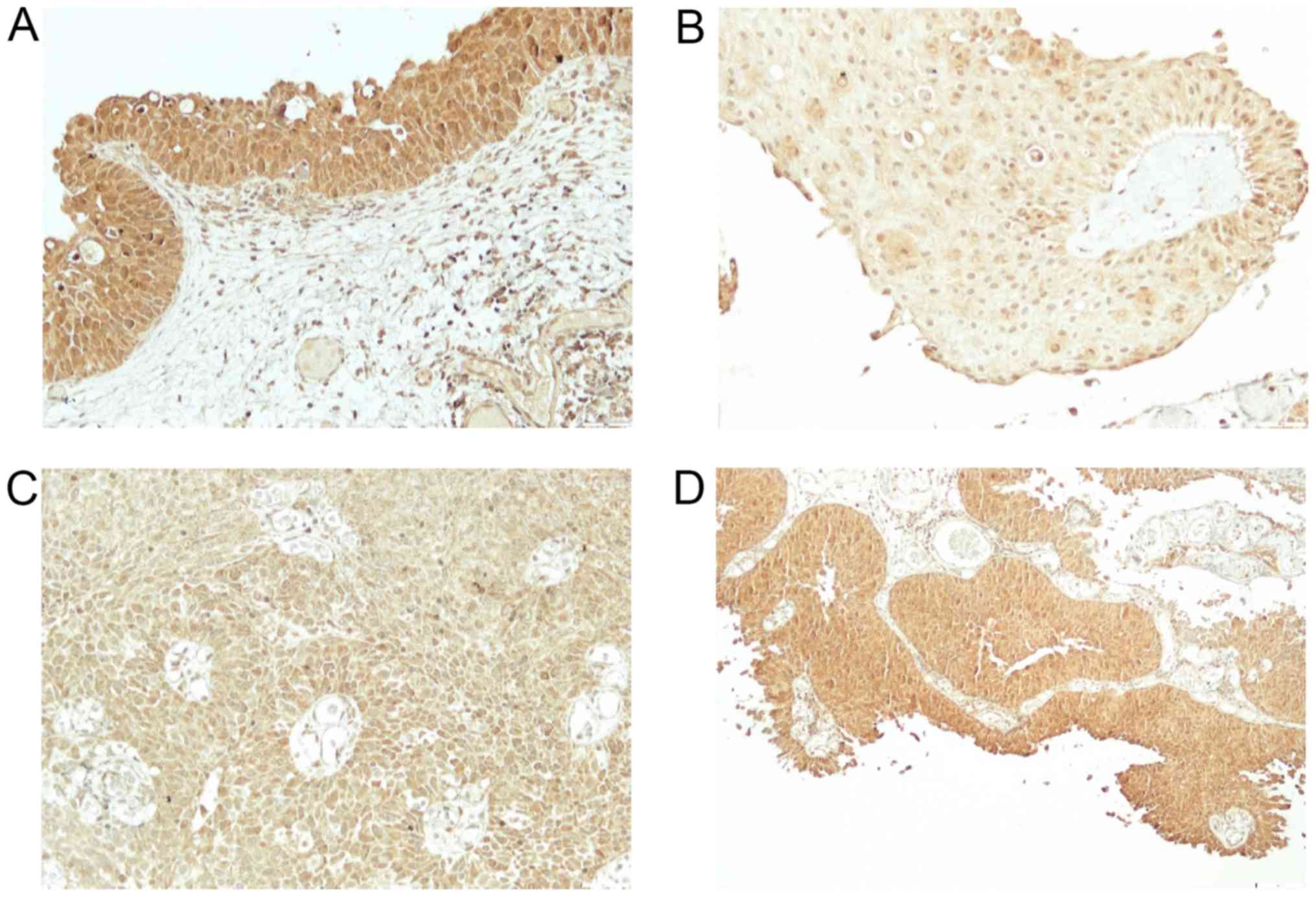
bladder tissue from 2 to 3 (2.9) (Fig.
1A) and in cancer tissue from 1–3 (2.5) (Fig. 1B-D), BMP-4 in normal bladder tissue
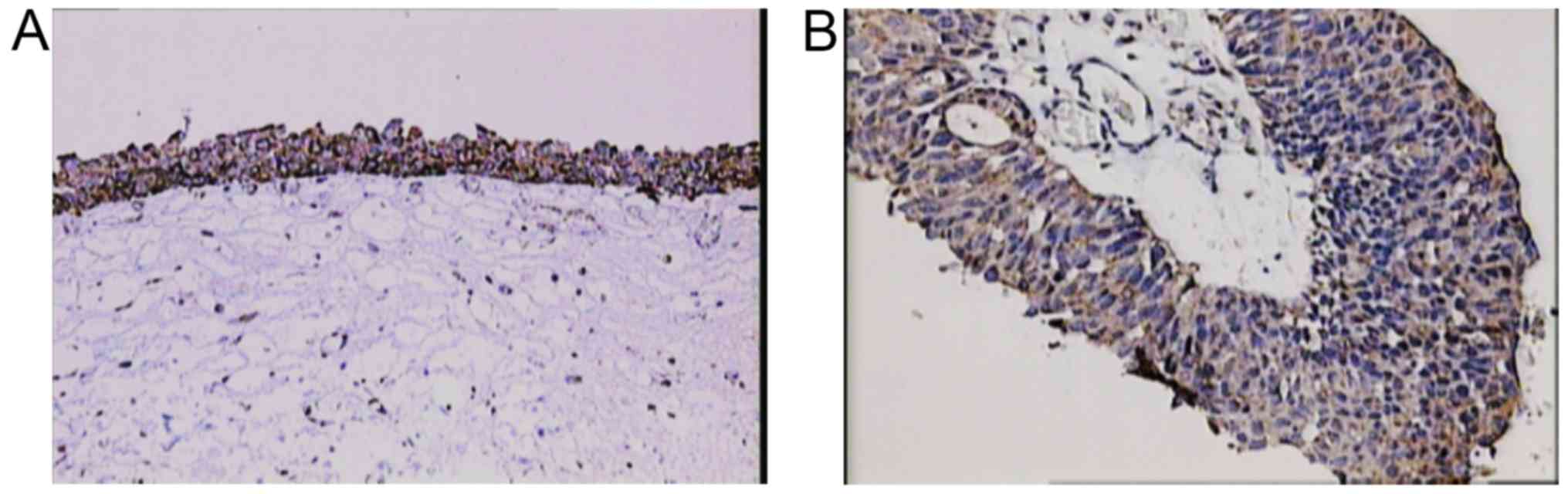
from 1–2 (1.1) (Fig. 2A) and in
cancer tissue from 0–3 (0.9) (Fig.
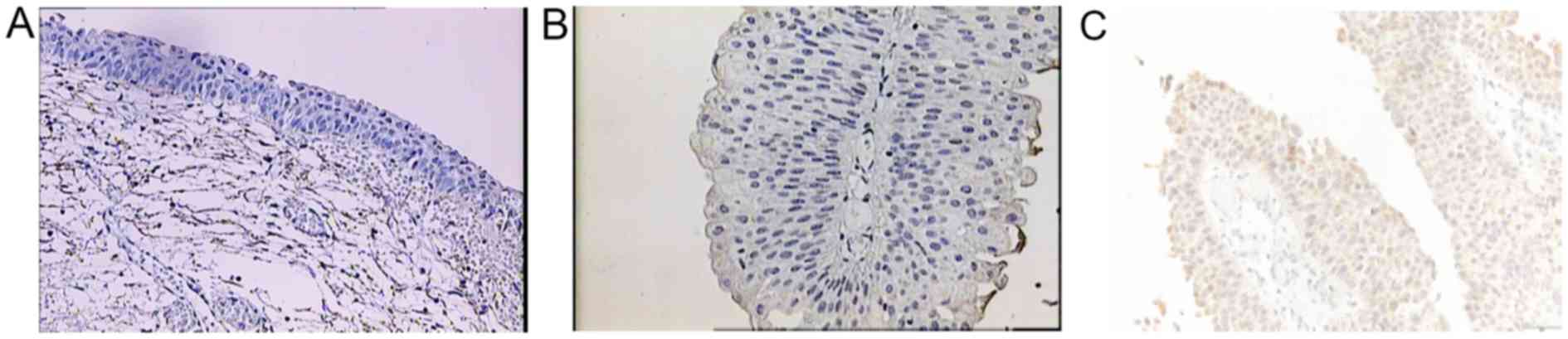
2B), BMP-6 in normal bladder tissue from 0–1 (0.9) (Fig. 3A) and in cancer tissue from 0–2 (0.3)
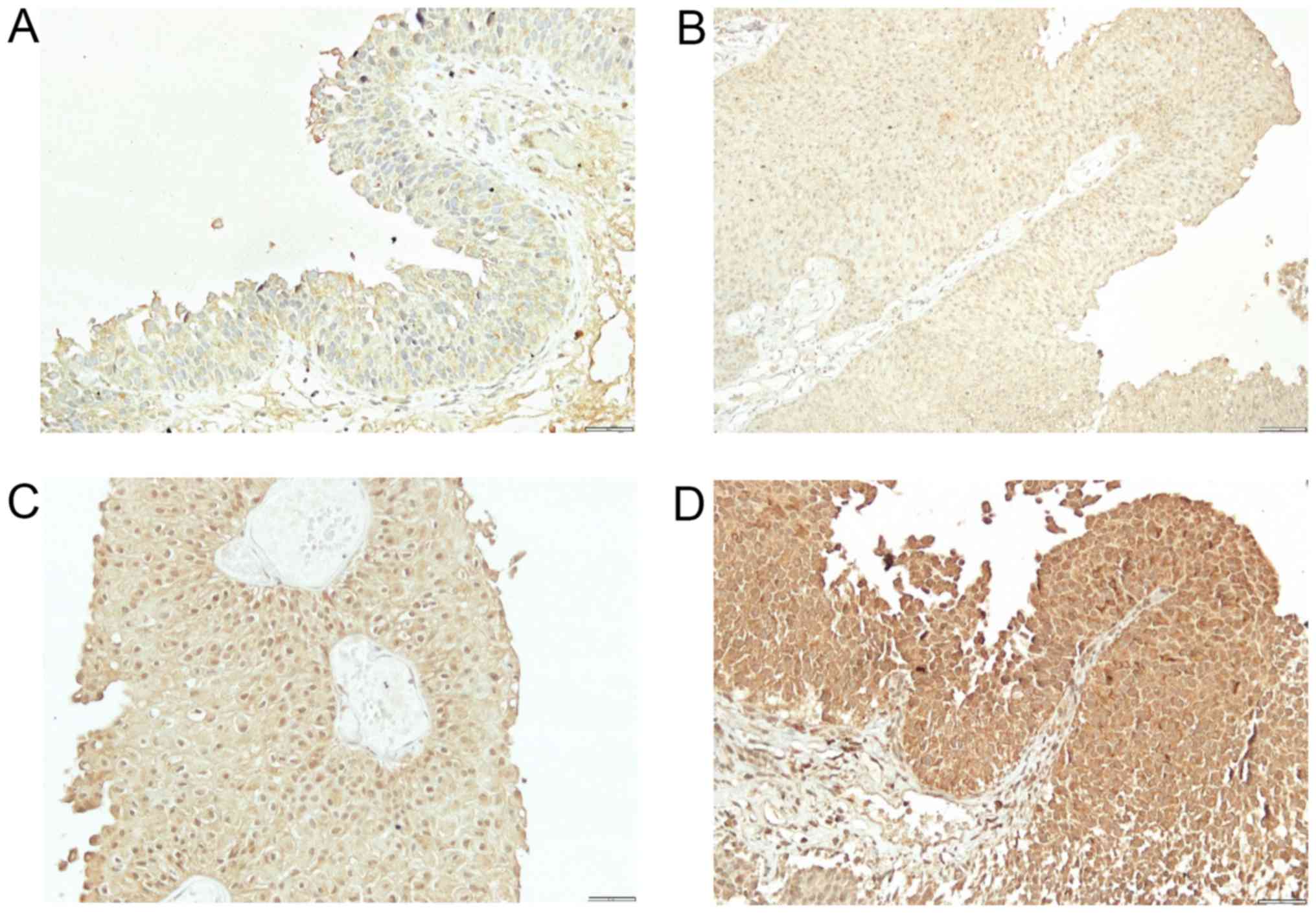
(Fig. 3B), BMP-7 in normal bladder
tissue from 2–3 (2.7) (Fig. 4A) and
in bladder cancer tissue from 0–3 (0.4) (Fig. 4B-D). Comparison of expression levels
in normal tissue and in cancer tissue revealed statistically
significant changes of BMP-2 (P<0.01), BMP-6 (P<0.01) and
BMP-7 (P<0.01) but not BMP-4 (P=0.32) (Table II). In normal tissue and in tumor
samples, the staining of BMP proteins was confined within the
cytoplasm, magnification 20× and for BMP-7 in cancer tissue
40×.
 | Table I.Patient characteristics and
pathohistological results. |
Table I.
Patient characteristics and
pathohistological results.
| Characteristics | Total n (%) |
|---|
| Mean age (range),
years | 65.5 (39–84) |
| Previous TUR |
|
| Yes | 24 (34) |
| No | 47 (66) |
| Tumor grade |
|
| Low | 38 (54) |
| High | 33 (46) |
| Tumor stage |
|
| Ta | 28 (39) |
| T1 | 43 (61) |
| No. of tumors,
average (range) | 2.3 [1-multiple
(≥8)] |
| Size of tumor in cm,
average (range) | 4 (1–10) |
| Mean follow-up,
months (range) | 33 (5–67) |
| Tumor recurrence |
|
| No | 37 (52) |
| Yes | 34 (48) |
| Mean no. of recurring
tumors, n (range) | 1.6 (1–4) |
 | Table II.Levels of BMP-2, −4, −6 and −7
expression in normal and cancer tissues. |
Table II.
Levels of BMP-2, −4, −6 and −7
expression in normal and cancer tissues.
| BMP type | Normal tissues | Cancer tissues | P-value |
|---|
| BMP-2 levels | 2–3 (2.9±0.32) | 1–3 (2.5±0.56) | <0.01 |
| BMP-4 levels | 1–2 (1.1±0.32) | 0–3 (0.9±0.95) | 0.32 |
| BMP-6 levels | 0–1 (0.9±0.32) | 0–2 (0.3±0.54) | <0.01 |
| BMP-7 levels | 2–3 (2.7±0.01) | 0–3 (0.4±0.80) | <0.01 |
The average number of tumors in the bladder was 2.3
[ranging from 1 to multiple tumors (≥8)], (Table I). By comparing the number of tumors
in the bladder and BMP expression we found that only the decrease
in BMP-7 was significant and almost statistically significant
(P=0.07). For other BMP proteins no significant change was found
[BMP-2 (P=0.93), BMP-4 (P=0.99), BMP-6 (P=0.89)]. When we divided
the subjects according to the number of tumors in the bladder into
those with 1 and those with >1 tumors, the results were similar:
BMP-7 (P=0.06), BMP-2 (P=0.55), BMP-4 (P=0.5) and BMP-6 (P=0.86).
The average tumor size in the bladder was 4 cm (from 1 to 10),
(Table I). The tumor size and BMP
expression did not show any correlation [BMP-2 (P=0.49), BMP-4
(P=0.63), BMP-6 (P=0.92), BMP-7 (P=0.95)]. When we divided the
subjects according to the tumor size into two groups: Patients with
tumors up to 3 cm, and patients with tumors ≥3 cm, no statistically
significant change was found either: BMP-2 (P=0.92), BMP-4
(P=0.56), BMP-6 (P=0.17) and BMP-7 (P=0.54). Since 24 patients
(34%) had a previous TUR for bladder cancer, we compared BMP
proteins expression in this group and in the group of patients
without any previous history of bladder cancer. Time from previous
TUR ranged from 4 to 234 months (mean 9). For BMP-2 expression,
patients who had <50% of cells positive compared to those with
≥50% have a significantly shorter time period from previous surgery
for bladder cancer, i.e. 5 vs. 13 months (P<0.01). Loss of BMP-7
positivity was also significantly related with a shorter period of
time from previous surgery, i.e. 6 vs. 22 months (P<0.01),
(Table III). When comparing
patients with previous recurrence ≤1 year, and those with tumor
reappearance >1 year, eleven patients had recurrence in a period
≤1 year while in 13 patients more than a year passed between the
previous tumor and the tumor from which the samples for this study
were obtained. However, the size of the two patient groups was too
small for a valid statistical analysis.
 | Table III.BMP-2 and −7 levels and time to tumor
recurrence in months and number of recurrent tumors. |
Table III.
BMP-2 and −7 levels and time to tumor
recurrence in months and number of recurrent tumors.
|
| Immunostaining
score |
|
|---|
|
|
|
|
|---|
| Expression
group | 0–2 | 3 | P-value |
|---|
| Time from previous
TUR, months |
|
|
|
| BMP-2
positive | 5 | 13 | <0.01 |
| BMP-7
positive | 6 | 22 | <0.01 |
| First recurrence,
months |
|
|
|
| BMP-2
positive | 5 | 8 | <0.01 |
| BMP-7
positive | 6 | 9 | <0.01 |
| No. of recurrent
tumors |
|
|
|
| BMP-2
positive | 0.5 | 1.1 | 0.015 |
| BMP-7
positive | 0.6 | 1.2 | 0.019 |
For patients exhibiting BMP-2 positive tumors with
<50% of positive cells, a shorter time to first recurrence and a
higher number of tumor recurrence in the follow-up period was
observed than in patients with ≥50% of positive cells (5 vs. 8
months, P<0.01; 0.5 vs. 1.1 P=0.015; respectively). In BMP-7
patients without any expression (i.e. patients who lost their BMP-7
high positivity found in normal tissue) there was a significant
difference in time elapsed to primary recurrence in comparison to
patients with BMP-7 positive samples, i.e. 6 vs. 9 months
(P<0.01), as well as in the number of tumor recurrences (0.6 vs.
1.2) in the follow-up period (P=0.019), (Table III). We found no correlation for
BMP-4 and BMP-6.
We did not find any statistically significant
difference between the levels of BMP expression and tumor presence
in lamina (positive vs. negative) (BMP-2 P=0.96, BMP-4 P=0.82,
BMP-6 P=0.755, BMP-7 P=0.084). It is however worth mentioning that
our results for BMP-7 almost reached statistical significance: This
difference may possibly be proven on a larger sample. No
statistically significant results were found for tumor grade (low
vs. high) and BMP expression (BMP-2, P=0.74, BMP-4, P=0.48, BMP-6
P=0.85, BMP-7 P=0.71). The changes in tumor grade and stage were
not significant either, most probably because of the small number
of such patients in comparison to patients who showed no change,
i.e. 11 vs. 60.
Discussion
Numerous studies have linked members of the BMP
family, BMP antagonists, and BMP receptors to cancer (11–24).
However, available data on involvement of BMP family members in
cancerogenesis are conflicting. Different members of the BMP group
induce different effects in various types of cancer. For example a
decrease of BMP-3 and an increase of BMP-4 have been related to
progression and poor prognosis of breast cancer (25,26).
Increased levels of BMP-6 and −7 have been correlated with bone
metastases in prostate cancer (27,28)
while the loss of BMP and BMP receptors has been associated with a
higher tumor grade and pathological stage, increased rate of
recurrence and a lower survival rate (29). Since BMPs are members of TGN-β, which
has in general inhibitory characteristics on malignant cells, the
same effect would be expected in bladder cancer cells. However,
expression of BMP-9 in bladder cancer tissue has recently been
established. Given that the up regulation of BMP-9 promotes
proliferation and migration of bladder cancer cells, it could be
used as a novel marker of tumor aggressiveness (13).
We have shown that there is a significant change: A
loss of BMP proteins in cancer tissue when compared to the normal
tissue. Although this loss is probably an important event in tumor
development, it is not clear what causes it. BMP-2 expression has
been shown in bladder cancer cell line (T24) (30) as well as in NMIBC, MIBC and
metastatic bladder cancer in progressive scale. By contrast, we
have found high levels of expression in normal (for BMP-2 and −7)
tissue and a decrease of expression levels in some NMIBC, but in
more than 50% of our samples the level of BMP-2 expression remained
high. Moreover, the patients with a reduced number of BMP-2
positive cells (<50%) also displayed a significantly shorter
time from previous TUR if they underwent it. They also had a
shorter time to first recurrence as well as a higher number of
recurrent tumors, although we did not detect any significant change
in tumor stage and grade related to BMP levels. The same results
were observed in BMP-7, but not in BMP-4 and −6. Likewise, using
real-time polymerase chain reaction, Kuzaka et al (31) have found BMP-2 and BMP-7
downregulation in infiltrating urothelial carcinoma, while BMP-4
was downregulated in non-invasive tumors. Furthermore, they have
shown that BMP-2 and BMP-7 correlated with prolonged time to
recurrence (31). Regarding BMP-4,
we have also shown low, although not significant, downregulation in
our group of NMIBC. On the other hand, using a human bladder cancer
patient samples, Martínez et al (32) have recently shown, increased
expression of BMP-4 in advanced and undifferentiated tumors.
Although we can only speculate about the reasons for these
observations, different members of the BMP group probably have
diverse roles in normal and in tumor tissue. Furthermore,
dissimilar results from different studies can be related to choice
of methodology and cancer sample variants used for detection of BMP
expression.
Also, changes in BMP expression levels in bladder
cancer can most likely be related to a significant heterogeneity
within the subgroups of TCC (33).
Kim et al (12) have also shown that tissue samples
from TCC frequently loss expression of BMP receptor and that
overexpression of BMP receptor in BMP resistant cell line leads to
a restoration of BMP signaling and a decreased rate of tumor
growth. These data as well as high levels of expression (for BMP-2
and −7) found in the normal healthy bladder tissue suggest that BMP
may play an important protective role in the urinary bladder.
Furthermore, a significant loss of BMP protein in cancer tissue can
be related to an increased number of and a shorter time period for
tumor recurrence, as we have shown in our study. Our results have
to be verified on larger number of samples and with additional
molecular medicine methods, such as polymerase chain reaction for
detecting BMP messenger ribonucleic acid and Western blot for
protein detection in tissue samples. However, we have shown that
there is a correlation between the loss of BMP-2 and −7 and tumor
recurrence. This finding is important for the treatment and
follow-up of patients with NMIBC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets generated and/or analyzed during the
present study are available from the corresponding author on
reasonable request.
Authors' contributions
TH conceived and designed the project and protocol,
collected the tissue samples, analysed the data and wrote the
manuscript. ZK, PK and NBJ conceived and designed the project and
protocol, and edited the manuscript. AES conceived and designed the
project and protocol. MB analyzed the data and edited the
manuscript. MC and DT analyzed the tissue samples and data.
Ethics approval and consent to
participate
The present study was revised and approved by the
Ethics Committee of the University Hospital Center Zagreb (Zagreb,
Croatia). Written informed consent for the use of their tissue was
obtained from all participants.
Patient consent for publication
Written informed consent was obtained from all
individual participants included in the study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
American Cancer Society: Key statistics
for bladder cancer. https://www.cancer.org/cancer/bladder-cancer/about/key-statistics.htmlMarch
1–2019
|
|
2
|
Freedman ND, Silverman DT, Hollenbeck AR,
Schatzkin A and Abnet CC: Association between smoking and risk of
bladder cancer among men and women. JAMA. 306:737–745. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pesch B, Taeger D, Johnen G, Gawrych K,
Bonberg N, Schwentner C, Wellhäusser H, Kluckert M, Leng G,
Nasterlack M, et al: Screening for bladder cancer with urinary
tumor markers in chemical workers with exposure to aromatic amines.
Int Arch Occup Environ Health. 87:715–724. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
M Babjuk M, Burger M, Zigeuner R, Shariat
S, Van Rhijn B, Compérat E, Sylvester R, Kaasinen E, Böhle A,
Palou J and Rouprêt M: Guidelines on Non-muscle-invasive Bladder
Cancer (TaT1 and CIS). http://www.uroweb.org/gls/pdf/05_TaT1_Bladder_Cancer_LR.pdf2013.PubMed/NCBI
|
|
5
|
Sylvester RJ, van der Meijden AP,
Oosterlinck W, Witjes JA, Bouffioux C, Denis L, Newling DW and
Kurth K: Predicting recurrence and progression in individual
patients with stage Ta T1 bladder cancer using EORTC risk tables: A
combined analysis of 2596 patients from seven EORTC trials. Eur
Urol. 49:466–475. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fernandez-Gomez J, Madero R, Solsona E,
Unda M, Martinez-Piñeiro L, Gonzalez M, Portillo J, Ojea A, Pertusa
C, Rodriguez-Molina J, et al: Predicting nonmuscle invasive bladder
cancer recurrence and progression in patients treated with bacillus
Calmette-Guerin: The CUETO scoring model. J Urol. 182:2195–2203.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Isharwal S and Konety B: Non-muscle
invasive bladder cancer risk stratification. Indian J Urol.
31:289–296. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wozney JM, Rosen V, Celeste AJ, Mitsock
LM, Whitters MJ, Kriz RW, Hewick RM and Wang EA: Novel regulators
of bone formation: Molecular clones and activites. Science.
242:1528–1534. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sampath TK, Maliakal JC, Hauschka PV,
Jones WK, Sasak H, Tucker RF, White KH, Coughlin JE, Tucker MM,
Pang RH, et al: Recombinant human osteogenic protein-1 (hOP-1)
induces new bone formation in vivo with a specific activity
comparable with natural bovine osteogenic protein and stimulates
osteoblast proliferation and differentiation in vitro. J Biol Chem.
267:20352–20362. 1992.PubMed/NCBI
|
|
10
|
Hogan BL: Bone morphogenic proteins:
Multifunctional regulators of vertebrate development. Genes Dev.
10:1580–1594. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim IY, Lee DH, Ahn HJ, Tokunaga H, Song
W, Devereaux LM, Jin D, Sampath TK and Morton RA: Expression of
bone morphogenetic protein receptors type-IA, -IB and II correlates
with tumor grade in human prostate cancer tissues. Cancer Res.
60:2840–2844. 2000.PubMed/NCBI
|
|
12
|
Kim IY, Lee DH, Lee DK, Kim WJ, Kim MM,
Morton RA, Lerner SP and Kim SJ: Restoration of bone morphogenic
protein receptor type II Expression leads to a decreased rate of
tumor growth in bladder transitional cell carcinoma cell lines
TSU-Pr1. Cancer Res. 64:7355–7360. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gou L, Liu M, Xia J, Wan Q, Jiang Y, Sun
S, Tang M, Zhou L, He T and Zhang Y: BMP9 promotes the
proliferation and migration of bladder cancer cells through
up-regulating lncRNA UCA1. Int J Mol Sci. 19(pii): E11162018.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Basic-Jukic N, Radic-Antolic M, Hudolin T,
Coric M, Zadro R, Pasini J, Kastelan Z and Kes P:
Immunolocalization and mRNA expression of bone morphogenetic
protein-6 in human clear cell renal carcinoma. Kidney Blood Press
Res. 32:445–450. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Basic-Jukic N, Hudolin T, Radic-Antolic M,
Coric M, Zadro R, Kastelan Z, Pasini J, Bandic-Pavlovic D and Kes
P: Bone morphogenetic protein-7 expression is down-regulated in
human clear cell renal carcinoma. J Nephrol. 24:91–97. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hung TT, Wang H, Kingsley EA, Risbridger
GP and Russell PJ: Molecular profiling of bladder cancer:
Involvement of the TGF-beta pathway in bladder cancer progression.
Cancer Lett. 265:27–38. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Komai Y, Morimoto S, Saito K, Urushibara
M, Sakai K and Ikeda S: Possible involvement of bone morphogenetic
protein 2 in heterotopic ossification in metastatic lesion from
urothelial carcinoma of bladder. Int J Urol. 13:1126–1128. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zaravinos A, Lambrou GI, Boulalas I,
Delakas D and Spandidos DA: Identification of common differentially
expressed genes in urinary bladder cancer. PLoS One. 6:e181352011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang ZJ, Liu FX, Yang YS, Yang X and Zhu
GX: Expression of bone-morphogenetic protein 2 and tumor necrosis
factor α correlates with bone metastases in bladder urothelial
carcinoma. Ann Diagn Pathol. 17:51–53. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Alarmo EL, Rauta J, Kauraniemi P, Karhu R,
Kuukasjärvi T and Kallioniemi A: Bone morphogenetic protein 7 is
widely overexpressed in primary breast cancer. Genes Chromosomes
Cancer. 45:411–419. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Haudenschild DR, Palmer SM, Moseley TA,
You Z and Reddi AH: Bone morphogenetic protein (BMP)-6 signaling
and BMP antagonist noggin in prostate cancer. Cancer Res.
64:8276–8284. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Franzen A and Heldin NE: BMP-7-induced
cell cycle arrest of anaplastic thyroid carcinoma cells via
p21(CIP1) and p27(KIP1). Biochem Biophys Res Commun. 285:773–781.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yanagita M: BMP antagonists: Their roles
in development and involvement in patophysiology. Cytokin Growth
Factor Rev. 16:309–317. 2005. View Article : Google Scholar
|
|
24
|
Deng H, Makizumi R, Ravikumar TS, Dong H,
Yang W and Yang WL: Bone morphogenetic protein-4 is overexpressed
in colonic adenocarcinomas and promotes migration and invasion of
HCT116 cells. Exp Cell Res. 313:1033–1044. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Davies SR, Watkins G, Douglas-Jones A,
Mansel RE and Jiang WG: Bone morphogenetic proteins 1 to 7 in human
breast cancer, expression pattern and clinical/prognostic
relevance. J Exp Ther Oncol. 7:327–338. 2008.PubMed/NCBI
|
|
26
|
Alarmo EL, Kuukasjaävi T, Karhu R and
Kallioniemi A: A comprehensive expression survey of bone
morphogenic proteins in breast cancer highlights the importance of
BMP4 and BMP7. Breast Cancer Res Treat. 103:239–246. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Autzen P, Robson CN, Bjartell A, Malcolm
AJ, Johnson MI, Neal DE and Hamdy FC: Bone morphogenic protein 6 in
skeletal metastases from prostate cancer and other common human
malignancies. Br J Cancer. 78:1219–1223. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Masuda H, Fukabori Y, Nakano K, Takezawa
Y, Csuzuki T and Yamanaka H: Increased expression of bone
morphogenic protein-7 in bone metastatic prostate cancer. Prostate.
54:268–274. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim IY, Lee DH, Ahn HJ, Ahn HJ, Kim MM,
Kim SJ and Morton RA: Loss of expression of bone morphogenic
protein receptor type II in human prostate cancer cells. Oncogene.
23:7651–7659. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hatakeyama S, Gao YH, Nemoto-Ohara Y,
Kataoka H and Satoh M: Expression of bone morphogenic proteins of
human neoplastic epithelial cells. Biochem Mol Biol Int.
42:497–505. 1997.PubMed/NCBI
|
|
31
|
Kuzaka B, Janiak M, Włodarski KH,
Radziszewski P and Włodarski PK: Expression of bone morphogenetic
protein-2 and −7 in urinary bladder cancer predicts time to tumor
recurrence. Arch Med Sci. 11:378–384. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Martínez VG, Rubio C, Martínez-Fernández
M, Segovia C, López-Calderón F, Garín MI, Teijeira A,
Munera-Maravilla E, Varas A, Sacedón R, et al: BMP4 induces M2
macrophage polarization and favors tumor progression in bladder
cancer. Clin Cancer Res. 23:7388–7399. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kim IY and Kim SJ: Role of bone
morphogenic proteins in transitional cell carcinoma cells. Cancer
Lett. 241:118–123. 2006. View Article : Google Scholar : PubMed/NCBI
|