Introduction
According to the Global Cancer Statistics 2018,
bladder cancer is the tenth most common form of cancer and the
ninth leading cause of cancer-associated mortality worldwide
(1). At initial diagnosis, ~70% of
patients with bladder cancer exhibit non-muscle invasive bladder
cancer; however, over a 5-year period the risk of recurrence has
been identified to vary between 30 and 80%, and ~15% of cases of
bladder cancer progress to muscle invasion (2). Therefore, early diagnosis and long-term
surveillance of patients with bladder cancer are crucial. The
current diagnostic and postoperative surveillance strategies are
based on the combination of cystoscopy and voided urine cytology
(3). Although these two methods are
regarded as the gold standard for the diagnosis of bladder cancer,
they have certain disadvantages. Cystoscopy, which is an invasive
procedure, results in high costs for the healthcare system and the
discomfort of patients (4).
Additionally, the symptoms and their duration after cystoscopy
induced pain during urination (50%), increased urinary frequency
(37%), visible hematuria (19%) and infection (3%) (5,6).
Furthermore, this procedure has been demonstrated to exhibit low
sensitivity in the detection of carcinoma in situ, and
tumors may be missed due to its operator-dependent effectiveness
(7). Overall sensitivity of urine
cytology has been identified to range between 28 and 100%, with a
median of 44%; however, this procedure exhibits high sensitivity
for detecting high-grade tumors and low sensitivity for low-grade
tumors (range, 4–31%) (8). In
addition, negative cytology does not exclude the presence of a
tumor as cytological results are patient-dependent and may be
hindered by several factors, including low cellular yield, urinary
tract infections and stones (9).
Great efforts have been made to develop new tests
with high diagnostic efficiency and reproducibility and low cost
for the non-invasive detection of bladder cancer. To date, numerous
potential urinary biomarkers have been suggested and the use of
molecular biomarkers for bladder cancer surveillance has
demonstrated potential clinical applicability (4,10).
Examples of these biomarkers include nuclear matrix protein 22,
survivin, matrix metallopeptidase 9, bladder tumor antigen,
cytokeratin, urinary bladder carcinoma antigen and Cyfra 21-1
(11).
Survivin is the smallest inhibitor of apoptosis
protein with a single amino-terminal BIR domain and
carboxy-terminal Coiled Coil domain (12). Survivin is a critical regulator of
mitosis, and an inhibitor of apoptosis, which promotes the
proliferation of tumor cells, induces angiogenesis and thus
increases the invasion capacity of tumors (13). The expression of survivin is
undetectable in terminally differentiated and mature tissues but is
highly expressed in common types of human cancer, including lung,
colon, pancreas, prostate and breast (14) cancer. Therefore, it is considered to
be a new tumor marker, which may be useful in the diagnosis of
human cancer (12). Swana et
al (15) reported that survivin
was expressed in 78% of patients with bladder cancer, as detected
by immunohistochemistry (IHC), but was absent in normal bladder
urothelium. Smith et al (16)
detected the expression of survivin protein and mRNA in urine
samples from patients with bladder cancer by Bio-Dot immunoassay
and reverse transcription-PCR (RT-PCR), respectively, in 2001. In
the following years, certain studies assessed the detection of
survivin protein in urine samples using IHC, ELISA or Bio-Dot
immunoassay as a means of diagnosing bladder cancer. The detection
of urinary survivin expression has been identified by Bio-Dot
immunoassay to be an accurate diagnostic method for bladder cancer
that retains its efficiency regardless of tumor stage and grade
(17). In addition to the survivin
protein, the survivin gene has gradually gained interest as a
marker for the diagnosis and treatment of bladder cancer. An
increasing number of studies have examined the expression of
survivin mRNA in urine by RT-PCR for the diagnosis of bladder
cancer. A meta-analysis by Liang et al (18) concluded that both survivin protein
and mRNA may be used as biomarkers for bladder cancer detection,
and survivin RNA exhibited higher accuracy compared with survivin
protein. In addition, numerous studies have demonstrated the
various accuracy of RT-PCR detection of urinary survivin mRNA
expression in the diagnosis of bladder cancer. Weikert et al
(19) reported a sensitivity of
68.6% and a specificity of 100% was identified in 53 patients with
bladder cancer. Pu et al (20) reported a sensitivity of 90.4% and a
specificity of 96.6% for the diagnosis of bladder cancer. Eissa
et al (21) reported a
sensitivity of 76.1% and a specificity of 95.0% in 86 patients.
The aim of the present meta-analysis was to review
and summarize the results of previous experimental studies
confirming the potential diagnostic value of urinary survivin mRNA
as a marker for bladder cancer, and to compare this test by RT-PCR
with traditional cytology. In addition, the present study aimed to
assess the quality of published studies.
Materials and methods
Search strategy
The present meta-analysis was performed according to
the Preferred Reporting Items for Systematic Reviews and
Meta-Analyses guidelines (22).
Scientific databases, including PubMed, Web of Science, Cochrane
Library and China National Knowledge Infrastructure (CNKI), were
comprehensively searched for publications between January 2001 and
January 2019 to identify studies on the use of urinary survivin
mRNA expression and urine cytology in the diagnosis of bladder
cancer. The published literature search was conducted in English
and restricted to original research studies. Published studies in
the CNKI database were searched using Chinese-language characters,
since this database contains research papers published in Chinese.
The following terms, which are Medical Subject Headings key words,
were searched in the text, title or abstract of relevant studies:
‘Bladder cancer’ or ‘carcinoma of bladder’ or ‘urothelial carcinoma
of the urinary tract’ and ‘survivin’. Similar publications
identified in the reference lists of the retrieved studies were
also obtained.
Selection criteria
The retrieved studies were independently reviewed by
two reviewers, who agreed on which studies were eligible for the
present meta-analysis; discrepancies were discussed and resolved by
consensus. The following inclusion criteria were applied to the
published studies retrieved by the database search: i) Studies
published in English or Chinese; ii) studies that included a 2×2
contingency table; iii) urinary survivin mRNA expression detected
by RT-PCR; iv) urine cytology as a comparison test and v)
cystoscopy and/or histopathology used as the gold standard.
Furthermore, the following exclusion criteria were applied: i) Case
reports, case series and review studies; ii) animal or cell
experiments; iii) use of immunohistochemical staining or western
blot detection of survivin; iv) patients with urinary tract tumors
other than bladder cancer or v) incomplete clinical data.
Data extraction and quality
assessment
The following primary outcome data were extracted
from the studies included in the meta-analysis: i) True positives
(TP); ii) false positives (FP); iii) false negatives (FN); iv) true
negatives (TN); and v) the total number of patients enrolled in
each study. Additional data included: i) The name of the first
author; ii) publication year; iii) country; iv) study design and v)
gold standard. The Quality Assessment of Diagnostic Accuracy
Studies 2 (QUADAS-2) tool (23) was
used to rate the quality of each of the included studies. This
method consisted of four components, the selection of cases, trials
to be assessed, gold standards and the flowcharts and progress of
cases. Each of the assessments comprised seven items and the
corresponding responses contained the terms ‘yes’, ‘no’ or
‘uncertainty’. Positive answers (‘yes’) meant that the risk bias of
a study was considered low, whereas negative (‘no’) and uncertain
(‘uncertainty’) answers meant that the risk of bias was high.
Statistical analysis
Data analysis was performed using the midas
(24–27) and metan [version 1.85; (28)] packages in Stata/MP (version 15.0;
StataCorp, LLC). Revman 5.3 (version 5.3; The Nordic Cochrane
Centre, The Cochrane Collaboration) was used for quality
assessment. The sensitivity (Sen), specificity (Spe), positive
likelihood ratios (PLRs), negative likelihood ratios (NLRs) and
corresponding 95% confidence intervals (CIs) were calculated using
the TP, FP, FN and TN values, which were extracted from each study
prior to data pooling. The summary receiver operating
characteristic curve (SROC) was constructed based on a bivariate
regression approach and the pooled estimate for sensitivity and
specificity was subsequently calculated. The diagnostic odds ratio
(DOR) with 95% CI and Youden's index (γ=Sensitivity +
Specificity-1) were also calculated. In addition, Fagan nomograms
were generated to evaluate the clinical utility of the two
diagnostic methods. Heterogeneity among the reports was assessed by
the χ2 test (Cochran Q test) and the I2
statistic. The DerSimonian Laird method for pooled analyses was
used for I2 values >50%. Statistical heterogeneity
was considered to be low when I2 was 25–49%, moderate
when I2 was 50–74% and high when I2 was
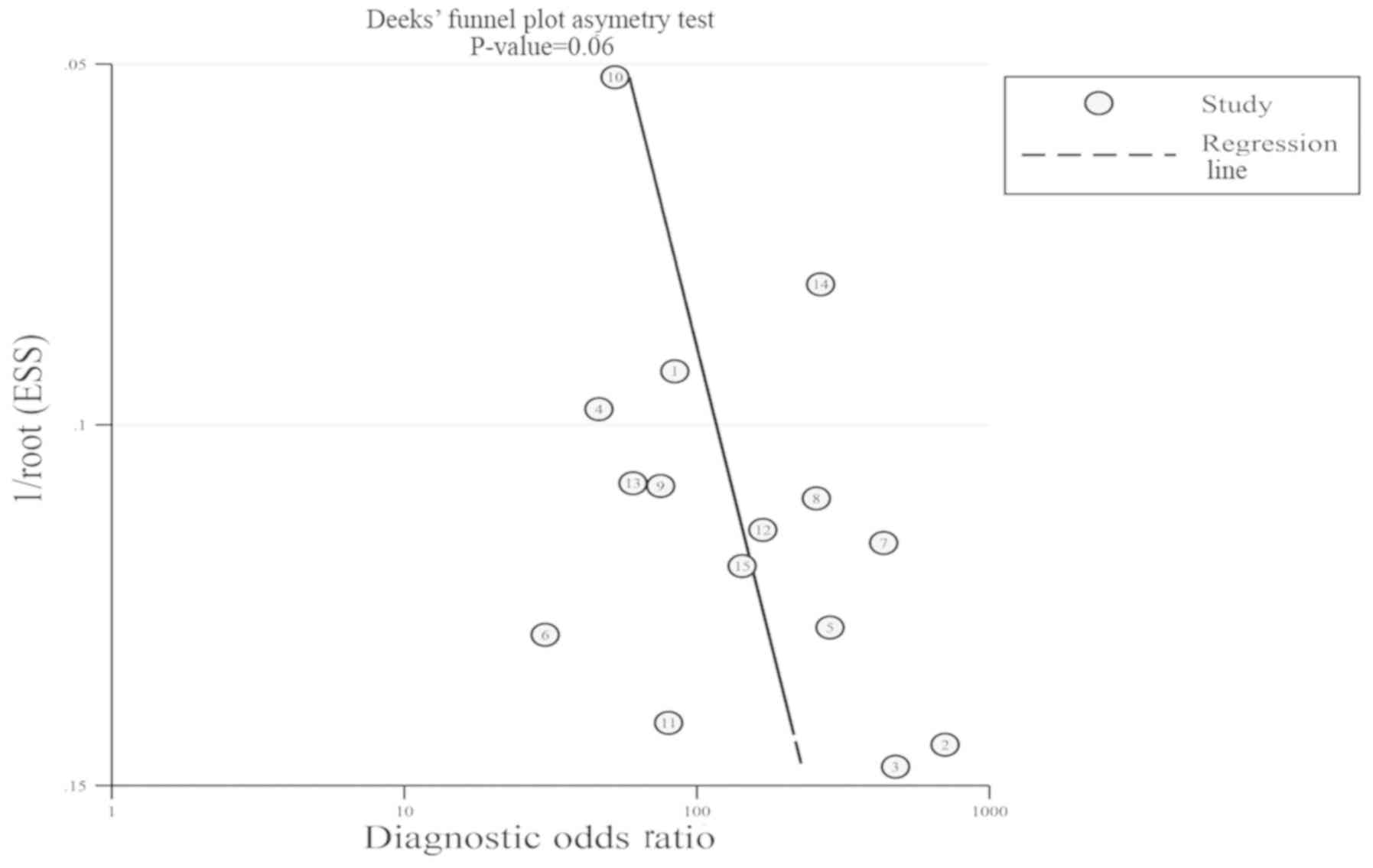
>75 (29). In addition, Deeks'
funnel plot asymmetry test based on parametric linear regression
was used to identify the possibility of publication bias (25). As the primary aim of the present
study was to assess the accuracy of urinary survivin mRNA
expression in the early diagnosis of bladder cancer, Deeks' funnel
plot was generated only for urinary survivin mRNA.
To compare urinary survivin mRNA expression with
urine cytology, data of the pathological grade, stage and pooled
sensitivity were collected. In addition, a paired χ2
test was performed for prime diagnostic indicators, sensitivity and
specificity. A Z test was used to analyze the area under the curve
(AUC) and Youden's index. P<0.05 was considered to indicate a
statistically significant difference.
Results
Search results and selected
studies
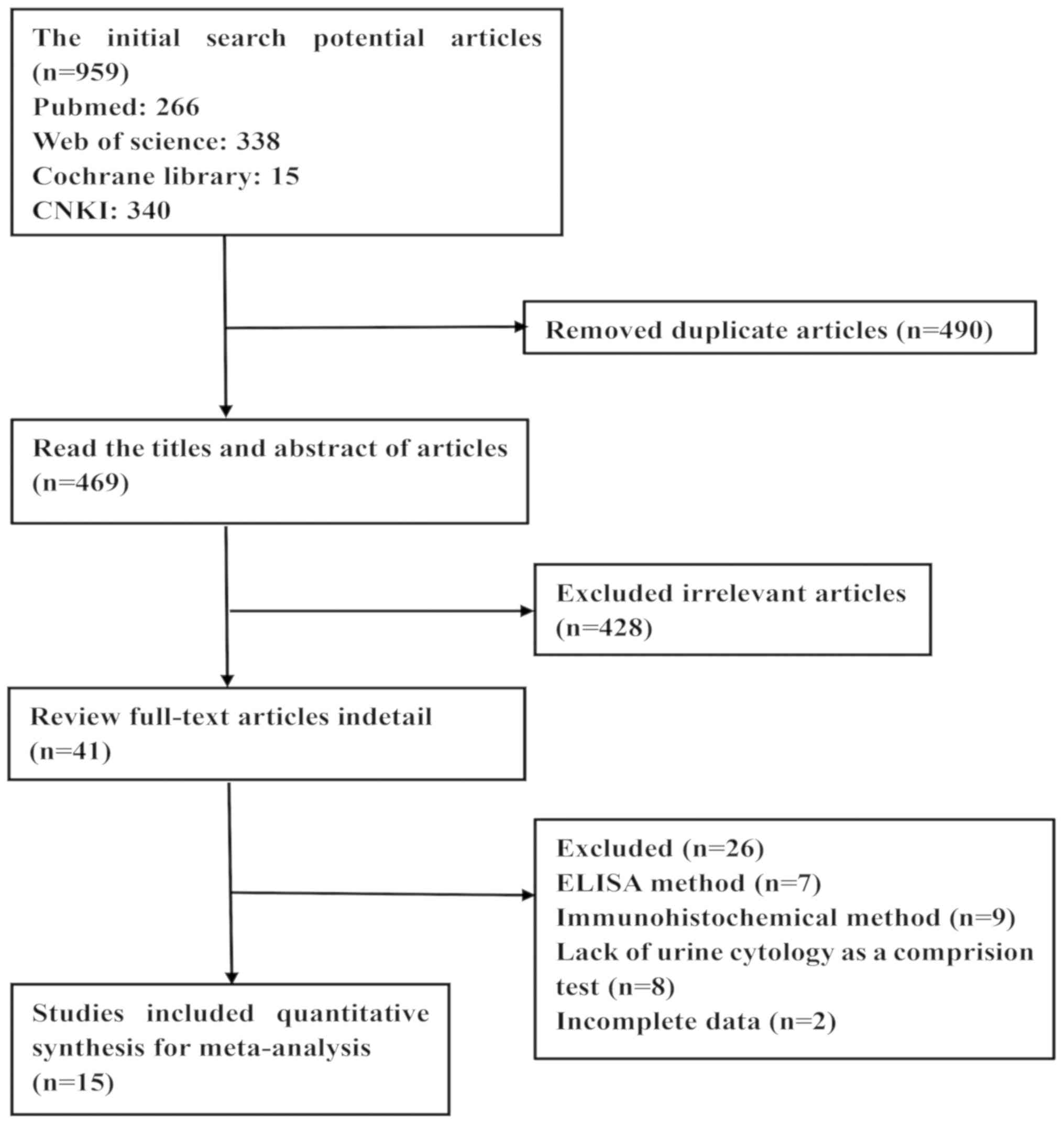
The results of the current meta-analysis selection
process are presented in Fig. 1. The
initial search resulted in 959 studies, 490 of which were
duplicates and were thus excluded. A total of two reviewers read
the titles and abstracts of the remaining 469 studies; 428
irrelevant studies were removed by consensus. A total of 41
potentially eligible studies were selected; following reading the
full text of each study and analyzing the results, 26 studies were
excluded due to a lack of complete data or incomplete descriptions
of the trials. A total of 15 eligible studies were included in the
meta-analysis.
Included study characteristics
The main characteristics of the included studies are
presented in Table I. The studies
were from Germany (19), Kuwait
(30), Portugal (31), Egypt (21,32,33) and
China (20,34–41), and
eight of them were published in English. A total of 1,624 patients
were included in the studies, 951 of which were diagnosed with
bladder cancer. The remaining 673 control participants comprised
healthy participants and patients with benign prostate hyperplasia,
urinary tract infection, urethral stricture or urolithiasis and
benign epithelial neoplasm of the bladder.
 | Table I.Characteristics of the studies
included in the meta-analysis. |
Table I.
Characteristics of the studies
included in the meta-analysis.
|
|
|
| Urinary survivin
mRNA expression | Urine cytology |
|
|---|
|
|
|
|
|
|
|
|---|
| Author, year | Country | Gold standard | TP | FP | FN | TN | TP | FP | FN | TN | (Refs.) |
|---|
| Weikert et
al, 2005 | Germany | Histopathology | 24 | 0 | 11 | 33 | 11 | 1 | 24 | 32 | (19) |
| Pu et al,
2008 | China | Histopathology | 104 | 2 | 11 | 56 | 53 | 0 | 62 | 58 | (20) |
| Pina-Cabral et
al, 2007 | Portugal | Histopathology | 20 | 0 | 10 | 20 | 9 | 0 | 21 | 20 | (31) |
| Al-Maghrebi et
al, 2012 | Kuwait | Cystoscopy and
histopathology | 70 | 1 | 10 | 24 | 32 | 1 | 48 | 24 | (30) |
| Eissa et al,
2013 | Egypt | Cystoscopy and
histopathology | 35 | 2 | 11 | 38 | 23 | 0 | 23 | 40 | (21) |
| Eissa et al,
2010 | Egypt | Histopathology | 126 | 12 | 40 | 200 | 80 | 0 | 86 | 212 | (32) |
| Eissa et al,
2010 | Egypt | Histopathology | 33 | 2 | 9 | 41 | 12 | 0 | 30 | 43 | (33) |
| Jiang et al,
2006 | China | Histopathology | 32 | 2 | 3 | 48 | 18 | 0 | 17 | 50 | (34) |
| Lin et al,
2007 | China | Histopathology | 45 | 1 | 3 | 29 | 14 | 0 | 34 | 30 | (35) |
| Liu et al,
2009 | China | Histopathology | 53 | 4 | 7 | 16 | 15 | 0 | 45 | 20 | (36) |
| Pu et al,
2008 | China | Histopathology | 60 | 1 | 4 | 19 | 26 | 0 | 38 | 20 | (37) |
| Wan et al,
2008 | China | Cystoscopy and
histopathology | 60 | 4 | 12 | 37 | 23 | 0 | 49 | 41 | (38) |
| Wang et al,
2004 | China | Histopathology | 38 | 0 | 2 | 15 | 27 | 0 | 13 | 15 | (39) |
| Wang et al,
2006 | China | Cystoscopy and
histopathology | 47 | 1 | 1 | 15 | 28 | 0 | 20 | 16 | (40) |
| Zhang et al,
2005 | China | Histopathology | 59 | 3 | 11 | 47 | 34 | 0 | 36 | 50 | (41) |
Quality assessment of the included
studies
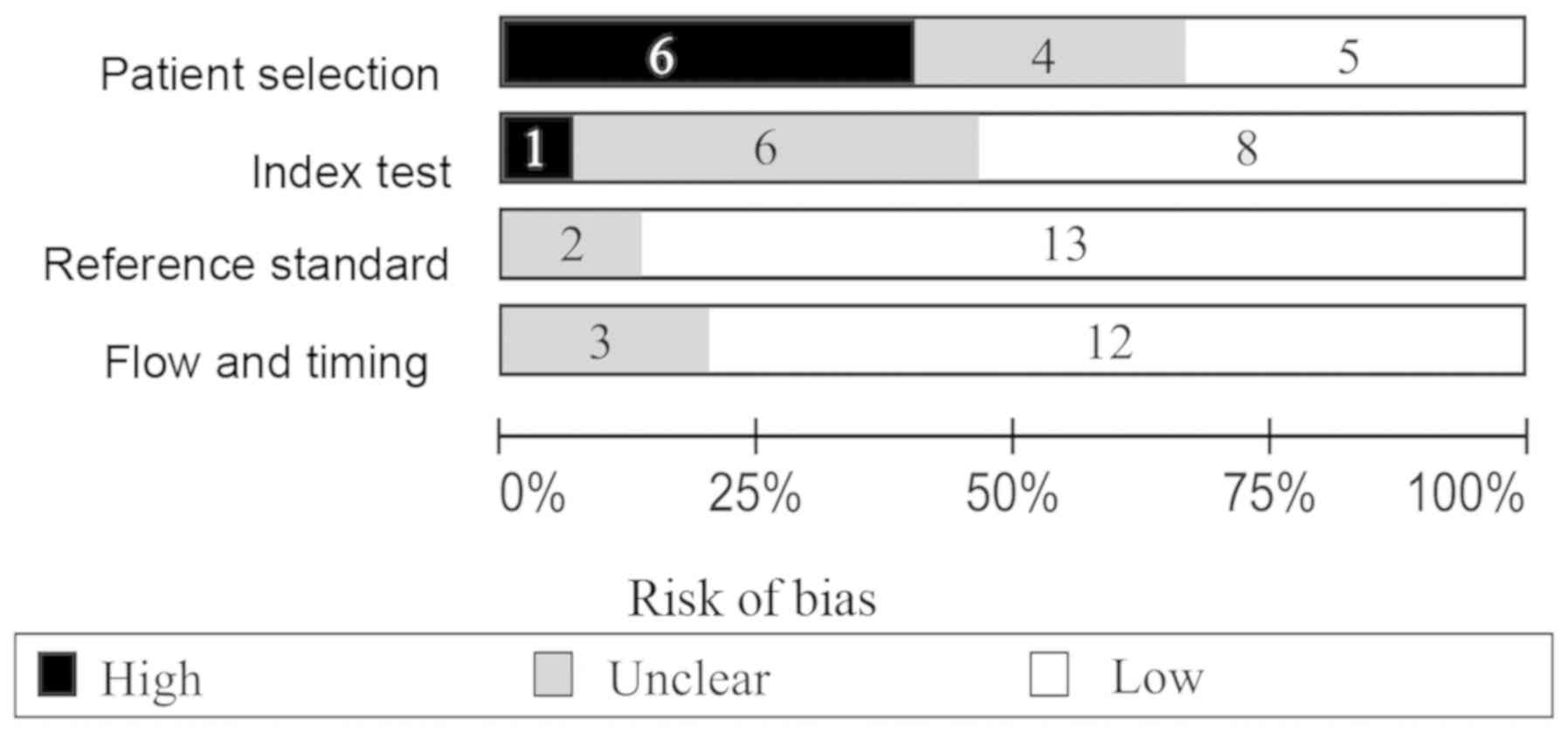
The quality assessment results of the included
studies are presented in Fig. 2.
Regarding patient selection, certain studies were identified to
exhibit a high risk of bias since healthy patients were used as the
control group, whereas others used non-malignant diseases of the
urinary system as controls. The index test in the QUADAS-2 tool was
associated with a high risk of bias, as study thresholds, reagents
and procedure were often different. Regarding the reference
standard, low risk of bias was identified. The flow and timing
characteristic were associated with a low risk of bias.
Pooled data for all included
studies
Table II presents
the pairwise comparisons of prime diagnostic indicators between
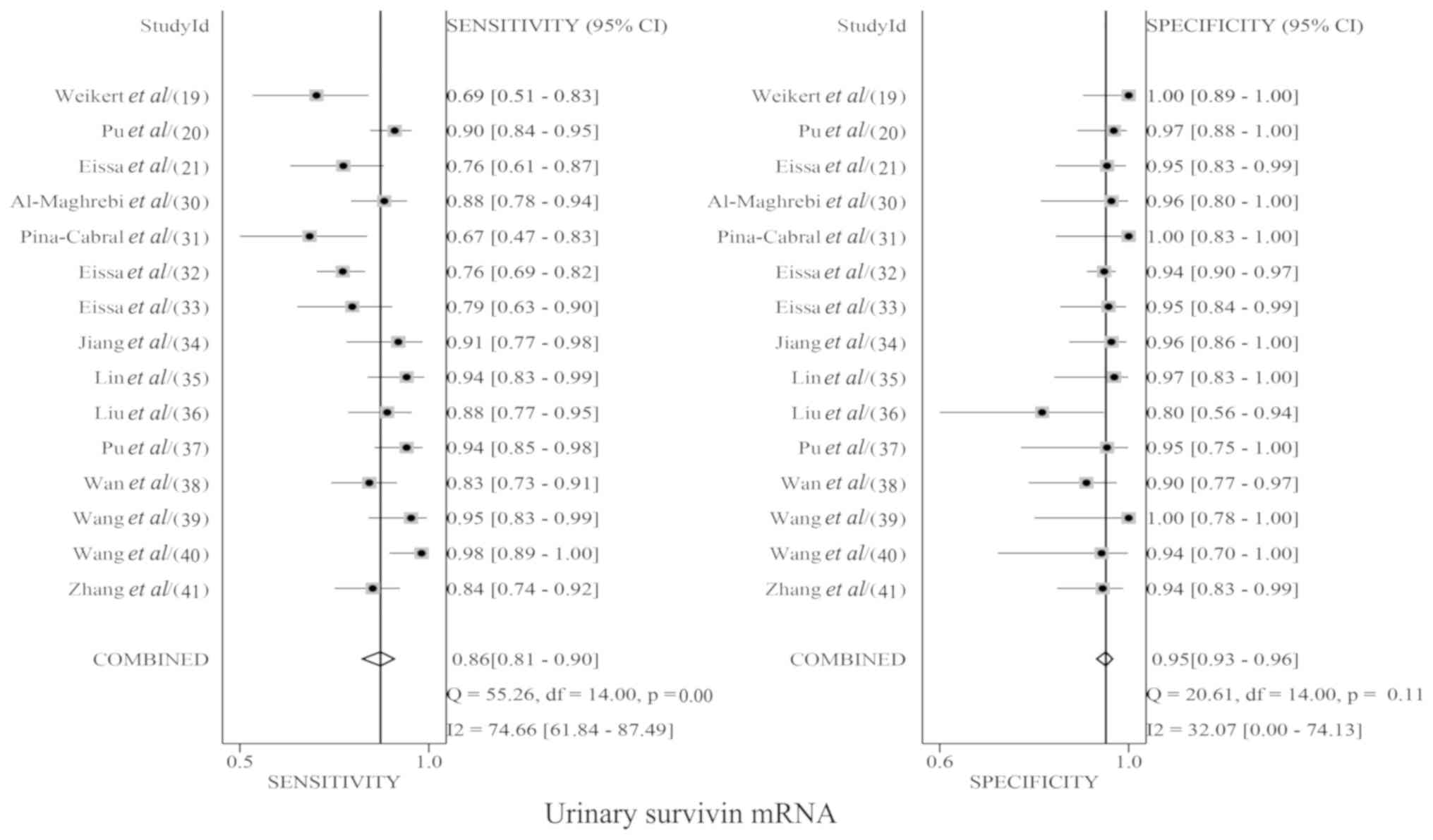
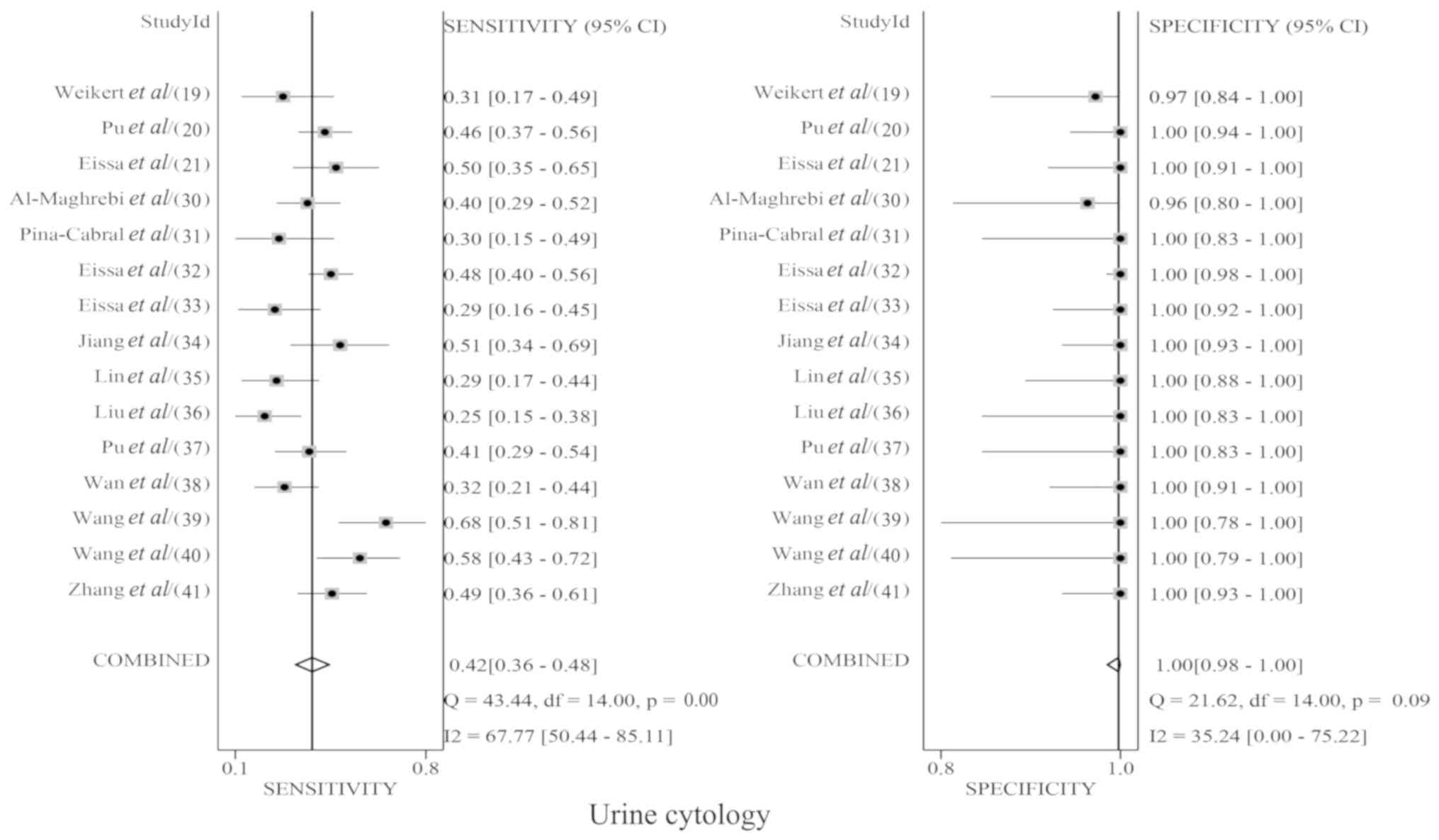
urinary survivin mRNA expression and urine cytology. The pooled Sen
for urinary survivin mRNA expression and urine cytology were 0.86
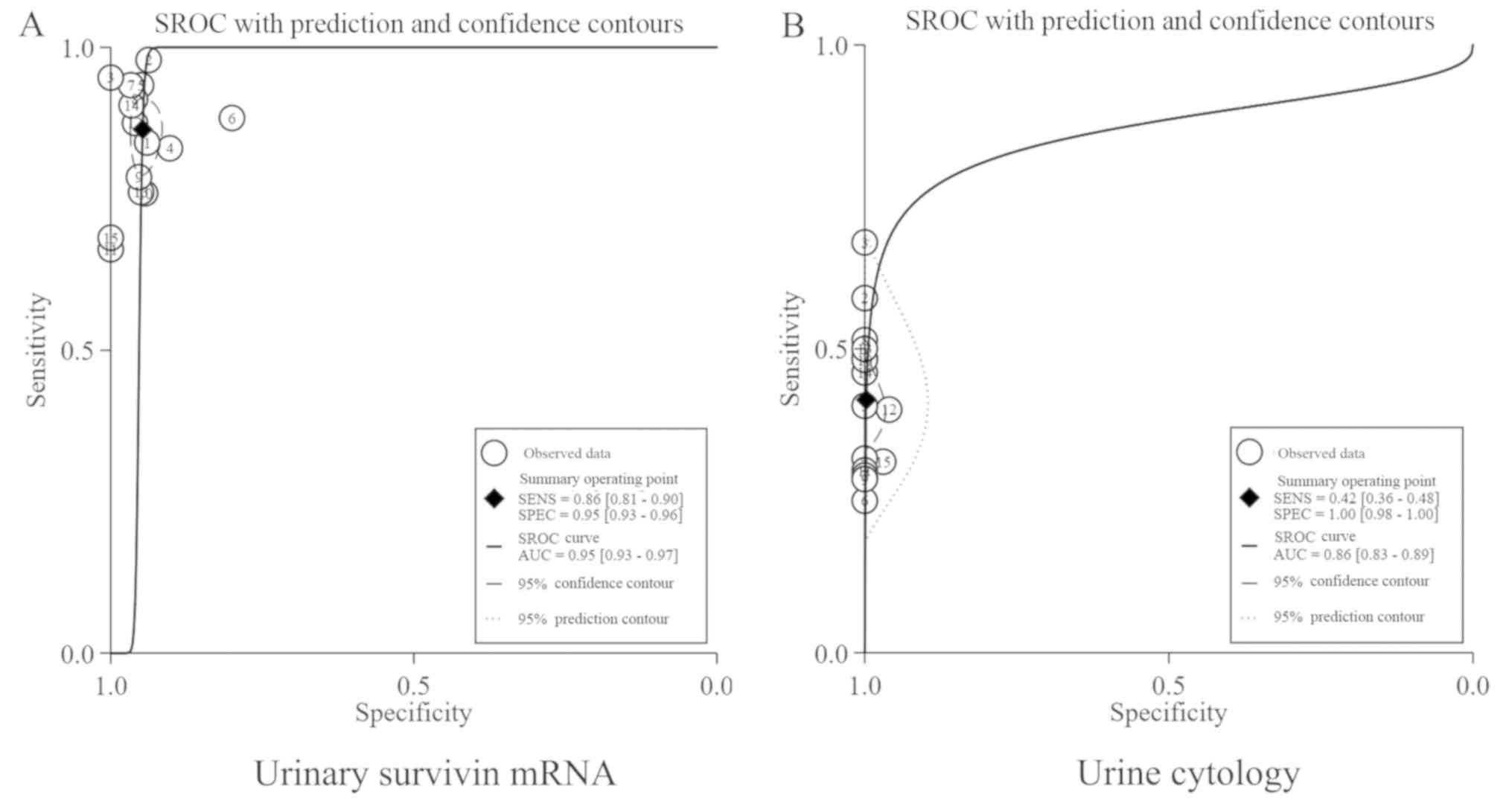
(95% CI, 0.81–0.90; Fig. 3) and 0.42
(95% CI, 0.36–0.48; Fig. 4),
respectively; the pooled Spe for urinary survivin mRNA expression
and urine cytology were 0.95 (95% CI, 0.93–0.96; Fig. 3) and 1.00 (95% CI, 0.98–1.00;
Fig. 4), respectively. The areas
under the curve (AUC) for urinary survivin mRNA expression and
urine cytology were 0.95 (95% CI, 0.93–0.97; Fig. 5A) and 0.86 (95% CI, 0.83–0.89;
Fig. 5B). The summary estimates of
DOR for urinary survivin mRNA expression and urine cytology were
83.42 (95% CI, 49.70–139.96) and 42.00 (95% CI, 22.95–76.89),
respectively; the pooled PLRs for urinary survivin mRNA expression
and urine cytology were 16.4 (95% CI, 11.6–23.2) and 178.5 (95% CI,
25.1–1269.7) and the pooled NLRs were 0.14 (95% CI, 0.10–0.20) and
0.59 (95% CI, 0.53–0.65), respectively (Table II).
 | Table II.Comparisons of prime diagnostic
indicators between the detection of urinary survivin mRNA
expression and urine cytology. |
Table II.
Comparisons of prime diagnostic
indicators between the detection of urinary survivin mRNA
expression and urine cytology.
| Parameter | Urine survivin mRNA
(95% CI) | Urine cytology (95%
CI) | P-value |
|---|
| Pooled Sen | 0.86 | (0.81–0.90) | 0.42 | (0.36–0.48) | <0.01 |
| Grade |
|
|
|
|
|
| G1 | 0.79 | (0.73–0.85) | 0.05 | (0.01–0.12) | <0.01 |
| G2 | 0.84 | (0.79–0.88) | 0.35 | (0.26–0.44) | <0.01 |
| G3 | 0.84 | (0.76–0.90) | 0.79 | (0.66–0.88) | >0.05 |
| Pooled Spe | 0.95 | (0.93–0.96) | 1.00 | (0.98–1.00) | >0.05 |
| γ | 0.81 |
| 0.42 |
| <0.05 |
| PLR | 16.40 | (11.60–23.20) | 178.50 |
(25.10–1269.70) | – |
| NLR | 0.14 | (0.10–0.20) | 0.59 | (0.53–0.65) | – |
| DOR | 88.99 | (57.35–138.08) | 40.39 | (20.13–81.06) | – |
| SAUC | 0.95 | (0.93–0.97) | 0.86 | (0.83–0.89) | >0.05 |
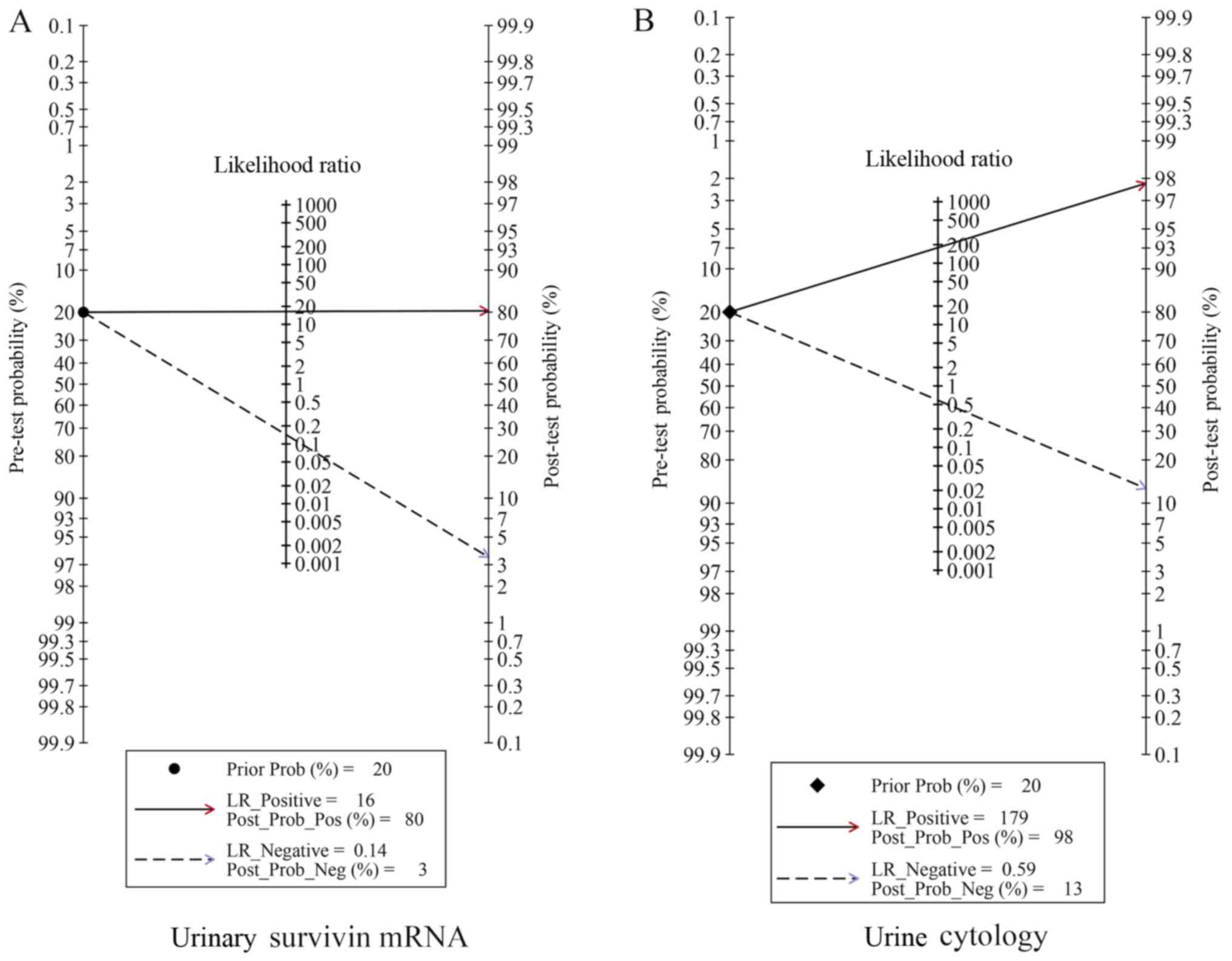
Fagan plot analysis was used to evaluate the
differences in clinical utility between urinary survivin mRNA
expression and urine cytology for the diagnosis of bladder cancer.
Regarding the detection of urinary survivin mRNA expression, the
probability of bladder cancer increased from 20 to 80% when the
test was positive and decreased to 3% when the results were
negative (Fig. 6A). For urine
cytology, the probability of bladder cancer increased from 20 to
98% when the results were positive and decreased to 13% when the
results were negative. The potential publication bias was evaluated
by Deeks' funnel plot (Fig. 7).
Discussion
Bladder cancer is a complex type of cancer with
variable biological and clinical characteristics that has the
tendency to recur and progress (42). Cystoscopy is ‘gold standard’
diagnostic procedure for bladder cancer, which can be invasive,
expensive, and increase risk for urologic disease in patients
(43,44). Although urine cytology has high
specificity (range, 85–100%), it is associated with low overall
sensitivity (range, 11–76%) depending on tumor grade and is not
suitable for preliminary screening in the diagnosis of bladder
cancer (10). Survivin, which is a
potential biomarker for urinary cancer, appears promising and worth
validating in a prospective study with regards to its diagnostic
and prognostic ability and clinical relevance (4,10,45).
Shariat et al (17) have
demonstrated that urinary survivin protein may be a predictor of
high-grade bladder tumors and Weikert et al (19) have detected survivin mRNA expression
in urine samples from 68.6% of patients with bladder cancer.
Currently, there are several methods available to detect survivin
expression, including IHC, ELISA and RT-PCR (46). Although IHC is regarded as a gold
standard for the diagnosis of cancer, its use is restricted by the
fact that tissue specimens are not easily obtained. Since survivin
is a non-secreted short-lived protein, certain methods to detect
its expression are based on the abundance of malignant cells in the
urine sediment (47,48). Although ELISA is considered a highly
accurate method, it is limited to a certain extent due to being
time-consuming and exhibiting poor uniformity (46). In addition, protein-based assays are
limited by insufficient antibody specificity and detection
sensitivity lower compared with that of RT-PCR assays (49). RT-PCR exhibits very high sensitivity
and is a well-established method able to exponentially amplify and
quantify minuscule amounts of nucleic acids in malignant cells in
the urinary sediment. Compared with RT-PCR, ELISA exhibits lower
sensitivity (0.75; 95% CI, 0.71–0.79) (18). The present study demonstrated that
RT-PCR based assays may have high sensitivity, especially for the
detection of the early stages of bladder cancer.
The aim of the present study was to perform a
meta-analysis of published literature investigating urinary
survivin mRNA expression detection by RT-PCR compared with urine
cytology in the diagnosis of bladder cancer. The results
demonstrated good diagnostic accuracy of urinary survivin mRNA
expression and urine cytology for bladder cancer, but they showed
their own different characteristics. The pooled sensitivity of
urinary survivin mRNA expression was higher compared with that of
urine cytology, and the difference was statistically significant in
the diagnosis of grade 1 and 2 bladder tumors. These findings
revealed that the expression of survivin may be associated with the
degree of malignancy of bladder cancer, although its role in tumor
metastasis and progression remains unclear. Regarding pooled
specificity, urine cytology was higher compared with urinary
survivin mRNA expression; as for SROC, urinary survivin mRNA
expression was higher, but neither of these parameters were
observed to exhibit statistically significant differences. Pooled
PLRs >10 and pooled NLRs <0.1 were considered to provide
convincing diagnostic evidence. PLRs >5 provide strong
diagnostic evidence to rule in diagnoses; NLRs <0.2 provide
strong diagnostic evidence to rule out diagnoses, in the majority
of cases (50). In the present
study, the NLR was 0.59 for urine cytology, which suggested that if
the outcome for this method was negative, 59% of the patients may
still have bladder cancer. However, the NLR for urinary survivin
mRNA expression was 0.14, indicating that 14% of patients may be
misdiagnosed. Based on the assessed parameters from the retrieved
studies, including Sen, Spe, AUC, PLR, NLR and DOR, the results of
the present study demonstrated that survivin mRNA exhibited
potential as a biomarker for the diagnosis of bladder cancer.
The present meta-analysis has several limitations.
First, the results demonstrated that the heterogeneity between
studies should not be ignored. Despite performing the subgroup and
sensitivity analysis, heterogeneity was still detected. The
DerSimonian Laird method was used for pooled analyses. The
heterogeneity of the pooled studies was low to moderate in all
analyses. This limitation is difficult to discuss since the
individual studies in this meta-analysis contained different
control groups. In previous studies, non-malignant diseases of the
urinary system were assigned as the control groups (37,38). In
two of the studies, healthy participants were assigned to the
control groups (30,31). In other studies, healthy participants
and non-malignant diseases of the urinary system were assigned as
the control groups (19–21, 32–36,
39–41). Certain studies focused on diagnostics
(16,21,30,32,33),
whereas others focused on the use of urinary mRNA expression as a
tumor marker; however, there is not sufficient data to predict the
recurrence of bladder cancer (19,31). The
pooled sensitivity and specificity for urinary survivin mRNA
expression were 0.86 and 0.94, respectively, indicating that the
urinary survivin mRNA expression analysis via RT-PCR was accurate.
Only studies written in English and Chinese were included; studies
published in other languages were excluded, which may have led to
further bias in this study. The experimental equipment and reagents
of each laboratory and the threshold setting of urinary survivin
mRNA expression may also vary.
Compared with traditional urine cytology, RT-PCR has
the following advantages: i) Easy collection of urine samples; ii)
high diagnostic accuracy; iii) low rate of missed diagnoses; and
iv) simple procedure without discomfort for the patient.
Furthermore, an important advantage of detecting urinary survivin
mRNA expression using RT-PCR is its high sensitivity.
In summary, urinary survivin mRNA expression may
serve as a screening marker for the diagnosis of bladder cancer.
However, prior to its application in the clinical setting, a large
prospective study is required in order to verify the accuracy of
the RT-PCR assay and identify the optimal cut-off value
clinical.
Acknowledgements
Not applicable.
Funding
The present study was funded by the Foundation of
Yongchuan Hospital of Chongqing Medical University (grant nos.
YJLCX201621 and 20180313).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JZ, YQY and LF designed the present study. YQY and
LL analyzed the data and prepared the figures. LF and YXY performed
the literature research and selected the relevant studies. LF and
YQY wrote and revised the initial manuscript. All authors reviewed
and approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Witjes JA and Hendricksen K: Intravesical
pharmacotherapy for non-muscle-invasive bladder cancer: A critical
analysis of currently available drugs, treatment schedules, and
long-term results. Eur Urol. 53:45–52. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Babjuk M, Burger M, Zigeuner R, Shariat
SF, van Rhijn BW, Compérat E, Sylvester RJ, Kaasinen E, Böhle A,
Palou Redorta J, et al: EAU guidelines on non-muscle-invasive
urothelial carcinoma of the bladder: Update 2013. Eur Urol.
64:639–653. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kamat AM, Hegarty PK, Gee JR, Clark PE,
Svatek RS, Hegarty N, Shariat SF, Xylinas E, Schmitz-Dräger BJ,
Lotan Y, et al: ICUD-EAU International Consultation on bladder
cancer 2012: Screening, diagnosis, and molecular markers. Eur Urol.
63:4–15. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Burke DM, Shackley DC and O'Reilly PH: The
community-based morbidity of flexible cystoscopy. BJU Int.
89:347–349. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Biardeau X, Lam O, Ba V, Campeau L and
Corcos J: Prospective evaluation of anxiety, pain, and
embarrassment associated with cystoscopy and urodynamic testing in
clinical practice. Can Urol Assoc J. 11:104–110. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Raitanen MP, Leppilahti M, Tuhkanen K,
Forssel T, Nylund P and Tammela T; FinnBladder Group, : Routine
follow-up cystoscopy in detection of recurrence in patients being
monitored for bladder cancer. Ann Chir Gynaecol. 90:261–265.
2001.PubMed/NCBI
|
|
8
|
Lotan Y and Roehrborn CG: Sensitivity and
specificity of commonly available bladder tumour markers versus
cytology: Results of a comprehensive literature review and
meta-analyses. Urology. 61:109–118. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Oeyen E, Hoekx L, De Wachter S, Baldewijns
M, Ameye F and Mertens I: Bladder cancer diagnosis and follow-up:
The current status and possible role of extracellular vesicles. Int
J Mol Sci. 20(pii): E8212019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lokeshwar VB, Habuchi T, Grossman HB,
Murphy WM, Hautmann SH, Hemstreet GP III, Bono AV, Getzenberg RH,
Goebell P, Schmitz-Dräger BJ, et al: Bladder tumour markers beyond
cytology: International consensus panel on bladder tumour markers.
Urology. 66 (6 Suppl 1):35–63. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
D'Costa JJ, Goldsmith JC, Wilson JS, Bryan
RT and Ward DG: A systematic review of the diagnostic and
prognostic value of urinary protein biomarkers in urothelial
bladder cancer. Bladder Cancer. 2:301–317. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Garg H, Suri P, Gupta JC, Talwar GP and
Dubey S: Survivin: A unique target for tumour therapy. Cancer Cell
Int. 16:492016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jacob NK, Cooley JV, Shirai K and
Chakravarti A: Survivin splice variants are not essential for
mitotic progression or inhibition of apoptosis induced by
doxorubicin and radiation. Onco Targets Ther. 5:7–20. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ambrosini G, Adida C and Altieri DC: A
novel anti-apoptosis gene, survivin, expressed in cancer and
lymphoma. Nat Med. 3:917–921. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Swana HS, Grossman D, Anthony JN, Weiss RM
and Altieri DC: Tumour content of the antiapoptosis molecule
survivin and recurrence of bladder cancer. N Engl J Med.
341:452–453. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Smith SD, Wheeler MA, Plescia J, Colberg
JW, Weiss RM and Altieri DC: Urine detection of survivin and
diagnosis of bladder cancer. JAMA. 285:324–328. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shariat SF, Casella R, Khoddami SM,
Hernandez G, Sulser T, Gasser TC and Lerner SP: Urine detection of
survivin is a sensitive marker for the noninvasive diagnosis of
bladder cancer. J Urol. 171:626–630. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liang Z, Xin R, Yu Y, Wang R, Wang C and
Liu X: Diagnostic value of urinary survivin as a biomarker for
bladder cancer: A systematic review and meta-analysis of published
studies. World J Urol. 36:1373–1381. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Weikert S, Christoph F, Schrader M, Krause
H, Miller K and Muller M: Quantitative analysis of survivin mRNA
expression in urine and tumour tissue of bladder cancer patients
and its potential relevance for disease detection and prognosis.
Int J Cancer. 116:100–104. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pu XY, Wang ZP, Chen YR, Wang XH, Wu YL
and Wang HP: The value of combined use of survivin, cytokeratin 20
and mucin 7 mRNA for bladder cancer detection in voided urine. J
Cancer Res Clin Oncol. 134:659–665. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Eissa S, Badr S, Elhamid SA, Helmy AS,
Nour M and Esmat M: The value of combined use of survivin mRNA and
matrix metalloproteinase 2 and 9 for bladder cancer detection in
voided urine. Dis Markers. 34:57–62. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Moher D, Liberati A, Tetzlaff J, Altman DG
and Group P: Preferred reporting items for systematic reviews and
meta-analyses: The PRISMA statement. PLoS Med. 6:e10000972009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Whiting PF, Rutjes AW, Westwood ME,
Mallett S, Deeks JJ, Reitsma JB, Leeflang MM, Sterne JA and Bossuyt
PM; QUADAS-2 Group, : QUADAS-2: A revised tool for the quality
assessment of diagnostic accuracy studies. Ann Intern Med.
155:529–536. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Skupski DW, Rosenberg CR and Eglinton GS:
Intrapartum fetal stimulation tests: A meta-analysis. Obstet
Gynecol. 99:129–134. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Deeks JJ, Macaskill P and Irwig L: The
performance of tests of publication bias and other sample size
effects in systematic reviews of diagnostic test accuracy was
assessed. J Clin Epidemiol. 58:882–893. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Glas AS, Lijmer JG, Prins MH, Bonsel GJ
and Bossuyt PM: The diagnostic odds ratio: A single indicator of
test performance. J Clin Epidemiol. 56:1129–1135. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Reitsma JB, Glas AS, Rutjes AW, Scholten
RJ, Bossuyt PM and Zwinderman AH: Bivariate analysis of sensitivity
and specificity produces informative summary measures in diagnostic
reviews. J Clin Epidemiol. 58:982–990. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Higgins JP, Thompson SG, Deeks JJ and
Altman DG: Measuring inconsistency in meta-analyses. BMJ.
327:557–560. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ye X, Xiao H, Chen B and Zhang S: Accuracy
of lung ultrasonography versus chest radiography for the diagnosis
of adult community-acquired pneumonia: Review of the literature and
meta-analysis. PLoS One. 10:e01300662015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Al-Maghrebi M, Kehinde EO, Kapila K and
Anim JT: Urinary survivin mRNA expression and urinary nuclear
matrix protein 22 BladderChek® and urine cytology in the
detection of transitional cell carcinoma of the bladder. Med Princ
Pract. 21:295–297. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pina-Cabral L, Santos L, Mesquita B, Amaro
T, Magalhães S and Criado B: Detection of survivin mRNA in urine of
patients with superficial urothelial cell carcinomas. Clin Transl
Oncol. 9:731–736. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Eissa S, Swellam M, Shehata H, El-Khouly
IM, El-Zayat T and El-Ahmady O: Expression of HYAL1 and survivin
RNA as diagnostic molecular markers for bladder cancer. J Urol.
183:493–498. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Eissa S, Shabayek MI, Ismail MF, El-Allawy
RM and Hamdy MA: Diagnostic evaluation of apoptosis inhibitory gene
and tissue inhibitor matrix metalloproteinase-2 in patients with
bladder cancer. IUBMB Life. 62:394–399. 2010.PubMed/NCBI
|
|
34
|
Jiang G, Zhang JH, Jian RJ and Chen ZD:
Clinical significance of survivin mRNA level and hyaluronic acid
level detection of patients suffered from bladder transitional cell
carcinomas. Sichuan Med J. 11:1162–1164. 2006.
|
|
35
|
Lin Y, Han ZH and Liu T: The clinical
significance of urinary survivin mRNA detection for bladder
transitional cell carcinoma. Zhejiang Journal of Integrated
Traditional Chinese and Western Medicine. 17:21–22. 2007.
|
|
36
|
Liu JG, Yang JY and Wei W: The value of
combined detection of urinary cell keratin 19, nuclear matrix
protein 22 and survivin in the early diagnosis of bladder cancer.
China Foreign Medical Treatment. 28:1682009.
|
|
37
|
Pu XY, Wang ZP, Chen YR, Wu YL, Wang HP
and Wang XH: Combined use of uirnary bladder cancer antigen,
hyaluronic aeid and survivin for the detection of bladder cancer.
Chin J Urol. 27:970–973. 2008.
|
|
38
|
Wan JH, Jin FS, Xiang D, Hu B and Gao F:
Value of cytokeratin 20 and survivin in the diagnosis of bladder
tumour. J Clin Res. 25:428–431. 2008.
|
|
39
|
Wang L, Zeng FQ, Liao GY and Chen FM:
Detection of survivin in exfoliated urothelial cells of bladder
cancer. J Clin Urol. 19:489–490. 2004.
|
|
40
|
Wang ZH, Hu ZQ, Ye Q, Ye ZQ, Cai D, Yang
N, Liu H, Zhuang QY, Yang WM, et al: Clinical application of
survivin detection in urothelial cells of patients with
transitional cell carcinoma of bladder. Chin J Exper Surg.
23:959–961. 2006.
|
|
41
|
Zhang WX, Zhen S and Zhen T: Diagnosis of
bladder cancer by detection of survivin and minichromosome
maitence5 protein in urine sediment. Chin J Urol. 26:233–236.
2005.
|
|
42
|
Nicolazzo C, Busetto GM, Del Giudice F,
Sperduti I, Giannarelli D, Gradilone A, Gazzaniga P, de Berardinis
E and Raimondi C: The long-term prognostic value of survivin
expressing circulating tumor cells in patients with high-risk
non-muscle invasive bladder cancer (NMIBC). J Cancer Res Clin
Oncol. 143:1971–1976. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Gogalic S, Sauer U, Doppler S and
Preininger C: Bladder cancer biomarker array to detect aberrant
levels of proteins in urine. Analyst. 140:724–735. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Herr HW: The risk of urinary tract
infection after flexible cystoscopy in patients with bladder tumor
who did not receive prophylactic antibiotics. J Urol. 193:548–551.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Eissa S, Kassim SK, Labib RA, El-Khouly
IM, Ghaffer TM, Sadek M, Razek OA and El-Ahmady O: Detection of
bladder carcinoma by combined testing of urine for hyaluronidase
and cytokeratin 20 RNAs. Cancer. 103:1356–1362. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chang Y, Xu J and Zhang Q: Microplate
magnetic chemiluminescence immunoassay for detecting urinary
survivin in bladder cancer. Oncol Lett. 14:4043–4052. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Altieri DC: Survivin, versatile modulation
of cell division and apoptosis in cancer. Oncogene. 22:8581–8589.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kitsukawa S, Aoyagi T, Noda K, Ito T,
Yamamoto Y, Hosoda S, Otsuru N and Matsumoto T: Quantitative
analysis of survivin mRNA expression in bladder transitional cell
carcinomas. Hinyokika Kiyo. 54:101–106. 2008.PubMed/NCBI
|
|
49
|
Moussa O, Abol-Enein H, Bissada NK, Keane
T, Ghoneim MA and Watson DK: Evaluation of survivin reverse
transcriptase-polymerase chain reaction for noninvasive detection
of bladder cancer. J Urol. 175:2312–2316. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Pormohammad A, Riahi SM, Nasiri MJ, Fallah
F, Aghazadeh M, Doustdar F and Pouriran R: Diagnostic test accuracy
of adenosine deaminase for tuberculous meningitis: A systematic
review and meta-analysis. J Infect. 74:545–554. 2017. View Article : Google Scholar : PubMed/NCBI
|