Introduction
Prostate cancer is a highly prevalent malignancy and
represents the second leading cause of cancer-related mortality in
elderly men (1,2). Moreover, it is estimated that there
would be 161,360 new prostate cancer diagnoses and 26,730 prostate
cancer-associated mortalities in 2015 (1,2). The
molecular heterogeneity of prostate cancer can make its early
diagnosis and treatment problematic; thus, the identification of
accurate molecular biomarkers and potential therapeutic targets (at
various disease stages) may result in improved patient outcome
(3,4). It is therefore important to investigate
the molecular mechanisms underpinning the development and
progression of prostate cancer, as this may catalyze the
identification of novel therapeutic targets (5,6).
Contactin-1 (CNTN-1), a member of the immunoglobulin
superfamily is a glycosylphosphatidylinositol (GPI)-anchored
neuronal membrane protein that facilitates cell adhesion (7,8). It may
also influence the formation of axon connections in the developing
nervous system (7). Mikami et
al (7) also reported that CNTN-1
was a functional receptor for neuroregulatory chondroitin
sulfate-E. Additionally, Lamprianou et al (8) discovered a complex (formed from CNTN-1
and protein tyrosine phosphatase receptor type Z1) that mediated
the development of oligodendrocyte precursor cells. CNTN-1 is
upregulated in several common types of human cancer, and promotes
the progression of lung (9) and
gastric cancer (10), and esophageal
(11) and oral squamous cell
carcinoma (12). For example, the
upregulation of CNTN-1 expression is correlated with more advanced
clinical stages and lymph node metastasis in patients with
esophageal squamous cell carcinoma (11). Moreover, CNTN-1 expression is
upregulated in oral squamous cell carcinoma, and is associated with
lymph node metastasis, as well as a poor prognosis (12). Su et al (9) discovered that the knockdown of CNTN-1
expression inhibited the invasion and metastasis of lung
adenocarcinoma, suggesting that it may represent a promising
therapeutic target for the treatment of patients with the
disease.
Furthermore, Yan et al (13) reported that the knockdown of CNTN1
inhibited stem-like, cell-mediated tumor initiation in prostate
cancer. It was also reported that the overexpression of CNTN1
promoted cellular invasion in vitro, as well as enhancing
xenograft tumor formation and lung metastasis in vivo
(13). Taken together, these
findings suggest that CNTN-1 may promote prostate cancer
progression. The present study aimed to investigate the clinical
significance of CNTN-1 expression in prostate cancer progression,
and to determine the mechanism of CNTN-1 regulation of the
malignant phenotypes of prostate cancer cells.
Materials and methods
Tissue collection
A total of 56 prostate cancer tissues and matched
adjacent paracancerous tissues were obtained from patients with
primary prostate cancer at the First Affiliated Hospital of Jishou
University (Jishou, China) between April 2011 and September 2013
and stored at −80°C until use. The patients were aged between 58
and 79 years (mean age, 66.5 years). The clinicopathological
features of all patients are presented in Table I. Follow-up occurred for 60 months
after surgery by phone calls. Written informed consent was obtained
from all patients prior to surgery, and the experimental procedures
were approved by the Ethics Committee of the First Affiliated
Hospital of Jishou University.
 | Table I.Association between CNTN-1 expression
and the clinicopathological characteristics of patients with
prostate cancer. |
Table I.
Association between CNTN-1 expression
and the clinicopathological characteristics of patients with
prostate cancer.
| Variable | Cases (n=56) | Low CNTN-1
(n=31) | High CNTN-1
(n=25) | P-value |
|---|
| Age |
|
|
| 0.784 |
|
<65 | 19 | 11 | 8 |
|
| ≥65 | 37 | 20 | 17 |
|
| Tumor size |
|
|
| 0.015a |
| ≤2
cm | 21 | 16 | 5 |
|
| >2
cm | 35 | 15 | 20 |
|
| Gleason score |
|
|
| 0.130 |
| ≤6 | 17 | 12 | 5 |
|
|
>6 | 39 | 19 | 20 |
|
| Tumor stage |
|
|
| 0.004b |
|
I–II | 38 | 26 | 12 |
|
|
III–IV | 18 | 5 | 13 |
|
| Lymph node
metastasis |
|
|
| 0.022a |
| No | 34 | 23 | 11 |
|
|
Yes | 22 | 8 | 14 |
|
| Distant
metastasis |
|
|
| 0.006b |
| No | 45 | 29 | 16 |
|
|
Yes | 11 | 2 | 9 |
|
Cell culture and transfection
The human prostate cell lines PC3 and LNCaP were
obtained from the American Type Culture Collection. PC3 cells were
cultured in 6-well plates (1×105 cells/well) Dulbecco's
modified Eagle's medium (DMEM) and LNCaP cells were cultured in
RPMI-1640 medium, both supplemented with 10% FBS (all Thermo Fisher
Scientific, Inc.); the cells were maintained at 37°C in a
humidified atmosphere with 5% CO2. Subsequently, both
cell types were transiently transfected with either 100 nM negative
control (NC) or 100 nM CNTN-1 shRNA (both Shanghai GenePharma Co.,
Ltd.) using Lipofectamine™ 2000 reagent (Thermo Fisher Scientific,
Inc.), according to the manufacturer's protocol. The shRNA
sequences were as follows: NC; 5′-UUCUCCGAACGUGUCACGUTT-3′, and
CNTN-1; 5′-GGUCCUUCAAUGGCUAUGUTT-3′. Subsequent experiments were
conducted at 48 h post-transfection.
Reverse transcription-quantitative
(RT-q)PCR
Total RNA was extracted from the tissues and cell
lines using TRIzol® reagent (Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol. The RevertAid First
Strand cDNA Synthesis kit (Thermo Fisher Scientific, Inc.) was used
to reverse transcribe the RNA into cDNA according to the
manufacturer's protocol, for which the primer sequences are as
follows: CNTN-1 forward, 5′-TGTTCAGCAAATTCATCCCA-3′ and reverse,
5′-TCTACCCACTCAGGGAATGC-3′; and GAPDH forward,
5′-ACGGATTTGGTCGTATTGGGCG-3′; and reverse,
5′-CTCCTGGAAGATGGTGATGG-3′. ABI Power SYBR® Green PCR
Master mix (Thermo Fisher Scientific, Inc.) was subsequently used
to perform qPCR according to the manufacturer's protocol. The
reaction conditions were 95°C for 3 min, followed by 35 cycles of
95°C for 15 sec, 58°C for 15 sec, and 72°C for 15 sec. The relative
expression levels were quantified using the 2−∆∆Cq
method (14) and normalized to those
of GADPH.
Cell proliferation assay
Transfected cells (5×104 cells per well)
were seeded into 96-well plates, and cultured at 37°C for 0, 24, 48
or 72 h. Subsequently, 10 µl CCK-8 solution (Dojindo Molecular
Technologies, Inc.) was added to each well, and the cells were
incubated at 37°C for 4 h. The absorbance at 450 nm was measured
using a microplate reader (Thermo Labsystems).
Colony formation assays
Transfected cells (1×103 cells/well) were
seeded into 12-well plates and cultured for 7 days. Crystal violet
(0.1%; Thermo Fisher Scientific) was then used to stain the cells
before images were captured using a light microscope
(magnification, ×200). The number of colonies was determined using
ImageJ (v. 1.46; National Institutes of health).
Wound-healing assay
Transfected cells (5×105 cells/well) were
seeded into 6-well plates, and cultured at 37°C until ~100%
confluence was achieved. A sterile 200-µl pipette tip was used to
scratch a wound line in each well. The transfected cells were
washed twice with Dulbecco's phosphate-buffered saline (Gibco;
Thermo Fisher Scientific) and resuspended in serum-free DMEM,
before being incubated at 37°C for 24 h. Cells were then imaged at
0 and 24 h using a light microscope (magnification, ×40). The wound
closure between the 0 to 24 h time points was measured using ImageJ
(v. 1.8; NIH) and relative wound closure was determined.
Cell invasion assay
Transfected cells (5×104 cells/well) were
resuspended in serum-free DMEM and seeded into the upper chamber of
8-µm Transwell inserts (BD Biosciences), which had been pre-coated
with Matrigel (BD Biosciences) at 37°C for 30 min. The lower
chamber was plated with DMEM supplemented with 10% FBS, and the
cells were incubated at 37°C for 24 h. The cells were then fixed
using 4% paraformaldehyde at room temperature for 30 min, and then
stained using crystal violet at room temperature for 5 min, before
being imaged under a light microscope (magnification, ×100). The
number of invading cells was counted in five random non-overlapping
fields.
Western blotting
Total protein was extracted from the transfected
cells using RIPA buffer (Thermo Fisher Scientific Inc.). The total
protein was quantified using a BCA method with a Pierce BCA Protein
assay kit (Thermo Fisher Scientific Inc.). The proteins (50
µg/lane) were separated by 10% SDS-PAGE gel, and transferred onto
PVDF membranes (Thermo Fisher Scientific Inc.). The membranes were
blocked using 5% non-fat milk at room temperature for 3 h, and then
incubated with rabbit anti-human antibodies against: CNTN-1 (1:500;
cat. no. ab66265), E-cadherin (1:250; cat. no. ab133597),
N-cadherin (1:500; cat. no. ab76011), vimentin (1:200; cat. no.
ab92547), phosphorylated (p)-PI3K (1:200; cat. no. ab182651), PI3K
(1:200; cat. no. ab191606), p-AKT (1:250; cat. no. ab81283), AKT
(1:500; cat. no. ab235958) and GAPDH (1:500; cat. no. ab8245) at
room temperature for 3 h, followed by further incubation with horse
radish peroxidase-conjugated goat anti-rabbit secondary antibody
(1:5,000; cat. no. ab6721) at room temperature for 1 h. All
antibodies were purchased from Abcam. The protein bands were
visualized using the Pierce™ ECL western blotting substrate (Thermo
Fisher Scientific, Inc.) and ImageJ software (v. 1.46; National
Institutes of health) was used to conduct densitometric
analysis.
Statistical analysis
All experiments were repeated ≥3 times. The data are
presented as the mean ± SD and were analyzed using SPSS (v.20.0;
IBM Corp.). Differences between 2 groups were analyzed using the
paired or unpaired Student's t-test, and differences between
multiple groups were analyzed using one-way ANOVA followed by
Tukey's post hoc test. The log-rank test was used to compare
patient survival times between high- and low-CNTN1 expression
groups, and the χ2 test was used to analyze the results
presented in Table I. P<0.05 was
considered to indicate a statistically significant difference.
Results
Upregulation of CNTN-1 is associated
with prostate cancer progression
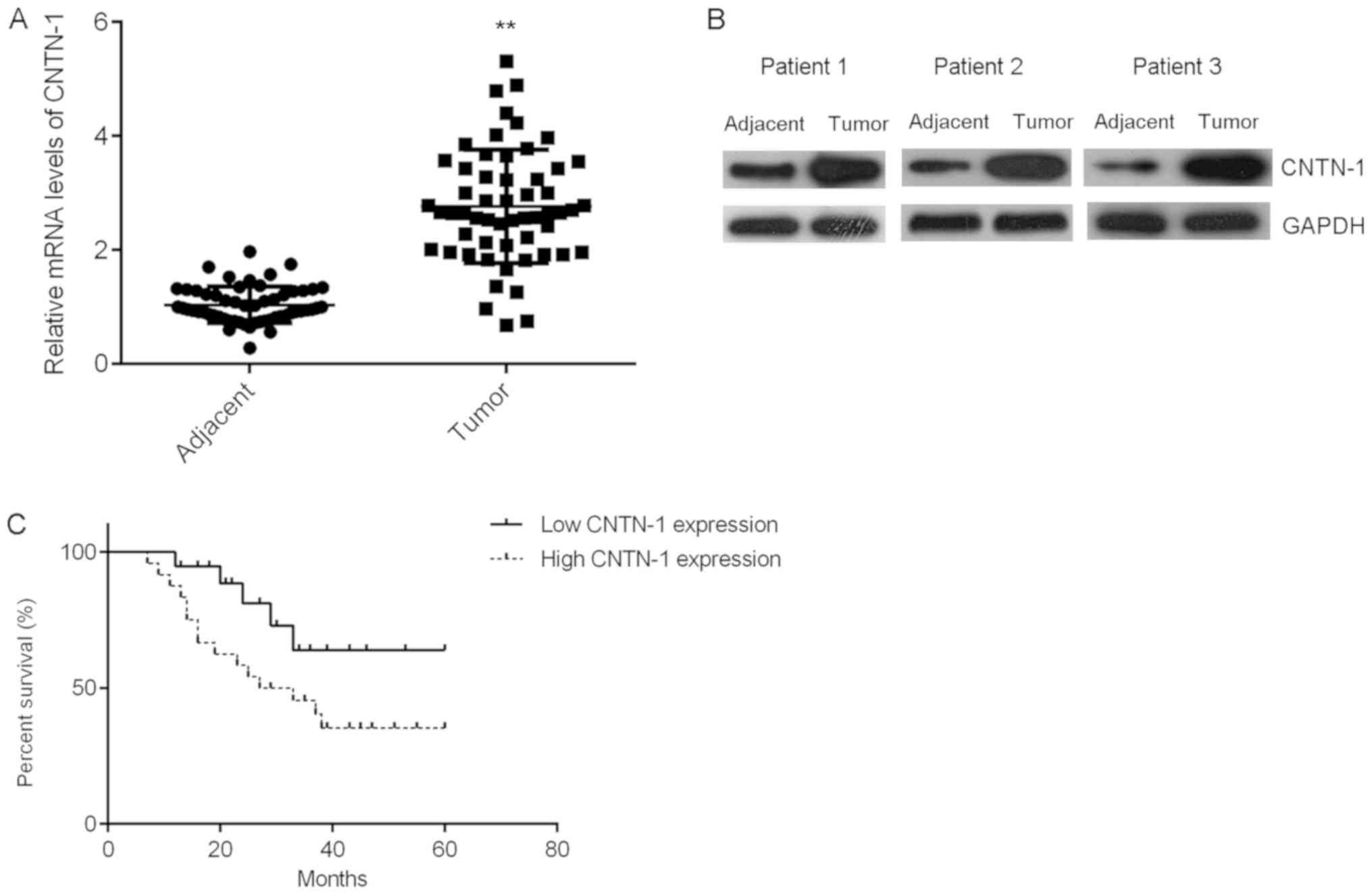
In the present study, the expression levels of
CNTN-1 in prostate cancer tissues were compared with those of
matched adjacent paracancerous tissues. The mRNA and protein
expression levels of CNTN-1 were significantly higher in prostate
cancer tissues than in the corresponding adjacent tissues (Fig. 1A and B; P<0.01). Patients were
then divided into high- and low-CNTN-1 expression groups, and the
clinical significance of CNTN-1 expression in the progression of
prostate cancer was investigated. The high-expression group was
significantly associated with a larger tumor size, more advanced
tumor state and risk of metastasis (Table I). Moreover, it was determined that
the high-CNTN-1 expression level group exhibited shorter overall
survival times compared with the low-expression group, suggesting
that the upregulation of CNTN-1 may predict poor prognosis in
patients with prostate cancer (Fig.
1C).
Knockdown of CNTN-1 inhibits prostate
cancer cell proliferation
The influence of CNTN-1 expression on prostate
cancer progression was also examined in vitro. To
investigate whether CNTN-1 expression was upregulated in prostate
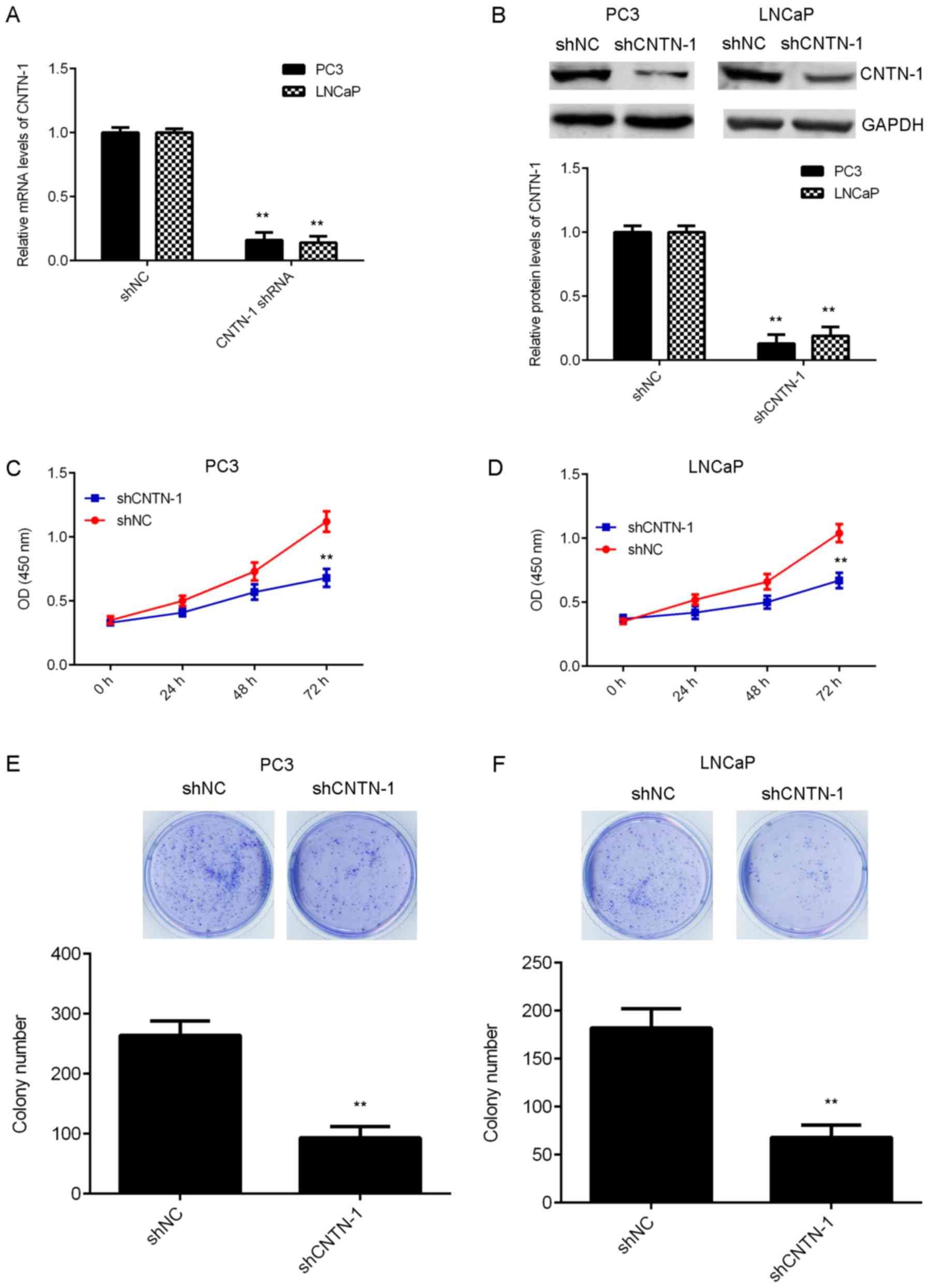
cancer cells, cells were transfected with shRNA to knockdown CNTN-1
expression. Following transfection, the mRNA and protein levels of
CNTN-1 were significantly reduced when compared with those of the
control group. However, transfection with NC shRNA did not affect
CNTN-1 expression in prostate cancer cells (Fig. 2A and B; P<0.01). The function of
CNTN-1 in prostate cancer cell proliferation was then investigated.
As shown in Fig. 2C-E,
CNTN-1-knockdown significantly inhibited the proliferation and
colony formation of prostate cancer cells (P<0.01), suggesting
that CNTN-1 promotes prostate cancer cell proliferation.
CNTN-1-knockdown suppresses the
migration and invasion abilities of prostate cancer cells
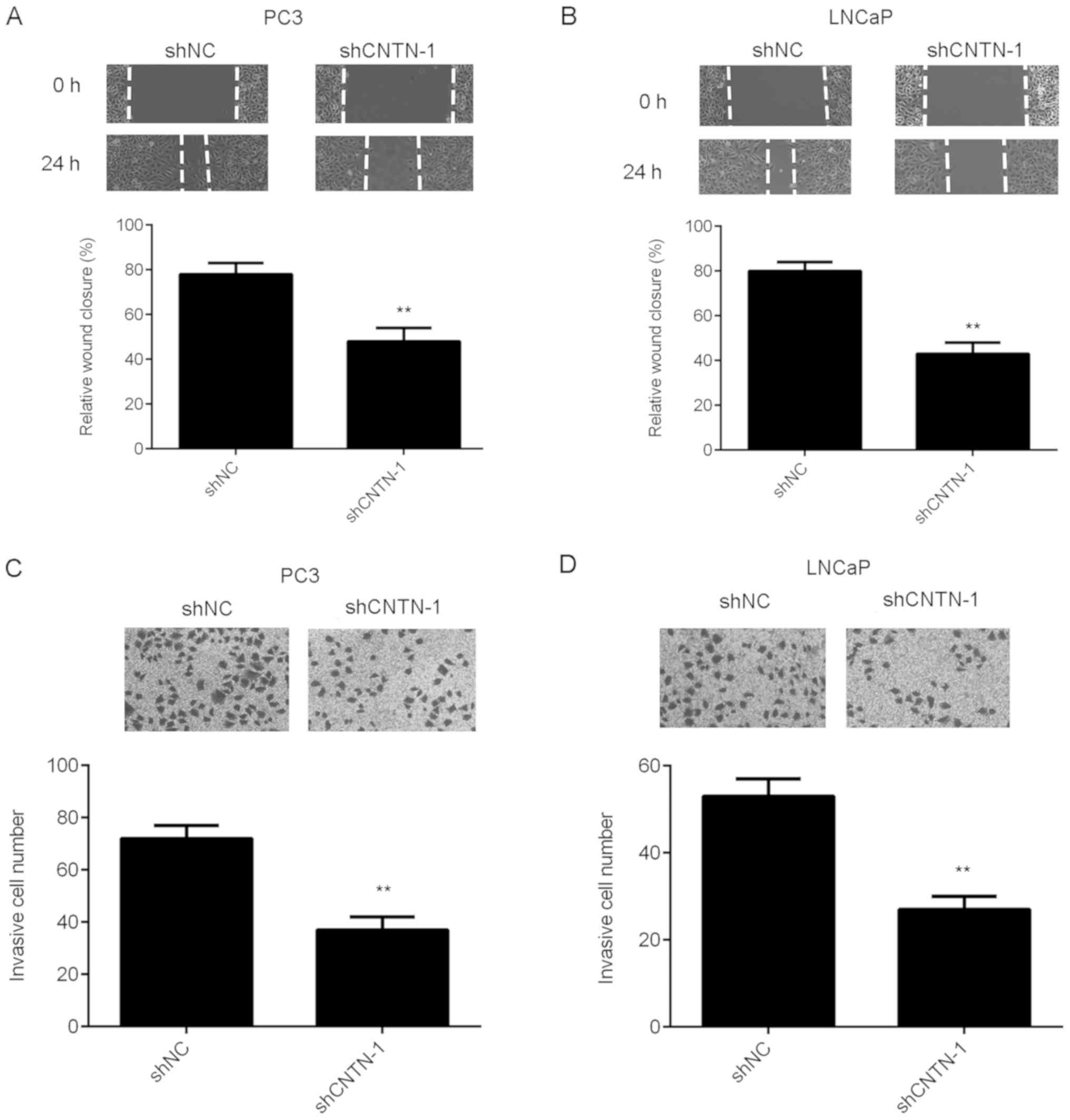
To further characterize the function of CNTN-1 in
prostate cancer metastasis, wound-healing and Transwell assays were
performed to examine the effects of CNTN-1-knockdown on the
migration and invasiveness of prostate cancer cells. As shown in
Fig. 3A and B, prostate cancer cell
migration was significantly inhibited in the CNTN-1-knockdown
group, compared with the control group (P<0.01). Knockdown of
CNTN-1 significantly suppressed the invasive capacity of PC3 and
LNCaP cells (Fig. 3C and D;
P<0.01). Therefore, CNTN-1 is suggested to promote the
regulation of migration and invasion in prostate cancer cells.
Inhibition of CNTN-1 represses
epithelial-mesenchymal transition (EMT) and PI3K/AKT signaling in
prostate cancer cells
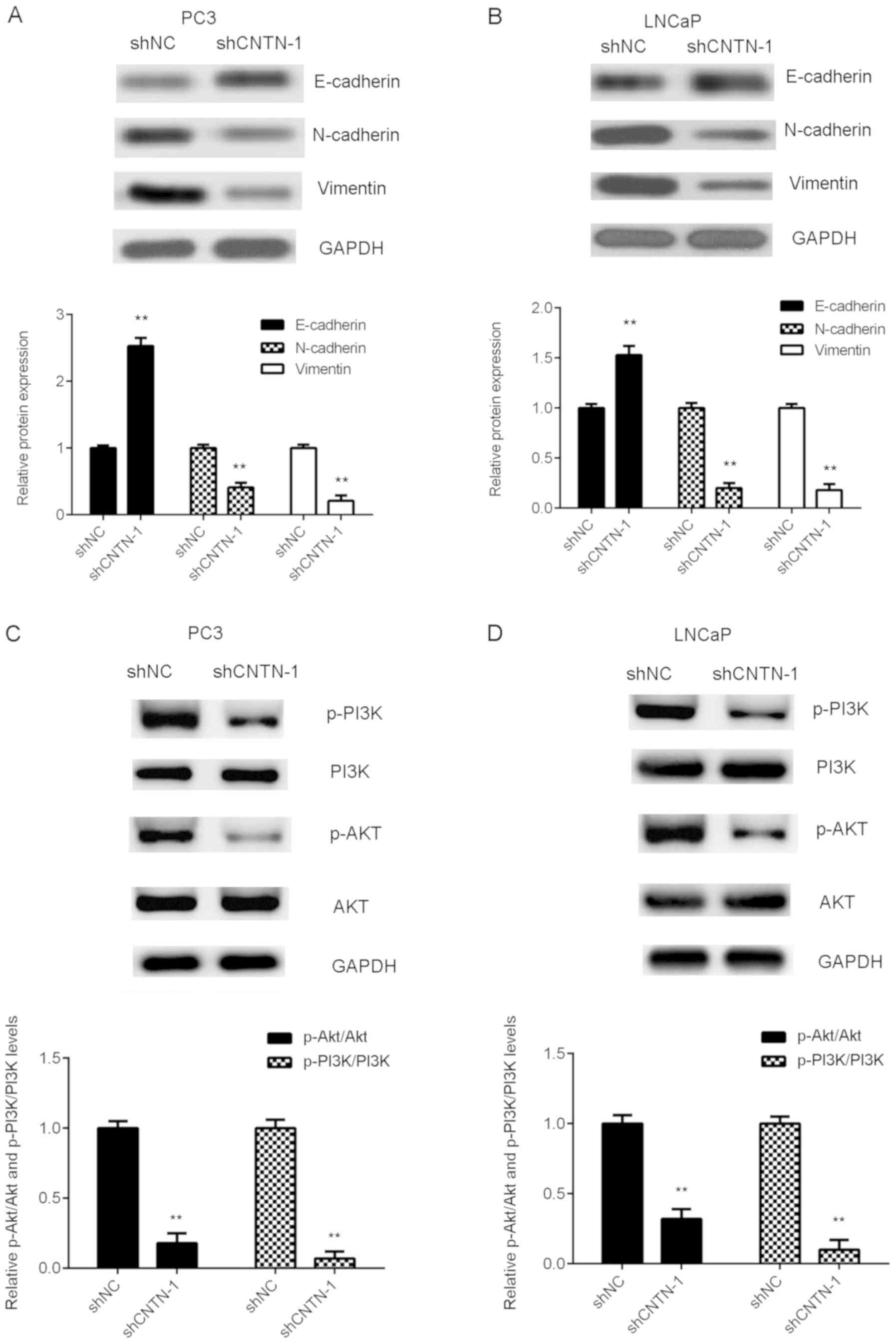
The effect of CNTN-1-knockdown on EMT (a mechanism
facilitating cancer cell migration and invasion) in prostate cancer
cells was also investigated. As exhibited in Fig. 4A and B, knockdown of CNTN-1
significantly increased the expression of E-cadherin, but decreased
the protein levels of N-cadherin and Vimentin in prostate cancer
cells (P<0.01), indicating that EMT was inhibited. Therefore, it
was suggested that CNTN-1-knockdown may suppress prostate cancer
metastasis via the inhibition of EMT.
The molecular mechanism of CNTN-1 in prostate cancer
progression was then investigated. PI3K/AKT signaling has been
reported to influence prostate tumor growth and metastasis.
Therefore, the function of CNTN-1 in the regulation of the PI3K/AKT
signaling pathway was assessed. As indicated in Fig. 4C and D, the expression levels of
phosphorylated PI3K and AKT were significantly reduced in the
shCNTN-1 group compared with those the shNC group, indicating that
CNTN-1-knockdown reduced PI3K/AKT signaling in prostate cancer
cells (P<0.01). Therefore, the results of the current study
suggest that the PI3K/AKT signaling pathway may be involved in the
function of CNTN-1, by regulating the malignant phenotypes of
prostate cancer cells.
Discussion
The function and clinical significance of CNTN-1
expression in prostate cancer has not yet been fully elucidated. In
the present study, it was observed that the expression level of
CNTN-1 was significantly higher in prostate cancer tissues compared
with adjacent paracancerous tissues. Moreover, high expression of
CNTN-1 was positively correlated with cancer progression, as well
as poor prognosis in patients with prostate cancer.
CNTN-1-knockdown resulted in significant inhibitory effects on
prostate cancer cell proliferation, colony formation, migration and
invasiveness. Moreover, the CNTN-1-knockdown inhibited EMT and
modulated PI3K/AKT signaling in prostate cancer cells.
CNTN-1 has been discovered to promote prostate
cancer cell invasion in vitro, as well as tumor growth and
lung metastasis in vivo (13). In the present study, the expression
pattern and function of CNTN-1 in prostate cancer was investigated,
and the data suggested that the expression levels of CNTN-1 were
significantly higher in prostate cancer tissues compared with those
in adjacent paracancerous tissues. Moreover, upregulation of CNTN-1
expression was significantly associated with a larger tumor size, a
more advanced clinical stage and metastasis in patients with
prostate cancer. Consistent with the results of the present study,
Yan et al (13) reported that
the expression level of CNTN-1 was significantly higher in prostate
cancer cells from primary tumors, lymph nodes and bone metastases,
compared with paracancerous prostate gland tissues. The present
study indicated that patients with high-CNTN-1 expression exhibited
shorter overall survival times when compared with the
low-expression group, suggesting that CNTN-1 expression may be used
as a predictive biomarker of prostate cancer. Yan et al also
showed that following radical prostatectomy, CNTN1 expression was
associated with a shorter recurrence-free survival time in patients
with prostate cancer (13). In the
current study, transfection with shRNA was used to knockdown the
expression of CNTN-1 in prostate cancer cell lines, which resulted
in significantly reduced proliferation, colony formation, migration
and invasiveness. This further supports the hypothesis that CNTN-1
promotes the progression of prostate cancer, and suggests that
CNTN-1 may represent a promising therapeutic target for the
treatment of the disease. Yan et al (13) used the DU145 cell line to study the
function of CNTN-1 in vitro. In the present study, two
different cell lines (PC3 and LNCaP) were used, providing further
validation of these results (13).
Moreover, in the study conducted by Yan et al (13), only CNTN-1 cell invasion was
investigated. In the present study wound-healing assays were
performed to further elucidate the function of CNTN-1 in prostate
cancer cell migration.
EMT is tightly regulated by several internal and
external stimuli that orchestrate the transition from an
epithelial-like to a mesenchymal phenotype (15–17). EMT
facilitates tumor cell invasiveness and metastatic capacity, and is
thus a principal mediator of cancer progression and metastasis
(18–20). Yan et al (13) only detected the expression of
E-cadherin, and thus did not reveal the function of CNTN-1 in EMT
in prostate cancer. In the present study, elucidation of the role
of CNTN-1 in EMT was a key objective. Thus, the effect of
CNTN-1-knockdown significantly increased the expression of
E-cadherin, while inhibiting the expression of N-cadherin and
vimentin in prostate cancer cells, indicating that EMT was
suppressed. The current findings indicated that the CNTN-1-mediated
promotion of prostate cancer cell migration and invasion might be
the result of EMT regulation.
The PI3K/AKT signaling pathway promotes tumor cell
proliferation, migration and invasion, and EMT in multiple human
cancers (21–24). In the present study, it was
discovered that CNTN-1-knockdown significantly inhibited the
PI3K/AKT signaling pathway in prostate cancer cells. Similar
findings have also been reported in lung cancer. For instance,
Zhang et al (25) reported
that CNTN-1- enhanced chemoresistance in lung adenocarcinoma via
the promotion of EMT, by activating the PI3K/AKT signaling pathway.
Moreover, Yan et al reported that CNTN-1 inhibited
E-cadherin expression via the activation of AKT in lung cancer
(26). Therefore, the interaction
between CNTN-1 and the PI3K/AKT signaling pathway may represent a
commonality shared by multiple cancer types. Animal experiments may
help to further clarify the exact function, and validate the
regulatory mechanisms of CNTN-1 in prostate cancer, in
vivo.
In summary, the present study demonstrated that
CNTN-1 was significantly upregulated in prostate cancer compared
with adjacent paracancerous tissues, and that the knockdown of
CNTN-1 inhibited proliferation, migration, invasiveness and EMT in
prostate cancer cells. Taken together, the results suggest that
CNTN-1 may represent a potential therapeutic target for the
treatment of prostate cancer.
Acknowledgements
Not applicable.
Funding
Not applicable.
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
HL and BW designed the study and wrote the
manuscript. BW, XY, TZ, HD, TW and SZ performed all of the
experiments. BY conducted the statistical analysis. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of First Affiliated Hospital of Jishou University,
Jishou, China. Written informed consent was obtained from each
participant, prior to surgery.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Misawa A and Inoue S: Estrogen-related
receptors in breast cancer and prostate cancer. Front Endocrinol
(Lausanne). 6:832015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mao Y, Li K, Lu L, Si-Tu J, Lu M and Gao
X: Overexpression of Cdc20 in clinically localized prostate cancer:
Relation to high Gleason score and biochemical recurrence after
laparoscopic radical prostatectomy. Cancer Biomark. 16:351–358.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xiong W, Huang C, Deng H, Jian C, Zen C,
Ye K, Zhong Z, Zhao X and Zhu L: Oncogenic non-coding RNA NEAT1
promotes the prostate cancer cell growth through the SRC3/IGF1R/AKT
pathway. Int J Biochem Cell Biol. 94:125–132. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Guo W, Keener AL, Jing Y, Cai L, Ai J,
Zhang J, Fisher AL, Fu G and Wang Z: FOXA1 modulates EAF2
regulation of AR transcriptional activity, cell proliferation, and
migration in prostate cancer cells. Prostate. 75:976–987. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mikami T, Yasunaga D and Kitagawa H:
Contactin-1 is a functional receptor for neuroregulatory
chondroitin sulfate-E. J Biol Chem. 284:4494–4499. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lamprianou S, Chatzopoulou E, Thomas JL,
Bouyain S and Harroch S: A complex between contactin-1 and the
protein tyrosine phosphatase PTPRZ controls the development of
oligodendrocyte precursor cells. Proc Natl Acad Sci USA.
108:17498–17503. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Su JL, Yang CY, Shih JY, Wei LH, Hsieh CY,
Jeng YM, Wang MY, Yang PC and Kuo ML: Knockdown of contactin-1
expression suppresses invasion and metastasis of lung
adenocarcinoma. Cancer Res. 66:2553–2561. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yu JW, Wu SH, Lu RQ, Wu JG, Ni XC, Zhou
GC, Jiang HG, Zheng LH, Li XQ, Du GY and Jiang BJ: Expression and
significances of contactin-1 in human gastric cancer. Gastroenterol
Res Pract. 2013:2102052013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu P, Chen S, Wu W, Liu B, Shen W, Wang
F, He X and Zhang S: Contactin-1 (CNTN-1) overexpression is
correlated with advanced clinical stage and lymph node metastasis
in oesophageal squamous cell carcinomas. Jpn J Clin Oncol.
42:612–618. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu HM, Cao W, Ye D, Ren GX, Wu YN and Guo
W: Contactin 1 (CNTN1) expression associates with regional lymph
node metastasis and is a novel predictor of prognosis in patients
with oral squamous cell carcinoma. Mol Med Rep. 6:265–270.
2012.PubMed/NCBI
|
|
13
|
Yan J, Ojo D, Kapoor A, Lin X, Pinthus JH,
Aziz T, Bismar TA, Wei F, Wong N, De Melo J, et al: Neural cell
adhesion protein CNTN1 promotes the metastatic progression of
prostate cancer. Cancer Res. 76:1603–1614. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Singh M, Yelle N, Venugopal C and Singh
SK: EMT: Mechanisms and therapeutic implications. Pharmacol Ther.
182:80–94. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Smith BN and Bhowmick NA: Role of EMT in
metastasis and therapy resistance. J Clin Med. 5(pii): E172016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Beuran M, Negoi I, Paun S, Ion AD, Bleotu
C, Negoi RI and Hostiuc S: The epithelial to mesenchymal transition
in pancreatic cancer: A systematic review. Pancreatology.
15:217–225. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu Y, Sarkissyan M and Vadgama JV:
Epithelial-mesenchymal transition and breast cancer. J Clin Med.
5(pii): E132016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jiang Z, Song Q, Zeng R, Li J, Li J, Lin
X, Chen X, Zhang J and Zheng Y: MicroRNA-218 inhibits EMT,
migration and invasion by targeting SFMBT1 and DCUN1D1 in cervical
cancer. Oncotarget. 7:45622–45636. 2016.PubMed/NCBI
|
|
20
|
Barrette K, Van Kelst S, Wouters J,
Marasigan V, Fieuws S, Agostinis P, van den Oord J and Garmyn M:
Epithelial-mesenchymal transition during invasion of cutaneous
squamous cell carcinoma is paralleled by AKT activation. Br J
Dermatol. 171:1014–1021. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sathe A and Nawroth R: Targeting the
PI3K/AKT/mTOR pathway in bladder cancer. Methods Mol Biol.
1655:335–350. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Murthy D, Attri KS and Singh PK:
Phosphoinositide 3-kinase signaling pathway in pancreatic ductal
adenocarcinoma progression, pathogenesis, and therapeutics. Front
Physiol. 9:3352018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhao HF, Wang J, Shao W, Wu CP, Chen ZP,
To ST and Li WP: Recent advances in the use of PI3K inhibitors for
glioblastoma multiforme: Current preclinical and clinical
development. Mol Cancer. 16:1002017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang J, Yu XH, Yan YG, Wang C and Wang
WJ: PI3K/Akt signaling in osteosarcoma. Clin Chim Acta.
444:182–192. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang R, Sun S, Ji F, Liu C, Lin H, Xie L,
Yang H, Tang W, Zhou Y, Xu J and Li P: CNTN-1 enhances
chemoresistance in human lung adenocarcinoma through induction of
epithelial-mesenchymal transition by targeting the PI3K/Akt
pathway. Cell Physiol Biochem. 43:465–480. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yan J, Wong N, Hung C, Chen WX and Tang D:
Contactin-1 reduces E-cadherin expression via activating AKT in
lung cancer. PLoS One. 8:e654632013. View Article : Google Scholar : PubMed/NCBI
|