Introduction
Renal cell carcinoma (RCC) accounts for 2–3% of all
malignancies, with an annual increase of ~2% in the last 20 years
globally (1). Most new cases of RCC
are identified incidentally, and the improved detection is
attributed to the advancement in imaging examinations, such as
ultrasound (US) and computed tomography (CT). The vast majority of
newly diagnosed cases are small renal cell carcinoma, which is
classified clinically as stage T1a (cT1a) (2). The cT1a RCC is commonly associated with
favorable treatment outcomes, therefore, nephron-sparing
techniques, such as partial nephrectomy (PN), are recommended by
the European Association of Eurology guidelines on RCC (3). However, among those patients who
undergo PN for cT1a RCC, some may have their tumor upstaged to
pT3a, according to the final pathological examination results
(4), which causes a dilemma for
surgeons. On the basis of the 2009 Tumor-Node-Metastasis (TNM)
classification system, both perinephric fat invasion (PFI) and
renal sinus fat invasion (SFI) are defined as stage T3a for RCC
(5). However, there are still
controversies with regard to the prognosis of patients with T3a
RCC. An increasing number of studies have reported that patients
with stage T3a RCC and SFI have a significantly worse prognosis
compared with those with PFI (6–8). By
contrast, other studies have suggested no significant differences
in survival rates between patients with the two types of T3a RCC
(9,10). Thus, further investigations are
required in order to clarify the cause underlying the controversy.
The present study proposes a new sub-classification criterion for
pT3a RCC with SFI or PFI, in order to provide new insights on this
dispute.
Patients and methods
Patients
A retrospective analysis of the nephrectomy database
at The Second Hospital of Tianjin Medical University (Tianjin,
China), Binzhou Medical University Hospital (Binzhou, China) and
The People's Hospital of Liaocheng (Liaocheng, China) and
Yuhuangding Hospital of Qingdao University (Yantai, China) was
performed, after obtaining approval from the respective
Institutional Review Boards. In total, 2,765 patients were
included; each patient underwent PN for cT1a RCC between December
2001 and December 2015, and were diagnosed by US or CT methods. The
patients were excluded if they had familial or hereditary RCC,
solitary kidney RCC or synchronous RCC. Finally, a total of 127
cases were recruited for this cohort. All patients provided written
informed consent, and this study was conducted in accordance with
the Declaration of Helsinki.
Clinicopathological analysis
Following PN, surgical specimens were fixed with 10%
formalin for 24 h at room temperature. Subsequently, ink was used
to stain the edges of the specimens for one minute at room
temperature, and was fixed using 4% paraffin. The RCC specimens
were routinely sliced at a thickness of ~3 µm and stained with
hematoxylin-eosin 10 min at room temperature. The pathological
examination was carried out by two professional genitourinary
pathologists using and light microscope. The sections were
classified according to the 2009 TNM classification system.
Follow-up observation
Postoperative follow-up was conducted based on the
analysis of kidney and chest CT images 6 months after surgery, and
then annually until 5 years after surgery, followed by one
examination every 2 years. Magnetic resonance imaging or bone scans
were applied when clinically indicated. Moreover, local recurrence
of tumors, regional lymph nodes, and distant tissue and organ
metastases were monitored.
Identification of sample size
Based on our pilot data, the sample size was
estimated at a study power of 75% and a significance level of 5%
using the PASS11 software (NCSS, LLC). A sample size of at least 56
patients per group was suggested.
Statistical analysis
Pearson's χ2 and Fisher exact text were
performed to assess the categorical variables. The numerical
variables were analyzed using the Mann-Whitney U test. Kaplan-Meier
curves were plotted to estimate recurrence-free survival
probabilities, using the log-rank test. Multivariate cox regression
analysis was also applied to identify predictors of recurrence. All
statistical analyses were performed using the SPSS20 software (IBM
Corp.). P<0.05 was used to indicate a statistically significant
difference.
Results
In total, 1,659 males and 1,106 females with a
median age of 63 years (range, 38–82 years) underwent PN for a
solitary cT1 RCC. According to the pathological examination
results, 127 cases were upstaged to pT3a, showing a rate of 4.59%,
including 55 SFI cases and 72 PFI cases. The characteristics of the
127 selected patients are listed in Table I. Based on the analysis of the sample
sections that were pathologically determined as cT1a, a new
sub-classification criteria for pT3a RCC with SFI or PFI was
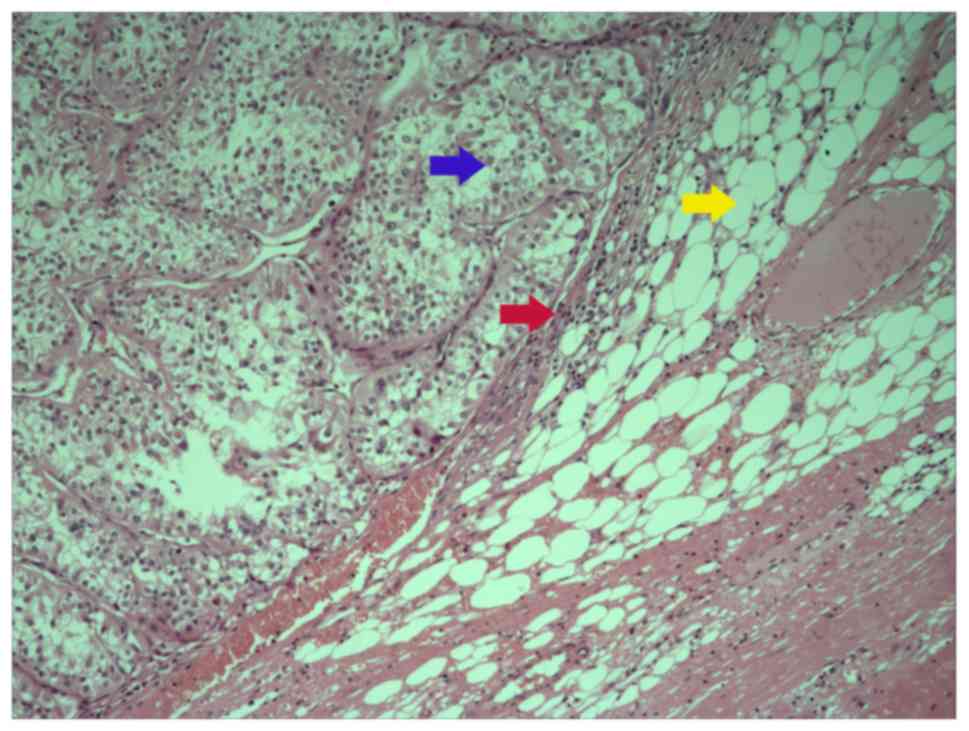
generated: i) Type A, renal tumor invades the pseudo-capsule and is
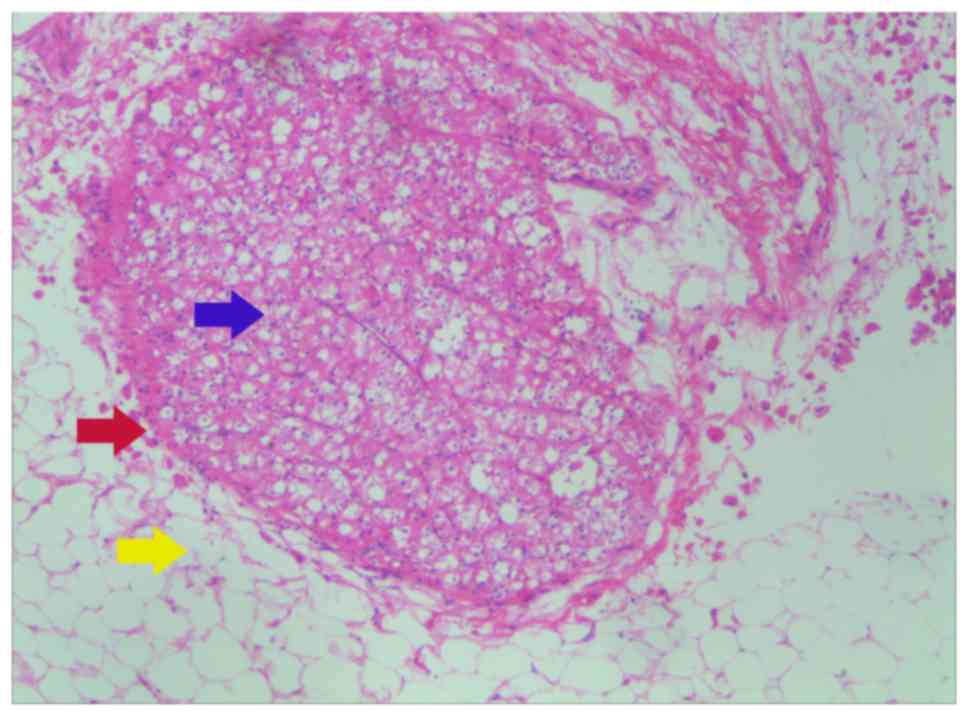
in contact with the perinephric adipose tissues directly (Fig. 1); type B, the tumor protrudes into
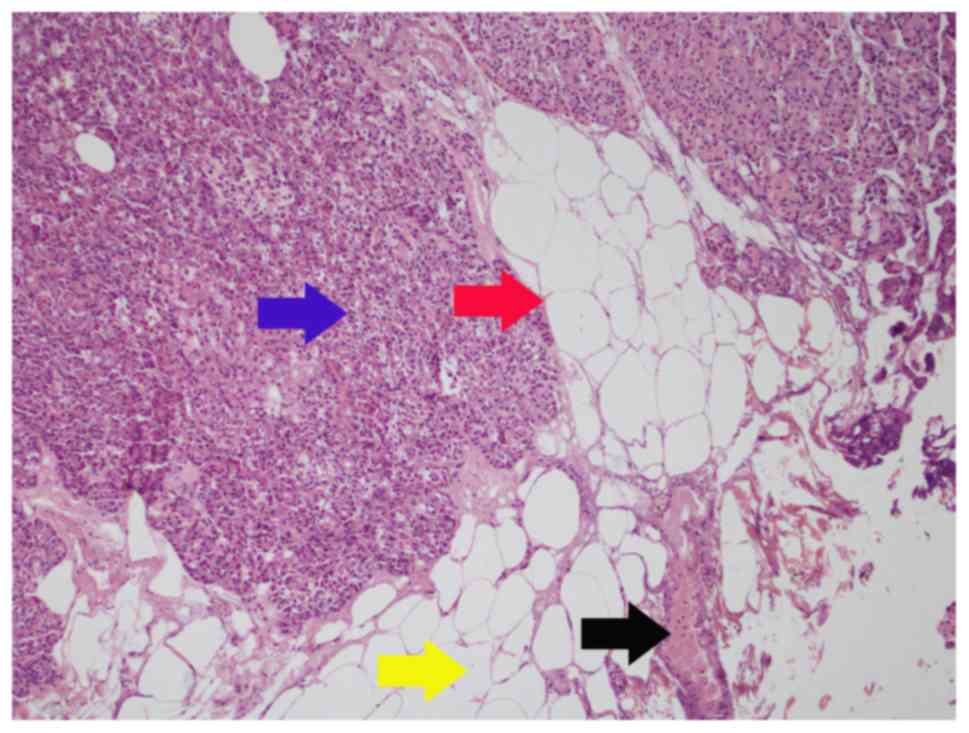
the perinephric adipose tissues like a tongue (Fig. 2); type C, tumor nodules distribute in
perinephric adipose tissues (Fig.
3).
 | Table I.Patient characteristics (n=127). |
Table I.
Patient characteristics (n=127).
| Variable | SFI | PFI | P-value |
|---|
| Patients, n | 55 | 72 |
|
| Sex, n |
|
| 0.37 |
| Male | 27 | 42 |
|
| Female | 28 | 30 |
|
| Age, years |
|
| 0.62 |
| Median | 64 | 61 |
|
| IQR | 38–79 | 49–82 |
|
| Surgical approach,
n |
|
| 0.26 |
| Open | 22 | 21 |
|
| Laparoscopic | 33 | 51 |
|
| Pathological tumor
size, cm |
|
| 0.22 |
| Median | 3.2 | 3.5 |
|
| IQR | 2.1–3.7 | 2.5–3.8 |
|
| Laterality, n |
|
| 0.86 |
| Left | 30 | 37 |
|
| Right | 25 | 35 |
|
| Tumor histological
type, n |
|
| 0.72 |
| Clear cell | 30 | 36 |
|
| Non-clear cell | 25 | 36 |
|
| R.E.N.A.L.
scorea |
|
| 0.13 |
| Median | 6.2 | 5.5 |
|
| IQR | 4–7 | 4–6 |
|
| Fuhrman grade, n |
|
| 0.85 |
| Low (I–II) | 20 | 25 |
|
| High (III–IV) | 35 | 47 |
|
| Margin status, n |
|
| 0.73 |
| Positive | 3 | 6 |
|
| Negative | 52 | 66 |
|
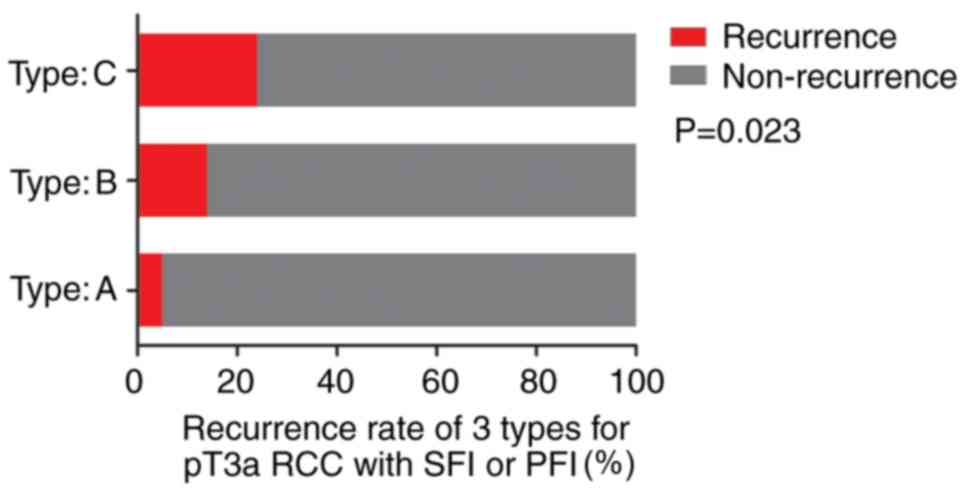
There were 57, 29 and 41 patients in the three
subtypes, respectively, with 3 (1 with SFI and 2 with PFI), 4 (2
with SFI and 2 with PFI) and 10 (4 with SFI and 6 with PFI) cases
of recurrences during follow-up. Notably, 2 patients demonstrated
distant metastases, both of which were of type C, showing a
statistically significant difference in the recurrence rate
(P=0.023; Fig. 4).
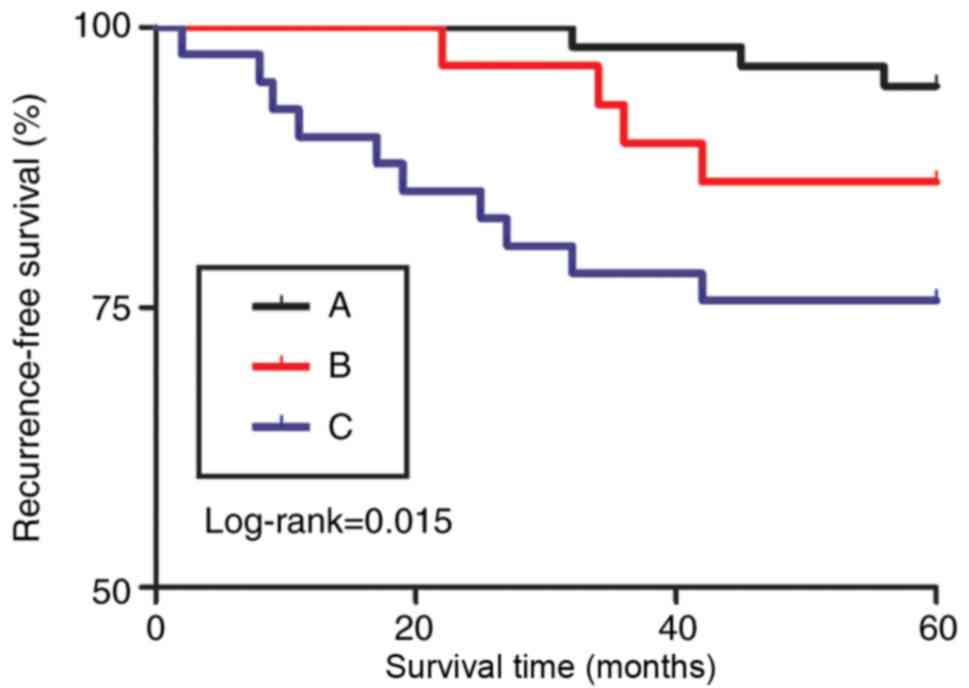
Kaplan-Meier curves also demonstrated consistent
results of the recurrence rates (P=0.015; Fig. 5). For PN, the difference in the
recurrence rates of SFI and PFI in the 3 types was not
statistically significant (Fig.
S1). In the multivariate cox regression analyses for predictors
of disease recurrence adjusted for age, sex, surgery approach,
tumor size, histology, Fuhrman grade (11), margin status and the
sub-classification presented in this study, the occurrence was
independently associated with sub-classification (P=0.021; Tables II; and P=0.009; SI).
 | Table II.Multivariate Cox regression analysis
for predictors of disease recurrence. |
Table II.
Multivariate Cox regression analysis
for predictors of disease recurrence.
| Variable | HR | 95% Confidence
interval | P-value |
|---|
| Sex |
|
|
|
|
Female | Ref. |
|
|
| Male | 0.947 | 0.660–1.358 | 0.767 |
| Age | 1.001 | 0.984–1.018 | 0.926 |
| Laterality |
|
|
|
|
Left | Ref. |
|
|
|
Right | 0.956 | 0.663–1.379 | 0.811 |
| Surgical
approach |
|
|
|
|
Open | Ref. |
|
|
|
Laparoscopic | 1.084 | 0.737–1.594 | 0.682 |
| Tumor
size | 1.073 | 0.500–2.303 | 0.856 |
| Histological
type |
|
|
|
|
Non-clear cell | Ref. |
|
|
| Clear
cell | 1.093 | 0.757–1.577 | 0.635 |
| Fuhrman grade |
|
|
|
| Low
(I–II) | Ref. |
|
|
| High
(III–IV) | 0.935 | 0.641–1.364 | 0.728 |
| Margin status |
|
|
|
|
Negative | Ref. |
|
|
|
Positive | 0.977 | 0.491–1.944 | 0.947 |
|
Sub-classification |
|
|
|
| Type
A | Ref. |
|
|
| Type
B | 0.970 | 0.645–1.460 | 0.884 |
| Type
C | 2.032 | 1.121–3.686 | 0.021 |
Discussion
It is recognized that both PFI and SFI belong to the
T3a stage in RCC, since the preoperative imaging examination cannot
distinguish the micro-tumor invasion of perinephric fat. Srivastava
et al (12) assessed 28,854
RCC patients undergoing PN, and found that 4.2% of patients with
cT1a tumors were upstaged to pT3a between 1998 and 2003. A number
of other studies reported a similar value, such as those by Gorin
et al (13), Weight et
al (14) and Jeong et al
(15), which reported the rates of
3.9, 4.8 and 5.7%, respectively. The rate in the present study was
4.5%, which was similar to that of the aforementioned studies. It
is apparent that although the pathological upstaging of RCC after
surgery is rare, it still occurs, and thus, further studies on this
phenomenon are required.
Only a few relevant studies could be retrieved on
the two types of pathological findings of fat invasion (SFI and
PFI) in T3a RCC. Bonsib et al (16) suggested that SFI could lead to a
worse prognosis, from an anatomical perspective. The reasons were
as follows: Firstly, there is no renal capsule surrounding the
renal sinus. As the capsule serves as a barrier to extra-renal
spread, tumors with SFI are more likely to spread. Secondly, the
renal sinus is rich in veins and lymphatic vessels. Conversely, the
density of micrangium and lymphatics in perinephric fat is much
lower than that in the sinus fat, and thus, the occurrence of SFI
increases the opportunity for tumor invasion. Another study
involving 205 patients with RCC, including 162 patients with PFI
and 43 patients with SFI, reported death rates of 59% (n=95) and
72% (n=31) during follow-up, respectively. This indicated that the
occurrence of SFI was more likely to cause death from RCC compared
with that of PFI [risk ratio (RR), 1.63; 95% CI, 1.09–2.46;
P=0.018)] (6). However, Margulis
et al (10) found that there
was no difference between SFI and PFI with regard to 5-year
cancer-specific survival rate (50.8 and 54.1%, respectively;
P=0.782). The study involved a total of 365 patients with pT3a RCC,
among whom 166 cases were diagnosed with SFI and 199 with PFI,
after a mean follow-up time of 33.5 months (range, 6.1–158.6
months). Patients with SFI and PFI therefore had similar clinical
and pathological characteristics, and tumors with SFI were more
likely to involve the urothelial collecting system, due to the
proximity to the renal pelvis, rather than distant metastasis.
The cause of conflicting results should be further
explored, as there may be other factors contributing to the
different outcomes. In the present study, after thorough
examination of the pathological findings by microscopy, T3a RCC fat
invasion with SFI and PFI was divided into three subtypes, as
aforementioned. We speculate that the aforementioned conflicts may
be attributed to failure to distinguish between these three
situations.
The first subtype is characterized by a tumor that
invades the pseudo-capsule and contacts with the perinephric
adipose tissues directly. In the present study, among the 127
patients, 57 were classified as this subtype. During the follow-up,
only 3 patients relapsed. Consequently, if the adipose tissue
covering the tumor surface can be removed during PN, the tumor can
be removed completely and the recurrence rate could be reduced. A
previous study, including 1,367 patients with small RCC, reported
the 5-year recurrence-free survival rates of patients with pT1a RCC
and pT3a RCC as 98.0 and 95.2%, respectively (P=0.521) (17). Thus, patients with pT3a RCC at the
pT1a stage have similar oncological outcomes following PN,
suggesting that PN is suitable for T3a RCC (17). Oh et al (18) analyzed the records of 3,567 patients
who underwent PN and the observations made during a median 43-month
follow-up period, and found that PN offers similar recurrence-free
survival outcomes compared with radical nephrectomy in patients
with cT1a but not pT3a RCC. In the present study, the T3a RCC
classified into type A may also be attributed to the possibility of
PN in T3a RCC reported in aforementioned literatures. Whether it is
SFI or PFI, the tumor can be removed completely during PN.
Renal tumors that have protruded into the
perinephric adipose tissues like a tongue are defined as the second
subtype. During surgery, if the surface adipose tissues are
improperly removed, the residual tumor that has protruded into the
surrounding fat may lead to the occurrence of a positive surgical
margin, which may result in tumor recurrence and even tumor
metastasis (19). Therefore, during
PN, surgeons should not excessively remove the fat on the surface
of the tumor to prevent the positive surgical margin, particularly
in the case of irregularly shaped tumors.
The third pathological subtype is a rare type
characterized by tumor nodules that are distributed in the
perinephric adipose tissues. The tumor nodules are like islands
distributed among the perinephric or the renal sinus adipose
tissues. After PN, despite the complete removal of adipose tissues
covering the kidney surface, it is still difficult to completely
remove the tumor nodules that are scattered in the perinephric fat,
as they are invisible to the naked eye. Thus, a radical resection
should be performed for this subtype. However, since the tumor
nodules cannot be detected by imaging before surgery, cT1a RCC is
commonly treated by PN. Therefore, close follow-up is recommended
with the upstaging of pT3a RCC in this type. Radical surgery should
be performed immediately upon the recurrence after surgery.
The present study has several potential limitations.
The study is retrospective with a relatively small sample size from
four institutions. Prospective, large sample size studies are
required for further research. Secondly, no differences were
observed between low-grade (grades 1–2) and high-grade (grades 3–4)
tumors in the present study (P=0.728), although another study has
demonstrated similar results (17).
The most widely used RCC assessment is the Fuhrman grading system
(11) released in 1982. Although
widely used, the grading system was based solely on the analysis of
103 renal cancer cases, of which only 85 were followed up, and did
not consider the histological classification of the RCC. In
practical applications, the grading system has problems, such as
difficulty in interpretation and poor repeatability. Therefore, the
system was replaced by the International Society of Urologic
Pathologists (ISUP) grade in 2016 (20). The ISUP grade will be used in future
studies. Thirdly, due to the small sample size, tumors were broadly
divided into clear cell and ‘non-clear cell’ categories, which may
introduce bias.
Regardless of the aforementioned limitations, the
present study demonstrated the association between the
classification of fat invasion in T3a RCC and tumor recurrence
after PN, for cT1a RCC upstaging to pT3a. We recommend that the
pathological examination results of patients with RCC should be
carefully evaluated and patients with pT3a RCC should be assessed
again individually with regards to fat invasion.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was supported by the Tianjin Municipal
Natural Science Foundation (grant no. 17JCYBJC26000) and the
Shandong Provincial Medical Health Technology Development Project
(grant no. 2014WS0189).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
GL and DSZ conceived and directed the project. DSZ
contributed to the writing of the manuscript. DSZ, GL and TG
analyzed the data. DSZ, JYC, YHL, CZ and ZQL collected the clinical
data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study protocols were approved by the Ethical
Committee Review Board of the Second Hospital of Tianjin Medical
University (Tianjin, China). All participants provided written
informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferlay J, Steliarova-Foucher E,
Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, Forman D and
Bray F: Cancer incidence and mortality patterns in Europe estimates
for 40 countries in 2012. Eur J Cancer. 49:1374–1403. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Laguna MP, Algaba F, Cadeddu J, Clayman R,
Gill I, Gueglio G, Hohenfellner M, Joyce A, Landman J, Lee B, et
al: Current patterns of presentation and treatment of renal masses
A clinical research office of the endourological society
prospective study. J Endourol. 28:861–170. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ljungberg B, Bensalah K, Canfield S,
Dabestani S, Hofmann F, Hora M, Kuczyk MA, Lam T, Marconi L,
Merseburger AS, et al: EAU guidelines on renal cell carcinoma 2014
update. Eur Urol. 67:913–924. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ramaswamy K, Kheterpal E, Pham H, Mohan S,
Stifelman M, Taneja S and Huang WC: Significance of pathologic T3a
upstaging in clinical T1 renal masses undergoing nephrectomy. Clin
Genitourin Cancer. 13:344–349. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Martinez-Salamanca JI, Huang WC, Millan I,
Bertini R, Bianco FJ, Carballido JA, Ciancio G, Hernández C,
Herranz F, Haferkamp A, et al: Prognostic impact of the 2009
UICC/AJCC TNM staging system for renal cell carcinoma with venous
extension. Eur Urol. 59:120–127. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Thompson RH, Leibovich BC, Cheville JC,
Webster WS, Lohse CM, Kwon ED, Frank I, Zincke H and Blute ML: Is
renal sinus fat invasion the same as perinephric fat invasion for
pT3a renal cell carcinoma. J Urol. 174:1218–1221. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen K, Lee BL, Huang HH, Tan BY, Lee LS,
Ng LG, Lau W and Yuen JS: Tumor size and Fuhrman grade further
enhance the prognostic impact of perinephric fat invasion and renal
vein extension in T3a staging of renal cell carcinoma. Int J Urol.
24:51–58. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang Z, Yu C, Velet L, Li Y, Jiang L and
Zhou F: The difference in prognosis between renal sinus fat and
perinephric fat invasion for pT3a renal cell carcinoma A
meta-analysis. PLoS One. 11:e01494202016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mouracade P, Dagenais J, Chavali JS, Kara
O, Nelson RJ, Maurice MJ, Reese J, Rini BI and Kaouk JH:
Perinephric and sinus fat invasion in stage pT3a tumors managed by
partial nephrectomy. Clin Genitourin Cancer. 16:e1077–e1082. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Margulis V, Tamboli P, Matin SF, Meisner
M, Swanson DA and Wood CG: Location of extrarenal tumor extension
does not impact survival of patients with pT3a renal cell
carcinoma. J Urol. 178:1878–1882. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fuhrman SA, Lasky LC and Limas C:
Prognostic significance of morphologic parameters in renal cell
carcinoma. Am J Surg Pathol. 6:655–663. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Srivastava A, Patel HD, Joice GA,
Semerjian A, Gorin MA, Johnson MH, Allaf ME and Pierorazio PM:
Incidence of T3a up-staging and survival after partial nephrectomy
Size-stratified rates and implications for prognosis. Urol Oncol.
36:12.e7–12.e13. 2018. View Article : Google Scholar
|
|
13
|
Gorin MA, Ball MW, Pierorazio PM, Tanagho
YS, Bhayani SB, Kaouk JH, Rogers CG, Stifelman MD, Khalifeh A,
Kumar R, et al: Outcomes and predictors of clinical T1 to
pathological T3a tumor up-staging after robotic partial nephrectomy
A multi-institutional analysis. J Urol. 190:1907–1911. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Weight CJ, Lythgoe C, Unnikrishnan R, Lane
BR, Campbell SC and Fergany AF: Partial nephrectomy does not
compromise survival in patients with pathologic upstaging to
pT2/pT3 or high-grade renal tumors compared with radical
nephrectomy. Urology. 77:1142–1146. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jeong SH, Kim JK, Park J, Jeon HJ, Yoon
MY, Jeong CW, Ku JH, Kim HH and Kwak C: Pathological T3a upstaging
of clinical T1 renal cell carcinoma outcomes according to surgical
technique and predictors of upstaging. PLoS One. 11:e01661832016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bonsib SM, Gibson D, Mhoon M and Greene
GF: Renal sinus involvement in renal cell carcinomas. Am J Surg
Pathol. 24:451–458. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee C, You D, Yoo S, Song C, Hong B, Hong
JH, Ahn H and Kim CS: Oncological outcomes of patients with
incidental pathological T3a stage small renal cell carcinoma after
partial nephrectomy. J Cancer Res Clin Oncol. 142:1651–1657. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Oh JJ, Byun SS, Lee SE, Hong SK, Lee ES,
Kim HH, Kwak C, Ku JH, Jeong CW, Kim YJ, et al: Partial nephrectomy
versus radical nephrectomy for non-metastatic pathological T3a
renal cell carcinoma A multi-institutional comparative analysis.
Int J Urol. 21:352–357. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Park M, Shim M, Kim M, Song C, Kim CS and
Ahn H: Prognostic heterogeneity in T3aN0M0 renal cell carcinoma
according to the site of invasion. Urol Oncol. 35:458.e17–458.e22.
2017. View Article : Google Scholar
|
|
20
|
Moch H: The WHO/ISUP grading system for
renal carcinoma. Der Pathologe. 37:355–360. 2016.(In German).
View Article : Google Scholar : PubMed/NCBI
|