Introduction
Bladder cancer (BC) is a common malignancy with an
incidence rate four times higher in males than in females (1,2). In
2018, GLOBOCAN statistics revealed a total of 549,393 new cases of
bladder cancer, accounting for ~3.0% of all cancer cases, and
resulting in ~199,922 mortalities (2.1% of all cancer-associated
mortalities) worldwide (3). Tobacco
smoking is a major risk factor for BC (4). Besides that, other factors, such as
infections of Schistosoma haematobium, are also closely
associated with the tumorigenesis of BC (1,4).
However, the molecular pathogenesis of BC is yet to be elucidated
(5), and this represents a major
challenge in the development of novel preventative and therapeutic
approaches.
Genetic alterations are frequently observed in
patients with BC (6,7). Rho associated coiled-coil containing
protein kinase 2 (ROCK2) is a serine/threonine kinase that serves a
critical role in smooth muscle contraction and cytokinesis
(8). ROCK2 is also implicated in
cancer biology and influences the metastasis of BC via the
promotion of cancer cell migration and invasiveness (9,10). In
effect, inactivation of ROCK2 signaling represents a promising
target for cancer treatment (9). It
is well established that certain miRNAs, such as miR-381, serve
tumor-suppressive roles via the inhibition of ROCK2 (11). Epidermal growth factor
receptor-antisense RNA 1 (EGFR-AS1) is a recently identified
oncogenic lncRNA in several types of cancer, such as liver cancer
(12). In the present study,
bioinformatics analysis indicated that EGFR-AS1 may interact with
miR-381 by forming base pairing between the complementary
sequences. Therefore, it was hypothesized that EGFR-AS1 may
interact with miR-381 and indirectly regulate ROCK2, thereby
influencing the progression BC. Therefore, the current study was
performed to investigate the possible interactions between EGFR-AS1
and miR-381 in BC and explore the consequent effects on the
expression level of ROCK2.
Materials and methods
Patients
A total of 145 patients with BC were admitted to The
Second People's Hospital of Liaocheng (Linqing, China) between
January 2011 and April 2014. Of these patients, 70 cases [52 males
and 18 females; 53.1±6.0 (SEM) years; range, 41–67 years]. The
present study was approved by the Ethics Committee of The Second
People's Hospital of Liaocheng (Linqing, China). Patients were
selected according to the following inclusion criteria: i) Newly
diagnosed BC cases; and ii) treatment was completed and 5 year
follow-up was conducted. The exclusion criteria were: i) Recurrent
cases of BC; ii) patients had been transferred from other
hospitals; iii) clinical disorders other than BC were diagnosed;
and iv) therapies had been initiated prior to patient admission.
All patients were informed of the design of experiments and the
potential publication of this paper, and written form informed
consent was provided by all participants. According to clinical
findings, all patients were staged in line with the guidelines
established by the American Joint Committee on Cancer (13). There were 8, 17, 21 and 24 cases at
stage I–IV, respectively.
BC tissue specimens and cell line
Under the guidance of MRI, bladder biopsy was
performed on all BC patients (prior to the initiation of any
therapies) to retrieve BC tumor tissues and adjacent paracancerous
tissues (collected ≤3 cm from the tumor border) from each BC
patient. All tissue samples were verified via histopathological
biopsy. Fresh tissues were stored in liquid nitrogen before
use.
The human BC cell line HT-1197 (American Type
Culture Collection) was used. Cells were cultured in a mixture of
10% FBS (Sigma-Aldrich; Merck KGaA) and 90% Eagle's Minimum
Essential Medium (Sigma-Aldrich; Merck KGaA). The culture
conditions were 95% humidity, 5% CO2 and 37°C.
Therapies and follow-up
Patients were subjected to different therapies
according to their conditions. Therapies included surgical
resection, chemotherapy, radiotherapy or targeted therapy.
Initiating at the day of admission, all patients were followed-up
for 5 years. The following patients were excluded from the survival
analysis: i) Patients who experienced mortality due to other
causes; ii) patients that were unwilling to complete the
follow-up.
RNA-RNA interaction prediction
The interactions between miR-381 and EGFR-AS1 by
base pairing were predicted by IntaRNA 2.0 (http://rna.informatik.uni-freiburg.de/IntaRNA/Input.jsp).
The sequence of EGFR-AS1 was used as the long sequence and the
sequence of miR-381 was used as the short sequence. All other
parameters were default.
Transient transfections
HT-1197 cells were harvested at ~80% confluency and
Lipofectamine 2000 (Sigma-Aldrich; Merck KGaA) was used to
transfect the following vectors or miRNAs into 1×106
cells: i) 10 nM EGFR-AS1 or ROCK2 expression vector (empty vector
as the negative control, NC); and ii) 50 nM miRNA
(5′-AGCGAGGUUGCCCUUUGUAUAU-3′) with NC miRNA
(5′-GUAGCUCGUGAUGCAUACGUGU-3′, targest no genes in the human
genome) as NC group. EGFR-AS1 and ROCK2 expression vectors were
constructed using pcDNA3.1 vector (Sangon Biotech Co., Ltd.). NC
miRNA and miR-381 mimic were purchased from Guangzhou Ribobio Co.,
Ltd. In all groups, untransfected cells were used as control cells.
Cells were harvested at 24 h post-transfection to be used in
subsequent analyses.
Total RNA extractions and
treatments
All tissue samples (0.05 g, both BC and non-tumor)
were ground in liquid nitrogen. Total RNAs in tissue samples and
1×105 HT-1197 cells (collected at 24 h
post-transfection) were extracted using RNAzol (Sigma-Aldrich;
Merck KGaA). In the precipitation step, 85% of ethanol was used to
harvest miRNAs. All RNA samples were digested with DNase I for 2 h
at 37°C to remove genomic DNAs.
Quantitative PCR (qPCR)
Total RNA was reverse transcribed using Tetro
Reverse Transcriptase (Bioline; Meridian Bioscience Inc.) following
manufacturer's protocol. To measure the expression levels of
EGFR-AS1 and ROCK2 mRNA, all qPCR assays were performed using TB
Green Advantage qPCR Premix from Clontech Laboratories, Inc.
following the manufacturer's protocol. GAPDH was used as an
endogenous control. The measure the expression level of mature
miR-381, the addition of poly (A) was performed, followed by miRNA
reverse transcription and qPCR assays. All these steps were
completed using All-in-One™ miRNA qRT-PCR Detection kit
(GeneCopoeia, Inc.), following manufacturer's protocol. The
following primer sequences were used: EGFR-AS1 forward,
5′-GGCCATCACGTAGGCTTCC-3′ and reverse, 5′-GCGTCTTCACCTGGAAGGG-3′;
ROCK2 forward, 5′-TGATTGGTGGTCTGTAG-3′ and reverse,
5′-CTGCCGTTTCTCTTATG-3′; and GADPH forward,
5′-ACAACTTTGGTATCGTGGAAG-3′ and reverse, 5′-GCCATCACGCCACAGTTT-3′.
Universal miRNA reverse primer and U6 primers were from the
All-in-One™ miRNA qRT-PCR Detection kit. All Cq values were
normalized using the 2−ΔΔCq method (14), and all PCR reactions were repeated 3
times.
Western blot analysis
To measure the expression levels of ROCK2 in HT-1197
cells, HT-1197 cells were harvested at 24 h post-transfection and
cells were counted. Total protein was extracted from
1×105 cells using RIPA solution, and quantified using a
bicinchoninic acid assay (both Sangon Biotech Co., Ltd.). Protein
denaturation was performed in boiling water for 10 min, followed by
SDS-PAGE electrophoresis, which was performed on a 12% gel with 30
µg of protein sample per lane. Subsequently, proteins were
transferred to PVDF membranes, and blocking was performed in 5%
non-fat milk with PBS (Sigma-Aldrich; Merck KGaA) for 2 h at room
temperature. The membranes were first incubated with rabbit
anti-ROCK2 (1:1,600; cat. no. ab71598) and anti-endogenous control
GAPDH (1:1,300; cat. no. ab37168; both Abcam) for 18 h at 4°C,
followed by incubation with goat HRP (IgG; 1:1,300; cat. no.
ab6721; Abcam) secondary antibody for 2 h at 22°C. After that,
membranes were incubated with an enhanced chemiluminescence kit
(Sigma-Aldrich; Merck KGaA) for 15 min at 22°C to develop the
signals. The Image J v1.46 software (National Institutes of Health)
was used to normalize signals.
Transwell assay
Transwell assays were performed to analyze the
effects of transfections on the invasion and migration of HT-1197
cells. To mimic in vivo invasion, Transwell membranes were
coated with Matrigel (Corning, Inc.) for 6 h at 37°C, prior to the
invasion assay. To prepare single-cell suspensions, 1 ml serum-free
Eagle's Minimum Essential Medium was used to resuspend
3×103 transfected cells. The suspension was then plated
in the upper Transwell chamber (96-well, 0.1 ml per well), and the
lower chamber was filled with a mixture of 80% Eagle's Minimum
Essential Medium and 20% FBS. Cells were cultivated under the
aforementioned conditions for 16 h. Subsequently, cells were
stained using 0.5% crystal violet (Sigma-Aldrich; Merck KGaA) at
22°C for 20 min. Cells were observed under a light microscope
(magnification ×40) and quantified using Image J v1.46
software.
Statistical analysis
Three biological replicates were included in each
experiment and mean values were calculated and used in all data
analyses. The GraphPad Prism 6 software (GraphPad Software, Inc.)
was used for statistical analysis. Associations were analyzed using
linear regression. To perform survival analysis, the 70 BC patients
were divided into high and low (both n=35) EGFR-AS1 level groups
with the median level of EGFR-AS1 in patients with BC used as the
cut-off value (4.23). The Kaplan-Meier plotter and the log-rank
test were used to plot and compare survival curves. Differences
were explored between two tissue types or among cell transfection
groups by paired Student's t-test and one-way ANOVA (followed by
Tukey's post-hoc test), respectively. The χ2 test was
used to compare clinical stages between 2 groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
EGFR-AS1 and ROCK2 mRNA were
upregulated and positively associated in BC
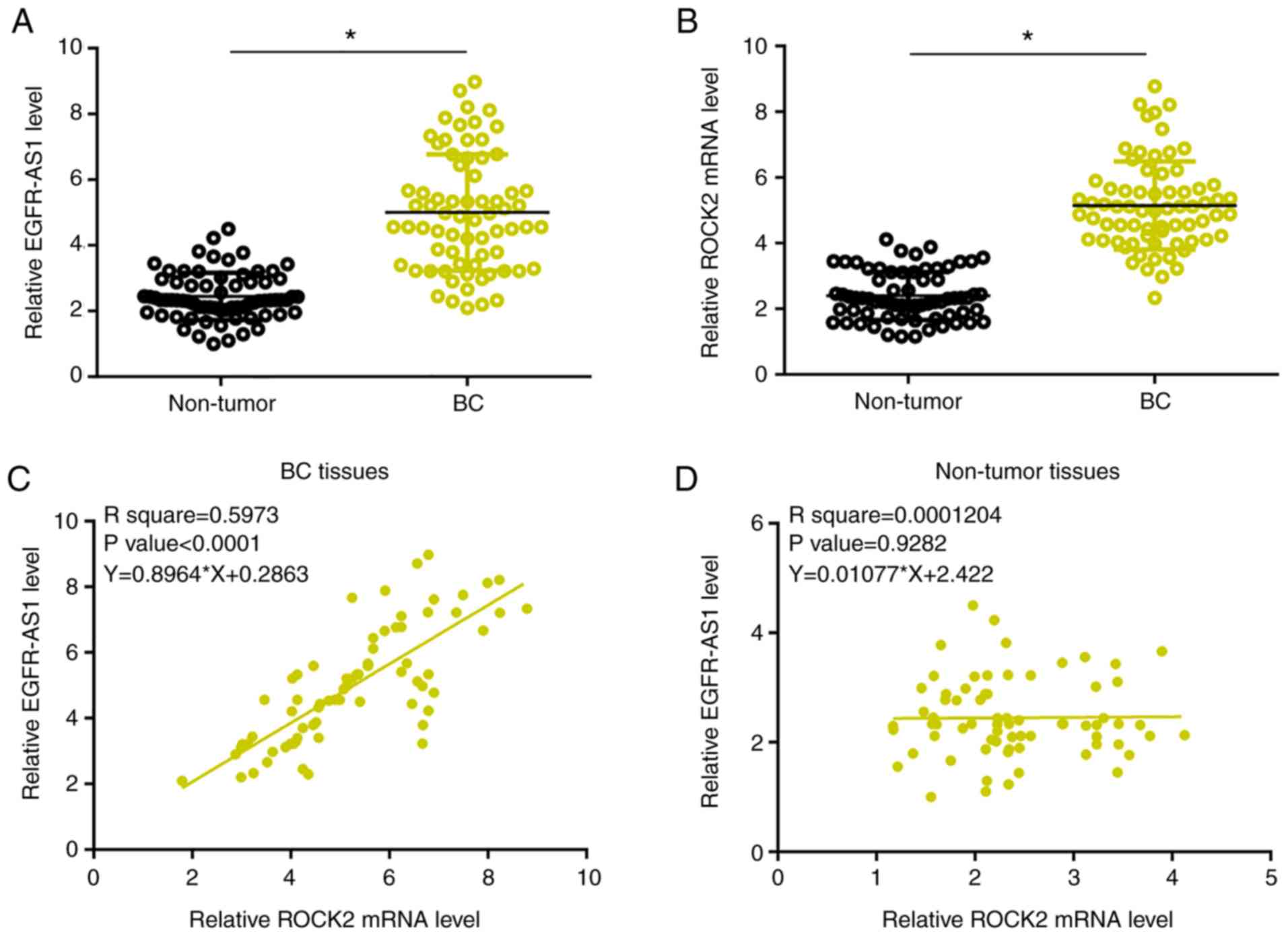
Expression levels of EGFR-AS1 and ROCK2 mRNA were
measured and compared between BC and adjacent paracancerous tissues
by performing qPCR and a paired t-test. Compared with paracancerous
tissues, significantly higher EGFR-AS1 (Fig. 1A) and ROCK2 mRNA levels were observed
in BC tissues (Fig. 1B; P<0.05).
Associations between EGFR-AS1 and ROCK2 mRNA expression were
analyzed using linear regression. Expression levels of EGFR-AS1
were significantly and positively associated with expression levels
of ROCK2 mRNA in BC tissues (P<0.0001; Fig. 1C). Moreover, the interaction between
them was not significant in the paracancerous tissues (Fig. 1D).
EGFR-AS1 may interact with miR-381 but
failed to regulate its expression
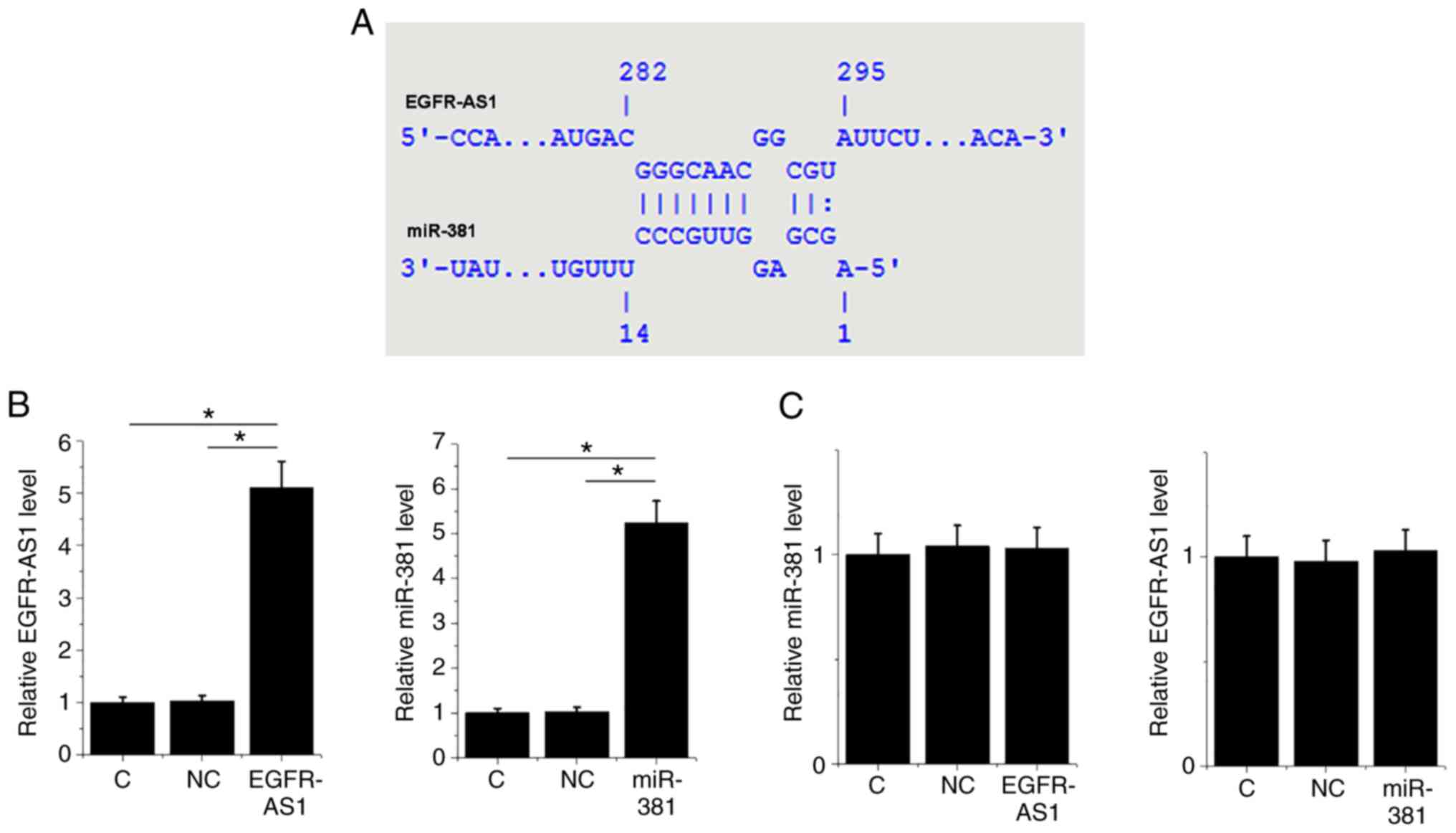
It is known that miR-381 is able to target ROCK2
(11). A bioinformatics analysis
performed using IntaRNA 2.0 (http://rna.informatik.uni-freiburg.de/IntaRNA/Input.jsp)
revealed that miR-381 can form a base pairing with EGFR-AS1
(Fig. 2A). To further analyze the
interactions between them, HT-1197 cells were transfected with
either a miR-381 mimic or EGFR-AS1 expression vector.
Overexpression of miR-381 and EGFR-AS1 was confirmed by qPCR at 24
h post-transfection. Compared with the control and NC groups,
expression levels of miR-381 and EGFR-AS1 mRNA were significantly
elevated post-transfection (Fig. 2B;
P<0.05). However, overexpression of miR-381 and EGFR-AS1 did not
influence the expression of each other (Fig. 2C).
EGFR-AS1 may sponge miR-381 to
upregulate ROCK2
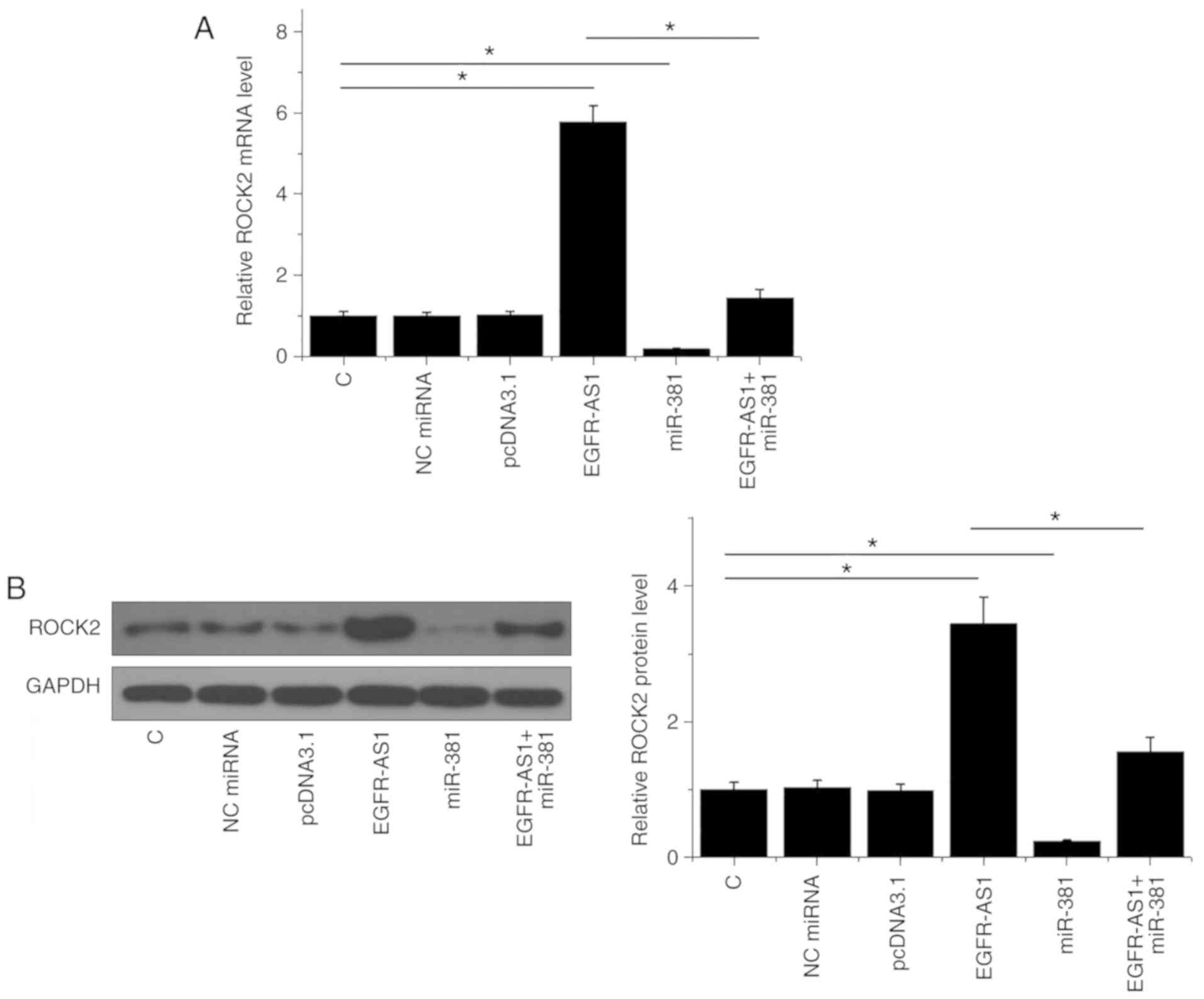
Western blotting and qPCR were performed to analyze
the effects of miR-381 and ROCK2 overexpression on ROCK expression
at the mRNA and protein level, respectively. Compared with the
control and NC (NC miRNA or empty pcDNA3.1) groups, EGFR-AS1
overexpression mediated the upregulation of ROCK2 at both mRNA
(P<0.05; Fig. 3A) and protein
(P<0.05; Fig. 3B) levels, while
miR-381 mediated the downregulation of ROCK2 and attenuated the
effects of EGFR-AS1 overexpression (P<0.05).
High expression levels of EGFR-AS1 in
BC predicted poor survival
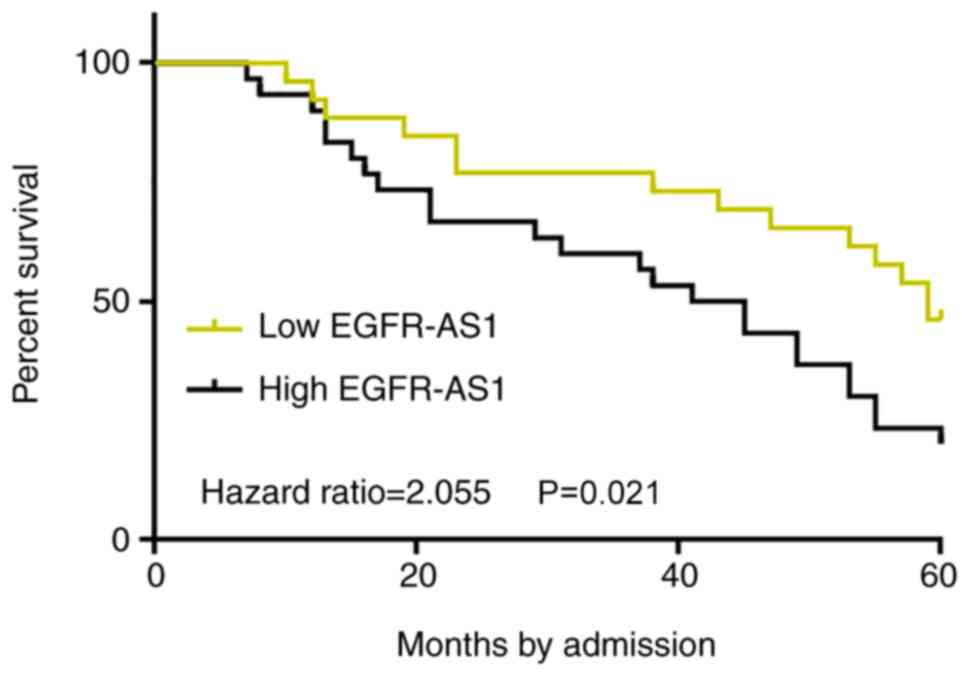
Survival curves were plotted and compared through
the aforementioned methods. Low EGFR-AS1 level group included 3,
10, 12 and 10 cases at stage I–IV, respectively. High EGFR-AS1
level group included 5, 7, 9 and 14 cases at stage I–IV,
respectively. No significant differences in clinical stages were
found between two groups (χ2 test, P=0.55). Treatments
are mainly determined by clinical stages. Therefore, it is likely
that no significant differences in therapeutic approaches would
exist between high and low EGFR-AS1 level groups. Notably, compared
with patients in the low-EGFR-AS1 expression level group, the
overall survival rate of patients in the high-EGFR-AS1 expression
level group was significantly lower (P=0.021; Fig. 4). The current data indicated that the
upregulation of EGFR-AS1 in patients with BC predicted poor
survival.
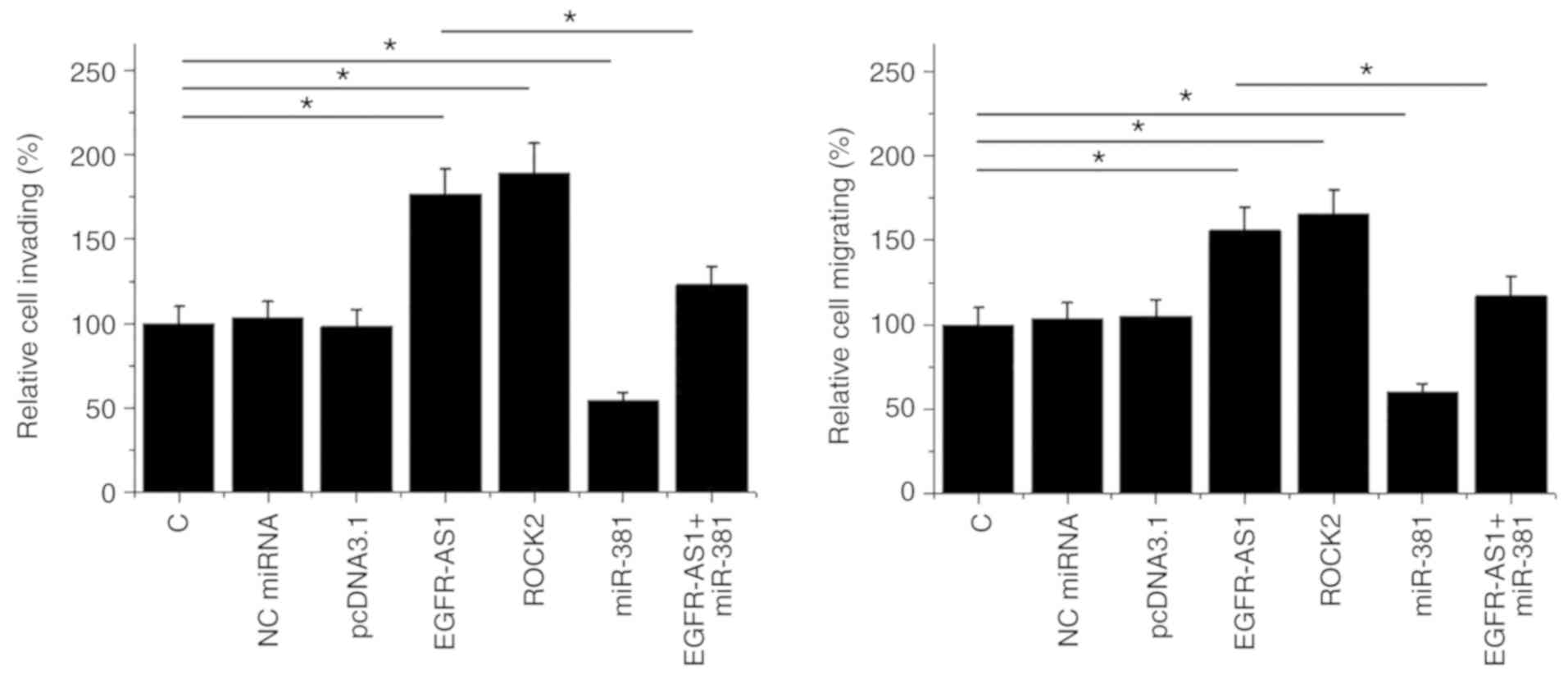
EGFR-AS1 promotes HT-1197 cell
invasion and migration via miR-381 and ROCK2
The effect of transfections on the invasion and
migration of HT-1197 cells were analyzed by Transwell assays. ROCK2
overexpression was achieved after transfection (Fig. S1). Compared with the control and NC
(NC miRNA or empty pcDNA3.1) groups, EGFR-AS1, and ROCK2
overexpression promoted cancer cell invasion (Fig. 5A) and migration (Fig. 5B). In addition, miR-381
overexpression played the opposite role and attenuated the effects
of EGFR-AS1 overexpression (P<0.05).
Discussion
The present study predominantly investigated the
role of EGFR-AS1 in BC. It was revealed that EGFR-AS1 was
upregulated in BC and predicted poor survival. In addition, it was
also determined that EGFR-AS1 may act as a sponge for miR-381 and
consequently upregulate ROCK2 expression, promoting cancer cell
invasion and migration abilities.
The roles of EGFR-AS1 have been investigated in
several types of malignancies. In both hepatocellular carcinoma and
gastric cancer, EGFR-AS1 is upregulated, and can in turn upregulate
the expression of epidermal growth factor receptor (EGFR) by
increasing the stability of EGFR mRNA, thereby promoting cancer
cell proliferation (12,15). Moreover, in squamous cell carcinoma
it was observed that EGFR-AS1 can mediate EGFR addiction and
regulate therapeutic responses (16). The present study is the first to
report the overexpression of EGFR-AS1 in BC. In addition,
accelerated cancer cell invasion and migration following EGFR-AS1
overexpression. Furthermore, high levels of EGFR-AS1 were found to
be significantly associated with the poor survival of BC patients.
The current data suggest that EGFR-AS1 serves as an oncogenic
lncRNA in BC. Notably, a preliminary CCK-8 assay revealed that
EGFR-AS1 exerted no significant effects on the proliferation of BC
cells (data not shown). Therefore, EGFR-AS1 may have different
functions dependent on the cancer type.
All previous studies on EGFR-AS1 focused on its
interactions with EGFR (12,15,16). In
the present study, it was revealed that EGFR-AS1 may form base
pairing with miR-381, which is a well-characterized
tumor-suppressive miRNA in many types of cancer, such as epithelial
ovarian cancer (17) and breast
cancer (18). In a recent study, Xie
et al (11) reported that
miR-381 can directly target ROCK2 to suppress the progression of
gastric cancer. In the current study an association was observed
between the downregulation of ROCK2 in BC cells and miR-381
overexpression, indicating that miR-381 may also target ROCK2 in
BC. It was also predicted that miR-381 may form base pairing with
EGFR-AS1. However, overexpression experiments revealed that miR-381
did not serve a regulatory role in the expression of EGFR-AS1.
Therefore, miR-381 may not target EGFR-AS1. Increasing evidence has
shown that lncRNAs may serve as the sponge of miRNAs to reduce
their role in targeting downstream genes (19). In the present study, EGFR-AS1 was
revealed to act as a sponge to miR-381, resulting in the
upregulation of ROCK2; however, a limitation in the study design
was the failure to analyze the direct interaction between EGFR-AS1
and miR-381 by dual-luciferase assay. Future studies should include
this assay to further strengthen the conclusions reported in the
current study. In addition, animal model experiments, such as tumor
xenograft experiments are needed to further analyze the effects of
EGFR-AS1 on tumor metastasis. In conclusion, EGFR-AS1 was
upregulated in BC and may upregulate ROCK2 by sponging miR-381 to
promote cancer cell invasion and migration.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SY designed the experiments. SY, XL and HC performed
experiments. XS and XZ collected and analyzed data. SY drafted the
manuscript. All authors approved the final version of the
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of The Second People's Hospital of Liaocheng (Linqing,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Antoni S, Ferlay J, Soerjomataram I, Znaor
A, Jemal A and Bray F: Bladder cancer incidence and mortality: A
global overview and recent trends. Eur Urol. 71:96–108. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kamat AM, Hahn NM, Efstathiou JA, Lerner
SP, Malmström PU, Choi W, Guo CC, Lotan Y and Kassouf W: Bladder
cancer. Lancet. 388:2796–2810. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Al-Zalabani AH, Stewart KJ, Wesselius AM,
Schols AM and Zeegers MP: Modifiable risk factors for the
prevention of bladder cancer: A systematic review of meta-analyses.
Eur J Epidemiol. 31:811–851. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Knowles MA and Hurst CD: Molecular biology
of bladder cancer: New insights into pathogenesis and clinical
diversity. Nat Rev Cancer. 15:25–41. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Choi W, Ochoa A, McConkey DJ, Aine M,
Höglund M, Kim WY, Real FX, Kiltie AE, Milsom I, Dyrskjøt L and
Lerner SP: Genetic alterations in the molecular subtypes of bladder
cancer: Illustration in the cancer genome atlas dataset. Eur Urol.
72:354–365. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li HT, Duymich CE, Weisenberger DJ and
Liang G: Genetic and epigenetic alterations in bladder cancer. Int
Neurourol J. 20 (Suppl 2):S84–S94. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Julian L and Olson MF: Rho-Associated
coiled-coil containing kinases (ROCK): Structure, regulation, and
functions. Small GTPases. 5:e298462014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rath N and Olson MF: Rho-associated
kinases in tumorigenesis: Re-Considering ROCK inhibition for cancer
therapy. EMBO Rep. 13:900–908. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kamai T, Tsujii T, Arai K, Takagi K, Asami
H, Ito Y and Oshima H: Significant association of Rho/ROCK pathway
with invasion and metastasis of bladder cancer. Clin Cancer Res.
9:2632–2641. 2003.PubMed/NCBI
|
|
11
|
Xie Y, Qi J, Zhu C, Zhao D and Liao G:
MiR-381 functions as a tumor suppressor in gastric cancer by
targeting ROCK2. Int J Clin Exp Pathol. 12:164–172. 2019.PubMed/NCBI
|
|
12
|
Qi HL, Li CS, Qian CW, Xiao YS, Yuan YF,
Liu QY and Liu ZS: The long noncoding RNA, EGFR-AS1, a target of
GHR, increases the expression of EGFR in hepatocellular carcinoma.
Tumuor Biol. 37:1079–1089. 2016. View Article : Google Scholar
|
|
13
|
Wang G and McKenney JK: Urinary bladder
pathology: World health organization classification and American
joint committee on cancer staging update. Arch Pathol Lab Med.
143:571–577. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delte Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hu J, Qian Y, Peng L, Ma L, Qiu T, Liu Y,
Li X and Chen X: Long noncoding RNA EGFR-AS1 promotes cell
proliferation by increasing EGFR mRNA stability in gastric. cancer.
Cell Physiol Biochem. 49:322–334. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tan DSW, Chong FT, Leong HS, Toh SY, Lau
DP, Kwang XL, Zhang X, Sundaram GM, Tan GS, Chang MM, et al: Long
noncoding RNA EGFR-AS1 mediates epidermal growth factor receptor
addiction and modulates treatment response in squamous cell
carcinoma. Nat Med. 23:1167–1175. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xia B, Li H, Yang S, Liu T and Lou G:
MiR-381 inhibits epithelial ovarian cancer malignancy via YY1
suppression. Tumuor Biol. 37:9157–9167. 2016. View Article : Google Scholar
|
|
18
|
Ming J, Zhou Y, Du J, Fan S, Pan B, Wang
Y, Fan L and Jiang J: miR-381 suppresses C/EBPα-dependent Cx43
expression in breast cancer cells. Biosci Rep. 35:e002662015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Thomson DW and Dinger ME: Endogenous
microRNA sponges: Evidence and controversy. Nat Rev Genet.
17:272–283. 2016. View Article : Google Scholar : PubMed/NCBI
|