Introduction
An increasing body of evidence indicates that
inflammation is one of the hallmarks of carcinogenesis, allowing
the growth and metastasis of tumors (1). The role of inflammation is two-fold: On
one hand, the tumor and antigens present on the surface of cells
can stimulate effector cell recruitment of the innate immune
system, resulting in the destruction of diseased tissue; on the
other hand, mere stimulation of the immune response is associated
with the development of inflammation, which can stimulate the
growth of a tumor through the production of proinflammatory
cytokines, thus creating a feedback loop (2). A special role in this process is played
by interleukin 1β (IL-1β), which promotes tumor progression,
neoangiogenesis, and metastasis (3).
Activation of IL-1β depends on the function of the proteolytic
enzyme, caspase-1, which is strictly regulated by multiprotein
complexes called inflammasomes.
Many endo- and exogenous factors can be involved in
the process of inflammasome activation. However, due to a dynamic
increase in non-melanoma skin cancers (NMSC) in the Caucasian
population, it has become necessary to determine how ultraviolet
radiation (UVR), which plays a key role in NMSC development, can
regulate the structure and function of inflammasomes. This review
presents a current overview of the inflammasome activation and
regulation of NLRP3 (NOD-Like Receptor Family Pyrin Domain
Containing 3) and NLRP1 (NOD-Like Receptor Family Pyrin Domain
Containing 1). Recent findings about the inflammasome-mediated
immune response after UVR exposition are also analyzed, and a
comparison of the structures and the regulation of NLRP3 and NLRP1
inflammasomes is made.
Inflammasomes: Mechanism of function depends
on structure
The immune system protects the human body against
various infections and tissue damage through the development of a
broad range of proteins that form multimeric active inflammasomes.
As part of an innate immune response, inflammasomes initiate
inflammation in response to cellular stress induced by
non-infective agents (e.g., reactive oxygen species and lysosomal
enzymes) commonly known as DAMPs (Danger Associated Molecular
Patterns) as well as pathogenic microbes known as PAMPs (Pathogen
Associated Molecular Patterns) (4).
The function of an inflammasome depends on its
structure. In general, the core of an inflammasome consists of
nucleotide-binding oligomerization domain-like receptors (NOD-like
receptors, NLRs). The binding of NLRP to ASC (apoptosis associated
with Speck-like protein) and procaspase-1 results in the formation
of a functional inflammasome and activates caspase-1 by
autocatalytic cleavage (5). Active
caspase-1 further processes pro-IL-1β and pro-IL-18 into their
mature and bioactive forms (IL-1β and IL-18), which induces the
secretion of subsequent factors that activate the inflammatory
process. The ASC protein is composed of two death-fold domains: one
pyrin domain (PYD) and one caspase activation and recruitment
domain (CARD). The PYD and CARD domains mediate homotypic
interactions with other proteins containing a PYD or a CARD domain.
The NLR family comprises 22 proteins that are present in humans;
however, not all of them can form an inflammasome. NLRP1, NLRP2,
NLRP3, NLRP6, NLRP7, NLRC4 (NLR family CARD domain-containing
protein 4), NLRP12, and AIM2 (absent in Melanoma 2) have been
reported to initiate the formation of inflammasomes (6).
In the last few years, important advances in the
understanding of NLRP1 and NLRP3 biology have been achieved. In
principle, NLR proteins, except for NLRP1, have a tripartite domain
organization which may contain PYD at the N-terminus to bind ASC;
nucleotide binding and an oligomerization domain (NACHT) in the
middle which are responsible for the oligomerization of the
inflammasome; and leucine-rich repeats (LRRs) at the C-terminus
which bind to PAMPs or DAMPs (Fig.
1) (5,7).
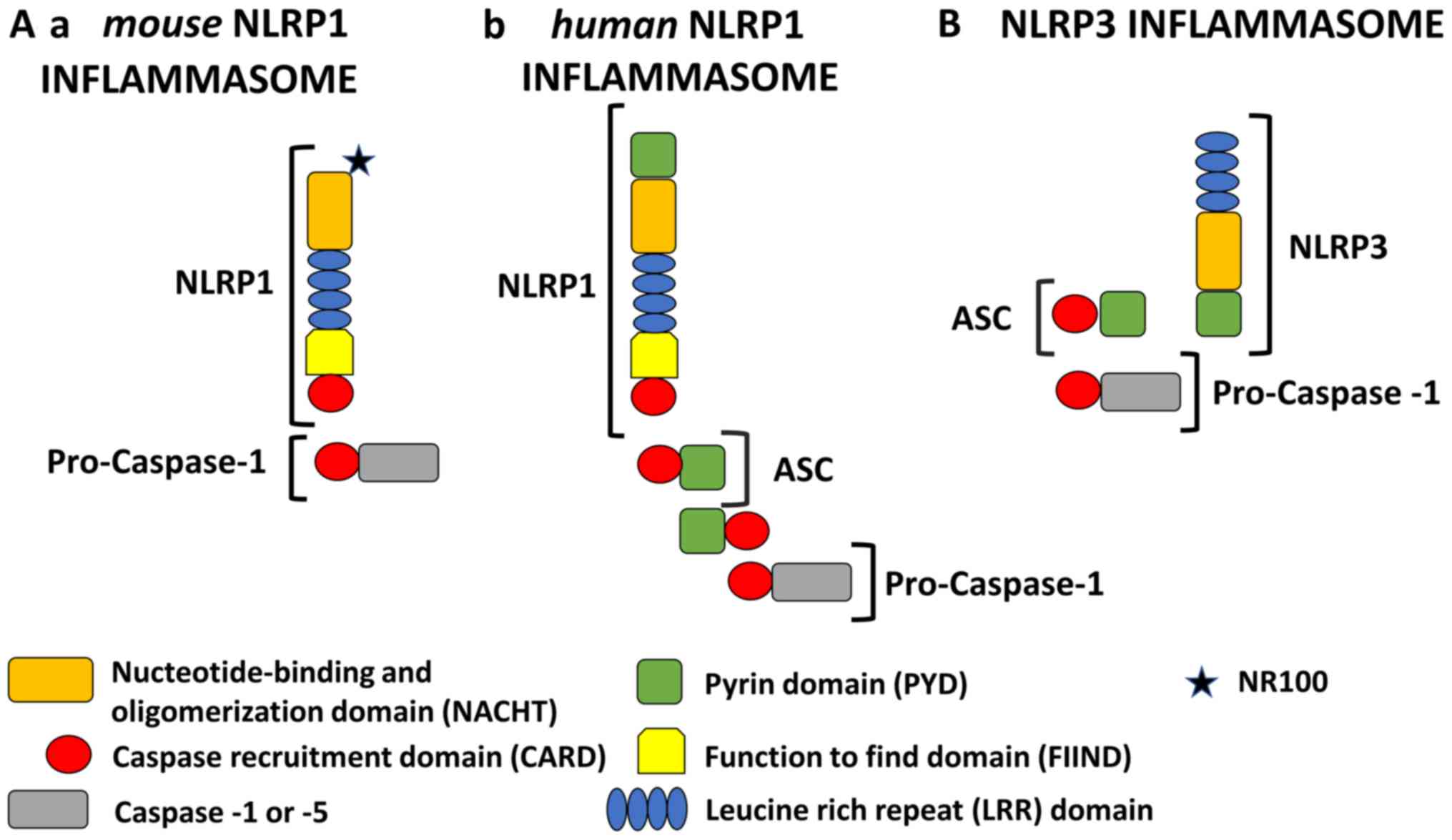 | Figure 1.Schematic representation of NLRP1 and
NLRP3 inflammasomes. All NLRP1 inflammasomes are composed of NACHT,
LRR, FIIND and CARD domains. Domain positions are indicated to
scale. The presence of FIIND and CARD domains at the C-terminus
makes NLRP1 stand out from all other members of the NLR family.
However, the NLRP1 inflammasome is quite different in murine and
human models. (A-a) Mouse NLRP1 inflammasome: In mice, there are
three paralogs of NLRP1 (Nlrp1a, -b, and -c) which contain an NR100
domain instead of the PYD found in humans. Mouse NLRP1 can activate
caspase-1 directly and ASC (apoptosis associated with Speck-like
protein) is not required. (A-b) Human NLRP1 inflammasome: Human
NLRP1 contains the N-terminal PYD. Its activity is dependent upon
ASC, which is associated with the C-terminal CARD domain. (B) NLRP3
inflammasome: The NLRP3 inflammasome consists of an NLRP3 protein,
an ASC adaptor, and procaspase-1. Upon stimulation, NLRP3 and ASC
adhere via their PYD domains, whereas ASC and caspase-1 adhere via
their CARD domains. Therefore, NLRP3 forms an ASC-dependent
inflammasome. NLRP1, NOD-like receptor family pyrin domain
containing 1; NLRP3, NOD-like receptor family pyrin domain
containing 3; NACHT, nucleotide-binding and oligomerization domain;
LRR, leucine rich repeat; FIIND, function to find domain; PYD,
pyrin domain; ASC, apoptosis associated with Speck-like
protein. |
In addition to these three domains, NLRP1 contains a
function-to-find domain (FIIND) and CARD. Moreover, the N-terminal
pyrin domain (PYD) is also found in human NLRP1 and other
non-rodent species but is absent in mice. The presence of two
effector domains (PYD and CARD) has caused confusion regarding the
role of each domain (Fig. 1A). For
this reason, initially, the human NLRP1 was considered to be unique
because it can activate caspase-1 through the CARD domain without
recruiting ASC (5). The initial
description of the NLRP1 inflammasome proposed also that the PYD is
important as an adapter protein for binding ASC and then recruiting
and activating caspase-1 (8).
Currently, most studies indicate that under some conditions,
caspase-1 can connect directly to the C-terminal CARD domain, but
only in murine NLRP1 (9–11). Interestingly, the direct activation
of caspase-1 by NLRP1 with a bypassed ASC does not result in the
proteolysis of caspase-1 which is still capable of producing mature
IL-1β (12,13). Moreover, the initial description of
the human NLRP1 inflammasome included NLRP1, ASC, caspase-1, and
caspase-5. However, the exact role of caspase-5 in the canonical
NLRP1 inflammasome activation still needs to be elucidated
(14).
Surprisingly, Finger et al (15) demonstrated that human NLRP1 activity
is dependent upon ASC which is associated with the C-terminal CARD
domain. Furthermore, it has been shown that human NLRP1 activity is
dependent upon autolytic cleavage in the FIIND domain (15). A recent study conducted by Yu et
al (16) redefined our
understanding of the role of the ASC protein in human NLRP1
function. It was concluded that the NLRP1 N-terminal domain (PYD in
humans) is autoinhibitory, while the C-terminal cleavage fragment
with the CARD domain engages an ASC dependent inflammasome.
NLRP inflammasomes are concerned with pyroptosis, a
recently described pathway of programmed cell death. Activated
caspase-1 results not only in processing and the release of
inflammatory cytokines (IL-1β, IL-18), but also in pyroptosis,
which causes a loss of plasma membrane integrity (17).
A recent study indicated that during an invasive
gram-negative bacterial infection, caspase-4/5 in humans and
caspase-11 in mice bind to cytosolic lipopolysaccharide (LPS),
promoting NLRP3 activation and forming a complex termed the
non-canonical inflammasome (18,19). The
non-canonical inflammasome also activates pyroptosis, but only
causes the processing of proinflammatory cytokines indirectly by
activating the canonical inflammasome through a not well-defined
mechanism (20). The NLRP1
inflammasome could also promote the activation of caspase-1 and the
subsequent activation and release of IL-1β as well as the
initiation of pyroptosis (Fig. 2)
(21).
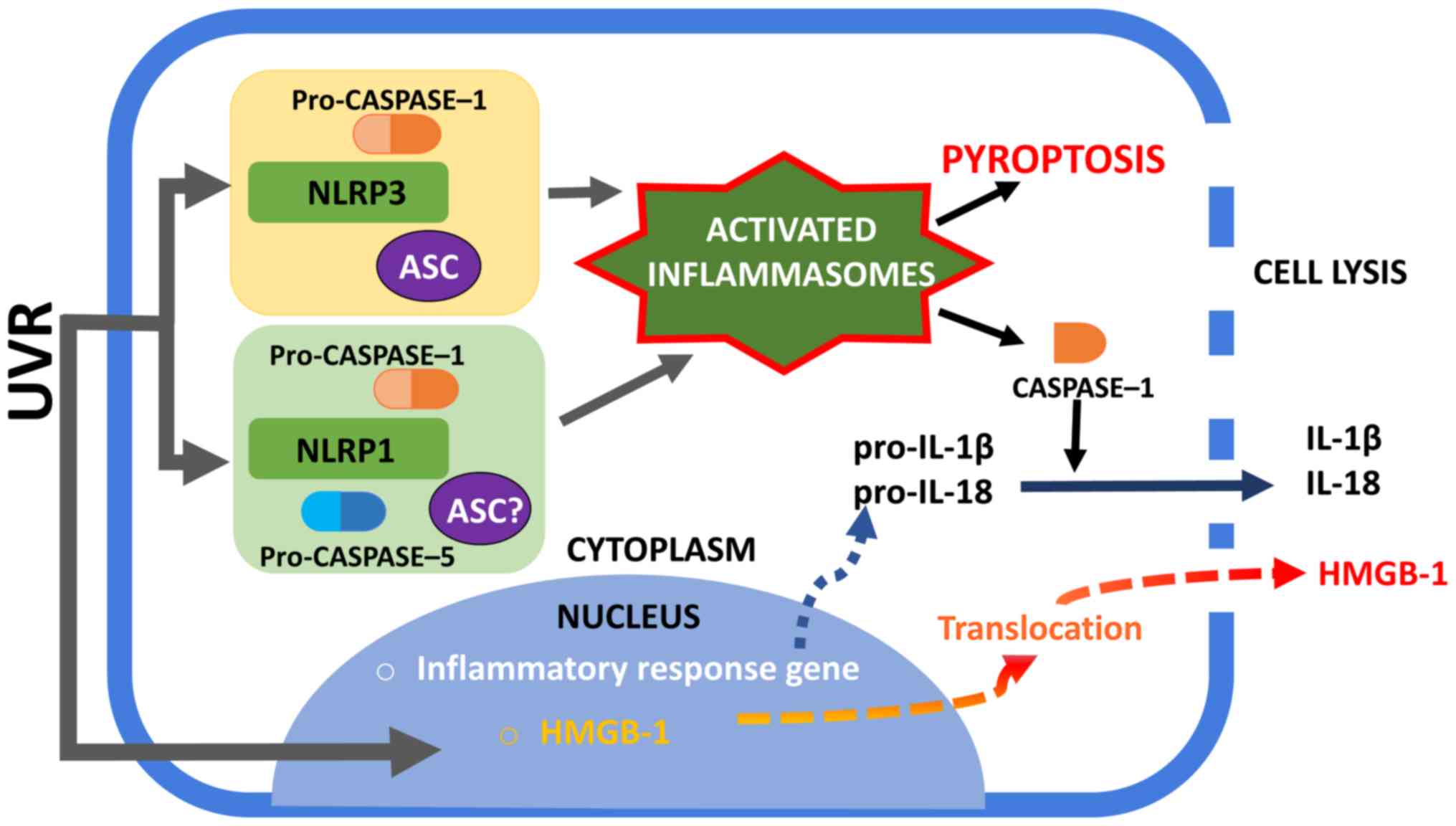 | Figure 2.Activation of NLRP1 and NLRP3
inflammasomes. UVR irradiation of human keratinocytes may trigger
the assembly of NLRP1 and NLRP3 inflammasomes. The NLRP1
inflammasome complex consists of caspase-1, ASC (which is not
required to form complexes in murine) and NLRP1. The exact role of
caspase-5 in NLRP1 inflammasome activation is unclear. The NLRP3
inflammasome is well characterized among the inflammasome complexes
and consists of NLRP3, ASC and caspase-1. An active caspase-1 form
is required to process pro-IL-1β and pro-IL-18 into mature forms
and to secrete them into the extracellular space. Furthermore,
inflammasome is associated with the unconventional secretion of
HMGB-1. Active caspase-1 can lead to cell pyroptosis with membrane
rupture and the release of alarmins, such as HMGB1. NLRP1, NOD-like
receptor family pyrin domain containing 1; NLRP3, NOD-like receptor
family pyrin domain containing 3; UVR, ultraviolet radiation; ASC,
apoptosis associated with Speck-like protein; HMGB-1, high mobility
group box protein 1; IL, interleukin. |
Influence of UVR on the activation of an
inflammasome
UVR represents one of the main environmental risks
and stress factors for the skin. Excessive exposure to UVR can
directly damage the DNA of dermal cells and, in addition, induces
inflammation of the skin that is commonly termed sunburn. At a
molecular level, this phenomenon is characterized by the activation
of inflammasomes and stress pathways that include nuclear factor
(NF)-κB. Both chronic and acute UVR exposures are potent complete
carcinogens which initiate and promote cancer development. Physical
and metabolic damage to the dermal cells caused by UVR exposition
causes the release and accumulation of endogenous cellular
components, extracellular DAMPs, which induce a ‘sterile’
inflammation. Different subtypes of NLR recognize specific DAMPs,
such as IL-1α and IL-33. These interleukins are two endogenous
molecules that are perceived to be potent ‘danger’ signals that
indicate the potential loss of epidermal barrier integrity
(4). Normally, IL-1α and IL-33 are
present in the nuclei involved in transcription modulation and are
released from cells under the influence of factors that
induce-dependent on the inflammasome-unconventional secretion. The
activation of an inflammasome is also associated with the
unconventional secretion of HMGB-1 (high mobility group box protein
1), which is an evolutionarily conserved protein with a broad
spectrum of actions. Inside cells, HMGB-1 is also found mainly in
the cell nuclei, where it participates, e.g., in replication and
DNA repair. However, when HMGB-1 is released into the extracellular
space, it becomes a proinflammatory cytokine which stimulates the
formation of new blood vessels, enhances cell migration, and
affects cell proliferation (Fig.
2).
UVR exposure stimulates keratinocytes to secrete an
abundant amount of pro-inflammatory IL-1-family proteins, namely,
IL-1α, IL-1β, IL-18, and IL-33 (6).
Under natural conditions, normal healthy skin contains low levels
of the inactive precursor forms of IL-18 and IL-1β that require the
presence of caspase-1 for maturation and secretion (4).
Previous research conducted in vitro
indicated the occurrence of inflammasome-dependent secretion of
mature IL-1β and IL-18 in human keratinocytes under the influence
of UVB radiation (22–25). Hasegawa et al (23) indicated, for the first time, that the
expression of the NLRP3 gene, like the production of IL-1β
and other inflammatory mediators, including IL-1α, IL-6, TNF-α, and
PGE2 in keratinocytes, increases under intensity-dependent UVB
radiation. In addition, immunofluorescence analysis research has
indicated that not only UV-irradiated human keratinocytes, but also
human epidermis, show elevated expression of NLRP3 (23). It is speculated that UVB-induced
NLRP3 inflammasome activation is the connection between nuclear DNA
damage responses and the production of various specific
inflammatory mediators (23).
So far, it has been proven that some genetic changes
in the NLRP family, that cause excessive activation of
inflammasomes, predispose an individual to the formation of
inflammatory skin lesions, such as psoriasis, atopic dermatitis,
and vitiligo (26–28). As for other constitutively expressed
NLRPs, mutations in NLRP1 are associated with skin diseases
(28).
Based on the fact that keratinocytes show the
constitutive expression of NLRP1, presumably, this NLR is likely to
be the first responder to DAMP induced by UV-damage. Although NLRP1
is characterized by the activation of both caspase-1 and caspase-5,
which in human cells can activate IL-1β (8). However, the knockdown of only caspase-5
in keratinocytes does not significantly affect the secretion of
IL-1β. This finding suggests that inflammatory processes in
keratinocytes caused by the action of UV-irradiation do not require
caspase-5. This has been confirmed by the fact that various stimuli
in a range of cell types can activate various NLR components
(29). Active NLRP1 forms
self-oligomers by binding to procaspase-1 and ASC (29). The mouse NLRP1 homologue, unlike the
human one, lacks the PYD domain and is therefore unable to bind
ASC. Bcl-2 (B-cell lymphoma 2) and Bcl-XL (B-cell
lymphoma-extra-large) are anti-apoptotic proteins, which, in normal
resting cells, bind to NLRP1, thus suppressing caspase-1 activation
and the secretion of Il-1β. In some analyses, it has been shown
that muramyl dipeptide (MDP) releases anti-apoptotic proteins from
NLRP1, which results in inflammasome activation and the formation
of oligomers (30). UVB irradiated
keratinocytes show decreased levels of these anti-apoptotic
proteins (Bcl-2 and Bcl-XL), therefore suggesting that this is a
possible mechanism by which UV-induced NLRP1 activation may occur
(31).
Nevertheless, Hasegawa et al (23) found that UVB-induced IL-1β production
in human keratinocytes is selectively mediated by NLRP3, but not by
NLRP1, which suggests that individual NLRPs might be specifically
activated, depending on the cutaneous pathological status.
On the other hand, the results presented in 2018 by
Sand et al (32) revealed
that UVB irradiation activates NLRP1 inflammasomes in human primary
keratinocytes, resulting in the secretion of large amounts of IL-1β
and IL-18. Furthermore, they revealed that human fibroblasts do not
secrete mature IL-1β or IL-18. However, these data confirm that our
knowledge regarding the role of NLRP3 and NLRP1 inflammasome
activation in UV-induced inflammation is still limited.
NLRP3 and skin carcinogenesis
Thus far, NLRP3 is the best described inflammasome
that is involved in the pathogenesis of cryopyrin-associated
periodic syndromes caused by mutations in the gene encoding the
cytoplasmic NLRP3 associated with exacerbated IL-1β production
(33). Other inflammatory diseases
connected with increases in NLRP3 inflammasome activity that are
usually associated with the polymorphism of NLRP3 have also been
noted. NLRP3 inflammasomes have been implicated in the development
of major diseases such as gout, type 2 diabetes, and
obesity-induced insulin resistance (34). Moreover, the NLRP3 inflammasome is
increasingly being suspected of playing a major role in other human
pathologies such as asbestosis, Alzheimer's disease, and
Parkinson's disease (35). The NLRP3
inflammasome is also an emerging key player in the development and
progression of various cancers; however, its exact role in
tumorigenesis remains unknown. Recent studies have shown that both
the overexpression and constitutive activation of NLRP3 could
contribute to the development and progression of various cancers
including malignant melanoma (36),
breast (5), and lung cancers
(37). Furthermore, the NLRP3
inflammasome functions as a negative regulator of tumorigenesis
during colitis-associated cancer (38). Thus, in those cancers NLRP3 works in
conjunction with a cell or tissue.
Previous studies have indicated that the NLRP3
inflammasome together with interleukin-1 may also promote the
growth of skin cancers. In studies carried out on a mouse model,
Drexler et al (39) observed
that mice with interleukin-1 or caspase-1 receptor knockout rarely
develop dimethylbenzanthracene (DMBA) and
tetradecanoylphorbolacetate (TPA)-induced skin tumors compared to
wild-type mice. The knockout of the NLRP3 gene itself also
caused a reduction in the number of skin tumors. These observations
indicate that the NLRP3-dependent production of IL-1β may
contribute to the growth of skin cancers.
Confirming this phenomenon has come from research on
the adaptor protein of the NLRP3 inflammasome, ASC. The effect of
ASC on several inflammasomes including NLRP3, has also been
assessed in the formation of skin cancers. Deficiencies in ASC in
myeloid cells in the murine model were shown to result in a
significantly lower frequency of skin tumor occurrence induced by
chemical agents (DMBA/TPA) (39).
Interestingly, the ablation of ASC in keratinocytes in a mouse
model caused the development of a greater number of tumors compared
with controls. The obtained results proved that ASC suppresses
tumor development in keratinocytes but promotes carcinogenesis in
myeloid cells (39). Furthermore, it
was found that human cutaneous squamous cell carcinoma (SCC)
involves the loss of ASC protein expression.
Okamoto et al (3) produced interesting results. They showed
that in cell lines derived from advanced malignant melanomas, there
was a constitutive activation of the NLRP3 inflammasome which
resulted in the oversecretion of IL-1β. In cell lines derived from
early forms of malignant melanoma, IL-1β secretion followed IL-1R
activation. Furthermore, the production of IL-1β was inhibited by
caspase-1 suppression or IL-1R receptor blockade. As suggested by
the authors, this autocrine feedback loop may be responsible for
the clinical phenotype of melanoma and its progression.
In an independent study, in a model of
chemical-induced carcinogenesis, Melvyn et al (40) showed that mice lacking NLRP3 have a
significantly reduced tumor burden than control wild-type mice.
Their data suggest that NLRP3 is an important suppressor of natural
killer cell-mediated control of metastases and carcinogenesis.
NLRP1 and skin carcinogenesis
In contrast to NLRP3, NLRP1 has not been precisely
analyzed. Recent studies have proven that the overexpression of
NLRP1 promotes malignant melanoma progression. Zhai et al
(41) found that NLRP1 can bind to
caspase −2 and −9 in melanoma, thus preventing the interaction of
apoptotic caspases with other activating proteins. They
demonstrated that NLRP1 can negatively regulate apoptosis in
malignant melanoma cells. Therefore, it is suspected that NLRP1 may
inhibit the caspase-2/9-mediated apoptotic pathway. Another
important link between the activation of inflammasomes and skin
carcinogenesis has come from studies on polymorphisms within the
gene for NLRP1 in multiple self-healing palmoplantar carcinoma
(MSPC) and keratosis lichenoides chronical (KLC), whose symptoms
include various ulcerative, hyperkeratotic nodular lesions on the
surfaces of the hands and soles, histologically resembling
well-differentiated SCC, as well as an increased predisposition to
the development of SCC. Studies have shown that in both diseases,
there is a gain-of-function mutation in the NLRP1 gene.
Under normal healthy conditions, the pyridine domain and LRR NLRP1
interact with each other, making it impossible to oligomerize the
inflammasome. Mutations in the NLRP1 gene cause changes in
the structures of these domains, resulting in the formation of
oligomers of NLRP1 and the activation of inflammasomes. As a
result, in the cell lines of keratinocytes gathered from patients
suffering from MSPC and KLC, continuous production and secretion of
IL-1 occurs (42). Consequently, it
will be important to determine how inflammasomes drive tumor
progression in future studies.
Therapies that target inflammasomes
In recent years, therapies with inflammasomes have
been significantly improved to overcome human diseases such as
cancer. As the activation of these multimeric cytosol complexes has
been revealed in diverse human pathologies, common approaches have
been set to dampen or even reduce inflammation. Most reagents that
particularly target the inflammasome, caspase-1, and IL-1 have been
developed and analyzed in various diseases.
Recently, much attention has been paid to the
development of compounds to inhibit the IL-1β signaling activities
as another approach for inflammasome inhibition in cancer
treatment. IL-1 is a pivotal proinflammatory cytokine that
stimulates angiogenesis and promotes tumor growth and metastatic
dissemination, which is composed of two molecular species (IL-1α
and IL-1β). Elaraj et al (43) revealed in the study carried out by
real time quantitative reverse transcriptase PCR that several
melanoma and squamous cell cancers and cell lines indicated the
gene expression of IL-1α and IL-1β.
Previous studies have indicated that IL-1 also
stimulates melanoma tumor growth in vivo and moreover,
increased intercellular adhesion molecule-1 (ICAM-1) on the cancer
cells (44). The IL-1 receptor
antagonist (IL-1Ra) blocks these effects, thereby inhibiting the
proliferation of skin tumor cell lines (45,46),
inhibiting the growth of mouse melanoma cells (47), and reducing the in vivo growth
and metastatic potential of human melanoma xenografts which
constitutively produce IL-1 (48).
Anakinra is a recombinant human IL-1 receptor antagonist and is
regarded as a biological agent that blocks the inflammatory effects
of IL-1. Moreover, anti-IL-1Ra therapy significantly decreases the
expression of ICAM-1 molecules. Triozzi et al (49) indicated that IL-1Ra modifies the
response to macroscopic melanoma and could additionally increase
the antitumor activity of the classical cytostatic chemotherapy
used in melanoma treatment. As IL-1 has also been connected with
the clinical toxicity of several classical chemotherapeutics used
in melanoma therapy, the combination of IL-1Ra with chemotherapy
has also been suggested to improve treatment outcomes (50–53).
However, currently, based on the use of immunotherapy to treat
melanoma, classic cytostatic agents are used very rarely and mainly
in subsequent treatment lines; therefore, this ability of IL-1Ra
does not actually apply. Interestingly, IL-1 has been suggested to
play a crucial role in UV-induced dermal collagen degradation which
is responsible for premature skin photoaging.
It has been revealed that Anakinra reduces matrix
metalloproteinase-1 (MMP-1) production, which is the main enzyme
that breaks down the interstitial collagen types I, II, and III,
and can be used for the prevention and treatment of photoaging
(54). Anakinra is currently
approved and is being used clinically for the treatment of
rheumatoid arthritis. What is more, this drug is currently used
mostly in autoinflammatory conditions.
NLRP3 inflammasome activation with the cleavage of
caspase-1 leads to auto-inflammation through the secretion and
maturation of the pro-inflammatory cytokines IL-1β and IL-18 in
melanoma cells (55). Thymoquinone
can block caspase-1 by inhibiting the NLRP3 inflammasome and thus
preventing the caspase-1-induced proteolytic maturation and
secretion of IL-1β and IL-18. It is the main bioactive constituent
of the essential oil extracted from Nigella sativa seeds, which has
been shown to have very promising pharmacological properties, such
as antioxidant and anti-inflammatory efficacy (56–58). In
many studies, it has also been reported that thymoquinone may
possess anticancer effects such as the inhibition of cell
proliferation, the generation of reactive oxygen species (ROS),
cell cycle arrest, the induction of apoptosis, and inhibition of
metastasis and angiogenesis (59).
Ahmad et al (60) first
demonstrated the exact antitumor function of thymoquinone and
revealed that inhibition of the NLRP3 inflammasome by thymoquinone
substance blocked cell migration and prevented melanoma cell
metastasis in an in vivo model. These same authors also
showed the inhibitory effect of metastasis through the inhibition
of NLRP3, with a consequent reduction in the synthesis of IL-1 and
IL-18 in an in vivo mouse model. Thymoquinone, as an
antioxidant agent, may also be able to inhibit NLRP3 by reducing
the production of ROS. Thus, thymoquinone has the potential to be
used as a chemotherapeutic agent in combination with existing
therapies for the treatment of melanoma.
Another interesting study was conducted by Ellis
et al (61), who reported
that physiological doses of epigallocate-chin-3-gallate, a
polyphenol found in green tea, inhibits the effect of metastatic
melanoma on the proliferation of human cell strains by inhibiting
NLRP1, but not NLRP3 (61). Tsai
et al (62) also demonstrated
the role of green tea polyphenols in the inhibition of the NLRP3
inflammasome in nephritis during lupus. To the best of our
knowledge, there have been no other reports on the effectiveness of
other dietary agents on the reduction of metastasis by the
inhibition of the NLRP3 inflammasome.
Great hope for revolutions in treatment rest on
oridonin (ORI), one of the most important active Chinese medicinal
components isolated from Rabdosia rubescens, which has been
shown to be a potent anti-metastatic agent. Notably, oridonin shows
a broad-spectrum of anti-proliferative and anti-cancer activities
in various types of tumor (63–68) as
well as in melanoma (69,70). Wang et al (69) indicated that ORI induces human
melanoma A375-S2 cell death (69).
In another independent study, Gu et al (70) reported that ORI impairs the survival
capability and proliferation of melanoma cells through the
induction of apoptosis (70). As the
mechanisms underlying the inhibiting effects of ORI in the
metastasis of melanoma cells remain unclear, many authors have
investigated this topic. Li et al (63,64)
proposed that ORI could effectively inhibit migration as well as
invasion and adhesion by inhibiting epithelial-mesenchymal
transition (EMT) in melanoma cells through inhibiting the
phosphorylation of phosphoiniositide-3 kinase (PI3K), Protein
kinase B (Akt), and glycogen synthase kinase 3β (GSK-3β) in the
presence of transforming growth factor β1 (TGF-β1). Recent studies
have also indicated that oridonin is a specific inhibitor for the
NLRP3 inflammasome which forms a covalent bond with the NACHT
domain of NLRP3, thereby inhibiting NLRP3 inflammasome assembly and
activation (71).
Despite the fact that, in recent years, great
advances have been achieved in the knowledge regarding
inflammasomes in cancer, there are still many questions to be
answered. It is still unclear as to why inflammasomes and their
components have a dual, ambivalent role in cancer development that
depends on the existing tissues and the cancer stage. Moreover, the
regulatory mechanisms of inflammasomes between different NLR
members as well as by other signaling molecules and their
interactions in tumor development are still unclear. Further
studies are required to confirm the molecular mechanisms for the
activation or inhibition of inflammasomes in the tumor
microenvironment. Identification of the regulatory factors for the
specific inflammasome may allow the determination of biomarkers for
cancer prognosis as well as identifying therapeutic targets against
various tumors.
There are still many knowledge gaps in the function
mechanisms of inflammasomes in carcinogenesis. Better understanding
of their signaling pathways and regulation may contribute to
advances in human cancer prevention and treatment.
Conclusions
It is highly probable that there is a causal
relationship between the activation of inflammasomes and skin
carcinogenesis. However, despite many studies, this process has not
been accurately described. So far, most of our knowledge about the
functional importance of NLRP3 and NLRP1 inflammasomes in skin
cancer has come from mouse models. Mice are nocturnal animals whose
skin is protected by fur and who are only occasionally exposed to
UVB radiation. This fact is particularly important when considering
the profound differences between murine and human skin. Therefore,
the results obtained in mouse models need to be interpreted very
carefully. Furthermore, it has been revealed that the expression of
inflammasome proteins is significantly lower in murine
keratinocytes than in humans.
Furthermore, the effect of UV radiation on the
activation of NLRP1 and NLRP3 inflammasomes has not been well
researched. Some research has focused on the role of UVB in the
process of inflammasome activation in keratinocytes. However, of
the solar UVR reaching the surface of the Earth, about 5% is UVB,
and 95% is UVA. Although UVA radiation is not directly absorbed by
the DNA of epidermal cells, it can damage its structure through
reactive oxygen species. This leads to so-called oxidative DNA
damage as well as to the development of tumors. NOD-like receptors
strongly react to ROS-induced cellular stress, thus resulting in
the formation of inflammasomes. Cell damage by UV radiation may
result in the activation of processes that are responsible for the
repair of potentially damaged genetic material. By default, the
mechanism of response to DNA damage (DDR) is a factor that prevents
its possible transformation into cancer. Therefore, future
investigation of the relationship between the UV-induced activation
of inflammasome and DDR is necessary to allow for a better
characterization of the processes underlying skin
carcinogenesis.
The present study highlighted the recent advances in
the understanding of the inflammasomes involved in skin
carcinogenesis and may contribute to the development of future
therapies involving the modulation of inflammasome responses for
anticancer treatment.
Acknowledgements
Not applicable.
Funding
The present study was funded by Medical University
of Lodz (project no. 503/5-064-01/503-1) and The National Centre of
Science (grant nos. 2017/25/N/NZ5/02064 and
2017/27/B/NZ5/02011).
Availability of data and materials
Data sharing is not applicable to this article, as
no datasets were generated or analyzed during the current
study.
Authors' contributions
MC, IAB and AL contributed to the conception of the
study. MC and IAB performed the investigation. MC, IAB and KW wrote
and prepared the original draft of the manuscript. MC, JN and AL
wrote, reviewed and edited the manuscript. JN and AL supervised the
study.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kantono M and Guo B: Inflammasomes and
cancer: The dynamic role of the inflammasome in tumor development.
Front Immunol. 8:11322017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Okamoto M, Liu W, Luo Y, Tanaka A, Cai X,
Norris DA, Dinarello CA and Fujita M: Constitutively active
inflammasome in human melanoma cells mediating autoinflammation via
caspase-1 processing and secretion of interleukin-1beta. J Biol
Chem. 285:6477–6488. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nasti TH and Timares L: Inflammasome
activation of IL-1 family mediators in response to cutaneous
photodamage. Photochem Photobiol. 88:1111–1125. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rathinam VAK and Fitzgerald KA:
Inflammasome complexes: Emerging mechanisms and effector functions.
Cell. 165:792–800. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
de Zoete MR, Palm NW, Zhu S and Flavell
RA: Inflammasomes. Cold Spring Harb Perspect Biol. 6:a0162872014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lamkanfi M: Emerging inflammasome effector
mechanisms. Nat Rev Immunol. 11:213–220. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Martinon F, Burns K and Tschopp J: The
inflammasome: A molecular platform triggering activation of
inflammatory caspases and processing of proIL-beta. Mol Cell.
10:417–426. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Frew BC, Joag VR and Mogridge J:
Proteolytic processing of Nlrp1b is required for inflammasome
activity. PLoS Pathog. 8:e10026592012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nour AM, Yeung YG, Santambrogio L, Boyden
ED, Stanley ER and Brojatsch J: Anthrax lethal toxin triggers the
formation of a membrane-associated inflammasome complex in murine
macrophages. Infect Immun. 77:1262–1271. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Faustin B, Lartigue L, Bruey JM, Luciano
F, Sergienko E, Bailly-Maitre B, Volkmann N, Hanein D, Rouiller I
and Reed JC: Reconstituted NALP1 inflammasome reveals two-step
mechanism of caspase-1 activation. Mol Cell. 25:713–724. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Van Opdenbosch N, Gurung P, Vande Walle L,
Fossoul A, Kanneganti TD and Lamkanfi M: Activation of the NLRP1b
inflammasome independently of ASC-mediated caspase-1
autoproteolysis and speck formation. Nat Commun. 5:32092014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Guey B, Bodnar M, Manié SN, Tardivel A and
Petrilli V: Caspase-1 autoproteolysis is differentially required
for NLRP1b and NLRP3 inflammasome function. Proc Natl Acad Sci USA.
111:17254–17259. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bauernfeind F and Ablasser A:
Inflammasomes: Current understanding and open questions. Cell Mol
Life Sci. 68:765–783. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Finger JN, Lich JD, Dare LC, Cook MN,
Brown KK, Duraiswami C, Bertin J and Gough PJ: Autolytic
proteolysis within the function to find domain (FIIND) is required
for NLRP1 inflammasome activity. J Biol Chem. 287:25030–25037.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yu CH, Moecking J, Geyer M and Masters SL:
Mechanisms of NLRP1 mediated autoinflammatory disease in humans and
mice. J Mol Biol. 430:142–152. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lamkanfi M and Dixit VM: Mechanisms and
functions of inflammasomes. Cell. 157:1013–1022. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kayagaki N, Warming S, Lamkanfi M, Vande
Walle L, Louie S, Dong J, Newton K, Qu Y, Liu J, Heldens S, et al:
Non-canonical inflammasome activation targets caspase-11. Nature.
479:117–121. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shi J, Zhao Y, Wang Y, Gao W, Ding J, Li
P, Hu L and Shao F: Inflammatory caspases are innate immune
receptors for intracellular LPS. Nature. 514:187–192. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu X and Lieberman J: A mechanistic
understanding of pyroptosis: The fiery death triggered by invasive
infection. Adv Immunol. 135:81–117. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhao D, Wu Y, Zhuang J, Xu C and Zhang F:
Activation of NLRP1 and NLRP3 inflammasomes contributed to cyclic
stretch-induced pyroptosis and release of IL-1β in human
periodontal ligament cells. Oncotarget. 7:68292–68302. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Feldmeyer L, Keller M, Niklaus G, Hohl D,
Werner S and Beer HD: The inflammasome mediates UVB-induced
activation and secretion of interleukin-1beta by keratinocytes.
Curr Biol. 17:1140–1145. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hasegawa T, Nakashima M and Suzuki Y:
Nuclear DNA damage-triggered NLRP3 inflammasome activation promotes
UVB-induced inflammatory responses in human keratinocytes. Biochem
Biophys Res Commun. 477:329–335. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Strittmatter GE, Sand J, Sauter M,
Seyffert M, Steigerwald R, Fraefel C, Smola S, French LE and Beer
HD: IFN-γ primes keratinocytes for HSV-1-induced inflammasome
activation. J Invest Dermatol. 136:610–620. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Reinholz M, Kawakami Y, Salzer S, Kreuter
A, Dombrowski Y, Koglin S, Kresse S, Ruzicka T and Schauber J:
HPV16 activates the AIM2 inflammasome in keratinocytes. Arch
Dermatol Res. 305:723–732. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ekman AK, Verma D, Fredrikson M, Bivik C
and Enerbäck C: Genetic variations of NLRP1: susceptibility in
psoriasis. Br J Dermatol. 171:1517–1520. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bivik C, Verma D, Winge MC, Lieden A,
Bradley M, Rosdahl I and Söderkvist P: Genetic variation in the
inflammasome and atopic dermatitis susceptibility. J Invest
Dermatol. 133:2486–2489. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Levandowski CB, Mailloux CM, Ferrara TM,
Gowan K, Ben S, Jin Y, McFann KK, Holland PJ, Fain PR, Dinarello CA
and Spritz RA: NLRP1 haplo-types associated with vitiligo and
autoimmunity increase interleukin-1β processing via the NLRP1
inflammasome. Proc Natl Acad Sci USA. 110:2952e29562013. View Article : Google Scholar
|
|
29
|
Faustin B and Reed JC: Sunburned skin
activates inflammasomes. Trends Cell Biol. 18:4–8. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Faustin B, Chen Y, Zhai D, Le Negrate G,
Lartigue L, Satterthwait A and Reed JC: Mechanism of Bcl-2 and
Bcl-X(L) inhibition of NLRP1 inflammasome: Loop domain-dependent
suppression of ATP binding and oligomerization. Proc Natl Acad Sci
USA. 106:3935–3940. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Reagan-Shaw S, Breur J and Ahmad N:
Enhancement of UVB radiation-mediated apoptosis by sanguinarine in
HaCaT human immortalized keratinocytes. Mol Cancer Ther. 5:418–429.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sand J, Haertel E, Biedermann T, Contassot
E, Reichmann E, French LE, Werner S and Beer HD: Expression of
inflammasome proteins and inflammasome activation occurs in human,
but not in murine keratinocytes. Cell Death Dis. 9:242018.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gurung P and Kanneganti TD:
Autoinflammatory skin disorders: The inflammasome in focus. Trends
Mol Med. 22:545–564. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu-Bryan R: Intracellular innate immunity
in gouty arthritis: Role of NALP3 inflammasome. Immunol Cell Biol.
88:20–23. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shi F, Kouadir M and Yang Y: NALP3
inflammasome activation in protein misfolding diseases. Life Sci.
135:9–14. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu W, Luo Y, Dunn JH, Norris DA,
Dinarello CA and Fujita M: Dual role of apoptosis-associated
speck-like protein containing a CARD (ASC) in tumorigenesis of
human melanoma. J Invest Dermatol. 133:518–527. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang Y, Kong H, Zeng X, Liu W, Wang Z, Yan
X, Wang H and Xie W: Activation of NLRP3 inflammasome enhances the
proliferation and migration of A549 lung cancer cells. Oncol Rep.
35:2053–2064. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Allen IC, TeKippe EM, Woodford RM, Uronis
JM, Holl EK, Rogers AB, Herfarth HH, Jobin C and Ting JP: The NLRP3
inflammasome functions as a negative regulator of tumorigenesis
during colitis-associated cancer. J Exp Med. 207:1045–1056. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Drexler SK, Bonsignore L, Masin M,
Tardivel A, Jackstadt R, Hermeking H, Schneider P, Gross O, Tschopp
J and Yazdi AS: Tissue-specific opposing functions of the
inflammasome adaptor ASC in the regulation of epithelial skin
carcinogenesis. Proc Natl Acad Sci. 109:18384–18389. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Melvin JC, Holmberg L, Rohrmann S, Loda M
and Van Hemelrijck M: Serum lipid profiles and cancer risk in the
context of obesity: Four meta-analyses. J Cancer Epidemiol.
2013:8238492013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhai Z, Liu W, Kaur M, Luo Y, Domenico J,
Samson JM, Shellman YG, Norris DA, Dinarello CA, Spritz RA and
Fujita M: NLRP1 promotes tumor growth by enhancing inflammasome
activation and suppressing apoptosis in metastatic melanoma.
Oncogene. 36:3820–3830. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhong FL, Mamaï O, Sborgi L, Boussofara L,
Hopkins R, Robinson K, Szeverényi I, Takeichi T, Balaji R, Lau A,
et al: Germline NLRP1 mutations cause skin inflammatory and cancer
susceptibility syndromes via inflammasome activation. Cell.
167:187–202.e17. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Elaraj DM, Weinreich DM, Varghese S,
Puhlmann M, Hewitt SM, Carroll SM, Feldman ED, Turner EM and
Alexander HR: The role of interleukin 1 in growth and metastasis of
human cancer xenografts. Clin Cancer Res. 12:1088–1096. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Pazyar N, Feily A and Yaghoobi R: An
overview of interleukin-1 receptor antagonist, anakinra, in the
treatment of cutaneous diseases. Curr Clin Pharmacol. 7:271–275.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
La E, Rundhaug JE and Fischer SM: Role of
intracellular interleukin-1 antagonist in skin carcinogenesis. Mol
Carcinog. 30:218–223. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Bar D, Apte RN, Voronov E, Dinarello CA
and Cohen S: A continuous delivery system of IL-1 receptor
antagonist reduces angiogenesis and inhibits tumor development.
FASEB J. 18:161–163. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
McKenzie RC, Oran A, Dinarello CA and
Sauder DN: Interleukin-1 receptor antagonist inhibits subcutaneous
B 16 melanoma growth in vivo. Anticancer Res. 16:437–441.
1996.PubMed/NCBI
|
|
48
|
Weinreich DM, Elaraj DM, Puhlmann M,
Hewitt SM, Carroll NM, Feldman ED, Turner EM, Spiess PJ and
Alexander HR: Effect of interleukin 1 receptor antagonist gene
transduction on human melanoma xenografts in nude mice. Cancer Res.
63:5957–5961. 2003.PubMed/NCBI
|
|
49
|
Triozzi PL and Aldrich W: Effects of
interleukin-1 receptor antagonist and chemotherapy on host-tumor
interactions in established melanoma. Anticancer Res. 30:345–354.
2010.PubMed/NCBI
|
|
50
|
Hoshino T, Okamoto M, Sakazaki Y, Kato S,
Young HA and Aizawa H: Role of proinflammatory cytokine IL-18 and
IL-1beta in bleomycin-induced lung injury in humans and mice. Am J
Respir Cell Mol Biol. 41:661–670. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ledeboer A, Jekich BM, Sloane EM, Mahoney
JH, Langer SJ, Milligan ED, Martin D, Maier SF, Johnson KW,
Leinwand LA, et al: Intrathecal interleukin-10 gene therapy
attenuates paclitaxel-induced mechanical allodynia and
proinflammatory cytokine expression in dorsal root ganglia in rats.
Brain Behav Immun. 21:686–698. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Twining CM, Sloane EM, Milligan ED, Chacur
M, Martin D, Poole S, Marsh H, Maier SF and Watkins LR:
Peri-sciatic proinflammatory cytokines, reactive oxygen species,
and complement induce mirror-image neuropathic pain in rats. Pain.
110:299–309. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Surguladze D, Deevi D, Claros N, Corcoran
E, Wang W, Plym MJ, Wu Y, Doody J, Mauro DJ, Witte L, et al: Tumor
necrosis factor-alpha and interleukin-1 antagonists alleviate
inflammatory skin changes associated with epidermal growth factor
receptor antibody therapy in mice. Cancer Res. 69:5643–5647. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wang X, Bi Z, Chu W and Wan Y: IL-1
receptor antagonist attenuates MAP kinase/AP-1 activation and MMP1
expression in UVA-irradiated human fibroblasts induced by culture
medium from UVB-irradiated human skin keratinocytes. Int J Mol Med.
16:1117–1124. 2005.PubMed/NCBI
|
|
55
|
van de Veerdonk FL, Netea MG, Dinarello CA
and Joosten LA: Inflammasome activation and IL-1β and IL-18
processing during infection. Trends Immunol. 32:110–116. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Abdelmeguid NE, Fakhoury R, Kamal SM and
Al Wafai RJ: Effects of Nigella sativa and thymoquinone on
biochemical and subcellular changes in pancreatic β-cells of
streptozotocin-induced diabetic rats. J Diabetes. 2:256–266. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Kanter M: Nigella sativa and derived
thymoquinone prevents hippocampal neurodegeneration after chronic
toluene exposure in rats. Neurochem Res. 33:579–588. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Woo CC, Kumar AP, Sethi G and Tan KH:
Thymoquinone: Potential cure for inflammatory disorders and cancer.
Biochem Pharmacol. 83:443–451. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Banerjee S, Padhye S, Azmi A, Wang Z,
Philip PA, Kucuk O, Sarkar FH and Mohammad RM: Review on molecular
and therapeutic potential of thymoquinone in cancer. Nutr Cancer.
62:938–946. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Ahmad I, Muneer KM, Tamimi IA, Chang ME,
Ata MO and Yusuf N: Thymoquinone suppresses metastasis of melanoma
cells by inhibition of NLRP3 inflammasome. Toxicol Appl Pharmacol.
270:70–76. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Ellis LZ, Liu W, Luo Y, Okamoto M, Qu D,
Dunn JH and Fujita M: Green tea polyphenol
epigallocatechin-3-gallate suppresses melanoma growth by inhibiting
inflammasome and IL-1β secretion. Biochem Biophys Res Commun.
414:551–556. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Tsai PY, Ka SM, Chang JM, Chen HC, Shui
HA, Li CY, Hua KF, Chang WL, Huang JJ, Yang SS and Chen A:
Epigallocatechin-3-gallate prevents lupus nephritis development in
mice via enhancing the Nrf2 antioxidant pathway and inhibiting
NLRP3 inflammasome activation. Free Radic Biol Med. 51:744–754.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Li D, Han T, Liao J, Hu X, Xu S, Tian K,
Gu X, Cheng K, Li Z, Hua H and Xu J: Oridonin, a promising
ent-Kaurane diterpenoid lead compound. Int J Mol Sci. 17:E13952016.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Li D, Han T, Xu S, Zhou T, Tian K, Hu X,
Cheng K, Li Z, Hua H and Xu J: Antitumor and antibacterial
derivatives of oridonin: A main composition of Dong-Ling-Cao.
Molecules. 21:E5752016. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Lu J, Chen X, Qu S, Yao B, Xu Y, Wu J, Jin
Y and Ma C: Oridonin induces G2/M cell cycle arrest and
apoptosis via the PI3K/Akt signaling pathway in hormone-independent
prostate cancer cells. Oncol Lett. 13:2838–2846. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Wang XH, Zhang SF, Bao JT and Liu FY:
Oridonin synergizes with Nutlin-3 in osteosarcoma cells by
modulating the levels of multiple Bcl-2 family proteins. Tumour
Biol. 39:10104283177016382017.PubMed/NCBI
|
|
67
|
Xia S, Zhang X, Li C and Guan H: Oridonin
inhibits breast cancer growth and metastasis through blocking the
Notch signaling. Saudi Pharm J. 25:638–643. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Zhang Y, Wang L, Zi Y, Zhang L, Guo Y and
Huang Y: Oridonin effectively reverses the drug resistance of
cisplatin involving induction of cell apoptosis and inhibition of
MMP expression in human acute myeloid leukemia cells. Saudi J Biol
Sci. 24:678–686. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Wang HJ, Li D, Yang FY, Tashiro S, Onodera
S and Ikejima T: Oridonin induces human melanoma A375-S2 cell death
partially through inhibiting insulin-like growth factor 1 receptor
signaling. J Asian Nat Prod Res. 10:787–798. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Gu Z, Wang X, Qi R, Wei L, Huo Y, Ma Y,
Shi L, Chang Y, Li and Zhou L: Oridonin induces apoptosis in uveal
melanoma cells by upregulation of Bim and downregulation of fatty
acid synthase. Biochem Biophys Res Commun. 457:187–193. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
He H and Jiang Y: Oridonin is a covalent
NLRP3 inhibitor with strong anti-inflammasome activity. Nat Commun.
9:25502018. View Article : Google Scholar : PubMed/NCBI
|
















