Introduction
Pancreatic cancer is one of the most lethal human
malignancies worldwide (1). To date,
the causes underlying pancreatic cancer are still unknown, although
certain risk factors have been identified. The possible risk
factors for pancreatic cancer include sex (male), age (≥50),
smoking, alcohol abuse, obesity, physical activities, diabetes,
chronic pancreatitis and genetic alterations (2). Patients diagnosed with pancreatic
cancer typically have a poor prognosis (1,2).
Although mortality from the most common types of cancer has
declined in the past few decades, the mortality of patients with
pancreatic cancer remains high with a 5-year survival rate of only
6% (1). Surgical resection is the
only potential curative therapy for pancreatic cancer. In the
majority of cases, the disease has already metastasized to distant
organs at the time of diagnosis (1,2).
Gemcitabine (dFdC) is a deoxycytidine nucleoside analog, whose
anti-proliferative properties are dependent on several inhibitory
actions on DNA synthesis, blocking cell cycle progression at the
G1/S-phase boundary (3).
Gemcitabine has been recognized as the standard first-line
chemotherapy drug for pancreatic cancer. However, a previous study
showed that gemcitabine can increase patient median survival by
only 6 months (4). It is therefore
crucial to develop novel therapeutic options and effective
treatments for this disease.
It has been demonstrated that the PI3K/Akt pathway
is excessively activated in various types of cancer, including
pancreatic cancer (5). Furthermore,
up to 60% of pancreatic cancer tissues and most pancreatic cancer
cell lines exhibit increased Akt activity (6). Previous studies have reported that Akt
is a major mediator of cell survival and apoptosis through the
regulation of pro-survival and antiapoptotic proteins, including
Bcl-XL and NF-κB in both normal and neoplastic cells (7,8).
Modulation of the Akt signaling pathway may therefore be considered
a promising therapeutic approach for the treatment of pancreatic
cancer.
MK-2206 is an allosteric Akt inhibitor that has been
approved as an anti-cancer agent and that is administered orally in
patients with cancer (such as renal cell carcinoma, parotid
adenocarcinoma and colorectal cancer) (9). Previous studies have demonstrated that
MK-2206 can suppress tumor growth (such as renal cell carcinoma,
parotid adenocarcinoma and colorectal cancer) and enhance the
antitumor efficacy of conventional chemotherapeutic agents,
including docetaxel and carboplatin (10,11).
However, to the best of our knowledge, only a few studies (10,11) have
investigated the effect of MK-2206 on pancreatic cancer. The
present study aimed to evaluate the antitumor effect of MK-2206 in
pancreatic cancer cell lines in order to determine whether it may
be considered as a promising therapeutic agent for the treatment of
patients with pancreatic cancer.
Materials and methods
Cell culture
The human pancreatic cancer lines Mia PaCa-2,
Panc-1, BxPC-3, AsPC-1, Capan-2, CFPAC-1 and SW1990 were obtained
from the American Type Culture Collection. All cell lines (Mia
PaCa-2, Panc-1, Capan-2, CFPAC-1 and SW1990) were routinely
maintained in Dulbecco's modified Eagle's medium (HyClone; GE
Healthcare Life Sciences) or RPMI-1640 medium (HyClone; GE
Healthcare Life Sciences) (BxPC-3 and AsPC-1) containing 10% FBS
(Gibco; Thermo Fisher Scientific, Inc.) in a humidified atmosphere
of 5% CO2 at 37°C.
Reagents and antibodies
MK-2206 and gemcitabine were purchased from Selleck
Chemicals. MK-2206 was dissolved in DMSO according to the
instruction. Gemcitabine was dissolved in water according to the
instruction. The primary antibodies against phosphorylated (p)-Akt
(ser473) (cat. no. 3787), total (T)-Akt (cat. no. 4691) and β-actin
(cat. no. 12262) were purchased from Cell Signaling Technology,
Inc. Anti-rabbit IgG, HRP-linked secondary antibody (cat. no. 7074)
were purchased from Cell Signaling Technology, Inc.
Western blotting
Pancreatic cells were lysed with 2X SDS lysis
buffer. Bicinchoninic acid (EpiZyme) was used to quantify proteins.
The amount of proteins loaded per lane was 20 µg. Proteins were
separated by SDS-PAGE (separating gel concentration, 10%; stacking
gel concentration, 10%) and transferred onto nitrocellulose
membranes (Axygen; Corning, Inc.). Membranes were blocked with 5%
milk in Tris-buffered saline (TBS) for 1 h at room temperature, and
incubated overnight with the primary antibodies (p-Akt (1:1,000),
T-Akt (1:1,000) and β-actin (1:1,000) at 4°C. After incubation with
HRP-conjugated secondary antibodies (anti-rabbit secondary antibody
for p-Akt and T-Akt, 1:5,000) for 1 h at room temperature, the
membranes were washed 3 times with TBS for 10 min. Bands were
detected using enhanced chemiluminescence detection kit (EMD
Millipore).
Cell viability assay
Mia PaCa-2 and Panc-1 cells were seeded in 96-well
plates at a density of 5×103 cells/well. After 24 h, the
medium was replaced by various concentrations of drugs, and the
cells were incubated for another 48 h. Cell viability was
determined using Cell Counting Kit-8 (CCK-8; Dojindo Molecular
Technologies, Inc.). The amount of CCK-8 reagent added per well was
10 µl. Cells were cultured in a humidified atmosphere of 5%
CO2 and 95% air at 37°C. Absorbance was measured at 450
nm using a microplate reader.
Colony-formation assay
To assess the effect of MK-2206 on the colony
formation of Mia PaCa-2 and Panc-1 cell lines, cells were seeded in
3.5 cm dishes at a density of 1×103 cells/dish. The
medium was renewed every three days until visible colonies were
formed for one week. Cells were fixed with anhydrous methanol for
15 min and stained with 1% crystal violet for 10 min at room
temperature. Colonies were captured with a camera.
Apoptosis detection
The apoptotic rates of Mia PaCa-2 and Panc-1 cell
lines following treatment with MK-2206 were assessed by flow
cytometry with Annexin-V/propidium iodide (PI) staining according
to the manufacturer's instructions (cat. no. FA101-01; TransGen
Biotech). Apoptotic rates were subsequently determined using a flow
cytometer (LSRFortessa X-20; BD Biosciences).
Statistical analysis
All data were presented as the means ± standard
deviation. Three independent repeats were conducted for each
experiment. Comparison between the groups was calculated using a
two-tailed Student's t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
MK-2206 inhibits Akt activation in
pancreatic cancer cell lines
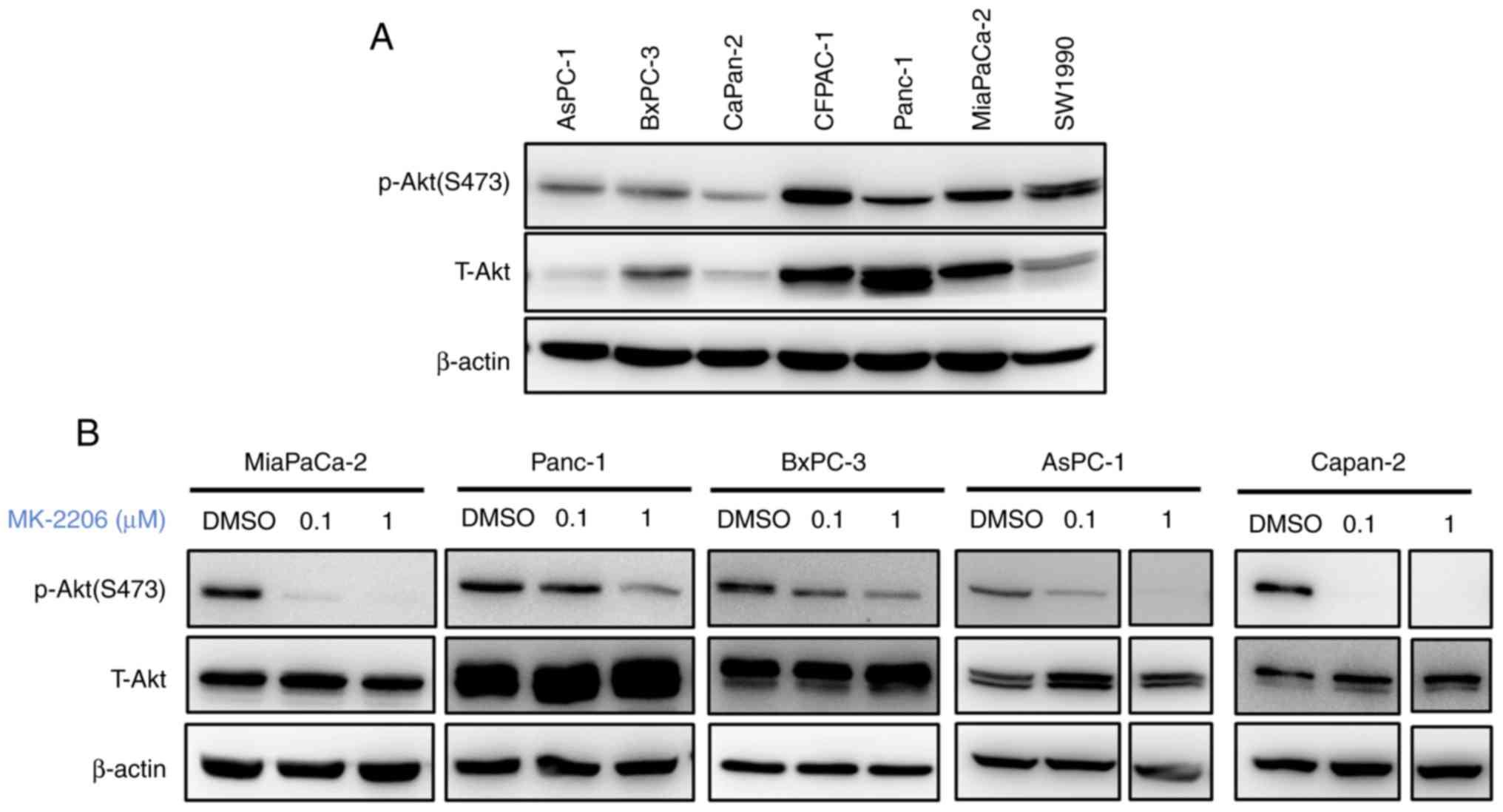
The expression of p-Akt was examined in human
pancreatic cancer cell lines. The results demonstrated that
different levels of Akt were activated in different pancreatic
caner cell lines (Fig. 1A). The
effect of MK-2206 on Akt phosphorylation was subsequently examined.
Treatment with MK-2206 was performed at 0.1 and 1 µM for 48 h. As
presented in Fig. 1B, MK-2206
treatment reduced the expression p-Akt in all pancreatic cancer
cell lines. No changes in the levels of total Akt protein were
observed. These results demonstrated that MK-2206 inhibited Akt
phosphorylation in pancreatic cancer cells.
MK-2206 inhibits the proliferation and
induces the apoptosis of pancreatic cancer cells
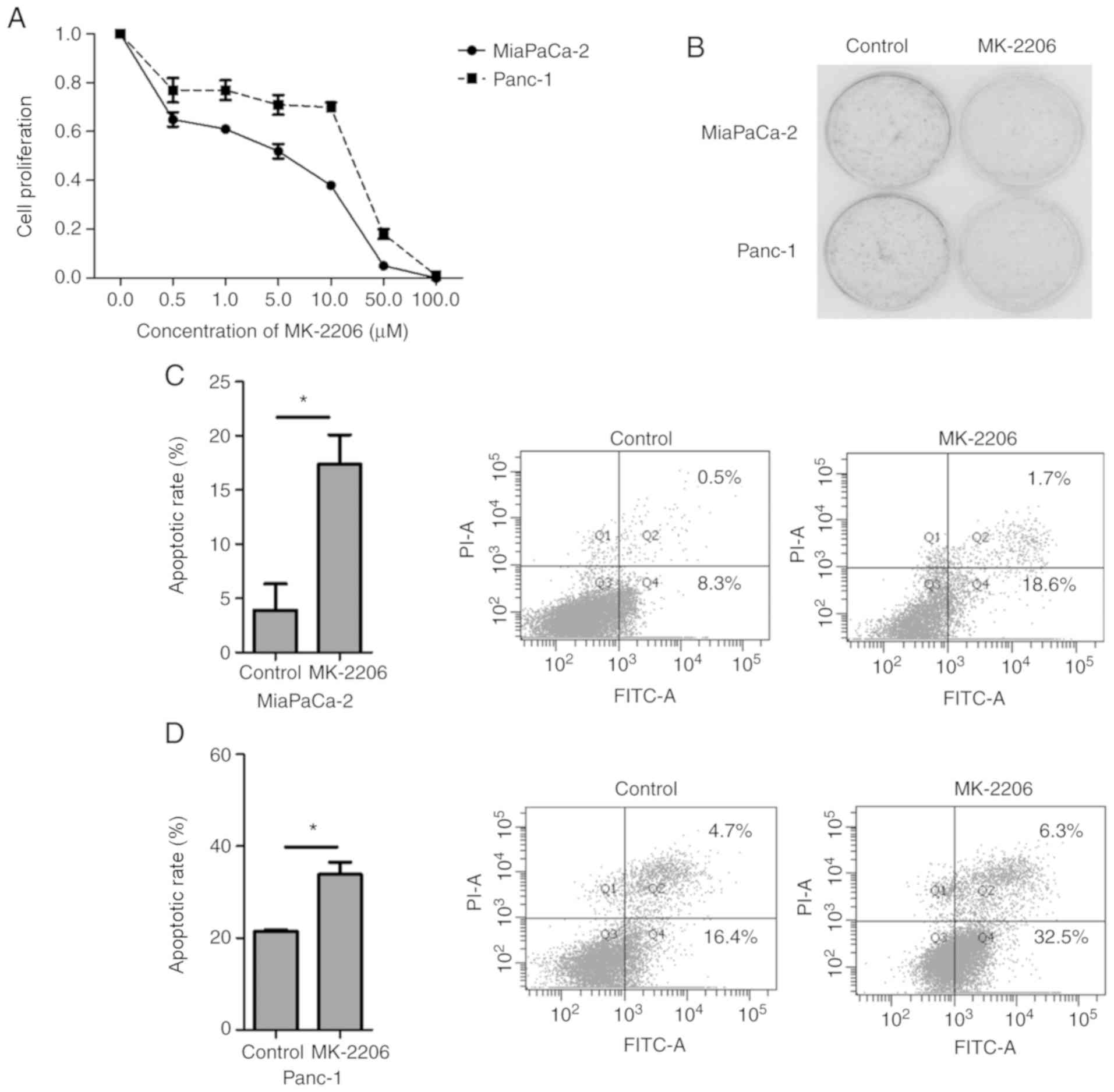
CCK-8 assay was performed to examine the effects of
MK-2206 on Mia PaCa-2 and Panc-1 cell proliferation. As presented
in Fig. 2A, MK-2206 inhibited cell
proliferation of human cancer cell lines. Furthermore, colony
formation assay was performed to assess the effects of MK-2206 on
the viability of Mia PaCa-2 and Panc-1 cells. The results
demonstrated that the number of colonies formed following treatment
with MK-2206 was reduced in both cell lines (Fig. 2B).
To assess MK-2206-induced apoptosis, Mia PaCa-2 and
Panc-1 cells were treated with DMSO (0.1%) or 1 µM MK-2206 for 48
h, and the percentage of annexin V-PI positive cells was
determined. The results demonstrated that treatment with MK-2206
for 48 h significantly induced apoptosis in the two pancreatic
cancer cell lines (Fig. 2C and
D).
MK-2206 increases the cytotoxic
effects of gemcitabine in human pancreatic cancer cells
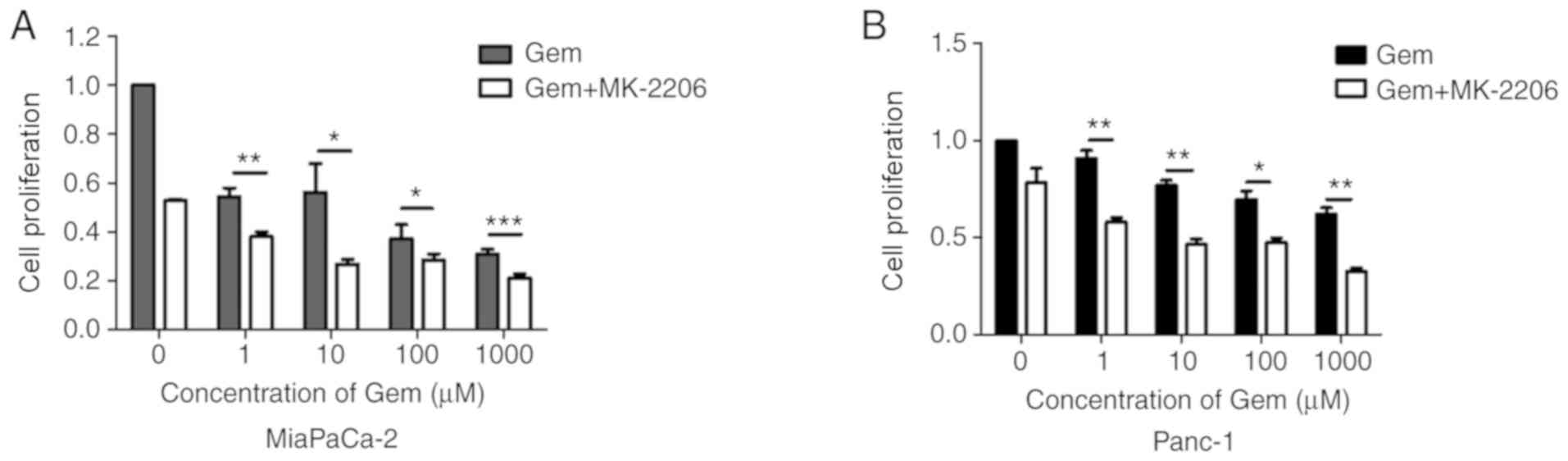
Gemcitabine has been extensively used in the
treatment of pancreatic cancer (3).
As presented in Fig. 3A and B,
MK-2206 and gemcitabine co-treatment for 48 h significantly
decreased cell proliferation compared with the gemcitabine and DMSO
group. These data suggested that MK-2206 may enhance the
cytotoxicity induced by gemcitabine in pancreatic cancer cells.
MK-2206 inhibits gemcitabine-induced
Akt activation in pancreatic cancer cell lines
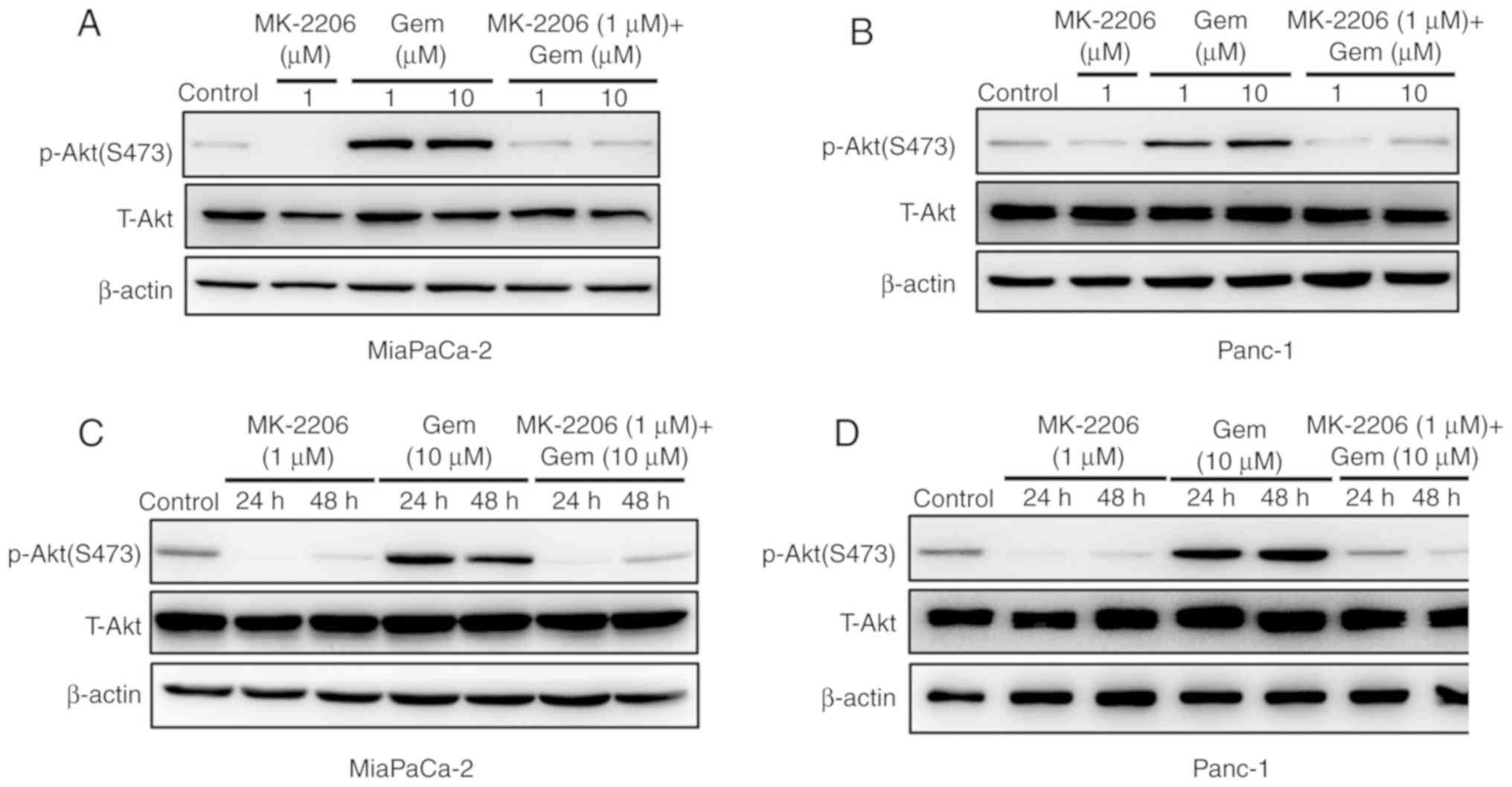
A previous study demonstrated that gemcitabine
induces Akt activation in pancreatic cancer cells (12). Activation of the Akt survival pathway
may therefore block the therapeutic efficacy of gemcitabine
(10). To investigate whether
MK-2206 may inhibit gemcitabine-induced Akt activation in
pancreatic cancer cells, p-Akt expression in Mia PaCa-2 and Panc-1
cell lines was assessed following treatment with gemcitabine alone
(1 or 10 µM) or in combination with 1 µM MK-2206 for 24 and 48 h.
The results demonstrated that the expression of p-Akt (Ser473) was
increased after treatment with 1 and 10 µM gemcitabine for 48 h in
Mia PaCa-2 and Panc-1 cell lines. However, gemcitabine-induced Akt
phosphorylation was inhibited by 1 µM MK-2206 in these two
pancreatic cancer cell lines (Fig. 4A
and B). In addition, 10 µM gemcitabine treatment for 24 and 48
h increased Akt phosphorylation in Mia PaCa-2 and Panc-1 cell
lines, and gemcitabine-induced Akt phosphorylation was inhibited by
1 µM MK-2206 treatment for 24 and 48 h in these two pancreatic
cancer cell lines (Fig. 4C and
D).
Discussion
MK-2206 is a highly selective allosteric inhibitor
of Akt that binds to the pleckstrin-homology domain of Akt,
inducing a conformational change that prevents Akt localization to
the plasma membrane and its subsequent activation (13). In the present study, human pancreatic
cancer cell lines Mia PaCa-2, Panc-1, CFPAC-1 and SW1990 exhibited
higher p-Akt expression compared with BxPC-3, AsPC-1 and Capan-2
cell lines. In addition, a previous study from our laboratory
demonstrated that Mia PaCa-2 and Panc-1 cell lines were more
resistant to gemcitabine compared with CFPAC-1 and SW1990 cell
lines (data not shown). Thus, Mia PaCa-2 and Panc-1 cell lines were
selected for subsequent experiments in the present study. The
results of the present study demonstrated that MK-2206 decreased
p-Akt expression and reduced cell proliferation and colony
formation in Mia PaCa-2 and Panc-1 cell lines. In addition, MK-2206
stimulated apoptosis in Mia PaCa-2 and Panc-1 cells. These results
were consistent with previous studies on other types of cancer,
including gastric cancer, neuroblastoma and thyroid cancer
(14–16).
It is worth noting that the inhibition rate of
MK-2206 in pancreatic cancer cells in Fig. 3A and B disagree with those of
Fig. 2. This may have occurred due
to the long-term use of the cell lines, as cellular viability
decreases and cell senescence level increases with the extension of
the passage number.
Gemcitabine, used alone or in combination with other
therapeutic agents, is the first-line chemotherapy strategy for the
treatment of locally advanced or metastatic pancreatic cancer
(4,17). However, most patients do not respond
well and develop chemoresistance to gemcitabine (18). It has been reported that gemcitabine
can activate the Akt signaling pathway in pancreatic cancer cells
via the overproduction of reactive oxygen species, which reduces
the anti-tumor responses and inhibits the therapeutic efficacy of
gemcitabine (12). In the present
study, MK-2206 treatment in combination with gemcitabine abolished
the gemcitabine-induced Akt activation and increased the cytotoxic
effect of gemcitabine. These findings were consistent with a
previous study reporting that MK-2206 can sensitize human cancer
cells to numerous chemotherapeutic agents, including doxorubicin
and fluorouracil (10). Previous
clinical phase 1 trial showed that MK-2206 combination with
carboplatin and paclitaxel, docetaxel, or erlotinib, was
well-tolerated at doses that inhibit Akt signaling (19). Furthermore, a number of ongoing or
completed phase 2 trials have demonstrated that numerous tumor
types are responsive to MK-2206 alone or in combination with other
drugs (20,21). Combining gemcitabine with MK-2206 may
therefore be considered as a promising therapeutic strategy for the
treatment of patients with pancreatic cancer.
In conclusion, the results of the present study
demonstrated that MK-2206 inhibited Akt phosphorylation and
attenuated pancreatic cancer cell proliferation. In addition, the
combination of MK-2206 with gemcitabine enhanced pancreatic cancer
cell proliferation inhibition, which may have been due to the
MK-2206-mediated inhibition of gemcitabine-induced Akt activation.
It is worth noting that the toxicity of MK-2206 on normal human
pancreatic ductal epithelium cells was not examined in the present
study. A previous study reported that MK-2206 was well tolerated in
patients and that its toxic and side effects were acceptable
(9). The combination of MK-2206 with
gemcitabine may therefore help overcome chemoresistance in
pancreatic cancer. Further in vivo investigation using
patient-derived xenograft models is required to confirm these
findings.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural
Science Foundation of China (grant no. 81372640) and the Shanghai
Sailing Program (grant no. 17YF1415700).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZW and GL conceived and designed the experiments. GL
interpreted the data, performed the statistical analysis and
analyzed the results. ZW and GL wrote the manuscript. ZW and ZQ
revised the manuscript. ZQ made substantial contributions to
conception and design of the study. All authors read and approved
the final version of the manuscript and agreed to be accountable
for all aspects of the research in ensuring that the accuracy of
integrity of any part of the work are appropriately investigated
and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kamisawa T, Wood LD, Itoi T and Takaori K:
Pancreatic cancer. Lancet. 388:73–85. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang Q, Zeng L, Chen Y, Lian G, Qian C,
Chen S, Li J and Huang K: Pancreatic cancer epidemiology,
detection, and management. Gastroenterol Res Pract. 2016:8962321.
2016. View Article : Google Scholar
|
|
3
|
Zeng S, Pöttler M, Lan B, Grützmann R,
Pilarsky C and Yang H: Chemoresistance in pancreatic cancer. Int J
Mol Sci. 20:E45042019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Oettle H, Post S, Neuhaus P, Gellert K,
Langrehr J, Ridwelski K, Schramm H, Fahlke J, Zuelke C, Burkart C,
et al: Adjuvant chemotherapy with gemcitabine vs observation in
patients undergoing curative-intent resection of pancreatic cancer:
A randomized controlled trial. JAMA. 297:267–277. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Manning BD and Toker A: AKT/PKB signaling:
Navigating the network. Cell. 169:381–405. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Arlt A, Müerköster SS and Schäfer H:
Targeting apoptosis pathways in pancreatic cancer. Cancer Lett.
332:346–358. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Engelman JA, Luo J and Cantley LC: The
evolution of phosphatidylinositol 3-kinases as regulators of growth
and metabolism. Nat Rev Genet. 7:606–619. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang XJ and Jia SS: Fisetin inhibits
laryngeal carcinoma through regulation of AKT/NF-κB/mTOR and ERK1/2
signaling pathways. Biomed Pharmacother. 83:1164–1174. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yap TA, Yan L, Patnaik A, Fearen I, Olmos
D, Papadopoulos K, Baird RD, Delgado L, Taylor A, Lupinacci L, et
al: First-in-man clinical trial of the oral pan-AKT inhibitor
MK-2206 in patients with advanced solid tumors. J Clin Oncol.
29:4688–4701. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hirai H, Sootome H, Nakatsuru Y, Miyama K,
Taguchi S, Tsujioka K, Ueno Y, Hatch H, Majumder PK, Pan BS and
Kotani H: MK-2206, an allosteric Akt inhibitor, enhances antitumor
efficacy by standard chemotherapeutic agents or molecular targeted
drugs in vitro and in vivo. Mol Cancer Ther. 9:1956–1967. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lin YH, Chen BYH, Lai WT, Wu SF, Guh JH,
Cheng AL and Hsu LC: The Akt inhibitor MK-2206 enhances the
cytotoxicity of paclitaxel (Taxol) and cisplatin in ovarian cancer
cells. Naunyn Schmiedebergs Arch Pharmacol. 388:19–31. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Arora S, Bhardwaj A, Singh S, Srivastava
SK, McClellan S, Nirodi CS, Piazza GA, Grizzle WE, Owen LB and
Singh AP: An undesired effect of chemotherapy: Gemcitabine promotes
pancreatic cancer cell invasiveness through reactive oxygen
species-dependent, nuclear factor κB- and hypoxia-inducible factor
1α-mediated up-regulation of CXCR4. J Biol Chem. 288:21197–21207.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Okuzumi T, Fiedler D, Zhang C, Gray DC,
Aizenstein B, Hoffman R and Shokat KM: Inhibitor hijacking of Akt
activation. Nat Chem Biol. 5:484–493. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jin P, Wong CC, Mei S, He X, Qian Y and
Sun L: MK-2206 co-treatment with 5-fluorouracil or doxorubicin
enhances chemosensitivity and apoptosis in gastric cancer by
attenuation of Akt phosphorylation. OncoTargets Ther. 9:4387–4396.
2016. View Article : Google Scholar
|
|
15
|
Li Z, Yan S, Attayan N, Ramalingam S and
Thiele CJ: Combination of an allosteric Akt Inhibitor MK-2206 with
etoposide or rapamycin enhances the antitumor growth effect in
neuroblastoma. Clin Cancer Res. 18:3603–3615. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Burke JF, Schlosser L, Harrison AD,
Kunnimalaiyaan M and Chen H: MK-2206 causes growth suppression and
reduces neuroendocrine tumor marker production in medullary thyroid
cancer through Akt inhibition. Ann Surg Oncol. 20:3862–3868. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Burris HA III, Moore MJ, Andersen J, Green
MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo
AM, Tarassoff P, et al: Improvements in survival and clinical
benefit with gemcitabine as first-line therapy for patients with
advanced pancreas cancer: A randomized trial. J Clin Oncol.
15:2403–2413. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Amrutkar M and Gladhaug IP: Pancreatic
cancer chemoresistance to gemcitabine. Cancers. 9:157–180. 2017.
View Article : Google Scholar
|
|
19
|
Molife LR, Yan L, Vitfell-Rasmussen J,
Zernhelt AM, Sullivan DM, Cassier PA, Chen E, Biondo A, Tetteh E,
Siu LL, et al: Phase 1 trial of the oral AKT inhibitor MK-2206 plus
carboplatin/paclitaxel, docetaxel, or erlotinib in patients with
advanced solid tumors. J Hematol Oncol. 7:12014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lara PN Jr, Longmate J, Mack PC, Kelly K,
Socinski MA, Salgia R, Gitlitz B, Li T, Koczywas M, Reckamp KL and
Gandara DR: Phase II study of the AKT inhibitor MK-2206 plus
erlotinib in patients with advanced non-small cell lung cancer who
previously progressed on erlotinib. Clin Cancer Res. 21:4321–4326.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ramanathan RK, McDonough SL, Kennecke HF,
Iqbal S, Baranda JC, Seery TE, Lim HJ, Hezel AF, Vaccaro GM and
Blanke CD: Phase 2 study of MK-2206, an allosteric inhibitor of
AKT, as second-line therapy for advanced gastric and
gastroesophageal junction cancer: A SWOG cooperative group trial (S
1005). Cancer. 121:2193–2197. 2015. View Article : Google Scholar : PubMed/NCBI
|