Introduction
Endometrial cancer (EC) represents the most frequent
gynecologic tumor in developed countries (1). The majority of patients presents with
early stage, well-differentiated, limited myometrial invasive
tumors and favorable prognosis, but a subset of women recurs and do
not survive from the disease (2). EC
is hormone-dependent and, like breast cancer, can express markers
such as estrogen receptors (ERs), progesterone receptors (PRs) and
oncoprotein c-erbB-2 (HER2) (3,4). Breast
cancer can be characterized into several subgroups based on
immunohistochemistry: Luminal A (ER+ and/or
PR+, HER2−, low Ki67); luminal B (ER and/or
PR+, HER2−, high Ki67); non-luminal
(ER−, PR−, and HER2+); and triple
negatives (ER−, PR−, and HER2−)
(5). This classification finds
clinical application in terms of prognosis and treatment
personalization. Since HER2+ and triple negative (TN)
subtypes show a poor prognosis, patients are treated with more
aggressive treatments (chemotherapy and immunotherapy), both in
adjuvant and in metastatic disease (6–8).
Particularly, triple negative phenotype (TNP) is often associated
with unfavorable pathological features such as high nuclear grade,
high proliferation rate, increased risk to show distant metastasis
at the diagnosis or to recur after surgery. The absence of ER, PR
and HER2 influence the poor response to treatments currently
available causing poor prognosis of this subgroup of patients
(9). Furthermore, no therapeutic
targets have yet been established in this subgroup of breast cancer
(10). Ongoing studies are
focalizing on target therapy. For example, PARP inhibitors have
demonstrated a potential benefit in association with chemotherapy
in patients affected by TN breast cancer (11). EC molecular subtypes based on ER, PR
and HER2 status have proven to differ in terms of prognosis and
clinicopathological data: ER and PR expression leads to a more
favorable prognosis, while overexpression of p53, HER2 and
epidermal growth factor receptor (EGFR) all predict a poor
prognosis (3,4,12–14).
Nevertheless, currently the choice of treatment in EC is not
conditioned by molecular features of the tumor.
The aim of our study is to estimate the prognostic
value of TNP in EC related to pathological and clinical
characteristics.
Materials and methods
This retrospective study includes two hundred and
twenty patients diagnosed with EC at the Guglielmo da Saliceto
hospital of Piacenza (Northern Italy) between January 1, 2000 and
December 31, 2010. All patients underwent total abdominal
hysterectomy with or without bilateral salpingo-oophorectomy. Only
patients with tissue available for staging and histological review
were included; fifty eight cases of EC were excluded because biopsy
was not followed by hysterectomy. Postoperatively, some only three
patients received adjuvant treatment, one triple negative
endometrial cancer (TNEC) and two non-TNECs. Follow-up information
was available until December 2016; the follow-up period ranged from
a minimum of 55 to a maximum of 170 months. A database with
demographic, clinical and pathologic information for each patient
was created. The study was approved by the Institutional Review
Board of Hospital of Piacenza, and conducted according to the
Declaration of Helsinki. Pathologist performed specimens review of
all endometrial cancers, redefining the histologic type, grade,
depth of myometrial invasion, and immunohistochemical expression of
ER, PR, HER2 and Ki67. The same tissue from formalin-fixed,
paraffin-embedded donor blocks were precisely arrayed into a new
recipient paraffin block (35×20 mm). Paraffin-embedded tissue was
cut at 3 µm thickness and placed on positively charged slides.
Slides were placed in a 60°C oven for 1 h, cooled, then
deparaffinized. Immunohistochemical techniques can be used to
demonstrate the presence of antigens in tissue. PR clone 16 and ER
clone 6F11 antibodies were specifically optimized for use in a
dedicated automation system (Leica Biosystems Ltd., Newcastle, UK),
in combination with Bond Polymer Refine Detection, a novel
controlled polymerization technology to prepare polymeric
HRP-linker antibody conjugates. This detection system avoids the
use of streptavidin and biotin, and therefore eliminates
nonspecific staining as a result of endogenous biotin. The system
is based on consecutive application of: i) Specimen, incubated with
hydrogen peroxide to quench endogenous peroxidase activity; ii)
ready-to-use primary PR (or ER) antibody; iii) post-primary
antibody solution, which enhances the penetration of subsequent
polymer reagents; iv) poly-HRP anti-mouse/rabbit IgG reagent,
localizing the primary antibody; and v) the chromogenic substrate
3,3′-diaminobenzidine (DAB), allowing the revelation of the
antibody complex via a brown precipitate. A blue counterstain of
cell nuclei is provided by Hematoxylin coloration. For ER and PR,
immunohistochemical results were evaluated both as a percentage of
nuclear staining and as intensity of staining. The results were
recorded as 3+ for strong or weak nuclear staining in >50% of
cells, 2+ for strong or weak nuclear staining in 10 to 50% of
cells, and 1+ for strong or weak nuclear staining in <10%. Cases
with no evidence of nuclear staining, or only rare scattered
positive cells, were recorded as negative (0). HER2 expression was
performed using Pathway HER2 CB11 clone) on the Leica automated
system (Leica Biosystems Ltd.) according to the manufacturer's
recommended protocol. Positive tissue controls are used to indicate
correctly prepared tissues and proper staining techniques. One
positive tissue control should be included for each set of test
conditions in each staining run. The score was recorded as 3+ for
complete strong membrane staining in >10% of tumor cells, 2+ for
complete moderate membrane staining in >10%, 1+ for incomplete
staining or complete staining in <10% of tumor cells, and 0 for
no staining or staining without a membranous pattern. Biomarker
expression for ER, PR and HER2 was designated as positive for 3+
and 2+, and negative for 1+ and 0. In most cases, we used
fluorescence in situ hybridization (FISH) for the
determination of HER2 gene amplification status when the test
results were borderline (2+). HER2 receptors receive signals that
stimulate the growth of cancer cells. Tumors staining negative for
ER, PR, and HER2 were designated as TNP and those with one or more
positive stains were designated as non-TNPs. All 220 cases of EC
underwent comprehensive surgery and were therefore were stratified
as low, intermediate and high risk according to the European
Society of Medical Oncology (ESMO) guidelines, which are based on
both pathological and surgical staging. Risk groups were related to
the expression of ER, PR and HER2.
Statistical analysis
Pearson's Chi-square (χ2) and Fisher's
exact test were used to evaluate the association of TNP cases with
several variables associated with a worse prognosis.
Progression-free survival (PFS) and overall survival (OS) were
analyzed with Kaplan-Meier curves. This function uses the
Kaplan-Meier procedure to estimate the survival function. All
patients were set to a standard starting time (t0), and
cases were censored as they quit follow-up. The log-rank test was
used to compare the groups (TNEC vs. non-TNEC). All tests were
two-tailed, and the P<0.0001 was considered to indicate a
statistically significant difference.
Results
Two hundred and twenty patients were included in our
study. All patients were Caucasian. The median age at the diagnosis
was 67 years (range, 36–89). One hundred and ninety-nine showed
endometrioid histotype (90.5%) and 21 were high risk histological
type, 11 with papillar serous type (5%), 9 clear cell (4%), and 1
carcinosarcoma (0.5%). Eighty-five (38.6%) patients were placed in
the G1 grading class, 87 (39.5%) were G2 and 48 (21.9%) G3.
Ninety-five cases (43.2%) showed deep myometrial invasion (>50%)
and 105 (56.8%) low invasion <50%. Sixty-four (29%) patients
showed low Ki67 (≤30%) and 156 (71%) Ki67 >30%. Regarding stage
at the diagnosis, 173 cases (78.6%) were staged in according to the
revised 2009 FIGO (International Federation of Gynecology and
Obstetrics) staging system for EC, resulting in 18 (8.2%) stage II,
8 (3.7%) III, and 21 (9.5%) IV.
Twenty-six (12%) patients showed a TNP. The
clinicopathological characteristics of the two groups of patients
included in the study are summarized in Table I. TN cases had a higher percentage of
grade 3 (42.3 vs. 19%), high risk histology (34.6 vs. 6.2%),
advanced stage (38.5 vs. 9.8%) and high grade disease (42.3 vs.
28.8%) compared to the non-TN subgroup. In this pattern of patient
the deep myometrial invasion, lymph node metastasis and cervical
involvement were similar between two groups. Relapses were
significantly higher in the TN patients group (39.1 vs. 12.3%).
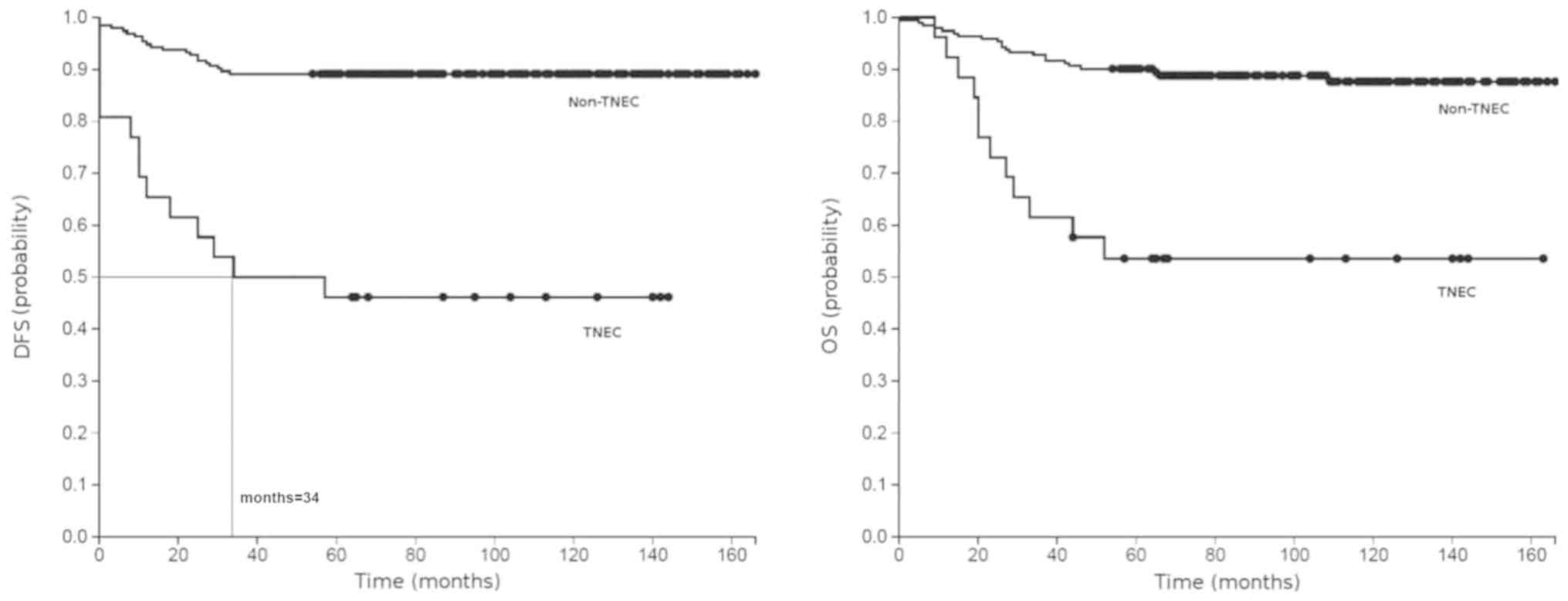
Outcome was also more favorable for non-TN cases (Table II). Kaplan-Meier plots showed
significantly shorter PFS and OS in TN patients compared to non-TN
cases (log-rank test, P<0.0001; Fig.
1). We could only calculate the median disease-free survival
(DFS) for TNECs (34 months), as other estimates did not reach 0.5.
The 5-year OS rate was 34.8% in TNPs compared to 64.7% in control
group.
 | Table I.Clinicopathological features of triple
negative endometrial cancer (TNEC) and non-triple negative
endometrial cancer (NON-TNEC). |
Table I.
Clinicopathological features of triple
negative endometrial cancer (TNEC) and non-triple negative
endometrial cancer (NON-TNEC).
|
| TNEC + non-TNEC
(n=220) | TNEC (n=26) | Non-TNEC (n=194) |
|
|---|
|
|
|
|
|
|
|---|
| Variable | n | % | n | % | n | % | P-value |
|---|
| Median age at
diagnosis | 67 | 66 | 71 |
|
|
|
|
| Patients
<65 | 87 | 39.50 | 5 | 19.20 | 82 | 42.30 | 0.0004 |
| Patients
≥65 | 133 | 60.50 | 21 | 80.80 | 112 | 57.70 |
|
| Stage |
| I | 173 | 78.60 | 15 | 57.70 | 158 | 81.40 | <0.0001 |
| II | 18 | 8.20 | 1 | 3.80 | 17 | 8.80 |
|
| III | 8 | 3.60 | 3 | 11.50 | 5 | 2.60 |
|
| IV | 21 | 9.50 | 7 | 26.90 | 14 | 7.20 |
|
| Histology |
|
Endometriod | 199 | 90.50 | 17 | 65.40 | 182 | 93.80 | <0.0001 |
| Clear
cell | 9 | 4.10 | 4 | 15.40 | 5 | 2.60 |
|
|
Serous | 11 | 5.00 | 5 | 19.20 | 6 | 3.10 |
|
|
Carcinosarcoma | 1 | 0.50 | 0 | 0.00 | 1 | 0.50 |
|
| Grading |
| G1 | 85 | 38.60 | 4 | 15.40 | 81 | 41.80 | <0.0001 |
| G2 | 87 | 39.50 | 11 | 42.30 | 76 | 39.20 |
|
| G3 | 48 | 21.80 | 11 | 42.30 | 37 | 19.10 |
|
| Myometrial
invasion |
|
<50% | 125 | 56.80 | 14 | 53.80 | 111 | 57.20 | 0.6727 |
|
>50% | 95 | 43.20 | 12 | 46.20 | 83 | 42.80 |
|
| Early | 191 | 86.80 | 16 | 61.50 | 175 | 90.20 | <0.0001 |
| Advanced | 29 | 13.20 | 10 | 38.50 | 19 | 9.80 |
|
| Risk group |
| Low to
intermediatea | 153 | 69.50 | 15 | 57.70 | 138 | 71.10 | 0.057 |
|
Highb | 67 | 30.50 | 11 | 42.30 | 56 | 28.90 |
|
 | Table II.Outcome of TNEC and non-TNEC. |
Table II.
Outcome of TNEC and non-TNEC.
|
| TNEC + non-TNEC
(n=212) | TNEC (n=23) | Non-TNEC
(n=189) |
|
|---|
|
|
|
|
|
|
|---|
| Outcome | n | % | n | % | n | % | P-value |
|---|
| Alive | 165 | 77.80 | 10 | 43.50 | 155 | 82.00 | <0.0001 |
| Deceased | 47 | 22.20 | 13 | 56.50 | 34 | 18.00 |
|
| Alive without
disease | 158 | 74.50 | 9 | 39.10 | 149 | 78.80 | <0.0001 |
| Alive with
disease | 7 | 3.30 | 1 | 4.30 | 6 | 3.20 |
|
| Deceased (from
disease) | 34 | 16.00 | 12 | 52.20 | 22 | 11.60 |
|
| Deceased (other
cause) | 13 | 6.10 | 1 | 4.30 | 12 | 6.30 |
|
Discussion
The term ‘triple negative’ (TN) is used to define a
specific subtype of breast cancer, which is characterized by
absence of ER, PR, and HER2 expression (9). This subgroup of breast cancers is
clinically more aggressive than other subgroups, causing poor
prognosis with low response to therapies and short-term survival of
patients (10,11). TNP is poorly investigated in other
types of tumors, although some authors report that the loss of
estrogen, progesterone, and HER2 receptors predict poor prognosis
also in gynecological cancers. In EC, TNPs are observed in ~15–20%
of patients and are related to unfavorable pathological features
such as high risk histological type, deeper myometrial invasion,
higher histological grade and clinical staging, and shorter
survival (12–15). For example, Kothari et al
reported that in a group of patients affected by EC the TNP was
associated with advanced stage, high grade, and high risk
histology, as well as poor survival, as compared to non-TNPs
(16). A similar percentage of TNs
observed in ovarian, endometrial, and breast cancers, may suggest a
similar pathogenesis for these neoplasms (17). Our research, comparing the difference
in clinicopathological parameters between TNEC and non-TNEC cases,
confirms that the TNP has prognostic significance in EC. In our
cohort, 12% of EC cases showed a TNP, which is consistent with
previous studies. Very few patients underwent adjuvant chemotherapy
and radiotherapy after surgical resection, and they were equally
distributed between the two groups of TNECs and non-TNECs.
Therefore, different treatments did not affect PFS and OS. Our data
revealed that most TNECs showed high-grade features, such as
advanced stage, high clinical grade, and high risk histology, as
well as poor survival. This was also confirmed by other authors.
TNEC cases show shorter progression-free and OS than non-TNEC
cases, thus indicating that TNP may be an independent prognostic
factor for progression-free and OS in EC. Although TN cases account
for only a minority of patients among EC, this subgroup should be
regarded as a clinically important subtype owing to its aggressive
clinicopathological characteristics. Confirming the correlation
between TNP and poor prognostic factors is important, as TNP in
endometrial tumors could potentially predict a lack of response to
specific therapies. This was widely described for TN breast
cancers, which are not responsive to antiestrogens or trastuzumab
(7,9,11).
Studies continue to be conducted to find the best approaches to
treat triple-negative tumors. Recent clinical trials are trying to
investigate whether some targeted therapies are effective against
triple-negative breast cancer (18).
These treatments are aimed to different and novel targets. The
specific role of some mutations in possible driver genes for these
tumors is also under investigation. BRCA-1 mutations, which are
present in a subgroup of TN breast cancer, deprive constitutively
tumor cells of a DNA repair mechanism, increase platinum
sensitivity, and seem to sensitize cells to the therapy with poly
ADP-ribose polymerase (PARP-1) inhibitors (19). The mutation of PTEN gene leads to the
loss of the suppressing effects of its encoded protein on the
PI3-K/AKT pathway, and consequently to an increased activity of
mTOR protein kinase, thus interfering with the cell cycle and
apoptosis of tumor cell. Abnormal PTEN expression seems to improve
the cellular response to therapies with mTOR inhibitors. PTEN gene
mutations are also described in EC, as associated with positive
prognostic factors (favorable histological type, lower histological
grade, absence of myometrial invasion, and lower clinical staging).
These mutations are also related to tumor response to chemotherapy
(20,21). Clinical studies are being conducted
on PARP-1 inhibitors and other drugs which can block mTOR and
PI3K/AKT pathways. These studies are performed in all types of TN
tumors, due to their similar pathogenesis and the equal role of DNA
repair pathways (22). Many TN
breast cancer cells overexpress epidermal growth factor receptors
(EGFR), which receive signals that stimulate the growth of the
cancer. Drugs targeting EGFR could block growth signals on the
cancer cells also in EC (23). Other
studies showed that a higher CD151 expression in TN breast cancers
is associated with shorter survival time and poor prognosis, but
data on the expression levels of these receptors are limited. A
recent study examined the relation between tumor related
macrophages and the TNEC. In this study, a higher percentage of
tumor related macrophages was found in TN cancers (24). Due to their aggressive profiles and
poor prognosis, TN cancers are important objects of research and
may prove to benefit from individualized target therapies.
In conclusion, TNP EC represent a subset of EC with
a worse prognosis with a shorter PFS and OS. Our results reported
here are confirmed in other studies, the evaluation of ER, PR and
HER-2 should be therefore integrated into the prognostic factor of
EC and this submit of TNP EC be considered a potential target for
possible experimental treatment.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
RP and LC designed and supervised the trial. RP, CC,
AMR, AU, FB, MP, CDN and LC were responsible for patients and data
collection. RP and CC analyzed the data. All authors read and
approved the manuscript and agree to be accountable for all aspects
of the research in ensuring that the accuracy or integrity of any
part of the work are appropriately investigated and resolved.
Ethics approval consent to participate
The present study was approved by the Local Ethics
Committee of Piacenza General Hospital. Informed consent was
previously obtained from each patient.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interest.
Glossary
Abbreviations
Abbreviations:
|
DFS
|
disease-free survival
|
|
EC
|
endometrial cancer
|
|
ER
|
estrogen receptor
|
|
EGFR
|
epidermal growth factor receptor
|
|
ESMO
|
European Society of Medical
Oncology
|
|
FIGO
|
Internazional Federation of Gynecology
and Obstetrics
|
|
FISH
|
fluorescence in situ
hybridization
|
|
OS
|
overall survival
|
|
PFS
|
progression-free survival
|
|
PR
|
progesterone receptor
|
|
TNEC
|
triple negative endometrial cancer
|
|
TNP
|
triple negative phenotype
|
References
|
1
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer statistics, 2009. CA Cancer J Clin. 59:225–249.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Colombo N, Preti E, Landoni F, Carinelli
S, Colombo A, Marini C and Sessa C; ESMO Guidelines Working Group,
: Endometrial cancer: ESMO clinical practice guidelines for
diagnosis, treatment and follow-up. Ann Oncol. 24 (Suppl
6):vi33–vi38. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kounelis S, Kapranos N, Kouri E, Coppola
D, Papadaki H and Jones MW: Immunohistochemical profile of
endometrial adenocarcinoma: A study of 61 cases and review of the
literature. Mod Pathol. 13:379–388. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Talhouk A, McConechy MK, Leung S, Li-Chang
HH, Kwon JS, Melnyk N, Yang W, Senz J, Boyd N, Karnezis AN, et al:
A clinically applicable molecular-based classification for
endometrial cancers. Br J Cancer. 113:299–310. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wu T, Wang Y, Jiang R, Lu X and Tian J: A
pathways-based prediction model for classifying breast cancer
subtypes. Oncotarget. 8:58809–58822. 2017.PubMed/NCBI
|
|
6
|
Jahn B, Rochau U, Kurzthaler C, Hubalek M,
Miksad R, Sroczynski G, Paulden M, Bundo M, Stenehjem D, Brixner D,
et al: Personalized treatment of women with early breast cancer: A
risk-group specific cost-effectiveness analysis of adjuvant
chemotherapy accounting for companion prognostic tests OncotypeDX
and Adjuvant!Online. BMC Cancer. 17:6852017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Prat A, Pineda E, Adamo B, Galván P,
Fernández A, Gaba L, Díez M, Viladot M, Arance A and Muñoz M:
Clinical implications of the intrinsic molecular subtypes of breast
cancer. Breast. 24 (Suppl 2):S26–S35. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dai X, Li T, Bai Z, Yang Y, Liu X, Zhan J
and Shi B: Breast cancer intrinsic subtype classification, clinical
use and future trends. Am J Cancer Res. 5:2929–2943.
2015.PubMed/NCBI
|
|
9
|
Oakman C, Viale G and Di Leo A: Management
of triple negative breast cancer. Breast. 19:312–321. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Trivers KF, Lund MJ, Porter PL, Liff JM,
Flagg EW, Coates RJ and Eley JW: The epidemiology of
triple-negative breast cancer, including race. Cancer Causes
Control. 20:1071–1082. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Anders CK and Carey LA: Biology,
metastatic patterns, and treatment of patients with triple-negative
breast cancer. Clin Breast Cancer. 9 (Suppl 2):S73–S81. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shabani N, Kuhn C, Kunze S, Schulze S,
Mayr D, Dian D, Gingelmaier A, Schindlback C, Willgeroth F, Sommer
H, et al: Prognostic significance of oestrogen receptor alpha
(ERalpha) and beta (ERbeta), progesterone receptor A (PR-A) and B
(PR-B) in endometrial carcinomas. Eur J Cancer. 43:2434–2444. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Khalifa MA, Mannel RS, Haraway SD, Walker
J and Min KV: Expression of EGFR, HER2/neu, P53, and PCNA in
endometrioid, serous papillary, and clear cell endometrial
adenocarcinomas. Gynecol Oncol. 53:84–92. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Samarnthai N, Hall K and Yeh IT: Molecular
profiling of endometrial malignancies. Obstet Gynecol Int.
2010:1623632010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Trovik J, Wik E, Werner HM, Krakstad C,
Helland H, Vandenput I, Njolstad TS, Stefansson IM, Marcickiewicz
J, Tingulstad S, et al: Hormone receptor loss in endometrial
carcinoma curettage predicts lymph node metastasis and poor outcome
in prospective multicentre trial. Eur J Cancer. 49:3431–3441. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kothari R, Morrison C, Richardson D,
Seward S, O'Malley D, Copeland L, Fowler J and Cohn DE: The
prognostic significance of the triple negative phenotype in
endometrial cancer. Gynecol Oncol. 118:172–175. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu N, Wang X and Sheng X: The
clinicopathological characteristics of ‘triple-negative’ epithelial
ovarian cancer. J Clin Pathol. 63:240–243. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bulsa M and Urasińska E: Triple negative
endometrial cancer. Ginekol Pol. 88:212–214. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Domagala P, Huzarski T, Lubinski J, Gugala
K and Domagala W: PARP-1 expression in breast cancer including
BRCA1-associated, triple negative and basal-like tumors: Possible
implications for PARP-1 inhibitor therapy. Breast Cancer Res Treat.
127:861–869. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dedes KJ, Wetterskog D, Mendes-Pereira AM,
Natrajan R, Lambros MB, Geyer FC, Vatcheva R, Savage K, Mackay A,
Lord CJ, et al: PTEN deficiency in endometrioid endometrial
adenocarcinomas predicts sensitivity to PARP inhibitors. Sci Transl
Med. 2:53ra752010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhou C, Bae-Jump VL, Whang YE, Gehrig PA
and Boggess JF: The PTEN tumor suppressor inhibits telomerase
activity in endometrial cancer cells by decreasing hTERT mRNA
levels. Gynecol Oncol. 101:305–310. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Łapińska-Szumczyk SM, Supernat AM,
Majewska HI, Gulczyński J, Biernat W, Wydra D and Żaczek AJ:
Immunohistochemical characterisation of molecular subtypes in
endometrial cancer. Int J Clin Exp Med. 8:21981–21990.
2015.PubMed/NCBI
|
|
23
|
Jiang XF, Tang QL, Shen XM, Li HG, Chen
LH, Wang XY, Luo X, Lin ZQ and Jiang GY: Tumor-associated
macrophages, epidermal growth factor receptor correlated with the
triple negative phenotype in endometrial endometrioid
adenocarcinoma. Pathol Res Pract. 208:730–735. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kwon MJ, Park S, Choi JY, Oh E, Kim YJ,
Park YH, Cho EY, Know MJ, Nam SJ, Im YH, et al: Clinical
significance of CD151 overexpression in subtypes of invasive breast
cancer. Br J Cancer. 106:923–930. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Colombo N, Creutzberg C, Amant F, Bosse T,
González-Martín A, Ledermann J, Marth C, Nout R, Querleu D, Mirza
MR, et al: ESMO-ESGO-ESTRO consensus conference on endometrial
cancer: Diagnosis, treatment and follow-up. Ann Oncol. 27:16–41.
2016. View Article : Google Scholar : PubMed/NCBI
|