Introduction
Stereotactic body radiotherapy (SBRT), which was
introduced by Blomgren et al in 1991 (1), aims to concentrate the radiation dose
to the tumor while minimizing that to the surrounding normal
tissues by accurately focusing multiple radiation beams to small
target volumes. SBRT in early-stage lung cancer yields excellent
local control (LC) with low toxicity (2–8).
While the standard treatment for peripheral stage I
non-small cell lung carcinoma (NSCLC) is surgery, SBRT is sometimes
recommended to patients who have poor pulmonary function with
chronic obstructive pulmonary disease (COPD), asthma or
interstitial pneumonia, or those who have previously undergone lung
cancer surgery. Studies on SBRT treatment in patients with lung
cancer and normal or poor pulmonary function reported that the
technique may cause severe radiation pneumonitis or pulmonary
toxicity, despite being generally safe (5,8,9). Patients with radiation pneumonitis and
poor pulmonary function may develop lethal toxicity (10). Several studies have focused on
patients with poor pulmonary function. Of 176 patients with severe
COPD, Palma et al (11)
reported that six (3%) exhibited grade 3 toxicity, concluding that
SBRT for lung cancer patients with severe COPD was well-tolerated
with acceptable toxicity. In a systematic review focusing on
treatment-associated toxicity in patients with early-stage NSCLC
and coexisting interstitial pneumonia, Chen et al (12) demonstrated a consistently high level
of SBRT-associated mortality (16.7%) and interstitial
pneumonia-specific toxicity (18.8%). Thus, curative treatment
including SBRT should be considered in the context of high
toxicity. However, the safety of SBRT in patients with lung cancer
and coexisting poor pulmonary function remains unclear. The present
study aimed to retrospectively evaluate the safety and
effectiveness of linear accelerator-based SBRT, and the prognostic
factors of overall survival (OS) were investigated in patients with
peripheral stage I lung cancer and poor pulmonary function.
Materials and methods
Patients
This study was approved by the Institutional Review
Board of Osaka Rosai hospital (Sakai, Japan, approval no. 18D093g)
and was conducted in accordance with the Declaration of Helsinki.
This study was a retrospective evaluation of all patients with
stage I lung cancer treated with SBRT at Osaka Rosai Hospital
between May 2003 and December 2009. Of these patients, 95 patients
presented with poor pulmonary function, based on pretreatment
spirometry testing; these patients were included in the study and
their data were retrospectively analyzed. The median age was 76
years (range, 60–90 years). The present study included 67 male
(71%) and 28 female (29%) patients. In 80 patients, the disease was
confirmed by histology or cytology, whereas clinical diagnosis of
the apparent tumor growth was performed using serial CT in the
remaining 15 patients. All tumors were classified according to the
International Union Against Cancer tumor-node-metastasis
classification (7th edition) (13).
Patients' performance status was assessed by Karnofsky performance
status (KPS) (14). The KPS
describes a patient's functional status in 11 categories ranging
from 100 (no symptoms) to 0 (death). A KPS score of 80–100 is
defined as ‘able to carry on normal activity and to work. No
special care is needed’.
Poor pulmonary function was defined as a forced
expiratory volume %/sec (FEV1/FVC) <70% or percentage of vital
capacity (%VC) <80% in the pretreatment spirometry test. If the
FEV1/FVC was <70%, the patient was classified as having
obstructive dysfunction. If the %VC was <80%, the patient was
classified as having restrictive dysfunction. If the FEV1/FVC was
<70% and %VC was <80%, the patient was classified as having
mixed obstructive and restrictive dysfunction. Central tumor
location was defined as that within 2 cm of the proximal bronchial
tree, heart, great vessels, trachea or other mediastinal
structures.
Treatment planning and delivery
All patients were immobilized in an individually
shaped vacuum bag that covered them from the head to the pelvis.
Each patient who, if necessary, was equipped with abdominal
compression, underwent CT simulation with 5-mm thickness and a 5-mm
interval under free breathing of 3–5 l/min oxygen. For
three-dimensional treatment planning, the RPS700U software
(Shimadzu Corporation) was used. The gross tumor volume (GTV) was
delineated in the lung window. The GTV with a 5-mm margin was
defined as the clinical target volume. Additional 5–6-mm margins
for tumor motion were added in the cranio-caudal direction for
internal target volume (ITV) creation. A uniform margin of 5 mm was
used to calculate the planning target volume (PTV) from the ITV.
Treatment plans were designed using 24–25 non-coplanar, irregularly
shaped isocentric beams. Beam shaping was performed using a
multi-leaf collimator with 1 cm width at the isocenter. The total
dose was administered to the isocenter and conformably enclosed the
PTV with the 90% isodose line. The prescribed doses were 50 Gy/4
fractions (Fr; 68 patients, 71.6%), 50 Gy/5 Fr (20 patients,
21.1%), 40 Gy/4 Fr (3 patients, 3.2%), 50 Gy/8 Fr (2 patients,
2.1%), 60 Gy/8 Fr (1 patient, 1.1%) and 48 Gy/4 Fr (1 patient,
1.1%). The dose constraints were applied as follows: Esophagus
V40Gy <1 cm3; bronchus V40Gy
<10 cm3; pulmonary artery V40Gy <1
cm3, V40Gy <10 cm3; lung
V20Gy ≤20%, V15Gy ≤25%; and spinal cord
Dmax <25 Gy (3).
For treatment delivery, a Mitsubishi EXL-15DP linear
accelerator (Mitsubishi Electric Corporation) was used with mainly
10-MV energy photon beans, occasionally using a 4-MV mixed beam to
avoid regions receiving a higher than prescribed dose. The linear
accelerator could not provide a 6-MV X-ray that is often used for
lung SBRT. Each patient was immobilized and treated under free
breathing of 3–5 l/min oxygen in the same manner as during the CT
simulation. Usually, 5–6 fields were irradiated per day, amounting
to 24–25 fields in total in order to improve the conformality and
to reduce the dosage to the organs at risk. In each treatment
session, the beam's eye view of each port was monitored, and the
tumor location was confirmed using a TheraView electronic portal
imaging device (Cablon Medical B.V.).
Follow-up
Follow-up examinations were performed in all
patients. The first examination was 1 month after treatment;
subsequently, the patients were followed up 3, 6 and 12 months
after treatment, and every 6 months thereafter. Each appointment
included a spiral CT scan (slice thickness, 5 mm) and a clinical
examination. If necessary, positron emission tomography was
performed.
All adverse effects were graded according to the
National Cancer Institute's Common Terminology Criteria for Adverse
Events (version 3.0) (15). The
information on toxicities of grade ≥2 was collected. All patients
with interstitial pneumonia were diagnosed by pulmonologists using
a bronchoscope, typical images and symptoms.
Statistical analysis
The statistical evaluation of LC, OS and
cancer-specific survival (CSS) was performed using the Kaplan-Meier
method. LC was defined as the duration from irradiation
commencement to local tumor regrowth in the PTV or the last
follow-up. OS was defined as the duration from irradiation
commencement to death or the last follow-up. CSS was defined as the
time interval from irradiation commencement to cancer-associated
death or the last follow-up. Log-rank tests were used for the
comparison of two survival curves. Univariate analysis using
Fisher's exact test for discrete variables or Mann-Whitney U test
for continuous variables were used to explore potential prognostic
indicators of grade ≥2 radiation pneumonitis. In addition,
univariate analyses using a log-rank test to determine the CSS and
OS prognostic indicators we performed. All patients were divided
into subgroups according to their median age, GTV, VC and FEV1/FVC.
For forced expiratory volume/sec (FEV1.0), patients were divided
into groups according to the Japanese Oncology Group Study 0403
criteria (9). P<0.05 was used to
indicate a statistically significant results. All statistical
analyses were conducted using the JMP statistical software (version
14.0; SAS Institute, Inc.).
Results
Patient characteristics
The characteristics of the patients included in the
present study are summarized in Table
I. The median follow-up period was 34 months (range, 1–89
months). A total of 87 patients (92%) had an inoperable status due
to poor pulmonary function and the presence of other comorbidities.
The remaining eight patients (8%) were considered to be operable
but refused surgery. A total of 57 patients (60%) had stage IA
(T1aN0M0 or T1bN0M0) and 38 (40%) had stage IB (T2aN0M0) lung
cancer. Regarding ventilatory impairment, 47 (49%), 22 (22%) and 26
(27%) patients were classified as having obstructive dysfunction,
restrictive dysfunction and mixed obstructive and restrictive
dysfunction, respectively. The median FEV1/FVC value in patients
with obstructive dysfunction during pretreatment spirometry testing
was 58.6%. The median %VC in patients with restrictive dysfunction
was 68.7%. The median FEV1/FVC and %VC in patients with mixed
obstructive and restrictive dysfunction were 51.9 and 67.7%,
respectively. One patient (1%) had interstitial pneumonitis.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Factors | Value or number
(%) |
|---|
| Age, median (range)
years | 76 (60–90) |
| Sex |
|
| Male | 67 (71) |
|
Female | 28 (29) |
| KPS |
|
| 90 | 1 (1) |
| 80 | 64 (67) |
| 70 | 15 (16) |
| 60 | 3 (3) |
| 50 | 11 (12) |
| 40 | 1 (1) |
| Smoking status |
|
| Current
or previous | 79 (83) |
|
Never | 12 (13) |
|
Unknown | 4 (4) |
| Interstitial
pneumonia |
|
| Yes | 1 (1) |
| No | 94 (99) |
| Treatment status |
|
| Initial
treatment | 90 (95) |
|
Recurrence or residual cancer
after surgery | 5 (5) |
| Operable |
|
| Yes | 8 (8) |
| No | 87 (92) |
| Clinical stage |
|
| cT1aN0M0,
stage IA | 32 (34) |
| cT1bN0M0,
stage IA | 25 (26) |
| cT2aN0M0,
stage IB | 38 (40) |
| Histology of primary
lung cancer |
|
|
Adenocarcinoma | 47 (49) |
| Squamous
cell carcinoma | 29 (31) |
| Small
cell carcinoma | 4 (4) |
|
Unknown | 15 (16) |
| VC, median (range)
cm3 | 2,230
(710–4,290) |
| FEV1.0, median
(range) cm2 | 1,220
(410–2,550) |
| Pattern of
ventilatory impairment |
|
|
Obstructive dysfunction | 47 (49) |
|
Restrictive dysfunction | 22 (22) |
| Mixed
dysfunction | 26 (27) |
| Total dose, median
(range) Gy | 50 (40–60) |
| GTV, median (range)
ml | 15.6
(3.1–87.7) |
Adverse effects
During the follow-up period, four patients (4%)
experienced grade ≥3 toxicities. One patient (1%), who had already
received home oxygen therapy (HOT) due to interstitial pneumonia
prior to SBRT, developed grade 5 radiation pneumonitis, and one
(1%) developed grade 3 radiation pneumonitis (Table II). In total, two patients (2%)
experienced grade ≥3 radiation pneumonitis. Grade 3 chest wall pain
was observed in one patient (1%); one patient (1%) who received
re-irradiation using SBRT developed grade 5 hemoptysis. For this
patient, the first SBRT was performed to the right S1 lung tumor,
which did not include the trachea and the bronchus. After 57
months, recurrence was detected in the marginal zone of the initial
SBRT. The recurrent tumor was located close to the pulmonary hilum.
The second SBRT, which was used to treat the right S6 lung tumor
with 60 Gy/8 Fr and the 95% dose line, partially included the right
main bronchus. Consequently, grade 5 hemoptysis occurred following
the second treatment with SBRT.
 | Table II.Adverse effects. |
Table II.
Adverse effects.
|
| Grade, n (%) |
|
|---|
|
|
|
|
|---|
| Variables | 2 | 3 | 4 | 5 | Total, n (%) |
|---|
| Dermatitis | 1 (1) | 0 | 0 | 0 | 1 (1) |
| Pneumonitis | 3 (3) | 1 (1) | 0 | 1 (1) | 5 (5) |
| Chest wall
pain | 2 (2) | 1 (1) | 0 | 0 | 3 (3) |
| Hemoptysis | 0 | 0 | 0 | 1 (1) | 1 (1) |
In the course of the clinical follow-up, 14 patients
(14.7%) eventually required HOT due to radiation pneumonitis in one
patient and pulmonary disease deterioration in 13 patients. The
average time until the introduction of HOT following SBRT was 25.8
months (range, 0.5–66.0 months). Of the 14 patients, eight survived
for >12 months following HOT introduction.
Univariate analysis was performed to identify the
potential risk factors of grade ≥2 radiation pneumonitis across the
different subgroups (Tables SI and
SII). However, none of the patient,
tumor or treatment characteristics were identified as risk factors.
In addition, none of the factors observed in the pretreatment
pulmonary function test were detected as significant risk
factors.
Local control and survival
All patients completed the planned treatment. During
the follow-up, 48 patients (51%) died; 22 succumbed to cancer, 24
died of unrelated causes and two patients died of
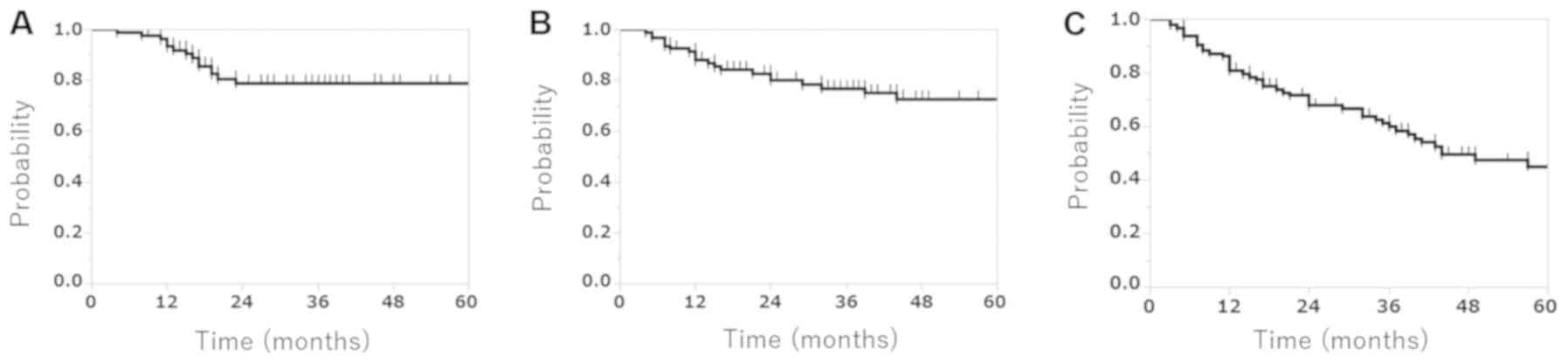
treatment-associated causes. The 3-year LC, CSS and OS rates were
78.8 (95% CI, 67.2–87.15%), 76.8 (95% CI, 66.2–84.8%) and 59.9%
(95% CI, 49.1–69.7%), respectively (Fig.
1). The median OS time was 34.0 months.
Considering clinical stage, the 3-year LC rate was
84.4% for Stage IA and 69.7% for Stage IB disease. The 3-year CSS
rate was 80.3% for Stage IA and 71.0% for Stage IB disease. The
3-year OS rate was 57.0% for Stage IA and 45.1% for Stage IB
disease (data not shown).
Prognostic factors
Univariate analyses were performed to identify
potential prognostic factors of CSS and OS among the different
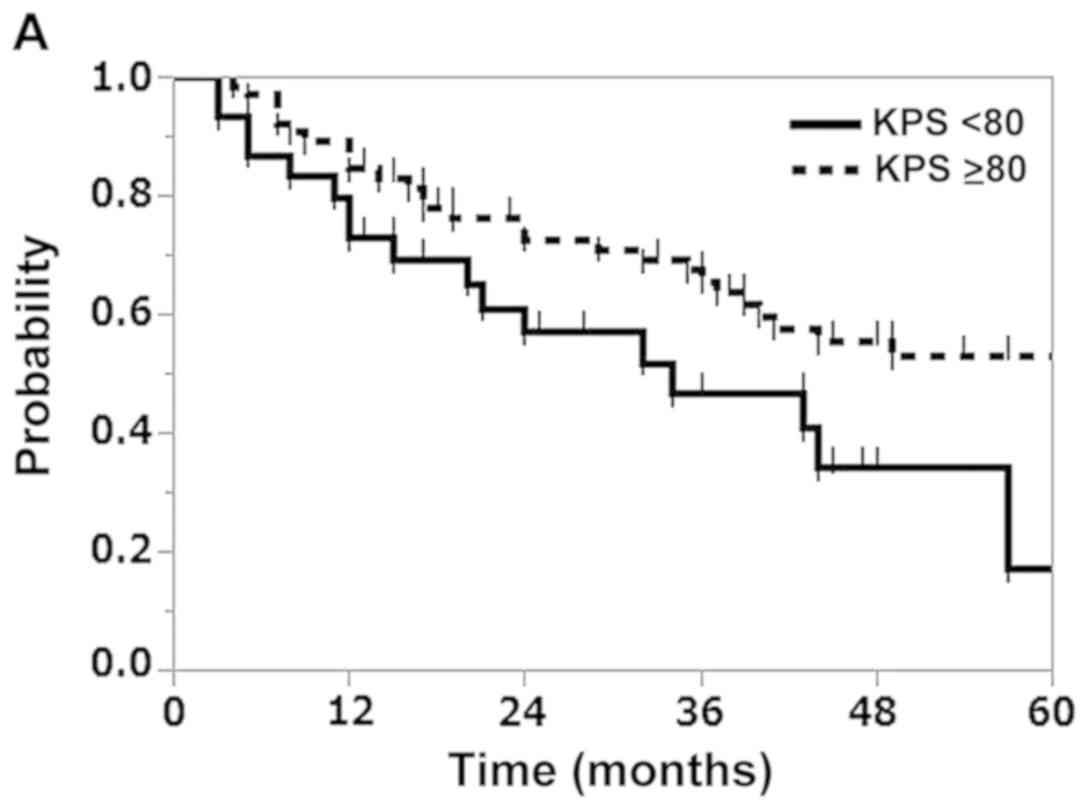
subgroups. The results revealed that the KPS was a significant
predictor of OS (P=0.037), and that the histology of primary lung
cancer was a significant predictor of CSS and OS (P<0.001 and
P=0.003, respectively; Table III).
In addition, the 3-year OS rates of patients with a KPS score
<80 and those with a score ≥80 were 46.6 and 65.5%, respectively
(Fig. 2A). The 3-year CSS rates of
patients with adenocarcinoma, squamous cell carcinoma and small
cell carcinoma were 81.3, 90.7 and 0%, respectively (Fig. 2B). The 3-year OS rates of patients
with adenocarcinoma, squamous cell carcinoma and small cell
carcinoma were 68.7, 66.0 and 0%, respectively (Fig. 2C). Although the associations between
prognosis and pulmonary variables such as FEV1.0, VC and FEV1/FVC
were also evaluated, no significant differences were
identified.
 | Table III.Univariate analysis of
cancer-specific survival and overall survival. |
Table III.
Univariate analysis of
cancer-specific survival and overall survival.
| Factor | No. of
patients | Cancer-specific
survival P-value | Overall survival
P-value |
|---|
| Age, years |
|
0.766 | 0.338 |
|
≥76 | 50 |
|
|
|
<76 | 45 |
|
|
| Sex |
|
0.589 | 0.362 |
|
Male | 67 |
|
|
|
Female | 28 |
|
|
| KPS |
|
0.858 | 0.037 |
|
<80 | 30 |
|
|
|
≥80 | 65 |
|
|
| Smoking status |
|
0.310 | 0.775 |
| Current
or previous | 79 |
|
|
|
Never | 12 |
|
|
| Operable |
|
0.996 | 0.285 |
|
Yes | 8 |
|
|
| No | 85 |
|
|
| Clinical stage |
|
0.223 | 1.000 |
| IA | 57 |
|
|
| IB | 38 |
|
|
| Histology of
primary lung cancer |
| <0.001 | 0.003 |
|
Adenocarcinoma | 47 |
|
|
|
Squamous cell carcinoma | 29 |
|
|
| Small
cell carcinoma | 4 |
|
|
| Pattern of
ventilatory impairment |
|
|
|
|
Obstructive dysfunction | 47 |
0.697 | 0.700 |
|
Restrictive dysfunction | 22 |
|
|
| Mixed
dysfunction | 26 |
0.497 | 0.510 |
| GTV, cm3 |
|
|
|
|
≥15.6 | 48 |
|
|
|
<15.6 | 47 |
|
|
| FEV1.0, cm3 |
|
0.669 | 0.979 |
|
>700 | 69 |
|
|
|
≤700 | 26 |
|
|
| VC, cm3 |
|
0.981 | 0.214 |
| >2,
282 | 43 |
|
|
| ≤2,
282 | 52 |
|
|
| FEV1/FVC, % |
|
0.219 | 0.188 |
|
>59.1 | 47 |
|
|
|
≤59.1 | 48 |
|
|
Discussion
To the best of our knowledge, although several
studies have focused on SBRT for patients with stage I lung cancer
and poor pulmonary function, the safety and efficacy of the
technique have not been clarified (16–18). The
results of the present study demonstrated that SBRT is effective in
patients with lung cancer and poor pulmonary function with
acceptable toxicity. A KPS score ≥80 was found to indicate
favorable prognosis, and SBRT may be an effective and safe
treatment option with a KPS score ≥80 among patients with poor
pulmonary function.
In the present study, one patient (1.0%) who
received re-irradiation by SBRT developed grade 5 hemoptysis. In
this patient, the recurrent tumor was located close to the
pulmonary hilum in the marginal zone of the initial SBRT.
Consequently, the patient succumbed to hemoptysis due to tracheal
hemorrhage. Subsequently, re-irradiation close to the pulmonary
hilum was not performed for the other patients.
Radiation pneumonitis is the most commonly observed
adverse effect following SBRT for lung cancer. Several studies
using SBRT have reported that grade ≥3 radiation pneumonitis is
detected in 1.8–14.5% of patients, although these studies include
patients with normal pulmonary function (5,8,9). Of 176 patients with severe COPD, Palma
et al (11) reported that
three patients (2%) had grade 3 radiation pneumonitis. Chen et
al (12) reported an occurrence
rate of grade ≥3 radiation pneumonitis or acute exacerbation of
interstitial pneumonia in 18.8% of patients with lung cancer and
coexisting interstitial pneumonia. The focus of the present study
was on patients with lung cancer and poor pulmonary function, and
only 2% of the patients developed grade ≥3 radiation pneumonitis,
although one patient (1%) with HOT due to interstitial pneumonia
prior to SBRT developed grade 5 radiation pneumonitis. This result
suggested that the incidence of severe radiation pneumonitis
following SBRT in patients with lung cancer and poor pulmonary
function is similar to that in patients with normal pulmonary
function or COPD. Guckenberger et al (16) analyzed the influence of pretreatment
pulmonary function on pulmonary toxicity following SBRT for
early-stage NSCLC; no significant associations between any of the
pretreatment pulmonary function parameters and the risk of either
grade ≥2 or ≥3 radiation pneumonitis were observed. Similarly, the
univariate analysis of potential risk factors of grade ≥2 radiation
pneumonitis in terms of average pulmonary function and dosimetric
parameters in the present study indicated that none of the factors
in the pretreatment pulmonary function test were significant risk
factors, suggesting that SBRT use in patients with lung cancer and
poor pulmonary function is relatively safe.
The reported 3-year LC and OS rates following SBRT
for peripheral stage I lung cancer were 89–97.6 and 47–69%,
respectively (5,8,9,11). In addition, LC and OS depend on
clinical stage or operability (8,19).
Shibamoto et al (19)
reported that the 3-year LC rate was 86% for Stage IA and 73% for
Stage IB disease, and the 3-year OS rate was 74% for operable and
59% for inoperable patients. The results of the present study
revealed that the 3-year LC rate was 78.8% for all patients, 84.4%
for those with Stage IA disease and 69.7% for those with Stage IB
disease. The 3-year OS rate was 59.9%, although all 95 patients had
poor pulmonary function, of which 87 patients (92%) had an
inoperable status. LC in the present study was slightly worse
compared with previous findings, although the OS was consistent
with the aforementioned studies. The reason for the lower LC is not
clear; however, it was hypothesized that in patients with low
pulmonary function, the tumor may be hypoxic and
radio-resistant.
Previous studies on SBRT for lung cancer have
identified several prognostic indicators such as T classification
and COPD severity (8,11). The univariate analysis in the present
study identified KPS score as a significant predictor of OS
(P=0.037), and primary lung cancer histology was a significant
predictor of CSS (P<0.001) and OS (P=0.003). This may arise from
the fact that patients with a low KPS score generally have poor
prognoses, or that small cell lung carcinoma is more aggressive
compared with NSCLC. In addition, no significant differences were
identified between prognoses and pulmonary variables such as
FEV1.0, VC and FEV1/FVC in the univariate analysis, consistent with
previous studies (17,18). The results of the present study
indicated that SBRT may be effective in patients with peripheral
stage I lung cancer and poor pulmonary function.
The present study had several limitations. Firstly,
this study was performed using a single-center retrospective
design. Therefore, the possibility of selection bias cannot be
eliminated. Secondly, the study sample size was limited. Finally,
the total doses and fractionation varied (48–60 Gy in 4–8 Fr),
which may have influenced tumor control, toxicity occurrence and
severity.
SBRT for patients with peripheral stage I lung
cancer and poor pulmonary function may be an effective treatment
with acceptable toxicity, and a KPS score ≥80 may indicate good
prognosis.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
AK, KH and MC conducted this study and collected the
data. YK collected the data. OS, YK and KO conducted the data
analysis. AK and KH wrote the manuscript. YK and KO interpreted and
analyzed the data. All authors have read and approved the
manuscript.
Ethics approval and consent to
participate
This study was approved by the Institutional Review
Board of Osaka Rosai hospital (Sakai, Japan; approval no. 18D093g)
and was conducted in accordance with the Declaration of
Helsinki.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
KPS
|
Karnofsky performance status
|
|
GTV
|
gross tumor volume
|
|
CTV
|
clinical target volume
|
|
ITV
|
internal target volume
|
|
PTV
|
planning target volume
|
|
Fr
|
fraction
|
|
FEV1.0
|
forced expiratory volume/sec
|
|
VC
|
vital capacity
|
|
FEV1/FVC
|
forced expiratory volume %/sec
|
|
LC
|
local control
|
|
OS
|
overall survival
|
|
CSS
|
cancer-specific survival
|
|
COPD
|
chronic obstructive pulmonary
disease
|
|
SBRT
|
stereotactic body radiotherapy
|
|
%VC
|
a percentage of vital capacity
|
|
NSCLC
|
non-small cell lung carcinoma
|
|
HOT
|
home oxygen therapy
|
References
|
1
|
Blomgren H, Lax I, Näslund I and Svanström
R: Stereotactic high dose fraction radiation therapy of
extracranial tumors using an accelerator. Clinical experience of
the first thirty-one patients. Acta Oncol. 34:861–870. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Uematsu M, Shioda A, Suda A, Fukui T,
Ozeki Y, Hama Y, Wong JR and Kusano S: Computed tomography-guided
frameless stereotactic radiotherapy for stage I non-small cell lung
cancer: A 5-year experience. Int J Radiat Oncol Biol Phys.
51:666–670. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nagata Y, Takayama K, Matsuo Y, Norihisa
Y, Mizowaki T, Sakamoto T, Sakamoto M, Mitsumori M, Shibuya K,
Araki N, et al: Clinical outcomes of a phase I/II study of 48 Gy of
stereotactic body radiotherapy in 4 fractions for primary lung
cancer using a stereotactic body frame. Int J Radiat Oncol Biol
Phys. 63:1427–1431. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Onimaru R, Shirato H, Shimizu S, Kitamura
K, Xu B, Fukumoto S, Chang TC, Fujita K, Oita M, Miyasaka K, et al:
Tolerance of organs at risk in small-volume, hypofractionated,
image-guided radiotherapy for primary and metastatic lung cancers.
Int J Radiat Oncol Biol Phys. 56:126–135. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Timmerman R, Paulus R, Galvin J, Michalski
J, Straube W, Bradley J, Fakiris A, Bezjak A, Videtic G, Johnstone
D, et al: Stereotactic body radiation therapy for inoperable early
stage lung cancer. JAMA. 303:1070–1076. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wulf J, Haedinger U, Oppitz U, Thiele W,
Mueller G and Flentje M: Stereotactic radiotherapy for primary lung
cancer and pulmonary metastases: A noninvasive treatment approach
in medically inoperable patients. Int J Radiat Oncol Biol Phys.
60:186–196. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xia T, Li H, Sun Q, Wang Y, Fan N, Yu Y,
Li P and Chang JY: Promising clinical outcome of stereotactic body
radiation therapy for patients with inoperable Stage I/II
non-small-cell lung cancer. Int J Radiat Oncol Biol Phys.
66:117–125. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Baumann P, Nyman J, Hoyer M, Wennberg B,
Gagliardi G, Lax I, Drugge N, Ekberg L, Friesland S, Johansson K-A,
et al: Outcome in a prospective phase II trial of medically
inoperable stage I non-small-cell lung cancer patients treated with
stereotactic body radiotherapy. J Clin Oncol. 27:3290–3296. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nagata Y, Hiraoka M, Shibata T, Onishi H,
Kokubo M, Karasawa K, Shioyama Y, Onimaru R, Kozuka T, Kunieda E,
et al: Prospective trial of stereotactic body radiation therapy for
both operable and inoperable T1N0M0 non-small cell lung cancer:
Japan Clinical Oncology Group Study JCOG0403. Int J Radiat Oncol
Biol Phys. 93:989–996. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nagata Y and Kimura T: Stereotactic body
radiotherapy (SBRT) for Stage I lung cancer. Jpn J Clin Oncol.
48:405–409. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Palma D, Lagerwaard F, Rodrigues G,
Haasbeek C and Senan S: Curative treatment of Stage I
non-small-cell lung cancer in patients with severe COPD:
Stereotactic radiotherapy outcomes and systematic review. Int J
Radiat Oncol Biol Phys. 82:1149–1156. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen H, Senan S, Nossent EJ, Boldt RG,
Warner A, Palma DA and Louie AV: Treatment-related toxicity in
patients with early-stage non-small cell lung cancer and coexisting
interstitial lung disease: A systematic review. Int J Radiat Oncol
Biol Phys. 98:622–631. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
International Union Against Cancer: TNM
Classification of Malignant Tumours. Sobin LH, Gospodarowicz MK and
Wittekind C: 7th. Wiley-Blackwell Inc.; New York, NY: 2009
|
|
14
|
Péus D, Newcomb N and Hofer S: Appraisal
of the Karnofsky Performance Status and proposal of a simple
algorithmic system for its evaluation. BMC Med Inform Decis Mak.
13:722013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
U.S. Department of Health and Human
Services, National Institutes of Health National Cancer Institute,
. Common Terminology Criteria for Adverse Events (CTCAE). Version
4.0. https://www.eortc.be/services/doc/ctc/CTCAE_4.03_2010-06-14_QuickReference_5×7.pdfMay
28–2009
|
|
16
|
Guckenberger M, Kestin LL, Hope AJ,
Belderbos J, Werner-Wasik M, Yan D, Sonke J-J, Bissonnette JP,
Wilbert J, Xiao Y, et al: Is there a lower limit of pretreatment
pulmonary function for safe and effective stereotactic body
radiotherapy for early-stage non-small cell lung cancer? J Thorac
Oncol. 7:542–551. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Henderson M, McGarry R, Yiannoutsos C,
Fakiris A, Hoopes D, Williams M and Timmerman R: Baseline pulmonary
function as a predictor for survival and decline in pulmonary
function over time in patients undergoing stereotactic body
radiotherapy for the treatment of stage I non-small-cell lung
cancer. Int J Radiat Oncol Biol Phys. 72:404–409. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Stone B, Mangona VS, Johnson MD, Ye H and
Grills IS: Changes in Pulmonary Function Following Image-Guided
Stereotactic Lung Radiotherapy: Neither Lower Baseline Nor
Post-SBRT Pulmonary Function Are Associated with Worse Overall
Survival. J Thorac Oncol. 10:1762–1769. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shibamoto Y, Hashizume C, Baba F, Ayakawa
S, Manabe Y, Nagai A, Miyakawa A, Murai T, Iwata H, Mori Y, et al:
Stereotactic body radiotherapy using a radiobiology-based regimen
for stage I nonsmall cell lung cancer: A multicenter study. Cancer.
118:2078–2084. 2012. View Article : Google Scholar : PubMed/NCBI
|