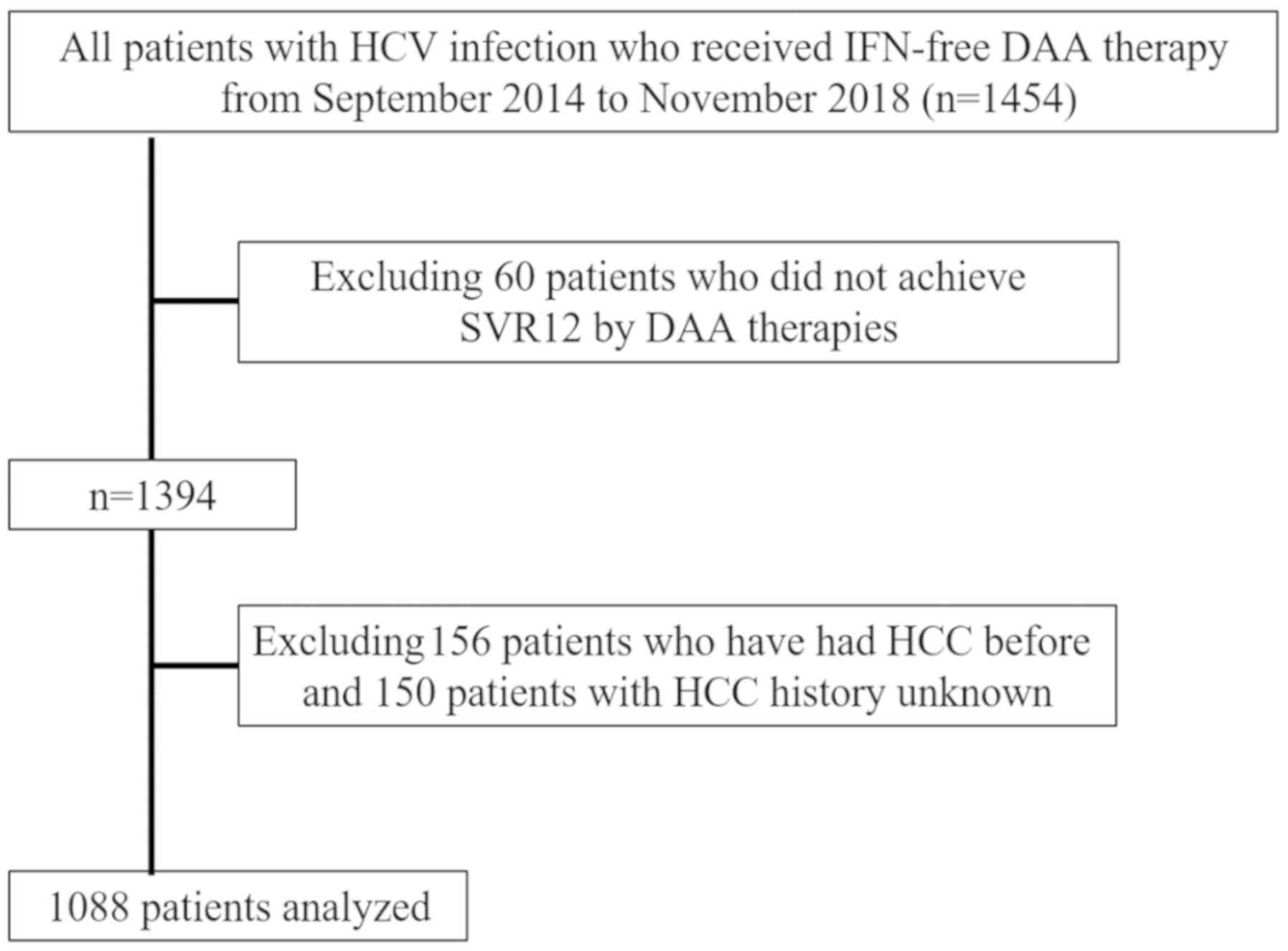
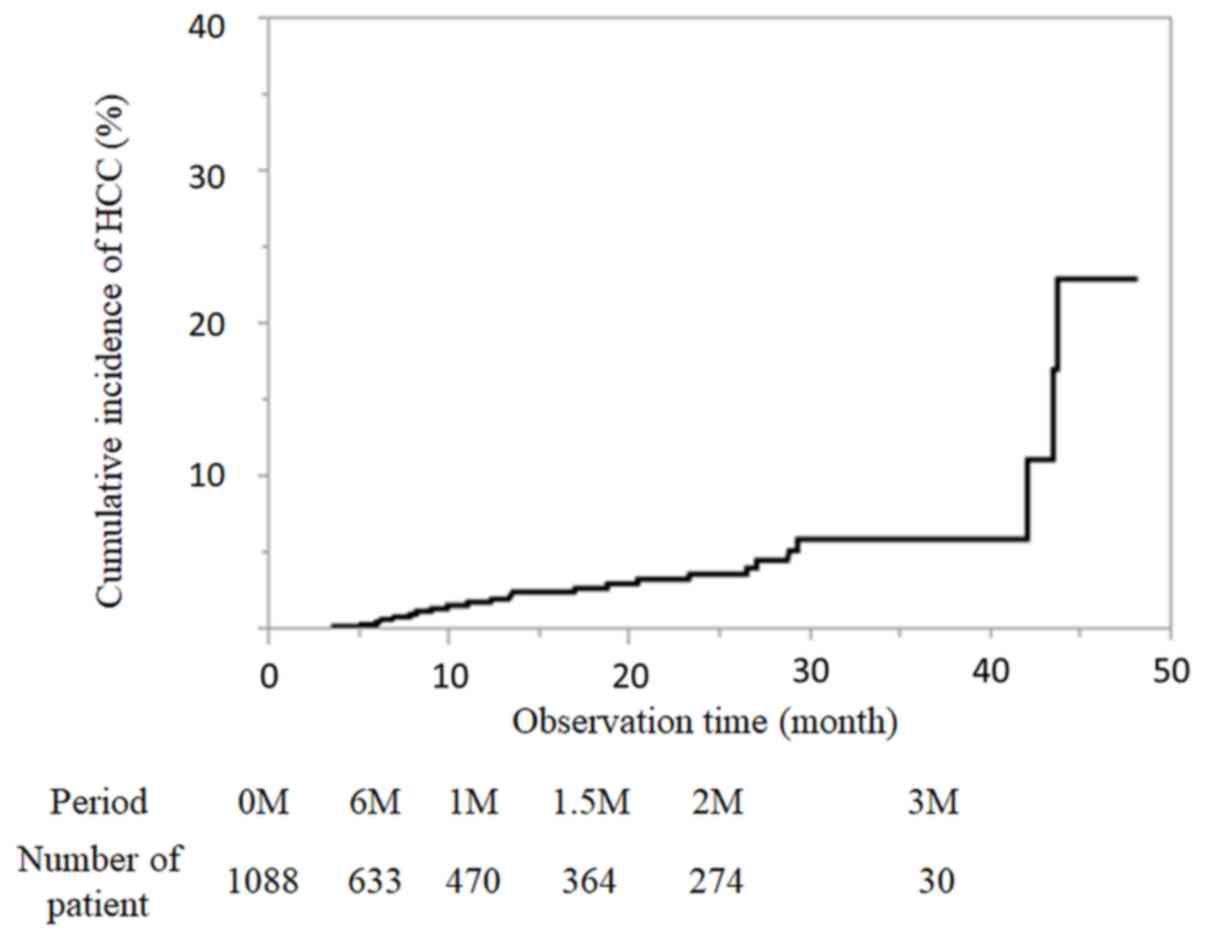
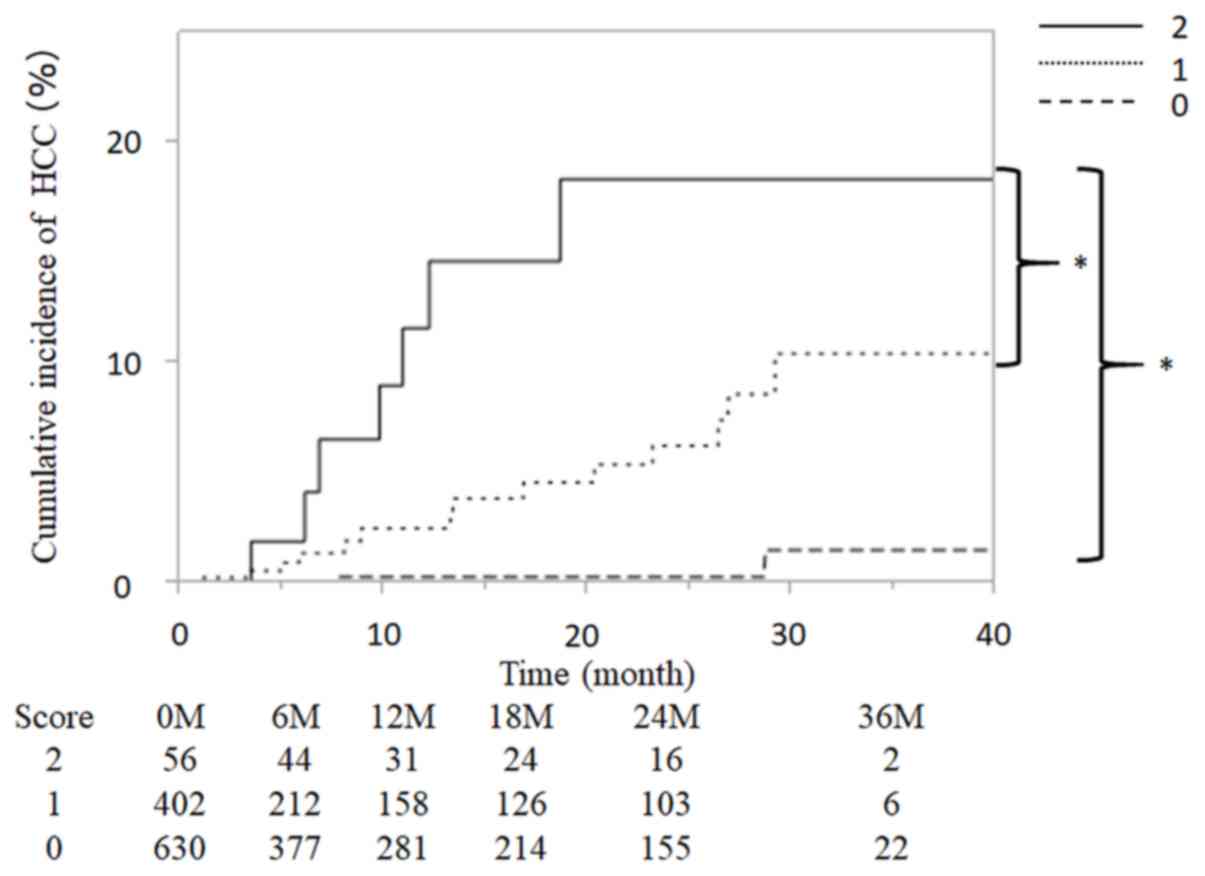
|
1
|
Lauer GM and Walker BD: Hepatitis C virus
infection. N Engl J Med. 345:41–52. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fried MW, Shiffman ML, Reddy KR, Smith C,
Marinos G, Gonçales FL Jr, Häussinger D, Diago M, Carosi G,
Dhumeaux D, et al: Peginterferon alfa-2a plus ribavirin for chronic
hepatitis C virus infection. N Engl J Med. 347:975–982. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hadziyannis SJ, Sette H Jr, Morgan TR,
Balan V, Diago M, Marcellin P, Ramadori G, Bodenheimer H Jr,
Bernstein D, Rizzetto M, et al: Peginterferon-alpha2a and ribavirin
combination therapy in chronic hepatitis C: A randomized study of
treatment duration and ribavirin dose. Ann Intern Med. 140:346–355.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Manns MP, McHutchison JG, Gordon SC,
Rustgi VK, Shiffman M, Reindollar R, Goodman ZD, Koury K, Ling M
and Albrecht JK: Peginterferon alfa-2b plus ribavirin compared with
interferon alfa-2b plus ribavirin for initial treatment of chronic
hepatitis C: A randomised trial. Lancet. 358:958–965. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fried MW: Side effects of therapy of
hepatitis C and their management. Hepatology. 36 (5 Suppl
1):S237–S244. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Omata M, Nishiguchi S, Ueno Y, Mochizuki
H, Izumi N, Ikeda F, Toyoda H, Yokosuka O, Nirei K, Genda T, et al:
Sofosbuvir plus ribavirin in Japanese patients with chronic
genotype 2 HCV infection: An open-label, phase 3 trial. J Viral
Hepat. 21:762–768. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ogawa E, Furusyo N, Nomura H, Dohmen K,
Higashi N, Takahashi K, Kawano A, Azuma K, Satoh T, Nakamuta M, et
al: NS5A resistance-associated variants undermine the effectiveness
of ledipasvir and sofosbuvir for cirrhotic patients infected with
HCV genotype 1b. J Gastroenterol. 52:845–854. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Poordad F, Felizarta F, Asatryan A,
Sulkowski MS, Reindollar RW, Landis CS, Gordon SC, Flamm SL, Fried
MW, Bernstein DE, et al: Glecaprevir and pibrentasvir for 12 weeks
for hepatitis C virus genotype 1 infection and prior direct-acting
antiviral treatment. Hepatology. 66:389–397. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Toyoda H, Kumada T, Tada T, Shimada N,
Takaguchi K, Senoh T, Tsuji K, Tachi Y, Hiraoka A, Ishikawa T, et
al: Efficacy and tolerability of an IFN-free regimen with DCV/ASV
for elderly patients infected with HCV genotype 1B. J Hepatol.
66:521–527. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Toyoda H, Chayama K, Suzuki F, Sato K,
Atarashi T, Watanabe T, Atsukawa M, Naganuma A, Notsumata K, Osaki
Y, et al: Efficacy and safety of glecaprevir/pibrentasvir in
Japanese patients with chronic genotype 2 hepatitis C virus
infection. Hepatology. 67:505–513. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Suda G, Kurosaki M, Itakura J, Izumi N,
Uchida Y, Mochida S, Hasebe C, Abe M, Haga H, Ueno Y, et al: Safety
and efficacy of elbasvir and grazoprevir in Japanese hemodialysis
patients with genotype 1b hepatitis C virus infection. J
Gastroenterol. 54:78–86. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Asahina Y, Tsuchiya K, Nishimura T,
Muraoka M, Suzuki Y, Tamaki N, Yasui Y, Hosokawa T, Ueda K,
Nakanishi H, et al: alpha-fetoprotein levels after interferon
therapy and risk of hepatocarcinogenesis in chronic hepatitis C.
Hepatology. 58:1253–1262. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hirakawa M, Ikeda K, Arase Y, Kawamura Y,
Yatsuji H, Hosaka T, Sezaki H, Akuta N, Kobayashi M, Saitoh S, et
al: Hepatocarcinogenesis following HCV RNA eradication by
interferon in chronic hepatitis patients. Intern Med. 47:1637–1643.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Conti F, Buonfiglioli F, Scuteri A, Crespi
C, Bolondi L, Caraceni P, Foschi FG, Lenzi M, Mazzella G, Verucchi
G, et al: Early occurrence and recurrence of hepatocellular
carcinoma in HCV-related cirrhosis treated with direct-acting
antivirals. J Hepatol. 65:727–733. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kanwal F, Kramer J, Asch SM, Chayanupatkul
M, Cao Y and El-Serag HB: Risk of hepatocellular cancer in HCV
patients treated with direct-acting antiviral agents.
Gastroenterology. 153:996–1005.e1. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bruix J and Sherman M; Practice Guidelines
Committee, American Association for the Study of Liver Diseases, :
Management of hepatocellular carcinoma. Hepatology. 42:1208–1236.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hiraoka A, Hiasa Y, Onji M and Michitaka
K: New contrast enhanced ultrasonography agent: Impact of Sonazoid
on radiofrequency ablation. J Gastroenterol Hepatol. 26:616–618.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kudo M, Kitano M, Sakurai T and Nishida N:
General rules for the clinical and pathological study of primary
liver cancer, nationwide follow-up survey and clinical practice
guidelines: The outstanding achievements of the liver cancer study
group of Japan. Dig Dis. 33:765–770. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hung CH, Lu SN, Wang JH, Lee CM, Chen TM,
Tung HD, Chen CH, Huang WS and Changchien CS: Correlation between
ultrasonographic and pathologic diagnoses of hepatitis B and C
virus-related cirrhosis. J Gastroenterol. 38:153–157. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sterling RK, Lissen E, Clumeck N, Sola R,
Correa MC, Montaner J, S Sulkowski M, Torriani FJ, Dieterich DT,
Thomas DL, et al: Development of a simple noninvasive index to
predict significant fibrosis in patients with HIV/HCV coinfection.
Hepatology. 43:1317–1325. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vallet-Pichard A, Mallet V, Nalpas B,
Verkarre V, Nalpas A, Dhalluin-Venier V, Fontaine H and Pol S:
FIB-4: An inexpensive and accurate marker of fibrosis in HCV
infection. comparison with liver biopsy and fibrotest. Hepatology.
46:32–36. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ikeda M, Fujiyama S, Tanaka M, Sata M, Ide
T, Yatsuhashi H and Watanabe H: Risk factors for development of
hepatocellular carcinoma in patients with chronic hepatitis C after
sustained response to interferon. J Gastroenterol. 40:148–156.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tokita H, Fukui H, Tanaka A, Kamitsukasa
H, Yagura M, Harada H and Okamoto H: Risk factors for the
development of hepatocellular carcinoma among patients with chronic
hepatitis C who achieved a sustained virological response to
interferon therapy. J Gastroenterol Hepatol. 20:752–758. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tanaka A, Uegaki S, Kurihara H, Aida K,
Mikami M, Nagashima I, Shiga J and Takikawa H: Hepatic steatosis as
a possible risk factor for the development of hepatocellular
carcinoma after eradication of hepatitis C virus with antiviral
therapy in patients with chronic hepatitis C. World J
Gastroenterol. 13:5180–5187. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ioannou GN, Green PK and Berry K: HCV
eradication induced by direct-acting antiviral agents reduces the
risk of hepatocellular carcinoma. J Hepatol. S0168-8278(17)32273-0.
2017.(Epub ahead of print).
|
|
26
|
Cammà C, Giunta M, Andreone P and Craxì A:
Interferon and prevention of hepatocellular carcinoma in viral
cirrhosis: An evidence-based approach. J Hepatol. 34:593–602. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mazzaferro V, Romito R, Schiavo M, Mariani
L, Camerini T, Bhoori S, Capussotti L, Calise F, Pellicci R, Belli
G, et al: Prevention of hepatocellular carcinoma recurrence with
alpha-interferon after liver resection in HCV cirrhosis.
Hepatology. 44:1543–1554. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Arase Y, Kobayashi M, Suzuki F, Suzuki Y,
Kawamura Y, Akuta N, Kobayashi M, Sezaki H, Saito S, Hosaka T, et
al: Effect of type 2 diabetes on risk for malignancies includes
hepatocellular carcinoma in chronic hepatitis C. Hepatology.
57:964–973. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ogawa E, Furusyo N, Kajiwara E, Takahashi
K, Nomura H, Maruyama T, Tanabe Y, Satoh T, Nakamuta M, Kotoh K, et
al: Efficacy of pegylated interferon alpha-2b and ribavirin
treatment on the risk of hepatocellular carcinoma in patients with
chronic hepatitis C: A prospective, multicenter study. J Hepatol.
58:495–501. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chang KC, Hung CH, Lu SN, Wang JH, Lee CM,
Chen CH, Yen MF, Lin SC, Yen YH, Tsai MC, et al: A novel predictive
score for hepatocellular carcinoma development in patients with
chronic hepatitis C after sustained response to pegylated
interferon and ribavirin combination therapy. J Antimicrob
Chemother. 67:2766–2772. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Watanabe T, Tokumoto Y, Joko K, Michitaka
K, Horiike N, Tanaka Y, Tada F, Kisaka Y, Nakanishi S, Yamauchi K,
et al: Predictors of hepatocellular carcinoma occurrence after
direct-acting antiviral therapy in patients with hepatitis C virus
infection. Hepatol Res. 49:136–146. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Asahina Y, Tsuchiya K, Tamaki N, Hirayama
I, Tanaka T, Sato M, Yasui Y, Hosokawa T, Ueda K, Kuzuya T, et al:
Effect of aging on risk for hepatocellular carcinoma in chronic
hepatitis C virus infection. Hepatology. 52:518–527. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
El-Serag HB and Rudolph KL: Hepatocellular
carcinoma: Epidemiology and molecular carcinogenesis.
Gastroenterology. 132:2557–2576. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hiraoka A, Kumada T, Ogawa C, Kariyama K,
Morita M, Nouso K, Toyoda H, Tada T, Ochi M, Murakami T, et al:
Proposed a simple score for recommendation of scheduled
ultrasonography surveillance for hepatocellular carcinoma after
direct acting antivirals: Multicenter analysis. J Gastroenterol
Hepatol. 34:436–441. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Toyoda H, Tada T, Takaguchi K, Senoh T,
Shimada N, Hiraoka A, Michitaka K, Ishikawa T and Kumada T:
Differences in background characteristics of patients with chronic
hepatitis C who achieved sustained virologic response with
interferon-free versus interferon-based therapy and the risk of
developing hepatocellular carcinoma after eradication of hepatitis
C virus in Japan. J Viral Hepat. 24:472–476. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kudo M, Chung H and Osaki Y: Prognostic
staging system for hepatocellular carcinoma (CLIP score): Its value
and limitations, and a proposal for a new staging system, the Japan
integrated staging score (JIS score). J Gastroenterol. 38:207–215.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hiraoka A, Kumada T, Kudo M, Hirooka M,
Tsuji K, Itobayashi E, Kariyama K, Ishikawa T, Tajiri K, Ochi H, et
al: Albumin-bilirubin (ALBI) grade as part of the evidence-based
clinical practice guideline for HCC of the Japan society of
hepatology: A comparison with the liver damage and child-pugh
classifications. Liver Cancer. 6:204–215. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Villani R, Facciorusso A, Bellanti F,
Tamborra R, Piscazzi A, Landriscina M, Vendemiale G and Serviddio
G: DAAs rapidly reduce inflammation but increase serum VEGF Level:
A rationale for tumor risk during anti-HCV treatment. PLoS One.
11:e01679342016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Toyoda H, Kumada T, Tada T, Mizuno K, Sone
Y, Akita T, Tanaka J and Johnson PJ: The impact of HCV eradication
by direct-acting antivirals on the transition of precancerous
hepatic nodules to HCC: A prospective observational study. Liver
Int. 39:448–454. 2019. View Article : Google Scholar : PubMed/NCBI
|