Introduction
Hepatitis C virus (HCV) infection is a risk factor
for the occurrence of liver cirrhosis (LC) and hepatocellular
carcinoma (HCC) (1). The combination
of interferon (IFN) and ribavirin has been used as a standard
treatment of HCV infection; however, the cure rate remains
unsatisfactory (~50%) (2–4). Furthermore, IFN cannot be administered
to elderly patients or to patients with LC and low platelet count,
due to the risk of numerous adverse events, including high fever,
general fatigue, depression, interstitial pneumonia and decreased
platelet count (5). As a result, the
number of patients who can benefit from IFN treatment is small.
Direct acting antivirals (DAA) have been developed against HCV
infection, and IFN-free DAA treatment has dramatically improved the
cure rate of patients to >90% (6–11). In
addition, this treatment is safe to use, due to mild adverse events
compared with IFN treatment, for patients with HCV (6–11).
It has been previously reported that IFN can
suppress hepatic carcinogenesis (12,13).
However, since numerous elderly patients with HCV and patients with
LC have received IFN-free DAA treatment, there is some concern
regarding the potential increase in hepatic carcinogenesis
occurrence following treatment. It has been demonstrated that
DAA-induced sustained virological response (SVR) does not reduce
the short-term occurrence of HCC in patients following successful
DAA treatment (14). Conversely, a
retrospective cohort study of 22,500 patients with HCV treated with
DAA demonstrated that HCC occurrence is significantly diminished in
patients with SVR compared with those without (15).
However, a method to predict the risk of HCC in
HCV-infected patients achieving SVR by IFN-free DAA treatment
remains to be determined. SVR was categorized as undetectable HCV
RNA (target not detected) at week 12 after the end of treatment
(SVR12) (6–11). The establishment of a simple scoring
system using general clinical data to predict the risk of HCC after
SVR12 is crucial. The present study proposed one scoring system for
the prediction of HCC occurrence in patients who achieved SVR
following IFN-free DAA treatment.
Materials and methods
Patients
This project was a multicenter study, which included
six institutions (Kagawa University Hospital, Kagawa Prefectural
Central Hospital, Takamatsu Red Cross Hospital, Mitoyo General
Hospital, Kagawa Rosai Hospital and Yashima General Hospital) in
Kagawa, Japan (All Kagawa Liver Disease Group Study). A total of
1,454 patients with HCV infection who received IFN-free DAA
treatment between September 2014 and November 2018 were included in
the present study. The study population included 742 men and 712
women. The median age of patients at the start of DAA treatment was
68 years (range, 59–76 years). The present study was conducted in
accordance with the guidelines of the Declaration of Helsinki and
was approved by the Ethics Committee of Kagawa University, Faculty
of Medicine (approval no. Heisei-27-174). The requirement for
informed consent from the participants was waived because of the
retrospective nature of the study.
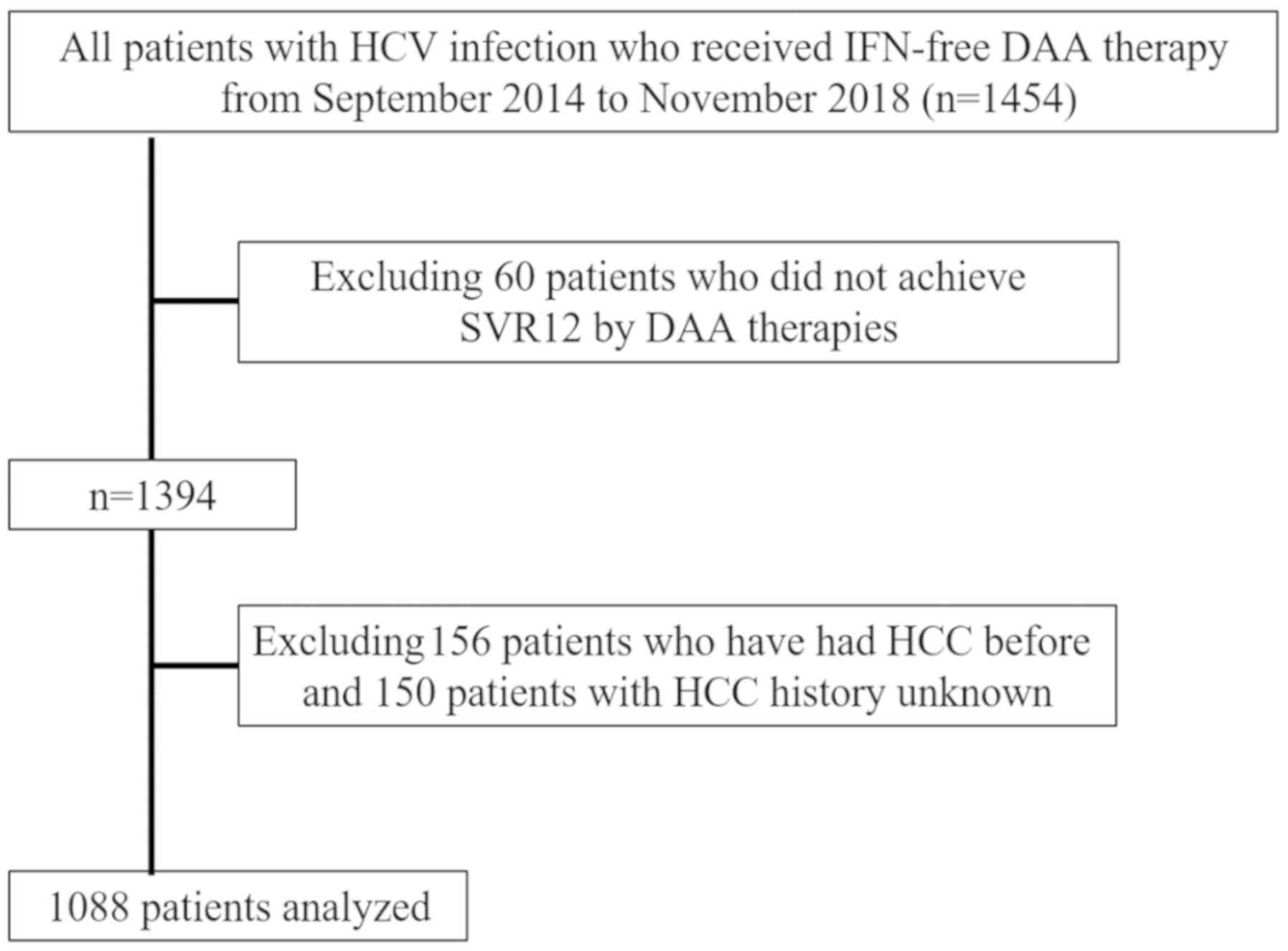
The exclusion criteria were as follows: i) Patients
co-infected with hepatitis B virus or human immunodeficiency virus,
or patients with other liver diseases, including primary biliary
cholangitis and autoimmune hepatitis; ii) patients with
decompensated cirrhosis, since IFN-free DAA treatment was not
approved for these patients in Japan; and iii) patients with a
previous history of HCC or patients who did not achieve SVR12
following DAA treatment. A total of 1,088 patients who achieved
SVR12 following IFN-free DAA treatment were finally included in the
present study (Fig. 1). Of the 1088
patients: 258 were treated with daclatasvir and asunaprevir for 24
weeks; 316 patients were treated with sofosbuvir (SOF) and
ledipasvir for 12 weeks; 244 patients were treated with SOF and
ribavirin for 12 weeks; 86 patients were treated with elbasvir and
grazoprevir for 12 weeks; 91 patients were treated with ombitasvir,
paritaprevir and ritonavir for 12 weeks; and the remaining 93
patients were treated with glecaprevir and pibrentasvir for 8–12
weeks. None of the patients enrolled in the present study received
multiple courses of DAA treatment, due to recurrence or
non-response to initial treatment. Each patient was examined by
ultrasonography (US) or CT to exclude the presence of HCC at the
onset of DAA administration. Serum samples for HCV RNA measurements
were collected at screening; treatment weeks 1, 2, 4, 8 and 12 (or
early discontinuation); and posttreatment weeks 4, 8, 12, and 24
(or early discontinuation). HCV-RNA was extracted from 200 µl of
serum sample using the Qiagen mRNeasy serum-plasma kit (Qiagen)
according to the manufacturer's instructions. By using a
commercially available device, a TaqMan polymerase chain reaction
method (COBAS AmpliPrep/COBAS TaqMan HCV test version 2; Roche
Molecular Diagnostics; lower limit of quantification, 1.6
log10 IU/ml; lower limit of detection, 1.2
log10 IU/ml) was used to detect HCV RNA, according to
the manufacturer's instructions. HCV Genotyping test was also
detected with the commercial VERSANT HCV Genotype 2.0 assay (LiPA
2.0; Siemens Healthineers) and HCV sequencing for detection of six
HCV genotypes 1a, 1b, 2, 3, 4, and 6 in the serum samples was
conducted according to the manufacturer's instructions.
HCC surveillance after obtaining
SVR12
The blood was collected from patients before, during
(at weeks 2, 4, 8 and 12), and after (at weeks 4, 8 and 12)
treatment. The serum samples were prepared according to the
manufacturer's instructions. The serum samples were not stored
before analysis. US was performed at the time of SVR12 and twice a
year after this. US examination was performed by experienced
hepatologists or sonographers at each institution. The serum levels
of α-fetoprotein (AFP) were measured every 1–6 months. If HCC was
suspected following US examination or as a result of elevated AFP
level, contrast enhanced US (CEUS), dynamic CT or
gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic
acid-enhanced magnetic resonance imaging (EOB-MRI) were performed
for further evaluation of HCC. For the diagnosis of HCC, the 2005
Guidelines of the American Association for the Study of Liver
Disease (16) were adopted. HCC was
diagnosed based on the findings from dynamic CT, EOB-MRI and/or
CEUS using perflubutane (Sonazoid™; Daiichi Sankyo Co., Ltd.)
(17). Typical HCC pattern imaging
criteria were defined as follows: i) Hypervascularity defined as
focal lesion hyperattenuation relative to the liver during the
arterial phase of each imaging method, and wash-out appearance was
observed during the portal and parenchymal phase; and ii) tumors
were revealed as defects in the post vascular phase of CEUS or the
hepatobiliary phase of EOB-MRI. TNM stage was determined according
to the HCC staging report in the Liver Cancer Study Group of Japan,
6th Edition (18). Diagnosis of LC
was performed in accordance with clinical, experimental, abdominal
US and/or histological findings (19). In addition, the fibrosis-4 (FIB-4)
index was used to evaluate fibrosis clinically before and after DAA
treatment. FIB-4 index was calculated using the following formula:
FIB-4 index=[aspartate aminotransferase (AST; IU/l) × age (years) /
platelet count (109/l) × alanine aminotransferase (ALT;
IU/l)1/2]. A cut-off value of 3.25 was used for
prediction of severe fibrosis (20,21).
Statistical analysis
Data are presented as the mean. Statistical analyses
were performed using the Kaplan-Meier method, log-rank test, ROC
analysis and Cox hazard analysis using JMP statistical software
(version 11.0 for Windows; SAS Institute, Inc.). All P-values were
derived from two-tailed tests, and P<0.05 was considered to
indicate a statistically significant difference. Receiver operating
characteristic (ROC) and area under the curve (AUC) values were
calculated to define cut-off values for risk factors of HCC
occurrence.
Results
Patient characteristics
A total of 1,454 patients started IFN-free DAA
treatment during the study period and 1,088 patients were enrolled
in the present study after applying the exclusion criteria.
Baseline characteristics of the patients are presented in Table I. The study population included 545
men and 543 women. The median age of patients at the start of DAA
treatment was 68 years (range, 58–75 years). The numbers of
patients with chronic hepatitis (CH) and LC were 897 and 191,
respectively. Only 683 cases were reported regarding the presence
or absence of diabetes mellitus.
 | Table I.Baseline characteristics of patients
included in the present study. |
Table I.
Baseline characteristics of patients
included in the present study.
| Characteristic | Value |
|---|
| Sex, n
(male/female) | 545/543 |
| Age, years
(range) | 68 (58–75) |
| CH/LC, n | 897/191 |
| HCV serotype, n
(1/2 or 3) | 779/309 |
| AST, IU/l
(range) | 37 (27–59) |
| ALT, IU/l
(range) | 35 (23–60) |
| Total bilirubin,
mg/dl (range) | 0.7
(0.6–0.9) |
| Albumin, g/dl
(range) | 4.1
(3.8–4.3) |
| Hemoglobin, g/dl
(range) | 13.5
(12.4–20.1) |
| White blood cell
count,/µl (range) | 4,850
(3,820–5,950) |
| Platelet count,
×104/µl (range) | 15.8
(11.6–20.1) |
| AFP, ng/ml
(range) | 4.4 (3–8) |
| Total cholesterol,
mg/dl (range) | 168.5
(148.8–190) |
| FIB-4 (range) | 2.94
(1.85–4.63) |
| APRI (range) | 0.86
(0.50–1.55) |
| WFA-M2BP, COI
(range) | 1.59
(0.99–2.42) |
| Diabetes mellitus,
n, no/yes (n=683) | 572/111 |
| Body mass index,
kg/m2 (range) | 22.7
(20.6–25.1) |
| HCV-RNA, log
copies/ml (range) | 6.1 (5.5–6.5) |
| DAA therapy, n (ASV
+DCV/SOF + LDV/SOF+RBV/ERB + GZR/OBV + PTV + r / GLE + PIB) |
258/316/244/86/91/93 |
Cumulative incidence rate of HCC
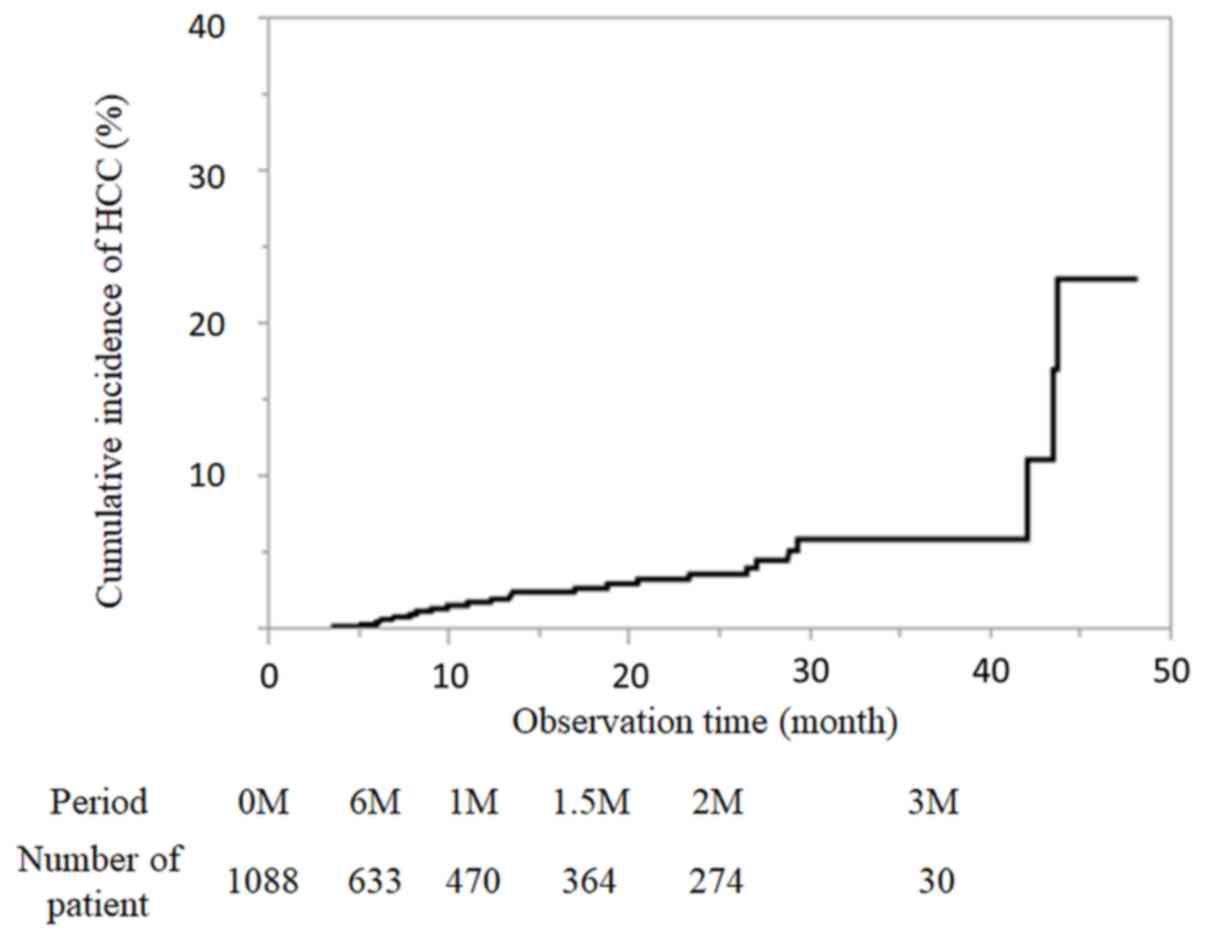
The median observation time was 13.8 months after
the end of DAA treatment. There were many patients 3–6 months after
the end of treatment, and 633 patients could be evaluated half a
year later. During the present study, 26 patients developed HCC.
The incidence of HCC was 0.61, 1.88, 2.82, 3.71 and 6% at 6, 12,
18, 24 and 36 months after DAA treatment by the Kaplan-Meier
method, respectively (Fig. 2). The
number of patients followed-up at each time point is presented in
Fig. 2.
Characteristics of patients who
developed de novo HCC following DAA treatment
The characteristics of the 26 patients who developed
de novo HCC after DAA treatment are presented in Table II. Among these patients, 15 were men
and 11 were women (median age, 72 years; range, 66–79 years).
Furthermore, the numbers of CH and LC cases were 16 and 10,
respectively. Median values for serum levels of AFP and
des-gamma-carboxy prothrombin were 6.5 ng/ml and 28 mAU/ml,
respectively. Among the 26 patients, eight had non-alcoholic fatty
liver disease, eight were alcohol consumers and seven had diabetes
mellitus. Six of the patients with diabetes mellitus were treated
for the condition. The median size of the maximum tumor diameter
was 13 mm and the median number of tumors was one.
 | Table II.Characteristics of patients who
developed HCC newly after DAA treatment. |
Table II.
Characteristics of patients who
developed HCC newly after DAA treatment.
| Characteristic | Value |
|---|
| Sex, n
(male/female) | 15/11 |
| Age, years
(range) | 72 (66–79) |
| CH/LC, n | 16/10 |
| HCV serotype (1/2
or 3) | 24/2 |
| AFP, ng/ml
(range) | 6.5 (5–22) |
| AFP-L3, %
(range) | 0.85 (0.5–3.8) |
| DCP, mAU/ml
(range) | 28 (17.5–66) |
| NAFLD, n,
no/yes | 18/8 |
| Alcohol, n,
none/drinking | 18/8 |
| Diabetes mellitus,
n, no/yes | 19/7 |
| Drugs used in
diabetes mellitus |
|
| Dipeptidyl
peptidase-4 inhibitor | 1 |
| Sulfonylurea | 2 |
| Metformin | 3 |
| Insulin
preparation | 3 |
| No medication | 1 |
| Number of
tumors | 1 (1–2) |
| Maximum tumor
diameter, mm (range) | 13 (10.75–18) |
Risk factors for HCC occurrence
following DAA treatment
Potential prognostic factors for HCC occurrence
included HCV serotype, sex, age, body mass index and blood test
data at both the start and end of DAA treatment (ALT, AST, total
bilirubin, albumin, platelet count, AFP, total cholesterol serum
levels and FIB-4 index; Table
III).
 | Table III.Factors associated with HCC
occurrence after DAA treatment in patients included in the present
study (n=1,088). |
Table III.
Factors associated with HCC
occurrence after DAA treatment in patients included in the present
study (n=1,088).
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Factor | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| HCV serotype, 1 vs.
others | 2.5198 | 0.8662–10.683 | 0.0950 | 1.3602 | 0.4127–6.1675 | 0.6363 |
| Sex, male vs.
female | 1.6772 | 0.7732–3.7544 | 0.1905 |
|
|
|
| Age, for every
year | 1.0729 | 1.0286–1.1253 | 0.0006 | 1.0814 | 0.4127–1.1517 | 0.0044 |
| Body mass index,
for every kg/m2 | 1.0156 | 0.9390–1.0352 | 0.8617 |
|
|
|
| Diabetes mellitus,
yes vs. no | 2.4271 | 0.7715–6.5522 | 0.1208 |
|
|
|
| History of
interferon-based therapy, yes vs. no | 1.2026 | 0.5088–2.6610 | 0.6608 |
|
|
|
| Before DAA
treatment |
|
|
|
|
|
|
| AST,
for every IU/l | 1.0016 | 0.9899–1.0097 | 0.7640 |
|
|
|
| ALT,
for every IU/l | 0.9966 | 0.9846–1.0038 | 0.4466 |
|
|
|
| Total
bilirubin, for every mg/dl | 1.4651 | 0.6076–2.1760 | 0.3257 |
|
|
|
|
Albumin, for every g/dl | 0.3022 | 0.1467–0.6677 | 0.0038 | 0.6493 | 0.1786–2.5285 | 0.0617 |
|
Platelet count, for every
1×104/µl | 0.9873 | 0.9784–0.9966 | 0.0002 | 0.7966 | 0.6234–1.0109 | 0.5269 |
| AFP,
for every ng/ml | 1.0004 | 0.9873–1.0052 | 0.9216 |
|
|
|
| FIB4,
for every 1 | 1.1795 | 1.0725–1.2705 | 0.0016 | 0.7774 | 0.5294–1.0515 | 0.1119 |
| After DAA
treatment |
|
|
|
|
|
|
| AST,
for every IU/l | 0.9985 | 0.9985–1.0127 | 0.8660 |
|
|
|
| ALT,
for every IU/l | 1.0008 | 0.9791–1.0136 | 0.9291 |
|
|
|
| Total
bilirubin, for every mg/dl | 1.0709 | 0.6376–1.5731 | 0.4053 |
|
|
|
|
Albumin, for every g/dl | 1.0042 | 0.8932–1.0160 | 0.8136 |
|
|
|
|
Platelet count, for every
1×104/µl | 0.9099 | 0.8536–0.9674 | 0.0022 | 1.0560 | 0.8605–1.2188 | 0.5688 |
| AFP,
for every ng/ml | 1.0497 | 1.0098–1.3828 | 0.0194 | 1.0486 | 1.0003–1.0900 | 0.0486 |
| FIB-4,
for every 1 | 1.0896 | 0.9799–1.1489 | 0.0938 | 1.0254 | 0.8105–1.1309 | 0.7582 |
The univariate analysis of the log-rank test
identified age (P=0.0006), albumin serum level before DAA treatment
(P=0.0038), platelet count before DAA treatment (P=0.0002), FIB-4
before DAA treatment (P=0.0016), platelet count after DAA treatment
(P=0.0022)and AFP serum level after DAA treatment (P=0.0194) as
risk factors for HCC. In addition, these results identified
serotype (P=0.0950) and FIB-4 after DAA treatment (P=0.0938) to
have the tendency as potential risk factors. Cox hazard analysis
was performed using HCV serotype, age, platelet count before and
after DAA treatment, FIB-4 before and after DAA treatment, albumin
before DAA treatment, and AFP serum level after DAA treatment. The
results demonstrated that age [hazard ratio (HR), 1.0814; 95% CI,
1.0814–1.1517; P=0.0044] and AFP serum level after DAA treatment
(HR, 1.0486; 95% CI, 1.0003–1.0900; P=0.0486) were significant risk
factors that may contribute to HCC occurrence after DAA treatment
(Table III).
ROC analysis and diagnostic value
According to the ROC value for HCC occurrence based
on age noted in 1,088 patients (sensitivity, 0.500; specificity,
0.7372; AUC, 0.6416), the cut-off value for age was set as 75 years
(data not shown). Furthermore, the cut-off value of AFP serum level
after DAA treatment was set as 6.0 ng/ml, based on the ROC value
for developing HCC based on AFP serum levels before DAA treatment
noted in 1,088 patients (sensitivity, 0.8400; specificity, 0.6315;
AUC, 0.743) (data not shown).
Determination of HCC risk score by
combining sex and AFP serum level at the start of DAA
treatment
The Cox hazard analysis was performed using age,
platelet count before and after DAA treatment, FIB-4 before and
after DAA treatment, albumin serum level before DAA treatment, AFP
serum level after DAA treatment grouped by cut-off values, and HCV
serotype (data not shown). Age ≥75 years (HR, 4.17; 95% CI,
1.5658–11.915; P=0.0043) and AFP serum level after DAA treatment
(HR, 2.99; 95% CI, 1.1002–8.6251; P=0.0318) were significant risk
factors contributing to HCC occurrence after DAA treatment
(Table IV). Therefore, based on the
results of the multivariate analysis, the scoring system to predict
occurrence of HCC should be based on two factors: Age and
post-treatment AFP serum level. The age groups of <75 and ≥75
years were set as 0 and 1 points, respectively and post-treatment
AFP serum level (ng/ml) <6 and ≥6 were set as 0 and 1 points,
respectively (Table IV). To
simplify the score of HCC occurrence, the sum of the points given
for each factor was considered as the score.
 | Table IV.Independent factors associated with
HCC occurrence after DAA treatment in Cox hazard analysis and
scores for prediction of HCC occurrence. |
Table IV.
Independent factors associated with
HCC occurrence after DAA treatment in Cox hazard analysis and
scores for prediction of HCC occurrence.
| Factor | HR | 95% CI | P-value | Score for
prediction of HCC occurrence (points) |
|---|
| Age |
|
|
|
|
| ≥75
years | 4.17 | 1.5658–11.915 | 0.0043 | 1 |
| <75
years | 1.00 |
|
| 0 |
| AFP after DAA
treatment |
|
|
|
|
| <6
ng/ml | 1.00 |
|
| 0 |
| ≥6
ng/ml | 2.99 | 1.1002–8.6251 | 0.0318 | 1 |
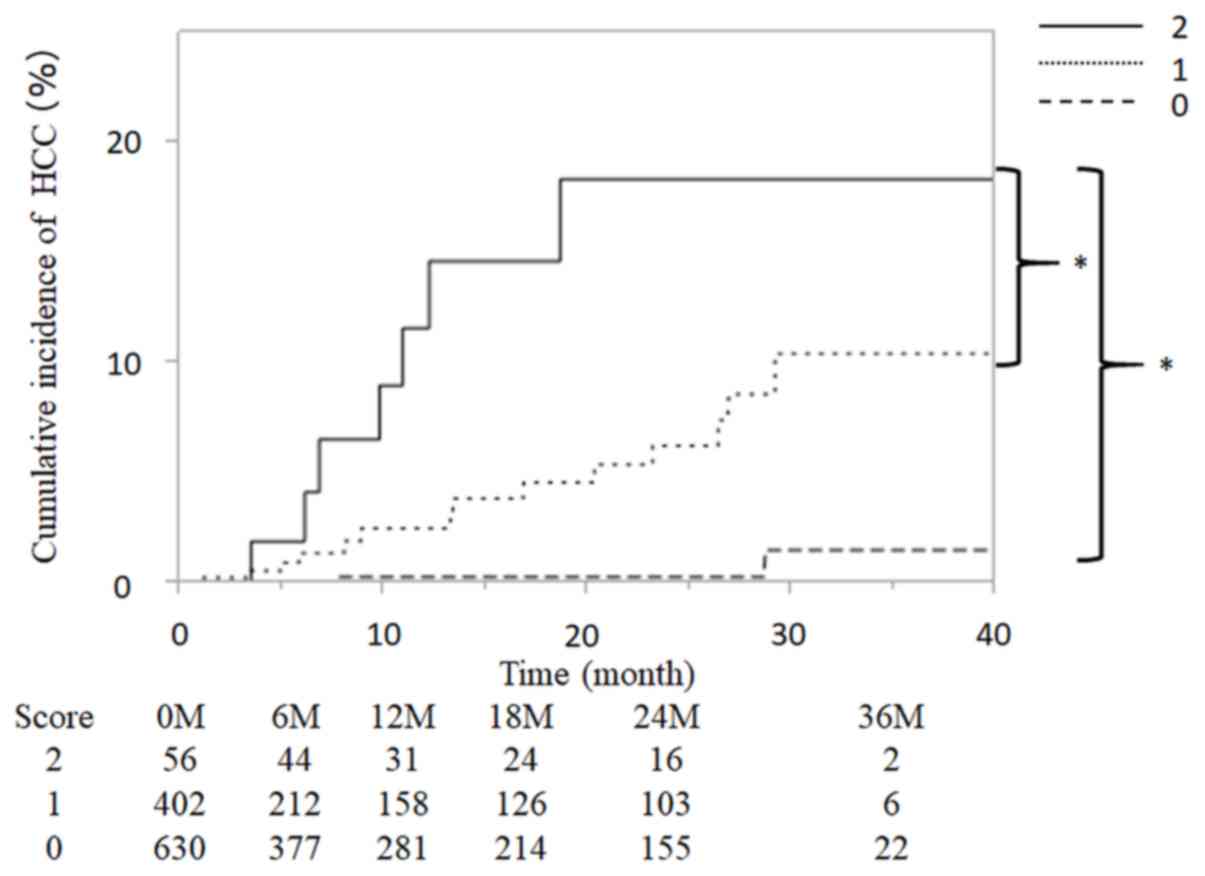
Patients included in the present study were scored
based on the aforementioned risk factors, giving total scores of 0,
1 or 2 for each patient, and were subsequently grouped as follows:
0 points (n=630), 1 point (n=402) and 2 points (n=56). Fig. 3 presents the cumulative incidence
curve of HCC occurrence for each group. The incidence of HCC was
0.00, 0.30, 0.30, 0.30 and 1.26% at 6, 12 18, 24 and 36 months,
respectively, in the 0 points group. The incidence of HCC was 0.71,
1.05, 1.58, 6.27 and 10.45% in the 1 point group. The incidence of
HCC was 2.88, 4.92, 11.61, 18.37 and 18.37%, respectively, in the 2
points group. In addition, the cumulative incidence of HCC was
increased significantly with higher predictive scores (P<0.001;
Fig. 3).
Discussion
HCC frequently occurs in patients with chronic HCV
infection who have received IFN-based treatment, even after
achieving SVR. It has been previously reported that AFP and ALT
levels after treatment are risk factors associated with HCC
occurrence (12). Furthermore, other
studies have demonstrated that risk factors for the occurrence of
HCC in patients who achieved SVR after IFN-based treatment are
elderly age, advanced liver fibrosis, fatty liver, male sex and
alcohol consumption (22–24). Although IFN-based treatment has been
the standard treatment for HCV-infected patients to date, it cannot
be used in elderly patients and in patients with advanced liver
fibrosis, due to the risks of IFN-related adverse events.
Recently, DAA has been introduced as a simple and
safe antiviral oral treatment for HCV infection. The application of
DAA has facilitated the eradication of HCV, even in elderly
patients and patients with LC (6–11). In
addition, it has been reported that eradication of HCV by DAA
treatment reduces the risk of HCC (25). However, there are few reports on the
incidence of HCC occurrence following DAA treatment in patients who
have achieved SVR, compared with IFN therapy. Previous studies have
demonstrated that IFN treatment reduces the incidence of HCC
(26,27). The 5-year cumulative incidence of HCC
is ~3% in patients who achieve SVR following IFN-based treatment
(22,28). However, in patients >65 years, the
5-year cumulative incidence of HCC is 6%, whereas patients with
cirrhosis presented with a high rate of 18.9% (12,29).
Therefore, it is crucial to identify and predict the incidence of
HCC after DAA treatment, which includes patients that are older and
have cirrhosis.
There are two possible reasons for the occurrence of
HCC after SVR. Firstly, HCC may have been missed following imaging
diagnosis before DAA administration. Secondly, HCC could develop
after DAA treatment. In the present study, all images obtained
following US, CT or MRI were repeatedly reviewed to prevent any
oversight and to exclude patients with HCC before starting DAA
treatment. The results of the present study demonstrated that the
12- and 36-months cumulative incidences of HCC were 1.88 and 6.00%,
respectively. Chang et al (30) reported that the 3-year cumulative
incidence of HCC in patients who received IFN treatment was 1.2%.
Furthermore, Watanabe et al (31) recently demonstrated that the 1- and
2-year cumulative incidences of HCC were 1.9 and 4.1%,
respectively. In addition, in the high-risk groups for HCC
occurrence, the 1- and 2-year cumulative incidences were 6.1 and
14.4%, respectively (31). The
results of these studies supported the data from the present study
demonstrating that the incidence of HCC at 3 years after DAA
treatment was higher than that after IFN treatment (11,22,26–31).
This difference in HCC incidence between DAA and IFN treatment may
be due to the inclusion of patients with LC in the study group.
The present study demonstrated that the 26 cases of
de novo HCC shared common characteristics (high age and
early stage HCC). Fatty liver, alcohol consumption and diabetes
mellitus have been suggested to be involved in HCC occurrence
(24,31); however, the hazard ratio of these
factors was not high in the present study. In addition, there was
no difference observed in HCC occurrence in patients following
diabetes treatment. Because the observation period until HCC
occurrence was short (13.8 months), alcohol consumption and
diabetes mellitus were not considered to be involved in HCC
occurrence. Instead, the present study suggested a cytological
origin of carcinogenesis before DAA treatment; however, long-term
observations indicated that these factors would gradually
contribute to liver carcinogenesis.
El-Serag et al demonstrated that older adults
are at higher risk of developing HCC (32). In the present study, univariate
analysis identified age, HCV serotype, albumin serum level,
platelet count and FIB-4 index before DAA treatment as risk factors
for HCC occurrence. In addition, platelet counts, AFP serum level
and FIB-4 index after DAA treatment were considered as risk factors
for HCC occurrence. Furthermore, following multivariate analysis,
age and AFP serum level after DAA treatment were identified as
independent risk factors for HCC occurrence. These results were
consistent with previous studies, which reported that elderly
patients have a higher risk of developing HCC after antiviral
treatment (22,23,33,34).
Elevated AFP serum level is recognized as one of the
common risk factors for HCC occurrence (31,35). In
the present study, AFP serum level after DAA treatment was
considered as a risk factor for HCC occurrence. Subsequently, the
cutoff value of AFP serum level after DAA treatment was established
as 6 ng/ml. In the present study, a simple scoring system was
proposed to predict the risk of HCC occurrence after HCV
eradication by DAA treatment. The HRs of age and AFP after DAA
treatment were similar. In order to establish a simple scoring
system, scores of 0 or 1 point for age <75 and ≥75 years,
respectively, were designated. The identified risk factors of age
(≥75 years) and AFP serum level after treatment (≥6.0 ng/ml) were
scored as 1 point, and each patient was scored for these risk
factors, giving a total of 0, 1 or 2 points. The 2-year incidence
of HCC was 0.30, 6.27 and 18.37% in the 0, 1 and 2 points groups,
respectively. The cumulative incidence of HCC increased
significantly with higher scores (P<0.001). Previous studies
have reported a stratification of HCC occurrence rate by scoring
with FIB-4 and AFP after SVR or sex (31,34).
However, compared with other reports, the scoring system
established in the present study is simple and concise, and uses
only two factors (advanced age and AFP levels after DAA treatment)
to predict the risk of HCC occurrence after SVR.
The prognosis of patients with HCC is known to be
dependent on tumor number, tumor size and liver function (36,37). It
has been demonstrated that VEGF expression is increased following
antiviral treatment, and may contribute to HCC occurrence after DAA
treatment (38). However, because
accurate quantification of VEGF is complicated, a simple method is
required for the early detection of HCC, particularly in high-risk
patients. Therefore, surveillance of HCC occurrence in patients who
have achieved successful HCV eradication by DAA treatment is
crucial in the presented simple scoring system. By identifying
patients with high risk of HCC occurrence after SVR, early
diagnosis could be more achievable. In the 2-points group
identified by the present scoring system, it may be necessary to
confirm non-hypervascular hyperpointense nodes with EOB-MRI before
DAA treatment (39). However, the
observation period was short in the present study, and a longer
observation period is required to validate the conclusions.
In conclusion, age ≥75 years and AFP ≥6 ng/ml after
DAA treatment were independent predictors for the development of
HCC in patients who achieved SVR after DAA treatment. The proposed
scoring system using these two factors may serve an important role
in determining the risk of HCC occurrence after DAA treatment.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JT designed the research and analyzed the patient
data. JT drafted the manuscript. JT, AsM, TeS, KeT, MN, KF, KO, TT,
TNo, HY, AT, ToS, TNa, CO, AkM and AD analyzed and interpreted the
data. SM, HK, TH, KoT and TM interpreted the data and revised the
manuscript critically for important intellectual content. All
authors were involved in data interpretation and drafting the
manuscript and have read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The present study conformed to the Clinical Research
Guidelines and was approved by the Ethics Committee of Kagawa
University, Faculty of Medicine (approval no. Heisei-27-174). The
requirement for informed consent from the participants was waived
because of the retrospective nature of the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
AFP
|
α-fetoprotein
|
|
CEUS
|
contrast enhanced ultrasonography
|
|
AUC
|
area under the curve
|
|
ALT
|
alanine aminotransferase
|
|
AST
|
aspartate aminotransferase
|
|
CH
|
chronic hepatitis
|
|
DAA
|
direct acting antivirals
|
|
EOB-MRI
|
gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid
enhanced magnetic resonance imaging
|
|
FIB-4
|
fibrosis-4
|
|
HCC
|
hepatocellular carcinoma
|
|
HCV
|
hepatitis C virus
|
|
HR
|
hazard ratio
|
|
IFN
|
interferon
|
|
LC
|
liver cirrhosis
|
|
ROC
|
receiver operating characteristic
|
|
SOF
|
sofosbuvir
|
|
SVR
|
sustained virological response
|
|
US
|
ultrasonography
|
|
VEGF
|
vascular endothelial growth factor
|
References
|
1
|
Lauer GM and Walker BD: Hepatitis C virus
infection. N Engl J Med. 345:41–52. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fried MW, Shiffman ML, Reddy KR, Smith C,
Marinos G, Gonçales FL Jr, Häussinger D, Diago M, Carosi G,
Dhumeaux D, et al: Peginterferon alfa-2a plus ribavirin for chronic
hepatitis C virus infection. N Engl J Med. 347:975–982. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hadziyannis SJ, Sette H Jr, Morgan TR,
Balan V, Diago M, Marcellin P, Ramadori G, Bodenheimer H Jr,
Bernstein D, Rizzetto M, et al: Peginterferon-alpha2a and ribavirin
combination therapy in chronic hepatitis C: A randomized study of
treatment duration and ribavirin dose. Ann Intern Med. 140:346–355.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Manns MP, McHutchison JG, Gordon SC,
Rustgi VK, Shiffman M, Reindollar R, Goodman ZD, Koury K, Ling M
and Albrecht JK: Peginterferon alfa-2b plus ribavirin compared with
interferon alfa-2b plus ribavirin for initial treatment of chronic
hepatitis C: A randomised trial. Lancet. 358:958–965. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fried MW: Side effects of therapy of
hepatitis C and their management. Hepatology. 36 (5 Suppl
1):S237–S244. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Omata M, Nishiguchi S, Ueno Y, Mochizuki
H, Izumi N, Ikeda F, Toyoda H, Yokosuka O, Nirei K, Genda T, et al:
Sofosbuvir plus ribavirin in Japanese patients with chronic
genotype 2 HCV infection: An open-label, phase 3 trial. J Viral
Hepat. 21:762–768. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ogawa E, Furusyo N, Nomura H, Dohmen K,
Higashi N, Takahashi K, Kawano A, Azuma K, Satoh T, Nakamuta M, et
al: NS5A resistance-associated variants undermine the effectiveness
of ledipasvir and sofosbuvir for cirrhotic patients infected with
HCV genotype 1b. J Gastroenterol. 52:845–854. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Poordad F, Felizarta F, Asatryan A,
Sulkowski MS, Reindollar RW, Landis CS, Gordon SC, Flamm SL, Fried
MW, Bernstein DE, et al: Glecaprevir and pibrentasvir for 12 weeks
for hepatitis C virus genotype 1 infection and prior direct-acting
antiviral treatment. Hepatology. 66:389–397. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Toyoda H, Kumada T, Tada T, Shimada N,
Takaguchi K, Senoh T, Tsuji K, Tachi Y, Hiraoka A, Ishikawa T, et
al: Efficacy and tolerability of an IFN-free regimen with DCV/ASV
for elderly patients infected with HCV genotype 1B. J Hepatol.
66:521–527. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Toyoda H, Chayama K, Suzuki F, Sato K,
Atarashi T, Watanabe T, Atsukawa M, Naganuma A, Notsumata K, Osaki
Y, et al: Efficacy and safety of glecaprevir/pibrentasvir in
Japanese patients with chronic genotype 2 hepatitis C virus
infection. Hepatology. 67:505–513. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Suda G, Kurosaki M, Itakura J, Izumi N,
Uchida Y, Mochida S, Hasebe C, Abe M, Haga H, Ueno Y, et al: Safety
and efficacy of elbasvir and grazoprevir in Japanese hemodialysis
patients with genotype 1b hepatitis C virus infection. J
Gastroenterol. 54:78–86. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Asahina Y, Tsuchiya K, Nishimura T,
Muraoka M, Suzuki Y, Tamaki N, Yasui Y, Hosokawa T, Ueda K,
Nakanishi H, et al: alpha-fetoprotein levels after interferon
therapy and risk of hepatocarcinogenesis in chronic hepatitis C.
Hepatology. 58:1253–1262. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hirakawa M, Ikeda K, Arase Y, Kawamura Y,
Yatsuji H, Hosaka T, Sezaki H, Akuta N, Kobayashi M, Saitoh S, et
al: Hepatocarcinogenesis following HCV RNA eradication by
interferon in chronic hepatitis patients. Intern Med. 47:1637–1643.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Conti F, Buonfiglioli F, Scuteri A, Crespi
C, Bolondi L, Caraceni P, Foschi FG, Lenzi M, Mazzella G, Verucchi
G, et al: Early occurrence and recurrence of hepatocellular
carcinoma in HCV-related cirrhosis treated with direct-acting
antivirals. J Hepatol. 65:727–733. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kanwal F, Kramer J, Asch SM, Chayanupatkul
M, Cao Y and El-Serag HB: Risk of hepatocellular cancer in HCV
patients treated with direct-acting antiviral agents.
Gastroenterology. 153:996–1005.e1. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bruix J and Sherman M; Practice Guidelines
Committee, American Association for the Study of Liver Diseases, :
Management of hepatocellular carcinoma. Hepatology. 42:1208–1236.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hiraoka A, Hiasa Y, Onji M and Michitaka
K: New contrast enhanced ultrasonography agent: Impact of Sonazoid
on radiofrequency ablation. J Gastroenterol Hepatol. 26:616–618.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kudo M, Kitano M, Sakurai T and Nishida N:
General rules for the clinical and pathological study of primary
liver cancer, nationwide follow-up survey and clinical practice
guidelines: The outstanding achievements of the liver cancer study
group of Japan. Dig Dis. 33:765–770. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hung CH, Lu SN, Wang JH, Lee CM, Chen TM,
Tung HD, Chen CH, Huang WS and Changchien CS: Correlation between
ultrasonographic and pathologic diagnoses of hepatitis B and C
virus-related cirrhosis. J Gastroenterol. 38:153–157. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sterling RK, Lissen E, Clumeck N, Sola R,
Correa MC, Montaner J, S Sulkowski M, Torriani FJ, Dieterich DT,
Thomas DL, et al: Development of a simple noninvasive index to
predict significant fibrosis in patients with HIV/HCV coinfection.
Hepatology. 43:1317–1325. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vallet-Pichard A, Mallet V, Nalpas B,
Verkarre V, Nalpas A, Dhalluin-Venier V, Fontaine H and Pol S:
FIB-4: An inexpensive and accurate marker of fibrosis in HCV
infection. comparison with liver biopsy and fibrotest. Hepatology.
46:32–36. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ikeda M, Fujiyama S, Tanaka M, Sata M, Ide
T, Yatsuhashi H and Watanabe H: Risk factors for development of
hepatocellular carcinoma in patients with chronic hepatitis C after
sustained response to interferon. J Gastroenterol. 40:148–156.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tokita H, Fukui H, Tanaka A, Kamitsukasa
H, Yagura M, Harada H and Okamoto H: Risk factors for the
development of hepatocellular carcinoma among patients with chronic
hepatitis C who achieved a sustained virological response to
interferon therapy. J Gastroenterol Hepatol. 20:752–758. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tanaka A, Uegaki S, Kurihara H, Aida K,
Mikami M, Nagashima I, Shiga J and Takikawa H: Hepatic steatosis as
a possible risk factor for the development of hepatocellular
carcinoma after eradication of hepatitis C virus with antiviral
therapy in patients with chronic hepatitis C. World J
Gastroenterol. 13:5180–5187. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ioannou GN, Green PK and Berry K: HCV
eradication induced by direct-acting antiviral agents reduces the
risk of hepatocellular carcinoma. J Hepatol. S0168-8278(17)32273-0.
2017.(Epub ahead of print).
|
|
26
|
Cammà C, Giunta M, Andreone P and Craxì A:
Interferon and prevention of hepatocellular carcinoma in viral
cirrhosis: An evidence-based approach. J Hepatol. 34:593–602. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mazzaferro V, Romito R, Schiavo M, Mariani
L, Camerini T, Bhoori S, Capussotti L, Calise F, Pellicci R, Belli
G, et al: Prevention of hepatocellular carcinoma recurrence with
alpha-interferon after liver resection in HCV cirrhosis.
Hepatology. 44:1543–1554. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Arase Y, Kobayashi M, Suzuki F, Suzuki Y,
Kawamura Y, Akuta N, Kobayashi M, Sezaki H, Saito S, Hosaka T, et
al: Effect of type 2 diabetes on risk for malignancies includes
hepatocellular carcinoma in chronic hepatitis C. Hepatology.
57:964–973. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ogawa E, Furusyo N, Kajiwara E, Takahashi
K, Nomura H, Maruyama T, Tanabe Y, Satoh T, Nakamuta M, Kotoh K, et
al: Efficacy of pegylated interferon alpha-2b and ribavirin
treatment on the risk of hepatocellular carcinoma in patients with
chronic hepatitis C: A prospective, multicenter study. J Hepatol.
58:495–501. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chang KC, Hung CH, Lu SN, Wang JH, Lee CM,
Chen CH, Yen MF, Lin SC, Yen YH, Tsai MC, et al: A novel predictive
score for hepatocellular carcinoma development in patients with
chronic hepatitis C after sustained response to pegylated
interferon and ribavirin combination therapy. J Antimicrob
Chemother. 67:2766–2772. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Watanabe T, Tokumoto Y, Joko K, Michitaka
K, Horiike N, Tanaka Y, Tada F, Kisaka Y, Nakanishi S, Yamauchi K,
et al: Predictors of hepatocellular carcinoma occurrence after
direct-acting antiviral therapy in patients with hepatitis C virus
infection. Hepatol Res. 49:136–146. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Asahina Y, Tsuchiya K, Tamaki N, Hirayama
I, Tanaka T, Sato M, Yasui Y, Hosokawa T, Ueda K, Kuzuya T, et al:
Effect of aging on risk for hepatocellular carcinoma in chronic
hepatitis C virus infection. Hepatology. 52:518–527. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
El-Serag HB and Rudolph KL: Hepatocellular
carcinoma: Epidemiology and molecular carcinogenesis.
Gastroenterology. 132:2557–2576. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hiraoka A, Kumada T, Ogawa C, Kariyama K,
Morita M, Nouso K, Toyoda H, Tada T, Ochi M, Murakami T, et al:
Proposed a simple score for recommendation of scheduled
ultrasonography surveillance for hepatocellular carcinoma after
direct acting antivirals: Multicenter analysis. J Gastroenterol
Hepatol. 34:436–441. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Toyoda H, Tada T, Takaguchi K, Senoh T,
Shimada N, Hiraoka A, Michitaka K, Ishikawa T and Kumada T:
Differences in background characteristics of patients with chronic
hepatitis C who achieved sustained virologic response with
interferon-free versus interferon-based therapy and the risk of
developing hepatocellular carcinoma after eradication of hepatitis
C virus in Japan. J Viral Hepat. 24:472–476. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kudo M, Chung H and Osaki Y: Prognostic
staging system for hepatocellular carcinoma (CLIP score): Its value
and limitations, and a proposal for a new staging system, the Japan
integrated staging score (JIS score). J Gastroenterol. 38:207–215.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hiraoka A, Kumada T, Kudo M, Hirooka M,
Tsuji K, Itobayashi E, Kariyama K, Ishikawa T, Tajiri K, Ochi H, et
al: Albumin-bilirubin (ALBI) grade as part of the evidence-based
clinical practice guideline for HCC of the Japan society of
hepatology: A comparison with the liver damage and child-pugh
classifications. Liver Cancer. 6:204–215. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Villani R, Facciorusso A, Bellanti F,
Tamborra R, Piscazzi A, Landriscina M, Vendemiale G and Serviddio
G: DAAs rapidly reduce inflammation but increase serum VEGF Level:
A rationale for tumor risk during anti-HCV treatment. PLoS One.
11:e01679342016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Toyoda H, Kumada T, Tada T, Mizuno K, Sone
Y, Akita T, Tanaka J and Johnson PJ: The impact of HCV eradication
by direct-acting antivirals on the transition of precancerous
hepatic nodules to HCC: A prospective observational study. Liver
Int. 39:448–454. 2019. View Article : Google Scholar : PubMed/NCBI
|