Introduction
Breast cancer is one of the most common malignant
tumors in women. In China, the incidence of breast cancer ranks
first and its mortality rate ranks second after that of lung cancer
(1). Clinical studies have
demonstrated that ~20–30% of early breast cancer will recur and
metastasize to become advanced breast cancer, that is, recurrent
metastatic breast cancer, while ~3–10% of breast cancer patients
have distant metastasis at the initial diagnosis, namely de
novo metastatic breast cancer (DnMBC) (2–4).
Patients with DnMBC often lose the opportunity of radical surgery
when they were diagnosed for the first time and their psychological
status and quality of life are seriously affected. At the same
time, because of its special clinicopathological characteristics
and since its proportion in advanced breast cancer patients is
increasing year by year, more and more clinicians are paying
attention to it (5). Prior reports
state that the clinicopathological features of DnMBC were greater
invasiveness. The proportion of multiple metastasis and visceral
metastasis was relatively high, and more patients had high
expression of Ki-67 and high histological grade (6). Although some progress has been made in
the treatment of MBC in the past decade, according to real-world
and clinical trial data, the 5-year survival is still low at ~25%
(6–9). The purpose of this retrospective study
was to analyze the clinicopathological characteristics of DnMBC and
to explore the prognostic factors, in order to provide guidance for
clinical diagnosis and treatment.
Patients and methods
Study population
Data for this study population were obtained from
Tianjin Medical University Cancer Hospital. A total of 1,890
patients with advanced breast cancer were treated between January
2008 to December 2017. Patient characteristics are presented in
Table I. A total of 171 patients
with DnMBC were screened out. The criteria for admission were as
follows: i) Female patients; ii) primary unilateral breast cancer
diagnosed by pathology or imaging with distant metastasis; iii)
relatively complete clinical and pathological data, including age
of onset, menopause, Ki-67 expression level, hormone receptor
status. Human epidermal growth factor receptor 2 (HER2) expression,
initial metastasis and treatment and iv) follow-up data are
complete. Case exclusion criteria: Male breast cancer, early or
locally advanced breast cancer, primary double breast cancer,
combined with other malignant tumors, incomplete clinical and
pathological data or lost follow-up. In order to adjust for
guaranteed time bias, patients only include DnMBC diagnosed early
by modern imaging and exclude those who diagnosed shortly after
surgery.
 | Table I.Baseline characteristics of patients
with de novo metastatic breast cancer. |
Table I.
Baseline characteristics of patients
with de novo metastatic breast cancer.
| Characteristic | Patient, n
(total=171) | % |
|---|
| Median age,
years | 53 | – |
| (range, years) | (23–77) |
|
| Menopausal
status |
|
|
|
Premenopausal | 74 | 43.3 |
|
Postmenopausal | 97 | 56.7 |
| Primary tumor
stage |
|
|
| T1 | 14 | 8.2 |
| T2 | 46 | 26.9 |
| T3 | 37 | 21.6 |
| T4 | 64 | 37.4 |
|
Unknown | 10 | 5.8 |
| Regional lymph node
stage |
|
|
| N0 | 10 | 5.8 |
| N1 | 30 | 17.5 |
| N2 | 40 | 23.4 |
| N3 | 81 | 47.4 |
|
Unknown | 10 | 5.8 |
| Estrogen-receptor
status |
|
|
|
Positive | 119 | 69.6 |
|
Negative | 52 | 30.4 |
|
Progesterone-receptor status |
|
|
|
Positive | 101 | 59.1 |
|
Negative | 70 | 40.9 |
| HER2 status |
|
|
|
Positive | 51 | 29.8 |
|
Negative | 120 | 70.2 |
| Subtype |
|
|
|
HER2+/HR± | 50 | 29.2 |
|
HER2−/HR+ | 97 | 56.7 |
|
HER2−/HR− | 24 | 14.0 |
| Ki-67 |
|
|
|
<20% | 13 | 7.6 |
|
≥20% | 117 | 68.4 |
|
Unknown | 41 | 24.0 |
| Visceral
metastasis |
|
|
| No | 95 | 55.6 |
|
Yes | 76 | 44.4 |
| Sites of first
metastasis |
|
|
|
Oligometastasis | 94 | 55.0 |
|
Bone | 54 | 31.6 |
|
Lung/pleura | 24 | 14.0 |
|
Liver | 15 | 8.8 |
|
Brain | 1 | 0.6 |
|
Polymetastasis | 77 | 45.0 |
| First-line
treatment |
|
|
|
Chemotherapy | 153 | 89.5 |
|
Surgery | 11 | 6.4 |
|
Endocrine | 1 | 0.6 |
|
Radiotherapy | 1 | 0.6 |
|
Unknown | 5 | 2.9 |
| Surgery |
|
|
|
Yes | 41 | 24.0 |
| No | 130 | 76.0 |
| First-line
chemotherapy |
|
|
|
Anthracycline-containing | 14 | 8.2 |
|
Taxane-containing | 48 | 28.1 |
|
Anthracycline+Taxanes | 100 | 58.5 |
|
Other | 9 | 5.2 |
| Trastuzumab for
HER2+ |
|
|
|
Yes | 33 | 64.7 |
| No | 18 | 35.3 |
| Family history of
cancer |
|
|
|
Yes | 22 | 12.9 |
| No | 149 | 87.1 |
Definition
Breast cancer staging is based on the TNM staging
guidelines of the seventh edition of the American Joint Committee
on Cancer. Estrogen receptor (ER), progesterone receptor (PR) and
HER2 were evaluated according to the scoring system recommended by
the American Society of Clinical Oncology and the College of
American Pathologists (10).
Estrogen and progesterone receptor status was assessed using
immunohistochemistry (IHC) staining of tissue sections, as
previously described (11,12). At least 1% nuclear positive tumor
cells were considered to be positive. ER and/or PR positive were
defined as hormone receptor (HR) positive BC. HER2 status by IHC
was positive if the score was 3+ and tumors with indeterminate (2+)
IHC scores were considered HER2 positive if amplified by
fluorescence in situ hybridization, as previously described
(11,13). The rest were classified as negative
for analysis. Patients who could not be operated on were clinically
staged by clinical palpation and imaging techniques such as
B-ultrasound and computed tomography. In this study, patients with
single organ metastasis and less than five metastatic lesions were
grouped into an oligometastasis group and the rest into a
polymetastasis group.
Data analysis
Patient characteristics were tabulated and compared
between groups using the chi-square test. Progression-free survival
(PFS) was defined as the time from the diagnosis of DnMBC to the
progression of the tumor or death. Overall survival (OS) was
defined as the time from the diagnosis of DnMBC to death by any
cause or the last visit. Survival curves were determined by the
Kaplan-Meier method and compared using the log-rank test. Values of
1-year and 3-year PFS; and 3 and 5-year OS were calculated.
Multivariate analysis was performed by Cox regression. All P-values
reported were two-sided and P<0.05 was considered to indicate a
statistically significant difference.
Results
Baseline patient characteristics
A total of 171 patients with DnMBC were screened out
of 1,890 patients with advanced breast cancer treated between
January 2008 to December 2017 in the authors' hospital. The
baseline characteristics are presented in Table I. Among the patients screened, the
median age at diagnosis was 53 years (range, 23–77). Regarding
primary tumor size, 14 cases (8.2%) were in stage T1, 46 (26.9%) in
stage T2, 37 (21.6%) in stage T3 and 64 (37.4%) in stage T4.
Regarding axillary lymph nodes, 10 cases (5.8%) were in stage N0,
30 (17.5%) in stage N1, 40 (23.4%) in stage N2 and 81 (47.4%) in
stage N3. A total of 119 (69.6%) were ER positive, 52 (30.4%) were
negative; 101 (59.1%) were PR positive and 70 (4%) were negative.
There were 51 cases (29.8%) with positive HER2 and 120 cases
(70.2%) with negative HER2, 13 cases (7.6%) with Ki-67 <20%, 117
cases (68.4%) with Ki-67 (>20%) and 77 cases (45%) with
extensive metastasis and 94 cases (55%) with oligometastasis, of
which 54 cases (31.6%) had single bone metastasis, 24 cases (14%)
had lung/pleura metastasis, 15 cases (8.8%) had liver metastasis,
and 1 (0.6%) case had brain metastasis.
In the present study, almost all of the pathological
types are invasive cancers and the pathological diagnosis of the
patients mainly came from breast tumor puncture specimens, which
may not be able to clearly reveal the pathological details of
invasive cancer due to the small amount of tissue contained in the
specimens.
Treatments
Of the 171 patients, 150 (87.7%) were treated with
chemotherapy initially and 162 (94.8%) were treated with taxane- or
anthracycline-based regimens in the first-line treatment, of which
100 cases (58.5%) were treated with the combination of
anthracyclines and taxanes. In HER2-positive patients, 33 (64.7%)
were treated with trastuzumab and 18 (35.3%) without it. Regarding
the operation, 41 patients (24.0%) received palliative surgery for
the primary tumor, of which 13 patients underwent surgery before
the rescue chemotherapy and the remaining 28 patients received
surgery after different cycles of chemotherapy. The average time to
surgery was 7.3 months (0–22 months) after the diagnosis of
DnMBC.
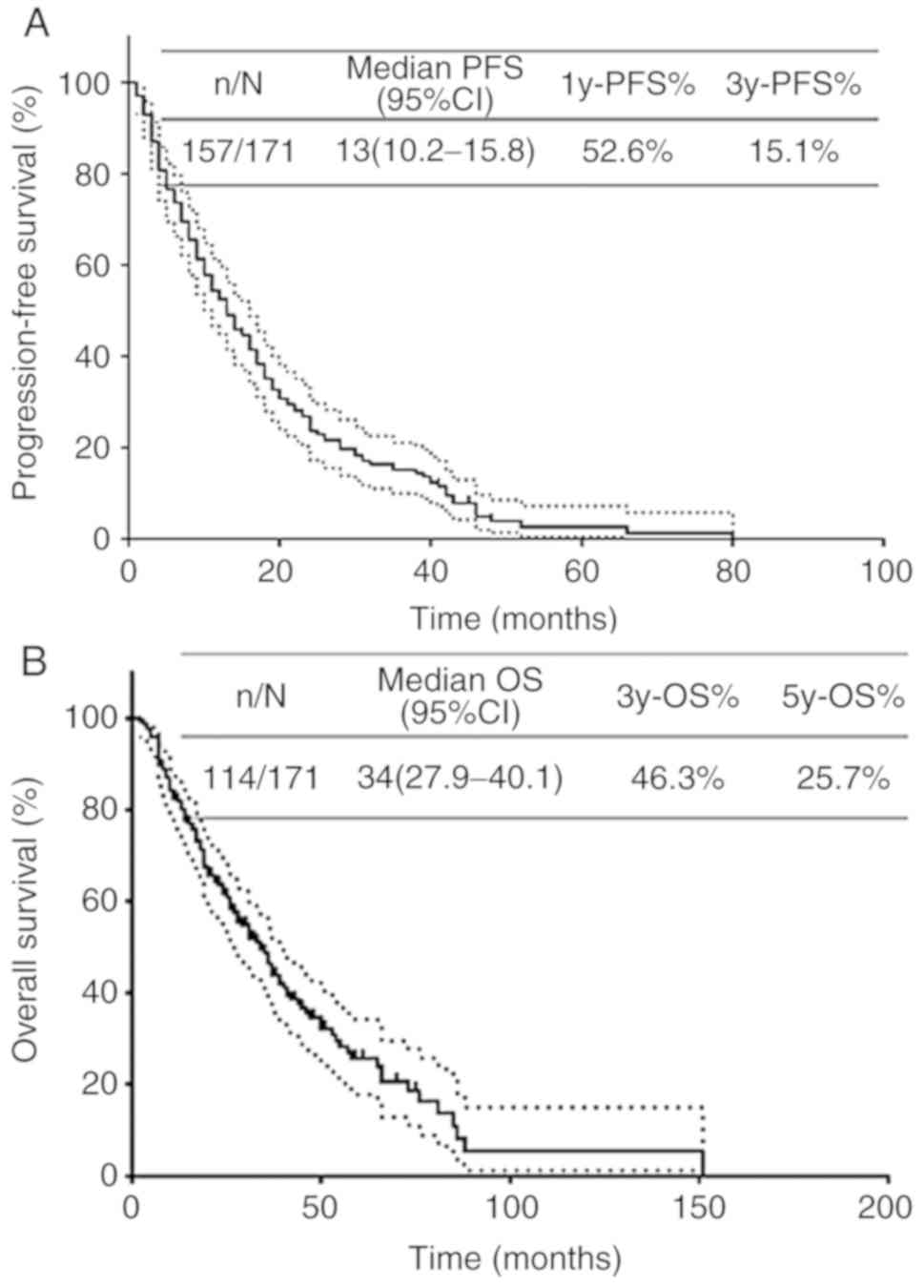
Survival estimates
The median follow-up time of 171 patients was 26
months (2–151 months). The survival rate was calculated using the
Kaplan-Meier method. The median PFS was 13 months (10.2–15.8
months), the median OS was 34 months (27.9–40.1 months), the 1-year
and 3-year PFS rates were 48.1% (48.0–48.2%) and 10.9%
(10.85–10.95%) respectively, and the 3-year and 5-year OS rates
were 46.3% (46.4%) and 25.7% (25.6–25.8%; Fig. 1).
Univariate and multivariate analysis
of predictors of survival
Univariate analysis showed that menstrual status, ER
status, PR status, HER2 expression, Ki-67 index, molecular typing,
initial metastasis and primary breast cancer surgery were
associated with PFS (P<0.05; Table
II). Primary tumor size, ER status, PR status, molecular
typing, initial metastasis, visceral metastasis, anti-HER2
treatment in HER2 positive patients and primary breast cancer
surgery were associated with OS (P<0.05; Table II).
 | Table II.Univariate analysis of PFS and
OS. |
Table II.
Univariate analysis of PFS and
OS.
|
|
| PFS |
|
| OS |
|
|
|---|
|
|
|
|
|
|
|
|
|
|---|
|
Characteristics |
| Median time, % | 1 y | 3 y | χ2 | P-value | Median time, % | 3 y | 5 y | χ2 | P-value |
|---|
| Age, years |
|
|
|
| 0.688 | 0.407 |
|
|
| 0.010 | 0.920 |
|
<40 | 30 | 14 | 53.3 | 21.8 |
|
| 31 | 40.9 | 25.6 |
|
|
|
≥40 | 141 | 13 | 52.5 | 13.5 |
|
| 34 | 47.5 | 25.3 |
|
|
| Menopausal
status |
|
|
|
| 6.210 | 0.013a |
|
|
| 2.840 | 0.092 |
|
Premenopausal | 74 | 17 | 62.1 | 19.7 |
|
| 36 | 49.4 | 35.2 |
|
|
|
Postmenopausal | 97 | 11 | 45.4 | 11.6 |
|
| 28 | 44.5 | 17.5 |
|
|
| Primary tumor
stage |
|
|
|
| 1.480 | 0.223 |
|
|
| 10.580 | 0.001a |
|
T1-2 | 60 | 16 | 63.3 | 16.5 |
|
| 42 | 53.4 | 37.9 |
|
|
|
T3-4 | 101 | 11 | 45.5 | 12.6 |
|
| 26 | 35.8 | 12.0 |
|
|
| Regional lymph node
stage |
|
|
|
| 1.180 | 0.278 |
|
|
| 0.870 | 0.350 |
|
N0-1 | 33 | 17 | 60.6 | 19.4 |
|
| 39 | 54.1 | 32.3 |
|
|
|
N2-3 | 90 | 14 | 53.3 | 13.5 |
|
| 34 | 43.9 | 21.6 |
|
|
| ER status |
|
|
|
| 17.130 |
<0.001a |
|
|
| 42.230 |
<0.001a |
|
Positive | 119 | 17 | 63.0 | 17.3 |
|
| 41 | 57.7 | 36.6 |
|
|
|
Negative | 52 | 7 | 28.8 | 9.6 |
|
| 14 | 21.2 | 0.0 |
|
|
| PR status |
|
|
|
| 11.230 | 0.001a |
|
|
| 14.730 |
<0.001a |
|
Positive | 101 | 17 | 63.3 | 19.0 |
|
| 41 | 58.4 | 37.2 |
|
|
|
Negative | 70 | 8 | 37.1 | 9.5 |
|
| 25 | 29.5 | 5.1 |
|
|
| HER2 status |
|
|
|
| 8.380 | 0.004a |
|
|
| 3.600 | 0.058 |
|
Positive | 51 | 9 | 35.1 | 8.9 |
|
| 26 | 25.9 | 19.4 |
|
|
|
Negative | 120 | 16 | 60.0 | 17.7 |
|
| 39 | 53.9 | 28.2 |
|
|
| Ki-67 |
|
|
|
| 4.610 | 0.032a |
|
|
| 3.530 | 0.060 |
|
<20% | 13 | 23 | 69.2 | 38.5 |
|
| 57 | 69.2 | 30.8 |
|
|
|
≥20% | 117 | 13 | 53.0 | 11.3 |
|
| 28 | 41.7 | 20.7 |
|
|
| Subtype |
|
|
|
| 19.100 |
<0.001a |
|
|
| 18.320 |
<0.001a |
|
HER2+/HR± | 50 | 9 | 35.8 | 9.1 |
|
| 26 | 26.4 | 19.8 |
|
|
|
HER2-/HR+ | 97 | 18 | 67.0 | 19.8 |
|
| 41 | 58.4 | 34.7 |
|
|
|
HER2-/HR- | 24 | 7 | 29.2 | 8.3 |
|
| 13 | 33.3 | 0.0 |
|
|
| Visceral
metastasis |
|
|
|
| 1.870 | 0.710 |
|
|
| 8.040 | 0.005a |
| No | 95 | 17 | 57.4 | 17.9 |
|
| 47 | 60.6 | 34.1 |
|
|
|
Yes | 76 | 12 | 49.5 | 13.2 |
|
| 28 | 36.5 | 20.9 |
|
|
| Sites of first
metastasis |
|
|
|
| 6.130 | 0.013a |
|
|
| 7.167 | 0.007a |
|
Oligometastasis | 94 | 16 | 54.3 | 21.5 |
|
| 41 | 54.0 | 33.6 |
|
|
|
Polymetastasis | 77 | 13 | 50.6 | 6.5 |
|
| 28 | 35.6 | 12.6 |
|
|
|
Oligometastasis |
|
|
|
| 4.430 | 0.219 |
|
|
| 5.850 | 0.119 |
|
Bone | 54 | 19 | 64.8 | 21.6 |
|
| 54 | 64.0 | 38.0 |
|
|
|
Lung/pleura | 24 | 8 | 50.0 | 25.0 |
|
| 29 | 45.8 | 34.4 |
|
|
|
Liver | 15 | 9 | 26.7 | 17.8 |
|
| 26 | 34.6 | 0.0 |
|
|
|
Brain | 1 | 4 |
0.0 | 0.0 |
|
| 19 | 0.0 | 0.0 |
|
|
| Surgery |
|
|
|
| 5.750 | 0.017a |
|
|
| 10.590 | 0.001a |
|
Yes | 41 | 20 | 70.7 | 25.3 |
|
| 57 | 66.5 | 45.0 |
|
|
| No | 130 | 11 | 46.9 | 11.7 |
|
| 28 | 39.4 | 16.7 |
|
|
| Trastuzumab for
HER2+ |
|
|
|
| 0.090 | 0.770 |
|
|
| 4.288 | 0.038a |
|
Yes | 33 | 10 | 44.8 | 8.4 |
|
| 31 | 36.2 | 24.1 |
|
|
| No | 18 | 8 | 33.3 | 11.1 |
|
| 16 | 14.7 | 14.7 |
|
|
| First-line
chemotherapy |
|
|
|
| 1.900 | 0.594 |
|
|
| 1.460 | 0.691 |
|
Anthracycline-containing | 14 | 9 | 50.0 | 17.9 |
|
| 29 | 46.3 | 23.1 |
|
|
|
Taxane-containing | 48 | 14 | 54.2 | 15.9 |
|
| 36 | 47.8 | 26.1 |
|
|
|
Anthracycline+Taxanes | 100 | 13 | 66.7 | 27.8 |
|
| 34 | 48.1 | 26.4 |
|
|
|
Other | 9 | 18 | 51.0 | 13.3 |
|
| 44 | 55.6 | 18.5 |
|
|
| Family history of
cancer |
|
|
|
| 0.100 | 0.750 |
|
|
| 0.080 | 0.770 |
|
Yes | 22 | 19 | 59.1 | 13.6 |
|
| 42 | 53.1 | 11.0 |
|
|
| No | 149 | 13 | 51.7 | 15.4 |
|
| 34 | 45.3 | 29.1 |
|
|
Tables III and
VI summarize the results of the
multivariable Cox proportional hazards model with hazard ratios
>1.0 indicating an increased risk of progression or death. ER
status and sites of first metastasis (oligometastasis vs.
polymetastasis) were highly significant independent predictors of
PFS in DnMBC. Women with negative ER status had 1.68 times higher
risk of progression compared to women with positive ER status [95%
confidence interval (CI) 1.12–2.53, P=0.013], women with
polymetastasis had 1.49 times higher risk of progression compared
to women with oligometastasis (95% CI 1.00–2.20, P=0.048). ER
status, primary tumor stage and surgical treatment of primary
tumors were highly significant independent predictors of OS in
DnMBC. Women with negative ER status had 3.11 times higher risk of
death compared to women with positive ER status (95% CI 2.06–4.70;
P<0.001), women without surgery of the primary tumor had 1.70
times had risk of death compared to women with surgical treatment
(95% CI 1.02–2.86; P=0.044) and women with tumor stage of T3-4 had
1.88 times higher risk of death compared to women with tumor stage
of T1-2 (95% CI 1.24–2.83, P=0.003).
 | Table III.Multivariate analysis of
progression-free survival. |
Table III.
Multivariate analysis of
progression-free survival.
|
Characteristics | HR | 95% CI | P-value |
|---|
| ER status |
|
| 0.013 |
|
ER+ | 1.00 |
|
|
|
ER- | 1.68 | 1.12–2.53 |
|
| Sites of first
metastasis |
|
| 0.048 |
|
Oligometastasis | 1.00 |
|
|
|
Polymetastasis | 1.49 | 1.00–2.20 |
|
 | Table VI.Associations between site of first
metastasis and surgery of primary tumor. |
Table VI.
Associations between site of first
metastasis and surgery of primary tumor.
| Variable | Oligometastasis, n
(%) | Polymetastasis, n
(%) | P-value |
|---|
| Surgery |
|
| <0.001 |
|
Yes | 33 (19.3) | 8 (4.7) |
|
| No | 61 (35.7) | 69 (40.4) |
|
Survival analysis of subgroups
Subgroup analysis was performed according to
different molecular subtypes, sites of first metastasis and
surgical treatment of the DnMBC patients.
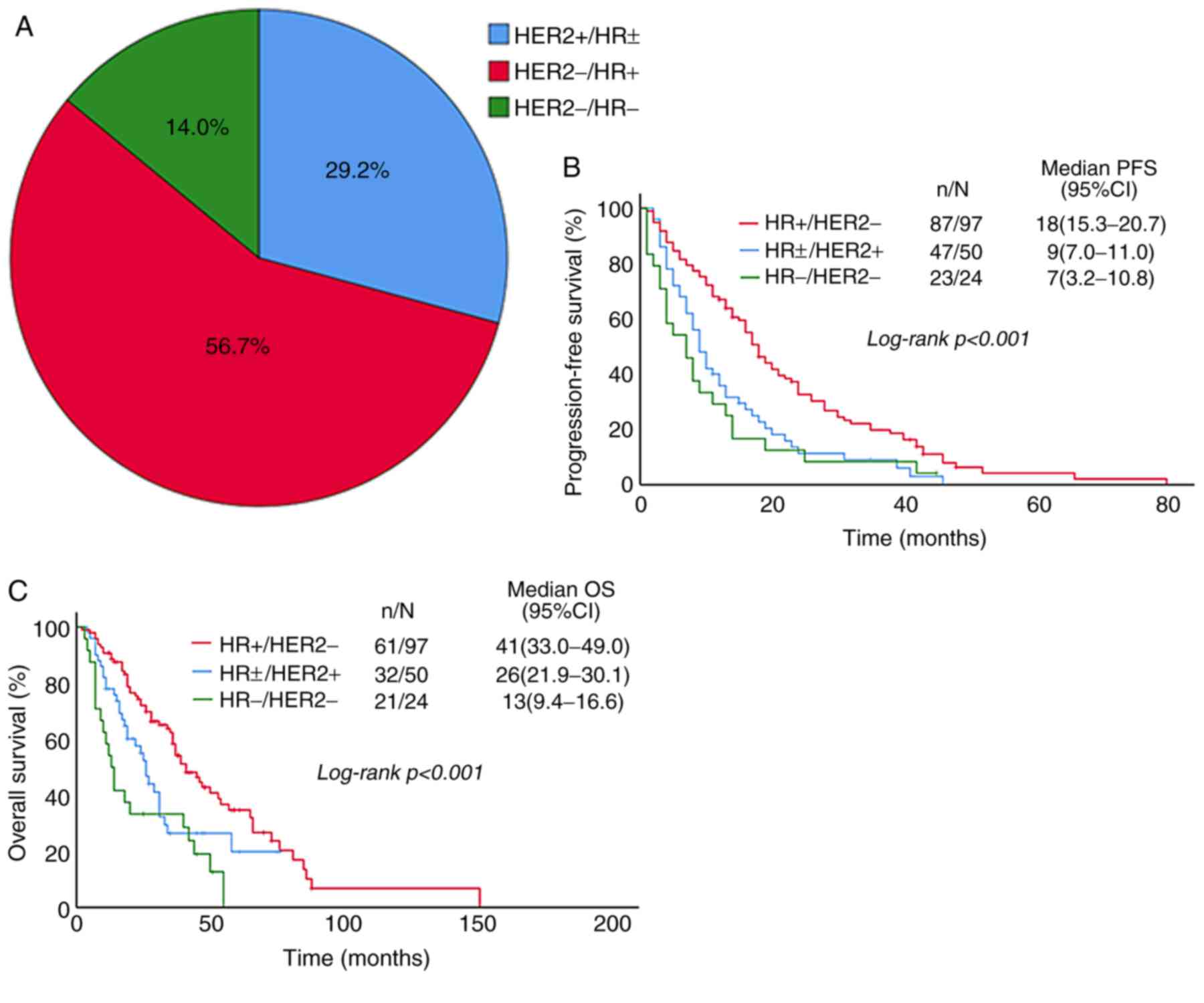
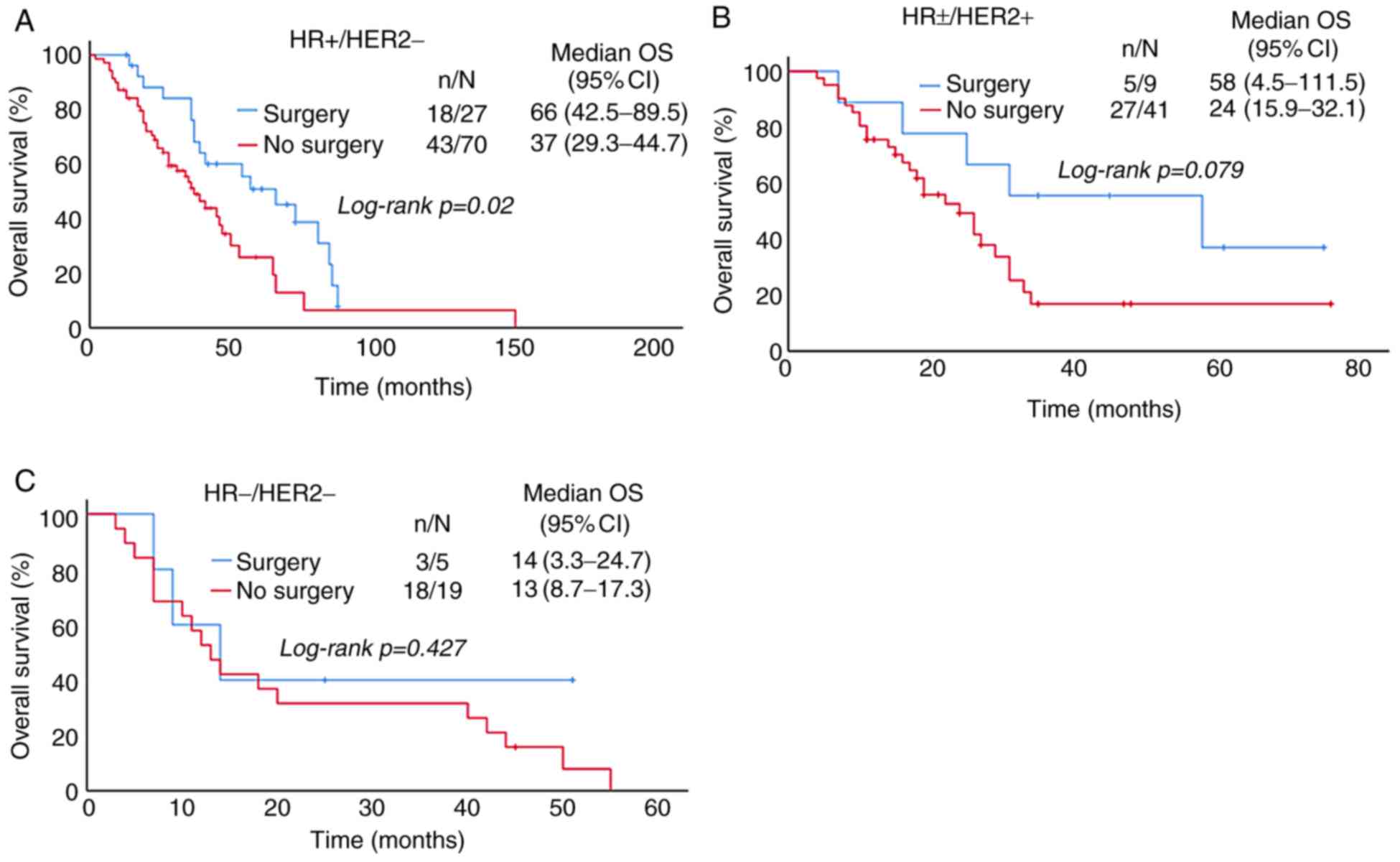
Patients were divided into HR+/HER2- (56.7%),
HR±/HER2+ (29.2%) and HR-/HER2- (14.0%) groups according to the
molecular subtype (Fig. 2). In these
groups, the median PFS was 16, 9, 7 months and the 1-year PFS rate
was 67.0, 35.8 and 29.2%, respectively (P<0.001; Table II). The median OS was 97, 50, 24
months and the 5-year OS was 34.7, 19.8 and 0.0%, respectively
(P<0.001; Table II). PFS and OS
in HR+/HER2- group were better than those in the other two groups
(Fig. 2B and C), and the difference
was statistically significant.
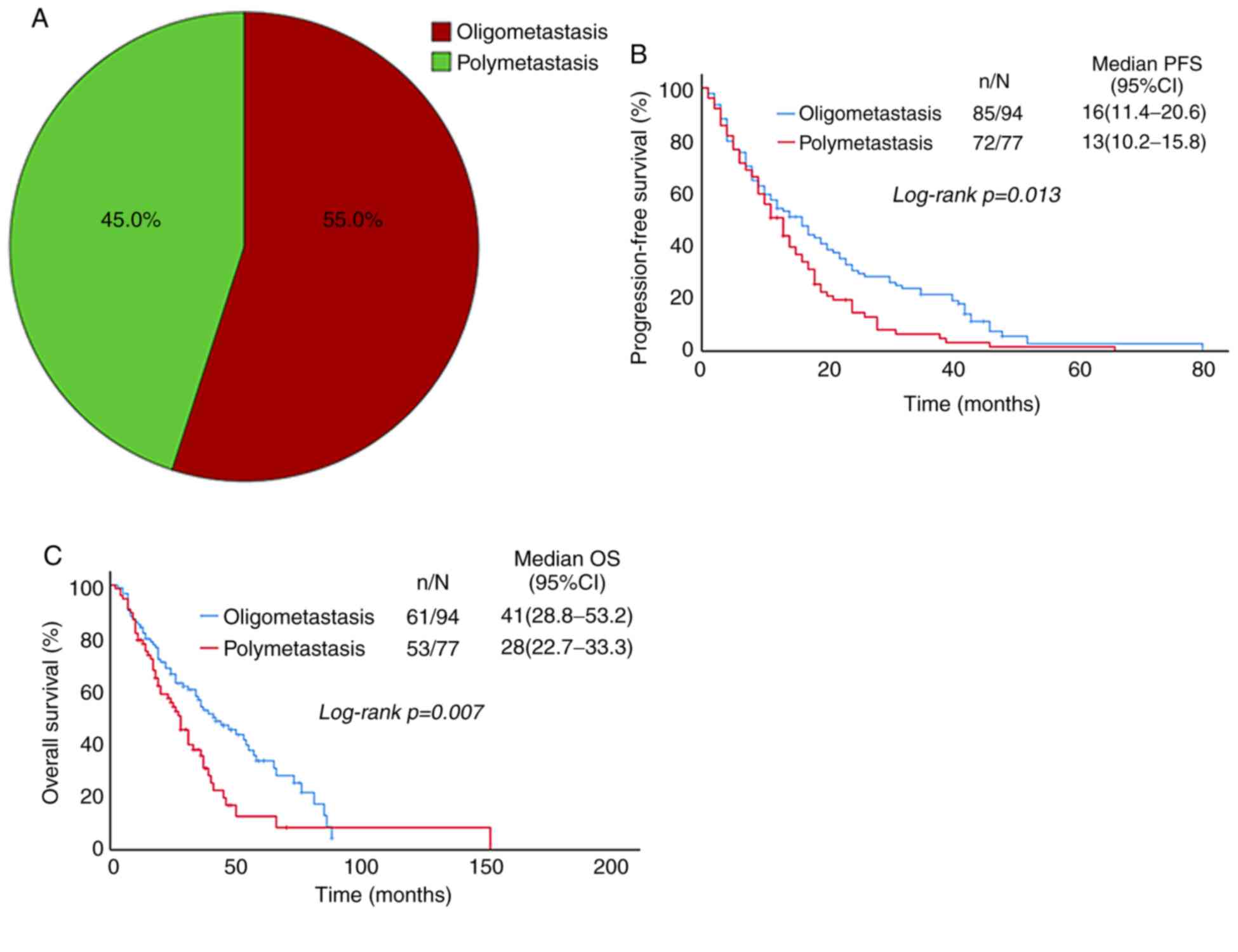
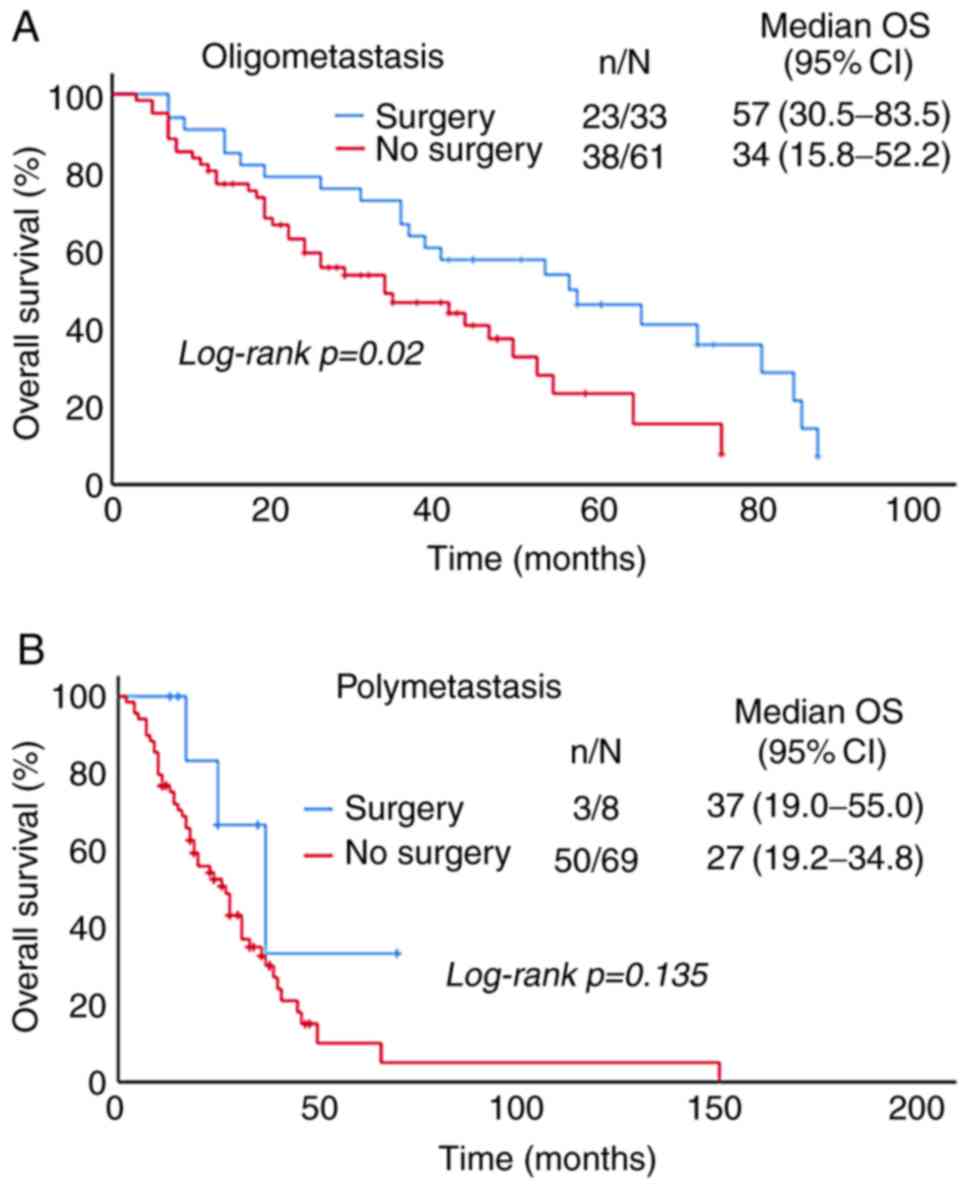
Patients were divided into oligometastasis (55%) and
polymetastasis (45%) groups according to the sites of first
metastasis (Fig. 3A). In these
groups, the median PFS was 16 and 13 months, and the 1-year PFS was
54.3 and 50.6%, respectively (P=0.013; Table II). The median OS was 41 and 28
months, and the 5-year OS was 33.6 and 12.6%, respectively
(P=0.007; Table II). PFS and OS in
the oligometastasis group were better than those in the
polymetastasis group (Fig. 3B and
C), and the difference was statistically significant.
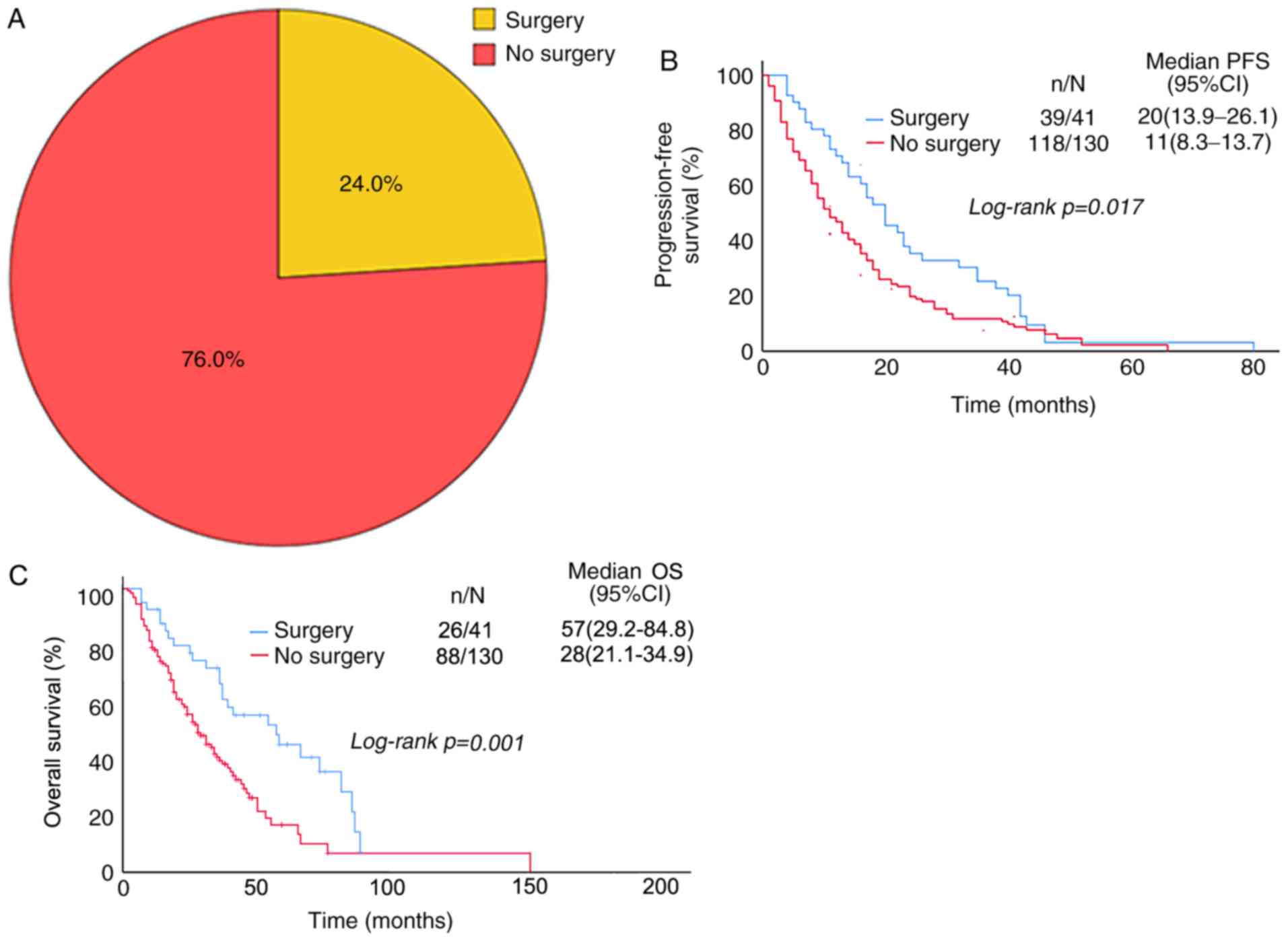
Patients were divided into the surgery group (24%)
and non-surgery group (76%) according to the surgical treatment
(Fig. 4A). In these groups, the
median PFS was 20 and 11 months, and the 1-year PFS was 70.7 and
46.9%, respectively (P=0.017; Table
II). The median OS was 57 and 28 months, and the 5-year OS was
45 and 16.7%, respectively (P=0.001; Table II). The PFS and OS in patients with
surgery of primary tumor were significantly better than in those
without surgery (Fig. 4B and C), and
the difference was statistically significant.
Associations between surgery and
molecular subtypes
Patients were divided into
HR+/HER2−, HR±/HER2+
and HR−/HER2- groups according to molecular subtype, and
were divided into surgery group and non-surgery group according to
the surgical treatment. The number of patients who underwent
surgical treatment was relatively higher in HR+/HER2-
group, but there was no significant difference (Table VI). In the HR+/HER2-
group, 27 cases (15.8%) were treated with surgery and 70 cases
(40.9%) without surgery, the OS of the patients who underwent
surgical treatment was longer than those without surgery, and the
difference was statistically significant. In the
HR±/HER2+ group, 9 cases (5.3%) were treated
with surgery and 41 cases (24%) without surgery, and the OS of the
surgery group was longer than that of the non-surgery group, but
there was no significant difference. In the HR-/HER2- group, 5
cases (2.9%) were treated with surgery and 19 cases (11.1%) without
surgery, and surgery had no effect on the OS (Fig. 5).
Associations between surgery and first
metastasis sites
Patients were divided into oligometastasis and
polymetastasis groups according to the sites of first metastasis.
The number of patients who underwent surgery was relatively large
in the oligometastasis group and the difference was statistically
significant (Table V). In the
oligometastasis group, 33 cases (19.3%) were treated with surgery
and 61 cases (35.7%) without it, the OS of the patients who
received surgical treatment was increased compared with those
without it and the difference was statistically significant. In the
polymetastasis group, 8 cases (4.7%) were treated with surgery with
surgery and 69 cases (40.4%) without it, and surgery had no effect
on OS (Fig. 6).
 | Table V.Associations between subtypes and
surgery of primary tumor surgery. |
Table V.
Associations between subtypes and
surgery of primary tumor surgery.
| Variable | HR+/HER2- n
(%) | HR±/HER2+ n
(%) | HR-/HER2- n
(%) | P-value |
|---|
| Surgery |
|
|
| 0.386 |
|
Yes | 27 (15.8) | 9 (5.3) | 5 (2.9) |
|
| No | 70 (40.9) | 41 (24.0) | 19 (11.1) |
|
Discussion
Cortesi et al (6) made a report based on 119 cases of DnMBC
from 2006 to 2009. It was found that their clinicopathological
features were more invasive. A total of ~45% of patients had
multiple metastases, 78.1% had Ki-67 >14%, 81.9% with
histological grade 3 tumor, 27.5% were HER2 positive, 59.7% had
initial chemotherapy and nearly 80% of patients preferred
anthracycline or taxane-based chemotherapy regimen. From the
analysis of 76 DnMBC cases from 2007 to 2011, Chen et al
(14) found that the positive rate
of ER, PR and HER2 were 69.7, 56.6, and 27.5%, respectively. The
clinicopathological features and treatment of 171 patients with
DnMBC between 2008 and 2017 were analyzed and it was found that the
positive rate of HER2 was higher at ~29.8%, the percentage of
postmenopausal patients and tumor stage T3-4 patients were higher,
68.4% of patients had Ki-67 ≥20%, 45% had polymetastasis, and 44.4%
with visceral metastasis, which confirmed that DnMBC expresses a
more aggressive phenotype. At the same time, the present study
found that, similar to studies done by Chen et al (14), the percentage of HR positive in
patients with DnMBC was high as well, of which 69.6% had positive
ER status and 59.1% had positive PR status, which may be associated
with better prognosis. Nearly 90% of the patients in this study
received initial chemotherapy and >90% of the patients chose
taxanes or anthracyclines as the first-line chemotherapy, of whom
58.5% were treated with the combination of the two drugs. It can be
seen that the proportion of chemotherapy is compared with the study
by Cortesi et al (6) and
there are relatively few studies on the treatment. Considering the
differences in traditional concepts among people in different
regions, the Chinese are pursuing active and comprehensive
treatment, while the Western population is pursuing a higher
quality of life, which may have led to the above-mentioned
differences in treatment.
Prior reports state that the median survival time of
patients with MBC was 2–3 years (7,9,15–18).
Andre et al (7) reported that
the median survival time of patients with DnMBC was 23 months in
1987–1993, compared with 29 months in 1994–2000, of which most
patients had single metastasis especially bone metastasis, >80%
had received chemotherapy and 51% had received local surgery or
radiation therapy. den Brok et al (18) reported that the median survival time
of patients with DnMBC (2001–2009) was 29 months, most of whom were
postmenopausal and >50 years old. Dawood et al (15) showed that the median survival time of
the patients with DnMBC was 39.2 months between 1992 and 2007.
Nearly 70% of the patients in the study were Caucasian and 40%
received surgical treatment for primary tumors, which may be
related to the relatively long survival time. In the present study,
the median survival time was 34 months and the 5-year OS rate was
25.7% (DnMBC from 2008 to 2017). According to the above reports, it
can be seen that with the continual emergence of new drugs and the
developments in treatment in recent years, the median survival time
of DnMBC is longer than before. The differences in individual
studies may be due to geographical, racial and therapeutic
factors.
In this study, univariate analysis showed that poor
prognosis was closely related to the following aspects: The primary
tumor stage T3-4, ER-negative, PR-negative, polymetastasis,
visceral metastasis, patients with positive HER2 status who did not
receive anti-HER2 therapy and no palliative surgery for primary
tumor. Multivariate analysis showed that ER-negative, primary tumor
stage T3-4 and no surgical treatment for primary tumor were
independent risk factors for poor prognosis of DnMBC. The results
were similar to that of previous studies. Andre et al
(7) pointed out that HR negative,
multiple metastasis and visceral involvement are risk factors for
poor prognosis in DnMBC. Cortesi et al (6) drew the conclusion that patients with
negative HR status who did not receive chemotherapy had a poor
prognosis.
There are different conclusions on whether surgical
treatment of primary tumors will benefit DnMBC. Some studies
(19–21) suggested that surgical resection of
primary tumors can improve the prognosis of patients with MBC. A
retrospective study conducted by Lambertini et al (21) on 113 HER2-positive DnMBC patients
showed that PFS and OS were significantly longer in patients who
underwent primary tumor surgery, had a lower number of metastatic
sites, had minor or no symptoms, received more commonly first-line
polychemotherapy treatment, and achieved higher benefit. In the
MF07-01 randomized trial (19) with
a median follow-up of 40 months, researchers drew a conclusion that
primary tumor surgery followed by chemotherapy could significantly
improve survival times compared with chemotherapy alone in DnMBC
and the median OS increased by ~9 months. This benefit was found
especially in patients who were ER positive, HER2 negative, had
simple bone metastasis or were younger than 55. Some studies
(22,23) indicated that primary tumor surgery
failed to bring survival benefit to MBC patients. A prospective
randomized controlled trial (NCT00193778) (22) showed that loco-regional treatment of
the primary tumor and axillary nodes had no impact on OS in
patients diagnosed with DnMBC, who had responded to frontline
chemotherapy. Another prospective trial, TBCRC 013777, (23) also pointed out that among patients
who responded to first-line chemotherapy, surgery did not affect
OS, irrespective of the tumor subtype. The present data found that
local surgical treatment prolonged the PFS and OS, and the 5-year
OS rate increased by 28.3%. Furthermore, surgical treatment of the
primary tumor was an independent prognostic factor of OS.
Considering the selection bias, the proportion of patients with the
HR+/HER2-subtype and oligometastasis, especially single bone
metastasis, is high in patients who underwent surgical treatment in
the current study, so its good prognosis does not exclude this
aspect.
Subgroup analysis: The prognosis of patients with
primary tumor surgery in different molecular types and sites of
first metastasis were analyzed. It was found that patients in the
HR+/HER2-group, compared with other subgroups, benefited from
surgical treatment and had a longer OS, which was statistically
significant. In addition, the effect of surgical treatment was more
obvious in the oligometastasis group and the difference was
statistically significant as well. Therefore, if patients are in
good health and able to undergo surgery, palliative resection of
the primary tumor may be considered in DnMBC patients with
oligometastasis and the HR+/HER2- subtype. So far, there are no
guidelines recommending routine surgical treatment for the primary
tumor of DnMBC. Prospective studies with large sample sizes and
need to be done to get more clinical data and experience.
Among the molecular subtypes, the post-metastasis OS
of MBC had different reports. It has been reported (24) that the longest survival time after
metastasis is Lumina subtype. Andre et al (7) speculated that patients with positive HR
status had the best prognosis. More reports (7,16,25–30)
showed that triple negative breast cancer (TNBC) had the worst
prognosis. In the present study, the
HR+/HER2− subtype had the best prognosis,
followed by HER2+/HR± and TNBC had the worst
prognosis. The poor prognosis of TNBC may be related to the lack of
corresponding hormone therapy and targeted therapy sites.
Hellman et al (31) first proposed the concept of
‘oligometastasis’ in 1995, as a special state between local
recurrence and widespread metastasis. One study (32) suggested that the biological
characteristics of the tumor at this stage were relatively stable,
the spreading ability was weak and the selectivity for metastatic
organs was high. There is no common definition for oligometastasis
and the number of metastatic lesions is generally defined as the
criterion. A number of studies (33–35)
defined the incidence of no more than five metastatic sites as
oligometastasis. The present study defined that patients with
single organ metastasis and <5 metastatic lesions were
classified into oligometastasis group, and the rest belonged to the
polymetastasis group. Compared with polymetastasis, patients with
oligometastasis had better PFS and OS, and the difference was
statistically significant. The present study also found that
surgical treatment notably improved prognosis in the
oligometastasis group, but the benefit was not reflected in the
polymetastasis group. It has been pointed out that some patients
with oligometastasis can survive for a long time by eliminating
oligometastasis through active local treatment such as surgery,
radiotherapy and radiofrequency ablation (34,35).
In conclusion, the clinicopathological
characteristics of DnMBC are being more invasive and have a higher
risk of progression. Palliative surgical treatment may improve the
prognosis of HR+/HER2− patients with
oligometastasis. Therefore, individualized treatment is
particularly important. A limitation of the present study was that
some subgroups were too small, thus statistical analysis was unable
to be performed. Therefore, trials with large samples are needed to
verify this conclusion and guide the treatment of DnMBC
further.
Acknowledgements
No applicable.
Funding
The present study was funded by the National Natural
Science Foundation of China (grant no. 81702636), the Anticancer
Key Technologies R&D Program of Tianjin (grant no.
12ZCDZSY16200), the Natural Science Foundation of Tianjin (grant
no. 18JCYBJC93500) and the Natural Science Foundation of Tianjin
(18JCYBJC91600).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
LZ designed the study, performed the research and
wrote the paper. ZL performed the research, analyzed data and wrote
the paper. JZ performed research and analyzed data. YW performed
research and checked the data. YZ helped performed research and
checked the data. ZT designed the study and analyzed data.
Ethics approval and consent to
participate
The present study was approved by the Medical Ethics
Committee of Tianjin and written informed consent was obtained from
all patients prior to the study start.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Badwe R, Hawaldar R, Nair N, Kaushik R,
Parmar V, Siddique S, Budrukkar A, Mittra I and Gupta S:
Locoregional treatment versus no treatment of the primary tumour in
metastatic breast cancer: An open-label randomised controlled
trial. Lancet Oncol. 16:1380–1388. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cardoso F, Spence D, Mertz S,
Corneliussen-James D, Sabelko K, Gralow J, Cardoso MJ, Peccatori F,
Paonessa D, Benares A, et al: Global analysis of
advanced/metastatic breast cancer: Decade report (2005–2015).
Breast. 39:131–138. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dawood S, Broglio K, Gonzalez-Angulo AM,
Buzdar AU, Hortobagyi GN and Giordano SH: Trends in survival over
the past two decades among white and black patients with newly
diagnosed stage IV breast cancer. J Clin Oncol. 26:4891–4898. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Malmgren JA, Mayer M, Atwood MK and Kaplan
HG: Differential presentation and survival of de novo and recurrent
metastatic breast cancer over time: 1990–2010. Breast Cancer Res
Treat. 167:579–590. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cortesi L, Toss A, Cirilli C, Marcheselli
L, Braghiroli B, Sebastiani F and Federico M: Twenty-years
experience with de novo metastatic breast cancer. Int J Cancer.
137:1417–1426. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Andre F, Slimane K, Bachelot T, Dunant A,
Namer M, Barrelier A, Kabbaj O, Spano JP, Marsiglia H, Rouzier R,
et al: Breast cancer with synchronous metastases: Trends in
survival during a 14-year period. J Clin Oncol. 22:3302–3308. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cortesi L, Cirilli C, Rashid I, Artioli E
and Federico M: Changes in survival from metastatic breast cancer
during the last twenty-years: A population based study in Northern
Italy. J Clin Oncol. 27:11252009.
|
|
9
|
Lobbezoo DJ, van Kampen RJ, Voogd AC,
Dercksen MW, van den Berkmortel F, Smilde TJ, van de Wouw AJ,
Peters FP, van Riel JM, Peters NA, et al: Prognosis of metastatic
breast cancer subtypes: The hormone receptor/HER2-positive subtype
is associated with the most favorable outcome. Breast Cancer Res
Treat. 141:507–514. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hammond ME, Hayes DF, Dowsett M, Allred
DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS,
Hayes M, et al: American society of clinical oncology/college of
American pathologists guideline recommendations for
immunohistochemical testing of estrogen and progesterone receptors
in breast cancer (unabridged version). Arch Pathol Lab Med.
134:e48–e72. 2010.PubMed/NCBI
|
|
11
|
Liu F, Lang R, Wei J, Fan Y, Cui L, Gu F,
Guo X, Pringle GA, Zhang X and Fu L: Increased expression of
SDF-1/CXCR4 is associated with lymph node metastasis of invasive
micropapillary carcinoma of the breast. Histopathology. 54:741–750.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cui LF, Guo XJ, Wei J, Liu FF, Fan Y, Lang
RG, Gu F, Zhang XM and Fu L: Overexpression of TNF-alpha and TNFRII
in invasive micropapillary carcinoma of the breast:
Clinicopathological correlations. Histopathology. 53:381–388. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Qian XL, Wen HY, Yang YL, Gu F, Guo XJ,
Liu FF, Zhang L, Zhang XM and Fu L: Assessment of dual-probe Her-2
fluorescent in situ hybridization in breast cancer by the 2013
ASCO/CAP guidelines produces more equivocal results than that by
the 2007 ASCO/CAP guidelines. Breast Cancer Res Treat. 159:31–39.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen L, Romond E, Chokshi S, Saeed H,
Hodskins J, Stevens M, Pasley G, Weiss H and Massarweh S: A
prognostic model of early breast cancer relapse after standard
adjuvant therapy and comparison with metastatic disease on initial
presentation. Breast Cancer Res Treat. 136:565–572. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dawood S, Broglio K, Ensor J, Hortobagyi
GN and Giordano SH: Survival differences among women with de novo
stage IV and relapsed breast cancer. Ann Oncol. 21:2169–2174. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vaz-Luis I, Lin NU, Keating NL, Barry WT,
Lii H, Winer EP and Freedman RA: Racial differences in outcomes for
patients with metastatic breast cancer by disease subtype. Breast
Cancer Res Treat. 151:697–707. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee ES, Jung SY, Kim JY, Kim JJ, Yoo TK,
Kim YG, Lee KS, Lee ES, Kim EK, Min JW, et al: Identifying the
potential long-term survivors among breast cancer patients with
distant metastasis. Ann Oncol. 27:828–833. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
den Brok WD, Speers CH, Gondara L, Baxter
E, Tyldesley SK and Lohrisch CA: Survival with metastatic breast
cancer based on initial presentation, de novo versus relapsed.
Breast Cancer Res Treat. 161:549–556. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Soran A, Ozmen V, Ozbas S, Karanlik H,
Muslumanoglu M, Igci A, Canturk Z, Utkan Z, Ozaslan C, Evrensel T,
et al: A randomized controlled trial evaluating resection of the
primary breast tumor in women presenting with de novo stage IV
breast cancer: Turkish Study (Protocol MF07-01). J Clin Oncol.
34:10052016. View Article : Google Scholar
|
|
20
|
Thomas A, Khan SA, Chrischilles EA and
Schroeder MC: Initial surgery and survival in stage IV breast
cancer in the United States, 1988–2011. JAMA Surg. 151:424–431.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lambertini M, Ferreira AR, Di Meglio A,
Poggio F, Puglisi F, Sottotetti F, Montemurro F, Poletto E,
Bernardo A, Risi E, et al: Patterns of care and clinical outcomes
of HER2-positive metastatic breast cancer patients with newly
diagnosed stage IV or recurrent disease undergoing first-line
trastuzumab-based therapy: A multicenter retrospective cohort
study. Clin Breast Cancer. 17:601–610.e2. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Badwe R, Parmar V, Hawaldar R, Nair N,
Kaushik R, Siddique S, Navale A, Budrukkar A, Mittra I and Gupta S:
Surgical removal of primary tumor and axillary lymph nodes in women
with metastatic breast cancer at first presentation: A randomized
controlled trial. Cancer Res. 73 (24 Suppl):S2–02. 2013.
|
|
23
|
King TA, Lyman J, Gonen M, Voci A, De Brot
M, Boafo C, Sing AP, Hwang ES, Alvarado MD, Liu MC, et al: A
prospective analysis of surgery and survival in stage IV breast
cancer: TBCRC 013. J Clin Oncol. 34:2359–2365. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Molnár IA, Molnár BÁ, Vízkeleti L, Fekete
K, Tamás J, Deák P, Szundi C, Székely B, Moldvay J, Vári-Kakas S,
et al: Breast carcinoma subtypes show different patterns of
metastatic behavior. Virchows Arch. 470:275–283. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lobbezoo DJ, van Kampen RJ, Voogd AC,
Dercksen MW, van den Berkmortel F, Smilde TJ, van de Wouw AJ,
Peters FP, van Riel JM, Peters NA, et al: Prognosis of metastatic
breast cancer: Are there differences between patients with de novo
and recurrent metastatic breast cancer. Br J Cancer. 112:1445–1451.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Savci-Heijink CD, Halfwerk H, Hooijer GK,
Horlings HM, Wesseling J and van de Vijver MJ: Retrospective
analysis of metastatic behaviour of breast cancer subtypes. Breast
Cancer Res Treat. 150:547–557. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tao L, Chu L, Wang LI, Moy L, Brammer M,
Song C, Green M, Kurian AW, Gomez SL and Clarke CA: Occurrence and
outcome of de novo metastatic breast cancer by subtype in a large,
diverse population. Cancer Causes Control. 27:1127–1138. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Vaz-Luis I, Lin NU, Keating NL, Barry WT,
Winer EP and Freedman RA: Factors associated with early mortality
among patients with de novo metastatic breast cancer: A
population-based study. Oncologist. 22:386–393. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang H, Zhang C, Zhang J, Kong L, Zhu H
and Yu J: The prognosis analysis of different metastasis pattern in
patients with different breast cancer subtypes: A SEER based study.
Oncotarget. 8:26368–26379. 2017.PubMed/NCBI
|
|
30
|
Wu SG, Li H, Tang LY, Sun JY, Zhang WW, Li
FY, Chen YX and He ZY: The effect of distant metastases sites on
survival in de novo stage-IV breast cancer: A SEER database
analysis. Tumour Biol. 39:10104283177050822017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hellman S and Weichselbaum RR:
Oligometastases. J Clin Oncol. 13:8–10. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Weichselbaum RR and Hellman S:
Oligometastases revisited. Nat Rev Clin Oncol. 8:378–382. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Milano MT, Katz AW, Schell MC, Philip A
and Okunieff P: Descriptive analysis of oligometastatic lesions
treated with curative-intent stereotactic body radiotherapy. Int J
Radiat Oncol Biol Phys. 72:1516–1522. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kobayashi T, Ichiba T, Sakuyama T, Arakawa
Y, Nagasaki E, Aiba K, Nogi H, Kawase K, Takeyama H, Toriumi Y, et
al: Possible clinical cure of metastatic breast cancer: Lessons
from our 30-year experience with oligometastatic breast cancer
patients and literature review. Breast Cancer. 19:218–237. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wong AC, Watson SP, Pitroda SP, Son CH,
Das LC, Stack ME, Uppal A, Oshima G, Khodarev NN, Salama JK, et al:
Clinical and molecular markers of long-term survival after
oligometastasis-directed stereotactic body radiotherapy (SBRT).
Cancer. 122:2242–2250. 2016. View Article : Google Scholar : PubMed/NCBI
|