Introduction
Hepatocellular carcinoma (HCC) is the sixth most
prevalent type of cancer and the second leading cause of
cancer-associated death worldwide, accounting for ~75% of the
primary liver cancer cases due to rapid disease progression and
poor prognosis (1). HCC is typically
diagnosed at an advanced stage, and as such, treatment options are
limited and the 5-year overall survival (OS) rate is only 3–5%
(2).
Advances in cancer immunotherapy, particularly the
development of immune checkpoint blockades (ICBs), have shown
clinical benefit and success in the treatment of several types of
cancer, including melanoma, lung cancer and HCC (3–5).
However, the clinical efficacy of ICB treatment is limited to a
subset of patients, due to high rates of drug resistance (6,7). Efforts
are being made to elucidate the underlying mechanisms of drug
efficacy and resistance to ICB treatment, which may help provide
individualized treatment and improved therapeutic strategies.
Programmed cell death ligand 1 (PD-L1) is the most commonly used
biomarker in anti-programmed death protein 1 (PD-1) therapy
(7,8), but has several limitations, for example
immunohistochemistry analysis of PD-L1 expression lacks a
standardized format. Biological and technical challenges, such as
heterogeneity of PD-L1 expression and variation in the affinities
of different anti-PD-L1 antibodies, further complicate PD-L1
expression level testing (8).
Advances in next-generation sequencing (NGS) has
identified tumor mutation burden (TMB) as a potential biomarker for
predicting the response of patients with multiple tumors to ICB
therapy (9,10). The underlying mechanisms of ICB
therapy involves the synthesis of large quantities of neoantigens
required for activating anti-tumor immune responses (11,12). A
recent study evaluated the efficacy of anti-PD-1 antibody treatment
combined with anti-angiogenesis therapy for patients with advanced
stage HCC, showing that the TMB of individuals who responded
favorably was significantly higher compared with patients who
showed a less favorable response. In addition, the combined
treatment strategy significantly improved the survival of patients
with HCC with a higher TMB (13).
Therefore, TMB may be a potentially novel predictor of survival for
patients with HCC undergoing ICB therapy. High TMB (TMB-H) is
frequently observed in patients with mismatch repair deficiency
(dMMR) and high microsatellite instability (MSI-H) (11,14,15).
TMB-H has also been identified in patients harboring driver gene
mutations associated with different types of cancer with a TMB-H,
including breast cancer antigen 2 in melanoma (16) and human epidermal growth factor
receptor (HER)-2 and HER3 in urological cancer (17). These findings have provided novel
insight into therapeutic strategies, such as combined therapy
consisting of ICBs and targeted molecular drugs. In the present
study, NGS was performed on a group of HCC tumor samples and
matching normal plasma samples were used to identify recurrent
mutations associated with TMB-H in patients with HCC.
Materials and methods
Patients and tissue samples
The present study was approved by The Institutional
Review Board of Peking University International Hospital (Xi'an,
China; approval no. 2016-045). A total of 81 patients with HCC from
Peking University International Hospital were enrolled into the
study between January 2015 and June 2018 and written informed
consent was obtained from all patients. The median age of patients
was 57 years (range, 30–85 years) and 30/81 patients (37.04%) were
over 60 years old. The majority of patients were male (71 males and
10 females) and clinical characteristics of patients are shown in
Table I. Patients had not received
ICB therapy at the time of specimen collection. HCC tissue and
paired plasma samples were collected to perform NGS (18). HCC formalin fixed paraffin embedded
(FFPE) samples were stored at room temperature, and were processed
within 72 h of collection. Peripheral blood was collected in EDTA
Vacutainer tubes (BD Diagnostics; Becton, Dickinson and Company) at
room temperature and processed within 4 h. Peripheral blood
lymphocytes (PBLs) and other blood cells were stored at −80°C.
 | Table I.Clinical characteristics of 81
patients with hepatocellular carcinoma. |
Table I.
Clinical characteristics of 81
patients with hepatocellular carcinoma.
| Characteristic | Patients, n
(%) |
|---|
| Age |
|
| <60
years | 48 (59.26) |
| ≥60
years | 30 (37.04) |
|
Unknown | 3 (3.70) |
| Sex |
|
|
Male | 71 (87.65) |
|
Female | 10 (12.35) |
DNA extraction from HCC samples
FFPE slides were stored at room temperature. Plasma
samples were centrifuged at 1,600 × g at 4°C for 10 min, then
transferred to new microcentrifuge tubes and centrifuged at 16,000
× g at 4°C for 10 min to remove the remaining cell debris. Germline
genomic DNA was extracted from PBLs using a DNeasy Blood and Tissue
kit according to the manufacturer's protocol (Qiagen GmbH). Genomic
DNA was extracted from FFPE samples using a Maxwell® RSC
DNA FFPE kit according to the manufacturer's protocol (Promega
Corporation). DNA concentration was estimated using a Qubit
fluorometer and a Qubit double stranded DNA high sensitivity
analysis kit according to the manufacturer's protocol (Invitrogen;
Thermo Fisher Scientific, Inc.).
NGS of HCC DNA
HCC tumor samples were analyzed using target capture
and NGS (18). Genomic DNA libraries
were constructed using a KAPA DNA Library kit (Kapa Biosystems;
Roche Diagnostics). The capture probe design was based on ~1.45 Mb
genomic regions of 1,021 genes frequently mutated in solid tumors
(coding sequence, 1 Mb). DNA sequencing was performed using HiSeq
3000 instrument according to the manufacturer's protocol (Illumina,
Inc.). Germline DNA extracted from PBLs was used as the non-cancer
associated component. Germline mutations of HCC tissues were
filtered out using the paired PBLs DNA from the same patient.
Sequencing data analysis
If the proportion of indeterminate bases in a read
accounted for >50%, or if the proportion of bases with BaseQ
<5 was >50%, then this was considered a low-quality read.
Terminal adaptor sequences and low-quality data were removed from
the raw data. Clean reads were mapped onto the human genome build
GRCh37 using Burrows-Wheeler Aligner (version 0.7.12-r1039)
(19). Picard (version 1.98) was
used to mark polymerase chain reaction (PCR) duplicates (http://broadinstitute.github.io/picard/). Single
nucleotide variants (SNVs) and insertions or deletions (indels)
were identified using MutTect2 (version 3.4–46-gbc02625 (20). Somatic copy number variations (CNVs)
were detected using CONTRA (version 2.0.8) (21). Structure variations (SVs) were
identified using split-read and discordant read-pair using in-house
methods (22). Capture baits for SVs
were designed according to selected exons and introns of the RET,
ALK, ROS1 and neurotrophic tyrosine kinase receptor type 1 (NTRK1)
oncogenes based on reported SVs (22).
Calculation of tissue TMB (tTMB)
tTMB was calculated after comprehensive genomic
profiling of tissue samples using the 1,021 gene panel on 1 Mb of
genomic coding region. The numbers of somatic, coding, SNVs and
short indels detected at a frequency of ≥3% were calculated as
tTMB, not including synonymous mutations. Patients harboring ≥7
mutations/Mb (the top quartile of tTMB distribution) were
classified as the high tTMB (tTMB-H) group and all others were
classified as low tTMB (tTMB-L) patients. The Cancer Genome Atlas
Liver Hepatocellular Carcinoma (TCGA-LIHC) database was used as the
validation set to verify the correlation between TMB and driver
genes and the value of predicting the clinical benefit. The data
was downloaded from the cBioPortal website (http://www.cbioportal.org/).
Statistical analysis
All statistical analyses were performed using
GraphPad Prism version 6.01 (GraphPad Software, Inc.). A
χ2 or Fisher's exact test (n<5) were used to compare
the mutation status of frequently mutated genes in the patients
classed as tTMB-H and tTMB-L. A Mann-Whitney U test was performed
to analyze the difference in tTMB between wild-type samples and
mutation samples of frequently mutated genes. Kaplan-Meier curves
were plotted to assess survival outcomes. Overall survival (OS) and
recurrence-free survival (RFS) were accessed using a log-rank test
in subgroups classified according to gene mutation. P<0.05 was
considered to indicate a statistically significant difference.
Results
Significantly altered genes in
HCC
A total of 81 HCC tumor samples were analyzed using
a 1,021-gene panel at 1,200 × average sequencing depth. Somatic
mutations included SNVs, short indels, CNVs and SVs. Overall, 506
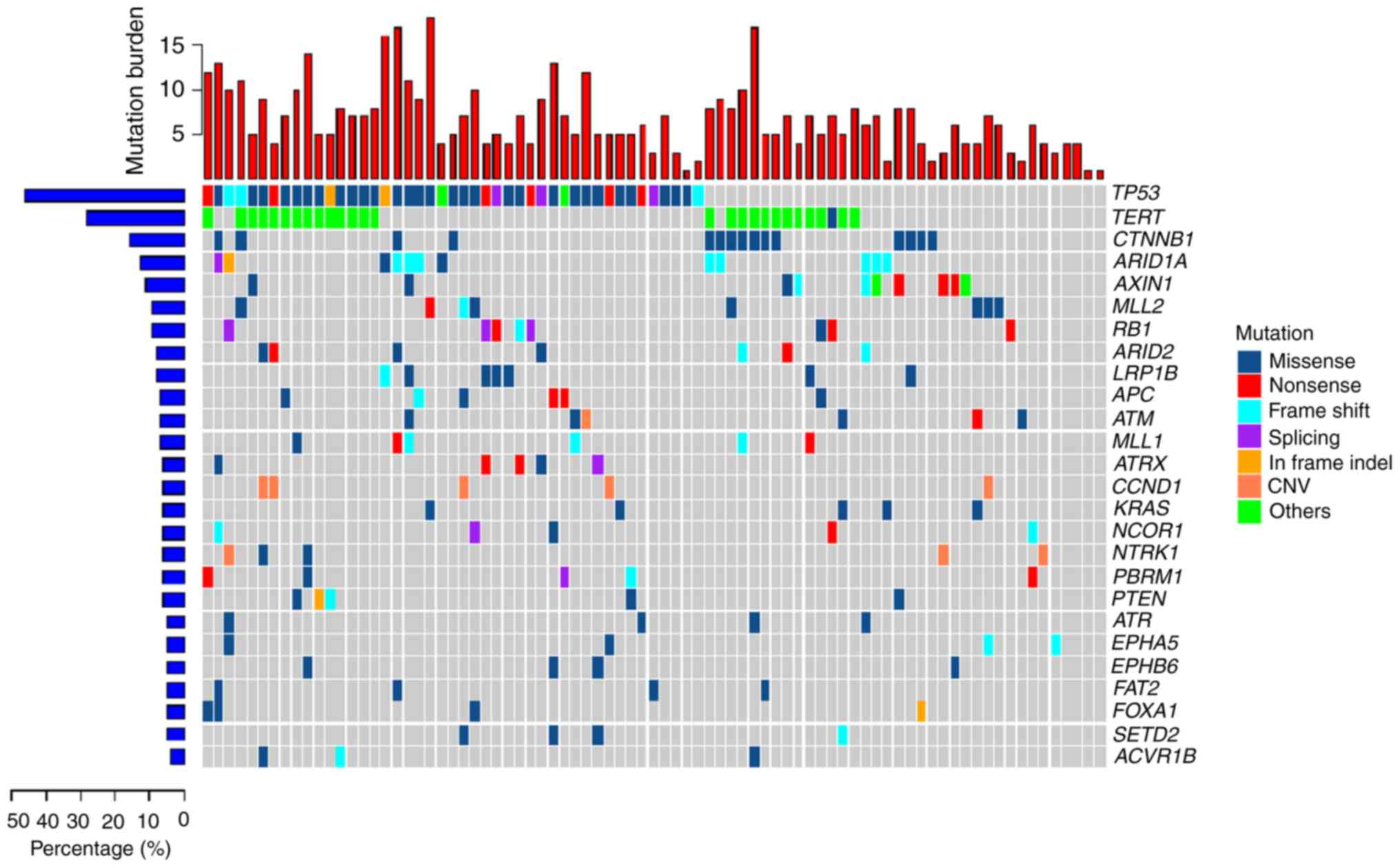
SNVs and 38 CNVs were found in the HCC tumor samples (Fig. 1). The most commonly mutated genes
were: Tumor protein 53 (TP53); telomerase reverse transcriptase
(TERT); Catenin beta 1 (CTNNB1); RB transcriptional co-repressor 1
(RB1); AT-rich interactive domain-containing protein (ARID)1A; axis
inhibition protein 1 (AXIN1); ARID2; cyclin D1 (CCND1); and
adenomatous polyposis coli (APC). The TP53 tumor suppressor gene
was the most frequently mutated (55.6% of samples) of all the
highly mutated genes in the cohort and RB1 gene was mutated in 9.9%
of the samples. Inactivation of the TP53-RB pathway was frequently
observed in the HCC samples. TERT, a crucial unit of the telomerase
complex (23), showed somatic
mutations in its promoter region 33.3%. CTNNB1, AXIN1 and APC,
which are important components of the wingless-type MMTV
integration site family (WNT) signaling pathway (23), showed alteration frequencies of 18.5,
12.4 and 7.4%, respectively. ATP-dependent nucleosome remodelers,
ARID1A and ARID2, were mutated in 14.8 and 8.6% of tumors,
respectively. CNV mutations were also commonly found in HCC tumors
samples and the most frequent CNV was a CCND1 amplification
(6.17%). CCND1 encodes cyclin D1, the major downstream target
factor of the WNT signaling pathway (23). CCND1 amplification was exclusively
mutated in the crucial components of the WNT signaling pathway,
including CTNNB1, AXIN1 and APC. Another CNV identified was an
amplification of NTRK1 in 3.7% of samples.
Distribution of tTMB in HCC
samples
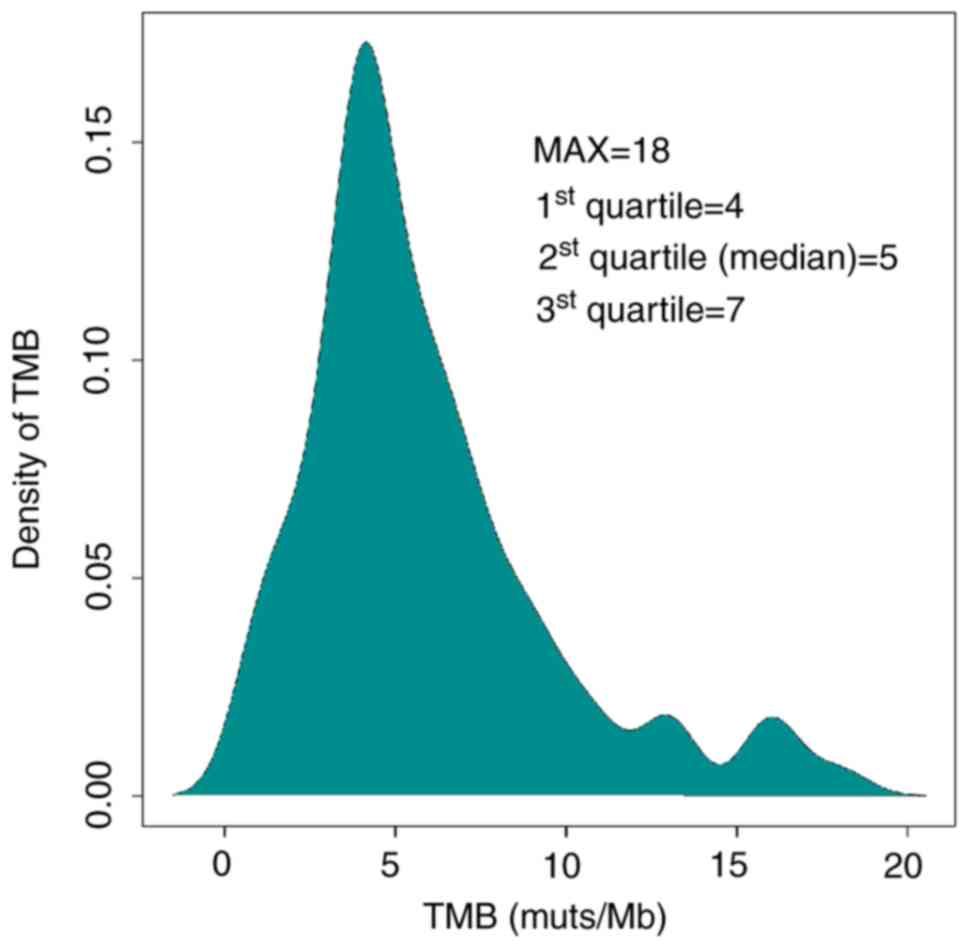
Calculation of tTMB was performed using a
hybridization-capture method and targeted ~1.45 Mb of the genomic
sequence (coding sequence, 1 Mb). tTMB was defined as the number of
somatic coding SNVs and indels occurring at a frequency of
≥3%/1×106 bases in the 1,021 gene panel. The median tTMB
of the HCC cohort was 5 mutations/megabase (muts/Mb). The top
quartile (7 muts/Mb) of TMB distribution was used as the cutoff
value to define TMB-H (Fig. 2). The
maximum of tTMB distribution was 18 muts per Mb. There were 26
patients with HCC and high tumor mutation burden. Subsequently, the
influenced factors of tTMB-H were analyzed.
Age and TMB distribution in HCC
HCC specimens were stratified according to age, in
order to analyze the differences in TMB distribution. From the age
of 30–70, there was an increasing trend of tTMB, however this was
not statistical significance. A slight decrease in tTMB was
observed in patients >70 years (Fig.
S1). These results are consistent with a previous study by
Podolskiy et al (24) which
demonstrated a similar relationship between human aging and the
development of tumor mutations.
Sex and TMB distribution in HCC
Stratification by sex showed that the median tTMB
values of male and female patients with HCC were 5 muts/Mb and 4
muts/Mb, respectively. The top quartile of tTMB in the male cohort
(7 muts/Mb) was higher compared with the female cohort (6.5
muts/Mb). No significant difference in TMB distribution was
observed between male and female HCC cohorts (P=0.6917; Fig. S2); however, this may be due to the
lower number of HCC specimens from females.
Frequently mutated genes in the TMB-H
cohort
To identify recurrently mutated genes in the TMB-H
cohort, HCC patients were classified into two groups: tTMB-H (≥7
Muts/Mb) and low tTMB (tTMB-L; <7 Muts/Mb). The results showed
that mutations in ARID1A, CTNNB1 and NCOR1 were more frequently
detected in HCC samples in the tTMB-H group compared with tTMB-L
group (P=0.0013, 0.0152 and 0.0347, respectively; Table II).
 | Table II.Gene mutation rates in a total of 81
patients with hepatocellular carcinoma, including 26 samples with
tTMB-H and 55 samples with tTMB-L. |
Table II.
Gene mutation rates in a total of 81
patients with hepatocellular carcinoma, including 26 samples with
tTMB-H and 55 samples with tTMB-L.
| Gene | tTMB-H, % | tTMB-L, % | P-value |
|---|
| TP53 | 65.38 | 50.91 | 0.2419 |
| TERT | 42.31 | 29.09 | 0.3135 |
| ARID1A | 34.62 | 5.45 | 0.0013b |
| CTNNB1 | 34.62 | 10.91 | 0.0152a |
| AXIN1 | 11.54 | 12.73 | 1 |
| MLL2 | 11.54 | 9.09 | 0.7071 |
| LRP1B | 11.54 | 7.27 | 0.6749 |
| RB1 | 7.69 | 10.91 | 1 |
| ARID2 | 15.38 | 5.45 | 0.2031 |
| APC | 11.54 | 5.45 | 0.3798 |
| MLL | 15.38 | 3.64 | 0.0803 |
| ATRX | 7.69 | 5.45 | 0.6539 |
| PTEN | 7.69 | 5.45 | 0.6539 |
| PBRM1 | 11.54 | 3.64 | 0.3211 |
| KRAS | 3.85 | 7.27 | 1 |
| ATM | 3.85 | 7.27 | 1 |
| NCOR1 | 15.38 | 1.82 | 0.0347a |
Association between gene mutations and
TMB distribution in HCC
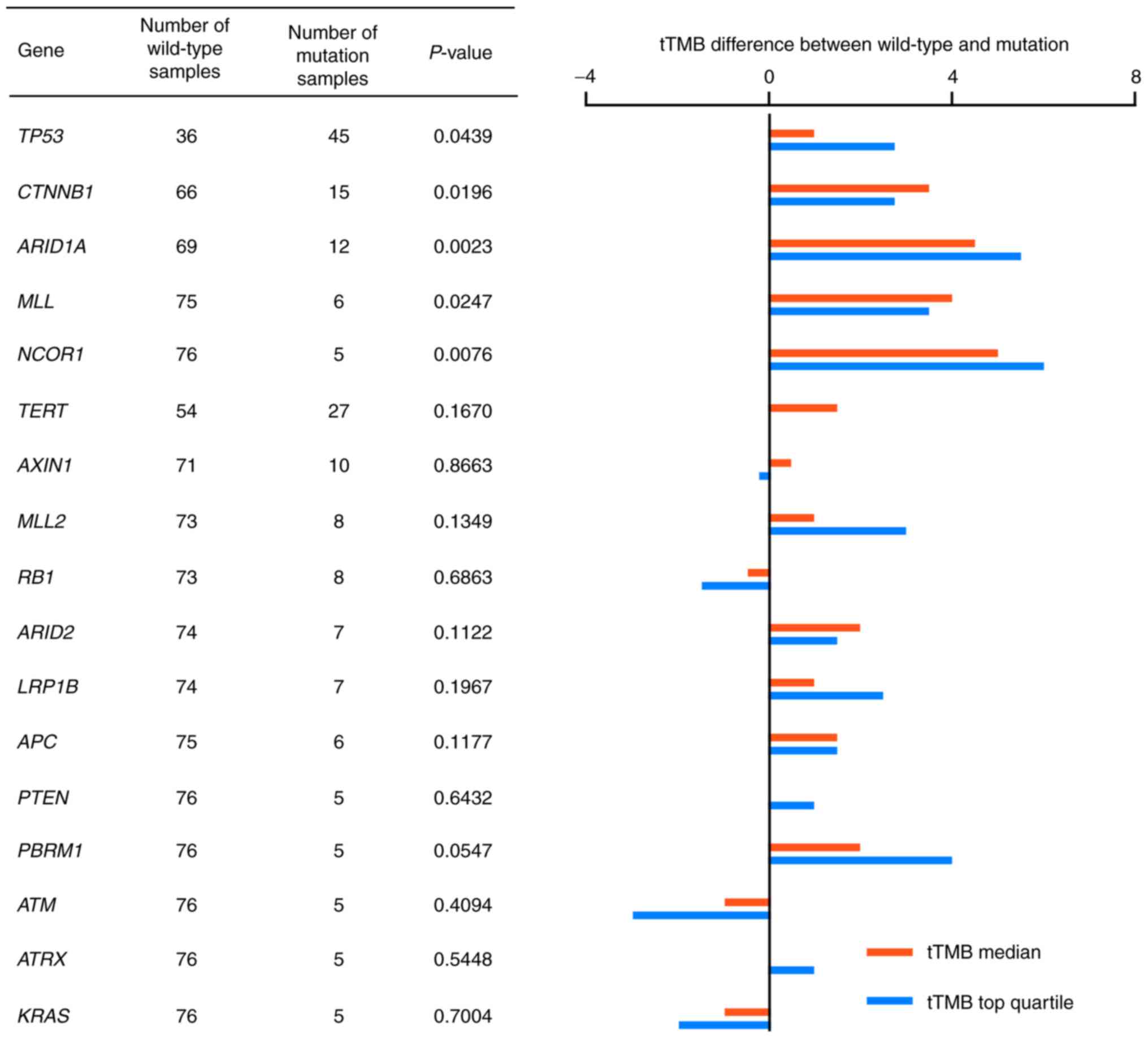
To further analyze the association between gene
mutations and TMB-H status in HCC, the tTMB distribution in HCC
samples was compared between the wild-type and mutated genotypes.
Frequently mutated genes, which were detected in >5 HCC tumor
cases were analyzed for TMB distribution. Median tTMB values and
the top quartile values of tTMB were compared in 18 genes. A total
of five genes were shown to be significantly different in tTMB
distribution between the wild-type and mutated genotypes: TP53;
CTNNB1; ARID1A; MLL; and NCOR1 (P=0.0439, 0.0196, 0.0023, 0.0247
and 0.0076, respectively; Fig. 3).
tTMB distribution is shown in Fig.
S3.
TMB distribution based on gene
mutation status in The Cancer Genome Atlas Liver Hepatocellular
Carcinoma database (TCGA-LIHC)
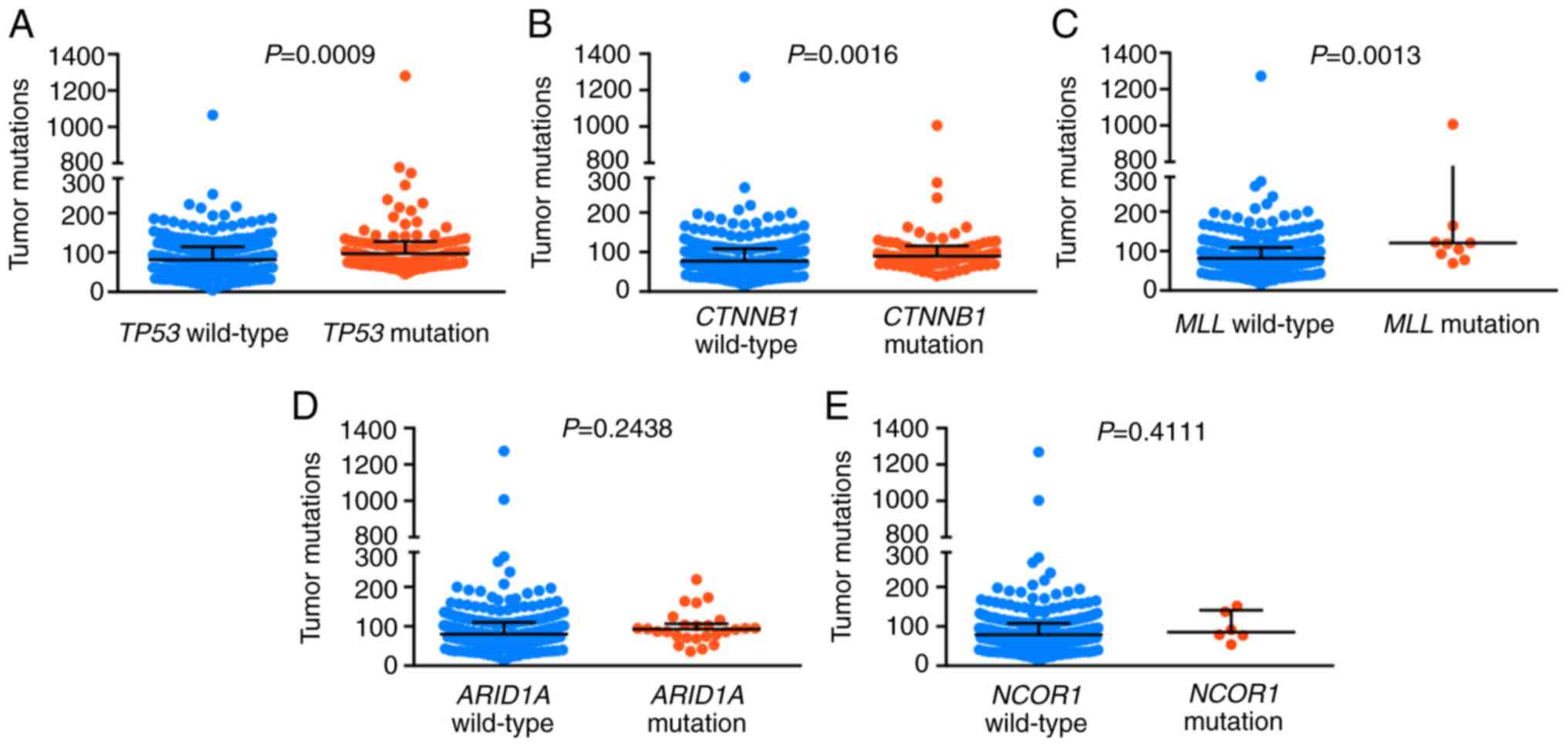
A retrospective analysis was performed using HCC
data obtained from the TCGA-LIHC database. A total of 363 HCC tumor
samples were included in the cohort and the data were obtained from
cBioPortal (cbioportal.org/). It was found that
mutations in TP53, CTNNB1 and MLL were positively correlated with
higher TMB (P=0.0009, 0.0016 and 0.0013, respectively; Fig. 4).
Frequently mutated genes predict
overall survival and RFS of HCC in TCGA-LIHC
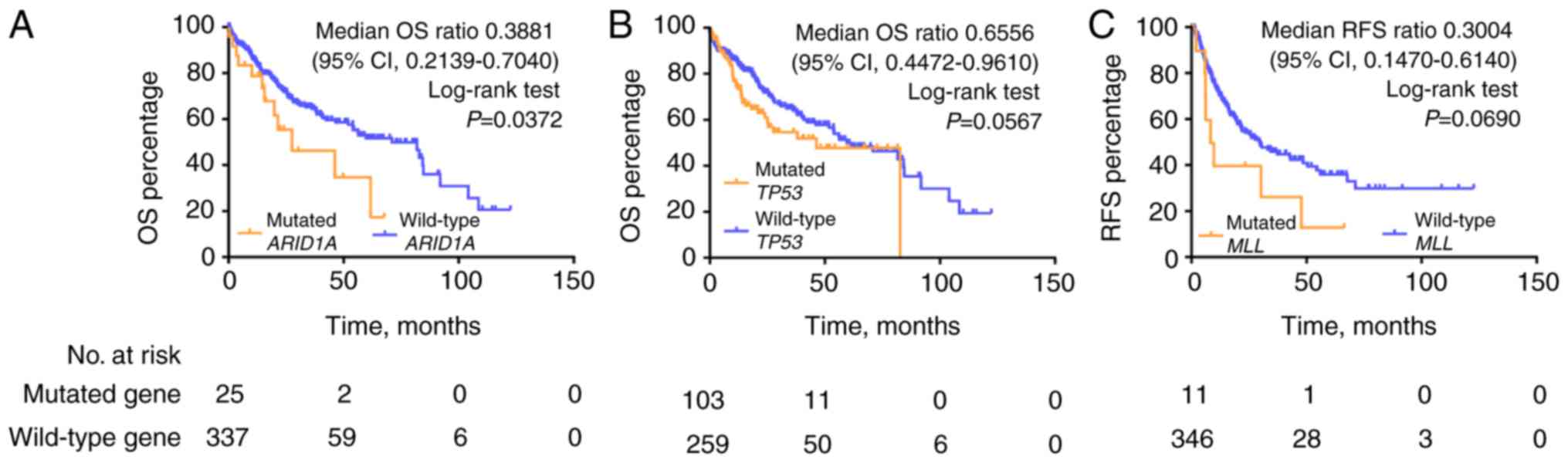
Kaplan-Meier analysis of OS demonstrated that the
ARID1A mutation was significantly correlated with poor survival
outcome in the TCGA HCC cohort [median OS (mOS), 27.57 vs. 71.03
months, ARID1A mutations vs. wild-type; mOS ratio, 0.3881, 95%
confidence interval (CI), 0.2139–0.7040; log-rank test, P=0.0372;
Fig. 5]. Mutations in TP53 predicted
a trend towards poor outcome (mOS 46.57 vs. 71.03 months, TP53
mutations vs. wild-type; log-rank test, P=0.0567). No statistically
significant differences in survival outcome were observed for
CTNNB1, MLL and NCOR1 mutations compared with the wild-type
genotypes in the TCGA HCC cohort (Fig.
S4). Kaplan-Meier analysis of RFS demonstrated that MLL
mutations were significantly correlated with poorer RFS compared
with the wild-type genotype (mRFS, 8.93 vs. 29.73 months; mRFS
ratio, 0.3004, 95% CI, 0.1470–0.6140; log-rank test, P=0.069,
Fig. 5).
Microsatellite instability status in
HCC
Only 1/81 HCC tumor samples (HCC057) in the present
study showed MSI-H. This sample also had TMB-H with a tTMB value of
9 muts/Mb, and one TP53 mutation and one ARID1A mutation (data not
shown).
Mutational status of genes that may
predict efficacy of ICB therapy in HCC
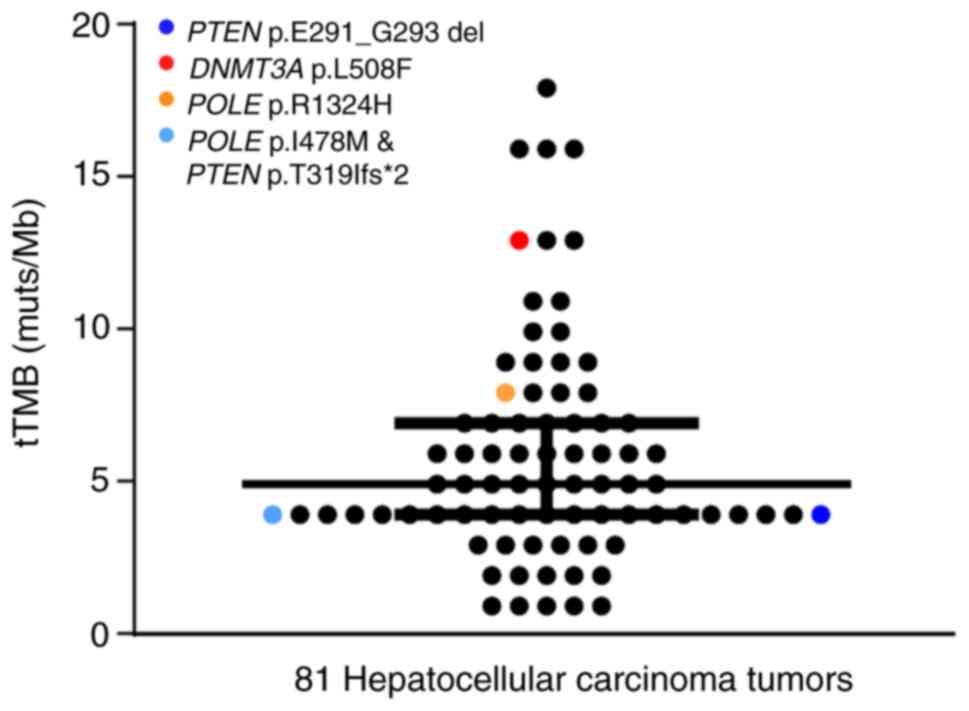
A total of 2/81 HCC patients (2.47%) possessed
mutations in POLE (R1324H and I478M; Table SI). The tTMB values of the R1324H
and I478 M mutations were 8 muts/Mb and 4 muts/Mb, respectively
(Fig. 6). Overall, 2 mutations were
observed in PTEN and 1 mutation was observed in DNMT3A. No
mutations were detected in MLH1, MLH2, PMS2, MSH6, POLD1, JAK1,
JAK2, B2M and STK11. Mutations in POLE and PTEN occurred in the
primary clonal mutation and the inactivating mutation in DNMT3A
occurred in the sub-clonal mutation.
Discussion
HCC is an aggressive disease and previous genetic
characterization of this disease has revealed significant
heterogeneity among tumors; even within a single tumor lesion, the
allelic frequency can be as low as 13% (25). Previous studies have identified
several signaling pathways, which are disrupted in HCC, including
the WNT, hypoxia-inducible factor 1, mechanistic target of
rapamycin, and several metabolic related pathways (26,27).
Actionable target genes found in other tumors, especially lung
adenocarcinoma, such as those encoding protein kinases and other
crucial enzymes are not significantly mutated in HCC (26–29). It
has been reported that no single protein kinase is mutated at a
frequency >5% in HCC (28,29). The
majority of targeted therapy (tyrosine kinase inhibitors or
monoclonal antibodies) for HCC has demonstrated minimal or no
clinical efficacy (30).
HCC typically manifests in patients with previous
liver damage, commonly caused by chronic hepatitis infection
(31). Chronic hepatitis and
subsequent inflammation are reported to induce immune evasion via
prolonged activation of the interferon γ signaling pathway
(32,33). The immune system serves an important
role in the development of HCC. Research has demonstrated that ICB
therapy may be an effective treatment strategy, which results in
effective and long-lasting responses in patients with HCC. For
example, in phase I/II of the CheckMate 040 trial evaluating the
safety and efficacy of nivolumab as a monotherapy in patients with
advanced HCC, the response rate was 20% and the disease control
rate was 64% (34). Although the
CheckMate 040 trial was non-randomized, it led to the approval of
nivolumab by the Food and Drug Administration (FDA) for the
treatment of HCC. Despite advances in ICB therapy, the clinical
efficacy of this treatment is limited due to drug resistance
(6,7). Even for melanoma, one of the tumors
most sensitive to ICB, ~60% of patients display primary resistance
and ~50% of individuals who respond favorably are likely to develop
acquired resistance after 3 years of treatment (6,7). To
provide individualized treatment, improve current treatment
modalities and to investigate potentially novel treatment
strategies, researchers have been trying to identify factors that
predict drug efficacy. Previous studies have reported tumor cell
mutations associated with drug efficacy observed in patients
undergoing ICB-based immunotherapy and that these mutations may
have value as biomarkers (35,36).
Mutations in MLH1, MLH2, PMS2 and MSH6 are associated with dMMR and
result in MSI (37). The FDA
approved MSI status as a biomarker for immunotherapy of
pan-cancerous species (38).
Mutations in POLE and POLD1 are associated with extremely high TMB
(39), and inactivation mutations in
JAK1, JAK2, B2M and PTEN are associated with immunotherapy
resistance (35,40). Meanwhile, mutations in DNMT3A are
associated with hyper-progression in immunotherapy (36). In the present study, the somatic
mutation landscape of HCC tumors was characterized, with a
particular focus on mutations associated with TMB status, to
improve our understanding of the role of the tumor cell intrinsic
factors on the efficacy of immunotherapy for HCC.
The genetic alterations identified in the present
study are consistent with previously published studies (23,41,42). The
results of the present study showed that most genes were mutated in
<20% of the samples analyzed, supporting the genetic
heterogeneity of HCC. Regarding tumor intrinsic genetic aberrations
which may be associated with the clinical efficacy of ICB therapy,
2 (2.47%) mutations in POLE, 2 mutations (2.47%) in PTEN, 1
mutation (1.23%) in DNMT3A were detected as well as only 1 sample
with MSI-H. No evidence of dMMR was observed in any of the samples.
It is possible that none of the factors investigated in the present
study, which have been studied in other tumor types (43,44), are
suitable targets for anti-HCC therapy. The underlying mechanisms of
immune evasion in HCC may be different compared with other types of
cancer. Overall, potential predictors of clinical efficacy of ICB
therapy in patients with HCC needs to be further explored.
The association between TMB and patient response to
ICB therapy was originally indicated by melanoma studies and
ICB-sensitive melanomas were found to comprise tumors with the
highest mutation burden (11,14). The
association between TMB and ICB efficacy was subsequently confirmed
in colorectal cancer (CRC). Only hyper-mutated CRCs with dMMR or
MSI-H tend to respond to ICB therapy (15). A recently published study evaluated
the efficacy of SHR-1210 anti-PD-1 antibody combined with the
anti-angiogenesis agent, apatinib, for patients with advanced
stages of tumors including adenocarcinoma of the stomach and
gastroesophageal function, and HCC (13). Among the 18 patients with HCC
enrolled in this study, the TMB of individuals who responded
favorably was significantly higher compared with those who did not
respond favorably, and this treatment significantly improved the
survival outcomes of patients with HCC who possessed a large number
of mutations (13). Therefore, TMB
is a potentially novel predictor of the efficacy of ICB therapy for
patients with HCC. This may be due to an increased number of
tumor-associated antigens or neoantigens expressed by cancer cells
with high TMB, which are critical for the activation of anti-tumor
immune responses. Increased numbers of neoantigens are known to
increase recruitment of various types of T cells, particularly CD8+
T lymphocytes. Tumor neoantigens that are generated by mutations,
especially frameshift-mutation-derived peptides have the highest
immunogenicity (45,46).
The DNA damage response (DDR) system is essential
for preserving genomic integrity by repairing damaged DNA (47). Dysfunction in this system may induce
MSI-H or hyper-mutational phenotypes and DDR deficiency is
considered to be a primary cause of TMB-H and MSI-H (47,48).
There are 8 pathways included in the DDR system, of which mismatch
repair (MMR) has been extensively studied and is a commonly used
predictor in a clinical setting (48,49). The
present study did not detect dMMR in any of the 81 samples that
were analyzed, including the sample that harbored the highest TMB
(18 muts/Mb); however, five genes, including TP53, CTNNB1, ARID1A,
MLL and NCOR1, were found to be significantly associated with
TMB-H. In addition, only 1/81 HCC samples showed MSI-H and TMB-H (9
muts/Mb). This sample also harbored a TP53 mutation and an ARID1A
mutation.
TP53 is one of the most frequently mutated genes in
HCC (23,42). It is a transcription factor
controlling the expression of genes involved in cell cycle arrest,
apoptosis and senescence in response to hypoxic stress, DNA damage
and oncogenic activation. TP53 is also a tumor suppressor gene,
functioning in the preservation of genomic integrity during
hypoxia, which is a common phenomenon in HCC (50,51).
ARID1A is also frequently mutated in HCC, as demonstrated in the
present study. ARID1A binds to other subunits such as BRG1/BRM,
forming a switch/sucrose non-fermentable chromatin remodeling
complex. This complex uses energy from ATP to mobilize nucleosomes
and to regulate DNA accessibility to various cellular machinery,
including DNA replication and DNA-damage repair machinery (52). In a proteomic screen, Shen et
al (53) found that ARID1A
interacts with MMR protein MSH2, recruiting MSH2 to chromatin
during DNA replication and promoting MMR. Conversely, ARID1A
inactivation compromised MMR and increased mutagenesis. ARID1A
deficiency was associated with an MSI genomic signature, a
predominant C>T mutation pattern and increased mutation load
across several types of cancer. Tumors formed using an
ARID1A-deficient ovarian cancer cell line in syngeneic mice
displayed increased mutation load (53). MLL belongs to the family of histone
H3 lysine 4 methyltransferases and is a chromatin regulatory enzyme
(25). NCOR1 also serves an
important role in regulating a variety of nuclear factors and in
chromatin remodeling (54). CTNNB1
encodes β-catenin, a subunit of the cadherin protein complex which
functions as a signaling molecule in the WNT signaling pathway and
regulates cellular proliferation (55). It is possible that factors which
influence genetic stability, facilitate DNA error generation or
regulate cell proliferation may all contribute to TMB-H. Further
studies are required to elucidate the underlying mechanisms
contributing to TMB-H development. The result of the present study
showing no association between ARID1A and NCOR1 with TMB in TCGA
cohort may be due to biased sampling from regional differences.
PD-L1 is the most commonly used clinical biomarker
for ICBs, but it has several limitations (8). The ability of NGS to reveal the TMB
status of patients provides another potential predictor of
ICB-therapy efficacy, as shown in clinical trials investigating
other tumor types (11,13–15).
Therefore, TMB-H may also serve as biomarker complementary to
PD-L1. However, several key questions need to be answered: How many
genes (the whole genome, targeted panel, or only expressed
mutations) should be included to define TMB status? What is the
optimal threshold for TMB-H? Is there a consensus between the
different diagnostic assays? Whether crucial driver gene mutations
associated with high mutation load could serve as potential
predictive biomarkers in patients with HCC treated with ICB
therapy? Further studies are required to establish uniform
diagnostic standards.
The present study has several limitations. First,
there was no cohort treated with ICB therapy. Second, it was only
demonstrated that gene mutations associated with TMB-H are present
in patients with HCC, but the underlying mechanisms of this
association remains to be investigated in vitro and in
vivo. In addition, the majority of patients enrolled in the
present study were male, accounting for 87.65% of the entire
cohort. This was higher compared with the sex distribution of
patients shown in a different study on HCC (56).
The present study provides novel insight into gene
signatures, which may predict the clinical efficacy of ICB therapy
in patients with HCC. The five genes identified showed recurrent
mutations which were significantly correlated with high mutation
load: TP53; CTNNB1; ARID1A; MLL; and NCOR1. These findings may
provide a novel understanding of the underlying mechanisms of HCC
to aid the development of therapeutic strategies, such as combined
therapy with ICBs and molecule-targeted drugs. Limitations of the
present study include the use of a small study cohort and the
retrospective nature of the analysis. Further investigation is
required to evaluate the association of TMB-H and crucial driver
gene with high mutation load and the potential of these genes as
predictive biomarkers of ICB therapy treatment in patients with
HCC.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Dr Michael J.
Overman (Department of Gastrointestinal Medical Oncology, The
University of Texas MD Anderson Cancer Center) for his advice
regarding the interpretation of the manuscript.
Funding
The present study was funded by The National Nature
Science Foundation of China (grant no. 81372632).
Availability of data and materials
The data that support the findings of the present
study are available from Geneplus-Beijing Institute, but
restrictions apply to the availability of these data, which were
used under license for the current study, and so are not publicly
available. Data are however available from the authors upon
reasonable request and with permission from Geneplus-Beijing
Institute.
Authors' contributions
LJ conceptualized the study. LL, ZW, XR designed the
study. Software was designed by WX. LL, WX and ZW collected the
data. LL, ZW, XD, YY, YG, YC and JW analyzed the data. LL, XR, XD,
XW, WX and CM obtained the resources necessary for the study. LL,
WX, LJ and XR wrote, reviewed and edited the manuscript. All
authors approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by The institutional
Review Board of Peking University International Hospital and
written informed consent was obtained from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
HCC
|
hepatocellular carcinoma
|
|
ICBs
|
immune checkpoint blockades
|
|
PD-1
|
programmed death protein 1
|
|
PD-L1
|
programmed cell death ligand 1
|
|
TMB
|
tumor mutation burden
|
|
TMB-H
|
high TMB
|
|
dMMR
|
mismatch repair deficiency
|
|
MSI-H
|
high microsatellite instability
|
|
SNVs
|
single nucleotide variants
|
|
indels
|
insertions or deletions
|
|
CNVs
|
copy number variants
|
|
SVs
|
structure variations
|
|
TP53
|
tumor protein 53
|
|
CTNNB1
|
Catenin®1
|
|
RB1
|
RB transcriptional co-repressor 1
|
|
AXIN1
|
axis inhibition protein 1
|
|
ARID
|
AT-rich interactive domain-containing
protein
|
|
CCND1
|
cyclin D1
|
|
APC
|
adenomatous polyposis coli
|
|
WNT
|
wingless-type MMTV integration site
family
|
|
TERT
|
telomerase reverse transcriptase
|
|
NTRK1
|
neurotrophic tyrosine kinase receptor
type 1
|
|
MLL
|
myeloid/lymphoid or mixed-lineage
leukemia
|
|
NCOR1
|
nuclear receptor co-repressor 1
|
|
tTMB
|
tissue TMB
|
|
TCGA-LIHC
|
Cancer Genome Atlas Liver
Hepatocellular Carcinoma
|
|
OS
|
overall survival
|
|
mOS
|
median OS
|
|
RFS
|
recurrence-free survival
|
|
mRFS
|
median RFS
|
|
FDA
|
Food and Drug Administration
|
|
CRC
|
colorectal cancer
|
|
DDR
|
DNA damage response
|
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yu SJ: A concise review of updated
guidelines regarding the management of hepatocellular carcinoma
around the world: 2010–2016. Clin Mol Hepatol. 22:7–17. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sprinzl MF and Galle PR: Current progress
in immunotherapy of hepatocellular carcinoma. J Hepatol.
66:482–484. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Topalian SL, Drake CG and Pardoll DM:
Immune checkpoint blockade: A common denominator approach to cancer
therapy. Cancer Cell. 27:450–461. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rotte A, Jin JY and Lemaire V: Mechanistic
overview of immune checkpoints to support the rational design of
their combinations in cancer immunotherapy. Ann Oncol. 29:71–83.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Topalian SL, Hodi FS, Brahmer JR,
Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD,
Sosman JA, Atkins MB, et al: Safety, activity, and immune
correlates of anti-PD-1 antibody in cancer. N Engl J Med.
366:2443–2454. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang Q and Wu X: Primary and acquired
resistance to PD-1/PD-L1 blockade in cancer treatment. Int
Immunopharmacol. 46:210–219. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhu J, Armstrong AJ, Friedlander TW, Kim
W, Pal SK, George DJ and Zhang T: Biomarkers of immunotherapy in
urothelial and renal cell carcinoma: PD-L1, tumor mutational
burden, and beyond. J Immunother Cancer. 6:42018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Overman MJ, Lonardi S, Wong KYM, Lenz HJ,
Gelsomino F, Aglietta M, Morse MA, Van Cutsem E, McDermott R, Hill
A, et al: Durable clinical benefit with nivolumab plus ipilimumab
in DNA mismatch repair-deficient/microsatellite instability-high
metastatic colorectal cancer. J Clin Oncol. 36:773–779. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yarchoan M, Hopkins A and Jaffee EM: Tumor
mutational burden and response rate to PD-1 inhibition. N Engl J
Med. 377:2500–2501. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schumacher TN and Schreiber RD:
Neoantigens in cancer immunotherapy. Science. 348:69–74. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rizvi NA, Hellmann MD, Snyder A, Kvistborg
P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS, et al: Cancer
immunology. Mutational landscape determines sensitivity to PD-1
blockade in non-small cell lung cancer. Science. 348:124–128. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu J, Zhang Y, Jia R, Yue C, Chang L, Liu
R, Zhang G, Zhao C, Zhang Y, Chen C, et al: Anti-PD-1 antibody
SHR-1210 combined with apatinib for advanced hepatocellular
carcinoma, gastric or esophagogastric junction cancer: An
open-label, dose escalation and expansion study. Clin Cancer Res.
25:515–523. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Alexandrov LB, Nik-Zainal S, Wedge DC,
Aparicio SA, Behjati S, Biankin AV, Bignell GR, Bolli N, Borg A,
Børresen-Dale AL, et al: Signatures of mutational processes in
human cancer. Nature. 500:415–421. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Le DT, Uram JN, Wang H, Bartlett BR,
Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, et
al: PD-1 blockade in tumors with mismatch-repair deficiency. N Engl
J Med. 372:2509–2520. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hugo W, Zaretsky JM, Sun L, Song C, Moreno
BH, Hu-Lieskovan S, Berent-Maoz B, Pang J, Chmielowski B, Cherry G,
et al: Genomic and transcriptomic features of response to Anti-PD-1
therapy in metastatic melanoma. Cell. 165:35–44. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pal SK, Agarwal N, Choueiri TK, Stephens
PJ, Ross JS, Miller VA, Ali SM, Chung J and Grivas P: Comparison of
tumor mutational burden (TMB) in relevant molecular subsets of
metastatic urothelial cancer (MUC). Ann Oncol. 28
(Suppl-5):v295–v329. 2017. View Article : Google Scholar
|
|
18
|
Lv X, Zhao M, Yi Y, Zhang L, Guan Y, Liu
T, Yang L, Chen R, Ma J and Yi X: Detection of rare mutations in
CtDNA using next generation sequencing. J Vis Exp. 2017.doi:
10.3791/56342. View
Article : Google Scholar
|
|
19
|
Li H and Durbin R: Fast and accurate short
read alignment with Burrows-Wheeler transform. Bioinformatics.
25:1754–1760. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cibulskis K, Lawrence MS, Carter SL,
Sivachenko A, Jaffe D, Sougnez C, Gabriel S, Meyerson M, Lander ES
and Getz G: Sensitive detection of somatic point mutations in
impure and heterogeneous cancer samples. Nat Biotechnol.
31:213–221. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li J, Lupat R, Amarasinghe KC, Thompson
ER, Doyle MA, Ryland GL, Tothill RW, Halgamuge SK, Campbell IG and
Gorringe KL: CONTRA: Copy number analysis for targeted
resequencing. Bioinformatics. 28:1307–1313. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nong J, Gong Y, Guan Y, Yi X, Yi Y, Chang
L, Yang L, Lv J, Guo Z, Jia H, et al: Circulating tumor DNA
analysis depicts subclonal architecture and genomic evolution of
small cell lung cancer. Nat Commun. 9:31142018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Totoki Y, Tatsuno K, Covington KR, Ueda H,
Creighton CJ, Kato M, Tsuji S, Donehower LA, Slagle BL, Nakamura H,
et al: Trans-ancestry mutational landscape of hepatocellular
carcinoma genomes. Nat Genet. 46:1267–1273. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Podolskiy DI, Lobanov AV, Kryukov GV and
Gladyshev VN: Analysis of cancer genomes reveals basic features of
human aging and its role in cancer development. Nat Commun.
7:121572016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tao Y, Ruan J, Yeh SH, Lu X, Wang Y, Zhai
W, Cai J, Ling S, Gong Q, Chong Z, et al: Rapid growth of a
hepatocellular carcinoma and the driving mutations revealed by
cell-population genetic analysis of whole-genome data. Proc Natl
Acad Sci USA. 108:12042–12047. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Breuhahn K, Gores G and Schirmacher P:
Strategies for hepatocellular carcinoma therapy and diagnostics:
Lessons learned from high throughput and profiling approaches.
Hepatology. 53:2112–2121. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zender L, Villanueva A, Tovar V, Sia D,
Chiang DY and Llovet JM: Cancer gene discovery in hepatocellular
carcinoma. J Hepatol. 52:921–929. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Guichard C, Amaddeo G, Imbeaud S, Ladeiro
Y, Pelletier L, Maad IB, Calderaro J, Bioulac-Sage P, Letexier M,
Degos F, et al: Integrated analysis of somatic mutations and focal
copy-number changes identifies key genes and pathways in
hepatocellular carcinoma. Nat Genet. 44:694–648. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fujimoto A, Totoki Y, Abe T, Boroevich KA,
Hosoda F, Nguyen HH, Aoki M, Hosono N, Kubo M, Miya F, et al:
Whole-genome sequencing of liver cancers identifies etiological
influences on mutation patterns and recurrent mutations in
chromatin regulators. Nat Genet. 44:760–764. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
da Motta Girardi D, Correa TS, Crosara
Teixeira M and Dos Santos Fernandes G: Hepatocellular carcinoma:
Review of targeted and immune therapies. J Gastrointest Cancer.
49:227–236. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gomaa AI, Khan SA, Toledano MB, Waked I
and Taylor-Robinson SD: Hepatocellular carcinoma: Epidemiology,
risk factors and pathogenesis. World J Gastroenterol. 14:4300–4308.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Elsegood CL, Tirnitz-Parker JE, Olynyk JK
and Yeoh GC: Immune checkpoint inhibition: Prospects for prevention
and therapy of hepatocellular carcinoma. Clin Transl Immunology.
6:e1612017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wieder T, Eigentler T, Brenner E and
Röcken M: Immune checkpoint blockade therapy. J Allergy Clin
Immunol. 142:1403–1414. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
El-Khoueiry AB, Sangro B, Yau T, Crocenzi
TS, Kudo M, Hsu C, Kim TY, Choo SP, Trojan J, Welling TH Rd, et al:
Nivolumab in patients with advanced hepatocellular carcinoma
(CheckMate 040): An open-label, non-comparative, phase 1/2 dose
escalation and expansion trial. Lancet. 389:2492–2502. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zaretsky JM, Garcia-Diaz A, Shin DS,
Escuin-Ordinas H, Hugo W, Hu-Lieskovan S, Torrejon DY,
Abril-Rodriguez G, Sandoval S, Barthly L, et al: Mutations
associated with acquired resistance to PD-1 blockade in melanoma. N
Engl J Med. 375:819–829. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kato S, Goodman A, Walavalkar V,
Barkauskas DA, Sharabi A and Kurzrock R: Hyperprogressors after
Immunotherapy: Analysis of Genomic Alterations associated with
accelerated growth rate. Clin Cancer Res. 23:4242–4250. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Llosa NJ, Cruise M, Tam A, Wicks EC,
Hechenbleikner EM, Taube JM, Blosser RL, Fan H, Wang H, Luber BS,
et al: The vigorous immune microenvironment of microsatellite
instable colon cancer is balanced by multiple counter-inhibitory
checkpoints. Cancer Discov. 5:43–51. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lemery S, Keegan P and Pazdur R: First FDA
approval agnostic of cancer site-when a biomarker defines the
indication. N Engl J Med. 377:1409–1412. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Campbell BB, Light N, Fabrizio D, Zatzman
M, Fuligni F, de Borja R, Davidson S, Edwards M, Elvin JA, Hodel
KP, et al: Comprehensive analysis of hypermutation in human cancer.
Cell. 171:1042–1056.e10. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Peng W, Chen JQ, Liu C, Malu S, Creasy C,
Tetzlaff MT, Xu C, McKenzie JA, Zhang C, Liang X, et al: Loss of
PTEN promotes resistance to t cell-mediated immunotherapy. Cancer
Discov. 6:202–216. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Cleary SP, Jeck WR, Zhao X, Chen K,
Selitsky SR, Savich GL, Tan TX, Wu MC, Getz G, Lawrence MS, et al:
Identification of driver genes in hepatocellular carcinoma by exome
sequencing. Hepatology. 58:1693–1702. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lee JS: The mutational landscape of
hepatocellular carcinoma. Clin Mol Hepatol. 21:220–229. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Braun DA, Burke KP and Van Allen EM:
Genomic approaches to understanding response and resistance to
immunotherapy. Clin Cancer Res. 22:5642–5650. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Teng F, Meng X, Kong L and Yu J: Progress
and challenges of predictive biomarkers of anti PD-1/PD-L1
immunotherapy: A systematic review. Cancer Lett. 414:166–173. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Giannakis M, Mu XJ, Shukla SA, Qian ZR,
Cohen O, Nishihara R, Bahl S, Cao Y, Amin-Mansour A, Yamauchi M, et
al: Genomic correlates of immune-cell infiltrates in colorectal
carcinoma. Cell Rep. 15:857–865. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Matsushita H, Vesely MD, Koboldt DC,
Rickert CG, Uppaluri R, Magrini VJ, Arthur CD, White JM, Chen YS,
Shea LK, et al: Cancer exome analysis reveals a T-cell-dependent
mechanism of cancer immunoediting. Nature. 482:400–404. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lee JK, Choi YL, Kwon M and Park PJ:
Mechanisms and consequences of cancer genome instability: Lessons
from genome sequencing studies. Annu Rev Pathol. 11:283–312. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wang Z, Zhao J, Wang G, Zhang F, Zhang Z,
Zhang F, Zhang Y, Dong H, Zhao X, Duan J, et al: Co-mutations in
DNA damage response pathways serve as potential biomarkers for
immune checkpoint blockade. Cancer Res. 78:6486–6496.
2018.PubMed/NCBI
|
|
49
|
Scarbrough PM, Weber RP, Iversen ES,
Brhane Y, Amos CI, Kraft P, Hung RJ, Sellers TA, Witte JS, Pharoah
P, et al: A cross-cancer genetic association analysis of the DNA
repair and DNA damage signaling pathways for lung, ovary, prostate,
breast, and colorectal cancer. Cancer Epidemiol Biomarkers Prev.
25:193–200. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Muller PA and Vousden KH: Mutant p53 in
cancer: New functions and therapeutic opportunities. Cancer Cell.
25:304–317. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Bieging KT, Mello SS and Attardi LD:
Unravelling mechanisms of p53-mediated tumor suppression. Nat Rev
Cancer. 14:359–370. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wilson BG and Roberts CW: SWI/SNF
nucleosome remodelers and cancer. Nat Rev Cancer. 11:481–492. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Shen J, Ju Z, Zhao W, Wang L, Peng Y, Ge
Z, Nagel ZD, Zou J, Wang C, Kapoor P, et al: ARID1A deficiency
promotes mutability and potentiates therapeutic antitumor immunity
unleashed by immune checkpoint blockade. Nat Med. 24:556–562. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Battaglia S, Maguire O and Campbell MJ:
Transcription factor co-repressors in cancer biology: Roles and
targeting. Int J Cancer. 126:2511–2519. 2010.PubMed/NCBI
|
|
55
|
Clevers H and Nusse R: Wnt/beta-catenin
signaling and disease. Cell. 149:1192–1205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Schulze K, Imbeaud S, Letouzé E,
Alexandrov LB, Calderaro J, Rebouissou S, Couchy G, Meiller C,
Shinde J, Soysouvanh F, et al: Exome sequencing of hepatocellular
carcinomas identifies new mutational signatures and potential
therapeutic targets. Nat Genet. 47:505–511. 2015. View Article : Google Scholar : PubMed/NCBI
|