Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most
common lymphoid haematopoietic malignancy, accounting for 30–40%
cases of non-Hodgkin's lymphoma (NHL) in developed countries
(1). The occurrence of DLBCL is even
higher in developing countries. The incidence is generally greater
than 40% (2,3). The age range of patients with DLBCL is
relatively wide, and the individual differences regarding the
prognosis are clear; the median age is 60–70 years. According to
the International Prognostic Index (IPI), those older than 60 years
are considered to have poor prognostic factors. DLBCL is a highly
invasive disease with a high incidence rate (4,5). DLBCL
can be divided into two subgroups, germinal centre B-cell-like
(GCB) lymphoma and non-GCB lymphoma, which have direct implications
on the prognosis of patients. The 5-year overall survival (OS) rate
of the GCB type was 76%, and the 5-year OS rate of the non-GCB type
was 34% (6). The IPI (7) is usually used to predict the prognosis
of patients with DLBCL, and has greatly helped clinical
decision-making. Changes in the expression of certain genes in the
tumour microenvironment will affect the development of tumours and
patient prognosis. E.g. CD10, BCL6, IRF4, FOXP1 and GCET1. The IPI
presents certain limitations. The IPI score depends on the
patient's age, general condition score, clinical stage, number of
sites outside the lymph nodes, and whether lactate dehydrogenase is
normal. With the development of molecular pathology, gene
rearrangements such as MYC have also become a characteristic
genetic change of lymphoma. It is therefore crucial to investigate
the pathogenesis of DLBCL and identify some prognostic factors of
DLBCL.
The transcription factor forkhead/wingeded-helix
protein 3 (Foxp3) is the most specific biological marker of
regulatory T-cells (Tregs) (8).
Foxp3 serves a crucial role in the differentiation of Tregs, which
maintain the body's immune homeostasis and possess
immunosuppressive functions (9,10). Tregs
express high levels of CD4 and CD25 and specifically express Foxp3;
however, the expression of interleukin-7 receptor (IL-7R), also
known as CD127, in Tregs is low or negative (11). IL-7R is a specific receptor for IL-7.
Foxp3 and IL-7R can regulate the expression of downstream target
genes by directly binding to the target gene promoter region or
synergizing with other transcription factors. Foxp3 and IL-7R
participate in multiple biological functions, including tumour cell
proliferation, apoptosis, invasion, metastasis and peripheral
angiogenesis (12).
The purpose of this study was to investigate the
prognostic value of Foxp3 and IL-7R protein expression in DLBCL
tumour tissues; their association with clinicopathological
characteristics, short-term efficacy and long-term survival of
patients with DLBCL was determined. The findings from the present
study may provide new bases for the clinical treatment
decision-making and determination of patients' prognosis.
Materials and methods
Clinical data
Clinicopathological data were selected from 208
patients with DLBCL, who did not receive any treatment prior to
surgery (surgical tumour resection and biopsy) at the Department of
Pathology, First Affiliated Hospital of Xinjiang Medical University
between January 2005 and December 2012. All patients were diagnosed
and classified by two senior pathologists (Dr Wenli Cui and Mr.
Zhiyin Feng; Department of Pathology, Τhe First Affiliated
Hospital, Xinjiang Medical University, Xinjiang, China) according
to the 2008 version of the World Health Organization ‘Pathology and
Genetics of Hematopoietic and Lymphoid Tissue Neoplasms’ (13). The clinicopathological
characteristics of patients, including age, sex, tumour site, mass,
size, type, clinical stage, IPI, performance status (PS), B
symptoms (includes fever, night sweats, and weight loss),
extra-ectopic sites, serum lactate dehydrogenase (LDH) level,
treatment status, treatment efficiency and follow-up results were
collected. The longest follow-up period was 91 months. This study
was approved by the Medical Ethics Committee of the First
Affiliated Hospital of Xinjiang Medical University. Written
informed consent was obtained from each participant prior to
biopsy.
Tissue microarray
HE sections of all patients were stored in the
hospital. First, the patient cases and pathology numbers were
retrieved from the HIS system. The corresponding slices and wax
blocks were subsequently collected. The tissues were formalin-fixed
and paraffin-embedded (FFPE). One TMA contained sections from 60
patients. All HE sections were analysed by two senior pathologists
who were blinded. Two representative regions were selected for each
tissue and were marked at the same position in the corresponding
wax block. The tissue of the target wax block was punched out
vertically by using a Beecher Tissue Arrayer (Beecher Instruments)
and embedded in an acceptor wax block to prepare an array
containing 120 tissues.
IHC
The EnVision two-step method was used to detect the
expression of Foxp3 and IL-7R proteins in tissue microarrays.
Samples from the microarray were serially sliced at a thickness of
4 µm and mounted on slides. The slides were placed in a baking oven
at 37°C overnight, dewaxed with xylene (for 20 min, twice),
dehydrated with ethanol gradient (concentrations: 100, 100, 95, 80
and 70%) and washed with tap water and distilled water. The slides
were subsequently incubated with 0.3% H2O2
for 10 at room temperature to eliminate endogenous peroxidase
activity. Antigen retrieval was performed using citrate repair
buffer (pH 6.0) in a 100°C oven for 20 min. After cooling, the
tissue chips were covered in goat serum (OriGene Technologies,
Inc.) and incubated at 37°C for 20 min. The sections were incubated
with primary antibodies against Foxp3 (cat. no. ab4728; 1:100;
Abcam) and IL-7R (cat. no. TA327014; 1:50; OriGene Technologies,
Inc.), which were added dropwise according to the manufacturer's
protocol, overnight at 4°C. The tissue chips were washed with PBS
and incubated with a secondary antibody that was added dropwise
(universal kit, PV-6000, undiluted; OriGene Technologies, Inc.) for
30 at 37°C. The sections were incubated with 3′-diaminobenzidine
(Bei Jing Zhong Shan Jin Qiao) for 5 at 23°C to develop the
staining. The reaction was stopped using tap water once the slides
were stained thoroughly. The slides were counterstained using
haematoxylin (14). Gradient ethanol
(Qingdao Jisskang Biotechnology Co., Ltd.; 70, 80, 95, 100 and
100%) was used to rapidly dehydrate the samples, transparent xylene
was applied, and neutral resin was used to seal the slide. IHC
staining was scored blindly by two independent pathologists based
on the proportion of positively stained tumour cells for Foxp3 and
IL-7R and the intensity of the staining. The immunoscores ranged
between 0 and 3 as follows: i) 0, no recognizable staining,
referred to as negative (−); ii) 1, slight staining, referred to as
weak positive (+); iii) 2, moderate staining, referred to as
moderate positive (++); and iv) 3, distinct staining, referred to
as strong positive (+++) High expression of Foxp3 and IL-7R was
defined as moderate positive staining (++) and strong positive
staining (+++) for Foxp3 and IL-7R, whereas low expression of Foxp3
and IL-7R was defined as negative (−) and weak positive (+)
staining. A light microscope (DM300; Leica Microsystems GmbH;
magnifications, ×4, ×10 and ×20) was used.
Statistical analysis
SPSS 17.0 software (SPSS, Inc.) was used for
statistical analysis. Clininopathological data were analysed by the
χ2 test. Correlation analysis was performed using the
Spearman correlation method. The Kaplan-Meier method was used to
evaluate the association between Foxp3 and IL-7R expression and
other clinicopathological characteristics and patient prognosis.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Clinicopathological data
Among the 208 patients with DLBCL, 124 were male and
84 were female (male:female ratio, 1.48:1). The patient age ranged
between 34 and 79 years, and the median age was 60 years. There
were 170 cases with a tumour diameter ≤10 cm, 22 cases with a
tumour diameter >10 cm and 16 cases without data on tumour
diameter. A total of 140 and 68 patients presented with intranodal
lymphoma and extranodal lymphoma, respectively. Among the 208
patients, 110 patients were diagnosed with GCB-type lymphoma, 95
had non-GCB-type lymphoma, and 3 harboured deletion mutations.
Overall, 125 patients survived, 43 patients died and 40 patients
were lost to follow-up.
IHC staining of Foxp3 and IL-7R
The results from IHC demonstrated that Foxp3 was
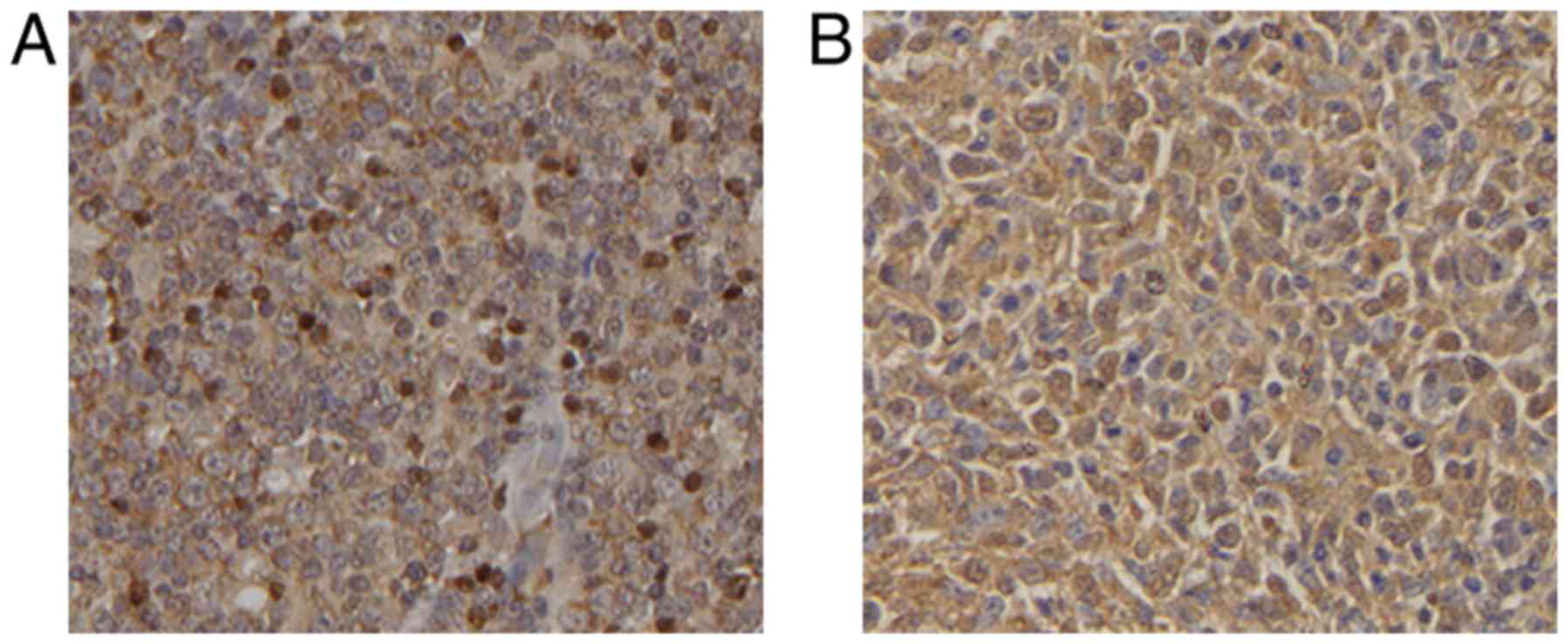
localized in the nucleus (Fig. 1A)
and IL-7R was located in the cytoplasm (Fig. 1B). Detection of Foxp3 expression in
the DLBCL tissue microarray revealed that scattered positively
stained lymphocytes were found both inside and outside of the
germinal centre, and presented with different levels of diffuse
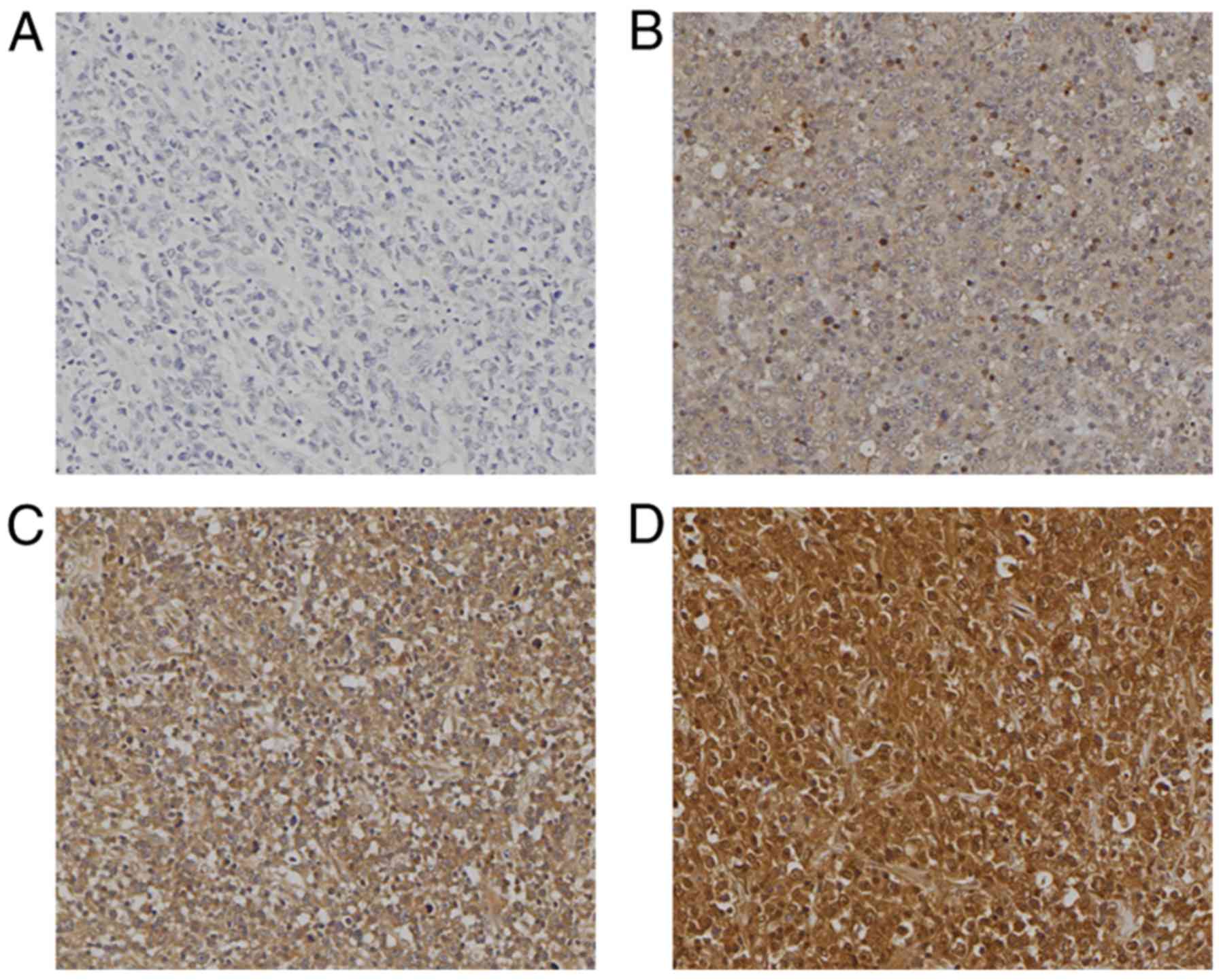
positive nuclear expression (Fig.
2). The positive staining rate was 65.7% (132/201 cases). The
strongly positive staining rate was 14.4% (29/201 cases), and high
expression was observed in 34.8% of cases (70/201 cases). Tissue
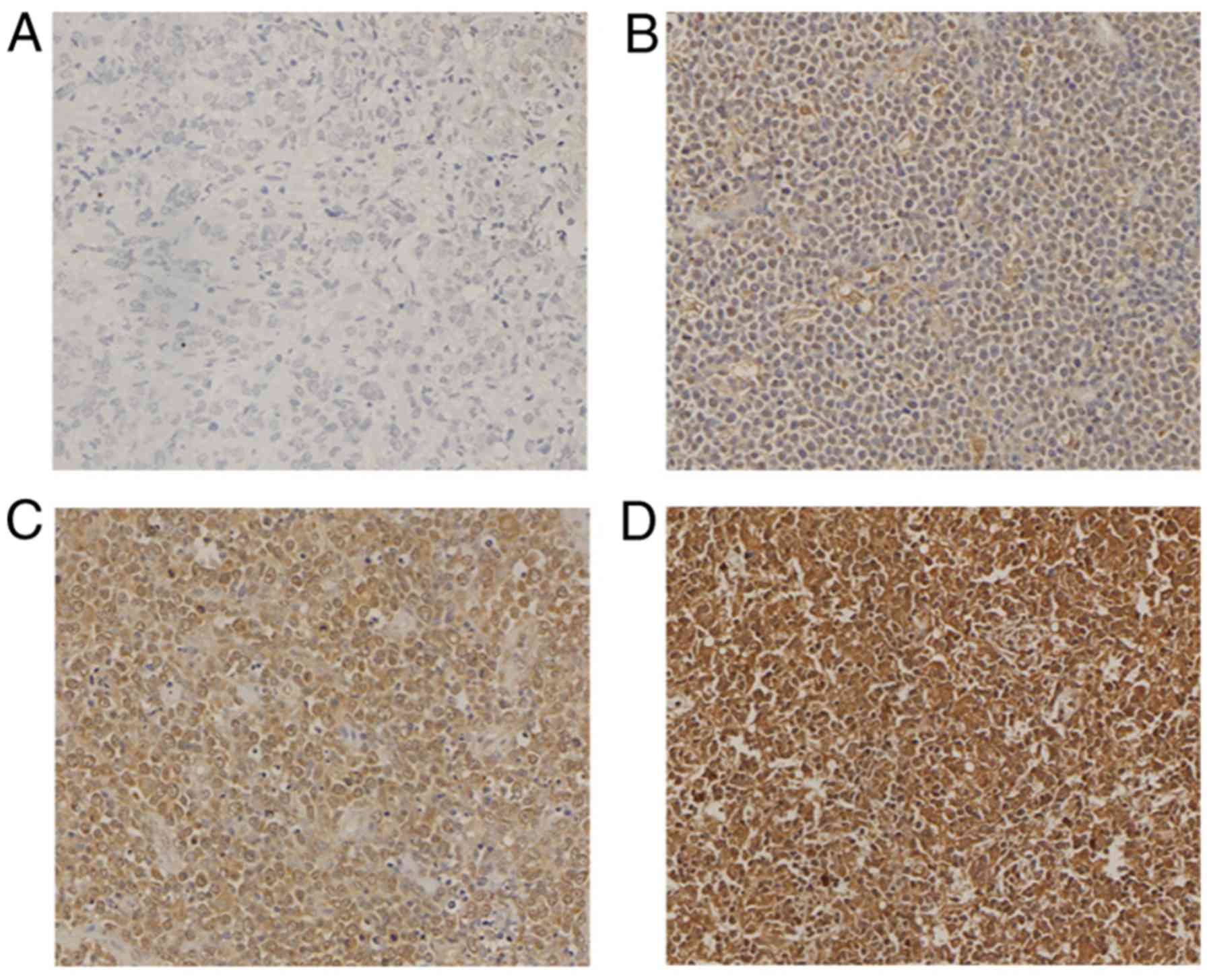
samples from 7 patients were missing. The expression of IL-7R in
the DLBCL tissue microarray was detected (Fig. 3). The positive expression rate was
94% (186/198 cases), strong positive staining was observed in
31.31% of the cases (62/198 cases), and a high expression rate was
observed in 68.2% of the cases (137/201 cases). Tissue samples from
10 patients were missing.
Association between Foxp3 and IL-7R
expression and patient clinicopathological characteristics
To determine the clinicopathological significance of
Foxp3 and IL-7R expression, the associations between Foxp3 and
IL-7R expression and patient clinicopathological characteristics,
including age, size, IPI score, PS, B symptoms and clinical stage,
were analysed using χ2 test. No significant association
was identified between Foxp3 expression and sex (P=0.464), age
(P=0.386), tumour size (P=0.528), tumour site (P=0.515), PS
(P=0.526), B symptoms (P=0.347), lymph node involvement >2
(P=0.383), LDH serum level (P=0.181) and treatment plan (P=0.218).
However, a statistically significant association was observed
between Foxp3 expression and tumour type (P=0.012), IPI score
(P=0.012), tumour stage (P=0.045) and treatment efficacy (P=0.032;
Table I). Furthermore, IL-7R
expression was not associated with sex (P=0.361), age (P=0.378),
tumour size (P=0.402), IPI score (P=0.099), PS (P=0.37), B symptoms
(P=0.088), tumour stage (P=0.212), lymph node involvement >2
(P=0.523), LDH serum level (P=0.331), treatment efficacy (P=0.552)
and treatment regimen (P=0.059). However, IL-7R expression was
associated with tumour type (P=0.001) and tumour site (P=0.008;
Table II). In addition, high Foxp3
expression was associated with non-GCB-type disease, IPI score
>0, stage 3 and 4 disease, and disease progression and stage
(PD+SD) (all P<0.05; Table I) in
patients with DLBCL. High expression of IL-7R was also associated
with extranodal lymphoma and non-GCB-type disease (P<0.05;
Table II).
 | Table I.Association between Foxp3 expression
and clinicopathological characteristics of patients with diffused
large B-cell lymphoma. |
Table I.
Association between Foxp3 expression
and clinicopathological characteristics of patients with diffused
large B-cell lymphoma.
|
|
| Foxp3
expression |
|
|
|---|
|
|
|
|
|
|
|---|
| Clinicopathological
characteristics | Number | High, n | Percentage | χ2 | P-value |
|---|
| Sex |
|
Male | 120 | 41 | 34.2 | 0.057 | 0.464 |
|
Female | 81 | 29 | 35.8 |
|
|
| Age, years |
|
≤60 | 123 | 41 | 33.3 | 0.192 | 0.386 |
|
>60 | 77 | 28 | 36.4 |
|
|
| Diameter, cm |
|
≤10 | 164 | 57 | 34.8 | 0.022 | 0.528 |
|
>10 | 22 | 8 | 36.4 |
|
|
| Location |
|
Intranodular | 136 | 47 | 34.6 | 0.013 | 0.515 |
|
Extranodular | 65 | 23 | 35.4 |
|
|
| Subtypes |
|
GCB | 105 | 29 | 27.6 | 5.857 | 0.012 |
|
Non-GCB | 93 | 41 | 44.1 |
|
|
| IPI |
| 0 | 104 | 28 | 26.9 | 5.857 | 0.012 |
|
>0 | 82 | 36 | 43.9 |
|
|
| Performance
state |
| ≤2
score | 165 | 59 | 35.8 | 0.022 | 0.526 |
| >2
score | 32 | 11 | 34.4 |
|
|
| B symptom |
| No | 99 | 37 | 37.4 | 0.294 | 0.347 |
|
Yes | 98 | 33 | 33.7 |
|
|
| Clinical stage |
| 1 and
2 | 112 | 33 | 29.5 | 3.453 | 0.045 |
| 3 and
4 | 75 | 32 | 42.7 |
|
|
| Extranodal
sites |
| ≤2 | 158 | 57 | 36.1 | 0.273 | 0.383 |
|
>2 | 29 | 9 | 31.0 |
|
|
| LDH, U/l |
|
≤240 | 103 | 32 | 31.1 | 1.141 | 0.181 |
|
>240 | 83 | 32 | 38.6 |
|
|
| Therapeutic
effect |
|
PR+CR | 90 | 31 | 34.4 | 4.488 | 0.032 |
|
PD+SD | 20 | 12 | 60.0 |
|
|
| R-CHOP
treatment |
| No | 94 | 37 | 39.4 | 0.881 | 0.218 |
|
Yes | 80 | 26 | 32.5 |
|
|
 | Table II.Association between IL-7R expression
and clinicopathological characteristics of patients with diffused
large B-cell lymphoma. |
Table II.
Association between IL-7R expression
and clinicopathological characteristics of patients with diffused
large B-cell lymphoma.
|
|
| IL-7R
expression |
|
|
|---|
|
|
|
|
|
|
|---|
| Clinicopathological
characteristics | Number | High, n | Percentage | χ2 | P-value |
|---|
| Sex |
|
Male | 118 | 80 | 67.8 | 0.267 | 0.361 |
|
Female | 80 | 57 | 71.3 |
|
|
| Age, years |
|
≤60 | 121 | 85 | 70.2 | 0.216 | 0.378 |
|
>60 | 76 | 51 | 67.1 |
|
|
| Size (diameter,
cm) |
|
≤10 | 162 | 109 | 67.3 | 0.263 | 0.402 |
|
>10 | 22 | 16 | 72.7 |
|
|
| Location |
|
Intranodular | 134 | 85 | 63.4 | 6.450 | 0.008a |
|
Extranodular | 64 | 52 | 81.3 |
|
|
| Subtypes |
|
GCB | 103 | 61 | 59.2 | 10.558 | 0.001a |
|
Non-GCB | 93 | 75 | 80.6 |
|
|
| IPI |
| 0 | 102 | 64 | 62.7 | 2.088 | 0.099 |
|
>0 | 81 | 59 | 72.8 |
|
|
| Performance
state |
| ≤2
score | 163 | 113 | 69.3 | 0.279 | 0.370 |
| >2
score | 31 | 20 | 64.5 |
|
|
| B symptom |
| No | 99 | 63 | 63.6 | 2.271 | 0.088 |
|
Yes | 95 | 70 | 73.7 |
|
|
| Clinical stage |
|
1,2 | 111 | 72 | 64.9 | 0.925 | 0.212 |
|
3,4 | 74 | 53 | 71.6 |
|
|
| Extra nodal
sites |
| ≤2 | 156 | 105 | 67.3 | 0.031 | 0.523 |
|
>2 | 29 | 20 | 69.0 |
|
|
| LDH, U/l |
|
≤240 | 101 | 66 | 65.3 | 0.356 | 0.331 |
|
>240 | 82 | 57 | 69.5 |
|
|
| Therapeutic
effect |
|
PR+CR | 90 | 57 | 63.3 | 0.020 | 0.552 |
|
PD+SD | 20 | 13 | 65.0 |
|
|
| R-CHOP
treatment |
| No | 95 | 69 | 72.6 | 2.969 | 0.059 |
|
Yes | 78 | 47 | 60.3 |
|
|
Influence of patient
clinicopathological characteristics, Foxp3 and IL-7R expression on
prognosis
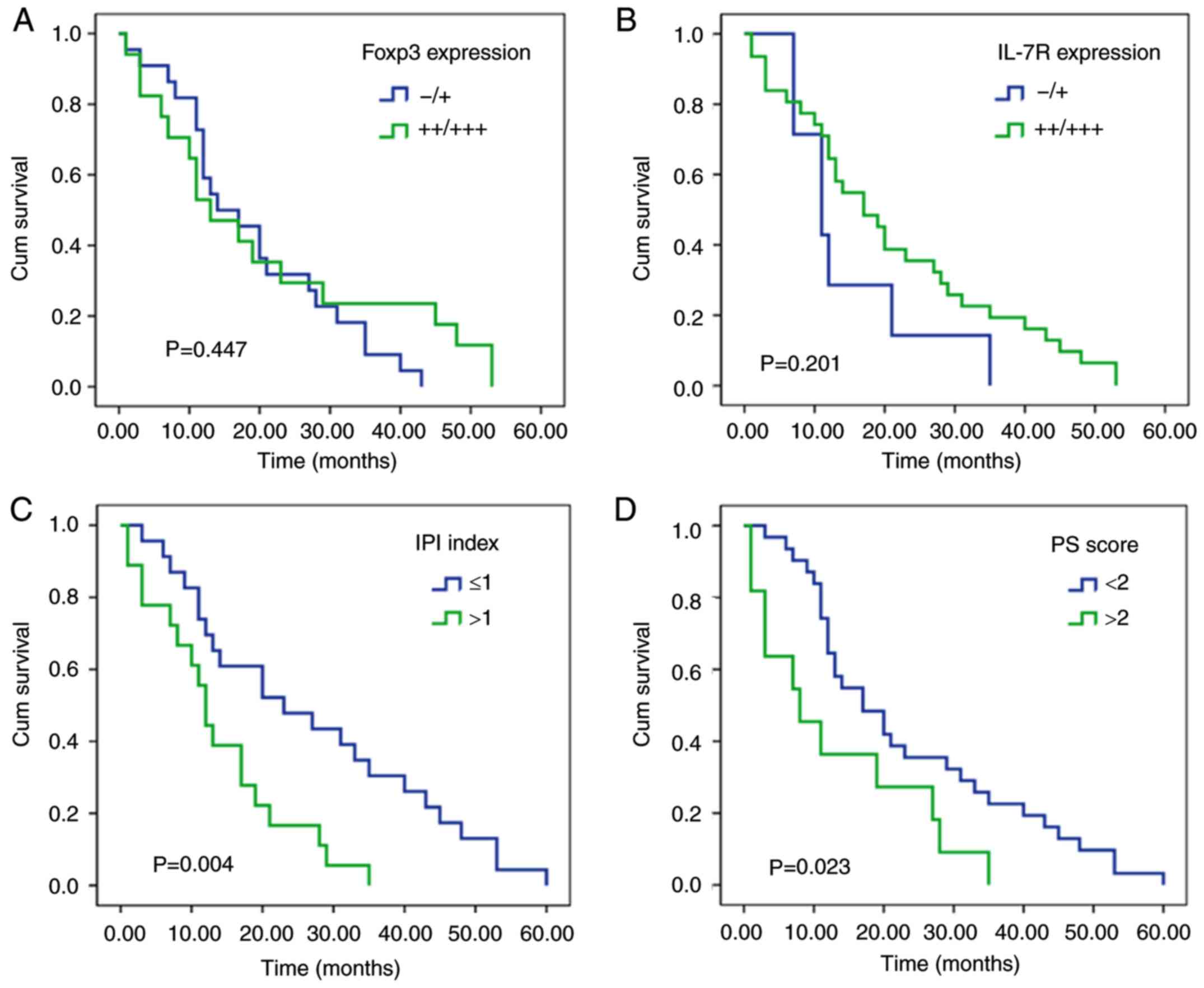
No significant associations were identified between
Foxp3 expression and the overall prognosis of patients using
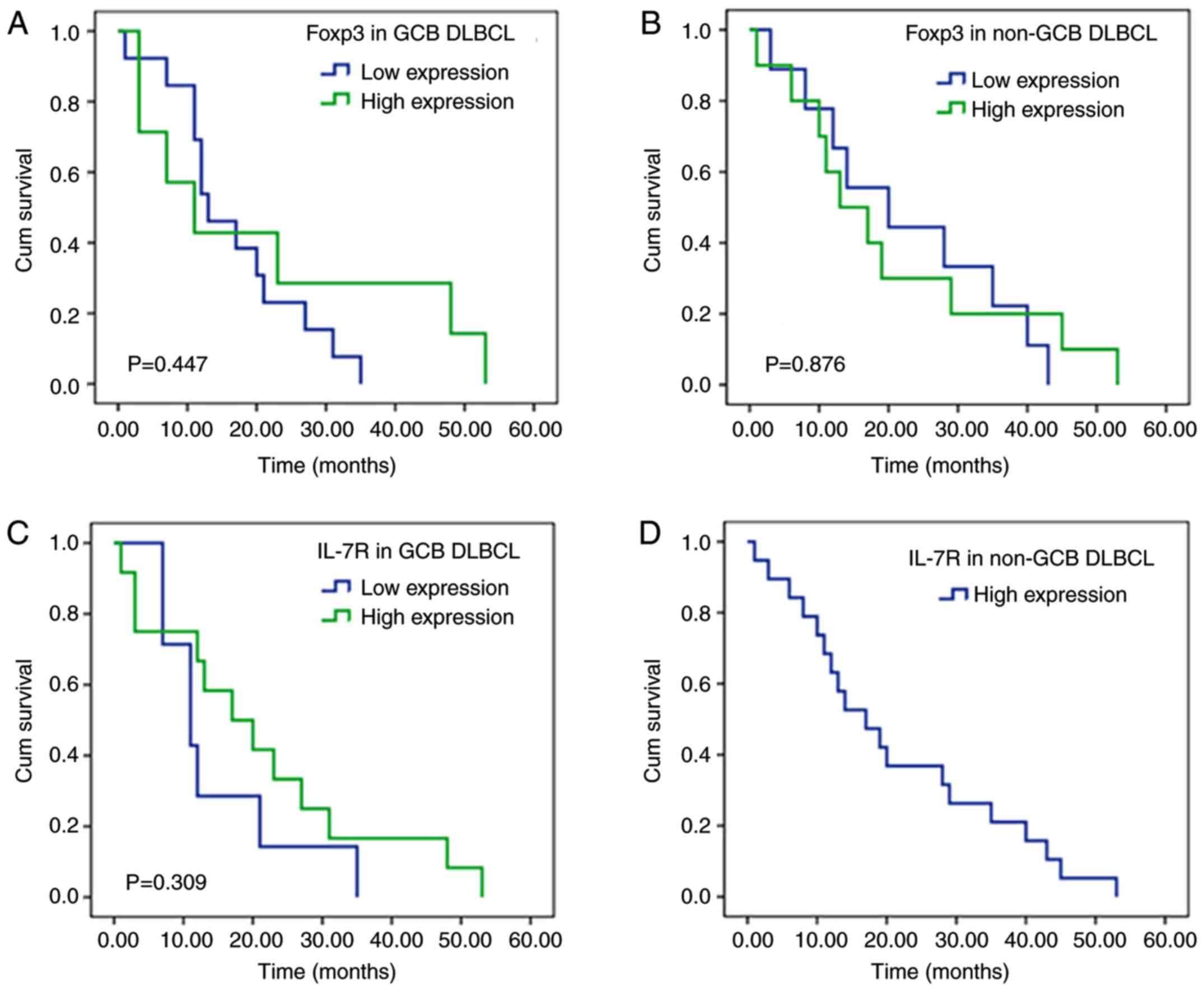
Kaplan-Meier survival curve analysis (P=0.447; Fig. 4A). IL-7R expression was also not
associated with overall prognosis (P=0.201; Fig. 4B). Subsequently, the association
between Foxp3 expression, IL-7R expression and the OS (cum
survival) of patients with GCB and non-GCB subtypes was determined;
however, the associations were not significant (Fig. 5). Kaplan-Meier survival curve
analysis demonstrated that male sex, IPI score >1, PS score
>2, presence of B symptoms, extranodal involvement in >2
sites and PS+SD were associated with short survival time (Fig. 4C-H). Spearman correlation analysis
demonstrated that expression levels of Foxp3 and IL-7R in patients
with DLBCL were weakly positively correlated (r=0.268; Table III).
 | Table III.Spearman correlation analysis of
Foxp3 and IL-7R. |
Table III.
Spearman correlation analysis of
Foxp3 and IL-7R.
|
| IL-7R
expression |
|
|
|---|
|
|
|
|
|
|---|
| Foxp3
expression | Low (%) | High (%) | R | P-value |
|---|
| Low | 51
(25.9) | 76 (38.6) | 0.268 | 0.000 |
| High | 10 (5.1) | 60 (30.5) |
|
|
Discussion
DLBCL is a lymphoid haematopoietic malignancy. The
occurrence and development of DLBCL are closely associated with
mutation or loss of regulatory factors (such as PI3K, AKT, P53,
MYC, IRF4, BCL-2 and BCL-6) involved in the cell cycle and
apoptosis (15). Tumour cell
survival depends on the interaction between multiple cells
(including tumor cells, T/B lymphocytes and dendritic cells) and
their cytokines in the tumour microenvironment (16,17).
Foxp3 belongs to the family of forkhead/winged-helix transcription
factors and is a characteristic marker located at the surface of
Tregs. Foxp3 is located on the X chromosome at Xp11.23. Its gene
contains 11 exons and 3 non-coding introns. Foxp3 is associated
with immune suppression and immune escape, and its abnormal
expression can also lead to autoimmune diseases (such as systemic
lupus erythematosus and X-linked autoimmunity-allergic
dysregulation syndrome) (18).
Previous studies have reported that Foxp3 is expressed by Tregs, as
well as in digestive tract tumours, breast cancer, pancreatic
cancer and nasopharyngeal carcinoma (19–22). The
number of genetic changes in Foxp3 is associated with the
occurrence and development of cervical cancer, kidney cancer, lung
adenocarcinoma (23), breast cancer
(24), colorectal cancer and
esophageal cancer (25). However,
the expression of Foxp3 in lymphoma remains unclear. Foxp3 has a
dual effect, and can upregulate or downregulate multiple oncogenes
in tumour cells, including ERBB2, SKP2, c-MYC, p21, and other
important cancer-associated genes, leading to transcriptional
activation or inhibition (26).
Previous studies have reported that elevated numbers of
Foxp3-positive cells in the tumour microenvironment indicate a good
prognosis for patients with DLBCL. In addition, the number of
Foxp3-positive cells in DLBCL is associated with patient prognosis
(25,27,28). The
results of the present study were similar. Detection of Foxp3 in
DLBCL tissue microarrays by IHC demonstrated that Foxp3 was not
only expressed in the tumour microenvironment (T lymphocytes) but
also presented with diffuse positive expression in DLBCL tumour
cells. The results of this study only proved that Foxp3 protein is
expressed in the nucleus of DLBCL tumour cells. However, its
expression on the surface of Tregs and on normal cells cannot be
explained. This study therefore hypothesized that in DLBCL, Foxp3
may be considered as a protein involved in Treg-independent tumour
immune escape mechanism. Foxp3 regulates the activation of
downstream target genes, which in turn allow tumour cells to evade
surveillance by the immune system (29). Foxp3 may serve as a biological marker
to predict tumour development and assess prognosis. Wang et
al (29) reported that the
tumour volume of the Foxp3 high expression group was significantly
increased in a subcutaneous tumour-bearing mouse model of
pancreatic cancer, and Foxp3 directly transcriptionally activated
the expression of the chemokine (C-C motif) ligand 5 (CCL5) in
tumour cells and recruited Foxp3-positive Tregs to the tumour
microenvironment. These phenomena subsequently inhibited cytotoxic
T cell-associated tumour destruction (29). When CCL5 inhibitors were applied, the
decreased number of Tregs in the high-Foxp3 expression group was
more apparent, and the tumour inhibition rate was higher compared
with the low-Foxp3 expression group (29). Furthermore, a previous study reported
that Foxp3 can upregulate the expression of transforming growth
factor-β in tumour cells, leading to the promotion of
epithelial-mesenchymal transition and the stimulation of tumour
cell proliferation, invasion and metastasis (30).
In the present study, according to the Hans
classification model (31), 208
cases of DLBCL were divided into GCB- and non-GCB-type cases. Foxp3
expression was detected by IHC. The results demonstrated that Foxp3
protein was expressed to different degrees in DLBCL tissues, with a
positive expression rate of 65.7% and a strong positive expression
rate of 14.4%. Subsequently, the association between Foxp3
expression and clinicopathological characteristics of patients was
analysed. High Foxp3 expression was associated with non-GCB-type
disease, IPI score >0), tumour stage 3 or 4, and PD+SD. These
results suggested that Foxp3 expression may be associated with poor
prognosis of patients with DLBCL. The results of the survival
analysis demonstrated that there was no difference in the survival
time between patients with high and low Foxp3 expression levels.
Nakayama et al (32) have
reported that a high infiltration of FOXP3-positive cells was
associated with a significantly better prognosis than patients with
low levels of FOXP3-positive cells for OS in DLBCL. In contrast, a
high infiltration of FOXP3/CTLA-4 double-positive cells was
significantly associated with a poor prognosis compared with
patients with low levels of FOXP3/CTLA-4 double-positive cells for
OS and progression-free survival. This result is inconsistent with
the findings the present study; this may be due to the variable
dilution of antibodies. Further investigation is therefore required
to determine whether Foxp3 may directly disturb tumour formation or
silence genes, affecting the occurrence, development and clinical
prognosis of DLBCL.
The IL-7R gene is located on chromosome 5q13
(33). Not only is it a member of
the erythropoietin family, but it is also a specific receptor for
IL-7. IL-R7 can stimulate haematopoietic cell proliferation and the
development of haematological malignancies, including leukaemia and
lymphoma (34,35). A study by Sasson et al
(36) demonstrated that IL-7R was
expressed on human pre-B, but not mature B-cells. Aberrant
expression of IL-7R contributes to B-cell oncogenesis, which is
consistent with the results of the present study. Furthermore,
tumour B-cells expressed IL-7R in DLBCL. Al-Rawi et al
(37) investigated the expression of
IL-7 and IL-7R in solid tumour tissues (breast cancer) and reported
that IL-7R expression is positive in cancer tissues and that IL-7R
expression is associated with tumour lymph node metastasis. Once
IL-7R binds to IL-7, new kinases are required to induce signalling.
As the intracellular domain of IL-7R lacks tyrosine kinase
activity, signalling is mainly induced through the activation of
Jak/STAT, PI3K and the Src family tyrosine kinases (38). The presence of IL-7 abrogated the
capacity of CD4+CD25+Foxp3+
regulatory T cells (Tregs) to suppress the proliferation of
conventional T cells in response to TCR activators, including
alloantigens and autoantigens. The removal of IL-7 restored the
suppressive function of Tregs. Preblocking of the IL-7R on the
Tregs also restored suppressor function, indicating that IL-7/IL-7R
directly affected Tregs function (39). Therefore, the present study
investigated the expression of Foxp3 and IL-7R in tumours and their
impact on patient OS. To investigate the role of IL-7R in DLBCL,
paraffin-embedded samples from 208 patients with DLBCL with
complete clinical medical records were examined by IHC. The results
demonstrated that IL-7R was highly expressed in DLBCL tissues,
which was similar to the results obtained by Al-Rawi et al
(37). The present study
demonstrated that IL-7R expression was higher in patients with
extranodal lymphoma compared with non-extranodal lymphoma
(P<0.05) and higher in patients with non-GCB-type disease
compared with those with GCB-type disease (P<0.05). IL-7R
expression may therefore be associated with tumour metastasis. The
results of the survival analysis demonstrated no significant
differences in the OS between patients with high and low IL-7R
expression. In addition, there was no association between IL-7R
expression and IPI >0 score (P=0.099), B symptoms (P=0.088) or
insensitivity to the R-CHOP (40)
protocol (P=0.059). However, a trend could be detected; when IPI
score was high, a B symptom was present, the patient was not
sensitive to R-CHOP and IL-7R expression was higher. These results
suggested that IL-7R may be involved in the poor prognosis of
patients with DLBCL. Further investigation with increased sample
size is required to verify these results.
Tregs express high levels of CD4 and CD25, express
Foxp3 specifically, and a low level or no IL-7R (11). Thus, IL-7R may also be a specific
marker of Tregs (36). The present
study demonstrated that Foxp3 and IL-7R was not only expressed on
the surface of Treg cells, but also presented with different
degrees of positive expression in DLBCL tissues. Furthermore, a
weak positive correlation between Foxp3 and IL-7R expression in
DLBCL tissues was identified. Foxp3 and IL-7R may represent a
tumour immune escape mechanism independent of Tregs. Foxp3 and
IL-7R regulate the activation of downstream target genes, leading
to the escape of tumour cells from the surveillance of the immune
system (29,38). The present study also analysed the
association between clinicopathological characteristics of patients
with DLBCL and their prognosis. Male sex, IPI index >1, PS score
>2, presence of a B symptom, extranodal involvement in >2
sites, and PD+SD were associated with decreased survival time. This
study demonstrated that Foxp3 and IL-7R exhibited different degrees
of positive expression in DLBCL tissues, and the difference was
statistically significant. The establishment of an
immunosuppressive microenvironment involving Foxp3 and IL-7R may
therefore represent one mechanism involved in DLBCL development.
The results from this study may provide novel targets for the
development of anti-tumour immunotherapy and help clinicians make
appropriate medication choices at an individual level. However, the
number of cases was limited. The expression of Foxp3 and IL-7R in
DLBCL and the clinicopathological parameters were analysed at an
in vitro tissue level. This study lacked the use of in
vivo mouse experiments and in vitro cell experiments to
confirm the findings.
In conclusion, the expression of Foxp3 and IL-7R in
DLBCL tissues was associated with tumour type. In addition, Foxp3
and IL-7R expression in patients with non-GCB-type disease was
significantly higher compared with patients with GCB-type disease.
The expression of Foxp3 and IL-7R may therefore help the
development of individualized treatment, prognostic prediction and
therapy stratification.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. NSFC 81560035), the
Science and Technology Talents Training Project of Xinjiang Uyghur
Autonomous Region (grant nos. qn2015bs011 and 2018Q047) and the
Science and Nature Foundation of Xinjiang Uyghur Autonomous Region
(grant no. 2014211C032).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YZ participated in the experiment, analysed the
data, and was a major contributor in writing the manuscript. WLC
interpreted the results of immunohistochemical experiments. ZYF
interpreted the data. JX and AG selected wax blocks and performed
immunohistochemistry experiments. WZ designed experimental
procedures. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Medical Ethics
Committee of the First Affiliated Hospital of Xinjiang Medical
University (approval no. IACUC-20150225-85). Written informed
consent was obtained from all participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Herreros B, Sanchez-Aguilera A and Piris
MA: Lymphoma microenvironment: Culprit or innocent? Leukemia.
22:49–58. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
A clinical evaluation of the International
Lymphoma Study Group classification of non-Hodgkin's lymphoma. The
Non-Hodgkin's Lymphoma Classification Project. Blood. 89:3909–3918.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Krol AD, le Cessie S, Snijder S,
Kluin-Nelemans JC, Kluin PM and Noordijk EM: Primary extranodal
non-Hodgkin's lymphoma (NHL): The impact of alternative definitions
tested in the Comprehensive cancer centre west population-based NHL
registry. Ann Oncol. 14:131–139. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chiu BC and Hou N: Epidemiology and
etiology of non-hodgkin lymphoma. Cancer Treat Res. 165:1–25. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hamlin PA, Satram-Hoang S, Reyes C, Hoang
KQ, Guduru SR and Skettino S: Treatment patterns and comparative
effectiveness in elderly diffuse large B-cell lymphoma patients: A
surveillance, epidemiology, and end results-medicare analysis.
Oncologist. 19:1249–1257. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Swerdlow SH, Campo E, Pileri SA, Harris
NL, Stein H, Siebert R, Advani R, Ghielmini M, Salles GA, Zelenetz
AD and Jaffe ES: The 2016 revision of the World Health Organization
classification of lymphoid neoplasms. Blood. 127:2375–2390. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yim SK, Yhim HY, Han YH, Jeon SY, Lee NR,
Song EK, Jeong HJ, Kim HS and Kwak JY: Early risk stratification
for diffuse large B-cell lymphoma integrating interim Deauville
score and International Prognostic Index. Ann Hematol.
98:2739–2748. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Farinha P, Al-Tourah A, Gill K, Klasa R,
Connors JM and Gascoyne RD: The architectural pattern of
FOXP3-positive T cells in follicular lymphoma is an independent
predictor of survival and histologic transformation. Blood.
115:289–295. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mailloux AW and Young MR: Regulatory
T-cell trafficking: From thymic development to tumor-induced immune
suppression. Crit Rev Immunol. 30:435–447. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang K and Vella AT: Regulatory T cells
and cancer: A two-sided story. Immunol Invest. 45:797–812. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee
MR, Zhu S, Gottlieb PA, Kapranov P, Gingeras TR, Fazekas de St
Groth B, et al: CD127 expression inversely correlates with FoxP3
and suppressive function of human CD4+ Treg cells. J Exp
Med. 203:1701–1711. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang H, Pan K and Xia JC: Interaction of
indoleamine-2,3-dioxyagnase and CD4+CD25+
regulatory T cells in tumor immune escape. Ai Zheng. 28:184–187.
2009.PubMed/NCBI
|
|
13
|
Campo E, Swerdlow SH, Harris NL, Pileri S,
Stein H and Jaffe ES: The 2008 WHO classification of lymphoid
neoplasms and beyond: Evolving concepts and practical applications.
Blood. 117:5019–5032. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cui W, Zheng S, Liu Z, Wang W, Cai Y, Bi
R, Cao B and Zhou X: PIK3CA expression in diffuse large B cell
lymphoma tissue and the effect of its knockdown in vitro. Onco
Targets Ther. 10:2239–2247. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cui W, Cai Y, Wang W, Liu Z, Wei P, Bi R,
Chen W, Sun M and Zhou X: Frequent copy number variations of
PI3K/AKT pathway and aberrant protein expressions of PI3K subunits
are associated with inferior survival in diffuse large B cell
lymphoma. J Transl Med. 12:102014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Keane C, Gould C, Jones K, Hamm D,
Talaulikar D, Ellis J, Vari F, Birch S, Han E, Wood P, et al: The
T-cell receptor repertoire influences the tumor microenvironment
and is associated with survival in aggressive B-cell lymphoma. Clin
Cancer Res. 23:1820–1828. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gomez-Gelvez JC, Salama ME, Perkins SL,
Leavitt M and Inamdar KV: Prognostic impact of tumor
microenvironment in diffuse large b-cell lymphoma uniformly treated
with R-chop chemotherapy. Am J Clin Pathol. 145:514–523. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang J, Wei B, Hu H, Liu F, Tu Y, Zhao M
and Wu D: Preliminary study on decreasing the expression of FOXP3
with miR-155 to inhibit diffuse large B-cell lymphoma. Oncol Lett.
14:1711–1718. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hinz S, Pagerols-Raluy L, Oberg HH,
Ammerpohl O, Grüssel S, Sipos B, Grützmann R, Pilarsky C,
Ungefroren H, Saeger HD, et al: Foxp3 expression in pancreatic
carcinoma cells as a novel mechanism of immune evasion in cancer.
Cancer Res. 67:8344–8350. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zuo T, Wang L, Morrison C, Chang X, Zhang
H, Li W, Liu Y, Wang Y, Liu X, Chan MW, et al: FOXP3 is an X-linked
breast cancer suppressor gene and an important repressor of the
HER-2/ErbB2 oncogene. Cell. 129:1275–1286. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang LH, Su L and Wang JT: Correlation
between elevated FOXP3 expression and increased lymph node
metastasis of gastric cancer. Chin Med J (Engl). 123:3545–3549.
2010.PubMed/NCBI
|
|
22
|
Triulzi T, Tagliabue E, Balsari A and
Casalini P: FOXP3 expression in tumor cells and implications for
cancer progression. J Cell Physiol. 228:30–35. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kinoshita F, Takada K, Yamada Y, Oku Y,
Kosai K, Ono Y, Tanaka K, Wakasu S, Oba T, Osoegawa A, et al:
Combined evaluation of tumor-infiltrating cd8 + and foxp3 +
lymphocytes provides accurate prognosis in stage ia lung
adenocarcinoma. Ann Surg Oncol. 262019.(Epub ahead of print).
|
|
24
|
Zhao X, Li Y, Wang X, Wu J, Yuan Y, Lv S
and Ren J: Synergistic association of FOXP3+ tumor
infiltrating lymphocytes with CCL20 expressions with poor prognosis
of primary breast cancer: A retrospective cohort study. Medicine
(Baltimore). 98:e184032019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shang B and Liu Y, Jiang SJ and Liu Y:
Prognostic value of tumor-infiltrating FoxP3+ regulatory
T cells in cancers: A systematic review and meta-analysis. Sci Rep.
5:151792015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Katoh H, Zheng P and Liu Y: Signalling
through FOXP3 as an X-linked tumor suppressor. Int J Biochem Cell
Biol. 42:1784–1787. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Won KY, Kim GY, Kim HK, Choi SIl, Kim SH,
Bae GE, Lim JU and Lim SJ: Tumoral FOXP3 expression is associated
with favorable clinicopathological variables and good prognosis in
gastric adenocarcinoma: The tumor suppressor function of tumoral
FOXP3 is related with the P21 expression in gastric adenocarcinoma.
Hum Pathol. 68:112–118. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hu G, Li Z and Wang S: Tumor-infiltrating
FoxP3(+) Tregs predict favorable outcome in colorectal cancer
patients: A meta-analysis. Oncotarget. 8:75361–75371.
2017.PubMed/NCBI
|
|
29
|
Wang X, Lang M, Zhao T, Feng X, Zheng C,
Huang C, Hao J, Dong J, Luo L and Li X: Cancer-FOXP3 directly
activated CCL5 to recruit FOXP3(+)Treg cells in pancreatic ductal
adenocarcinoma. Oncogene. 36:3048–3058. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang L, Xu J, Zhang X, Zhang Y, Wang L,
Huang X and Xu Z: The role of tumoral foxp3 on cell proliferation,
migration, and invasion in gastric cancer. Cell Physiol Biochem.
42:1739–1754. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Berglund M, Thunberg U, Amini RM, Book M,
Erlanson M, Linderoth J, Dictor M, Jerkeman M, Cavallin-Ståhl E,
Sundström C, et al: Evaluation of immunophenotype in diffuse large
B-cell lymphoma and its impact on prognosis. Mod Pathol.
18:1113–1120. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nakayama S, Yokote T, Akioka T, Hiraoka N,
Nishiwaki U, Miyoshi T, Iwaki K, Takayama A, Masuda Y, Hatooka J,
et al: Infiltration of effector regulatory T cells predicts poor
prognosis of diffuse large B-cell lymphoma, not otherwise
specified. Blood Adv. 1:486–493. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fry TJ and Mackall CL: The many faces of
IL-7: From lymphopoiesis to peripheral T cell maintenance. J
Immunol. 174:6571–6576. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Qin JZ, Zhang CL, Kamarashev J, Dummer R,
Burg G and Dobbeling U: Interleukin-7 and interleukin-15 regulate
the expression of the bcl-2 and c-myb genes in cutaneous T-cell
lymphoma cells. Blood. 98:2778–2783. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Takakuwa T, Nomura S, Matsuzuka F, Inoue H
and Aozasa K: Expression of interleukin-7 and its receptor in
thyroid lymphoma. Lab Invest. 80:1483–1490. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sasson SC, Smith S, Seddiki N, Zaunders
JJ, Bryant A, Koelsch KK, Weatherall C, Munier ML, McGinley C,
Yeung J, et al: IL-7 receptor is expressed on adult pre-B-cell
acute lymphoblastic leukemia and other B-cell derived neoplasms and
correlates with expression of proliferation and survival markers.
Cytokine. 50:58–68. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Al-Rawi MA, Mansel RE and Jiang WG:
Interleukin-7 (IL-7) and IL-7 receptor (IL-7R) signalling complex
in human solid tumours. Histol Histopathol. 18:911–923.
2003.PubMed/NCBI
|
|
38
|
Görgün G, van der Spek J, Cosenza L,
Menevse A and Foss F: Altered biological activity associated with
C-terminal modifications of IL-7. Cytokine. 20:17–22. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Heninger AK, Theil A, Wilhelm C, Petzold
C, Huebel N, Kretschmer K, Bonifacio E and Monti P: IL-7 abrogates
suppressive activity of human
CD4+CD25+FOXP3+ regulatory T cells
and allows expansion of alloreactive and autoreactive T cells. J
Immunol. 189:5649–5658. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Coiffier B and Sarkozy C: Diffuse large
B-cell lymphoma: R-CHOP failure-what to do? Hematology Am Soc
Hematol Educ Program. 2016:366–378. 2016. View Article : Google Scholar : PubMed/NCBI
|