Introduction
Liver cancer, primarily known as hepatocellular
carcinoma (HCC), is the fifth most common cancer worldwide.
Furthermore, >780,000 new cases of liver cancer were estimated
worldwide in 2012 (1). Various
injury factors, including hepatitis B virus (HBV) infection
(2), chronic aflatoxin intake
(3) and alcohol consumption
(4), could induce long-term chronic
inflammation and promote carcinogenesis. Therefore, inflammation
and inflammatory pathways play a role in the occurrence and
development of liver cancer, indicating that anti-inflammatory
cytokines or drugs will be a potential way for the prevention and
treatment of liver cancer (5).
The new interleukin anti-inflammatory cytokine IL-37
was first cloned in 2000 and officially named in 2010 (6). IL-37 exerted anti-inflammatory and
immunomodulatory effects by inhibiting a variety of
pro-inflammatory factors, including IL-6, IL-1α, IL-1β and TNF-α
(6). It has been reported that IL-37
can relieve the inflammatory response in a variety of diseases,
such as colitis (7), rheumatoid
arthritis (8), allergic disease
(9), leprosy (10), systemic lupus erythematous (11) and transplantation rejection (12). Certain studies have also demonstrated
that IL-37 can protect the liver from ischemia-reperfusion injury
(13) and inhibit T cell-dependent
liver injury (14). Given the close
association between liver inflammation and liver cancer, IL-37 may
play a crucial part in the occurrence and development of liver
cancer.
The role of IL-37 in liver cancer currently remains
unclear. In previous studies, IL-37 has been demonstrated to
inhibit the proliferation and invasion of cervical cancer cells
through STAT3, and promoted cell apoptosis through Bcl-2-like
protein 11 (15,16). In non-small cell lung cancer and
renal cell carcinoma, IL-37 played an anticancer role through STAT3
(17). In gallbladder carcinoma,
IL-37 acted as a tumor suppressor by inhibiting cell migration and
invasion (18). In another study,
IL-37 and CD66b+ TANs (tumor-associated neutrophils) were
considered as independent factors for evaluating the prognosis of
colorectal cancer (19). In HCC,
IL-37 was associated with CD57+ natural killer cells (20). However, to the best of our knowledge,
data remain limited in further understanding the role of IL-37 in
HCC. NF-κB functions as not only a transcription factor in the
process of inflammation but also an important regulator of
tumorigenesis in HCC. The present study focused on the significance
of IL-37 and NF-κB expression in HCC, paracancerous tissues (PT)
and liver cancer cell lines. The present study aimed to investigate
the expression of IL-37 in hepatocellular carcinoma, paracancerous
tissues and liver cancer cell lines, and the association between
IL-37 and NF-κB expression.
Materials and methods
Specimens
The present study was reviewed and approved by the
Medical Ethics Committee of Foshan First People's Hospital, Foshan,
China [approval no. L(2016)3]. All patients agreed to the use of
their samples in the present study and provided written informed
consent. All personal information will be kept confidential and not
disclosed. Slices of specimens of 65 HCC and 65 PT (2 cm from tumor
tissue) were collected from the Department of the Pathology of
Foshan First People's Hospital. Among 65 cases of HCC, there were
51 males and 14 females. Their ages ranged from 16–89, with an
average age of 48.8±10.5 years, and a median age of 51 years. There
were 17 cases of AFP negative (≤8.1 ng/ml) and 28 cases of AFP
positive. All subjects were diagnosed with HCC for the first time
and did not receive radiotherapy or chemotherapy. Patients with
diabetes, autoimmune diseases or other diagnosed serious or chronic
inflammatory diseases, including colitis or pneumonia, were
excluded from this study.
Materials
Human liver cancer cell lines, HepG2 and MHCC97H
were purchased from the Cell Bank of Typical Culture Preservation
Committee of Chinese Academy of Sciences (Shanghai, China), where
the cell lines were authenticated via STR profiling. IL-37 gene
(NM_014439) was cloned into a pEZ-M02 vector (Genecopoeia, Inc.) by
the manufacturer. Lipofectamine 3000 was purchased from Invitrogen;
Thermo Fisher Scientific, Inc. Rabbit anti-human IL-37 (cat. no.
ab153889), NF-κB (cat. no. D14E12) and GAPDH primary antibody (cat.
no. AB-P-R 001) were obtained from Abcam, Cell Signaling
Technology, Inc., and Goodhere Technology, respectively.
HRP-labeled goat anti-mouse IgG (cat. no. A0216), HRP-labeled Goat
Anti-Rabbit IgG (cat. no. A0208), ECL kit, BCA Protein Quantitative
kit, RIPA buffer, SDS-PAGE kit and DAPI staining kit were obtained
from Beyotime Biotechnology. PVDF membranes were purchased from EMD
Millipore.
Immunohistochemistry (IHC)
IHC was performed as described in a previous study
(21). Briefly, the
paraffin-embedded sections (4 µm thick) were dewaxed with xylene
and rehydrated using decreasing gradient of alcohol (100, 95, 85
and 75%). Antigen retrieval was performed under high pressure at
115°C for 6 min. The samples were incubated with primary antibodies
against IL-37 (1:100) and NF-κB (1:100) overnight at 4°C. Samples
were visualized following incubation with 3′-diaminobenzidine at
room temperature for 30–90 sec. In addition, sections were
counterstained with hematoxylin at room temperature for 3–10 min.
PBS served as a control instead of the primary antibody.
The following method was used for IHC analysis: Five
high-power visual fields (magnification ×40; under a light
microscope) with a large number of parenchymal cells were randomly
selected on each slice. Then, the strong positive, weak positive
and negative results were determined by the intensity of positive
staining and the percentage of positive cells. The intensity of
positive staining was: Score of 0, no coloring; score of 1, pale
yellow; score of 2, brown-yellow; score of 3, tan. The percentage
of positive cells was: Score of 1, the percentage of positive cells
<30%; score of 2, the percentage of positive cells ≤30–60%;
score of 3, the percentage of positive cells >60%. The final
results were determined by the sum of the scores of the intensity
of positive staining and percentage of positive cells: Score of 0,
negative (−); score of 2–3, weakly positive (+); score of 4–5,
moderately positive (++) and score of 6, strong positive (+++).
Cell culture and gene
transfection
Human liver cancer cells were cultured in Dulbecco's
modified Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific,
Inc.) supplemented with 10% fetal bovine serum (lot no. D213FD0254;
BBI Life Sciences Corporation), 100 mg/ml penicillin and 100 U/ml
streptomycin, and cultured in a wet incubator containing 5%
CO2 at 37°C. For gene transfection, pEZ-M02 or
pEZ-M02-IL-37 vector was transfected into cells by Lipofectamine
3000. PEZ-M02 was an empty vector and used as the control plasmid
for transfection. A total of 5×105 cells were
transfected with 1.5 µl/ml Lipofectamine 3000 and 3 µl/ml auxiliary
reagent (Invitrogen; Thermo Fisher Scientific, Inc.; cat. no.
L3000015), and 1.5 µg/ml plasmid. Cells were subsequently cultured
for 48 h and harvested for immunofluorescence (IF) and western blot
analyses.
IF
HepG2 and MHCC97H cells were seeded on cover glass
(5×104 cells/slide) previously sterilized (121°C, 0.12
MPa, 30 min) and soaked in 1× poly-L-lysine for 10 min. After 2
days, the cells were fixed with 100% methanol for 5 min at room
temperature. Then the cells were permeabilized with Triton X-100
for 10 min at room temperature and blocked in the animal non-immune
serum [reagent B, UltraSensitive™ SP (Mouse/Rabbit) IHC Kit from
MXB Biotechnologies] for 1 h at room temperature. The cells were
incubated with the primary antibodies against IL-37 (1:100) and
NF-κB (1:100) at 37°C for 1 h. Finally, cells were washed three
times with PBS and stained with Dylight 594-conjugated secondary
antibody (1:100; cat. no. A23420; Abbkine Scientific Co., Ltd.) for
1 h. Images were analyzed under a fluorescence microscope
(magnification ×40). Staining intensity was calculated using ImageJ
software (version 1.52p; National Institutes of Health).
Western blot analysis
HepG2 and MHCC97H cells were lysed in RIPA buffer
(cat. no. P0013C; Beyotime Institute of Biotechnology) on ice,
collected into tubes and centrifuged at 12,000 × g for 20 min at
4°C. The supernatants were then boiled and protein concentrations
were determined using a BCA assay. An equal amount of protein (200
µg per lane) was separated via SDS-PAGE (10% gel) and transferred
onto PVDF membranes. Membranes were blocked in PBS containing 5%
powdered milk and 0.05% Tween 20 for 1 h at 25°C. Membranes were
then incubated overnight at 4°C with antibodies against IL-37
(1:500), NF- κB (1:1,000), and GAPDH (1:500). After being washed
wish PBST, the membranes were incubated with goat anti-rabbit
horseradish peroxidase-conjugated secondary antibody (1:1,000; cat.
no. A0208; Beyotime Institute of Biotechnology) for 1 h and
visualized using ECL kit under the Amersham Imager 600 automated
chemiluminescence image analysis system (Roche Diagnostics). The
amount of target protein was calculated by gray scanning using
Image J software (version 1.52p, National Institutes of
Health).
Statistical analysis
The results were analyzed using SPSS software
(version 19.0; SPSS, Inc.) and expressed as the mean ± standard
deviation. The differences were compared by χ2 test,
student's t-test or Spearman correlation. P<0.001, P<0.01 or
P<0.05 were considered to indicate statistically significant
difference.
Results
Protein expression of IL-37 and NF-κB
in HCC and PT
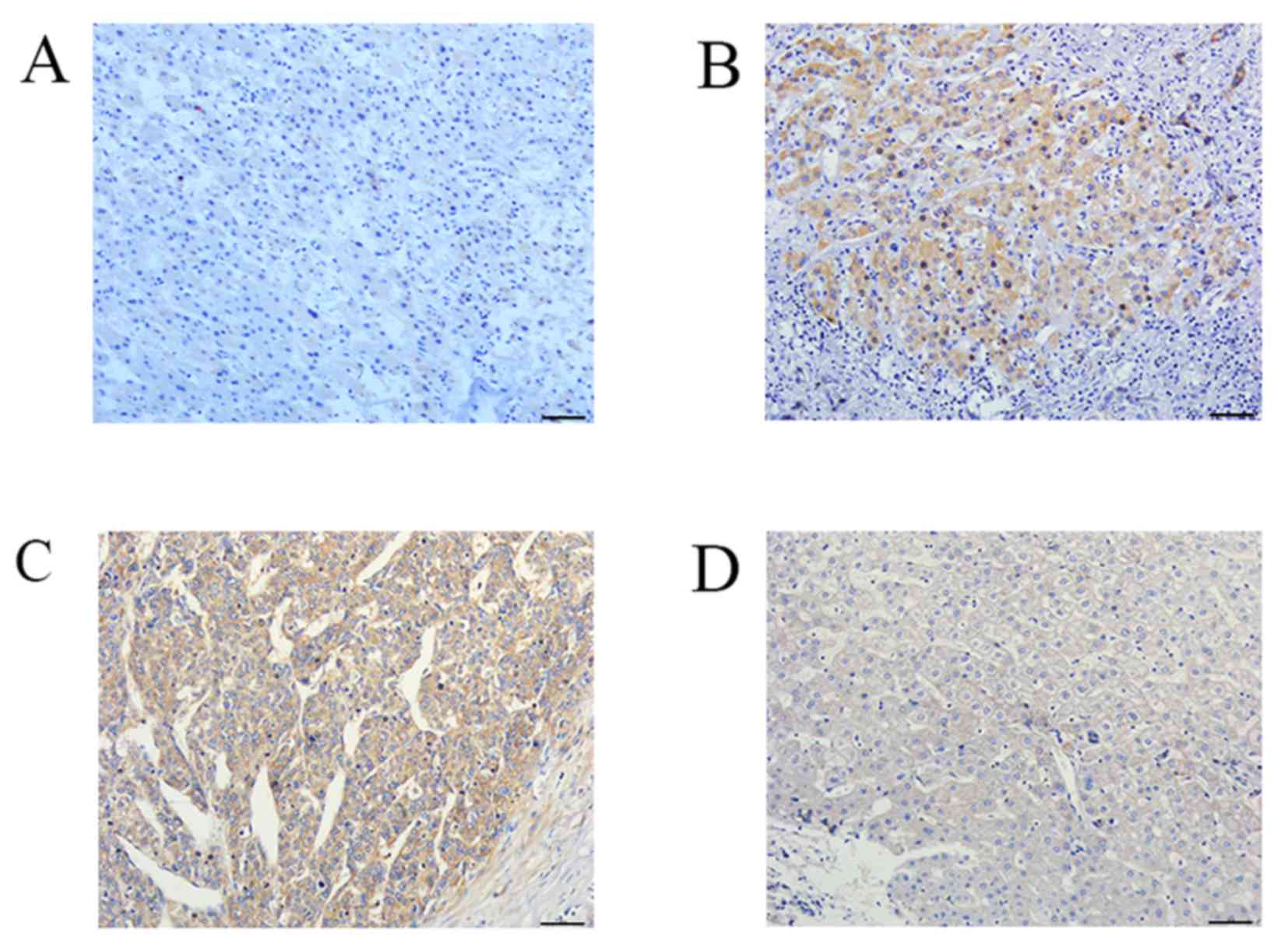
In HCC, IL-37 protein expression was at a low level
with weak positive staining (Fig.
1A). The positive rate was 21.5% (14/65). In PT, the moderately
positive staining of IL-37 protein was 27.7% (18/65), and the
strongly positive was 30.8% (20/65) (Fig. 1B). The positive rate was 81.5%
(53/65). There was a significant difference in the expression of
IL-37 between HCC and PT (χ2=55.05, P<0.001; Table I).
 | Table I.Expression of IL-37 protein in 65
cases of HCC and paracancerous tissues. |
Table I.
Expression of IL-37 protein in 65
cases of HCC and paracancerous tissues.
|
|
| IL-37 expression |
|---|
|
|
|
|
|---|
| Group | n (100%) | − | + | ++ | +++ | χ2 | P-value |
|---|
| HCC tissues | 65 | 51 (78.5) | 12 (18.5) | 0 (0.0) | 2 (3.1) | 55.05 | <0.001 |
| Paracancerous
tissue | 65 | 12 (18.5) | 18 (27.7) | 15 (23.1) | 20 (30.8) |
|
|
For NF-κB expression, strong positive expression in
HCC tissues (Fig. 1C) and weak
positive expression in PT (Fig. 1D)
was observed. NF-κB protein expression was observed in the
cytoplasm and nucleus. Notably, the expression rate of NF-κB
protein in both HCC and PT was 95.4% (62/65). However, the positive
intensity of NF-κB in HCC tissues was significantly different from
that in PT (χ2=25.966, P<0.001; Table II)
 | Table II.Expression of NF-κB protein in 65
cases of HCC and paracancerous tissues. |
Table II.
Expression of NF-κB protein in 65
cases of HCC and paracancerous tissues.
|
|
| NF-κB
expression |
|---|
|
|
|
|
|---|
| Group | n (100%) | − | + | ++ | +++ | χ2 | P-value |
|---|
| HCC tissues | 65 | 3 (4.6) | 13 (20.0) | 17 (26.2) | 32 (49.2) | 25.966 | <0.001 |
| Paracancerous
tissue | 65 | 3 (4.6) | 28 (43.1) | 28 (43.1) | 6 (9.2) |
|
|
Gene transfection of IL-37 in liver
cancer cell lines increases the protein expression of IL-37 and
decreases NF-κB through IF staining
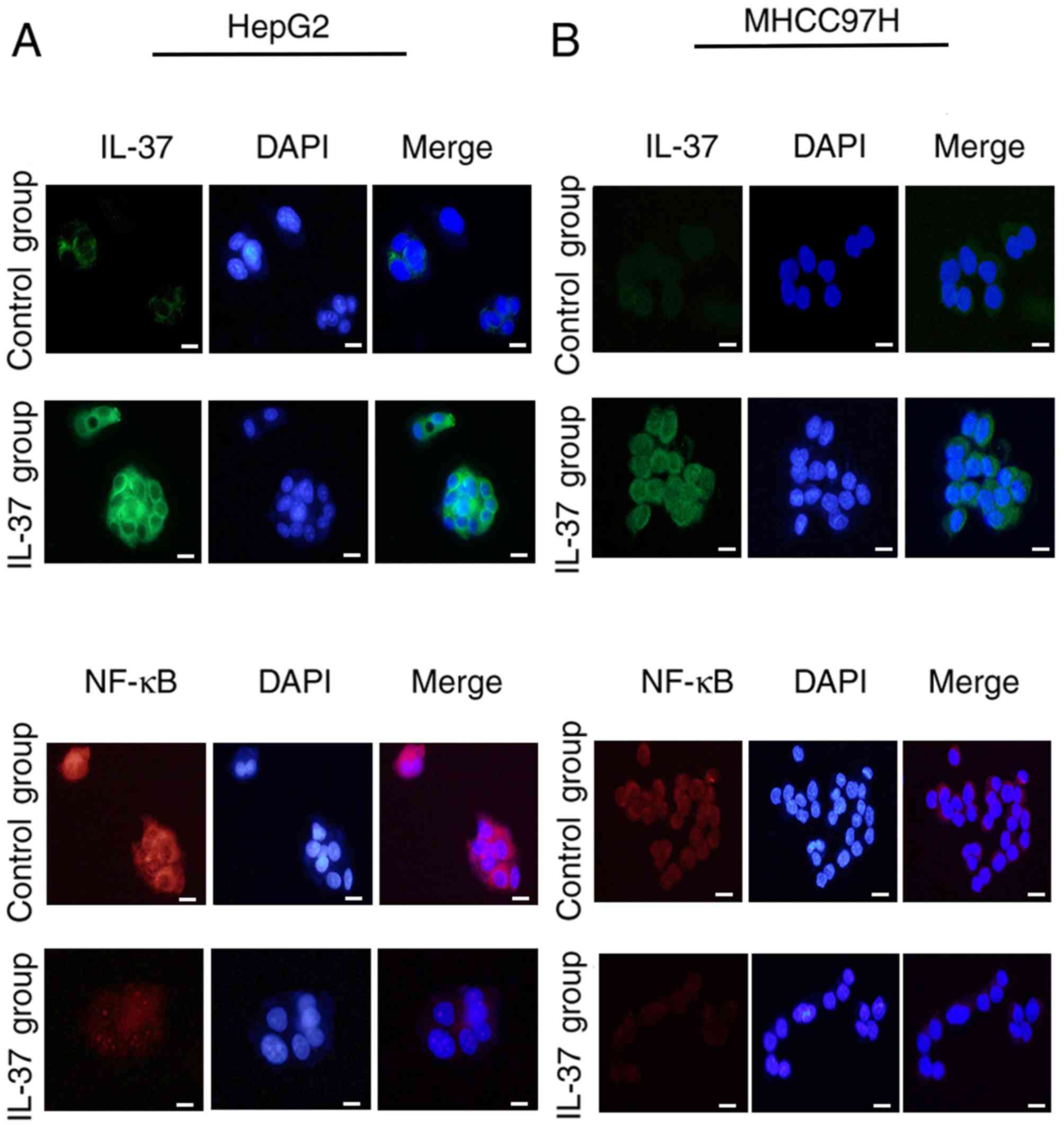
In order to assess whether the protein expression
levels of liver cancer cell lines was similar to that of the HCC
tissues, IF was performed in the present study. Weak positive IL-37
and strong positive NF-κB were observed in both of the two cell
lines (Fig. 2), which was consistent
with that of the IHC results. Furthermore, plasmids overexpressing
IL-37 were constructed and transfected into HepG2 (Fig. 2A) and MHCC97H cells (Fig. 2B). The results revealed that the
fluorescence intensity of IL-37 was significantly increased and
higher than that in the control group (Fig. 2A and B). The fluorescence intensity
of NF-κB was markedly decreased by IL-37 expression, indicating
that IL-37 inhibited the protein expression of NF-κB in both of the
two cell lines (Fig. 2A and B).
Association between expression of
IL-37 protein and clinical indexes in HCC and PT
The expression of IL-37 protein in HCC was
associated with serum AFP (χ2=6.522, P=0.039) but not
with other clinicopathological features (P>0.05; Table III). In AFP-negative HCC, the weak
positive and strong positive expression rates of IL-37 protein were
23.5% (4/17) and 11.8% (2/17), respectively. In AFP-positive HCC,
IL-37 expression was only weakly positive, with a positive rate of
16.7% (8/48). The expression of IL-37 protein in the PT was
associated with the sex of the individual (χ2=13.12,
P=0.003) and tumor size (χ2=7.996, P=0.045) but not with
other clinicopathological features (Table IV).
 | Table III.Association between the expression of
IL-37 protein and clinicopathological parameters in 65 cases of HCC
tissues. |
Table III.
Association between the expression of
IL-37 protein and clinicopathological parameters in 65 cases of HCC
tissues.
|
| IL-37
expression |
|---|
|
|
|
|---|
| Group | n (100%) | − | + | +++ | χ2 | P-value |
|---|
| Age |
|
|
|
| 1.177 | 0.634 |
| ≤55
years | 49 | 39 (79.6) | 8 (16.3) | 2 (4.1) |
|
|
| >55
years | 16 | 12 (75.0) | 4 (25.0) | 0 (0.0) |
|
|
| Sex |
|
|
|
| 2.259 | 0.357 |
|
Male | 51 | 38 (74.5) | 11 (21.6) | 2 (3.9) |
|
|
|
Female | 14 | 13 (92.9) | 1 (7.1) | 0 (0.0) |
|
|
| Tumor size |
|
|
|
| 1.548 | 0.434 |
| ≤2
cm | 17 | 15 (88.2) | 2 (11.8) | 0 (0.0) |
|
|
| >2
cm | 48 | 36 (75.0) | 10 (20.8) | 2 (4.2) |
|
|
| Serum AFP |
|
|
|
| 6.522 | 0.039 |
| ≤8.1
ng/ml | 17 | 11 (64.7) | 4 (23.5) | 2 (11.8) |
|
|
| >8.1
ng/ml | 48 | 40 (83.3) | 8 (16.7) | 0 (0.0) |
|
|
| HBsAg |
|
|
|
| 1.37 | 0.624 |
|
Negative | 12 | 9 (75.0) | 2 (16.7) | 1 (8.3) |
|
|
|
Positive | 53 | 42 (79.2) | 10 (18.9) | 1 (1.9) |
|
|
| Histological
differentiation |
|
|
|
| 1.978 | 0.719 |
|
Well | 16 | 13 (81.3) | 3 (18.8) | 0 (0.0) |
|
|
|
Moderatea | 43 | 34 (79.1) | 7 (16.3) | 2 (4.7) |
|
|
|
Poor | 6 | 4 (66.7) | 2 (33.3) | 0 (0.0) |
|
|
| BCLC stage |
|
|
|
| 3.174 | 0.28 |
| A | 26 | 19 (73.1) | 7 (26.9) | 0 (0.0) |
|
|
|
B&C | 39 | 32 (82.1) | 5 (12.8) | 2 (5.1) |
|
|
| Liver
cirrhosis |
|
|
|
| 2.928 | 0.331 |
|
Absence | 38 | 31 (81.6) | 5 (13.2) | 2 (5.3) |
|
|
|
Presence | 27 | 20 (74.1) | 7 (25.9) | 0 (0.0) |
|
|
| Vascular
infiltration |
|
|
|
| 1.112 | 0.659 |
|
Negative | 59 | 47 (79.6) | 10 (16.9) | 2 (3.4) |
|
|
|
Positive | 6 | 4 (66.7) | 2 (33.3) | 0 (0.0) |
|
|
 | Table IV.Association between the expression of
IL-37 protein and clinicopathological parameters in 65 cases of
paracancerous tissues. |
Table IV.
Association between the expression of
IL-37 protein and clinicopathological parameters in 65 cases of
paracancerous tissues.
|
| IL-37
expression |
|---|
|
|
|
|---|
| Group | N (100%) | − | + | ++ | +++ | χ2 | P-value |
|---|
| Age |
|
|
|
|
| 6.29 | <0.999 |
| ≤55
years | 49 | 6 (12.2) | 13 (26.5) | 13 (26.5) | 17 (34.7) |
|
|
| >55
years | 16 | 6 (37.5) | 5 (31.3) | 2 (12.5) | 3 (18.8) |
|
|
| Sex |
|
|
|
|
| 13.12 | 0.003 |
|
Male | 51 | 11 (21.6) | 9 (17.6) | 12 (23.5) | 19 (37.3) |
|
|
|
Female | 14 | 1 (7.1) | 9 (64.3) | 3 (21.4) | 1 (7.1) |
|
|
| Tumor size |
|
|
|
|
| 7.996 | 0.045 |
| ≤2
cm | 16 | 0 (0.0) | 8 (50.0) | 4 (25.0) | 4 (25.0) |
|
|
| >2
cm | 49 | 12 (24.5) | 10 (20.4) | 11 (22.4) | 16 (32.7) |
|
|
| Serum AFP |
|
|
|
|
| 1.257 | 0.774 |
| ≤8.1
ng/ml | 17 | 2 (11.8) | 4 (23.5) | 5 (29.4) | 6 (35.3) |
|
|
| >8.1
ng/ml | 48 | 10 (20.8) | 14 (29.2) | 10 (20.8) | 14 (29.2) |
|
|
| HBsAg |
|
|
|
|
| 0.894 | 0.873 |
|
Negative | 12 | 2 (16.7) | 3 (25.0) | 2 (16.7) | 5 (41.7) |
|
|
|
Positive | 53 | 10 (18.9) | 15 (28.3) | 13 (24.5) | 15 (28.3) |
|
|
| Histological
differentiation |
|
|
|
|
| 1.829 | 0.949 |
|
Well | 16 | 3 (18.8) | 5 (31.3) | 3 (18.8) | 5 (31.3) |
|
|
|
Moderate | 43 | 7 (16.3) | 11 (25.6) | 11 (25.6) | 14 (32.6) |
|
|
|
Poor | 6 | 2 (33.3) | 2 (33.3) | 1 (16.7) | 1 (16.7) |
|
|
| BCLC stage |
|
|
|
|
| 3.681 | 0.319 |
| A | 26 | 2 (7.7) | 9 (34.6) | 6 (23.1) | 9 (34.6) |
|
|
|
B&C | 39 | 10 (25.6) | 9 (23.1) | 9 (23.1) | 11 (28.2) |
|
|
| Liver
cirrhosis |
|
|
|
|
| 1.881 | 0.637 |
|
Absence | 38 | 6 (15.8) | 10 (26.3) | 11 (28.9) | 11 (28.9) |
|
|
|
Presence | 27 | 6 (22.2) | 8 (29.6) | 4 (14.8) | 9 (33.3) |
|
|
| Vascular
infiltration |
|
|
|
|
| 1.612 | 0.715 |
|
Negative | 59 | 12 (20.3) | 16 (27.1) | 13 (22.0) | 18 (30.5) |
|
|
|
Positive | 6 | 0 (0.0) | 2 (33.3) | 2 (33.3) | 2 (33.3) |
|
|
Association between NF-κB expression
and clinical indexes in HCC and PT
In HCC, there was no association between the
expression of NF-κB protein and the clinicopathological features of
patients with HCC (P>0.05; Table
V). In PT, the expression of NF-κB protein in PT was associated
with age (χ2=14.9, P=0.002), sex (χ2=13.15,
P=0.006) and BCLC stage, which is a clinical staging system for
liver cancer (22)
(χ2=9.048; P=0.02; Table
VI).
 | Table V.Association between the expression of
NF-κB protein and clinicopathological parameters in 65 cases of HCC
tissues. |
Table V.
Association between the expression of
NF-κB protein and clinicopathological parameters in 65 cases of HCC
tissues.
|
| NF-κB
expression |
|---|
|
|
|
|---|
| Group | N (100%) | − | + | ++ | +++ | χ2 | P-value |
|---|
| Age |
|
|
|
|
| 3.071 | 0.389 |
| ≤55
years | 49 | 2 (4.1) | 8 (16.30) | 12 (24.5) | 27 (55.1) |
|
|
| >55
years | 16 | 1 (6.3) | 5 (31.3) | 5 (31.3) | 5 (31.3) |
|
|
| Sex |
|
|
|
|
| 0.452 | 0.968 |
|
Male | 51 | 2 (3.9) | 10 (19.6) | 13 (25.5) | 26 (51.0) |
|
|
|
Female | 14 | 1 (7.1) | 3 (21.4) | 4 (28.6) | 6 (42.9) |
|
|
| Tumor size |
|
|
|
|
| 4.383 | 0.226 |
| ≤2
cm | 17 | 1 (5.9) | 1 (5.9) | 7 (41.2) | 8 (47.1) |
|
|
| >2
cm | 48 | 2 (4.2) | 12 (25.0) | 10 (20.8) | 24 (50.0) |
|
|
| Serum AFP |
|
|
|
|
| 1.352 | 0.727 |
| ≤8.1
ng/ml | 17 | 1 (5.9) | 2 (11.8) | 4 (23.5) | 10 (58.8) |
|
|
| >8.1
ng/ml | 48 | 2 (4.2) | 11 (22.9) | 13 (27.1) | 22 (45.8) |
|
|
| HBsAg |
|
|
|
|
| 2.496 | 0.48 |
|
Negative | 12 | 0 (0.0) | 4 (33.3) | 2 (16.7) | 6 (50.0) |
|
|
|
Positive | 53 | 3 (5.7) | 9 (17.0) | 15 (28.3) | 26 (49.1) |
|
|
| Histological
differentiation |
|
|
|
|
| 4.875 | 0.584 |
|
Well | 16 | 0 (0.0) | 3 (18.8) | 5 (31.3) | 8 (50.0) |
|
|
|
Moderate | 43 | 2 (4.7) | 10 (23.3) | 11 (25.6) | 20 (46.5) |
|
|
|
Poor | 6 | 1 (16.7) | 0 (0.0) | 1 (16.7) | 4 (66.7) |
|
|
| BCLC stage |
|
|
|
|
| 2.147 | 0.593 |
| A | 26 | 1 (3.8) | 3 (11.5) | 8 (30.8) | 14 (53.8) |
|
|
|
B&C | 39 | 2 (5.1) | 10 (25.6) | 9 (23.1) | 18 (46.2) |
|
|
| Liver
cirrhosis |
|
|
|
|
| 1.168 | 0.788 |
|
Absence | 38 | 1 (2.6) | 8 (21.1) | 11 (28.9) | 18 (47.4) |
|
|
|
Presence | 27 | 2 (7.4) | 5 (18.5) | 6 (22.2) | 18 (46.2) |
|
|
| Vascular
infiltration |
|
|
|
|
| 0.473 | 1 |
|
Negative | 59 | 3 (5.1) | 12 (20.3) | 15 (25.4) | 29 (49.2) |
|
|
|
Positive | 6 | 0 (0.0) | 1 (16.7) | 2 (33.3) | 3 (50.0) |
|
|
 | Table VI.Association between the expression of
NF-κB protein and clinicopathological parameters in paracancerous
tissues. |
Table VI.
Association between the expression of
NF-κB protein and clinicopathological parameters in paracancerous
tissues.
|
| NF-κB
expression |
|---|
|
|
|
|---|
| Group | N (100%) | − | + | ++ | +++ | χ2 | P-value |
|---|
| Age |
|
|
|
|
| 14.9 | 0.002 |
| ≤55
years | 49 | 0 (0.0) | 19 (38.8) | 26 (53.1) | 4 (8.2) |
|
|
| >55
years | 16 | 3 (18.8) | 9 (56.3) | 2 (12.5) | 2 (12.5) |
|
|
| Sex |
|
|
|
|
| 13.15 | 0.006 |
|
Male | 51 | 0 (0.0) | 25 (49.0) | 21 (41.2) | 5 (9.8) |
|
|
|
Female | 14 | 3 (21.4) | 3 (21.4) | 7 (50.0) | 1 (7.1) |
|
|
| Tumor size |
|
|
|
|
| 5.703 | 0.126 |
| ≤2
cm | 17 | 1 (5.9) | 11 (64.7) | 5 (29.4) | 0 (0.0) |
|
|
| >2
cm | 48 | 2 (4.2) | 17 (35.4) | 23 (47.9) | 6 (12.5) |
|
|
| Serum AFP |
|
|
|
|
| 5.703 | 0.126 |
| ≤8.1
ng/ml | 17 | 0 | 8 (47.1) | 6 (35.3) | 3 (17.6) |
|
|
| >8.1
ng/ml | 48 | 3 (6.3) | 20 (41.7) | 22 (45.8) | 6 (12.5) |
|
|
| HBsAg |
|
|
|
|
| 0.863 | 0.957 |
|
Negative | 12 | 0 | 6 (50.0) | 5 (41.7) | 1 (8.3) |
|
|
|
Positive | 53 | 3 (5.7) | 22 (41.5) | 23 (43.4) | 5 (9.4) |
|
|
| Histological
differentiation |
|
|
|
|
| 5.779 | 0.439 |
|
Well | 16 | 0 (0.0) | 9 (56.3) | 7 (43.8) | 0 (0.0) |
|
|
|
Moderate | 43 | 2 (4.7) | 17 (39.5) | 19 (44.2) | 5 (11.6) |
|
|
|
Poor | 6 | 1 (16.7) | 2 (33.3) | 2 (33.3) | 1 (16.7) |
|
|
| BCLC stage |
|
|
|
|
| 9.048 | 0.02 |
| A | 26 | 1 (3.8) | 17 (65.4) | 7 (26.9) | 1 (3.8) |
|
|
|
B&C | 39 | 2 (5.1) | 11 (28.2) | 21 (53.8) | 5 (12.8) |
|
|
| Liver
cirrhosis |
|
|
|
|
| 0.584 | 0.917 |
|
Absence | 38 | 2 (5.3) | 15 (39.5) | 17 (44.7) | 4 (10.5) |
|
|
|
Presence | 27 | 1 (3.7) | 13 (48.1) | 11 (40.7) | 2 (7.4) |
|
|
| Vascular
infiltration |
|
|
|
|
| 2.909 | 0.388 |
|
Negative | 59 | 2 (3.4) | 26 (44.1) | 25 (42.4) | 6 (10.2) |
|
|
|
Positive | 6 | 1 (16.7) | 2 (33.3) | 3 (50.0) | 0 |
|
|
Correlation between the protein
expression of IL-37 and NF-κB in HCC and PT
The results of the present study demonstrated that
there was a correlation between the protein expression of IL-37 and
NF-κB in HCC (r=−0.277, P=0.029), suggesting a negative correlation
between the two genes (Table VII).
However, there was no correlation between the protein expression of
IL-37 and NF-κB in PT (P>0.05, data not shown).
 | Table VII.Correlation between the protein
expression of IL-37 and NF-κB in HCC tissues. |
Table VII.
Correlation between the protein
expression of IL-37 and NF-κB in HCC tissues.
|
|
| NF-κB |
|---|
|
|
|
|
|---|
| IL-37 | n (100%) | − | + | ++ | +++ |
|---|
| − | 51 | 3 | 8 | 8 | 32 |
| + | 12 | 0 | 5 | 7 | 0 |
| +++ | 2 | 0 | 0 | 2 | 0 |
| r=−0.277 | P=0.029 |
|
|
|
|
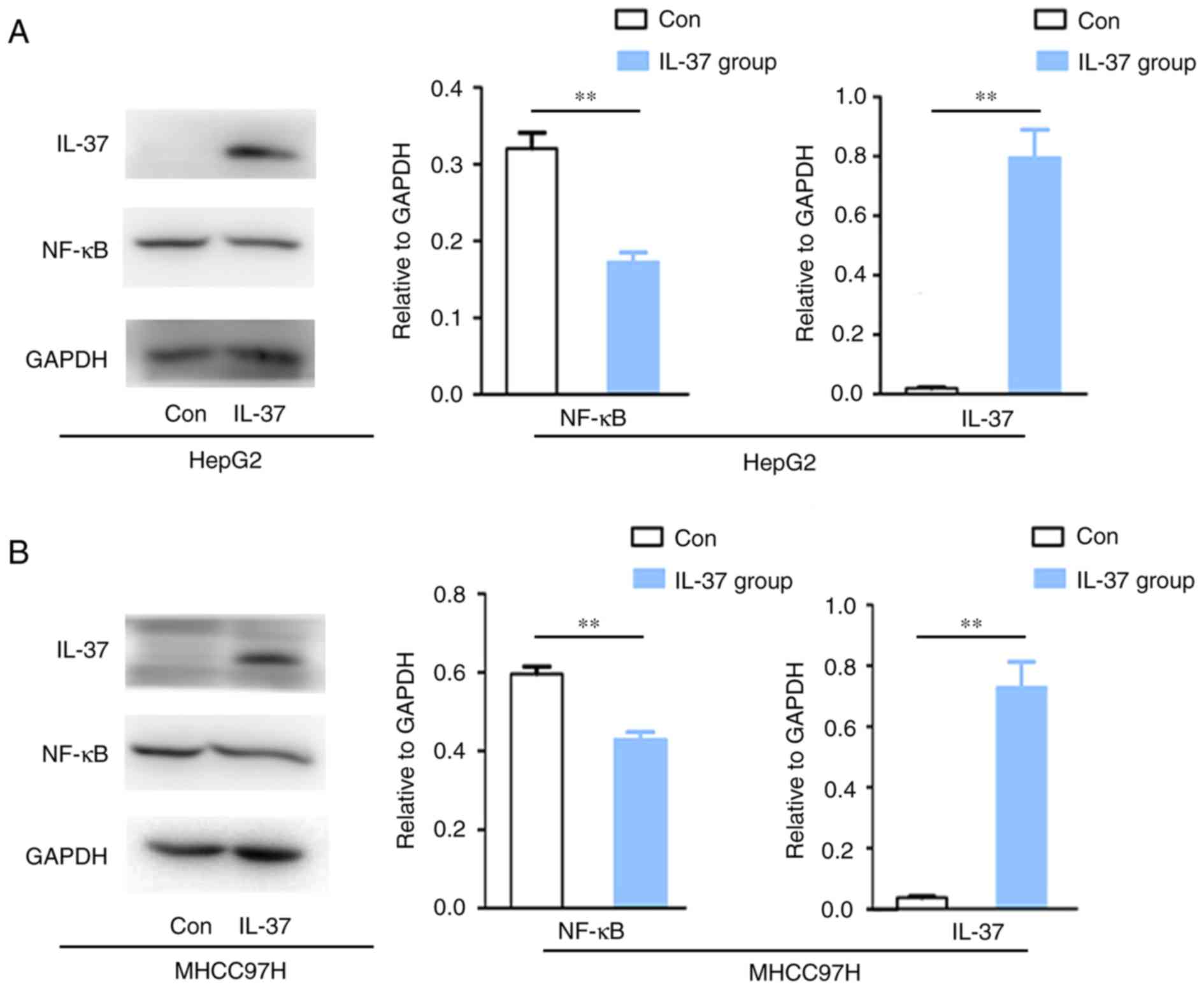
Overexpression of IL-37 inhibited
NF-κB protein expression in liver cancer cell lines
In order to further verify whether IL-37 modulates
the protein expression of NF-kB in liver cancer cells, gene
transfection of IL-37 and western blot analysis was performed.
Herein, IL-37 gene transfection upregulated IL-37 protein
expression in HepG2 and MHCC97H cells (Fig. 3). Overexpression of IL-37 inhibited
NF-κB protein expression by 56.50% in HepG2 cells (P<0.05;
Fig. 3A) and 30.52% in MHCC97H cells
(P<0.05; Fig. 3B).
Discussion
HBV infection involves the infection of the virus
itself and a series of pathological reactions induced by HBV
(2). The present study indicated
that there is no significant association between IL-37 and HBV
(HBsAg positive), but IL-37 may be associated with HBV
infection-induced immune responses through NF-kB. HBV infection
could trigger a rapid innate immune response through NF-κB
(23). The present study
demonstrated that IL-37 protein expression downregulated NF-kB
protein expression, indicating that IL-37 may play an inhibitory
role in HBV infection-induced immune responses through inhibition
of NF-kB. This hypothesis requires further research in the future
and was not the primary focus of the present study. The present
study mainly focused on the protein expression IL-37 in HCC, and
the results did provide new clues for understanding the development
of HCC.
The present study revealed a low expression of IL-37
in HCC, which was negatively associated with serum AFP. IL-37
expression is at a very low level in normal tissues and increases
markedly as a response to the inflammatory stimulation. The
expression of IL-37 is also very low in cancer tissues, such as
colorectal cancer (24) and
non-small cell lung cancer (25).
The results of the present study demonstrated that IL-37 expression
was high in PT cells and markedly decreased in liver cancer cells.
Notably, the present study revealed a negative association between
serum AFP and IL-37 of cancer tissues, but not in PT. AFP usually
increases with the deterioration of hepatocellular carcinogenesis
and is a relatively specific clinical index for the diagnosis of
primary liver cancer, but the specificity and sensitivity of
diagnosis are relatively low (26).
Some researchers have tried to combine AFP detection with some
interleukin detection for the diagnosis and prognosis of liver
cancer (27). In the present study,
serum AFP was revealed to be specifically associated with IL-37 of
cancerous tissues, but not PT. This phenomenon indicated that
combining the detection of AFP and cancerous IL-37 may improve the
sensitivity and accuracy for the diagnosis and prognostic judgment
of liver cancer.
There were 59 cases (59/65) that were associated
with HBV, hepatitis C virus or non-alcoholic steatohepatitis in
those 65 specimens. The results revealed that there was no
statistical difference in IL-37 expression (data not shown). In
addition, due to strict ethical requirements, it is often difficult
to collect large amounts of human tissues. In the present study,
the Pathology Department of Foshan First People's Hospital provided
65 specimens, and no wax tissues. There were two slices of each
specimen provided. Larger sample sizes are required in order to
further investigate the role of IL-37 and the associated mechanisms
in liver cancer.
There was a significant difference between the NF-κB
protein expression in cancer tissues and PT. The expression rate of
NF-κB in cancer tissues was markedly increased compared with that
in PT. This was consistent with that in a variety of tumors, such
as colorectal cancer, gastric cancer, prostate cancer and liver
cancer, in previous studies (28).
Furthermore, the correlation between IL-37 and NF-κB
expression in HCC tissues was unclear before, and therefore
analyzed in the present study. NF-κB expression was negatively
correlated with IL-37 expression in cancer tissues but not in PT.
Here, if was demonstrated that the decrease of IL-37 can increase
inflammation, and the increase of NF-κB can directly promote the
release and activation of inflammatory factors. These abnormal
changes may form positive feedback to promote the controllable
inflammation into uncontrollable inflammation, and finally caused
the malignant transformation of normal cells and the occurrence of
tumor.
In conclusion, IL-37 expression was negatively
correlated with NF-κB expression in both HCC tissue and liver
cancer cell lines. IL-37 may play a role in the development of
liver cancer.
Acknowledgements
The authors would like to thank Dr Haiyan Shi
(Pathology Department, Foshan First People's Hospital) who helped
to collect the specimens and provided the ethics certificate in her
hospital.
Funding
The present study was funded by the Science and
Technology Plan Project of Guangdong Province (grant no.
2016A020215147), the Natural Science Foundation of Guangdong
Province, China (grant no. 2018A030307026), the Medical Science and
Technology Research Fund of Guangdong province (grant no.
A2017605), and the National Natural Science Foundation of China
(grant no. 81302244).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
SW and PL wrote the manuscript and were responsible
for the examination and analysis. KW, LS, GZ and HG assisted with
the statistical analysis. KH, RL, ZD, SL, PO, YW and ZC performed
the experiments. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
The First People's Hospital of Foshan (Foshan, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wong MC, Jiang JY, Goggins WB, Liang M,
Fang Y, Fung FD, Leung C, Wang HH, Wong GL, Wong VW and Chan HL:
International incidence and mortality trends of liver cancer: A
global profile. Sci Rep. 7:458462017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cheuk-Fung Yip T, Wai-Sun Wong V, Lik-Yuen
Chan H, Tse YK, Pik-Shan Kong A, Long-Yan Lam K, Chung-Yan Lui G
and Lai-Hung Wong G: Effects of diabetes and glycemic control on
risk of hepatocellular carcinoma after seroclearance of hepatitis B
surface antigen. Clin Gastroenterol Hepatol. 16:765–773. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Saha Turna N and Wu F: Risk assessment of
aflatoxin-related liver cancer in Bangladesh. Food Additi Contam
Part A Chem Anal Control Expo Risk Assess. 36:320–326. 2019.
View Article : Google Scholar
|
|
4
|
Kawamura Y, Arase Y, Ikeda K, Akuta N,
Kobayashi M, Saitoh S, Suzuki F, Suzuki Y, Inao M, Mochida S and
Kumada H: Effects of alcohol consumption on hepatocarcinogenesis in
Japanese patients with fatty liver disease. Clin Gastroenterol
Hepatol. 14:597–605. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Taniguchi K and Karin M: NF-κB,
inflammation, immunity and cancer: Coming of age. Nat Rev Immunol.
18:309–324. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Banchereau J, Pascual V and O'garra A:
From IL-2 to IL-37: The expanding spectrum of anti-inflammatory
cytokines. Nat Immunol. 13:925–931. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang WQ, Dong K, Zhou L, Jiao GH, Zhu CZ,
Li WW, Yu G, Wu WT, Chen S, Sun ZN, et al: IL-37b gene transfer
enhances the therapeutic efficacy of mesenchumal stromal cells in
DSS-induced colitis mice. Acta Pharm Sin. 36:1377–1387. 2015.
View Article : Google Scholar
|
|
8
|
Wang L, Wang Y, Xia L, Shen H and Lu J:
Elevated frequency of IL-37-and IL-18Rα-positive T cells in the
peripheral blood of rheumatoid arthritis patients. Cytokine.
110:291–297. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang J, Shen Y, Li C, Liu C, Wang ZH, Li
YS, Ke X and Hu GH: IL-37 attenuates allergic process via
STAT6/STAT3 pathways in murine allergic rhinitis. Int
Immunopharmacol. 69:27–33. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
de Sousa JR, Prudente RL, Dias Junior LB,
Oliveira Carneiro FR, Sotto MN and Simoes Quaresma JA: IL-37 and
leprosy: A novel cytokine involved in the host response to
mycobacterium leprae infection. Cytokine. 106:89–94. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ye L, Ji L, Wen Z, Zhou Y, Hu D, Li Y, Yu
T, Chen B, Zhang J, Ding L, Du J, Huang Z, et al: IL-37 inhibits
the production of inflammatory cytokines in peripheral blood
mononuclear cells of patients with systemic lupus erythematosus:
Its correlation with disease activity. J Transl Med. 12:692014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang Y, Zhang ZX, Lian D, Haig A,
Bhattacharjee RN and Jevnikar AM: IL-37 inhibits IL-18-induced
tubular epithelial cell expression of pro-inflammatory cytokines
and renal ischemia-reperfusion injury. Kidney Int. 87:396–408.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bulau AM, Fink M, Maucksch C, Kappler R,
Mayr D, Wagner K and Bufler P: In vivo expression of interleukin-37
reduces local and systemic inflammation in concanavalin A-induced
hepatitis. ScientificWorldJournal. 11:2480–2490. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Feng XX, Chi G, Wang H, Gao Y, Chen Q, Ru
YX, Luo ZL, Yan W, Li PY, Liu M, et al: IL-37 suppresses the
sustained hepatic IFN-γ/TNF-α production and T cell-dependent liver
injury. Int Immunopharmacol. 69:184–193. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang S, An W, Yao Y, Chen R, Zheng X, Yang
W, Zhao Y, Hu X, Jiang E, Bie Y, et al: Interleukin 37 expression
inhibits STAT3 to suppress the proliferation and invasion of human
cervical cancer cells. J Cancer. 6:962–969. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ouyang P, An W, Chen R, Zhang H, Chen D,
Jiang E, Zhu W, Li P, Guo H, Chen Z and Wang S: IL-37 promotes cell
apoptosis in cervical cancer involving bim upregulation.
OncoTargets Ther. 12:2703–2712. 2019. View Article : Google Scholar
|
|
17
|
Jiang M, Wang Y, Zhang H, Ji Y, Zhao P,
Sun R and Zhang C: IL-37 inhibits invasion and metastasis in
nonsmall cell lung cancer by suppressing the IL-6/STAT3 signaling
pathway. Thoracic Cancer. 9:621–629. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu T, Xu B, Zhao G, Luo J and Luo C: IL-37
suppresses migration and invasion of gallbladder cancer cells
through inhibition of HIF-1α induced epithelial-mesenchymal
transition. Eur Rev Med Pharm Sci. 22:8179–8185. 2018.
|
|
19
|
Zhu B, Zhang P, Liu M, Jiang C, Liu H and
Fu J: Prognostic significance of CSN2, CD8, and MMR
status-associated nomograms in patients with colorectal cancer.
Transl Oncol. 11:1202–1212. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhao JJ, Pan QZ, Pan K, Weng DS, Wang QJ,
Li JJ, Lv L, Wang DD, Zheng HX, Jiang SS, et al: Interleukin-37
mediates the antitumor activity in hepatocellular carcinoma: Role
for CD57+ NK cells. Sci Rep. 4:51772014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li P, Guo H, Zhou G, Shi H, Li Z, Guan X,
Deng Z, Li S, Zhou S, Wang Y and Wang S: Increased ZNF84 expression
in cervical cancer. Arch Gynecol Obstet. 297:1525–1532. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chan AW, Kumada T, Toyoda H, Tada T, Chong
CC, Mo FK, Yeo W, Johnson PJ, Lai PB, Chan AT, et al: Integration
of albumin-bilirubin (ALBI) score into barcelona clinic liver
cancer (BCLC) system for hepatocellular carcinoma. J Gastroenterol
Hepatol. 31:1300–1306. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yoneda M, Hyun J, Jakubski S, Saito S,
Nakajima A, Schiff ER and Thomas E: Hepatitis B virus and DNA
stimulation trigger a rapid innate immune response through NF-κB. J
Immunol. 197:630–643. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yan X, Zhao J and Zhang R: Interleukin-37
mediates the antitumor activity in colon cancer through β-catenin
suppression. Oncotarget. 8:490642017.PubMed/NCBI
|
|
25
|
Ge G, Wang A, Yang J, Chen Y, Yang J, Li Y
and Xue Y: Interleukin-37 suppresses tumor growth through
inhibition of angiogenesis in non-small cell lung cancer. J Exp
Clin Cancer Res. 35:132016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ma H, Sun X, Chen L, Cheng W, Han XX, Zhao
B and He C: Multiplex immunochips for high-accuracy detection of
AFP-L3% based on surface-enhanced raman scattering: Implications
for early liver cancer diagnosis. Anal Chem. 89:8877–8883. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang JY, Li X, Gao L, Teng ZH and Liu WC:
Co-transfection of dendritic cells with AFP and IL-2 genes enhances
the induction of tumor antigen-specific antitumor immunity. Exp
Ther Med. 4:655–660. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xia Y, Shen S and Verma IM: NF-κB, an
active player in human cancers. Cancer Immunol Res. 2:823–830.
2014. View Article : Google Scholar : PubMed/NCBI
|