Introduction
Lung cancer is one of the most common and fatal
malignancies worldwide, and the histological subtype non-small cell
lung cancer (NSCLC) accounts for ~85% of all cases (1,2).
Although certain improvements have been made in surgical treatment,
chemotherapy and radiotherapy, the prognoses of patients with NSCLC
remain unsatisfactory (3,4); therefore, elucidation of novel
approaches for the prevention, early detection and treatment of
NSCLC are urgently required.
Centrosome-associated family proteins (CEPs) are the
active components of centrosomes and participate in centrosome
biogenesis and function (5).
Disrupted CEP expression can result in the detrimental duplication
of centrosomes, causing genomic instability and subsequent
carcinogenesis. Centrosomal protein 131 (CEP131), alternatively
named azacytidine-inducible-1, was initially identified in murine
spermatid as a pre-acrosome-localized protein, and was subsequently
identified more specifically as a novel centrosomal protein in a
large-scale proteomic screen (6–8). CEP131
is now understood to be an evolutionarily conserved centriolar
satellite protein that exerts a pivotal function in the development
and movement of cilia (9). In
addition, CEP131 has been demonstrated to be essential for the
maintenance of genome stability (10), suggesting a possible role in the
development and progression of cancer. Notably, it has been
demonstrated that CEP131 is involved in osteosarcoma and breast
carcinogenesis via interactions with 14-3-3 and ubiquitin specific
peptidase 9 X-linked, respectively (11,12).
Additionally, CEP131 exhibits oncogenic activity in hepatocellular
carcinoma (13). However, the
precise roles and molecular mechanisms of CEP131 in NSCLC remain
unclear.
The present study aimed to explore the role of
CEP131 in NSCLC transformation and progression. The present study
demonstrated that CEP131 overexpression in NSCLC tissues is
associated with advanced Tumor-Node-Metastasis (TNM) stage and
positive regional lymph node metastasis. Furthermore,
CEP131-knockdown was demonstrated to inhibit NSCLC cell
proliferation in vitro by inhibiting the ERK and AKT
signaling pathways and their downstream signals.
Materials and methods
Patients and specimens
The present study was approved by the Institutional
Review Board of Dalian Medical University. Written informed consent
was obtained from the patients. NSCLC tissue samples were obtained
from 91 patients (65 males and 26 females) who underwent complete
surgical excision of squamous cell carcinoma or adenocarcinoma at
the First Affiliated Hospital of Dalian Medical University (Dalian,
China) during the period between January 2014 and January 2016. No
neoadjuvant radiotherapy or chemotherapy was applied prior to
surgery. The mean age of the patients was 62 years (range, 39–83
years). Histological classification and lung cancer differentiation
were evaluated according to 2015 World Health Organization
classification criteria, and TNM staging of lung cancer was
performed according to the 2009 Union for International Cancer
Control standard (14).
Immunohistochemical staining
Surgically excised NSCLC specimens were fixed in 10%
neural formalin overnight at room temperature and then embedded in
paraffin. Subsequently, 4-µm-thick sections were prepared, and
immunostaining was performed using the UltraSensitive™ SP
kit (cat. no. KIT-9720; Fuzhou Maixin Biotech Co., Ltd.) according
to the manufacturer's protocol. Tissue sections were incubated with
a CEP131 rabbit polyclonal antibody (cat. no. ab84864, Abcam)
overnight at 4°C, and PBS was used as the negative control. All
tumor slides were randomly examined by two independent
investigators from the Department of Pathology of The First
Affiliated Hospital of Dalian Medical University (Dalian, China). A
total of 100 cells were examined at ×400 magnification in five
randomly selected fields of view per slide. CEP131 expression was
semi-quantitatively scored according to the percentage of
expressing cells and staining intensity. The staining intensity was
scored as 0 (negative), 1 (weak) or 2 (marked), and percentage
scores were allocated as 1 (1–25%), 2 (26–50%), 3 (51–75%) and 4
(76–100%). Scores for each tumor sample were multiplied to give a
final score between 0 and 8. CEP131 status was regarded as low
expression (score < 4) or high expression/overexpression (score
≥ 4).
Cell culture
The A549 and SPC-A-1 cell lines were purchased from
the Cell Bank of Type Culture Collection of the Chinese Academy of
Sciences. All cells were cultured in RPMI-1640 medium (Invitrogen;
Thermo Fisher Scientific, Inc.) supplemented with 10% fetal bovine
serum (Invitrogen; Thermo Fisher Scientific, Inc.), 100 IU/ml
penicillin (Sigma-Aldrich: Merck KGaA) and 100 µg/ml streptomycin
(Sigma-Aldrich; Merck KGaA) at 37°C and 5% CO2. Cells
were passaged every 2 days by trypsinization (0.25%; Invitrogen;
Thermo Fisher Scientific, Inc.).
Transfection
CEP131-small interfering RNA (siRNA; cat. no.
sc-94024) and negative control (NC)-siRNA (cat. no. sc-37007) were
obtained from Santa Cruz Biotechnology, Inc. A549 and SPC-A-1 cells
were transfected with these siRNAs using Lipofectamine®
3000 (Invitrogen; Thermo Fisher Scientific, Inc.), according to
efficiency by western blotting. The siRNA sequences used in the
experiment were: siRNA-CEP131, 5′-GGAGGAGAAGGCACGCCAATT-3′;
siRNA-NC, 5′-UUCUCCGAACGUGUCACGUTT-3′. Subsequent experiments were
performed using cells collected 48 h post-transfection.
MTT assay
Following transfection with CEP131 siRNA, A549 and
SPC-A-1 cells were cultured on 96-well plates, and cell viability
was quantitated after 4 days using the MTT assay. A 20-µl aliquot
of MTT (5 mg/ml) solution was added to each well and incubated for
4 h at 37°C. Following incubation, the resulting formazan crystals
were dissolved in DMSO (150 µl/well). The absorbance was measured
spectrophotometrically at a wavelength of 490 nm. Experiments were
performed in triplicate.
Flow cytometry
A549 and SPC-A-1 cells were seeded onto 6-cm dishes
at 30–50% confluence, and subsequently transfected with
CEP131-siRNA or NC-siRNA. Cells were harvested 48 h following
transfection and stained using the Cell Cycle Staining kit (cat.
no. WLA010; Wanleibio Co., Ltd.), according to the manufacturer's
protocol. DNA content was determined by flow cytometry (BD
Biosciences). Cell cycle analysis was modeled using Modfit version
3.2 (Verity Software House, Inc.). Each experiment was repeated
three times. The results are presented as the percentage of the
total cell count in different phases of the cell cycle, namely
G1, S and G2.
Western blotting
Total protein from the cell lines was extracted
using lysis buffer (Pierce; Thermo Fisher Scientific, Inc.), and
the protein concentration was determined by the BCA protein assay.
A concentration of 60 µg per sample was separated by 10% SDS-PAGE
and subsequently transferred to polyvinylidene fluoride (EMD
Millipore) membrane. The membranes were blocked with 5% non-fat
milk at room temperature for 1 h and incubated overnight at 4°C
with primary antibodies. All primary antibodies were provided by
Cell Signaling Technology (Danvers, MA) or Abcam (Cambridge, MA)
and the dilutions used in the current study are listed: CEP131
(cat. no. ab84864; 1:100), cyclinD1 (cat. no. CST#2922; 1:1,000),
cyclin E (cat. no. CST#4132; 1:1,000), cyclin-dependent kinase
(CDK) 2 (cat. no. CST#2546; 1:1,000), CDK4 (cat. no. CST#12790;
1:1,000), CDK6 (cat. no. CST#13331; 1:1,000), p21 (cat. no.
CST#2947; 1:1,000), p27 (cat. no. CST#3686; 1:1,000), MEK1 (cat.
no. CST#9122; 1:1,000), phosphorylated (p)-MEK1/2 (Ser-217/221)
(cat. no. CST#9121; 1:1,000), Erk1/2 (cat. no. CST#9102; 1:1,000),
p-Erk1/2 (Tyr202/Tyr204) (cat. no. CST#9101; 1:1,000), glycogen
synthase kinase-3β (GSK-3β) (cat. no. CST#9315; 1:1,000), p-GSK-3β
(ser-9) (cat. no. CST#9336; 1:1,000), p-Akt (Ser-473) (cat. no.
CST#4060; 1:1,000), p-PI3K (Tyr-458) (cat. no. CST#4228; 1:1,000),
PI3K (cat. no. CST#4292; 1:1,000), and GAPDH (cat. no. CST#5174;
1:1,000). Membranes were washed three times in PBS and subsequently
incubated with peroxidase-conjugated anti-mouse or anti-rabbit IgG
(cat. no. sc-2357 and sc-2005, respectively; both 1:4,000; both
Santa Cruz Biotechnology, Inc.) at 37°C for 2 h. Protein bands were
detected using ECL reagent (GE Healthcare) and processed using the
ImageJ software (version 1.46; National Institutes of Health).
Statistical analysis
All data were analyzed using SPSS 22.0 software (IBM
Corp.). A χ2 test was used for the evaluation of the
associations between CEP131 expression levels and patient
clinicopathological characteristics. A two-tailed Student's t-test
was employed to compare two independent groups. P<0.05 was
considered to indicate a statistically significant difference. All
experiments were performed in triplicate, and mean values were
calculated for data comparison. Data are expressed as the mean ±
standard deviation.
Results
CEP131 is highly expressed in NSCLC
tissues
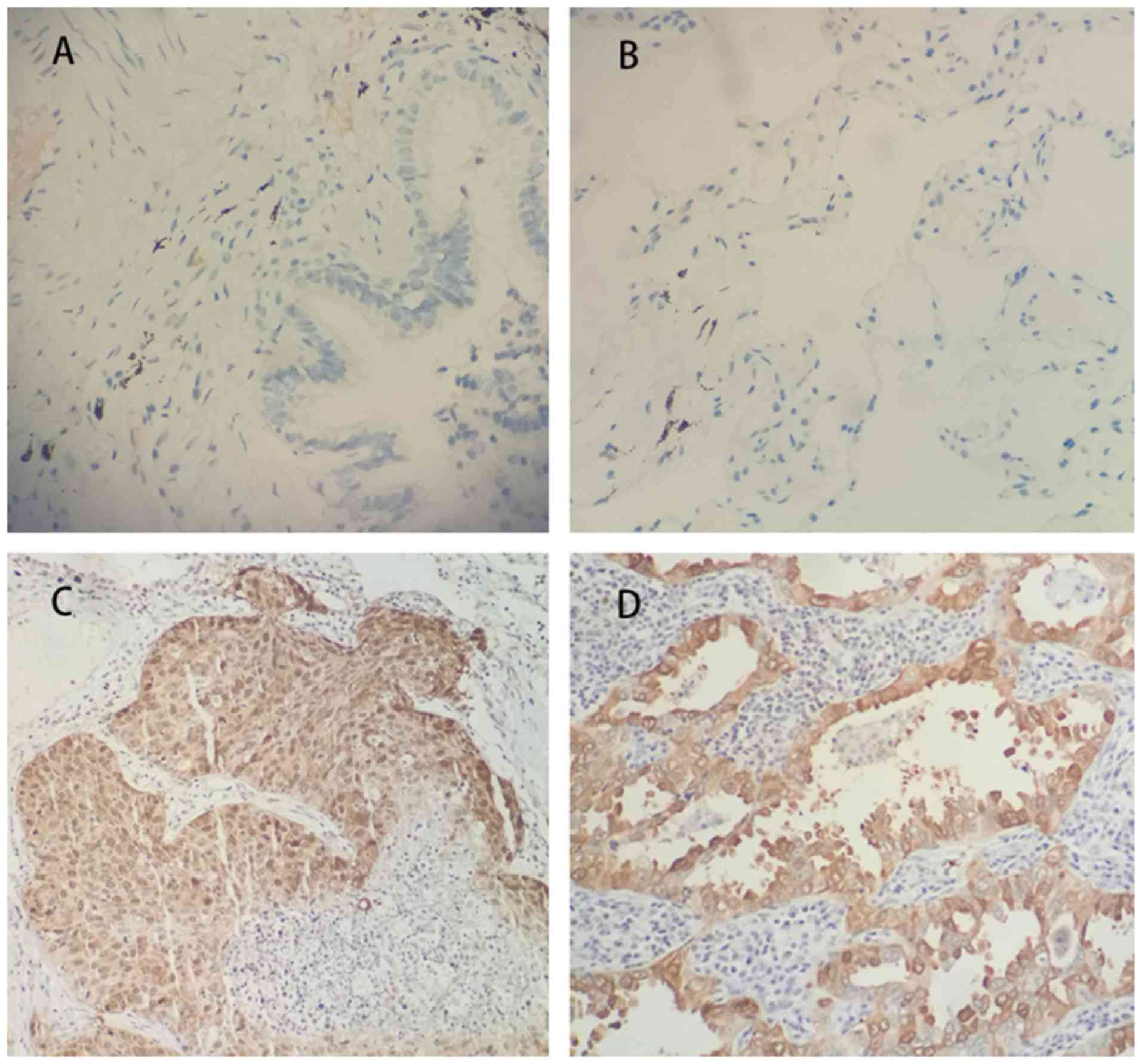
Immuno-histochemistry of 91 NSCLC tissues samples
and their corresponding normal tissue samples was performed, which
revealed that CEP131 expression levels were markedly higher in
malignant tissue when compared with normal lung epithelial cells.
Normal bronchial epithelial cells exhibited negative CEP131
staining (Fig. 1A and B); however,
CEP131 was overexpressed in cases of NSCLC, primarily in the
nucleus and cytoplasm (Fig. 1C and
D). As presented in Table I,
high CEP131 expression was observed in 63.7% (58/91) of cases. High
CEP131 expression was identified in 65.9% (27/41) of squamous cell
carcinoma cases and 62.0% (31/50) of adenocarcinoma cases. The
expression of CEP131 was identified to be significantly associated
with TNM stage (P=0.016) and nodal status (P=0.023).
 | Table I.Association of CEP131 expression and
clinicopathological parameters in 91 cases of NSCLC. |
Table I.
Association of CEP131 expression and
clinicopathological parameters in 91 cases of NSCLC.
|
|
| CEP131 |
|
|
|---|
|
|
|
|
|
|
|---|
| Characteristics | No. of patients | Low expression | High expression |
χ2-value | P-value |
|---|
| Age, years |
|
<60 | 32 | 13 | 19 | 0.406 | 0.524 |
| ≥60 | 59 | 20 | 39 |
|
|
| Sex |
| Male | 65 | 22 | 43 | 0.575 | 0.448 |
|
Female | 26 | 11 | 15 |
|
|
| Histology |
|
Adenocarcinoma | 50 | 19 | 31 | 0.145 | 0.704 |
| Squamous
cell carcinoma | 41 | 14 | 27 |
|
|
| Differentiation |
| Well +
Moderate | 60 | 23 | 37 | 0.326 | 0.568 |
| Poor | 31 | 10 | 21 |
|
|
| TNM stage |
| I | 54 | 25 | 29 | 5.784 | 0.016a |
|
II+III | 37 | 8 | 29 |
|
|
| Lymph node
metastasis |
|
Positive | 39 | 9 | 30 | 5.135 | 0.023a |
|
Negative | 52 | 24 | 28 |
|
|
CEP131-knockdown inhibits NSCLC cell
proliferation
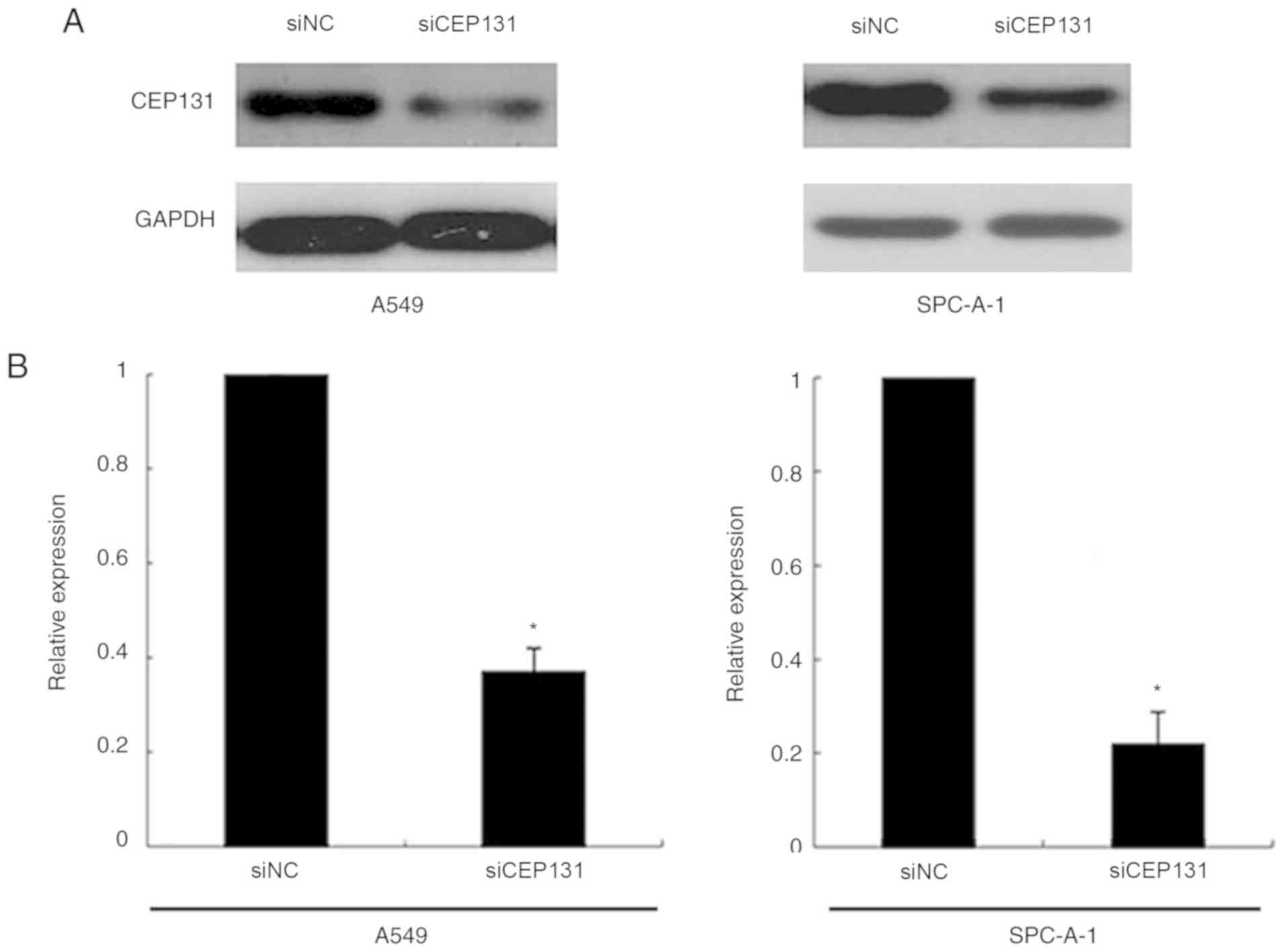
To examine the functional significance of CEP131 in
lung cancer cells, the present study utilized siRNA to knockdown
CEP131 expression in A549 and SPC-A-1 cell lines, both of which
exhibit moderate CEP131 protein expression (15). CEP131-specific siRNA substantially
reduced protein expression levels following 48 h of siRNA treatment
(Fig. 2A and B). The proliferation
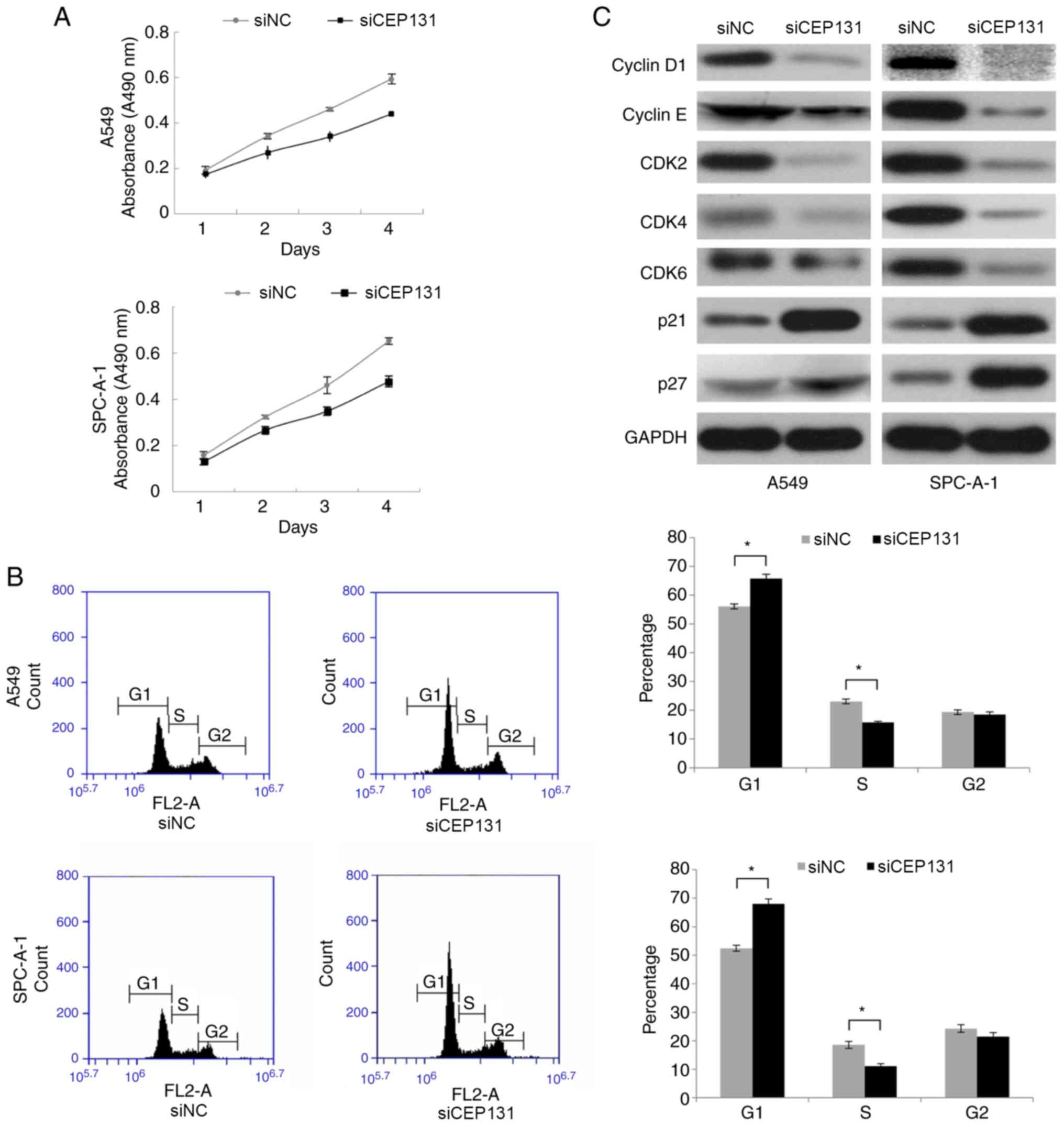
rate was determined by MTT assays. Following transfection with
CEP131 siRNA, the proliferation rate of A549 and SPC-A-1 cells was
markedly reduced when compared with the negative control (Fig. 3A). Taken together, these finding
suggest that CEP131 promotes lung cancer cell proliferation.
CEP131 regulates the cell cycle in
NSCLC cells
Since CEP131 knockdown inhibited cell proliferation
in A549 and SPC-A-1 cells, the present study subsequently analyzed
its effect on the cell cycle using flow cytometry. CEP131 knockdown
in A549 and SPC-A-1 cells significantly increased the percentage of
cells in the G1 phase but significantly decreased the percentage in
the S phase (Fig. 3B). No
significant changes were detected in the G2 phase. The present
study also examined the expression of cell cycle-associated
proteins in CEP131-downregulated cells. Western blotting analysis
revealed associations between CEP131 protein expression in NSCLC
tissue and that of cell cycle-related regulatory proteins,
including cyclin D1, cyclin E, CDK2, CDK4, CDK6, p21 and p27. These
data demonstrate that the expression levels of cyclin D1, cyclin E,
CDK2, CDK4, and CDK6 are reduced and those of p21 and p27 are
elevated in CEP131-downregulated cells (Fig. 3C). Taken together, these data suggest
that CEP131 promotes cell cycle progression via an increase in the
expression of cell cycle-promoting proteins and a reduction in the
expression of cell cycle-inhibiting proteins.
CEP131 promotes the cell cycle through
the ERK and AKT signaling pathways
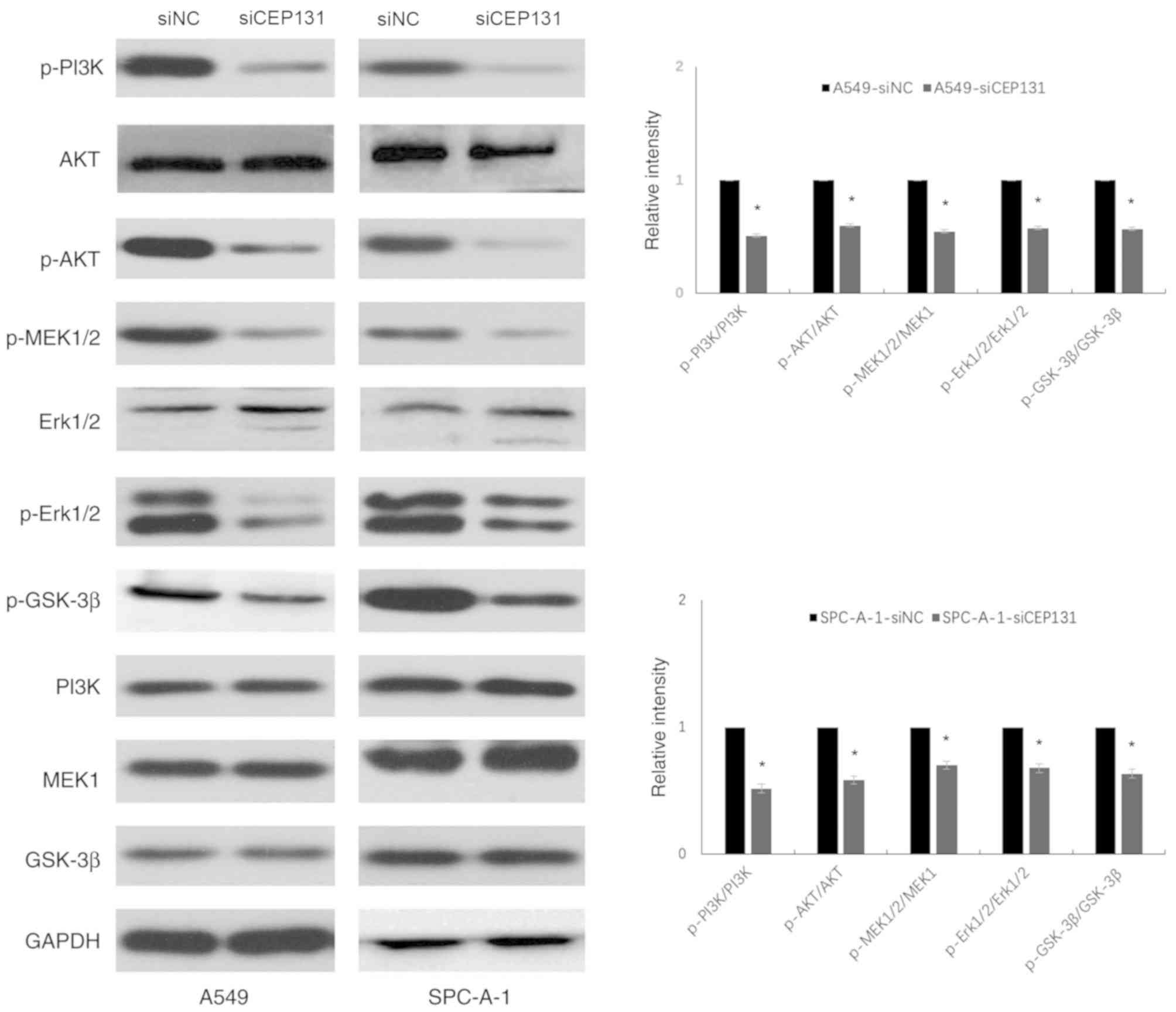
The ERK and PI3K/Akt signaling pathways are
well-known upstream factors that regulate the cell cycle (16,17). The
expression levels of ERK- and PI3K/Akt-related proteins were
detected by western blotting. Densitometric analysis was performed
on phospho-target protein bands and normalized to the total
expression of the corresponding protein and GAPDH (Fig. 4). Data are presented as the mean ±
standard deviation of three independent experiments. A value of 1
was arbitrarily assigned to the ratio obtained in the negative
control group. Knockdown of CEP131 expression in A549 and SPC-A-1
cells resulted in a significant decrease in the expression levels
of p-PI3K (Tyr458) (0.51±0.11; 0.52±0.08), p-Akt (Ser473)
(0.60±0.09; 0.58±0.12), p-MEK1/2 (Ser-217/221) (0.55±0.04;
0.70±0.03), p-Erk1/2 (Tyr202/Tyr204) (0.58±0.24; 0.68±0.16), and
p-GSK-3β (ser-9) (0.57±0.18; 0.63±0.09) compared with the negative
control (P<0.05; Table II).
These results demonstrate that CEP131 promotes NSCLC cell
proliferation, at least partly, via the activation of the ERK and
PI3K/Akt signaling pathways and their downstream effectors.
 | Table II.Ratio of phosphorylated protein
levels to total protein levels. |
Table II.
Ratio of phosphorylated protein
levels to total protein levels.
|
| A549 |
| SPC-A-1 |
|
|---|
|
|
|
|
|
|
|---|
|
Phosphorylated/total protein | siNC | siCEP131 | P-value | siNC | siCEP131 | P-value |
|---|
| p-AKT/AKT | 1 | 0.60±0.09 | 0.003a | 1 | 0.58±0.12 | 0.017a |
| p-MEK1/2/MEK1 | 1 | 0.55±0.04 | 0.008a | 1 | 0.70±0.03 | 0.013a |
|
p-GSK-3β/GSK-3β | 1 | 0.57±0.18 | 0.024a | 1 | 0.63±0.09 | 0.004a |
|
p-ERK1/2/ERK1/2 | 1 | 0.58±0.24 | 0.041a | 1 | 0.68±0.16 | 0.016a |
| p-PI3K/PI3K | 1 | 0.51±0.11 | 0.008a | 1 | 0.52±0.08 | 0.004a |
Discussion
CEP131 is a relatively recently discovered protein
whose expression pattern and role in NSCLC progression were
previously unknown. The present study demonstrated that CEP131 is
highly expressed in the nucleus and cytoplasm of NSCLC cells.
CEP131 overexpression was revealed to be associated with advanced
TNM stage and positive lymph node metastasis, but not with patient
age, sex, tumor differentiation or histopathology. Statistical
analyses demonstrated that no association exists between CEP131
expression and tumor differentiation grade in either adenocarcinoma
or squamous cell carcinoma. Results of the MTT and cell cycle
assays demonstrated that, in addition to regulating cell
proliferation and cell cycle progression, CEP131 also modulates the
expression levels of cell cycle-related proteins. The high
proliferation level of tumor cells is known to be associated with
increased cell-cycle progression, with cell cycle dysregulation
being a prevalent cancer characteristic (18–20).
Cell cycle distribution is tightly regulated by cyclins, CDKs and
cyclin-dependent kinase inhibitors (CDKIs) (21). Progression of the G1 phase is
primarily regulated by cyclin D1 and the G1/S transition is
enhanced by cyclin E (22,23). Cyclin D1 is one of the most important
cell cycle regulators during tumor development, which forms
functional kinase complexes with CDK4 and CDK6 to aid transition
from the G1 phase to the S phase (24,25).
Both p21 and p27 are CDKIs that participate in cell-cycle
regulation via the inhibition of cyclin-CDK complex activity in the
G1 phase (26). The present data
indicates that the downregulation of CEP131 induces G1/S cell cycle
arrest by inhibiting cyclins D1/E and CDK 2/4/6 and inducing
inhibitory p21/p27. Therefore, it was concluded that CEP131
regulates tumor proliferation via the cell cycle regulation.
The present study screened key signaling proteins,
such as members of the ERK and AKT pathways, which are known to be
involved in cell cycle progression and cell growth (27–30).
GSK-3β is a downstream regulator of the ERK and AKT pathways, the
activity of which can be inhibited by serine phosphorylation
mediated by AKT or ERK, inducing cell cycle transition from the G1
to the S phase (31,32). In the present study knockdown of
CEP131 expression decreased the expression of p-PI3K, p-Akt,
p-MEK1/2, p-Erk1/2 and p-GSK-3β. These results suggest that CEP131
promotes cell proliferation in NSCLC tissue, at least partly, via
the activation of the ERK and AKT pathways.
In conclusion, the present study demonstrated that
CEP131 expression is upregulated in NSCLC tissues. Additionally,
knockdown of CEP131 expression inhibits cell proliferation by
inhibiting the ERK and AKT signaling pathways in NSCLC cells.
Future studies should perform overexpression experiments in order
to confirm these results. Taken together, these findings indicate
that CEP131 may be a potential therapeutic target in NSCLC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
Study concept and design was undertaken by JW. SH
and LZ analyzed and interpreted the data. JW drafted the
manuscript. XY critically revised the manuscript for important
intellectual content and performed the statistical analysis.
Ethics approval and consent to
participate
Ethical approval was obtained from the Institutional
Review Board of Dalian Medical University (Dalian, China). Patients
included in the present study provided written informed
consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Baker MJ, Cooke M and Kazanietz MG:
Nuclear PKCl-ECT2-Rac1 and ribosome biogenesis: A novel axis in
lung tumorigenesis. Cancer Cell. 31:167–169. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wu DW, Chen TC, Huang HS and Lee H:
TC-N19, a novel dual inhibitor of EGFR and cMET, efficiently
overcomes EGFR-TKI resistance in non-small-cell lung cancer cells.
Cell Death Dis. 7:e22902016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang X, Yu X, Jiang G, Miao Y, Wang L,
Zhang Y, Liu Y, Fan C, Lin X, Dong Q, et al: Cytosolic TMEM88
promotes invasion and metastasis in lung cancer cells by binding
DVLS. Cancer Res. 75:4527–4537. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wei CC, Nie FQ, Jiang LL, Chen QN, Chen
ZY, Chen X, Pan X, Liu ZL, Lu BB and Wang ZX: The pseudogene
DUXAP10 promotes an aggressive phenotype through binding with LSD1
and repressing LATS2 and RRAD in non small cell lung cancer.
Oncotarget. 8:5233–5246. 2017.PubMed/NCBI
|
|
5
|
Kumar A, Rajendran V, Sethumadhavan R and
Purohit R: CEP proteins: The knights of centrosome dynasty.
Protoplasma. 250:965–983. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Aoto H, Tsuchida J, Nishina Y, Nishimune
Y, Asano A and Tajima S: Isolation of a novel cDNA that encodes a
protein localized to the pre-acrosome region of spermatids. Eur J
Biochem. 234:8–15. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Aoto H, Miyake Y, Nakamura M and Tajima S:
Genomic organization of the mouse AZ1 gene that encodes the protein
localized to preacrosomes of spermatids. Genomics. 40:138–141.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Andersen JS, Wilkinson CJ, Mayor T,
Mortensen P, Nigg EA and Mann M: Proteomic characterization of the
human centrosome by protein correlation profiling. Nature.
426:570–574. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ma L and Jarman AP: Dilatory is a
Drosophila protein related to AZI1 (CEP131) that is located at the
ciliary base and required for cilium formation. J Cell Sci.
124:2622–2630. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Staples CJ, Myers KN, Beveridge RD, Patil
AA, Lee AJ, Swanton C, Howell M, Boulton SJ and Collis SJ: The
centriolar satellite protein Cep131 is important for genome
stability. J Cell Sci. 125:4770–4779. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tollenaere MAX, Villumsen BH, Blasius M,
Nielsen JC, Wagner SA, Bartek J, Beli P, Mailand N and
Bekker-Jensen S: p38- and MK2-dependent signalling promotes
stress-induced centriolar satellite remodelling via
14-3-3-dependent sequestration of CEP131/AZI1. Nat Commun.
6:100752015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li X, Song N, Liu L, Liu X, Ding X, Song
X, Yang S, Shan L, Zhou X, Su D, et al: USP9X regulates centrosome
duplication and promotes breast carcinogenesis. Nat Commun.
8:148662017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu XH, Yang YF, Fang HY, Wang XH, Zhang
MF and Wu DC: CEP131 indicates poor prognosis and promotes cell
proliferation and migration in hepatocellular carcinoma. Int J
Biochem Cell Biol. 90:1–8. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Travis WD, Brambilla E, Nicholson AG,
Yatabe Y, Austin JHM, Beasley MB, Chirieac LR, Dacic S, Duhig E,
Flieder DB, et al: The 2015 World Health Organization
classification of lung tumors: Impact of genetic, clinical and
radiologic advances since the 2004 classification. J Thorac Oncol.
10:1243–1260. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang H, Yu Z, Huo S, Chen Z, Ou Z, Mai J,
Ding S and Zhang J: Overexpression of ELF3 facilitates cell growth
and metastasis through PI3K/Akt and ERK signaling pathways in
non-small cell lung cancer. Int J Bio Cell Bio. 94:98–106. 2018.
View Article : Google Scholar
|
|
16
|
Zhao J, Ou B, Han D, Wang P, Zong Y, Zhu
C, Liu D, Zheng M, Sun J, Feng H and Lu A: Tumor-derived CXCL5
promotes human colorectal cancer metastasis through activation of
the ERK/Elk-1/Snail and AKT/GSK3β/β-catenin pathways. Mol Cancer.
16:702017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang Y, Nie H, Zhao X, Qin Y and Gong X:
Bicyclol induces cell cycle arrest and autophagy in HepG2 human
hepatocellular carcinoma cells through the PI3K/AKT and
Ras/Raf/MEK/ERK pathways. BMC Cancer. 16:7422016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hall JR, Messenger ZJ, Tam HW, Phillips
SL, Recio L and Smart RC: Long noncoding RNA lincRNA-p21 is the
major mediator of UVB-induced and p53-dependent apoptosis in
keratinocytes. Cell Death Dis. 6:e17002015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sun L, Song L, Wan Q, Wu G, Li X, Wang Y,
Wang J, Liu Z, Zhong X, He X, et al: CMyc-mediated activation of
serine biosynthesis pathway is critical for cancer progression
under nutrient deprivation conditions. Cell Res. 25:429–444. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mizuno H, Nakanishi Y, Ishii N, Sarai A
and Kitada K: A signature-based method for indexing cell cycle
phase distribution from microarray profiles. BMC Genomics.
10:1372009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sherr CJ and Roberts JM: CDK inhibitors:
positive and negative regulators of G1-phase progression. Genes
Dev. 13:1501–1512. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tashima Y, Hamada H, Okamoto M and Hanai
T: Prediction of key factor controlling G1/S phase in the mammalian
cell cycle using system analysis. J Biosci Bioeng. 106:368–374.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sherr CJ: Mammalian G1 cyclins. Cell.
73:1059–1065. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Donnellan R and Chetty R: Cyclin D1 and
human neoplasia. Mol Pathol. 51:1–7. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zuryn A, Litwiniec A, Safiejko-Mroczka B,
Klimaszewska-Wiśniewska A, Gagat M, Krajewski A, Gackowska L and
Grzanka D: The effect of sulforaphane on the cell cycle, apoptosis
and expression of cyclin D1 and p21 in the A549 non-small cell lung
cancer cell line. Int J Oncol. 48:2521–2533. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang X, Wu J, Luo S, Lechler T and Zhang
JY: FRA1 promotes squamous cell carcinoma growth and metastasis
through distinct AKT and c-Jun dependent mechanisms. Oncotarget.
23:34371–34383. 2016.
|
|
27
|
Wang Y, Liu J, Cui J, Xing L, Wang J, Yan
X and Zhang X: ERK and p38 MAPK signalling pathways are involved in
ochratoxin A-induced G2 phase arrest in human gastric epithelium
cells. Toxicol Lett. 209:186–192. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shao Q, Han F, Peng S and He B: Nur77
inhibits oxLDL induced apoptosis of macrophages via the p38 MAPK
signalling pathway. Biochem Biophys Res Commun. 471:633–638. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chien CC, Wu MS, Shen SC, Ko CH, Chen CH,
Yang LL and Chen YC: Activation of JNK contributes to
evodiamineinduced apoptosis and G2/M arrest in human colorectal
carcinoma cells: A structure-activity study of evodiamine. PLoS
One. 9:e997292014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tsai WB, Aiba I, Long Y, Lin HK, Feun L,
Savaraj N and Kuo MT: Activation of Ras/PI3K/ERK pathway induces
c-Myc stabilization to upregulate argininosuccinate synthetase,
leading to arginine deiminase resistance in melanoma cells. Cancer
Res. 72:2622–2633. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Saiprasad G, Chitra P, Manikandan R and
Sudhandiran G: Hesperidin induces apoptosis and triggers autophagic
markers through inhibition of Aurora-A mediated
phosphoinositide-3-kinase/Akt/mammalian target of rapamycin and
glycogen synthase kinase-3 beta signalling cascades in experimental
colon carcinogenesis. Eur J Cancer. 50:2489–2507. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wen W, Ding J, Sun W, Fu J, Chen Y, Wu K,
Ning B, Han T, Huang L, Chen C, et al: Cyclin G1-mediated
epithelial-mesenchymal transition via phosphoinositide 3-kinase/Akt
signaling facilitates liver cancer progression. Hepatology.
55:1787–1798. 2012. View Article : Google Scholar : PubMed/NCBI
|