Introduction
Liver cancer, mainly hepatocellular carcinoma (HCC),
is one of the major causes of cancer mortality worldwide (1). A recent international survey estimated
that 841,080 new cases of liver cancer and 781,631
liver-cancer-related mortalities occurred worldwide in 2018;
notably, during the same period China alone accounted for
approximately one-half of the total number of new cases and
mortalities (1). In China, the
prevalence of hepatitis B virus (HBV) infection between 2005 and
2006 was >7% in the entire population, and >80% of all cases
of primary liver cancer were reportedly caused by chronic HBV
infection (2,3).
Owing to the development of various detection
techniques, the prognosis for liver cancer has improved
considerably; however, the improvement has been limited as liver
cancer progresses rapidly and is frequently detected in the
intermediate or advanced stage with portal vein tumor thrombus
(PVTT) or distant (extrahepatic) metastasis (4). Distant metastasis, commonly observed in
intermediate to advanced stage HCC, severely worsens the prognosis;
consequently, survival in patients with HCC and metastasis is
significantly shorter compared with that in patients with HCC
without metastasis (5–7). During progression, liver cancer
commonly invades the portal veins to form PVTT; the incidence rate
of PVTT is 44.0–62.2% (8). Studies
have reported that the presence of PVTT is an independent predictor
of distant metastasis and promotes distant metastasis of HCC
(5–7). Therefore, the mortality rates in
patients with HCC and PVTT are considerably higher compared with
those without PVTT. The prognosis is particularly poor in patients
with HCC-PVTT and metastasis (9).
Accurately predicting the metastasis of HCC with PVTT is crucial
for improving treatment outcomes and prognosis; however, to the
best of our knowledge, studies on HCC with PVTT metastasis
prediction are not currently available.
In the present study, independent risk factors for
distant metastasis of HCC with PVTT were identified to develop an
early risk warning system for distant metastasis. The results of
this study may facilitate early prediction and appropriate
treatment of distant metastasis of HBV-HCC with PVTT to improve the
prognosis of patients with intermediate and advanced HCC.
Materials and methods
Patients
Data for 182 patients who had received primary
diagnoses of HBV-HCC and PVTT and did not exhibit distant
metastasis (non-distant metastasis group), and 81 patients who had
received diagnoses of HBV-HCC, PVTT and distant metastasis (distant
metastasis group) between January 2012 and December 2014 at Beijing
Ditan Hospital, Capital Medical University (Beijing, China), were
retrospectively examined. In addition, data for 83 patients who had
received primary diagnoses of HBV-HCC and PVTT between January 2015
and June 2015 at the same hospital (validation cohort) were also
examined. Patients were included if they were 18–85 years old and
had received diagnoses of HBV-HCC and PVTT with vascular invasion.
Patients were excluded if they exhibited HBV-HCC and PVTT with
hepatitis A, C, D or E virus infection or non-hepatitis virus
infection, severe diseases (with functional insufficiency) of vital
organs (e.g. the heart, lung, kidney, brain or blood), severe
mental illness or metastatic HCC, or had incomplete clinical
information. This study was approved by the Ethics Committee of
Beijing Ditan Hospital, Capital Medical University. As this study
was retrospective, informed consent was not required from the
patients.
Diagnosis
HBV infection
According to the Prevention and Treatment Guidelines
of Hepatitis B (Version Year 2000) (5,10),
patients with a history of hepatitis B, those who tested positive
for hepatitis B surface antigen (HBsAg) for >6 months, and those
who continued to test positive for HBsAg and/or HBV DNA were
considered to exhibit a chronic HBV infection.
Primary HCC
Primary HCC was determined through histological and
radiological examination. In accordance with the gold standard,
specimens of surgically resected liver-occupying lesions and
metastatic lesions were examined histologically. According to the
radiological diagnosis standard for HCC (5), the patients whose CT images showed
vessel intake of contrast agent during the arterial phase, vessel
loss during the venous phase and advanced phase, and nodes with a
diameter >2 cm were considered to exhibit HCC based on the
results of a single radiological examination. However, if the
diameter of the nodes was 1–2 cm, the patients were considered to
exhibit HCC only when the results of two radiological examinations
were consistent (6).
PVTT
The presence of PVTT was determined by assessing the
filling defect of the portal trunk or branch, which was determined
using ultrasound B, computed tomography (CT), magnetic resonance
imaging (MRI) or digital subtraction angiography (DSA) examination.
Ultrasound examination identified abnormal echo filling (including
hypoecho, isoecho, hyperecho and mixed echo) in the entire portal
vein, widening of the inner diameter of the portal vein or
interruption of blood signals. CT examination indicated widened
portal veins with uneven inner density and filling defects, and
enhanced CT scans revealed tumor thrombus shadows with low density
but without enhancement by plain scanning. MRI scans demonstrated a
high signal shadow of the portal vein. DSA reports revealed filling
defects, total interruption and widening of the portal vein.
Distant metastasis
Patients with distant metastatic lesions in the
lung, lymph nodes, kidney and adrenal gland detected through
ultrasound B, CT or MRI examination were considered to exhibit
distant metastasis.
Statistical analysis
Clinical characteristics (including age, sex, tumor
multiplicity and thrombus site), blood parameters [red blood cell
(RBC) count, neutrophil to lymphocyte (N/L) ratio, platelet count
and platelet/lymphocyte (P/L) ratio], hepatic and kidney function
parameters [levels of aspartate aminotransferase (AST), γ-glutamyl
transpeptidase (GGT), alkaline phosphatase (ALP), direct bilirubin
and creatinine (Cr), as well as albumin/globulin (A/G) ratio], the
thrombin function parameter prothrombin activity (PTA), the HCC
indicator α-fetoprotein (AFP), levels of lactate dehydrogenase
(LDH) and C-reactive protein (CRP) were reviewed. Data were
analyzed using SPSS 20.0 software (IBM Corp.). Data with a normal
distribution are expressed as the mean ± standard deviation and
were compared between the metastasis and non-metastasis groups in
the modeling cohort using Student's t-test. Data with abnormal
distributions are expressed as the median ± interquartile range and
compared between the two groups by using the rank sum test.
Enumeration data are expressed as a frequency, and between-group
comparisons were conducted using the χ2 test. P<0.05
was considered to indicate a statistically significant
difference.
Variables with P<0.05 in the aforementioned
comparisons and those with high clinical significance in the
modeling cohort were included in a multivariate logistic regression
analysis. Variables that were significantly associated with distant
metastasis were determined, and regression coefficients were
calculated. Thus, a prediction model for distant metastasis was
developed. The prediction accuracy, specificity, sensitivity,
false-positive and false-negative values were determined by
receiver operating characteristic (ROC) curve analysis. For each
value mentioned above, only a single value instead of the mean ±
standard derivation is presented, and statistical comparisons
between the data for ROC curves were not performed. To validate
this model, the patients from both the modeling and validation
cohorts were assessed for the presence or absence of distant
metastasis using the aforementioned developed model, which was
verified by the clinical data.
Results
Patient basic characteristics
Data from 263 patients who had received primary
diagnoses of HBV-HCC and PVTT (modeling cohort) were
retrospectively reviewed for the development of a prediction model
for distant metastasis. Among them, 81 patients exhibited distant
metastasis (metastasis group), whereas 182 patients did not exhibit
distant metastasis (non-metastasis group). In addition, data from
83 patients with HBV-HCC and PVTT (comprising 47 patients without
metastasis and 36 patients with metastasis) were reviewed for the
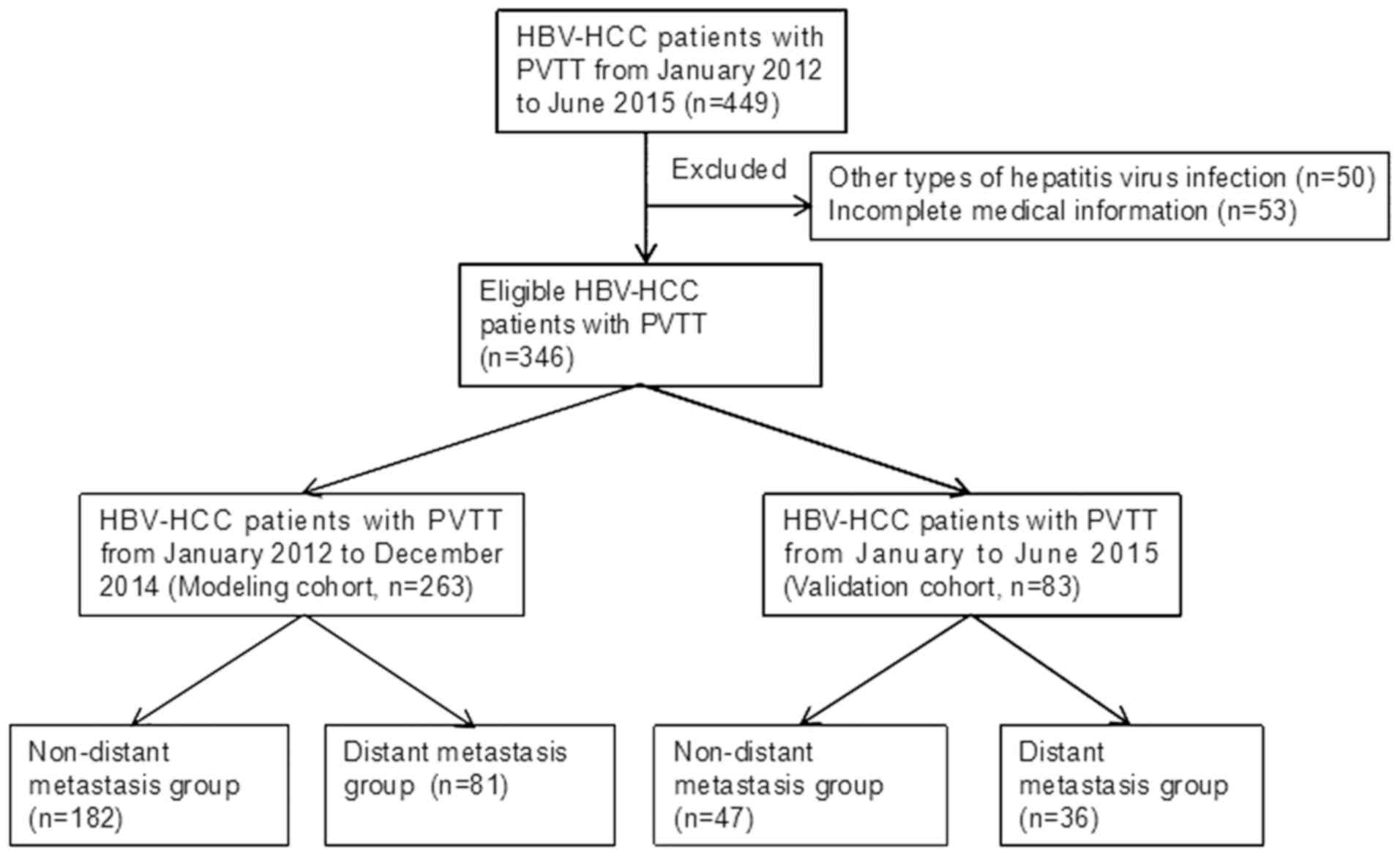
independent validation of the developed model (Fig. 1).
Establishment of the prediction model
of distant metastasis
In the modeling cohort, the metastasis and
non-metastasis groups differed significantly in tumor thrombus
site, blood platelet count, RBC count, P/L ratio, AST level, A/G
ratio, GGT, LDH, ALP, CRP and PTA level (P<0.05; Table I). Variables with P<0.2 after
univariate analysis were included in the multivariate logistic
analysis. Since an increase in the N/L ratio is related to the
progression and metastasis of liver cancer (11–13) and
serum AFP level is a biomarker for liver cancer, N/L and AFP with
P>0.05 after univariate analysis were also included in the
multivariate analysis. The results demonstrated that N/L ratio
≥2.31, RBC count ≥4.07×1012 cells/l, CRP level ≥7.02
mg/l, AST level ≥118.5 U/l and tumor thrombus site at branch were
significantly associated with distant metastasis of HBV-HCC and
PVTT (P<0.05; odds ratio >1.00; Table II).
 | Table I.Comparison between distant and
non-distant metastasis groups. |
Table I.
Comparison between distant and
non-distant metastasis groups.
| Variables | Non-distant
metastasis group (n=182) | Distant metastasis
group (n=81) | P-value |
|---|
| Age, years | 55.53±10.16 | 54.98±12.17 | 0.703 |
| Sex |
|
| 0.702 |
| Male, n
(%) | 155 (85.2) | 71 (87.7) |
|
| Female,
n (%) | 27 (14.8) | 10 (12.3) |
|
| Tumor multiplicity
(single/multiple) | 127/55 | 60/21 | 0.478 |
| Tumor thrombus
site |
|
| 0.013a |
| Branch,
n (%) | 68 (37.4) | 46 (56.8) |
|
|
Unilateral, n | 66 | 37 |
|
|
Bilateral, n | 2 | 9 |
|
| Trunk,
n (%) | 26 (14.3) | 7 (8.6) |
|
| Branch
+ trunk, n (%) | 88 (48.3) | 28 (34.6) |
|
| Blood platelet
count (×109 cells/l), M (IQR) | 94.30
(70.68–143.38) | 108.75
(78.90–189.90) | 0.041a |
| RBC count,
×1012 cells/l |
|
|
|
|
<4.07, n (%) | 124 (68.1) | 39 (50.0) | 0.006a |
| ≥4.07,
n (%) | 58 (31.9) | 39 (50.0) |
|
| N/L ratio |
|
|
|
|
<2.31, n (%) | 47 (25.8) | 14 (17.9) | 0.170 |
| ≥2.31,
n (%) | 135 (74.2) | 64 (82.1) |
|
| P/L ratio |
|
|
|
|
<3.16, n (%) | 53 (29.1) | 10 (12.8) | 0.005a |
| ≥3.16,
n (%) | 129 (70.9) | 68 (87.2) |
|
| AST (U/l), M
(IQR) | 71.10
(42.70–106.60) | 90.60
(54.00–189.40) | 0.033a |
| TBIL (µmol/l), M
(IQR) | 23.55
(14.15–43.03) | 27.70
(16.20–44.90) | 0.287 |
| A/G ratio |
|
|
|
|
<1.0, n (%) | 37 (20.3) | 32 (39.5) | 0.004a |
| ≥1.0, n
(%) | 145 (79.7) | 49 (60.5) |
|
| GGT (U/l), M
(IQR) | 126.15
(67.40–249.70) | 192.40
(115.70–309.70) | 0.003a |
| LDH (U/l), M
(IQR) | 196.20
(162.40–232.10) | 211.70
(178.10–259.00) | 0.020a |
| ALP (U/l), M
(IQR) | 131.60
(91.40–187.30) | 163.40
(119.20–245.20) | 0.002a |
| Creatinine
(µmol/l), M (IQR) | 65.00
(56.00–75.00) | 62.30
(56.00–72.00) | 0.386 |
| AFP, µg/l |
|
|
|
|
<1,000, n (%) | 93 (57.1) | 32 (45.7) | 0.112 |
| ≥1,000,
n (%) | 70 (42.9) | 38 (54.3) |
|
| CRP, mg/l |
|
|
|
|
<7.02, n (%) | 37 (25.3) | 4 (6.8) | 0.003a |
| ≥7.02,
n (%) | 109 (74.7) | 55 (93.2) |
|
| PTA, % |
|
|
|
|
<62.55, n (%) | 46 (26.4) | 12 (14.8) | 0.039a |
| ≥62.55
m, n (%) | 128 (73.6) | 69 (85.2) |
|
 | Table II.Multivariate analysis of the factors
associated with distant metastasis. |
Table II.
Multivariate analysis of the factors
associated with distant metastasis.
| Variables | Regression
coefficient | Assignment | Odds ratio | 95% CI | P-value |
|---|
| P/L ratio |
|
|
|
|
|
|
<3.16 |
| 0 | Normal |
|
|
|
≥3.16 |
| 1 | 2.209 | 0.479–10.191 | 0.310 |
| Blood platelet
count, ×109 cells/l |
|
|
|
|
|
|
<108 |
|
| Normal |
|
|
|
≥108 |
|
| 1.525 | 0.515–4.519 | 0.447 |
| N/L ratio |
|
|
|
|
|
|
<2.31 |
| 0 | Normal |
|
|
|
≥2.31 | 1.192 | 1 | 3.294 | 1.104–9.829 | 0.033a |
| RBC count,
×1012 cells/l |
|
|
|
|
|
|
<4.07 |
| 0 | Normal |
|
|
|
≥4.07 | 1.312 | 1 | 3.712 | 1.677–8.218 | 0.001a |
| A/G ratio |
|
|
|
|
|
|
<1.0 |
|
| Normal |
|
|
|
≥1.0 |
|
| 2.359 | 0.836–6.658 | 0.105 |
| LDH, U/l |
|
|
|
|
|
|
<218.6 |
|
| Normal |
|
|
|
≥218.6 |
|
| 0.851 | 0.358–2.022 | 0.715 |
| TBIL, µmol/l |
|
|
|
|
|
|
<12.9 |
|
| Normal |
|
|
|
≥12.9 |
|
| 1.842 | 0.666–5.100 | 0.239 |
| ALP, U/l |
|
|
|
|
|
|
<128.8 |
|
| Normal |
|
|
|
≥128.8 |
|
| 0.405 | 0.133–1.239 | 0.113 |
| CRP, mg/l |
|
|
|
|
|
|
<7.02 |
| 0 | Normal |
|
|
|
≥7.02 | 1.711 | 1 | 5.537 | 1.461–20.991 | 0.012a |
| AST, U/l |
|
|
|
|
|
|
<118.5 |
| 0 | Normal |
|
|
|
≥118.5 | 0.912 | 1 | 2.489 | 1.109–5.657 | 0.029a |
| GGT, U/l |
|
|
|
|
|
|
<100 |
|
| Normal |
|
|
|
≥100 |
|
| 2.450 | 0.716–8.384 | 0.153 |
| PTA |
|
|
|
|
|
|
<63 |
|
| Normal |
|
|
|
>63 |
|
| 2.282 | 0.759–6.859 | 0.142 |
| AFP, µg/l |
|
|
|
|
|
|
<1,000 |
|
| Normal |
|
|
|
≥1,000 |
|
| 1.578 | 0.898–2.771 | 0.113 |
| Tumor thrombus
site |
|
|
|
|
|
|
Branch | 0.553 | 1 | 1.739 | 1.152–2.547 | 0.007a |
|
Trunk |
| 2 |
|
|
|
| Branch
+ trunk |
| 3 |
|
|
|
Regression analysis was conducted to further assess
the factors associated with distant metastasis. Regression
coefficients were calculated for the analyzed factors (Table II). In addition, based on the
aforementioned regression analysis of these association factors,
the following formula was proposed to predict distant metastasis: Y
= −5.276 + 1.711 × CRP (mg/l) + 1.312 × RBC (×1012
cells/l) + 1.192 × N/L + 0.912 × AST (U/l) + 0.553 × tumor thrombus
site (branch, assignment of 1). The threshold value was −1.05. To
assess the formula for the prediction of distant metastasis, ROC
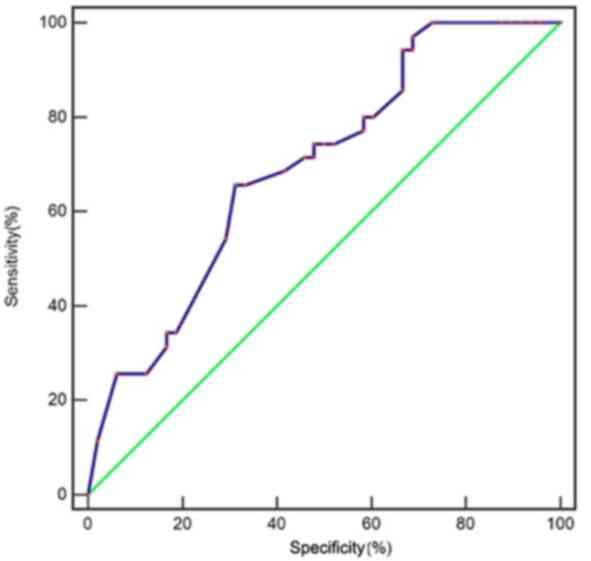
analysis was conducted. An area under the ROC curve (AUC) of 0.731
(95% CI, 0.649–0.776), Youden's index of 0.3247, sensitivity of
72.88%, specificity of 59.55%, false-positive value of 40.45% and
false-negative value of 27.12% were obtained (Fig. 2). In addition, the prediction
efficacy of the model [determined using a combination of five
factors: N/L ratio, RBC count, tumor thrombus site (branch,
assignment of 1), AST level and CRP level] was higher compared with
that of any individual factor. The AUC (representing accuracy) and
sensitivity of the model were higher compared with those of the
individual factors, although the specificity of the model was lower
compared with that of the individual factors (Table III; Fig.
2).
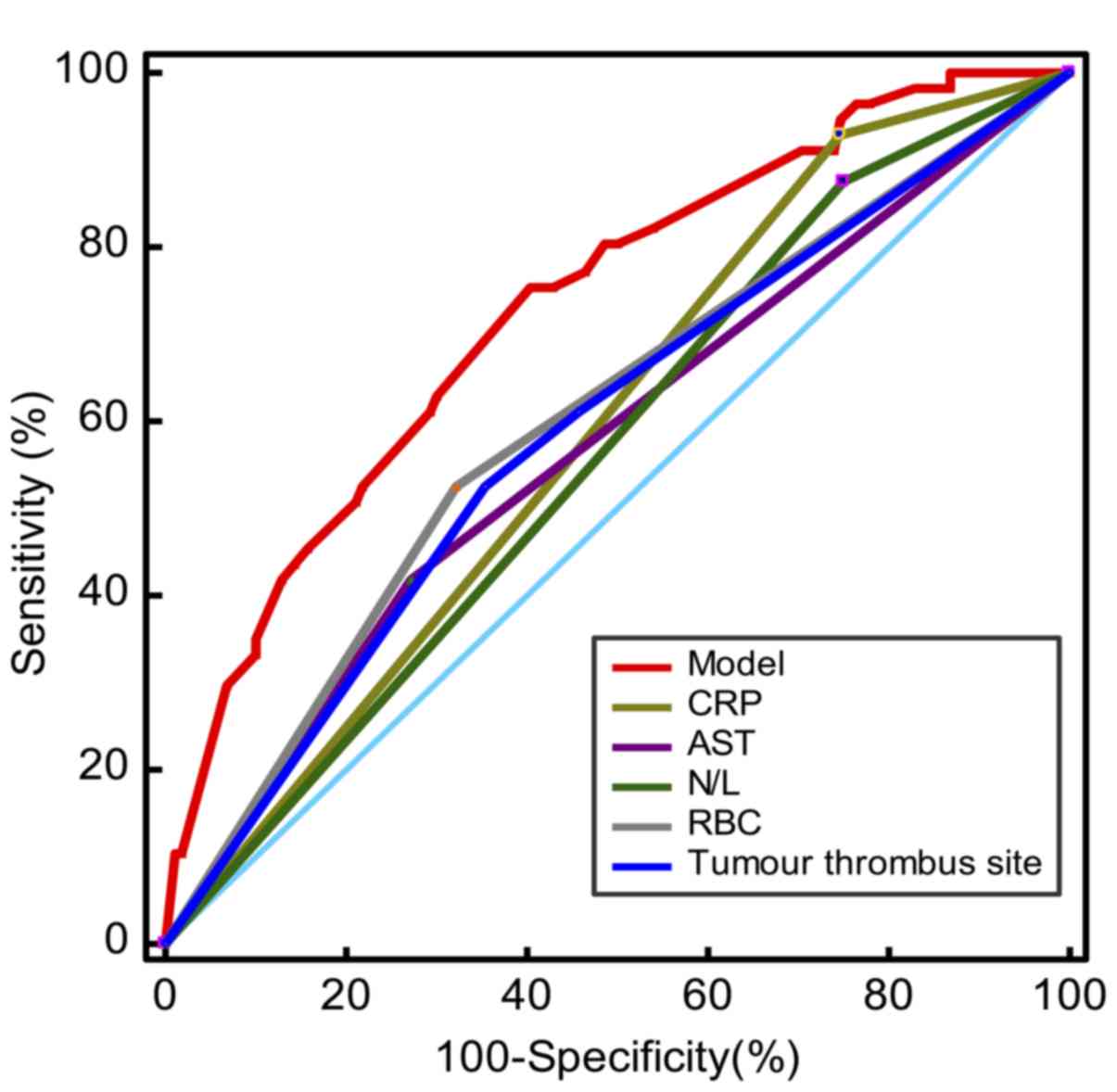 | Figure 2.ROC analysis of the established model
for distant metastasis in the modeling cohort. The red, dark blue,
green, yellow, brown, and gray curves indicate ROC curves of the
model, tumor thrombus site, N/L ratio, CRP level, AST level, and
RBC count, respectively. The light blue curve indicates the
reference line. ROC, receiver operating characteristic; N/L,
neutrophil to lymphocyte; CRP, C-reactive protein; AST, aspartate
aminotransferase; RBC, red blood cell. |
 | Table III.Receiver operating characteristic
curve analysis of various individual parameters and the established
model in the modeling cohort. |
Table III.
Receiver operating characteristic
curve analysis of various individual parameters and the established
model in the modeling cohort.
| Parameters | Accuracy (95%
CI) | Sensitivity
(%) | Specificity
(%) | False positive
value (1-specificity) (%) | False negative
value (1-sensitivity) (%) |
|---|
| CRP | 0.592
(0.549–0.686) | 33.54 | 90.24 |
9.76 | 66.46 |
| N/L ratio | 0.562
(0.483–0.608) | 32.16 | 77.05 | 22.95 | 67.84 |
| RBC count | 0.602
(0.519–0.642) | 40.21 | 76.07 | 23.93 | 59.88 |
| Tumor thrombus
site | 0.589
(0.522–0.645) | 40.35 | 76.51 | 23.49 | 59.65 |
| AST | 0.574
(0.531–0.653) | 43.75 | 74.86 | 25.14 | 56.25 |
| Model | 0.731
(0.649–0.776) | 72.88 | 59.55 | 40.55 | 27.12 |
Validation of the prediction model of
distant metastasis
To validate the established model, the data of the
83 patients (47 non-metastasis and 36 metastasis patients) with
HBV-HCC and PVTT in the validation cohort were reviewed. The
results of ROC curve analysis revealed an AUC of 0.695 (95% CI,
0.584–0.791), sensitivity of 65.71%, specificity of 68.75%,
false-positive value of 31.25% and false-negative value of 34.29%
in the validation cohort (Table IV;
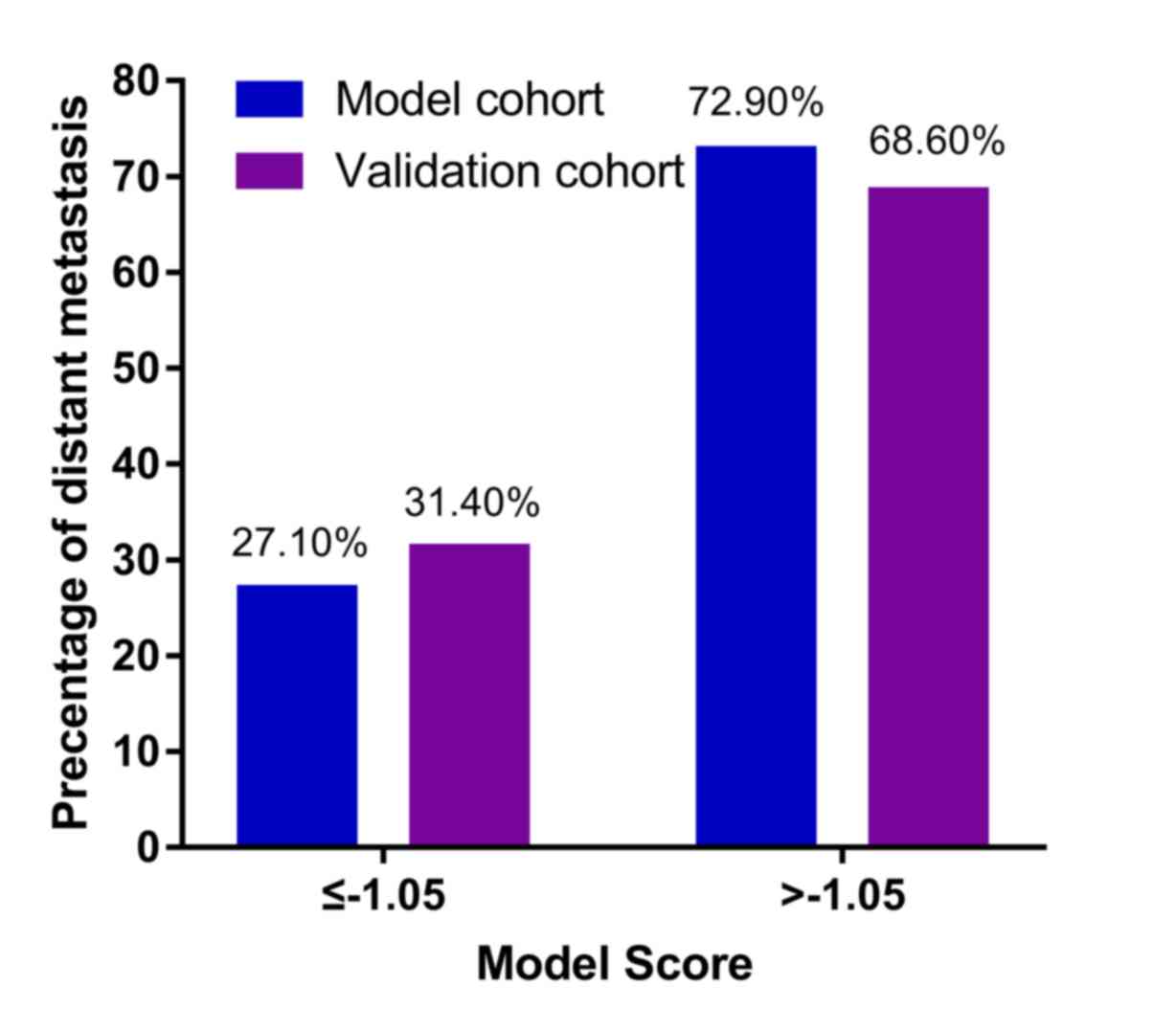
Fig. 3). At Y≤-1.05, the percentages
of patients with metastasis in the model and validation cohorts
were 27.10 and 31.40%, respectively, whereas at Y>-1.05, the
percentages of patients with metastasis increased significantly
(72.90 and 68.60% in the modeling and validation cohorts,
respectively; Fig. 4).
 | Table IV.Receiver operating characteristic
curve analysis of individual parameters and the established model
in the validation cohort. |
Table IV.
Receiver operating characteristic
curve analysis of individual parameters and the established model
in the validation cohort.
| Parameters | Accuracy (95%
CI) | Sensitivity(%) | Specificity(%) | False positive
value (1-specificity) (%) | False negative
value (1-sensitivity) (%) |
|---|
| CRP | 0.594
(0.481–0.701) | 77.14 | 41.67 | 58.33 | 22.86 |
| N/L ratio | 0.626
(0.531–0.730) | 85.71 | 39.58 | 60.42 | 14.29 |
| RBC count | 0.502
(0.390–0.641) | 40.00 | 60.42 | 39.58 | 60.00 |
| Tumor thrombus
site | 0.697
(0.584–0.795) | 62.86 | 75.56 | 24.44 | 37.14 |
| AST | 0.544
(0.431–0.654) | 94.29 | 14.58 | 85.42 | 5.71 |
| Model | 0.695
(0.584–0.791) | 65.71 | 68.75 | 31.25 | 34.29 |
Discussion
HCC commonly invades portal veins and causes PVTT
with an incidence of 44.0–62.2% (2).
PVTT promotes the metastasis of HCC (14). If not treated urgently, patients with
HCC with PVTT exhibit a median survival time of only 2.7–4.0
months; in addition, mortality in patients with PVTT is
considerably higher compared with that in patients without PVTT
(10,15,16). As
PVTT is independently associated with distant metastasis of HCC
(5–7), in patients with HCC with PVTT,
concomitant distant metastasis considerably increases the mortality
rate, although the presence of PVTT alone also leads to poor
prognosis. In addition, several treatment methods have been used to
improve prognosis for patients with HCC with PVTT. For example, a
recent study involving clinical data from 6,474 patients with HCC
with PVTT reported that liver resection treatment significantly
improved the median overall survival by 1.77 years compared with
non-resection treatments (9). In
addition, transarterial chemoembolization (TACE) combined with
γ-knife treatment improved survival rates in patients with HCC with
PVTT (17). By contrast, metastasis
is not easily treated and remains a major cause of treatment
failure. To improve the treatment outcomes and prognosis,
predicting distant metastasis of HCC with PVTT is crucial.
Variables such as high pretreatment platelet counts,
numerous or large tumors, microvascular invasion, incomplete
capsulation and high preoperative AFP are significantly associated
with distant metastasis in HCC (18–21);
however, prediction models for the metastasis of HCC with PVTT are
not currently available, to the best of our knowledge. In the
present study, commonly accessed clinical and biochemical
parameters were used to screen the risk factors significantly
associated with the distant metastasis of primary HCC with PVTT to
establish a convenient early warning system for distant metastasis.
The results demonstrated that several biochemical parameters,
including N/L ratio ≥2.31, RBC count ≥4.07×1012 cells/l,
CRP level ≥7.02 mg/l, AST level ≥118.5 U/l and tumor thrombus site
at branch, were significantly associated with distant metastasis of
primary HCC with PVTT. In addition, based on these results, an
early prediction system for distant metastasis was proposed, which
may be useful for the timely prevention and treatment of distant
metastasis of HBV-HCC with PVTT to prolong survival.
Tumor cells produce erythropoietin, which induces
the generation of neutrophils (22).
Neutrophil generation stimulates active oxygen and cytokines to
kill tumor cells; however, it simultaneously inhibits TNF-α
production and increases the production of interleukin-1 and
endothelial growth factor, which promotes the formation of tumor
vessels that results in distant metastasis (23,24).
Lymphocytes participate in the tumor immune response and induce
apoptosis of tumor cells; thus, they inhibit tumor development and
recurrence (25). A high N/L ratio
indicates that the number of neutrophils is higher compared with
that of lymphocytes; a high N/L ratio is considered a prognostic
factor for the progression and metastasis of liver cancer (11–13). In
the present study, the N/L ratio was significantly associated with
distant metastasis in intermediate to advanced HCC.
Thus far, few studies have addressed the role of RBC
count in the prognosis of cancer. A previous study demonstrated
that a low preoperative RBC count is an independent risk factor for
poor prognosis (overall survival) of primary liver cancer following
surgical treatment, which may be explained by the observation that
preoperative RBC counts indicate worse Child-Pugh grades in
patients, indicating the deterioration of liver function (26). The results of the present study
demonstrated that high RBC counts (≥4.07×1012 cells/l)
were independently associated with distant metastasis of HCC with
PVTT. Tumor-associated macrophages, which are crucial to the
progression and prognosis of cancer (27–29),
arise from the spleen (30).
Macrophages serve a crucial role in erythropoiesis under
pathological conditions (31–33) and
promote tumor growth, at least partially by stimulating tumor
stress-induced erythropoiesis in the spleen (33). This suggests that a high RBC count
may be associated with poor prognosis, which may explain the
positive association between high RBC counts and distant metastasis
of HCC with PVTT in the present study. The role of RBC count in the
prognosis of HCC with PVTT needs additional investigation.
CRP is synthesized in hepatic cells and rapidly
released into the plasma in the presence of tissue injury,
infection and malignant tumors (34,35). CRP
is commonly used as a systemic inflammatory marker and is
significantly associated with poor survival or recurrence of HCC
(36,37). CRP level has even been considered an
independent predictor of recurrence of HCC with PVTT (38). In the present study, a high CRP level
(≥7.02 mg/l) was an independent risk factor for distant metastasis
of HCC with PVTT.
A high AST level is considered to be an independent
predictor of poor survival of HCC after treatment (39,40), but
a correlation between AST and HCC metastasis has not been reported.
In the present study, high AST levels (≥118.5 U/l) were
significantly associated with distant metastasis in primary HCC
with PVTT.
A previous report demonstrated that in patients with
HCC, PVTT in the main trunk or the first branch caused a
significantly higher occurrence of intrahepatic metastasis compared
with that in other locations (41).
By contrast, the results of the present study revealed that PVTT in
the branch was significantly associated with distant metastasis of
HCC with PVTT. Further study is required to verify this result.
Several prediction models for distant metastasis of
HCC following treatment are available (9,42,43);
however, to the best of our knowledge, no prediction systems for
distant metastasis of HCC with PVTT have been reported thus far.
Routine laboratory parameters that are significantly associated
with distant metastasis, including N/L ratio ≥2.31, RBC count
≥4.07×1012 cells/l, CRP level ≥7.02 mg/l, AST level
≥118.5 U/l and tumor thrombus site at branch, were selected and
combined to develop an early risk warning model, Y=−5.276 + 1.711 ×
CRP (mg/l) + 1.312 × RBC (×1012 cells/l) + 1.192 × N/L +
0.912 × AST (U/l) + 0.553 × tumor thrombus site (branch), to
predict distant metastasis in patients with HBV-HCC and PVTT.
Patients with a high Y value (>-1.05) had a considerably higher
possibility of distant metastasis compared with those with a lower
Y value in the modeling and validation cohorts. In subsequent
studies with a sufficiently larger number of patients, the Y
threshold for the formula may be determined more accurately to
achieve a more accurate prediction of distant metastasis of HCC
with PVTT. This model may be beneficial for the identification of
patients with HCC with PVTT with a high risk of distant metastasis
to actively provide target treatment (such as liver resection,
TACE, TACE with sorafenib or radiotherapy) to prevent and treat
metastasis as early as possible. Early prediction of metastasis in
combination with current or future treatment methods for metastasis
may considerably improve the treatment outcomes and prognosis of
patients with intermediate to advanced HCC. To the best of our
knowledge, similar results have not been reported thus far. Of
note, all the variables included in the model are easily assessed
in the clinic, which suggests that the established model may be
clinically practical and convenient.
This study has several limitations. First, this was
a retrospective study involving medical records from only a single
medical center, which may have caused bias of patient selection and
incompleteness of patient clinical information. For example,
Child-Pugh scores are usually used to evaluate hepatic function,
whereas in the present study, TBIL, ALP and PTA, which similarly
reflect hepatic function, were used instead. Crucial factors
associated with metastasis or prognosis such as HBV markers (HBsAg
titers, HBV DNA levels, testing positive or negative for HBeAg and
receipt or non-receipt of HBV treatment), des-γ-carboxy
prothrombin, the site (intrahepatic or extrahepatic) of metastasis,
HCC treatment history (TACE, transcatheter arterial infusion or
chemotherapy) and the time interval between the evaluation of
clinical parameters and the diagnosis of distant metastasis were
not evaluated in the present study, as complete information for all
patients was not available. Consequently, these variables were not
included in the analysis. Additionally, the sample size of patients
with HCC with PVTT, particularly in the validation cohort, was
limited. In addition, diagnosis of HCC with PVTT was only based on
clinical imaging diagnosis without gold-standard criteria (such as
histological observation), which may have weakened the validation
of the established model based on the clinical information of the
modeling cohort. A prospective study with a larger sample size
conducted at multiple centers and with numerous and comprehensive
parameters, including Child-Pugh scores, HBV markers, des-γ-carboxy
prothrombin, the site of metastasis, HCC treatment history, the
time interval between the evaluation of clinical parameters and the
diagnosis of distant metastasis and survival data, is needed to
validate the results of the present study.
In conclusion, the present study revealed the risk
factors significantly associated with distant metastasis of HBV-HCC
and PVTT and established an early risk warning model for distant
metastasis. This model may facilitate the early prediction and
treatment of distant metastasis of HBV-HCC with PVTT to improve the
prognosis of patients.
Acknowledgements
Not applicable.
Funding
This study was supported by the Fund of Special
Research of Traditional Chinese Medicine in the Capital City (grant
no. 17ZY02), the Fund for Beijing Science and Technology
Development of Traditional Chinese Medicine (grant no. JJ2016-14)
and the Application of Clinical Features of the Capital City of the
Science and Technology Commission (grant no. Z171100001017082).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZY and YJ designed this study. XL and SZ collected,
assembled and analyzed data. ML and YZ analyzed and interpreted
data. ML and YZ drafted the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Beijing Ditan Hospital, Capital Medical University. As this study
was retrospective, informed consent was not required from the
patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liang X, Bi S, Yang W, Wang L, Cui G, Cui
F, Zhang Y, Liu J, Gong X, Chen Y, et al: Reprint of:
Epidemiological serosurvey of Hepatitis B in China-declining HBV
prevalence due to Hepatitis B vaccination. Vaccine. 31 (Suppl
9):J21–J28. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cui Y and Jia J: Update on epidemiology of
hepatitis B and C in China. J Gastroenterol Hepatol. 28 (Suppl
1):S7–S10. 2013. View Article : Google Scholar
|
|
4
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bruix J and Sherman M; Practice Guidelines
Committee, : American Association for the Study of Liver Diseases:
Management of hepatocellular carcinoma. Hepatology. 42:1208–1236.
2015. View Article : Google Scholar
|
|
6
|
Bruix J and Sherman M; American
Association for the Study of Liver Diseases, : Management of
hepatocellular carcinoma: An update. Hepatology. 53:1020–1022.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Addario L, Tritto G, Cavaglià E, Amodio F,
Giannelli E and Di Costanzo GG: Preserved liver function, portal
thrombosis and absence of oesophageal varices are risk factors for
metastasis of hepatocellular carcinoma. Dig Liver Dis. 43:319–324.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang ZM, Lai EC, Zhang C, Yu HW, Liu Z,
Wan BJ, Liu LM, Tian ZH, Deng H, Sun QH and Chen XP: The strategies
for treating primary hepatocellular carcinoma with portal vein
tumor thrombus. Int J Surg. 20:8–16. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kokudo T, Hasegawa K, Matsuyama Y,
Takayama T, Izumi N, Kadoya M, Kudo M, Ku Y, Sakamoto M, Nakashima
O, et al: Survival benefit of liver resection for hepatocellular
carcinoma associated with portal vein invasion. J Hepatol.
65:938–943. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
European Association for the Study of the
Liver. Electronic address, . easloffice@easloffice.eu; European
Association for the Study of the Liver: EASL 2017 clinical practice
guidelines on the management of hepatitis B virus infection. J
Hepatol. 67:370–398. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Okamura Y, Sugiura T, Ito T, Yamamoto Y,
Ashida R, Mori K and Uesaka K: Neutrophil to lymphocyte ratio as an
indicator of the malignant behaviour of hepatocellular carcinoma.
Br J Surg. 103:891–898. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lu SD, Wang YY, Peng NF, Peng YC, Zhong
JH, Qin HG, Xiang BD, You XM, Ma L and Li LQ: Preoperative ratio of
neutrophils to lymphocytes predicts postresection survival in
selected patients with early or intermediate stage hepatocellular
carcinoma. Medicine (Baltimore). 95:e27222016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu X, He L, Han J, Wang L, Li M, Jiang Y,
Wang X and Yang Z: Association of neutrophil-lymphocyte ratio and T
lymphocytes with the pathogenesis and progression of HBV-associated
primary liver cancer. PLoS One. 12:e01706052017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pawarode A, Voravud N, Sriuranpong V,
Kullavanijaya P and Patt YZ: Natural history of untreated primary
hepatocellular carcinoma: A retrospective study of 157 patients. Am
J Clin Oncol. 21:386–391. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tawada A, Chiba T, Ooka Y, Kanogawa N,
Motoyama T, Saito T, Ogasawara S, Suzuki E, Maruyama H, Kanai F, et
al: Efficacy of transarterial chemoembolization targeting portal
vein tumor thrombus in patients with hepatocellular carcinoma.
Anticancer Res. 34:4231–4237. 2014.PubMed/NCBI
|
|
16
|
Villa E, Moles A, Ferretti I, Buttafoco P,
Grottola A, Del Buono M, De Santis M and Manenti F: Natural history
of inoperable hepatocellular carcinoma: Estrogen receptors' status
in the tumor is the strongest prognostic factor for survival.
Hepatology. 32:233–238. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huang M, Lin Q, Wang H, Chen J, Bai M,
Wang L, Zhu K, Jiang Z, Guan S, Li Z, et al: Survival benefit of
chemoembolization plus Iodine125 seed implantation in unresectable
hepatitis B-related hepatocellular carcinoma with PVTT: A
retrospective matched cohort study. Eur Radiol. 26:3428–3436. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Byeon J, Cho EH, Kim SB and Choi DW:
Extrahepatic recurrence of hepatocellular carcinoma after curative
hepatic resection. Korean J Hepatobiliary Pancreat Surg. 16:93–97.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jun L, Zhenlin Y, Renyan G, Yizhou W,
Xuying W, Feng X, Yong X, Kui W, Jian L, Dong W, et al: Independent
factors and predictive score for extrahepatic metastasis of
hepatocellular carcinoma following curative hepatectomy.
Oncologist. 17:963–969. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Morimoto Y, Nouso K, Wada N, Takeuchi Y,
Kinugasa H, Miyahara K, Yasunaka T, Kuwaki K, Onishi H, Ikeda F, et
al: Involvement of platelets in extrahepatic metastasis of
hepatocellular carcinoma. Hepatol Res. 44:E353–E359. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee CH, Lin YJ, Lin CC, Yen CL, Shen CH,
Chang CJ and Hsieh SY: Pretreatment platelet count early predicts
extrahepatic metastasis of human hepatoma. Liver Int. 35:2327–2336.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jang WS, Cho KS, Kim MS, Yoon CY, Kang DH,
Kang YJ, Jeong WS, Ham WS and Choi YD: The prognostic significance
of postoperative neutrophil-to-lymphocyte ratio after radical
prostatectomy for localized prostate cancer. Oncotarget.
8:11778–11787. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Duan RD and Nilsson A: Metabolism of
sphingolipids in the gut and its relation to inflammation and
cancer development. Prog Lipid Res. 48:62–72. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Paramanathan A, Saxena A and Morris DL: A
systematic review and meta-analysis on the impact of pre-operative
neutrophil lymphocyte ratio on long term outcomes after curative
intent resection of solid tumours. Surg Oncol. 23:31–39. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Qin M, Brummel S, Singh KK, Fenton T and
Spector SA: Associations of host genetic variants on CD4+
lymphocyte count and plasma HIV-1 RNA in antiretroviral naïve
children. Pediatr Infect Dis J. 33:946–952. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xie X, Yao M, Chen X, Lu W, Lv Q, Wang K,
Zhang L and Lu F: Reduced red blood cell count predicts poor
survival after surgery in patients with primary liver cancer.
Medicine (Baltimore). 94:e5772015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Noy R and Pollard JW: Tumor-associated
macrophages: From mechanisms to therapy. Immunity. 41:49–61. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ries CH, Cannarile MA, Hoves S, Benz J,
Wartha K, Runza V, Rey-Giraud F, Pradel LP, Feuerhake F, Klaman I,
et al: Targeting tumor-associated macrophages with anti-CSF-1R
antibody reveals a strategy for cancer therapy. Cancer Cell.
25:846–859. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tang X: Tumor-associated macrophages as
potential diagnostic and prognostic biomarkers in breast cancer.
Cancer Lett. 332:3–10. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cortez-Retamozo V, Etzrodt M, Newton A,
Rauch PJ, Chudnovskiy A, Berger C, Ryan RJ, Iwamoto Y, Marinelli B,
Gorbatov R, et al: Origins of tumor-associated macrohages and
neutrophils. Proc Natl Acad Sci USA. 109:2491–2496. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chow A, Huggins M, Ahmed J, Hashimoto D,
Lucas D, Kunisaki Y, Pinho S, Leboeuf M, Noizat C, van Rooijen N,
et al: CD169+ macrophages provide a niche promoting erythropoiesis
under homeostasis and stress. Nat Med. 19:429–436. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ramos P, Casu C, Gardenghi S, Breda L,
Crielaard BJ, Guy E, Marongiu MF, Gupta R, Levine RL, Abdel-Wahab
O, et al: Macrophages support pathological erythropoiesis in
polycythemia vera and β-thalassemia. Nat Med. 19:437–445. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu M, Jin X, He X, Pan L, Zhang X and
Zhao Y: Macrophages support splenic erythropoiesis in 4T1
tumor-bearing mice. PLoS One. 10:e01219212015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Eklund CM: Proinflammatory cytokines in
CRP baseline regulation. Adv Clin Chem. 48:111–136. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Grivennikov SI, Greten FR and Karin M:
Immunity, inflammation and cancer. Cell. 140:883–899. 2000.
View Article : Google Scholar
|
|
36
|
Liu YB, Ying J, Kuang SJ, Jin HS, Yin Z,
Chang L, Yang H, Ou YL, Zheng JH, Zhang WD, et al: Elevated
preoperative serum Hs-CRP level as a prognostic factor in patients
who underwent resection for hepatocellular carcinoma. Medicine
(Baltimore). 94:e22092015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chan SL, Chan AW, Chan AK, Jian P, Mo F,
Chan CM, Mok K, Liu C, Chong CC, Chan AT, et al: Systematic
evaluation of circulating inflammatory markers forhepatocellular
carcinoma. Liver Int. 37:280–289. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kim JM, Kwon CH, Joh JW, Ko JS, Park JB,
Lee JH, Kim SJ, Paik SW and Park CK: C-reactive protein may be a
prognostic factor in hepatocellular carcinoma with malignant portal
vein invasion. World J Surg Oncol. 11:922013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang Q, Shang L, Zang Y, Chen X, Zhang L,
Wang Y, Wang L, Liu Y, Mao S and Shen Z: α-Fetoprotein is a
potential survival predictor in hepatocellular carcinoma patients
with hepatitis B selected for liver transplantation. Eur J
Gastroenterol Hepatol. 26:544–552. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liao W, Zhang J, Zhu Q, Qin L, Yao W, Lei
B, Shi W, Yuan S, Tahir SA, Jin J and He S: Preoperative
neutrophil-to-lymphocyte ratio as a new prognostic marker in
hepatocellular carcinoma after curative resection. Transl Oncol.
7:248–255. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Choi Y, Kim JW, Cha H, Han KH and Seong J:
Overall response of both intrahepatic tumor and portal vein tumor
thrombosis is a good prognostic factor for hepatocellular carcinoma
patients receiving concurrent chemoradiotherapy. J Radiat Res.
55:113–120. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Huang X, Wei W, Ya N, Zeng J, Zeng Y, Ma
C, Chi M, Wu Y, Li Y, Huang Y, et al: A mathematical model to
predict short-term recurrence and metastasis of primary
hepatocellular carcinoma larger than 10 cm in diameter.
Hepatogastroenterology. 60:225–230. 2013.PubMed/NCBI
|
|
43
|
Xiang ZL, Zeng ZC, Fan J, Wu WZ, He J,
Zeng HY and Tang ZY: A clinicopathological model to predict bone
metastasis in hepatocellular carcinoma. J Cancer Res Clin Oncol.
137:1791–1797. 2011. View Article : Google Scholar : PubMed/NCBI
|