Cancer remains one of the diseases with a poor
prognosis, which results in a high mortality rate (1). According to the 2019 American Cancer
Annual Report, approximately 1,762,450 new cases of cancer and
606,880 cancer-associated deaths were predicted in the United
States in 2019, with no significant improvement compared with the
previous year (1,2). The prevalence of cancer and associated
death statistics are also not optimistic in China; in 2015,
4,292,000 new cases of cancer were estimated and more than half of
the cases were predicted to result in cancer-associated death in
2016 (3). Enormous economic burdens
have been placed on nations and society in response to the
increasing numbers of patients with cancer. For example, health
care spending on patients with breast cancer in the United States
has increased in recent years and the cost of treating patients
with human epidermal growth factor receptor-2-positive breast
cancer is predicted to rise from $2.7 billion in 2012 to $3.6
billion in 2035 (4). Therefore,
cancer prevention and treatment is currently a major problem.
Identifying natural food ingredients with low toxicity and minimal
adverse side effects that can effectively target tumors may be
valuable to cancer prevention and early treatment.
Curcumin, also known as diacetylmethane, is a
bioactive lipophilic flavonoid polyphenol compound extracted from
turmeric, the rhizome of the ginger family. Curcumin is an
orange-yellow crystalline powder and its molecular weight is 368.38
(5). Curcumin is almost insoluble in
water but soluble in ethanol and dimethyl sulfoxide (5). In addition to the use of curcumin as a
food flavoring, curcumin has also been used in India and South Asia
to treat a variety of diseases, including fever, gastrointestinal
disorders, skin diseases, rheumatism, hepatitis, arthritis and
burns due to its non-toxic, anti-oxidant, anti-inflammatory and
anti-infection properties (6–9).
Previous findings have shown that curcumin also has
significant anticancer effects. Curcumin has been shown to inhibit
proliferation, invasion, metastasis and angiogenesis, weaken
chemoresistance and inhibits the immortalization of cancer cells.
Curcumin has been shown to modulate signaling pathways involving
Janus kinase (JAK)/signal transducers and activators of
transcription (STAT), phosphatidylinositol 3-kinase (PI3K)/protein
kinase B (Akt)/mammalian target of rapamycin (mTOR), Wnt/β-catenin,
vascular endothelial growth factor (VEGF)/vascular endothelial
growth factor receptor (VEGFR) and genes such as cyclin D1,
TP53, BAX, BCL-2, hTERT and MMPs (10–14);
these pathways have been confirmed to modulate the proliferation,
migration and invasion, cell cycle and cell apoptosis of tumor
cells (15–17).
During tumor progression, the tumor
immunosuppressive microenvironment is considered to serve a crucial
role in immune escape and subsequent progression of tumors
(18). In addition to curcumin
inhibiting tumors through the abovementioned mechanisms, curcumin
also enhances the anticancer immune response, remodels the tumor
immunosuppressive microenvironment and exerts influence on
lymphocyte infiltration, such as cytotoxic T cells (CTL) (19), Foxhead p3 (FOXP3)+ regulatory T cells
(FOXP3+Tregs) (20) and natural
killer (NK) cells (21), and the M1
to M2 macrophage transformation in the process of innate immunity
(22).
The present review briefly summarizes the important
roles of curcumin in the treatment of tumors, with a focus on the
functions of curcumin in tumor immunity regulation, and describes
recent clinical trials investigating the potential of curcumin in
cancer therapy.
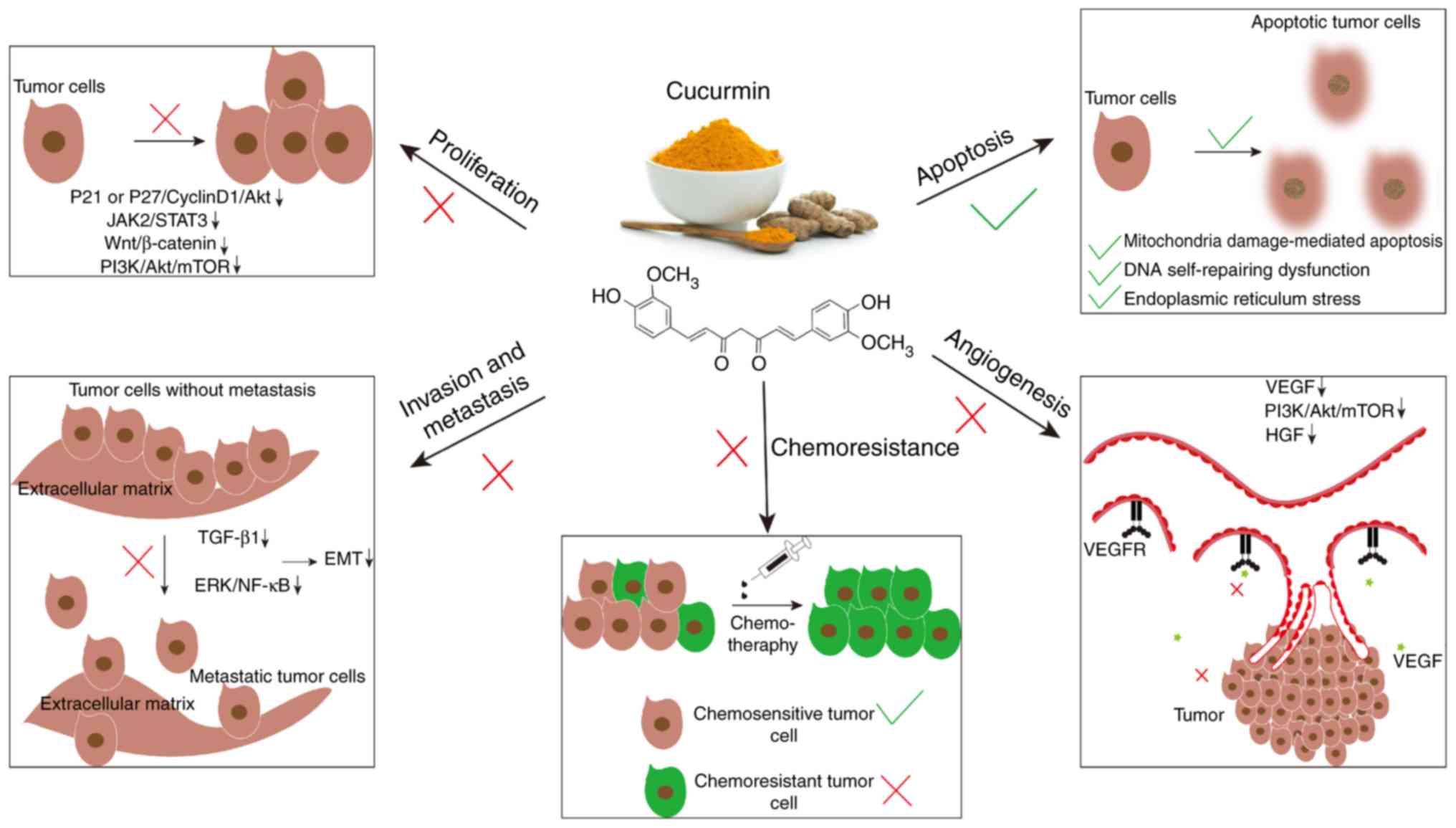
Curcumin can inhibit various malignant biological
behaviors of tumor cells, including tumor proliferation and growth,
invasion, metastasis, neoangiogenesis and chemoresistance, as
summarized in Fig. 1.
Previous studies have shown that curcumin can
regulate tumor proliferation and growth through multiple signal
pathways (10,23). Human trophoblastic surface antigen-2
(Trop2) is a cell surface glycoprotein, which serves an essential
role in tumor progression and tumorigenesis (24). A recent study in bladder cancer
showed that Trop2 abolished the increased expression of p27 caused
by curcumin, thereby mediating tumor proliferation (25). Therefore, Trop2 may be an
intermediate link in the inhibition of the tumor proliferation
signal pathway by curcumin, with potential for therapeutic targets.
However, curcumin has also been shown to inhibit pancreatic cancer
cells proliferation through p21 or the p27/Cyclin D1/PI3K/Akt
signaling pathway (26).
The Wnt/β-catenin signaling pathway is involved in
the mechanisms underlying the function of curcumin in hindering
tumor growth and proliferation. Wang et al (27) and Srivastava and Srivastava (28) demonstrated that curcumin inhibits the
proliferation of lung cancer cell lines. Wang et al
(27) also demonstrated that
curcumin inhibited tumor oxidative stress with subsequent
inactivation of the Wnt/β-catenin signaling pathway. Moreover,
phosphorylation-mediated inactivation of the JAK2/STAT3 signaling
pathway is also involved in the anti-proliferation effect of
curcumin on osteosarcoma cells (29). Interestingly, in lung cancer,
increased expression levels of long non-coding RNA (lncRNA) UCA1
counteracted the anticancer proliferation effects of curcumin,
suggesting that lncRNA UCA1 may be involved in curcumin-mediated
inactivation of Wnt and mTOR signaling pathways (30).
Apoptosis is the process of programmed cell death
that is caused by damage to DNA or other organelles, such as
mitochondria and endoplasmic reticulum, when cells are externally
stimulated (31). Apoptosis can be
induced by several exogenous pathways, including pathways mediated
by death receptor Fas and the tumor necrosis factor receptor family
(32,33), cytokine-mediated endogenous pathways
and caspase-12 activation caused by endoplasmic reticulum stress
(34). Curcumin is hypothesized to
mediate tumor cell apoptosis through these exogenous pathways.
Synthetic curcumin non-spherical mesoporous silica nanoparticles
can increase the carrying capacity and saturability of curcumin
(35). In-depth studies have shown
that curcumin binds apoptotic proteins, such as caspase-3 (36), phosphatase and tensin homolog deleted
from chromosome 10 and poly ADP-ribose polymerase (37), inducing mitochondrial damage and
thereby promoting tumor cell apoptosis (35). Another synthetic nanomaterial,
chitosan nanoparticles loaded with demethoxycurcumin in combination
with cisplatin, downregulates the expression levels of thymidine
phosphorylase required for DNA self-repairing pyrimidine salvage
pathways and induces apoptosis in non-small cell lung cancer
(NSCLC) cell lines (38). Moreover,
Wang et al (27) demonstrated
that in NSCLC, curcumin decreases the mitochondrial transmembrane
potential and increases the accumulation of reactive oxygen species
(ROS) in cells, inducing DNA and mitochondrial damage-mediated
apoptosis (39).
The invasion and metastasis ability of tumor cells
is a common cause of tumor treatment failure (40) and curcumin can significantly inhibit
these activities in tumor cells. A study of oral squamous cell
carcinoma showed that curcumin reduced cell adhesion and inhibited
proliferation of tumor cells with mesenchymal features (41). In addition, tumor growth factor-β1
(TGF-β1) is an important promoter of the epithelial-mesenchymal
transition (EMT) in tumor cells. In liver cancer, curcumin can
decreased the expression levels of TGF-β1, inhibit the
phosphorylation and nuclear translocation of Smad2, reduce the
specific binding of Smad2 to the Snail promoter, downregulate Snail
expression levels and inhibit EMT by competing with TGF-β1
(42). A moderate amount of ROS
accumulation has been reported to be beneficial to tumor
progression (43). Curcumin reversed
the effect of H2O2 and ROS on pancreatic
cancer cell invasion and metastasis by specifically inhibiting the
extracellular regulated protein kinase (ERK)/nuclear factor kappa-B
(NF-κB) signal pathway (44).
The intrinsic resistance and adaptive resistance of
tumors to chemotherapeutics are important reasons for chemotherapy
failure (50). Therefore,
identifying efficient chemotherapy sensitizers is important to
address this problem. Curcumin has been demonstrated to exert
specific functions in resensitizing certain types of cancer to
chemotherapy, such as colorectal cancer and ovarian cancer.
Chemotherapeutic drugs, such as irinotecan, 5-fluorouracil and
capecitabine, show a decline in efficacy during colorectal cancer
therapy and cancer stem cells (CSCs) are considered to influence
this process (51). Curcumin
increases the sensitivity of colon cancer cells to capecitabine by
inducing apoptosis of CSCs (51). In
addition, the combination of mitomycin C and curcumin downregulates
the expression levels of anti-apoptotic proteins Bcl-2 and Bcl-w
and induces the apoptosis of breast CSCs (52). Zhang et al (53) demonstrated that curcumin restores
lncRNA MEG3 function in ovarian cancer cells and exosomal vesicles
via demethylation, reduces the expression levels of miR-214, which
can induce cell survival and cisplatin resistance through targeting
the PTEN/Akt pathway (54).
In addition, post-transcriptional modifications of
some tumor-specific antigen genes, such as GIL1 in multiple myeloma
(67) and hPMS1 in oral squamous
cell carcinoma (68), may result in
their downregulated expression or complete silencing. The
cancer-testis family is expressed on a variety of cell surfaces and
hypomethylation of the cancer-testis family promoter facilitates
gene expression and thus expression on the tumor cell surface;
however, in contrast, demethylation of this promotor occurs in
tumor cells mediating tumor immune tolerance (69). DNA methyltransferase or histone
deacetylase activity can restore the gene expression of
tumor-specific antigens to a certain degree, mediating anticancer
immunity (70,71). However, Rosenthal et al
(72) indicated that
hypermethylation of gene promoters of mutant neoantigens was also a
possible mechanism leading to tumor immunoediting in early stage
NSCLC.
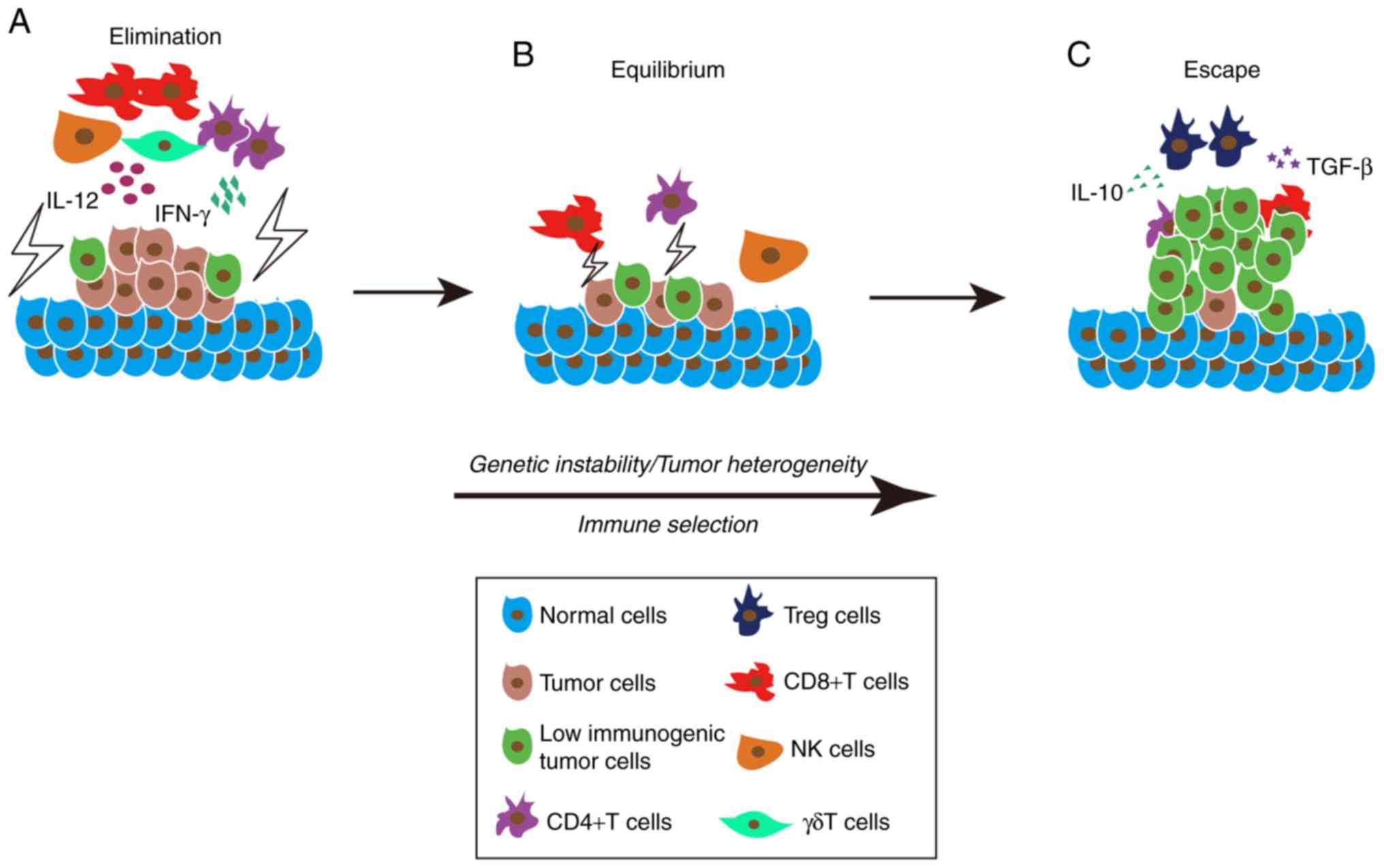
Increasing studies have shown that the interaction
between the immune microenvironment and tumor cells is a process of
dynamic equilibrium, which was called immunoediting by Dunn et
al (73). Immunoediting is the
entire process of editing and shaping the immunosuppressive
microenvironment and is also a Darwinian selection process.
Immunoediting consists of three phases: Elimination (also called
immunosurveillance); equilibrium (also called cancer dormancy); and
escape and collectively these are referred to as the 3 ‘E's’ of
immune editing (73). In the first
phase, a growing tumor recruits innate immune cells such as NK
cells and γδT cells, which mediate perforin, granzyme, FasL and
TraiL-dependent cytotoxic effects through releasing interferon-γ
(IFN-γ), IL-12 and other tumor suppressive cytokines (74,75). In
addition, antigen-presenting cells present tumor-specific antigens
to CD4+T cells thereby recruiting specific CD8+ T cells to kill
tumor cells (76). In the second
phase, adaptive immunity, rather than innate immunity, prevents
tumors from continuing to grow and confers immunogenicity to tumor
cells to mediate immune recognition (77). Tumor cells hijack the various
mechanisms mentioned above to inhibit the immune system killing
mechanisms allowing some of the less immunogenic tumor cells to
survive (77). In addition, tumor
cell instability caused by gene mutations also confers the ability
of some tumor cells to escape from immune recognition (78). Thus, tumor cells and the immune
system can reach an equilibrium state, and tumor proliferation and
tumor apoptosis also reach an equilibrium state (77). This balancing process may be inclined
toward tumor cell elimination or tumor cell escape depending on the
progression of tumor and may last for months to years (79). In the third phase, the tumor further
grows and becomes a clinically detectable tumor, which gradually
suppresses the immune system and shapes the immunosuppressive
microenvironment (80). Tumor cells
regulate the immune response of T cells and promote their apoptosis
through releasing immunosuppressive cytokines, such as TGF-β,
IL-10, galectin and indole 2,3-dioxygenase (81). In addition, intratumoral infiltration
of CD4+CD25+FOXP3+Tregs in tumor cells increases TGF-β and IL-10
expression levels, IL-2 expression level knockdown, and reduces
co-stimulation and activation of DCs (82). The three phases of immune editing
ultimately lead to the immune escape of tumor cells as summarized
in Fig. 2.
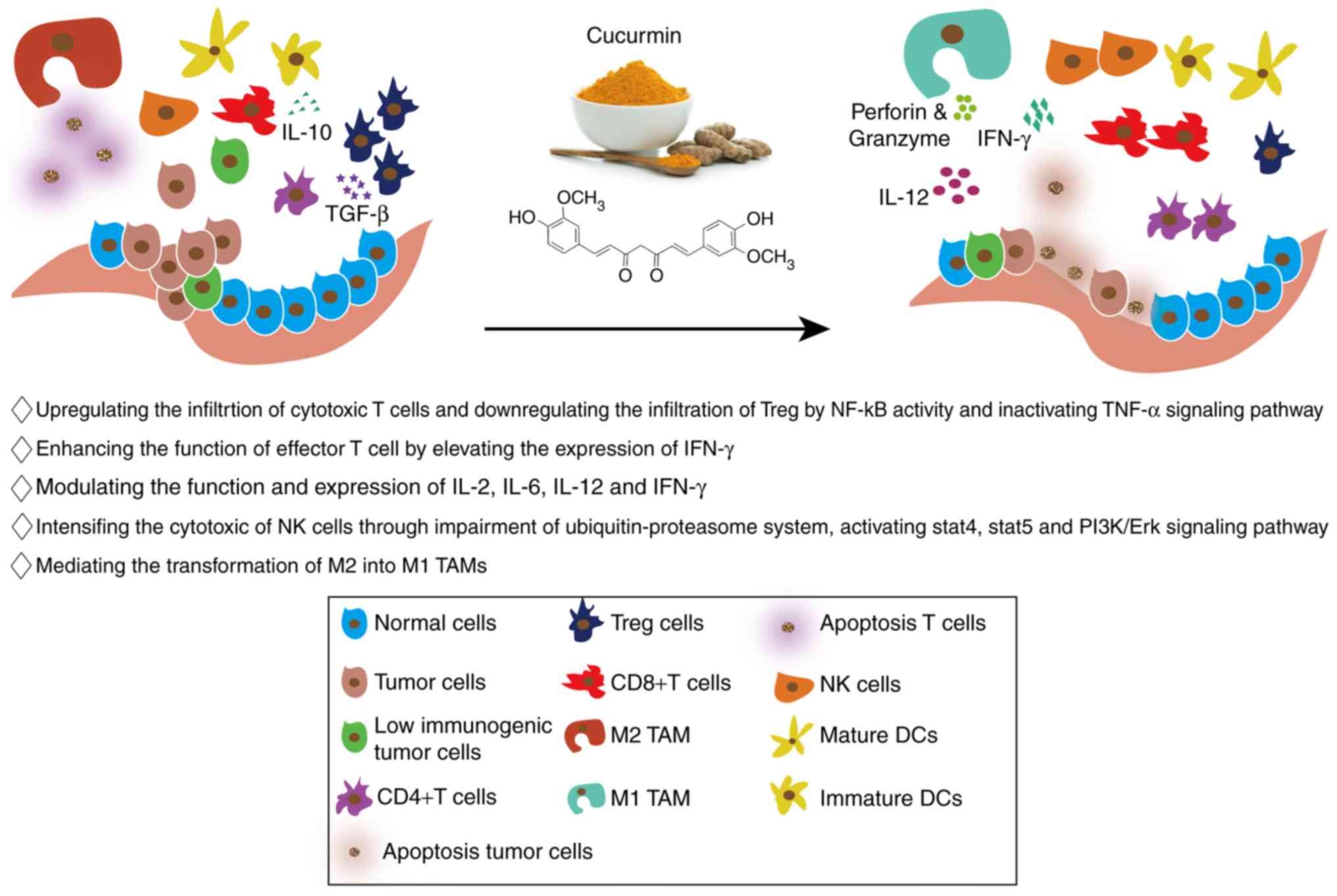
Curcumin not only inhibits tumors by affecting the
biological behaviors of tumor cells, but it also regulates the
composition of different components in the tumor immune
microenvironment, so that the immune microenvironment is conducive
to tumor killing (83). The detailed
roles of curcumin in tumor immunomodulation are shown in Fig. 3.
In the tumor immunosuppressive microenvironment, due
to the lack of effective presentation of tumor-specific antigens
and the influence of various inhibitory cytokines, a large number
of inactivated CD4+ and CD8+ T cells and CTLs undergo apoptosis
(78). Curcumin has been shown to
effectively reverse this process. During tumor generation, tumor
cells can induce atrophy of the thymus, reduce the production of
mature T cells and enable escape from the adaptive immune response.
Tumor cells induce apoptosis of T cells by interfering with the
production of NF-κB in T cells, making T cells susceptible to
TNF-α-mediated apoptosis (84).
Curcumin neutralizes oxidative stress of tumor cells, restores
NF-κB activity and reactivates the TNF-α signal pathway, thereby
enhancing the ability of T cells to resist apoptosis (84). Bhattacharyya et al (84) found that mechanisms of tumor cell
immune escape included loss of effector T cells and memory T cell
subsets, secretion of type II cytokines and increased proliferation
of Treg cells. In Ehrlich's ascites carcinoma-bearing nude mice
treated with curcumin, curcumin inhibited the apoptosis of T cells,
expanded the number of central memory cells and effector memory T
cells and disabled the functions of Treg cells, thus successfully
reversing the tumor immunosuppressive microenvironment (84). A clinical trial also demonstrated
that in patients with colon cancer, transcription and expression of
the FOXP3 gene was inhibited by curcumin, thereby permitting
the conversion of FOXP3+Treg cells into Th1 cells (85). Curcumin also abolished the induction
of IFN-γ secretion from CD4+ T cells by inhibiting Treg cell
function (85). Zou et al
(86) also demonstrated the
inhibition of Treg function by curcumin in lung cancer.
The effect of curcumin on T cells not only increases
the number of effector T cells or to induce the infiltration of
Treg cells, but more importantly, enhances the cell killing
function of effector T cells inhibited by tumor cells (87). A novel nanocurcumin significantly
increased the expression levels of co-stimulatory molecule CD86 on
the surface of DCs and decreased the levels of pro-inflammatory
factors secreted by effector T cells in vitro (87). Moreover, curcumin was also reported
to increased effector T cell-induced cytotoxicity against
esophageal cancer cells (87).
Adoptive T-cell therapy has become an important research field of
tumor immunotherapy. Autologous reinfusion of effector T cells,
which proliferate in vitro, can increase the number of
exogenous effector T cells and induce tumor apoptosis in humans
(88). However, due to the numerous
immunosuppressive cytokines in the tumor immunosuppressive
microenvironment, the efficacy of adoptive T-cell therapy is often
transient and prone to failure (89). In the E.G7 mouse T lymphoma model,
the combination of adaptive T-cell therapy and curcumin led to
increased intratumoral infiltration of CD8+ T cells at the end of
the course and curcumin increased the levels of IFN-γ secreted by
CD8+T cells (90). These results
showed that combination therapy had greater efficacy compared with
adaptive T-cell therapy alone. Different doses of curcumin were
administered to mice bearing tumors derived from the 3LL breast
cancer cell line and a low dose of curcumin led to delayed tumor
growth and prolonged survival of the mice. An in vitro study
also demonstrated that low doses of curcumin increased the number
of CD8+T cells and enhanced their IFN-γ secretion capacity
(91). Administration of high doses
of curcumin, however, reduced the number of CD8+ T cells,
indicating that the anti-tumor effect of curcumin may be
dose-dependent (91). Moreover, the
combination of an intravenously administered polyethylene glycol
curcumin coupler, rather than an oral formulation, and a
tyrosinase-related peptide vaccine for the treatment of melanoma
significantly increased the CTL response and IFN-γ expression
levels compared with the vaccine alone, while the combination
therapy also decreased the levels of tumor immunosuppressive cells,
such as marrow-derived suppressor cells and Treg cells (92).
During the gradual shaping of the tumor
immunosuppressive microenvironment, various cytokines are
considered to serve a crucial role in regulating the composition
and proportions of the cancer-promoting immune cell component and
the cancer-suppressing immune cell component (93). Curcumin, as a tumor inhibitor, not
only facilitates the secretion of cancer-promoting cytokines, but
also inhibits the secretion of cancer-suppressing cytokines,
thereby remodeling the tumor immunosuppressive microenvironment
(94). IL-2 plays a dual role,
stimulating and inhibiting the tumor immune response. Since
high-affinity IL-2 receptor (IL-2R) is highly expressed on the
surface of Treg cells, IL-2 is a tumor promoter activating Treg
cells (95). Curcumin directly
inhibits the interaction between IL-2 and IL-2Rα (CD25), thus
inhibiting the activation of Treg cells (96). A previous study reported that
curcumin inhibits tumor cell apoptosis by downregulating the levels
of the tumor immunosuppressive cytokine IL-10, resulting in
JAK/STAT signal pathway malfunction (97). Curcumin also downregulates the levels
of TGF-β and IL-10 in Treg cells, reduces their ability to
stimulated Treg cells and decreases the Treg cell infiltration in
tumors (86). An increased
expression level of IL-6 in triple-negative breast cancer is
considered to mediate the tumor immunosuppressive microenvironment,
leading to tumor vaccine therapy failure (98). Curcumin is a specific inhibitor of
IL-6, and the combination of curcumin and breast cancer vaccine
Listeriaat-Mage-b resulted in decreased IL-6 expression levels and
increased IL-12 and IFN-γ levels, resulting in greater anti-tumor
efficacy compared with using vaccine alone (99). However, Bill et al (100) showed different results. Although
previous reports showed that curcumin promoted tumor apoptosis
(36–38), curcumin-pretreated human A375
melanoma cells led to STAT1 phosphorylation inhibition and
decreased downstream IFN-γ and IFN-α expression levels. In
addition, co-culture of NK cells and these curcumin-pretreated
melanoma cells also reduced IL-12-induced IFN-γ expression, which
indicated curcumin may also inhibit the expression of
cancer-suppressing cytokines.
Curcumin not only influences adaptive immunity
through regulating T cell proliferation and apoptosis, but it also
influences NK cell-mediated innate immunity (101). In a study of breast cancer,
exosomes secreted by tumor cells abolished the activation of NK
cells via IL-2 by inhibiting the JAK3/STAT5 signal pathway
(102). Curcumin may inhibit the
cytotoxicity of exosomes to circulating immune cells by disrupting
the ubiquitin protease system and increasing the ubiquitinated
exosomal proteins that interfere the function of normal exosomal
proteins (102). Another study in
breast cancer showed that curcumin increased the expression of
CD16+ and CD56dim on the surface of NK-92 cells
(103). In addition, the effect of
curcumin on promoting cytotoxic ability of NK cells is also
associated with the activation of STAT4 and STAT5 in NK cells and
the suppression of pERK and PI3K expression in curcumin-induced
breast cancer cells (103). In a
study of pancreatic cancer, curcuminoids enhanced the ability of NK
cells to secrete IFN-γ and promoted the killing effect of NK cells
in pancreatic cancer cell lines (104).
Tumor-associated macrophages (TAMs) are classified
into inducible nitric oxide synthase (NOS) (+) M1 macrophages and
ARG1 (+) M2 macrophages according to their function following a
strict binary classification, which was proposed by Mills et
al (105). The former exhibit
tumor-suppressing functions while the latter show tumor-promoting
ability. M1 TAMs show high expression levels of IL-12 and low
expression levels of IL-10, whereas M2 TAMs exhibit the opposite
expression (106). In a study of
HPV+ tumors, injection of TriCurin, a synergistic formulation of
curcumin, resveratrol and epicatechin gallate into tumor-bearing
mice transformed ARGhigh, IL-10high,
iNOSlow, IL-12low M2 TAMs into
ARGlow, IL-10low, iNOShigh,
IL-12high M1 TAMs. Subsequently, M1 TAMs induced STAT1
and NF-κB (p65) expression, subsequently increasing the expression
levels of IL-12 and thereby activating NK cells and CTLs, resulting
in cytotoxicity (107). The same
mechanism of action was also demonstrated in malignant glioma
(108,109).
To address the low bioavailability of curcumin, a
recent clinical trial investigated the combination of
phospholipid-coated curcumin using nanotechnology and gemcitabine
(111). Although the patient
quality of life was not significantly improved during treatment,
the patient drug response rate reached 27.3%, which was higher
compared with the rate of 12% of gemcitabine alone, and the average
progression-free survival and overall survival also reached 8.4 and
10.2 months, respectively (111).
Therefore, curcumin combined with gemcitabine is may be a safe and
effective therapy for wider application for pancreatic cancer
treatment; however, before the combination of curcumin and
gemcitabine therapy can become routine therapy for pancreatic
cancer large multicentric randomized controlled trials need to be
conducted. The recent ongoing and completed clinical trials of
curcumin in diverse tumors are summarized in Table I by referring to clinicaltrials.gov.
Curcumin, a traditional food ingredient, has been
identified to serve versatile roles in the prevention and treatment
of several diseases (119–121). Curcumin has also been identified to
inhibit a variety of biological functions of tumors, including
growth, proliferation, invasion, metastasis, apoptosis,
angiogenesis and drug resistance of tumor cells (122). In the present review, highlighted
the role of the tumor immunosuppressive microenvironment in tumor
progression and the functions of curcumin in modulating and
remodeling the tumor immunosuppressive microenvironment have been
highlighted. Curcumin increases the number of effector T cells
(78), reduces the infiltration of
FOXP3+Tregs (85), inhibits
cytokine-induced apoptosis of effector T cells and the expression
of tumor immunosuppressive cytokines (87,94),
enhances NK cell cytotoxicity and mediates M2 TAM transformation to
M1 TAM (101,105), which can change the tumor
immunosuppressive microenvironment and lead to the killing of tumor
cells. Therefore, given the multiple functions and good biosafety
of curcumin in inhibiting tumor progression, as a tumor-preventing
food and tumor-assisted therapeutic drug, curcumin has potential in
tumor treatment potential in combination with multimodal anticancer
therapies. However, curcumin has several limitations in clinical
practice such as its low bioavailability, drug dose-effect
disproportion and poor water solubility (123). To address these problems, an
increasing numbers of studies are focusing on how to enhance the
tolerance of curcumin, improve the targeting of curcumin delivery,
overcome low bioavailability and obtain better therapeutic effects
(124,125). A variety of nano-curcumin
formulations have been introduced, which have improved the
anti-cancer effects of curcumin (126,127).
It is considered that curcumin will serve a more important role in
the prevention and treatment of cancer in the future.
Not applicable.
The present study was supported by The China Academy
of Medical Sciences Innovation Fund for Medical Sciences (grant no.
2016-I2M-3-019).
Not applicable.
In this review, the concept and design was conducted
by YW and JG. YW and JL drafted the manuscript. Tables and diagrams
were generated by JL and BJ. Critical revision of the manuscript
for important intellectual content was conducted by YW, JL and BJ.
JG provided the funding support. All authors read and approved the
final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tartari F, Santoni M, Pistelli M and
Berardi R: Healthcare cost of HER2-positive and negative breast
tumors in the United States (2012–2035). Cancer Treat Rev.
60:12–17. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Maheshwari RK, Singh AK, Gaddipati J and
Srimal RC: Multiple biological activities of curcumin: A short
review. Life Sci. 78:2081–2087. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jin TR: Curcumin and dietary polyphenol
research: Beyond drug discovery. Acta Pharmacol Sin. 39:779–786.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sa G and Das T: Anti cancer effects of
curcumin: Cycle of life and death. Cell Div. 3:142008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Saha S, Adhikary A, Bhattacharyya P, Das T
and Sa G: Death by design: Where curcumin sensitizes drug-resistant
tumours. Anticancer Res. 32:2567–2584. 2012.PubMed/NCBI
|
|
9
|
Aggarwal BB and Harikumar KB: Potential
therapeutic effects of curcumin, the anti-inflammatory agent,
against neurodegenerative, cardiovascular, pulmonary, metabolic,
autoimmune and neoplastic diseases. Int J Biochem Cell Biol.
41:40–59. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Choudhuri T, Pal S, Das T and Sa G:
Curcumin selectively induces apoptosis in deregulated cyclin
D1-expressed cells at G2 phase of cell cycle in a p53-dependent
manner. J Biol Chem. 280:20059–20068. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kunnumakkara AB, Anand P and Aggarwal BB:
Curcumin inhibits proliferation, invasion, angiogenesis and
metastasis of different cancers through interaction with multiple
cell signaling proteins. Cancer Lett. 269:199–225. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang JB, Qi LL, Zheng SD and Wu TX:
Curcumin induces apoptosis through the mitochondria-mediated
apoptotic pathway in HT-29 cells. J Zhejiang Univ Sci B. 10:93–102.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee JH and Chung IK: Curcumin inhibits
nuclear localization of telomerase by dissociating the Hsp90
co-chaperone p23 from hTERT. Cancer Lett. 290:76–86. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shehzad A and Lee YS: Molecular mechanisms
of curcumin action: Signal transduction. Biofactors. 39:27–36.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bagratuni T, Mavrianou N, Gavalas NG,
Tzannis K, Arapinis C, Liontos M, Christodoulou MI, Thomakos N,
Haidopoulos D, Rodolakis A, et al: JQ1 inhibits tumour growth in
combination with cisplatin and suppresses JAK/STAT signalling
pathway in ovarian cancer. Eur J Cancer. 126:125–135. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Su T, Huang L, Zhang N, Peng S, Li X, Wei
G, Zhai E, Zeng Z and Xu L: FGF14 functions as a tumor suppressor
through inhibiting PI3K/AKT/mTOR pathway in colorectal cancer. J
Cancer. 11:819–825. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rebouissou S and Nault JC: Advances in
molecular classification and precision oncology in hepatocellular
carcinoma. J Hepatol. 72:215–229. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vinay DS, Ryan EP, Pawelec G, Talib WH,
Stagg J, Elkord E, Lichtor T, Decker WK, Whelan RL, Kumara HMCS, et
al: Immune evasion in cancer: Mechanistic basis and therapeutic
strategies. Semin Cancer Biol. 35 (Suppl):S185–S198. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mukherjee S, Hussaini R, White R, Atwi D,
Fried A, Sampat S, Piao L, Pan Q and Banerjee P: TriCurin, a
synergistic formulation of curcumin, resveratrol, and epicatechin
gallate, repolarizes tumor-associated macrophages and triggers an
immune response to cause suppression of HPV+ tumors. Cancer Immunol
Immunother. 67:761–774. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bahrami A, Fereidouni M, Pirro M, Bianconi
V and Sahebkar A: Modulation of regulatory T cells by natural
products in cancer. Cancer Lett. 459:72–85. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pan P, Huang YW, Oshima K, Yearsley M,
Zhang J, Arnold M, Yu J and Wang LS: The immunomodulatory potential
of natural compounds in tumor-bearing mice and humans. Crit Rev
Food Sci Nutr. 59:992–1007. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schnekenburger M, Dicato M and Diederich
MF: Anticancer potential of naturally occurring immunoepigenetic
modulators: A promising avenue? Cancer. 125:1612–1628. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sun XD, Liu XE and Huang DS: Curcumin
induces apoptosis of triple-negative breast cancer cells by
inhibition of EGFR expression. Mol Med Rep. 6:1267–1270. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
McDougall AR, Tolcos M, Hooper SB, Cole TJ
and Wallace MJ: Trop2: From development to disease. Dev Dyn.
244:99–109. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang L, Yang G, Zhang R, Dong L, Chen H,
Bo J, Xue W and Huang Y: Curcumin inhibits cell proliferation and
motility via suppression of TROP2 in bladder cancer cells. Int J
Oncol. 53:515–526. 2018.PubMed/NCBI
|
|
26
|
Zhao Z, Li C, Xi H, Gao Y and Xu D:
Curcumin induces apoptosis in pancreatic cancer cells through the
induction of forkhead box O1 and inhibition of the PI3K/Akt
pathway. Mol Med Rep. 12:5415–5422. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang JY, Wang X, Wang XJ, Zheng BZ, Wang
Y, Wang X and Liang B: Curcumin inhibits the growth via
Wnt/β-catenin pathway in non-small-cell lung cancer cells. Eur Rev
Med Pharmacol Sci. 22:7492–7499. 2018.PubMed/NCBI
|
|
28
|
Srivastava NS and Srivastava RAK: Curcumin
and quercetin synergistically inhibit cancer cell proliferation in
multiple cancer cells and modulate Wnt/β-catenin signaling and
apoptotic pathways in A375 cells. Phytomedicine. 52:117–128. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sun Y, Liu L, Wang Y, He A, Hu H, Zhang J,
Han M and Huang Y: Curcumin inhibits the proliferation and invasion
of MG-63 cells through inactivation of the p-JAK2/p-STAT3 pathway.
Onco Targets Ther. 12:2011–2021. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang WH, Chen J, Zhang BR, Lu SJ, Wang F,
Peng L, Dai JH and Sun YZ: Curcumin inhibits proliferation and
enhances apoptosis in A549 cells by downregulating lncRNA UCA1.
Pharmazie. 73:402–407. 2018.PubMed/NCBI
|
|
31
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sato Y, Yoshino H, Tsuruga E and
Kashiwakura I: Fas ligand enhances apoptosis of human lung cancer
cells cotreated with RIG-I-like receptor agonist and radiation.
Curr Cancer Drug Targets. Jan 15–2020.(Epub ahead of print).
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lee KC, Lee KF, Tung SY, Huang WS, Lee LY,
Chen WP, Chen CC, Teng CC, Shen CH, Hsieh MC and Kuo HC: Induction
apoptosis of erinacine a in human colorectal cancer cells involving
the expression of TNFR, fas, and fas ligand via the JNK/p300/p50
signaling pathway with histone acetylation. Front Pharmacol.
10:11742019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mortezaee K, Salehi E, Mirtavoos-Mahyari
H, Motevaseli E, Najafi M, Farhood B, Rosengren RJ and Sahebkar A:
Mechanisms of apoptosis modulation by curcumin: Implications for
cancer therapy. J Cell Physiol. 234:12537–12550. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Harini L, Srivastava S, Gnanakumar GP,
Karthikeyan B, Ross C, Krishnakumar V, Kannan VR, Sundar K and
Kathiresan T: An ingenious non-spherical mesoporous silica
nanoparticle cargo with curcumin induces mitochondria-mediated
apoptosis in breast cancer (MCF-7) cells. Oncotarget. 10:1193–1208.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Moustakas A and Heldin CH: Non-Smad
TGF-beta signals. J Cell Sci. 118:3573–3584. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang X, Hang Y, Liu J, Hou Y, Wang N and
Wang M: Anticancer effect of curcumin inhibits cell growth through
miR-21/PTEN/Akt pathway in breast cancer cell. Oncol Lett.
13:4825–4831. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen YY, Lin YJ, Huang WT, Hung CC, Lin
HY, Tu YC, Liu DM, Lan SJ and Sheu MJ: Demethoxycurcumin-loaded
chitosan nanoparticle downregulates DNA repair pathway to improve
cisplatin-induced apoptosis in non-small cell lung cancer.
Molecules. 23(pii): E32172018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang C, Song X, Shang M, Zou W, Zhang M,
Wei H and Shao H: Curcumin exerts cytotoxicity dependent on
reactive oxygen species accumulation in non-small-cell lung cancer
cells. Future Oncol. 15:1243–1253. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tan C, Hu W, He Y, Zhang Y, Zhang G, Xu Y
and Tang J: Cytokine-mediated therapeutic resistance in breast
cancer. Cytokine. 108:151–159. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
de Campos PS, Matte BF, Diel LF, Jesus LH,
Bernardi L, Alves AM, Rados PV and Lamers ML: Low doses of curcuma
longa modulates cell migration and cell-cell adhesion. Phytother
Res. 31:1433–1440. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cao MT, Liu HF, Liu ZG, Xiao P, Chen JJ,
Tan Y, Jiang XX, Jiang ZC, Qiu Y, Huang HJ, et al: Curcumin
downregulates the expression of Snail via suppressing Smad2 pathway
to inhibit TGF-β1-induced epithelial-mesenchymal transitions in
hepatoma cells. Oncotarget. 8:108498–108508. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Li W, Ma Z, Ma J, Li X, Xu Q, Duan W, Chen
X, Lv Y, Zhou S, Wu E, et al: Hydrogen peroxide mediates
hyperglycemia-induced invasive activity via ERK and p38 MAPK in
human pancreatic cancer. Oncotarget. 6:31119–31133. 2015.PubMed/NCBI
|
|
44
|
Cao L, Liu J, Zhang L, Xiao X and Li W:
Curcumin inhibits H2O2-induced invasion and migration of human
pancreatic cancer via suppression of the ERK/NF-κB pathway. Oncol
Rep. 36:2245–2251. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Jászai J and Schmidt MHH: Trends and
challenges in tumor anti-angiogenic therapies. Cells. 8(pii):
E11022019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lin Z, Zhang Q and Luo W: Angiogenesis
inhibitors as therapeutic agents in cancer: Challenges and future
directions. Eur J Pharmacol. 793:76–81. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Norooznezhad AH and Norooznezhad F:
Cannabinoids: Possible agents for treatment of psoriasis via
suppression of angiogenesis and inflammation. Med Hypotheses.
99:15–18. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Saberi-Karimian M, Katsiki N, Caraglia M,
Boccellino M, Majeed M and Sahebkar A: Vascular endothelial growth
factor: An important molecular target of curcumin. Crit Rev Food
Sci Nutr. 59:299–312. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Jiao D, Wang J, Lu W, Tang X, Chen J, Mou
H and Chen QY: Curcumin inhibited HGF-induced EMT and angiogenesis
through regulating c-Met dependent PI3K/Akt/mTOR signaling pathways
in lung cancer. Mol Ther Oncolytics. 3:160182016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Liang C, Shi S, Meng Q, Liang D, Ji S,
Zhang B, Qin Y, Xu J, Ni Q and Yu X: Complex roles of the stroma in
the intrinsic resistance to gemcitabine in pancreatic cancer: Where
we are and where we are going. Exp Mol Med. 49:e4062017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Su P, Yang Y, Wang G, Chen X and Ju Y:
Curcumin attenuates resistance to irinotecan via induction of
apoptosis of cancer stem cells in chemoresistant colon cancer
cells. Int J Oncol. 53:1343–1353. 2018.PubMed/NCBI
|
|
52
|
Zhou QM, Sun Y, Lu YY, Zhang H, Chen QL
and Su SB: Curcumin reduces mitomycin C resistance in breast cancer
stem cells by regulating Bcl-2 family-mediated apoptosis. Cancer
Cell Int. 17:842017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zhang J, Liu J, Xu X and Li L: Curcumin
suppresses cisplatin resistance development partly via modulating
extracellular vesicle-mediated transfer of MEG3 and miR-214 in
ovarian cancer. Cancer Chemother Pharmacol. 79:479–87. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Yang H, Kong W, He L, Zhao JJ, O'Donnell
JD, Wang J, Wenham RM, Coppola D, Kruk PA, Nicosia SV and Cheng JQ:
MicroRNA expression profiling in human ovarian cancer: miR-214
induces cell survival and cisplatin resistance by targeting PTEN.
Cancer Res. 68:425–433. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Batista S, Gregório AC, Hanada Otake A,
Couto N and Costa-Silva B: The gastrointestinal tumor
microenvironment: An updated biological and clinical perspective. J
Oncol. 2019:62405052019. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
O'Donnell JS, Teng MWL and Smyth MJ:
Cancer immunoediting and resistance to T cell-based immunotherapy.
Nat Rev Clin Oncol. 16:151–167. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Wattenberg MM and Beatty GL: Overcoming
immunotherapeutic resistance by targeting the cancer inflammation
cycle. Semin Cancer Biol. Jan 15–2020.(Epub ahead of print).
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Dunn GP, Koebel CM and Schreiber RD:
Interferons, immunity and cancer immunoediting. Nat Rev Immunol.
6:836–848. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Lee JH, Choi SY, Jung NC, Song JY, Seo HG,
Lee HS and Lim DS: The effect of the tumor microenvironment and
tumor-derived metabolites on dendritic cell function. J Cancer.
11:769–775. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Galland S and Stamenkovic I: Mesenchymal
stromal cells in cancer: A review of their immunomodulatory
functions and dual effects on tumor progression. J Pathol. Oct
14–2019.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Swann JB and Smyth MJ: Immune surveillance
of tumors. J Clin Invest. 117:1137–1146. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Das T, Sa G, Paszkiewicz-Kozik E, Hilston
C, Molto L, Rayman P, Kudo D, Biswas K, Bukowski RM, Finke JH and
Tannenbaum CS: Renal cell carcinoma tumors induce T cell apoptosis
through receptor-dependent and receptor-independent pathways. J
Immunol. 180:4687–4696. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Snyder JT, Alexander-Miller MA, Berzofskyl
JA and Belyakov IM: Molecular mechanisms and biological
significance of CTL avidity. Curr HIV Res. 1:287–294. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Chen MC, Pangilinan CR and Lee CH:
Salmonella breaks tumor immune tolerance by downregulating tumor
programmed death-ligand 1 expression. Cancers (Basel). 12(pii):
E572019. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Sa G, Das T, Moon C, Hilston CM, Rayman
PA, Rini BI, Tannenbaum CS and Finke JH: GD3, an overexpressed
tumor-derived ganglioside, mediates the apoptosis of activated but
not resting T cells. Cancer Res. 69:3095–3104. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Rabinovich GA, Gabrilovich D and Sotomayor
EM: Immunosuppressive strategies that are mediated by tumor cells.
Annu Rev Immunol. 25:267–296. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Geng Y, Liu J, Xie Y, Jiang H, Zuo K, Li T
and Liu Z: Trichostatin A promotes GLI1 degradation and P21
expression in multiple myeloma cells. Cancer Manag Res.
10:2905–2914. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Wang Y, Zhou X, Song Y, Ji X, Zhang A,
Zhang G and Gao Z: The mismatch repair gene hPMS1 (human
postmeiotic segregation1) is down regulated in oral squamous cell
carcinoma. Gene. 524:28–34. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Fratta E, Coral S, Covre A, Parisi G,
Colizzi F, Danielli R, Nicolay HJ, Sigalotti L and Maio M: The
biology of cancer testis antigens: Putative function, regulation
and therapeutic potential. Mol Oncol. 5:164–182. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Liu M, Zhou J, Chen Z and Cheng AS:
Understanding the epigenetic regulation of tumours and their
microenvironments: Opportunities and problems for epigenetic
therapy. J Pathol. 241:10–24. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Dunn J and Rao S: Epigenetics and
immunotherapy: The current state of play. Mol Immunol. 87:227–239.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Rosenthal R, Cadieux EL, Salgado R, Bakir
MA, Moore DA, Hiley CT, Lund T, Tanić M, Reading JL, Joshi K, et
al: Neoantigen-directed immune escape in lung cancer evolution.
Nature. 567:479–485. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Dunn GP, Bruce AT, Ikeda H, Old LJ and
Schreiber RD: Cancer immunoediting: From immunosurveillance to
tumor escape. Nat Immunol. 3:991–998. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Mori S, Jewett A, Murakami-Mori K,
Cavalcanti M and Bonavida B: The participation of the Fas-mediated
cytotoxic pathway by natural killer cells is tumor-cell-dependent.
Cancer Immunol Immunother. 44:282–290. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Takeda K, Hayakawa Y, Smyth MJ, Kayagaki
N, Yamaguchi N, Kakuta S, Iwakura Y, Yagita H and Okumura K:
Involvement of tumor necrosis factor-related apoptosis-inducing
ligand in surveillance of tumor metastasis by liver natural killer
cells. Nat Med. 7:94–100. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
76
|
Street SE, Cretney E and Smyth MJ:
Perforin and interferon-gamma activities independently control
tumor initiation, growth, and metastasis. Blood. 97:192–197. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
MacKie RM, Reid R and Junor B: Fatal
melanoma transferred in a donated kidney 16 years after melanoma
surgery. N Engl J Med. 348:567–568. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Li W, Wang H, Ma Z, Zhang J, Ou-Yang W, Qi
Y and Liu J: Multi-omics analysis of microenvironment
characteristics and immune escape mechanisms of hepatocellular
carcinoma. Front Oncol. 9:10192019. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Kim R, Emi M and Tanabe K: Cancer
immunoediting from immune surveillance to immune escape.
Immunology. 121:1–14. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Itakura E, Huang RR, Wen DR, Paul E,
Wünsch PH and Cochran AJ: IL-10 expression by primary tumor cells
correlates with melanoma progression from radial to vertical growth
phase and development of metastatic competence. Mod Pathol.
24:801–809. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Brody JR, Costantino CL, Berger AC, Sato
T, Lisanti MP, Yeo CJ, Emmons RV and Witkiewicz AK: Expression of
indoleamine 2,3-dioxygenase in metastatic malignant melanoma
recruits regulatory T cells to avoid immune detection and affects
survival. Cell Cycle. 8:1930–1934. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Zou W: Immunosuppressive networks in the
tumour environment and their therapeutic relevance. Nat Rev Cancer.
5:263–274. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Bhattacharyya S, Mandal D, Sen GS, Pal S,
Banerjee S, Lahiry L, Finke JH, Tannenbaum CS, Das T and Sa G:
Tumor-induced oxidative stress perturbs nuclear factor-kappaB
activity-augmenting tumor necrosis factor-alpha-mediated T-cell
death: Protection by Curcumin. Cancer Res. 67:362–370. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Bhattacharyya S, Md Sakib Hossain D,
Mohanty S, Sankar Sen G, Chattopadhyay S, Banerjee S, Chakraborty
J, Das K, Sarkar D, Das T and Sa G: Curcumin reverses T
cell-mediated adaptive immune dysfunctions in tumor-bearing hosts.
Cell Mol Immunol. 7:306–315. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Xu B, Yu L and Zhao LZ: Curcumin up
regulates T helper 1 cells in patients with colon cancer. Am J
Transl Res. 9:1866–1875. 2017.PubMed/NCBI
|
|
86
|
Zou JY, Su CH, Luo HH, Lei YY, Zeng B, Zhu
HS and Chen ZG: Curcumin converts Foxp3+ regulatory T cells to T
helper 1 cells in patients with lung cancer. J Cell Biochem.
119:1420–1428. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Milano F, Mari L, van de Luijtgaarden W,
Parikh K, Calpe S and Krishnadath KK: Nano-curcumin inhibits
proliferation of esophageal adenocarcinoma cells and enhances the T
cell mediated immune response. Front Oncol. 3:1372013. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Rosenberg SA, Restifo NP, Yang JC, Morgan
RA and Dudley ME: Adoptive cell transfer: A clinical path to
effective cancer immunotherapy. Nat Rev Cancer. 8:299–308. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Hawkins RE, Gilham DE, Debets R, Eshhar Z,
Taylor N, Abken H and Schumacher TN; ATTACK Consortium, :
Development of adoptive cell therapy for cancer: A clinical
perspective. Hum Gene Ther. 21:665–672. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Chang YF, Chuang HY, Hsu CH, Liu RS,
Gambhir SS and Hwang JJ: Immunomodulation of curcumin on adoptive
therapy with T cell functional imaging in mice. Cancer Prev Res
(Phila). 5:444–452. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Luo F, Song X, Zhang Y and Chu Y: Low-dose
curcumin leads to the inhibition of tumor growth via enhancing
CTL-mediated antitumor immunity. Int Immunopharmacol. 11:1234–1240.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Lu Y, Miao L, Wang Y, Xu Z, Zhao Y, Shen
Y, Xiang G and Huang L: Curcumin micelles remodel tumor
microenvironment and enhance vaccine activity in an advanced
melanoma model. Mol Ther. 24:364–374. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Lin Y, Xu J and Lan H: Tumor-associated
macrophages in tumor metastasis: Biological roles and clinical
therapeutic applications. J Hematol Oncol. 12:762019. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Kim DH, Lee HG and Choi JM: Curcumin
Elevates TFH cells and germinal center B cell response
for antibody production in mice. Immune Netw. 19:e352019.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Shevach EM: Application of IL-2 therapy to
target T regulatory cell function. Trends Immunol. 33:626–632.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Oh JG, Hwang DJ and Heo TH: Direct
regulation of IL-2 by curcumin. Biochem Biophys Res Commun.
495:300–305. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Shiri S, Alizadeh AM, Baradaran B,
Farhanghi B, Shanehbandi D, Khodayari S, Khodayari H and Tavassoli
A: Dendrosomal curcumin suppresses metastatic breast cancer in mice
by changing m1/m2 macrophage balance in the tumor microenvironment.
Asian Pac J Cancer Prev. 16:3917–3922. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Xie Q, Yang Z, Huang X, Zhang Z, Li J, Ju
J, Zhang H and Ma J: Ilamycin C induces apoptosis and inhibits
migration and invasion in triple-negative breast cancer by
suppressing IL-6/STAT3 pathway. J Hematol Oncol. 12:602019.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Singh M, Ramos I, Asafu-Adjei D,
Quispe-Tintaya W, Chandra D, Jahangir A, Zang X, Aggarwal BB and
Gravekamp C: Curcumin improves the therapeutic efficacy of
Listeria(at)-Mage-b vaccine in correlation with improved T-cell
responses in blood of a triple-negative breast cancer model 4T1.
Cancer Med. 2:571–582. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Bill MA, Bakan C, Benson DM Jr, Fuchs J,
Young G and Lesinski GB: Curcumin induces proapoptotic effects
against human melanoma cells and modulates the cellular response to
immunotherapeutic cytokines. Mol Cancer Ther. 8:2726–2735. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Jin H, Jia Y, Yao Z, Huang J, Hao M, Yao
S, Lian N, Zhang F, Zhang C, Chen X, et al: Hepatic stellate cell
interferes with NK cell regulation of fibrogenesis via curcumin
induced senescence of hepatic stellate cell. Cell Signal. 33:79–85.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Zhang HG, Kim H, Liu C, Yu S, Wang J,
Grizzle WE, Kimberly RP and Barnes S: Curcumin reverses breast
tumor exosomes mediated immune suppression of NK cell tumor
cytotoxicity. Biochim Biophys Acta. 1773:1116–1123. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Lee HH and Cho H: Improved anti-cancer
effect of curcumin on breast cancer cells by increasing the
activity of natural killer cells. J Microbiol Biotechnol.
28:874–882. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Halder RC, Almasi A, Sagong B, Leung J,
Jewett A and Fiala M: Curcuminoids and ω-3 fatty acids with
anti-oxidants potentiate cytotoxicity of natural killer cells
against pancreatic ductal adenocarcinoma cells and inhibit
interferon γ production. Front Physiol. 22:6:1292015.
|
|
105
|
Mills CD, Kincaid K, Alt JM, Heilman MJ
and Hill AM: M-1/M-2 macrophages and the Th1/Th2 paradigm. J
Immunol. 164:6166–6173. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Murray PJ, Allen JE, Biswas SK, Fisher EA,
Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence
T, et al: Macrophage activation and polarization: Nomenclature and
experimental guidelines. Immunity. 41:14–20. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Mukherjee S, Hussaini R, White R, Atwi D,
Fried A, Sampat S, Piao L, Pan Q and Banerjee P: TriCurin, a
synergistic formulation of curcumin, resveratrol, and epicatechin
gallate, repolarizes tumor-associated macrophages and triggers an
immune response to cause suppression of HPV+ tumors. Cancer Immunol
Immunother. 67:761–774. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Mukherjee S, Fried A, Hussaini R, White R,
Baidoo J, Yalamanchi S and Banerjee P: Phytosomal curcumin causes
natural killer cell-dependent repolarization of glioblastoma (GBM)
tumor-associated microglia/macrophages and elimination of GBM and
GBM stem cells. J Exp Clin Cancer Res. 37:1682018. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Mukherjee S, Baidoo JNE, Sampat S, Mancuso
A, David L, Cohen LS, Zhou S and Banerjee P: Liposomal TriCurin, a
synergistic combination of curcumin, epicatechin gallate and
resveratrol, repolarizes tumor-associated microglia/macrophages,
and eliminates glioblastoma (GBM) and GBM stem cells. Molecules.
23(pii): E2012018. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Kuttan R, Sudheeran PC and Josph CD:
Turmeric and curcumin as topical agents in cancer therapy. Tumori.
73:29–31. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Pastorelli D, Fabricio ASC, Giovanis P,
D'Ippolito S, Fiduccia P, Soldà C, Buda A, Sperti C, Bardini R, Da
Dalt G, et al: Phytosome complex of curcumin as complementary
therapy of advanced pancreatic cancer improves safety and efficacy
of gemcitabine: Results of a prospective phase II trial. Pharmacol
Res. 132:72–79. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
James MI, Iwuji C, Irving G, Karmokar A,
Higgins JA, Griffin-Teal N, Thomas A, Greaves P, Cai H, Patel SR,
et al: Curcumin inhibits cancer stem cell phenotypes in ex vivo
models of colorectal liver metastases, and is clinically safe and
tolerable in combination with FOLFOX chemotherapy. Cancer Lett.
364:135–141. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Mahammedi H, Planchat E, Pouget M, Durando
X, Curé H, Guy L, Van-Praagh I, Savareux L, Atger M, Bayet-Robert
M, et al: The New Combination docetaxel, prednisone and curcumin in
patients with castration-resistant prostate cancer: A pilot phase
II study. Oncology. 90:69–78. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Ryan JL, Heckler CE, Ling M, Katz A,
Williams JP, Pentland AP and Morrow GR: Curcumin for radiation
dermatitis: A randomized, double-blind, placebo-controlled clinical
trial of thirty breast cancer patients. Radiat Res. 180:34–43.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Dhillon N, Aggarwal BB, Newman RA, Wolff
RA, Kunnumakkara AB, Abbruzzese JL, Ng CS, Badmaev V and Kurzrock
R: Phase II trial of curcumin in patients with advanced pancreatic
cancer. Clin Cancer Res. 14:4491–4499. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Saif MW: Is there a role for herbal
medicine in the treatment of pancreatic cancer? Highlights from the
‘44th ASCO Annual Meeting’. Chicago, IL, USA. May 30-June 3, 2008.
JOP. 9:403–407. 2008.PubMed/NCBI
|
|
117
|
Epelbaum R, Schaffer M, Vizel B, Badmaev V
and Bar-Sela G: Curcumin and gemcitabine in patients with advanced
pancreatic cancer. Nutr Cancer. 62:1137–1141. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Kanai M, Yoshimura K, Asada M, Imaizumi A,
Suzuki C, Matsumoto S, Nishimura T, Mori Y, Masui T, Kawaguchi Y,
et al: A phase I/II study of gemcitabine-based chemotherapy plus
curcumin for patients with gemcitabine-resistant pancreatic cancer.
Cancer Chemother Pharmacol. 68:157–164. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Wan Y, Liang Y, Liang F, Shen N, Shinozuka
K, Yu JT, Ran C, Quan Q, Tanzi RE and Zhang C: A curcumin analog
reduces levels of the Alzheimer's disease-associated amyloid-β
protein by modulating AβPP processing and autophagy. J Alzheimers
Dis. 72:761–771. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Rezzani R, Franco C and Rodella LF:
Curcumin as a therapeutic strategy in liver diseases. Nutrients.
11(pii): E24982019. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Fleenor BS, Carlini NA and Campbell MS:
Curcumin and arterial function in health and disease: Impact on
oxidative stress and inflammation. Curr Opin Clin Nutr Metab Care.
22:459–464. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Zhao S, Pi C, Ye Y, Zhao L and Wei Y:
Recent advances of analogues of curcumin for treatment of cancer.
Eur J Med Chem. 180:524–535. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Tønnesen HH, Másson M and Loftsson T:
Studies of curcumin and curcuminoids. XXVII. Cyclodextrin
complexation: Solubility, chemical and photochemical stability. Int
J Pharm. 244:127–135. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Li X, Uehara S, Sawangrat K, Morishita M,
Kusamori K, Katsumi H, Sakane T and Yamamoto A: Improvement of
intestinal absorption of curcumin by cyclodextrins and the
mechanisms underlying absorption enhancement. Int J Pharm.
535:340–349. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Bisht S, Feldmann G, Soni S, Ravi R,
Karikar C and Maitra A and Maitra A: Polymeric
nanoparticle-encapsulated curcumin (‘nanocurcumin’): A novel
strategy for human cancer therapy. J Nanobiotechnology. 5:32007.
View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Han W, Xie B, Li Y, Shi L, Wan J, Chen X
and Wang H: Orally deliverable nanotherapeutics for the synergistic
treatment of colitis-associated colorectal cancer. Theranostics.
9:7458–7473. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Guo F, Fu Q, Jin C, Ji X, Yan Q, Yang Q,
Wu D, Gao Y, Hong W, Li A and Yang G: Dual functional matrix
metalloproteinase-responsive curcumin-loaded nanoparticles for
tumor-targeted treatment. Drug Deliv. 26:1027–1038. 2019.
View Article : Google Scholar : PubMed/NCBI
|