Cervical Cancer (CC) is a highly aggressive tumor
and is one of the leading causes of cancer-associated mortality in
women, with an estimated 570,000 new cases and 311,000 deaths in
2018 worldwide (1). Women with CC
are considered to have a lower quality of life (2). The progression of CC, from normal
cervical mucosal epithelium to cervical intraepithelial neoplasia
(CIN) grade 1, 2, and 3, to CC (3)
is associated with persistent high-risk human papillomavirus (HPV)
infection (4). Furthermore, a number
of risk factors, including early sexual activity (5), multiple sexual partners (6), long-term use of oral contraceptives
(7), genetic factors [active
oncogenes, including PIK3CA (8),
ATAD2 (9) and CRNDE (10); tumor suppressor genes, including p53
(11), Ras association domain family
1 isoform A (12) and NOL7 (13)], tobacco use [current smoker, started
smoking age ≤15 years, smoking duration ≥30 years, ≥20
cigarettes/day (14)] and other
viral infections (such as HIV, herpes simplex virus (HSV) type II
and bacterial infections caused by Chlamydia trachomatis)
(15) have been associated with CC
progression.
The development of CC occurs over a number of years
and its complexity presents clinical challenges in patients
screening and treatment. Currently, The Bethesda System (35), which is a tool that is used to report
Pap smear results for cervical cytologic diagnoses, provides useful
data that allows research into the epidemiology, biology and
pathology of cervical lesions; however, its diagnostic value
remains poor (36). Instead, direct
biopsy remains the gold standard for diagnosis. Nevertheless,
invasive examinations may cause adverse psychological effects,
including anxiety, depression or distress (37). Surgery, chemotherapy and radiotherapy
(38) are the three major
therapeutic strategies in the treatment of CC; however, their uses
may be limited for various reasons. Surgery may be limited by the
status and stage of patients, including late stage or tolerance to
anesthesia (39), whereas
chemotherapy is limited due to the lack of sensitivity and the
development of drug resistance (40). In addition, radiotherapy can be
limited by the maximum tolerated dose to adjacent normal tissues
(41). Thus, it is essential to
understand the underlying molecular mechanisms in the initiation
and development of CC, in order to develop methods for its accurate
diagnosis and effective treatment. A number of studies have
reported that multiple genes [CXCL12 (42), FGFR4 (29) and SHH (43)], proteins [cyclin D1 (44), FOXO1 (45) and BASP1 (46)] and pathways [Toll-like signaling
pathway (47), VEGF signaling
pathway (48) and Wnt signaling
pathway (30)] are involved in the
natural progression of CC; however, few studies have investigated
the fundamental pathological molecular mechanisms in the
progression of CC (from normal, to CIN1, CIN2, CIN3, to cancer).
Thus, the specific pathological processes remain unclear.
The present study provided a systematic
investigation of the development of CC and further understanding of
the associations between the four phases of CC progression, and
thus revealed additional targets for the detection and treatment of
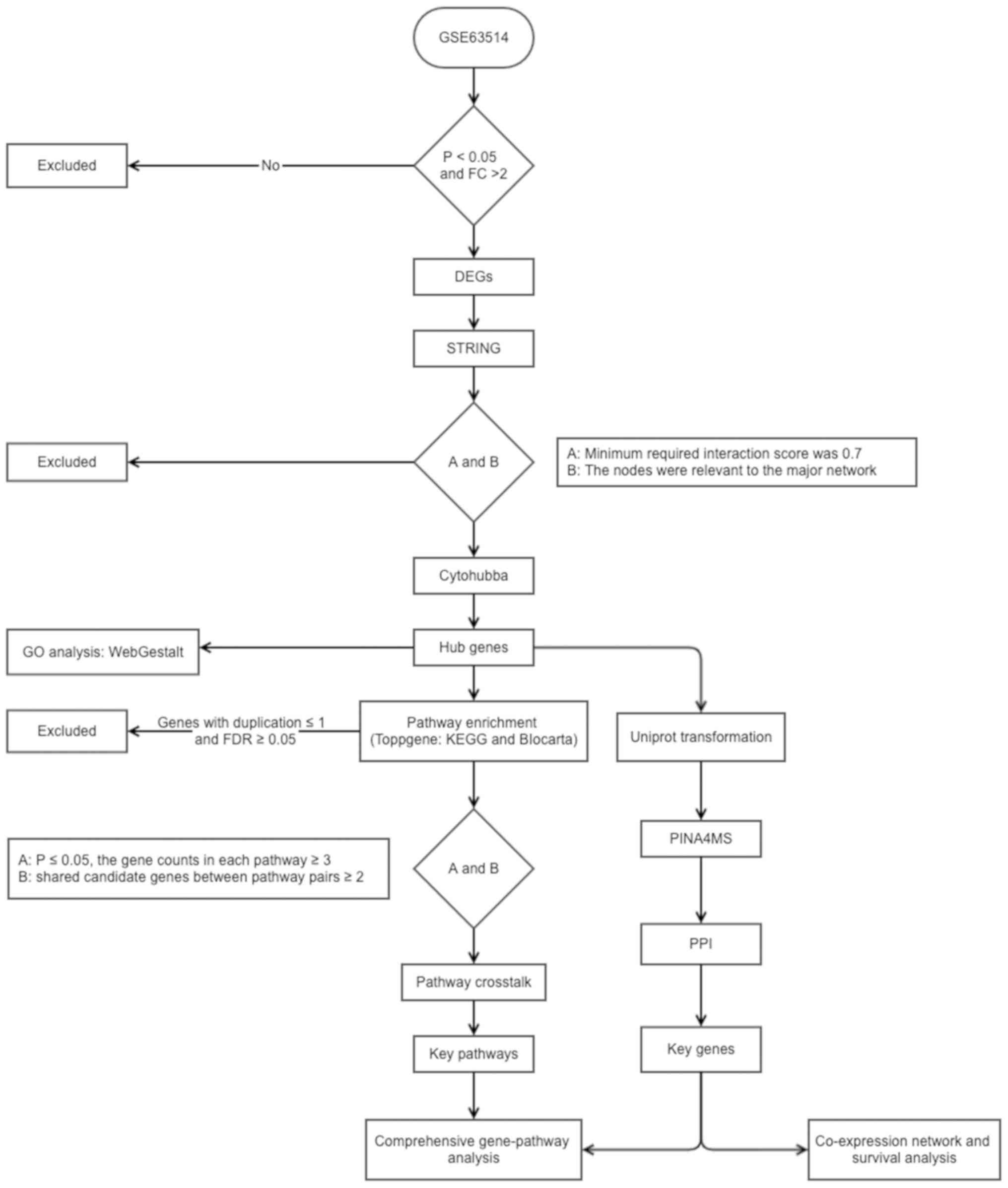
CC. A flow diagram of the present study is presented in Fig. 1.
The CC gene expression profile in the GSE63514
dataset, acquired using the GPL570 platform (Affymetrix Human
Genome U133 Plus 2.0 Array) provided by den Boon in 2015 (49), was downloaded from the GEO database
(https://www.ncbi.nlm.nih.gov/geo/).
The profile contained 128 cervical specimens, including: Normal
(n=24), CIN1 (n=14), CIN2 (n=22), CIN3 (n=40) and cancer (n=28)
samples. All samples were divided into four phases as follows:
Phase I, normal to CIN1; phase II, CIN1 to CIN2; phase III, CIN2 to
CIN3 and phase IV, CIN3 to cancer, and GEO2R tools (https://www.ncbi.nlm.nih.gov/geo/geo2r/)
(50) within the limma package
version 3.26.8 (51) were used to
screen the DEGs at the four phases. The criteria fold change (FC)
of expression >2 and P<0.05 were used to identify DEGs.
The functional features of the genes associated with
the four phases were examined using WebGestalt (55) and ToppGene (56). In WebGestalt, over-representation
analysis was selected as the enrichment method, Biological Process
in GO as the functional database, gene symbol as the gene ID type
and genome as the reference set for enrichment analysis. In
ToppGene, two frequently used databases, Kyoto Encyclopedia of
Genes and Genomes (KEGG; http://www.kegg.jp/) and BioCarta (https://www.biocarta.com/), were utilized to perform
pathway enrichment analysis, to improve the reliability of the
results. Pathways with a false discovery rate of P<0.05 were
considered to indicate significantly enriched pathways.
To determine the overall progression and further
detect an association between two pathways, the KEGG and BioCarta
databases were used to identify the upstream or downstream
associations between pathways. Furthermore, the nodal degree was
calculated using Centiscape (58) to
identify key nodes. According to Han et al (59), key nodes are considered as those with
a nodal degree ≥5.
To determine the molecular mechanisms and
associations between the key genes and pathways, the gene-pathway
network was constructed by examining the key pathways, in order to
determine which pathway contained at least one of the key
genes.
To identify the co-expression of key genes and their
impact on OS time, the LinkedOmics database (68) was used, which was based on TCGA
(69). The co-expression analysis
was performed using Pearson correlation and OS analysis was
assessed with Cox regression method. For survival analysis, samples
were divided by the median value of the investigated gene.
P<0.05 was considered to indicate a statistically significant
difference for both the co-expression correlation and OS time.
Analysis of the GSE63514 dataset using GEO2R, with a
criteria of ≥2 FC and P<0.05, identified a total of 3,446 DEGs
for the four phases as follows: 446 DEGs in phase I, of which 76
were upregulated and 370 were downregulaged; 382 DEGs in phase II,
of which 146 were upregulated and 236 were downregulated; 756 DEGs
in phase III, of which 435 were upregulated and 321 were
downregulated; 1,862 DEGs in phase IV, of which 816 were
upregulated and 1046 were downregulated.
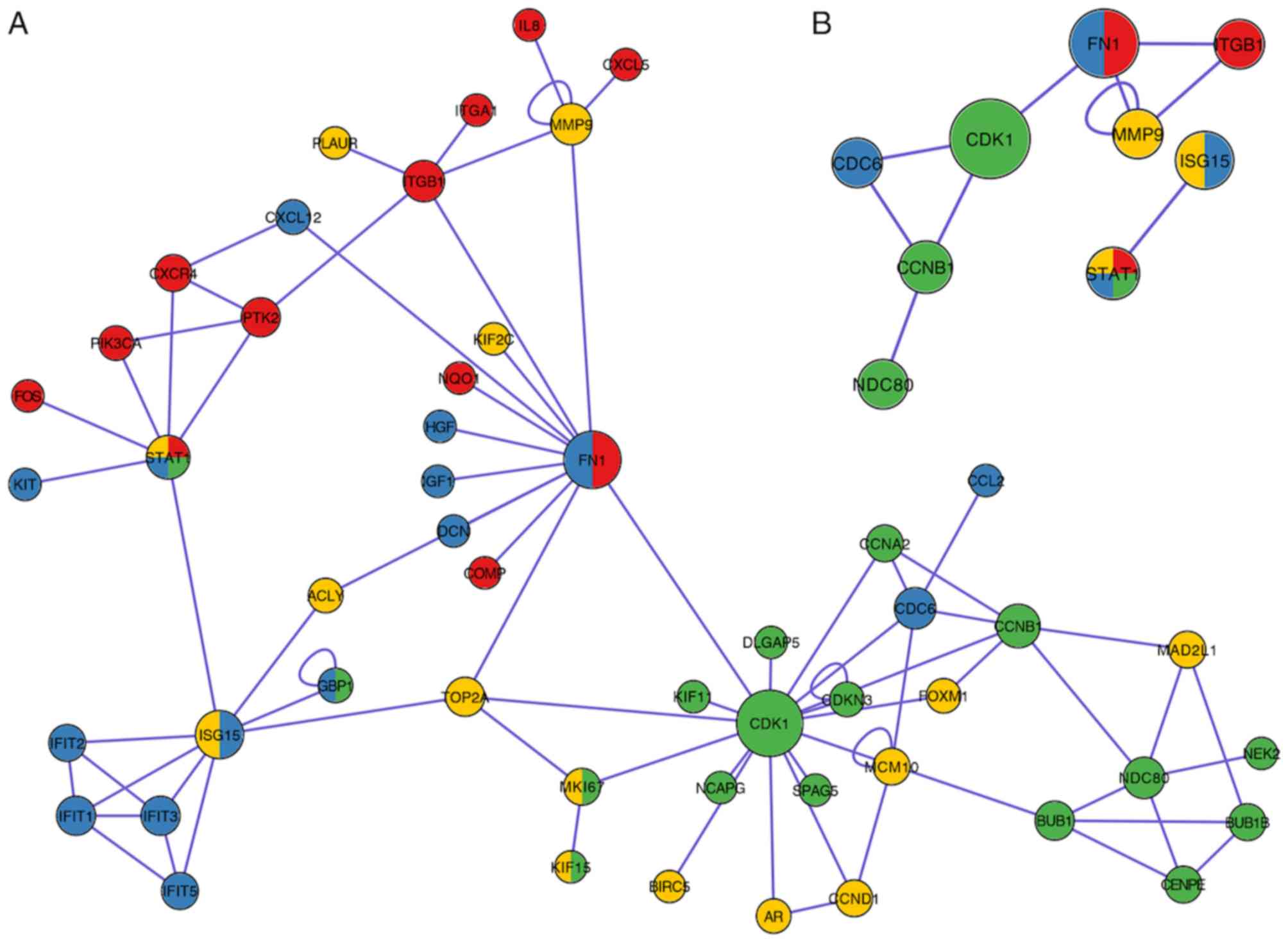
Following removal of 2,256 irrelevant genes (Phase
I, 265; Phase II, 197; Phase III, 603; Phase IV, 1191), 12
topological algorithms were used and the top 10 genes for each
method were extracted. A total of 107 genes that appeared at least
twice were conserved as hub genes, as presented in Table I. A total of 29 genes were identified
in phase I, among which five genes were members of the kinesin
family (KIF11, KIF15, KIF23, KIF4A and KIF14), and
five genes were associated with meiosis and the maturation of
oocytes [BUB1B (70),
BUB1 (71), CCNA2
(72), CCNB1 (72) and CDK1 (73)], as well as other genes associated
with inflammation and innate immune responses [STAT1
(74), GBP1 (75) and RHOA (76)]. A total of 25 hub genes were verified
in phase II, among which the involvement of seven
interferon-induced genes was identified (IFI44L, IFIT3, IFIF1,
IFIF5, IFI44, IFIT2 and IFI6), and several pattern
recognition receptor-associated genes [IRF7 (77), STAT1 (78) and CXCL10 (79)], as well as some genes involved in
invasion and metastasis of cancer cells [HGF (80), IGF1 (81), KIT (82), FN1 (83) and CXCL12 (84)]. A number of common cancer-associated
signaling pathway genes were identified in phase III [CCND1
(85), STAT1 (86) and VEGFA (87)]. A total of three C-X-C motif
chemokine ligands (CXCL8, CXCL11 and CXCL4), two
integrin subunits (ITGB1 and ITGA1), and one
mitogen-activated protein (MAPK12) were identified in phase
IV. Furthermore, PIK3CA (88)
and FOS (89) participated in
cancer-associated pathways in phase IV. The diversity of genes
within the four phases demonstrated that CC progression is a
complex process and its molecular mechanisms are not constant.
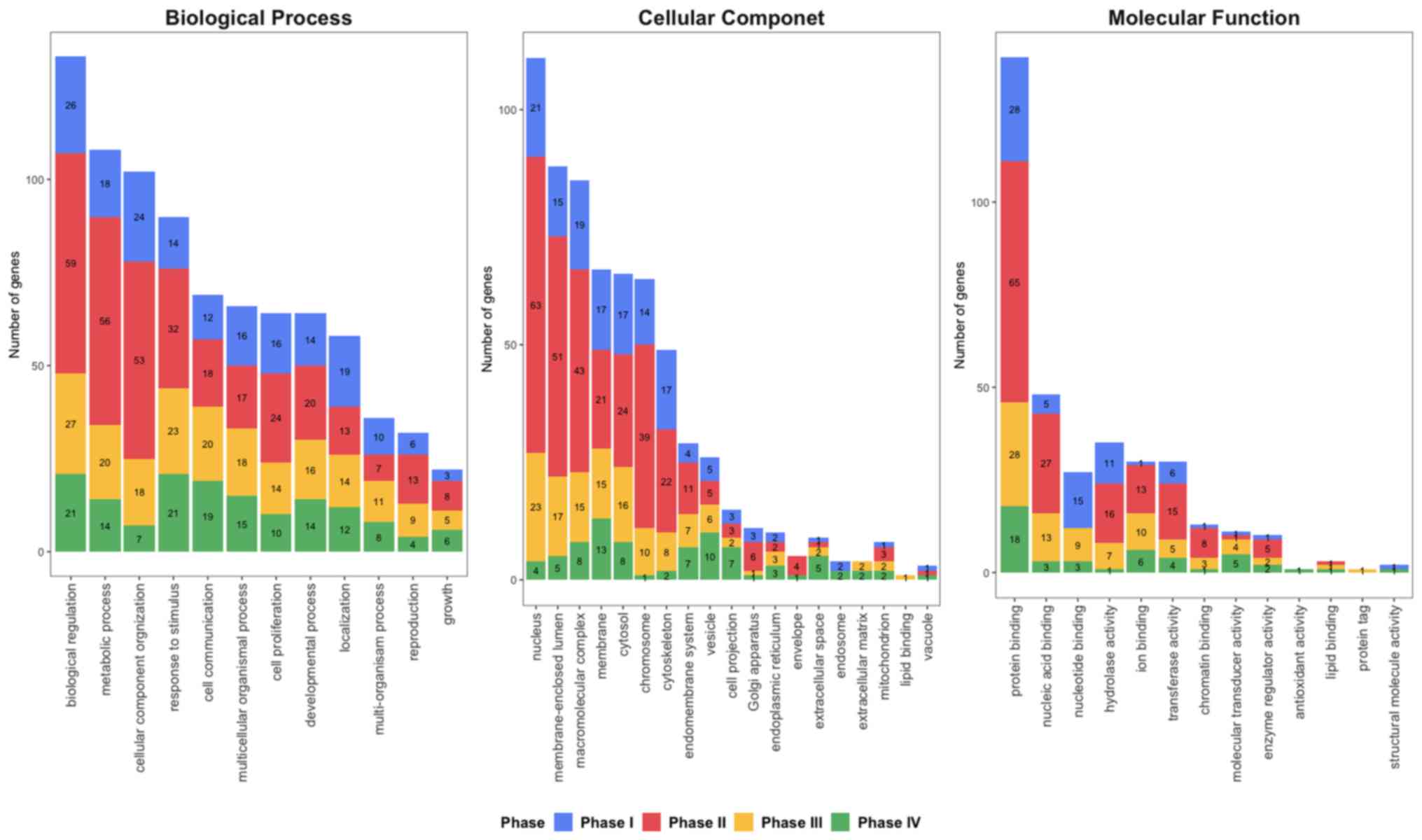
To further identify the biological functions and
locations of hub genes, GO enrichment analysis (90) was performed (Fig. 2). Hub genes were notably enriched in
‘biological regulation’, ‘metabolic process’ and ‘cellular
component organization’ in phase I and II, while ‘responses to
stimulus’ and ‘biological regulation’ were predominantly enriched
at phases III–IV in biological process. For the cellular
components, ‘nucleus’, ‘membrane-enclosed lumen’ and
‘macromolecular complex’ was enriched at phases I–III, while
‘chromosome’ and ‘membrane’ was identified in phases II and IV,
respectively. ‘Protein binding’ was enriched at all four phases for
Molecular Function. Furthermore, ‘nucleic acid binding’ and
‘hydrolase activity’ were enriched at phase II and III, while ‘ion
binding’ was enriched at phase III and IV.
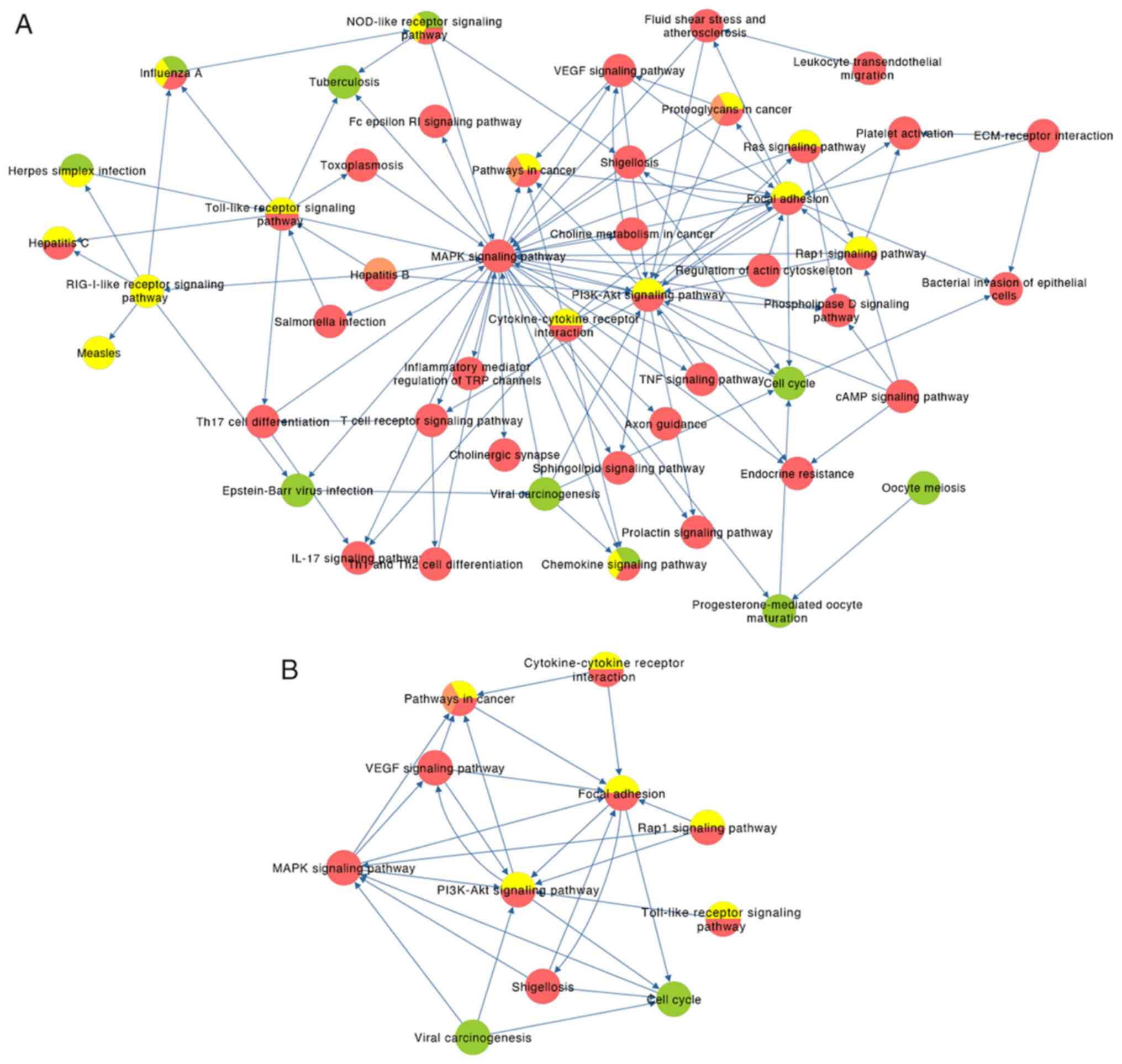
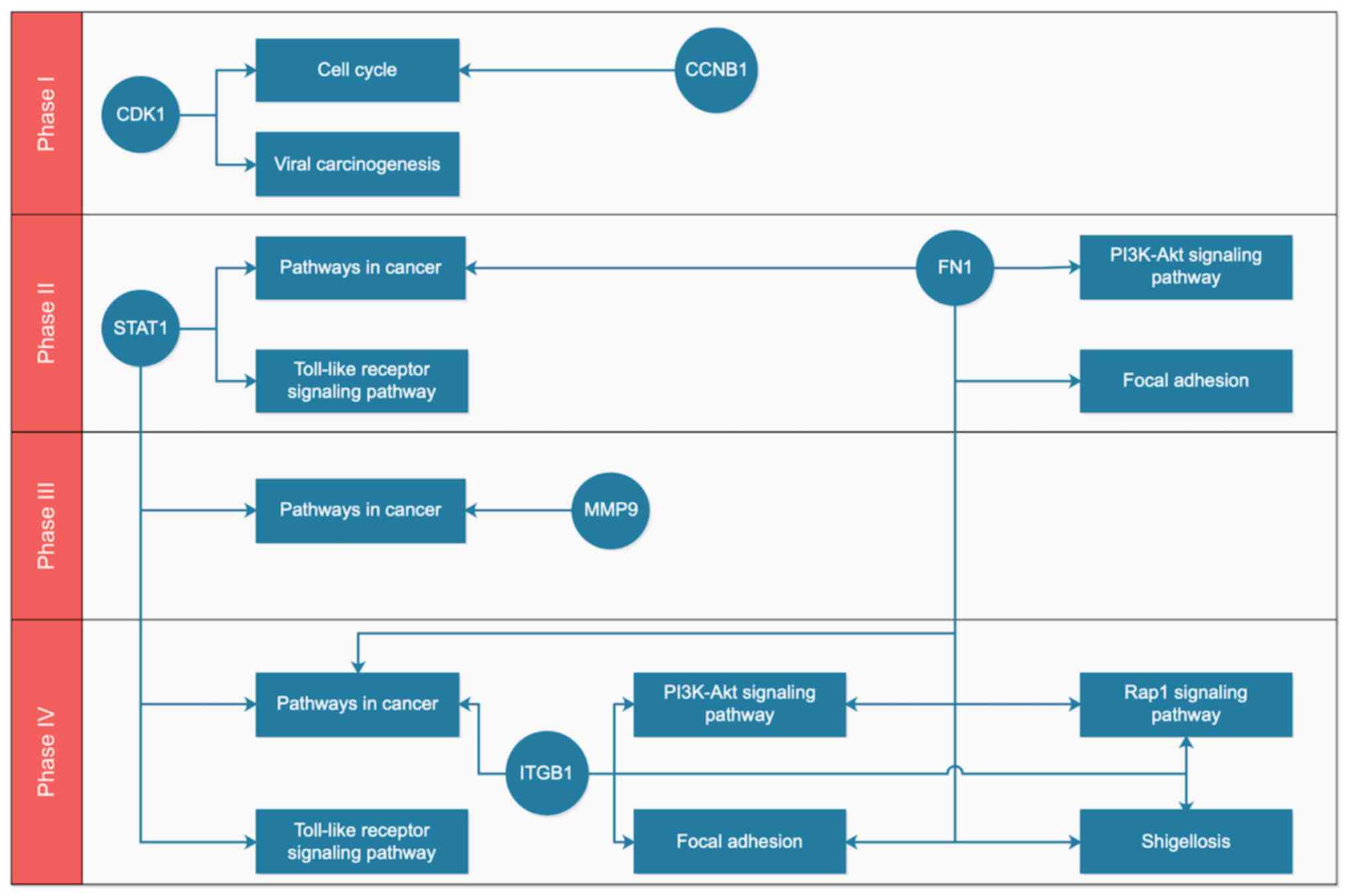
After mapping the key genes onto the key pathways
using the KEGG and BioCarta databases, a potential gene-pathway
flowchart, including eight key pathways and six key genes was
constructed (Fig. 5). The results
demonstrated the following: For phase I, CDK1 and
CCNB1 participated in the regulation of the cell cycle,
while CDK1 was also involved in viral carcinogenesis; for
phases II–IV, ‘pathways in cancer,’ ‘focal adhesion’ and ‘PI3K-Akt
signaling pathway’ were ranked the top three pathways according to
the number of genes involved; FN1, ITGB1 and MMP9 may
be associated with metastasis of tumor cells, and STAT1
participated in ‘pathways in cancer’ and ‘Toll-like receptor
signaling pathway’, which functioned at a phase IV.
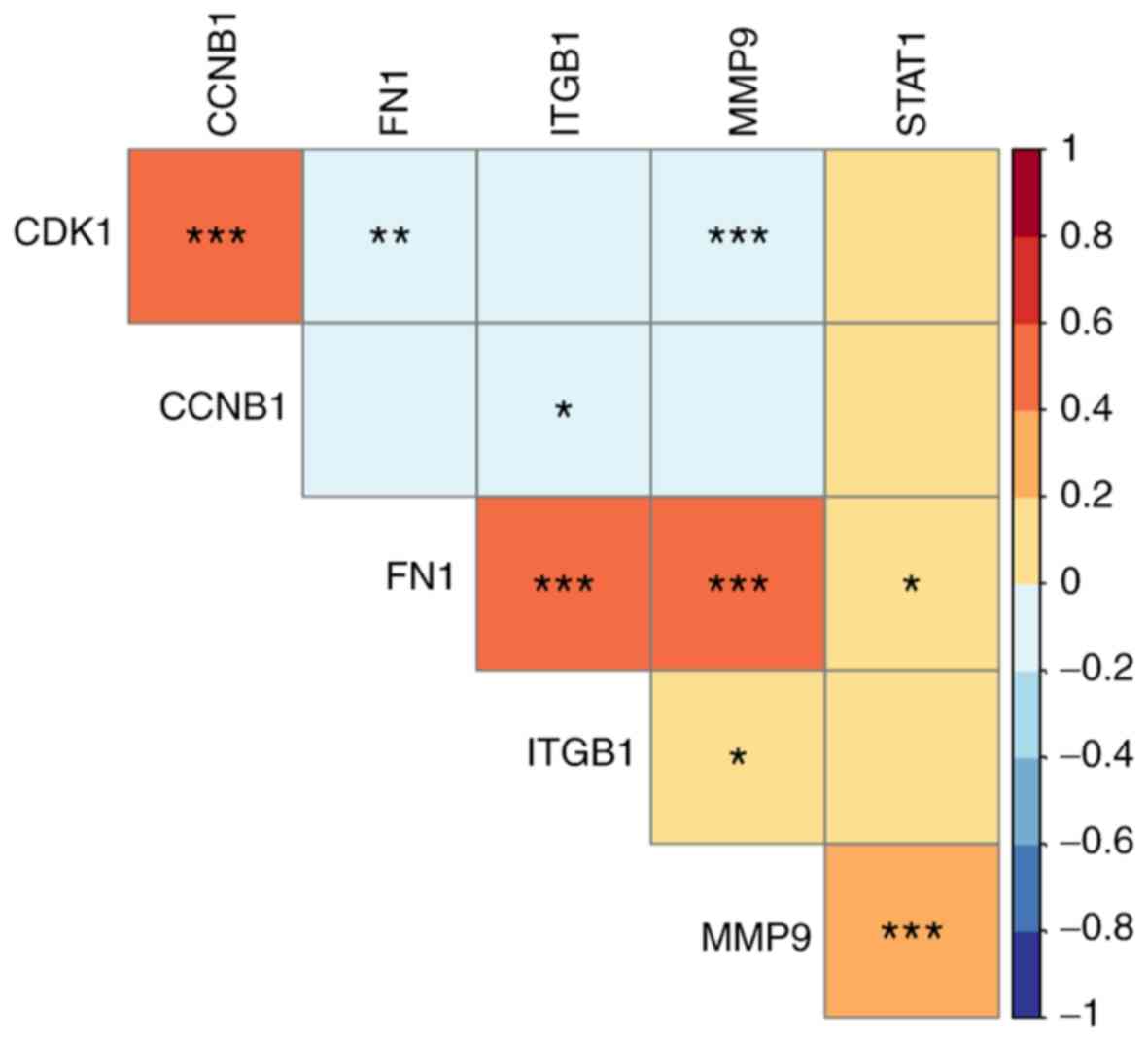
By mining the data from LinkedOmics, the results of
co-expression demonstrated that CDK1 had a significantly
positive correlation with CCNB1 (P<0.0001), but negative
correlation with FN1 (P=0.003) and MMP9 (P=0.001),
respectively (Fig. 6). CCNB1
demonstrated a significantly negative correlation with ITGB1
(P=0.047); however, FN1, ITGB1 and MMP9 indicated a
significantly positive correlation between each other (FN1 and
ITGB1, P<0.001; FN1 and MMP9, P<0.0001; ITGB1 and MMP9,
P=0.023). STAT1 was significantly positively correlated with
MMP9 (P<0.0001).
To date, the occurrence and development of CC is
hypothesized to be linked with persistent HPV infection (100); however, the specific molecular
mechanisms require further investigation. In addition, although a
number of studies are examining the molecular mechanisms of CC
(101–104), the detailed pathological process
remains unclear.
In the process of tumor invasion and metastasis,
cancer cells can bind to ligands of the extracellular matrix (ECM)
via integrins and degrade the basement membrane (BM) by secreting
proteases via the pathways of focal adhesion and the PI3K-Akt
signaling pathway (117). This
degradation is also the prerequisite for stromal infiltration and
cancer cell migration (118).
ITGB1 belongs to the integrin family and FN1 is the
ligand. The binding of ITGB1 and FN1 induces the
phosphorylation of tyrosine and directly affects cytoskeleton
reconstruction and signal transduction activities of the Ras-MAPK
signaling pathway via the RAP1 signaling pathway, which
initiates the expression of MMP genes (119). MMPs are a family of calcium and
zinc-dependent proteases that degrade a variety of components of
the ECM (120). Collagen type IV is
the main scaffold in the BM of the ECM and also the main substrate
of MMP9 (121). MMP9
can decompose the nestin in the BM to destroy the cells integrity
and promote the invasion and metastasis of cancer cells (122). MMP9 expression in
HPV-positive patients with CC is higher than in HPV-negative
patients (123). Cardeal et
al (124) reported that
MMP9 is upregulated in human keratinocytes expressing the
HPV16 E7 protein. This may be due to TIMP2, an inhibitor of
MMP9, which could be downregulated by HPV16 E7. It was also
demonstrated that HPV can directly regulate the activity of
MMP9 in lung cancer cells (125). There may be an association between
HPV infection and the MMP family, which may be beneficial in
the diagnosis of cervical precancerous lesions and CC as
MMP9 may be considered as a novel biomarker. However, the
specific molecular mechanisms require further investigation. As FN1
and ITGB1 were targets of miR-9-3p (126) and FN1 promoted migration and
invasion by upregulating MMP9 in cancer (127), it is not surprising that these
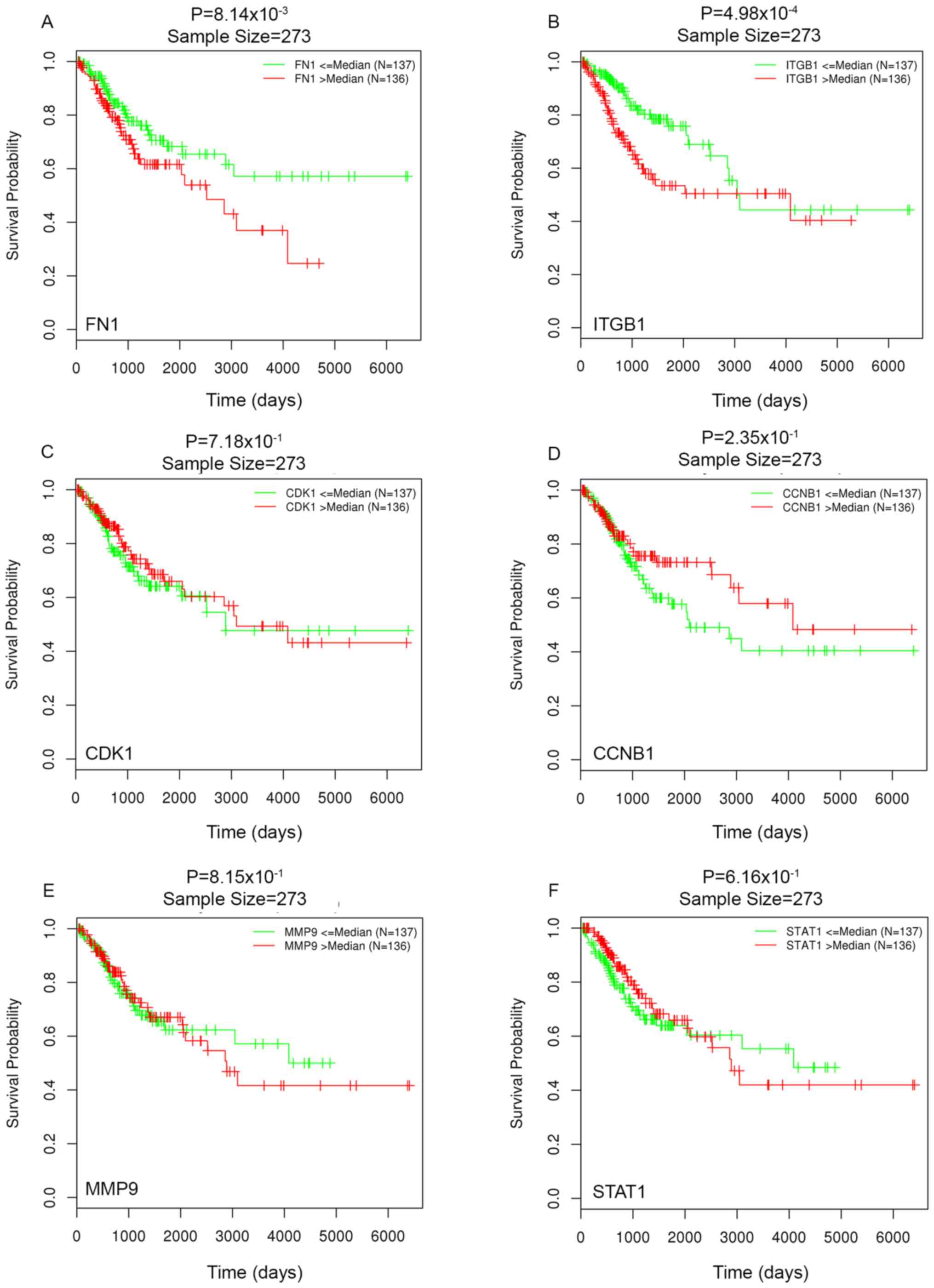
three genes are co-expressed as a reaction triplet. Furthermore,
since higher levels of FN1 and ITGB1 are
significantly associated with lower OS rate, these two genes may be
developed as novel prognostic factors for CC.
Not applicable.
No funding was received.
All data generated and/or analyzed during this
study are included in this published article. The datasets
generated and/or analyzed during the current study are available in
the GEO (https://www.ncbi.nlm.nih.gov/geo/) and LinkOmics
(http://www.linkedomics.org/)
repository.
YY, YF and WZ designed the present study and
drafted the initial manuscript. KW and YL performed the literature
review, acquired the data and performed the statistical analyses.
All authors have read and approved the manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Muliira RS, Salas AS and O'Brien B:
Quality of life among female cancer survivors in Africa: An
integrative literature review. Asia Pac J Oncol Nurs. 4:6–17. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gravitt PE and Winer RL: Natural history
of HPV infection across the lifespan: Role of viral latency.
Viruses. 9:E2672017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Walboomers JM, Jacobs MV, Manos MM, Bosch
FX, Kummer JA, Shah KV, Snijders PJ, Peto J, Meijer CJ and Muñoz N:
Human papillomavirus is a necessary cause of invasive cervical
cancer worldwide. J Pathol. 189:12–19. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Louie KS, de Sanjose S, Diaz M,
Castellsagué X, Herrero R, Meijer CJ, Shah K, Franceschi S, Muñoz N
and Bosch FX; International Agency for Research on Cancer
Multicenter Cervical Cancer Study Group, : Early age at first
sexual intercourse and early pregnancy are risk factors for
cervical cancer in developing countries. Br J Cancer.
100:1191–1197. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu ZC, Liu WD, Liu YH, Ye XH and Chen SD:
Multiple sexual partners as a potential independent risk factor for
cervical cancer: A meta-analysis of epidemiological studies. Asian
Pac J Cancer Prev. 16:3893–3900. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Smith JS, Green J, Berrington de Gonzalez
A, Appleby P, Peto J, Plummer M, Franceschi S and Beral V: Cervical
cancer and use of hormonal contraceptives: A systematic review.
Lancet. 361:1159–1167. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ma YY, Wei SJ, Lin YC, Lung JC, Chang TC,
Whang-Peng J, Liu JM, Yang DM, Yang WK and Shen CY: PIK3CA as an
oncogene in cervical cancer. Oncogene. 19:2739–2744. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zheng L, Li T, Zhang Y, Guo Y, Yao J, Dou
L and Guo K: Oncogene ATAD2 promotes cell proliferation, invasion
and migration in cervical cancer. Oncol Rep. 33:2337–2344. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bai X, Wang W, Zhao P, Wen J, Guo X, Shen
T, Shen J and Yang X: LncRNA CRNDE acts as an oncogene in cervical
cancer through sponging miR-183 to regulate CCNB1 expression.
Carcinogenesis. Oct 12–2019.(Epub ahead of print).
|
|
11
|
Bremer GL, Tieboschb AT, van der Putten
HW, de Haan J and Arends JW: p53 tumor suppressor gene protein
expression in cervical cancer: Relationship to prognosis. Eur J
Obstet Gynecol Reprod Biol. 63:55–59. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cohen Y, Singer G, Lavie O, Dong SM,
Beller U and Sidransky D: The RASSF1A tumor suppressor gene is
commonly inactivated in adenocarcinoma of the uterine cervix. Clin
Cancer Res. 9:2981–2984. 2003.PubMed/NCBI
|
|
13
|
Hasina R, Pontier AL, Fekete MJ, Martin
LE, Qi XM, Brigaudeau C, Pramanik R, Cline EI, Coignet LJ and
Lingen MW: NOL7 is a nucleolar candidate tumor suppressor gene in
cervical cancer that modulates the angiogenic phenotype. Oncogene.
25:588–598. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Roura E, Castellsagué X, Pawlita M,
Travier N, Waterboer T, Margall N, Bosch FX, de Sanjosé S, Dillner
J, Gram IT, et al: Smoking as a major risk factor for cervical
cancer and pre-cancer: Results from the EPIC cohort. Int J cancer.
135:453–466. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Husain RS and Ramakrishnan V: Global
variation of human papillomavirus genotypes and selected genes
involved in cervical malignancies. Ann Glob Health. 81:675–683.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Soto D, Song C and McLaughlin-Drubin ME:
Epigenetic alterations in human papillomavirus-associated cancers.
Viruses. 9:E2482017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mincheva A, Gissmann L and zur Hausen H:
Chromosomal integration sites of human papillomavirus DNA in three
cervical cancer cell lines mapped by in situ hybridization. Med
Microbiol Immunol. 176:245–256. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Senapati R, Senapati NN and Dwibedi B:
Molecular mechanisms of HPV mediated neoplastic progression. Infect
Agent Cancer. 11:592016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Münger K, Baldwin A, Edwards KM, Hayakawa
H, Nguyen CL, Owens M, Grace M and Huh K: Mechanisms of human
papillomavirus-induced oncogenesis. J Virol. 78:11451–11460. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Martinez-Zapien D, Ruiz FX, Poirson J,
Mitschler A, Ramirez J, Forster A, Cousido-Siah A, Masson M, Vande
Pol S, Podjarny A, et al: Structure of the E6/E6AP/p53 complex
required for HPV-mediated degradation of p53. Nature. 529:541–545.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Scheffner M, Huibregtse JM, Vierstra RD
and Howley PM: The HPV-16 E6 and E6-AP complex functions as a
ubiquitin-protein ligase in the ubiquitination of p53. Cell.
75:495–505. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sherr CJ: The Pezcoller lecture: Cancer
cell cycles revisited. Cancer Res. 60:3689–3695. 2000.PubMed/NCBI
|
|
23
|
Martin LG, Demers GW and Galloway DA:
Disruption of the G1/S transition in human papillomavirus type 16
E7-expressing human cells is associated with altered regulation of
cyclin E. J Virol. 72:975–985. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Löffler H, Fechter A, Matuszewska M,
Saffrich R, Mistrik M, Marhold J, Hornung C, Westermann F, Bartek J
and Krämer A: Cep63 recruits Cdk1 to the centrosome: Implications
for regulation of mitotic entry, centrosome amplification, and
genome maintenance. Cancer Res. 71:2129–2139. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Skyldberg B, Fujioka K, Hellström AC,
Sylvén L, Moberger B and Auer G: Human papillomavirus infection,
centrosome aberration, and genetic stability in cervical lesions.
Mod Pathol. 14:279–284. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Duensing S and Münger K: The human
papillomavirus type 16 E6 and E7 oncoproteins independently induce
numerical and structural chromosome instability. Cancer Res.
62:7075–7082. 2002.PubMed/NCBI
|
|
27
|
Chaiwongkot A, Niruthisard S, Kitkumthorn
N and Bhattarakosol P: Quantitative methylation analysis of human
papillomavirus 16 L1 gene reveals potential biomarker for cervical
cancer progression. Diagn Microbiol Infect Dis. 89:265–270. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sharma A, De R, Javed S, Srinivasan R, Pal
A and Bhattacharyya S: Sonic hedgehog pathway activation regulates
cervical cancer stem cell characteristics during epithelial to
mesenchymal transition. J Cell Physiol. Feb 4–2019.(Epub ahead of
print). View Article : Google Scholar
|
|
29
|
Li YP, Zhang L, Zou YL and Yu Y:
Association between FGFR4 gene polymorphism and high-risk HPV
infection cervical cancer. Asian Pac J Trop Med. 10:680–684. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang J and Gao Y: CCAT-1 promotes
proliferation and inhibits apoptosis of cervical cancer cells via
the Wnt signaling pathway. Oncotarget. 8:68059–68070.
2017.PubMed/NCBI
|
|
31
|
Mora-García ML, Ávila-Ibarra LR,
García-Rocha R, Weiss-Steider B, Hernández-Montes J, Don-López CA,
Gutiérrez-Serrano V, Titla-Vilchis IJ, Fuentes-Castañeda MC,
Monroy-Mora A, et al: Cervical cancer cells suppress effector
functions of cytotoxic T cells through the adenosinergic pathway.
Cell Immunol. 320:46–55. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Song ZC, Ding L, Ren ZY, Sun XS, Yang Q,
Wang L, Feng MJ, Liu CL and Wang JT: Effects of Src on cervical
cancer cells proliferation and apoptosis through ERK signal
transduction pathway. Zhonghua Liu Xing Bing Xue Za Zhi.
38:1246–1251. 2017.(In Chinese; Abstract available in Chinese from
the publisher). PubMed/NCBI
|
|
33
|
Xu Z, Zhou Y, Shi F, Cao Y, Dinh TLA, Wan
J and Zhao M: Investigation of differentially-expressed microRNAs
and genes in cervical cancer using an integrated bioinformatics
analysis. Oncol Lett. 13:2784–2790. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen SZ, Ma WL and Zheng WL: Screening for
cervical cancer-related genes and their bioinformatics analysis.
Nan Fang Yi Ke Da Xue Xue Bao. 28:585–588. 2008.(In Chinese).
PubMed/NCBI
|
|
35
|
Nayar R and Wilbur DC: The bethesda system
for reporting cervical cytology: A historical perspective. Acta
Cytol. 61:359–372. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shoji T, Takatori E, Takeuchi S, Yoshizaki
A, Uesugi N, Sugai T and Sugiyama T: Clinical significance of
atypical glandular cells in the bethesda system 2001: A comparison
with the histopathological diagnosis of surgically resected
specimens. Cancer Invest. 32:105–109. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
O'Connor M, Gallagher P, Waller J, Martin
CM, O'Leary JJ and Sharp L; Irish Cervical Screening Research
Consortium (CERVIVA), : Adverse psychological outcomes following
colposcopy and related procedures: A systematic review. BJOG.
123:24–38. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Haque N, Uddin AFMK, Dey BR, Islam F and
Goodman A: Challenges to cervical cancer treatment in Bangladesh:
The development of a women's cancer ward at Dhaka Medical College
Hospital. Gynecol Oncol Rep. 21:67–72. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Roque DR, Wysham WZ and Soper JT: The
surgical management of cervical cancer: An overview and literature
review. Obstet Gynecol Surv. 69:426–441. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Marin JJ, Romero MR, Blazquez AG, Herraez
E, Keck E and Briz O: Importance and limitations of chemotherapy
among the available treatments for gastrointestinal tumours.
Anticancer Agents Med Chem. 9:162–184. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chen HHW and Kuo MT: Improving
radiotherapy in cancer treatment: Promises and challenges.
Oncotarget. 8:62742–62758. 2017.PubMed/NCBI
|
|
42
|
Lecavalier-Barsoum M, Chaudary N, Han K,
Pintilie M, Hill RP and Milosevic M: Targeting CXCL12/CXCR4 and
myeloid cells to improve the therapeutic ratio in patient-derived
cervical cancer models treated with radio-chemotherapy. Br J
Cancer. 121:249–256. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhang F, Ren CC, Liu L, Chen YN, Yang L,
Zhang XA, Wang XM and Yu FJ: SHH gene silencing suppresses
epithelial-mesenchymal transition, proliferation, invasion, and
migration of cervical cancer cells by repressing the hedgehog
signaling pathway. J Cell Biochem. 119:3829–3842. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Gu J, Zhang X, Yang Z and Wang N:
Expression of cyclin D1 protein isoforms and its prognostic
significance in cervical cancer. Cancer Manag Res. 11:9073–9083.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chay DB, Han GH, Nam S, Cho H, Chung JY
and Hewitt SM: Forkhead box protein O1 (FOXO1) and paired box gene
3 (PAX3) overexpression is associated with poor prognosis in
patients with cervical cancer. Int J Clin Oncol. 24:1429–1439.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Tang H, Wang Y, Zhang B, Xiong S, Liu L,
Chen W, Tan G and Li H: High brain acid soluble protein 1(BASP1) is
a poor prognostic factor for cervical cancer and promotes tumor
growth. Cancer Cell Int. 17:972017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yang X, Cheng Y and Li C: The role of TLRs
in cervical cancer with HPV infection: A review. Signal Transduct
Target Ther. 2:170552017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Frumovitz M and Sood AK: Vascular
endothelial growth factor (VEGF) pathway as a therapeutic target in
gynecologic malignancies. Gynecol Oncol. 104:768–778. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
den Boon JA, Pyeon D, Wang SS, Horswill M,
Schiffman M, Sherman M, Zuna RE, Wang Z, Hewitt SM, Pearson R, et
al: Molecular transitions from papillomavirus infection to cervical
precancer and cancer: Role of stromal estrogen receptor signaling.
Proc Natl Acad Sci USA. 112:E3255–E3264. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Barrett T, Wilhite SE, Ledoux P,
Evangelista C, Kim IF, Tomashevsky M, Marshall KA, Phillippy KH,
Sherman PM, Holko M, et al: NCBI GEO: Archive for functional
genomics data sets-update. Nucleic Acids Res. 41:D991–D995. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ritchie ME, Phipson B, Wu D, Hu Y, Law CW,
Shi W and Smyth GK: Limma powers differential expression analyses
for RNA-sequencing and microarray studies. Nucleic Acids Res.
43:e472015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Szklarczyk D, Franceschini A, Wyder S,
Forslund K, Heller D, Huerta-Cepas J, Simonovic M, Roth A, Santos
A, Tsafou KP, et al: STRING v10: Protein-protein interaction
networks, integrated over the tree of life. Nucleic Acids Res.
43:D447–D452. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Shannon P, Markiel A, Ozier O, Baliga NS,
Wang JT, Ramage D, Amin N, Schwikowski B and Ideker T: Cytoscape: A
software environment for integrated models of biomolecular
interaction networks. Genome Res. 13:2498–2504. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Chin CH, Chen SH, Wu HH, Ho CW, Ko MT and
Lin CY: cytoHubba: Identifying hub objects and sub-networks from
complex interactome. BMC Syst Biol. 8 (Suppl 4):S112014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Wang J, Vasaikar S, Shi Z, Greer M and
Zhang B: WebGestalt 2017: A more comprehensive, powerful, flexible
and interactive gene set enrichment analysis toolkit. Nucleic Acids
Res. 45:W130–W137. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Chen J, Bardes EE, Aronow BJ and Jegga AG:
ToppGene Suite for gene list enrichment analysis and candidate gene
prioritization. Nucleic Acids Res. 37:W305–W311. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Hu Y, Pan Z, Hu Y, Zhang L and Wang J:
Network and pathway-based analyses of genes associated with
Parkinson's disease. Mol Neurobiol. 54:4452–4465. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Scardoni G, Petterlini M and Laudanna C:
Analyzing biological network parameters with CentiScaPe.
Bioinformatics. 25:2857–2859. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Han JD, Berlin N, Hao T, Goldberg DS,
Berriz GF, Zhang LV, Dupuy D, Walhout AJ, Cusick ME, Roth FP and
Vidal M: Evidence for dynamically organized modularity in the yeast
protein-protein interaction network. Nature. 430:88–93. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Kanwal A and Fazal S: Construction and
analysis of protein-protein interaction network correlated with
ankylosing spondylitis. Gene. 638:41–51. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Wu J, Vallenius T, Ovaska K, Westermarck
J, Mäkelä TP and Hautaniemi S: Integrated network analysis platform
for protein-protein interactions. Nat Methods. 6:75–77. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Kerrien S, Alam-Faruque Y, Aranda B,
Bancarz I, Bridge A, Derow C, Dimmer E, Feuermann M, Friedrichsen
A, Huntley R, et al: IntAct-source resource for molecular
interaction data. Nucleic Acids Res. 35:D561–D565. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Chatr-aryamontri A, Ceol A, Palazzi LM,
Nardelli G, Schneider MV, Castagnoli L and Cesareni G: MINT: The
Molecular INTeraction database. Nucleic Acids Res. 35:D572–D574.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Breitkreutz BJ, Stark C, Reguly T, Boucher
L, Breitkreutz A, Livstone M, Oughtred R, Lackner DH, Bähler J,
Wood V, et al: The BioGRID interaction database: 2008 update.
Nucleic Acids Res. 36:D637–D640. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Salwinski L, Miller CS, Smith AJ, Pettit
FK, Bowie JU and Eisenberg D: The database of interacting proteins:
2004 update. Nucleic Acids Res. 32:D449–D451. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Peri S, Navarro JD, Amanchy R, Kristiansen
TZ, Jonnalagadda CK, Surendranath V, Niranjan V, Muthusamy B,
Gandhi TK, Gronborg M, et al: Development of human protein
reference database as an initial platform for approaching systems
biology in humans. Genome Res. 13:2363–2371. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Güldener U, Münsterkötter M, Oesterheld M,
Pagel P, Ruepp A, Mewes HW and Stümpflen V: MPact: The MIPS protein
interaction resource on yeast. Nucleic Acids Res. 34:D436–D441.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Vasaikar SV, Straub P, Wang J and Zhang B:
LinkedOmics: Analyzing multi-omics data within and across 32 cancer
types. Nucleic Acids Res. 46:D956–D963. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Cancer Genome Atlas Research Network, ;
Weinstein JN, Collisson EA, Mills GB, Shaw KR, Ozenberger BA,
Ellrott K, Shmulevich I, Sander C and Stuart JM: The cancer genome
atlas pan-cancer analysis project. Nat Genet. 45:1113–1120. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Gasca S, Pellestor F, Assou S, Loup V,
Anahory T, Dechaud H, De Vos J and Hamamah S: Identifying new human
oocyte marker genes: A microarray approach. Reprod Biomed Online.
14:175–183. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Schwab MS, Roberts BT, Gross SD, Tunquist
BJ, Taieb FE, Lewellyn AL and Maller JL: Bub1 is activated by the
protein kinase p90(Rsk) during Xenopus oocyte maturation. Curr
Biol. 11:141–150. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Ouandaogo ZG, Frydman N, Hesters L, Assou
S, Haouzi D, Dechaud H, Frydman R and Hamamah S: Differences in
transcriptomic profiles of human cumulus cells isolated from
oocytes at GV, MI and MII stages after in vivo and in vitro oocyte
maturation. Hum Reprod. 27:2438–2447. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Li J, Qian WP and Sun QY: Cyclins
regulating oocyte meiotic cell cycle progression†. Biol Reprod.
101:878–881. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Kaplan MH: STAT signaling in inflammation.
JAKSTAT. 2:e241982013.PubMed/NCBI
|
|
75
|
Qiu X, Guo H, Yang J, Ji Y, Wu CS and Chen
X: Down-regulation of guanylate binding protein 1 causes
mitochondrial dysfunction and cellular senescence in macrophages.
Sci Rep. 8:16792018. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Bros M, Haas K, Moll L and Grabbe S: RhoA
as a key regulator of innate and adaptive immunity. Cells.
8:E7332019. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Erdely A, Antonini JM, Salmen-Muniz R,
Liston A, Hulderman T, Simeonova PP, Kashon ML, Li S, Gu JK, Stone
S, et al: Type I interferon and pattern recognition receptor
signaling following particulate matter inhalation. Part Fibre
Toxicol. 9:252012. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Luu K, Greenhill CJ, Majoros A, Decker T,
Jenkins BJ and Mansell A: STAT1 plays a role in TLR signal
transduction and inflammatory responses. Immunol Cell Biol.
92:761–769. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Smolen KK, Ruck CE, Fortuno ES III, Ho K,
Dimitriu P, Mohn WW, Speert DP, Cooper PJ, Esser M, Goetghebuer T,
et al: Pattern recognition receptor-mediated cytokine response in
infants across 4 continents. J Allergy Clin Immunol.
133:818–826.e4. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Xiang C, Chen J and Fu P: HGF/Met
signaling in cancer invasion: The impact on cytoskeleton
remodeling. Cancers (Basel). 9:E442017. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Lei T and Ling X: IGF-1 promotes the
growth and metastasis of hepatocellular carcinoma via the
inhibition of proteasome-mediated cathepsin B degradation. World J
Gastroenterol. 21:10137–10149. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Cho S, Kitadai Y, Yoshida S, Tanaka S,
Yoshihara M, Yoshida K and Chayama K: Deletion of the KIT gene is
associated with liver metastasis and poor prognosis in patients
with gastrointestinal stromal tumor in the stomach. Int J Oncol.
28:1361–1367. 2006.PubMed/NCBI
|
|
83
|
Li B, Shen W, Peng H, Li Y, Chen F, Zheng
L, Xu J and Jia L: Fibronectin 1 promotes melanoma proliferation
and metastasis by inhibiting apoptosis and regulating EMT. Onco
Targets Ther. 12:3207–3221. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Liu Y, Ren CC, Yang L, Xu YM and Chen YN:
Role of CXCL12-CXCR4 axis in ovarian cancer metastasis and
CXCL12-CXCR4 blockade with AMD3100 suppresses tumor cell migration
and invasion in vitro. J Cell Physiol. 234:3897–3909. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Chen Y, Jiang J, Zhao M, Luo X, Liang Z,
Zhen Y, Fu Q, Deng X, Lin X, Li L, et al: microRNA-374a suppresses
colon cancer progression by directly reducing CCND1 to inactivate
the PI3K/AKT pathway. Oncotarget. 7:41306–41319. 2016.PubMed/NCBI
|
|
86
|
Thomas SJ, Snowden JA, Zeidler MP and
Danson SJ: The role of JAK/STAT signalling in the pathogenesis,
prognosis and treatment of solid tumours. Br J Cancer. 113:365–371.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Stacker SA and Achen MG: The VEGF
signaling pathway in cancer: The road ahead. Chin J Cancer.
32:297–302. 2013.PubMed/NCBI
|
|
88
|
Shi X, Wang J, Lei Y, Cong C, Tan D and
Zhou X: Research progress on the PI3K/AKT signaling pathway in
gynecological cancer (Review). Mol Med Rep. 19:4529–4535.
2019.PubMed/NCBI
|
|
89
|
Li X, Jiang S and Tapping RI: Toll-like
receptor signaling in cell proliferation and survival. Cytokine.
49:1–9. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Gene Ontology Consortium: Gene ontology
consortium: Going forward. Nucleic Acids Res. 43:D1049–D1056. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Kawai T and Akira S: Toll-like receptor
and RIG-1-like receptor signaling. Ann N Y Acad Sci. 1143:1–20.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Gkretsi V and Stylianopoulos T: Cell
adhesion and matrix stiffness: Coordinating cancer cell invasion
and metastasis. Front Oncol. 8:1452018. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Zhang YL, Wang RC, Cheng K, Ring BZ and Su
L: Roles of Rap1 signaling in tumor cell migration and invasion.
Cancer Biol Med. 14:90–99. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Campbell PM and Der CJ: Oncogenic Ras and
its role in tumor cell invasion and metastasis. Semin Cancer Biol.
14:105–114. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Sun F, Wang J, Sun Q, Li F, Gao H, Xu L,
Zhang J, Sun X, Tian Y, Zhao Q, et al: Interleukin-8 promotes
integrin β3 upregulation and cell invasion through PI3K/Akt pathway
in hepatocellular carcinoma. J Exp Clin Cancer Res. 38:4492019.
View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Sanderson RD: Heparan sulfate
proteoglycans in invasion and metastasis. Semin Cell Dev Biol.
12:89–98. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Loo YM and Gale M Jr: Immune signaling by
RIG-I-like receptors. Immunity. 34:680–692. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Nyati KK and Prasad KN: Role of cytokines
and Toll-like receptors in the immunopathogenesis of Guillain-Barré
syndrome. Mediators Inflamm. 2014:7586392014. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Saxena M and Yeretssian G: NOD-Like
receptors: Master regulators of inflammation and cancer. Front
Immunol. 5:3272014. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Crosbie EJ, Einstein MH, Franceschi S and
Kitchener HC: Human papillomavirus and cervical cancer. Lancet.
382:889–899. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Balasubramaniam SD, Balakrishnan V, Oon CE
and Kaur G: Key molecular events in cervical cancer development.
Medicina (Kaunas). 55:E3842019. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Lin M, Ye M, Zhou J, Wang ZP and Zhu X:
Recent advances on the molecular mechanism of cervical
carcinogenesis based on systems biology technologies. Comput Struct
Biotechnol J. 17:241–250. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Ye J, Yin L, Xie P, Wu J, Huang J, Zhou G,
Xu H, Lu E and He X: Antiproliferative effects and molecular
mechanisms of troglitazone in human cervical cancer in vitro. Onco
Targets Ther. 8:1211–1218. 2015.PubMed/NCBI
|
|
104
|
Yugawa T and Kiyono T: Molecular
mechanisms of cervical carcinogenesis by high-risk human
papillomaviruses: Novel functions of E6 and E7 oncoproteins. Rev
Med Virol. 19:97–113. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Jones MC, Askari JA, Humphries JD and
Humphries MJ: Cell adhesion is regulated by CDK1 during the cell
cycle. J Cell Biol. 217:3203–3218. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Hochegger H, Dejsuphong D, Sonoda E,
Saberi A, Rajendra E, Kirk J, Hunt T and Takeda S: An essential
role for Cdk1 in S phase control is revealed via chemical genetics
in vertebrate cells. J Cell Biol. 178:257–268. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Vassilev LT: Cell cycle synchronization at
the G2/M phase border by reversible inhibition of CDK1. Cell Cycle.
5:2555–2556. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Shaikh F, Sanehi P and Rawal R: Molecular
screening of compounds to the predicted protein-protein interaction
site of Rb1-E7 with p53-E6 in HPV. Bioinformation. 8:607–612. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Yun J, Chae HD, Choy HE, Chung J, Yoo HS,
Han MH and Shin DY: p53 negatively regulates cdc2 transcription via
the CCAAT-binding NF-Y transcription factor. J Biol Chem.
274:29677–29682. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Lindqvist A, van Zon W, Karlsson Rosenthal
C and Wolthuis RM: Cyclin B1-Cdk1 activation continues after
centrosome separation to control mitotic progression. PLoS Biol.
5:e1232007. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Crasta K, Huang P, Morgan G, Winey M and
Surana U: Cdk1 regulates centrosome separation by restraining
proteolysis of microtubule-associated proteins. EMBO J.
25:2551–2563. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Fang Y, Yu H, Liang X, Xu J and Cai X:
Chk1-induced CCNB1 overexpression promotes cell proliferation and
tumor growth in human colorectal cancer. Cancer Biol Ther.
15:1268–1279. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Krek W and Nigg EA: Differential
phosphorylation of vertebrate p34cdc2 kinase at the G1/S and G2/M
transitions of the cell cycle: Identification of major
phosphorylation sites. EMBO J. 10:305–316. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Morgan DO: Principles of CDK regulation.
Nature. 374:131–134. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Banerjee NS, Wang HK, Broker TR and Chow
LT: Human papillomavirus (HPV) E7 induces prolonged G2 following S
phase reentry in differentiated human keratinocytes. J Biol Chem.
286:15473–15482. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Morgan EL, Wasson CW, Hanson L, Kealy D,
Pentland I, McGuire V, Scarpini C, Coleman N, Arthur JSC, Parish
JL, et al: STAT3 activation by E6 is essential for the
differentiation-dependent HPV18 life cycle. PLoS Pathog.
14:e10069752018. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Stewart DA, Cooper CR and Sikes RA:
Changes in extracellular matrix (ECM) and ECM-associated proteins
in the metastatic progression of prostate cancer. Reprod Biol
Endocrinol. 2:22004. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Chiu CF, Ho MY, Peng JM, Hung SW, Lee WH,
Liang CM and Liang SM: Raf activation by Ras and promotion of
cellular metastasis require phosphorylation of prohibitin in the
raft domain of the plasma membrane. Oncogene. 32:777–787. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Matter ML and Ruoslahti E: A signaling
pathway from the alpha5beta1 and alpha(v)beta3 integrins that
elevates bcl-2 transcription. J Biol Chem. 276:27757–27763. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Egeblad M and Werb Z: New functions for
the matrix metalloproteinases in cancer progression. Nat Rev
Cancer. 2:161–174. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
121
|
Sand JM, Larsen L, Hogaboam C, Martinez F,
Han M, Røssel Larsen M, Nawrocki A, Zheng Q, Karsdal MA and Leeming
DJ: MMP mediated degradation of type IV collagen alpha 1 and alpha
3 chains reflects basement membrane remodeling in experimental and
clinical fibrosis-validation of two novel biomarker assays. PLoS
One. 8:e849342013. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Bokhari AA, Baker TM, Dorjbal B, Waheed S,
Zahn CM, Hamilton CA, Maxwell GL and Syed V: Nestin suppression
attenuates invasive potential of endometrial cancer cells by
downregulating TGF-β signaling pathway. Oncotarget. 7:69733–69748.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
da Silva Cardeal LB, Brohem CA, Corrêa TC,
Winnischofer SM, Nakano F, Boccardo E, Villa LL, Sogayar MC and
Maria-Engler SS: Higher expression and activity of
metalloproteinases in human cervical carcinoma cell lines is
associated with HPV presence. Biochem Cell Biol. 84:713–719. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Cardeal LB, Boccardo E, Termini L,
Rabachini T, Andreoli MA, di Loreto C, Longatto Filho A, Villa LL
and Maria-Engler SS: HPV16 oncoproteins induce MMPs/RECK-TIMP-2
imbalance in primary keratinocytes: Possible implications in
cervical carcinogenesis. PLoS One. 7:e335852012. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Shiau MY, Fan LC, Yang SC, Tsao CH, Lee H,
Cheng YW, Lai LC and Chang YH: Human papillomavirus up-regulates
MMP-2 and MMP-9 expression and activity by inducing interleukin-8
in lung adenocarcinomas. PLoS One. 8:e544232013. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Ding Y, Pan Y, Liu S, Jiang F and Jiao J:
Elevation of MiR-9-3p suppresses the epithelial-mesenchymal
transition of nasopharyngeal carcinoma cells via down-regulating
FN1, ITGB1 and ITGAV. Cancer Biol Ther. 18:414–424. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Wang J, Deng L, Huang J, Cai R, Zhu X, Liu
F, Wang Q, Zhang J and Zheng Y: High expression of Fibronectin 1
suppresses apoptosis through the NF-κB pathway and is associated
with migration in nasopharyngeal carcinoma. Am J Transl Res.
9:4502–4511. 2017.PubMed/NCBI
|
|
128
|
Rajkumar T, Sabitha K, Vijayalakshmi N,
Shirley S, Bose MV, Gopal G and Selvaluxmy G: Identification and
validation of genes involved in cervical tumourigenesis. BMC
Cancer. 11:802011. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Koromilas AE and Sexl V: The tumor
suppressor function of STAT1 in breast cancer. JAKSTAT.
2:e233532013.PubMed/NCBI
|
|
130
|
Zhang Y and Liu Z: STAT1 in cancer: Friend
or foe? Discov Med. 24:19–29. 2017.PubMed/NCBI
|
|
131
|
Akram M, Kim KA, Kim ES, Shin YJ, Noh D,
Kim E, Kim JH, Majid A, Chang SY, Kim JK and Bae ON: Selective
inhibition of JAK2/STAT1 signaling and iNOS expression mediates the
anti-inflammatory effects of coniferyl aldehyde. Chem Biol
Interact. 256:102–110. 2016. View Article : Google Scholar : PubMed/NCBI
|