Introduction
A neuroendocrine neoplasm (NEN) is a type of
heterogeneous tumor, which was first reported and identified as
carcinoid by Oberndorfer in 1907. A NEN originates and spreads from
neuroendocrine cells and peptidergic neural crest Kulchitsky cells
(silver-addicted cells) (1). These
cells perform essential biochemical functions such as the uptake of
amine precursors, decarboxylation, and the release of bioactive
peptide hormones and nerve mediators (2). As the occurrence of gallbladder NEN
(GB-NEN) is low, it is difficult to obtain clear and large amounts
of data on the etiology, pathogenesis, standard treatment plan and
prognosis from large-scale studies conducted at multi-center
research centers. As there is no standard procedure to identify and
treat GB-NENs, the present study reviews the current clinical
characteristics, diagnosis and treatment regimens available for
patients with GB-NEN in order to better our understanding of the
condition.
Epidemiology
The incidence of NEN is rare in clinical practice,
it has been observed in only 115/100,000 cases worldwide in the
last decade, which accounts for 1.25% of all malignancies (3). The average age of onset is 60 years and
the majority of NENs are found in the gastrointestinal tract (66%),
followed by the lungs (31%) (4). A
hierarchical pattern of prevalence of NENs is found within the
gastrointestinal tract, with increasing numbers found from the
appendix, to the small intestine, to the rectum and finally to the
colon. Although the occurrence of NENs is rare in the stomach and
other parts of the gastrointestinal system, the ovaries, pancreas
and testicles can be affected; however, occurrence in the liver and
gallbladder is rare (5). Among all
gastrointestinal carcinoids, extrahepatic biliary carcinoids
account for 0.5–2.0% of cases and liver metastases remain common
(6,7). Surveillance Epidemiology and End Result
(SEER) research data indicate that GB-NENs account for 0.5% of all
NETs and 2.1% of all gallbladder tumors (8). There are only a few reports available
in the literature about GB-NENs and even fewer on cholecystic
neuroendocrine carcinoma. From previous studies, there were three
groups of large data reported, including two groups from Korea with
6 and 12 cases (9), respectively.
The third group was from China with 10 cases (10). In addition to the above 10 cases,
most of the literature in available in China about GB-NENs are
individual case reports.
Causes
To identify the root cause of the GB-NEN, a thorough
examination and understanding of the cellular nature of the disease
is important. In the process of examination, minimal neuroendocrine
cells can be found on the gallbladder mucosa, if any exist in this
space (11–13). Further GB-NEN etiological identifiers
may be as follows: i) The undifferentiated gallbladder stem cells
separate into neuroendocrine cells. ii) The presence of gallbladder
stones results in chronic inflammation of the gallbladder mucosa
causing intestinal epithelium or gastric metaplasia, which are
considered to be pathological metaplasia. In an advanced stage,
this inflammation produces neuroendocrine cells at the lesion site
eventually leading to the development of a GB-NEN (12,14,15).
iii) In certain situations, the gallbladder adenocarcinoma function
switches to a neuroendocrine one, resulting in chronic inflammation
of gallbladder tissues and formation of stones, further
exacerbating the other risk factors for gallbladder
adenocarcinoma.
Molecular mechanisms
Despite inadequate understanding of the etiology and
pathogenesis of GB-NENs, significant progress has been made in
elucidating the molecular and biological pathways involved. These
results encourage the creation of follow-up studies for the
identification of novel diagnostic and treatment procedures for the
disease. It has been confirmed in two previous studies (15,16) that
activation of the epidermal growth factor receptor (EGFR) can
upregulate downstream effector protein kinase B and extracellular
signal regulate kinase expression (Fig.
1). Concurrently, the target protein of rapamycin in human
cells regulates the growth, proliferation and motor activity of the
cells. The expression levels of this protein and the proliferation
index of the cells were determined to be positively correlated
(13). The high expression of the
aforementioned three molecules (the target protein of rapamycin,
protein kinase B and extracellular signal regulate kinase) resulted
in a poor prognosis in patients with GB-NENs (13). Scoazec described that angiogenesis
serves an important role in NEN etiology and pathogenesis (17). It is also possible to treat NEN and
prolong the survival of patients with the disease by blocking the
high expression of vascular endothelial growth factor (VEGF) and
its receptor. However, no specific mechanism backed by concrete
evidence has been discovered to date for GB-NENs.
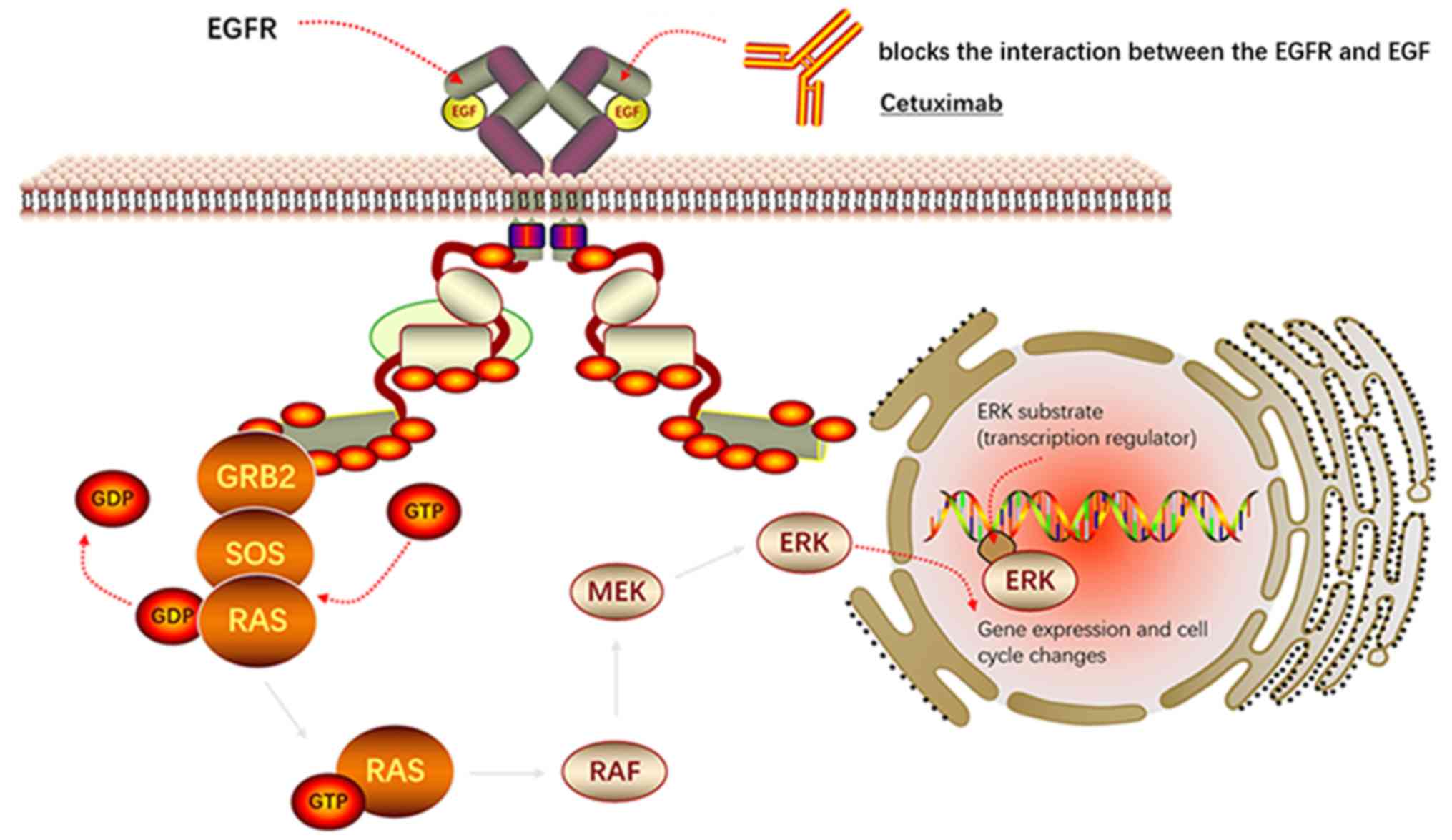 | Figure 1.Inhibitor (Cetuximab) of EGFR
signaling in GB-NEN. Cetuximab blocks the interaction between the
EGFR and EGF, thereby inhibiting the activation of downstream
RAS-ERK and thereby blocking the occurrence of GB-NEN. EGF is a
polypeptide molecule and EGFR is a typical receptor protein PTK.
The signal transduction process of EGFR is: i) EGFR forms a dimer
to change the conformation, PTK activity is enhanced, through
self-phosphorylation of the receptor intracellular tyrosine
residues phosphorylation. ii) EGFR phosphorylated by tyrosine binds
to GRB2. iii) GRB2 activates RAS by collecting SOS, a positive
regulator of RAS, promoting the release of GDP and binding to GTP.
iv) Activated RAS (RAS-GTP) acts on RAF to activate it. Activated
RAF acts on MEK, which acts on ERK to activate it. v) The activated
ERK is translocated to the nucleus, which affects the expression of
target genes and promotes the generation of GB-NEN. EGF, epidermal
growth factor; EGFR, epidermal growth factor receptor; GRB2, growth
factor receptor-bound protein 2; SOS, son of sevenless; GDP,
guanine diphosphate; GTP, guanine triphosphate; MEK,
mitogen-activated protein kinase; GB-NEN, gallbladder
neuroendocrine neoplasm. |
Pathological classification
There are three pathological classifications of
GB-NEN: Carcinoid or typical carcinoid (low malignancy), atypical
carcinoid (moderate malignancy) and small cell carcinoma (high
malignancy). These classifications are based on GB-NEN
histopathological structure, cell morphology and degree of
differentiation, mitotic combination, and necrotic and biological
behavior (18). Morphologically, the
majority of neuroendocrine tumor (NET) cells are small, cone-shaped
and polygonal, with no clear cell boundaries (18). GB-NEN cells have small nucleoli,
granular chromatin, and relatively consistent tumor cell
morphology, and are rich in interstitial blood vessels (19). According to the classification
criteria of digestive system tumors by the World Health
Organization in 2010, the well-differentiated NETs include grades
G1 and G2, and the poorly differentiated NET (G3) is defined as a
neuroendocrine carcinoma (NEC). The NEC is further classified based
on the tumor cell size into large or small. G1 contains both the
NEC and adenocarcinoma tumors and is therefore identified as a
mixed gland neuroendocrine carcinoma (18).
The extrahepatic bile ducts and the ampullary region
are more commonly observed in a GB-NEC of high malignancy, poor
prognosis and little to no pathological differentiation (20). The occurrence of early metastasis is
mainly identified through local infiltration and lymph node
metastasis (21). The lymph nodes,
liver, lungs and peritoneum are the most common metastasis sites of
small cell GB-NEC (22). The
American Cancer Institute Research reported 41 cases of gallbladder
NEC of varying pathological grades between 1973 and 2005. The data
revealed that high, medium and low/no differentiation pathological
grade accounted for 2.4, 7.3 and 89.7% of the cases, respectively
(8).
The most common histological finding reported in
gallbladder malignancy is adenocarcinoma (80–90%) followed by
undifferentiated carcinoma (10%) and then squamous cell
carcinoma/squamous adenocarcinoma (5%). Overall, the occurrence of
NEC is rare.
Clinical manifestations
According to case reports, the occurrence of GB-NEN
is higher among women (~68%) of cases identified (22,23). The
majority of these patients do not have any manifestations of
carcinoid syndrome (24). The
majority of NECs in the gallbladder and extrahepatic bile duct are
non-functional APUD (amine precursor uptake and decarboxylation)
tumors, with low or no functional endocrine granules in tumor cells
(25). Early clinical manifestations
of NEC are similar to those of other types of gallbladder cancer
and include abdominal distension pain, nausea and other
non-specific symptoms (26). In
order to distinguish from GB-NEC, symptoms specific to carcinoid
syndrome include spasmodic abdominal pain, flushes, edema,
wheezing, diarrhea and right heart valve disease, yet these account
for only 1.0% of those symptoms reported in patients with NEC
(26).
Diagnosis
The diagnosis of GB-NEN prior to surgery remains
difficult. Currently, tumor markers and imaging examinations, such
as ultrasound, computed tomography (CT) and magnetic resonance
imaging (MRI), are used. Limitations of the current techniques
hinder the proper diagnosis of patients with GB-NENs. For instance,
tumor markers that include carbohydrate antigen (CA)19-9,
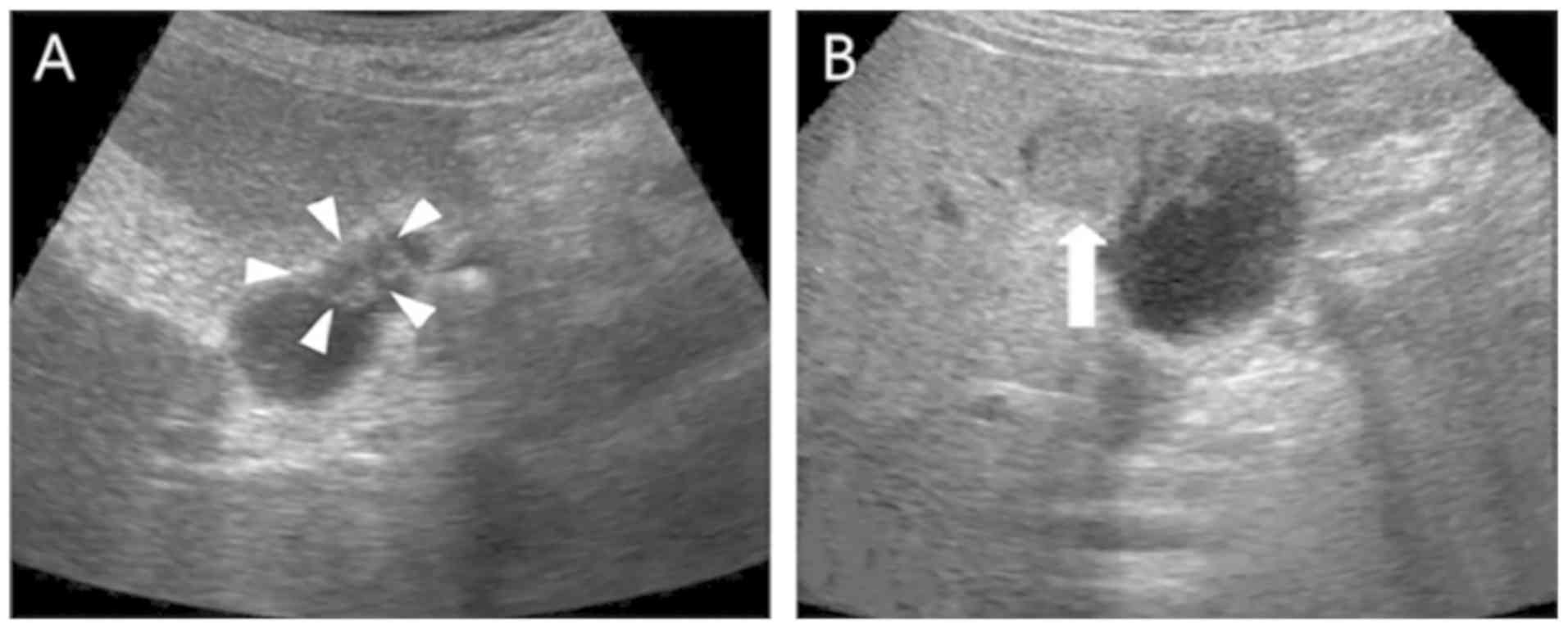
carcinoembryonic antigen and CA125 are often negative (27). Ultrasound examination detects only
the thickening of gallbladder walls, gallbladder swelling-type
lesions and a low echoic nodule (Fig.
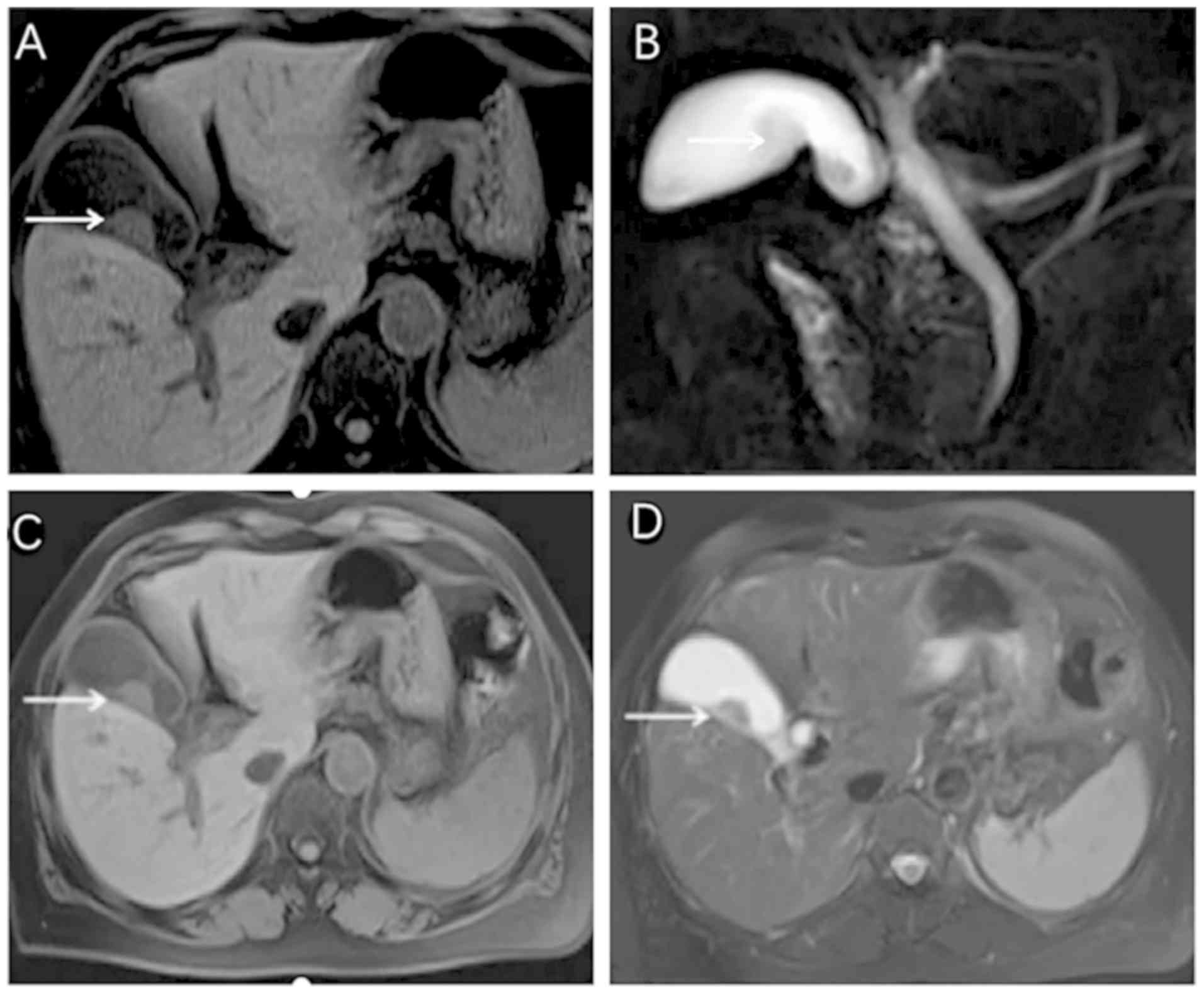
2). CT and MRI examination generate impressions indicating that
GB-NEN typically presents a thickening of the cystic wall on one
side, with the mass protruding into or out of the cavity (Fig. 3). These scans also indicate a visible
necrotic shadow in larger lesions (28). In studies examining the results of CT
and MRI scans in GB-NEN, it was noted that after the enhancement of
GB-NEN, the lesion continued to strengthen and the enhancement was
slightly more obvious compared with that of adenocarcinoma
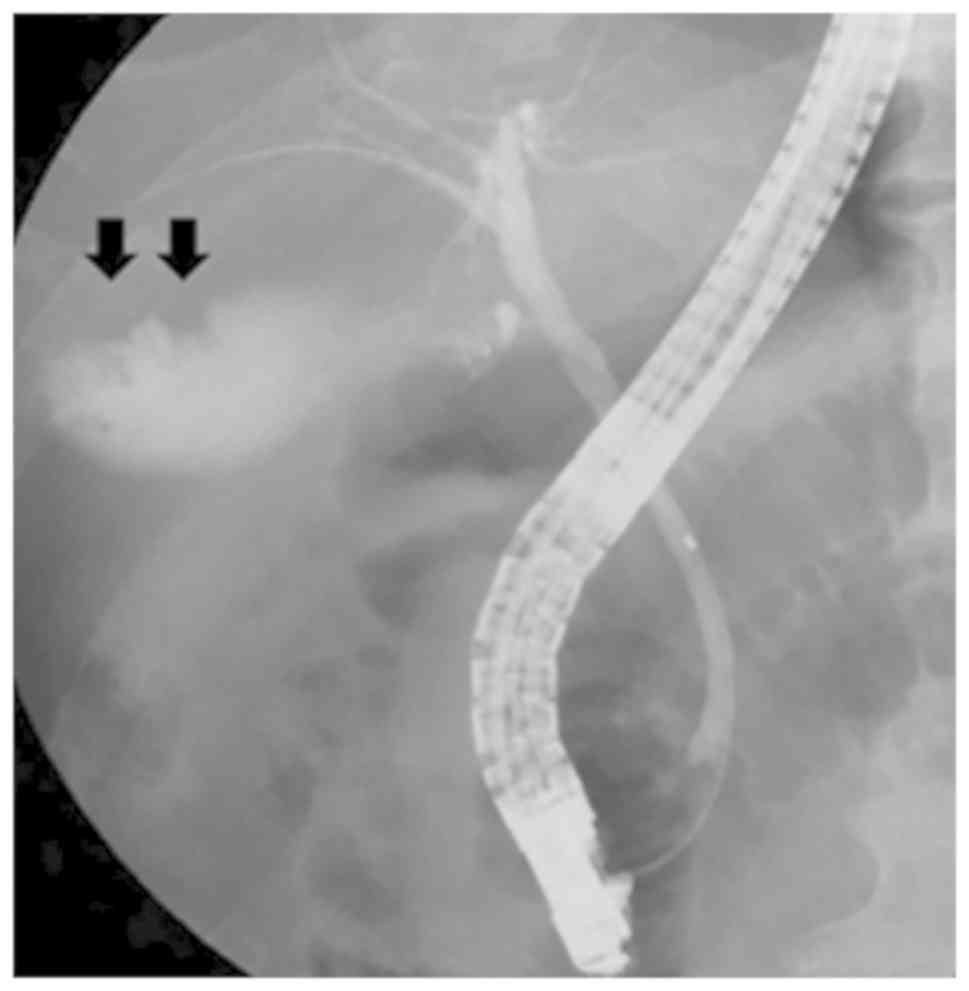
(29). Endoscopic retrograde
cholangiography revealed the defect of the tumor in the gallbladder
(Fig. 4). However, the presence of
lymph node metastasis around the gallbladder and retroperitoneum is
difficult to distinguish from the other gallbladder tumors
(30). Imaging examinations provide
significant results only when used at an early stage and then
provide aid in establishing a treatment plan (31).
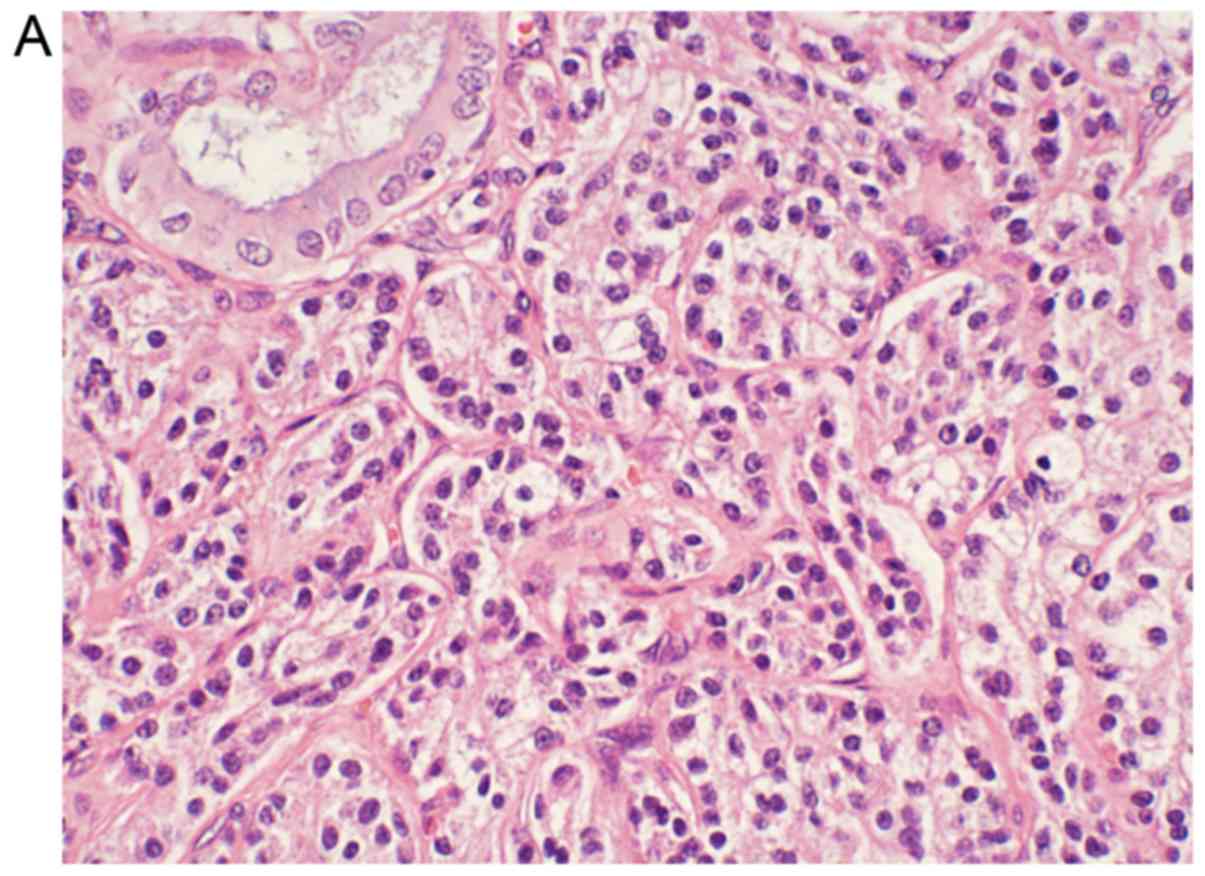
Diagnostic comparisons indicate that
immunohistochemical staining is the most effective tool for the
diagnosis of GB-NENs. Immunohistochemistry is divided into two
parts (32). First, neuroendocrine
cell markers such as neuron-specific enolase (NSE), synaptophysin
(Syn), pheochromin-A (CHG-A), protein gene product and Rankine are
positively detected (Fig. 5).
Second, amines and amine hormones such as adrenocorticotropic
hormone, growth hormone, human chorionic gonadotropin,
5-hydroxytryptamine, vasoactive polypeptide, insulin, gastrin,
somatostatin, pancreatic polypeptide and calcitonin can
simultaneously trigger expression of other hormones (33). Among all of the previously mentioned
hormones CHG-A, Syn and NSE have the highest specificity (34). A study has demonstrated that CHG-A is
a substance released by secretory particles in neuroendocrine cells
to confer their secretory characteristics (35). Evidence demonstrated that the serum
CHG-A level in 60–80% of patients with NEC of the digestive system
was higher compared with that of normal patients (36). Therefore, the serum CHG-A test has
the highest significance in the diagnosis of GB-NEN. Monier et
al (37) further suggested that
the urine detection of 5-hydroxyindole-acetic acid (5-HIAA) may aid
in the diagnosis of GB-NEN. However, in this study, the positive
rate reads were low due to the insufficiency or non-secretion of
5-HIAA in some patients with GB-NEN.
Differential diagnosis
Examination of patients presenting with GB-NEN
symptoms and markers can identify differential disorders such as
cholestasis, gallbladder polyps, gallbladder adenomyosis and
gallbladder adenoma. Apart from the pathological
immunohistochemical examinations, contrast-enhanced ultrasonography
has differential clinical significance (38). Case reports using contrast-enhanced
ultrasonography have revealed that biliary sludge is not enhanced
(39). In these case reports, the
gallbladder polyp was enhanced with grape-like fine pedicles and
the three-layer structure of gallbladder wall was clear (40). For adenomyosis of the gallbladder,
unenhanced vesiculoid echocardiography was observed and the inner
and serous membranes of the gallbladder were intact (41). Gallbladder adenoma resulted in
delayed enhancement that was identifiable by ‘fast in and slow out’
through the complete three-layer structure of the gallbladder wall
(38). In conclusion,
contrast-enhanced ultrasonography can improve the appearance of
gallbladder carcinoma and facilitate an early differential
diagnosis.
Treatment options
Surgical treatment
Advantages and disadvantages of the various
treatment options discussed are listed in Table I: Due to its complexity, gallbladder
cancer must be treated surgically by experienced biliary tract
physicians and pathologists (42).
GB-NEC is characterized by high malignancy, early lymphatic
metastasis (the N2 lymph node metastasis rate is significantly
higher compared with that of patients with adenocarcinoma in the
same period) and a poor prognosis when compared with all other
types of gallbladder cancer (20).
Radical resection is considered to be the most effective and
preferred method of surgical treatment for patients with GB-NEC
(43). The purpose of a radical
resection is to eliminate lesions, confirm a clear diagnosis,
provide a basis for post-operative comprehensive treatment and
improve the quality of life of affected patients (33). Surgical methods include simple,
radical and expanded radical cholecystectomy, whereby the choice of
surgical type is often discussed between the medical professional
and patient (44). The progress made
in recent years to expand the time period during which radical
resection can be performed, including R0 resection for GB-NEC, has
increased the overall long-term survival time of patients (31). With these promising results, expanded
radical prostatectomy should also be attempted (45). Only under situations where the tumor
invades the mucosa, submucosa or muscularis for GB-NET is simple
cholecystectomy feasible (46). In
the case of late-stage occurrence without distant metastasis,
cholecystectomy combined with local liver resection and lymph node
clearance is an option for obtaining a good surgical margin
(47). The aforementioned study
strongly recommends performing radical resection to the maximum
possible extent even when liver metastases are limited. If radical
resection is not feasible, then volume reduction surgery must be
considered as an effective follow-up treatment to improve the
quality of life of patients with GB-NENs (48).
 | Table I.Comparison of advantages and
disadvantages of different gallbladder neuroendocrine neoplasm
treatment options. |
Table I.
Comparison of advantages and
disadvantages of different gallbladder neuroendocrine neoplasm
treatment options.
| Treatment
options | Advantages | Disadvantages |
|---|
| Surgical
treatment | 1. Is the most
effective treatment | 1. Needs
experienced biliary physicians and pathologists |
|
| 2. Eliminates
lesions completely | 2. Cholecystectomy
alone is only feasible if the tumor invades the mucosa, submucosa
or muscularis |
|
| 3. Makes the
diagnosis clear |
|
|
| 4. Provides the
basis for postoperative comprehensive treatment |
|
|
| 5. Improves the
quality of life and the overall long-term survival rate |
|
| Chemotherapy | 1. An important
alternative therapy (for patients who are not suitable for
surgery) | 1. Has limited
effect for tumors with high differentiation and slow growth |
|
| 2. Prolongs the
survival of patients significantly | 2. No unified
standard chemotherapy treatment available currently |
| Molecular targeted
therapy | Prolongs
progression-free survival and overall survival times | No molecular
targeted drug therapy has been widely recognized and used |
| Treatment with
somatostatin analogs | 1. Inhibits tumor
progression | Only effective with
positive expression of the somatostatin receptor |
|
| 2. Improves
symptoms and the overall prognosis of patients |
|
| Radiotherapy and
interventional therapy | 1. Inhibits tumor
growth | The clinical
efficacy and safety have not been determined yet and need further
study |
|
| 2. Interventional
embolization of the hepatic artery is an effective method to treat
hepatic metastasis |
|
Chemotherapy
Patients with GB-NENs who are medically unfit for
surgery must be given chemotherapy, a significant alternate
treatment method (49). GB-NEC is
highly invasive and develops early lymph node metastasis, hence
surgery followed by radiotherapy and chemotherapy is recommended to
help prolong the survival period of such patients (50). In the case of NEC with high
differentiation and slow growth, the effect of chemotherapy has
been observed to be limited (51).
For rapidly growing tumors, chemotherapy response rates range from
20–60% worldwide (52). Based on the
differing degrees of tumor differentiation, the most commonly used
chemotherapy drugs include streptozotocin, 5-fluorouracil,
adriamycin, cisplatin and etoposide (11). Due to the clinical incidence of
GB-NEN being low, there are few associated studies available, which
results in the lack of a unified standard chemotherapy program.
Available studies have demonstrated that oxaliplatin plus
gemcitabine is the most effective chemotherapy regimen for
gallbladder cancer at present; however, cholecystic NEC has a poor
response to this treatment. Therefore, chemotherapy drugs advocated
for gastrointestinal and cholecystic NEC include etoposidem,
cisplatin and Adriamycin (11).
Associated case reports demonstrated that the post-operative use of
gemcitabine, docetaxel, or cisplatin combined with cisplatin,
sunitinib and docetaxel, respectively, resulted in a longer
survival time in patients with GB-NENs (53,54).
Inoue et al (55) reported
that following combination treatment with cisplatin and irinotecan,
the tumor size in a patient with GB-NEC was significantly reduced
and the tumor-free survival period was significantly prolonged
compared with cisplatin alone. In addition, in another study, it
was reported that the response rate to etoposide and cisplatin
increased up to 50–56% in poorly differentiated and rapidly growing
NEC (56). In the cases
investigated, a medium dose of 3–6 million units of α-interferon
was used 3–7 times a week as adjuvant therapy for NEC (56).
Molecular targeted therapy
To date, there is no molecular targeted drug therapy
of note for the treatment of patients with GB-NENs. However, there
are limited drug therapies available under review. A study has
reported progression of the disease coupled with an increased level
of VEGF in the blood of patients with GB-NEN (57). This indicates that VEGF-mediated
neovascularization plays an important role in the occurrence,
progression, metastasis and recurrence of GB-NEN. Raymond et
al (58) and Yao et al
(59) confirmed that the targeted
drug sunitinib extends the progression-free survival (P<0.001)
and the overall survival (P=0.02) rate of pancreatic NEN patients
by resisting the binding of the vascular endothelial growth factor
and platelet-derived growth factor receptors to the corresponding
ligand. To develop this as an effective treatment option, the
mechanism by which sunitinib acts in patients with GB-NEN requires
detailed investigation.
Treatment with somatostatin
analogs
Caplin et al (60) conducted a randomized control trial
where the progression-free survival rate of patients with NEN
treated with a somatostatin analog (octreotide) was significantly
higher compared with that of the placebo group (P<0.05). Other
studies by Igaz (61) and Oberg
et al (62) confirmed the
significance of somatostatin analogs (octreotide) in inhibiting
tumor progression, and improving symptoms and overall patient
prognosis. Meanwhile, Oberg et al (62) also described that with the emergence
of long-acting drugs, patients are able treat themselves using
monthly injections. This treatment method is effective for patients
with positive expression of somatostatin receptor GB-NEN.
Radiotherapy and interventional
therapy
The molecular mechanisms that underpin the clinical
effect of radioactive isotopes of peptide receptors and the
somatostatin analogs are similar (63). Local radiotherapy inhibits tumor
growth through use of the radioactive isotopes Y90 or Lu177, with
good clinical tolerance in patients with GB-NEN (64). In addition, adjuvant treatment of
GB-NEN also includes interventional therapy, namely radiofrequency
ablation, seed implantation, arterial embolization and laser
hyperthermia. Among them, interventional embolization of the
hepatic artery is found to be effective for hepatic metastasis
(65). Embolization agents such as
anhydrous ethanol or chemotherapy drugs have also been used for
patients with GB-NEN; however, the clinical effects and safety of
the aforementioned treatment methods require further investigation
in detailed studies (66). In
clinical practice, it is suggested that targeted treatment is
performed in accordance with relevant guidelines and by keeping the
specific condition of the patient in mind (67).
Prevention
NEC is a slow-growing malignant tumor without any
specific clinical manifestations (68). Preoperative diagnosis often depends
on the typical oncoid syndrome. Since GB-NEN and oncoid syndrome
are not associated, patients typically visit the doctor only during
middle- or late-stage presentation with metastasis (44). Therefore, to increase the diagnosis
rate and prevent progression of GB-NEN, an appropriate treatment is
advised following complete routine examination for all patients
with chronic cholecystitis and cholecystolithiasis. The
presentation of GB-NEN is further complicated by the presence of
gallbladder stones (29).
Stimulation of the gallbladder wall by stones (69) and the occurrence of GB-NEN are
related. The complexity of the disease is further increased as
GB-NEN is also associated with small intestinal carcinoid. Thus,
regular examination is strongly recommended for the correct
diagnosis (5). Both gastrointestinal
and pulmonary carcinoid cancers result in calcification via
dystrophic calcification and endocrine hormone stimulation
(70). However, to the best of our
knowledge, there are no national or international cases of patients
with GB-NEN in which calcification has been reported, providing
some level of distinction.
Prognosis
The prognosis of GB-NEN depends on the pathological
type under investigation. Atypical carcinoid, low-differentiated
adenocarcinoma and small-cell carcinoma. The prognosis, in general,
is acceptable for patients with GB-NET, especially G1 disease, as
there is a low degree of clinical malignancy with no obvious early
metastasis. However, due to the high malignancy and rapid progress
of GB-NEC, lymph node and liver metastasis occur simultaneously at
diagnosis and lead to a poor prognosis in practice (71). A study has indicated that a lower
Ki-67 index with smaller tumor volume leads to a better prognosis
for GB-NEN (72). Conversely, the
prognosis is worse when the tumor sizes vary and the symptoms are
more complex (73). A study by the
Memorial Sloan Kettering Cancer Center demonstrated that the median
survival time of 13 patients with GB-NEC was only slightly shorter
compared with that of 435 patients with gallbladder cancer (9.8
months vs. 10.3 months, respectively) (14). Chiorean et al (11) demonstrated that GB-NEC 1-, 2- and
3-year survival rates were lower compared with those of other types
of gallbladder cancer (20 vs. 38%; 10 vs. 31%; 0 vs. 30.1%,
respectively) during the same period.
Conclusion
The current review demonstrates that GB-NEN is a
relatively rare gallbladder lesion, unique in presentation and
often relayed as a case study. Therefore, early detection, correct
diagnosis and reasonable treatment of such tumors will help in
extending the quality of life of affected patients. At present, the
origin of GB-NEN is unclear, the clinical manifestations are
atypical and the majority of laboratory and imaging examinations
provide no specificity. The diagnosis of GB-NEN depends on
pathological and immunohistochemical examinations utilizing markers
such as Syn, NSE and CHG-A. In terms of treatment options, surgical
treatment is the best choice and active multi-mode comprehensive
treatment such as chemoradiotherapy, targeted therapy and
somatostatin analogs also significantly prolong survival times in
patients with GB-NEN. This review has certain limitations. Due to
the low incidence and availability of studies, there is no uniform
standard treatment identified for treating GB-NEN. Therefore, we
suggest that treatment is provided on a case by case basis to
comprehensively integrate the advantages of various treatment
methods for providing targeted treatment and to maximize the
benefits for patients with GB-NEN in clinical practice.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81802805) and The
Jilin Province Health Technology Innovation Project (grant no.
2017J047).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CN, YL and BJ conceived and designed the study. CN,
SW, QG and XR performed the literature searches and drafted the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
GB-NEN
|
gallbladder neuroendocrine
neoplasm
|
|
VEGF
|
vascular endothelial growth factor
|
|
NET
|
neuroendocrine tumor
|
|
NEC
|
neuroendocrine carcinoma
|
|
CT
|
computed tomography
|
|
MRI
|
magnetic resonance imaging
|
|
NSE
|
neuron-specific enolase
|
|
Syn
|
synaptophysin
|
|
CHG-A
|
pheochromin-A
|
|
5-HIAA
|
5-hydroxyindole-acetic acid
|
References
|
1
|
Modlin IM, Lye KD and Kidd M: A 5-decade
analysis of 13715 carcinoid tumors. Cancer. 97:934–959. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Naito S, Naito M, Yamamoto N, Kume T,
Hosino S, Kinjyo Y, Naito Y, Naito H and Hasegawa S: Polypoid
gallbladder neuroendocrine tumor diagnosed as benign polyp before
surgery: A case report. Mol Clin Oncol. 12:225–229. 2020.PubMed/NCBI
|
|
3
|
Dogra VS and Poblete J: Metaatatic
carcinoid tumor in the liver. J Clin Ultrasound. 21:639–641. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gustafsson BI, Kidd M and Modlin IM:
Neuroendocrine tumors of the diffuse neuroendocrine system. Curr
Opin Oncol. 20:1–12. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Eltawil KM, Gustafsson BI, Kidd M and
Modlin IM: Neuroendocrine tumors of the gallbladder: An evaluation
and reassessment of management strategy. J Clin Gastroenterol.
44:687–695. 2010.PubMed/NCBI
|
|
6
|
Rothenstein J, Cleary SP, Pond GR, Dale D,
Gallinger S, Moore MJ, Brierley J and Siu LL: Neuroendocrine tumors
of the gastrointestinal tract: A decade of experience at the
princess margaret hospital. Am J Clin Oncol. 31:64–70. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Oberg K: Diagnostic work-up of
gastroenteropancreatic neuroendocrine tumors. Clinics (Sao Paulo).
67 (Suppl 1):S109–S112. 2012. View Article : Google Scholar
|
|
8
|
Yao JC, Hassan M, Phan A, Dagohoy C, Leary
C, Mares JE, Abdalla EK, Fleming JB, Vauthey JN, Rashid A and Evans
DB: One hundred years after ‘carcinoid’: Epidemiology of and
prognostic factors for neuroendocrine tumors in 35,825 cases in the
United States. J Clin Oncol. 26:3063–3072. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim J, Lee WJ, Lee SH, Lee KB, Ryu JK, Kim
YT, Kim SW, Yoon YB, Hwang JH, Han HS, et al: Clinical features of
20 patients with curatively resected biliary neuroendocrine
tumours. Dig Liver Dis. 43:965–970. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee JM, Hwang S, Lee SG, Lee YJ, Park KM,
Kim KH, Ahn CS, Kim MH, Lee SK and Kim MW: Neuroendocrine tumors of
the gallbladder: Twelve cases in a single institution.
Hepatogastroenterology. 57:1064–1068. 2010.PubMed/NCBI
|
|
11
|
Chiorean L, Bartos A, Pelau D, Iancu D,
Ciuleanu T, Buiga R, Oancea I, Mangrau A, Iancu C and Badea R:
Neuroendocrine tumor of gallbladder with liver and retroperitoneal
metastases and a good response to the chemotherapeutical treatment.
J Med Ultrason (2001). 42:271–276. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Adachi T, Haraguchi M, Irie J, Yoshimoto
T, Uehara R, Ito S, Tokai H, Noda K, Tada N, Hirabaru M, et al:
Gallbladder small cell carcinoma: A case report and literature
review. Surg Case Rep. 2:712016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yun SP, Shin N and Seo HI: Clinical
outcomes of small cell neuroendocrine carcinoma and adenocarcinoma
of the gallbladder. World J Gastroenterol. 21:269–275. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Duffy A, Capanu M, Abou-Alfa GK, Huitzil
D, Jarnagin W, Fong Y, D'Angelica M, Dematteo RP, Blumgart LH and
O'Reilly EM: Gallbladder cancer (GBC): 10-year experience at
memorial sloan-kettering cancer center (MSKCC). J Surg Oncol.
98:485–489. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Komori Y, Yada K, Ohta M, Uchida H,
Iwashita Y, Fukuzawa K, Kashima K, Yokoyama S, Inomata M and Kitano
S: Mammalian target of rapamycin signaling activation patterns in
pancreatic neuroendocrine tumors. J Hepatobiliary Pancreat Sci.
21:288–295. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Missiaglia E, Dalai I, Barbi S, Beghelli
S, Falconi M, della Peruta M, Piemonti L, Capurso G, Di Florio A,
delle Fave G, et al: Pancreatic endocrine tumors: Expression
profiling evidences a role for AKT-mTOR pathway. J Clin Oncol.
28:245–255. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Scoazec JY: Angiogenesis in neuroendocrine
tumors: Therapeutic applications. Neuroendocrinology. 97:45–56.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Luttges J: What's new? The 2010 WHO
classification for tumours of the pancreas. Pathologe. 32 (Suppl
2):S332–S336. 2011.(In German).
|
|
19
|
Bosman FT, Camelr F, Hruban RH and Theise
ND: World Health Organization classification of tumours of the
digestive system [M]. IARC Press; Lyon: pp. 195–334. 2010
|
|
20
|
Ayabe RI, Wach M, Ruff S, Martin S, Diggs
L, Wiemken T, Hinyard L, Davis JL, Luu C and Hernandez JM: Primary
gallbladder neuroendocrine tumors: Insights into a rare histology
using a large national database. Ann Surg Oncol. 26:3577–3585.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bae JS, Kim SH, Yoo J, Kim H and Han JK:
Differential and prognostic MRI features of gallbladder
neuroendocrine tumors and adenocarcinomas. Eur Radiol. Feb
5–2020.(Epub ahead of print). doi: 10.1007/s00330-019-06588-9.
View Article : Google Scholar
|
|
22
|
Moskal TL, Zhang PJ and Nava HR: Small
cell carcinoma of the gallbladder. J Surg Oncol. 70:54–59. 1999.
View Article : Google Scholar
|
|
23
|
Fujimoto G, Yamada S, Kusanagi H and
Uegami W: Rapidly growing neuroendocrine carcinoma of the
gallbladder: A case report. Radiol Case Rep. 15:259–265. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chakrabarti D, Qayoom S, Ghosh A and Gupta
R: Neuroendocrine carcinoma of the gall bladder in a young lady
presenting with upper abdominal heaviness: A common complaint and a
rare diagnosis. BMJ Case Rep. 12:e2296842019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Skalický A, Vištejnová L, Dubová M, Malkus
T, Skalický T and Troup O: Mixed neuroendocrine-non-neuroendocrine
carcinoma of gallbladder: Case report. World J Surg Oncol.
17:552019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shimono C, Suwa K, Sato M, Shirai S,
Yamada K, Nakamura Y and Makuuchi M: Large cell neuroendocrine
carcinoma of the gallbladder: Long survival achieved by multimodal
treatment. Int J Clin Oncol. 14:351–355. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Neyaz A, Husain N, Gupta S, Kumari S,
Arora A, Awasthi NP, Malhotra KP and Misra S: Investigation of
targetable predictive and prognostic markers in gallbladder
carcinoma. J Gastrointest Oncol. 9:111–125. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Adachi T, Haraguchi M, Irie J, Yoshimoto
T, Uehara R, Ito S, Tokai H, Noda K, Tada N, Hirabaru M, et al:
Gallbladder small cell carcinoma: A case report and literature
review. Surg Case Rep. 2:712016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nishigami T, Yamada M, Nakasho K, Yamamura
M, Satomi M, Uematsu K, Ri G, Mizuta T and Fukumoto H: Cacinoid
tumor of the gall bladder. Intern Med. 35:953–956. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Handra-Luca A: Gallbladder neuroendocrine
carcinoma: Metastasis or synchronous tumor? Minerva Chir.
73:619–620. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu W, Chen W, He X, Qu Q, Hong T and Li
B: Cholecystectomy with gallbladder bed cautery might be sufficient
for T1bN0M0 neuroendocrine carcinoma of gallbladders: Cases report
and literature review. Medicine (Baltimore). 96:e87782017.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hussain I, Sarvepalli D, Zafar H, Jehanzeb
S and Ullah W: Neuroendocrine tumor: A rare, aggressive tumor of
the gallbladder. Cureus. 11:e55712019.PubMed/NCBI
|
|
33
|
Liu W, Chen W, Chen J, Hong T, Li B, Qu Q
and He X: Neuroendocrine carcinoma of gallbladder: A case series
and literature review. Eur J Med Res. 24:82019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Soga J: Carcinoids and their variant
endocrinomas. An analysis of 11,842 reported cases. J Exp Clin
Cancer Res. 22:517–530. 2003.PubMed/NCBI
|
|
35
|
Yadav R, Jain D, Mathur SR and Iyer VK:
Cytomorphology of neuroendocrine tumours of the gallbladder.
Cytopathology. 27:97–102. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Peracchi M, Gebbia C, Basilisco G,
Quatrini M, Tarantino C, Vescarelli C, Massironi S and Conte D:
Plasma chromogranin A in patients with autoimmune chronic atrophic
gastritis, enterochromaffin-like cell lesions and gastric
carcinoids. Eur J Endocrinol. 152:443–448. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Monier A, Saloum N, Szmigielski W,
Alrashid A and Napaki SM: Neuroendocrine tumor of the gallbladder.
Pol J Radiol. 80:228–231. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Leem G, Chung MJ, Park JY, Bang S, Song
SY, Chung JB and Park SW: Clinical value of contrast-enhanced
harmonic endoscopic ultrasonography in the differential diagnosis
of pancreatic and gallbladder masses. Clin Endosc. 51:80–88. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sugimoto M, Takagi T, Suzuki R, Konno N,
Asama H, Watanabe K, Nakamura J, Kikuchi H, Waragai Y, Takasumi M,
et al: Contrast-enhanced harmonic endoscopic ultrasonography in
gallbladder cancer and pancreatic cancer. Fukushima J Med Sci.
63:39–45. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Reimão S, Francioni E, Bories E, Caillol
F, Pesenti C and Giovannini M: Endoscopic ultrasonography-guided
bi-lateral biliary drainage: A case series study. Endosc
Ultrasound. 3 (Suppl 1):S182014.PubMed/NCBI
|
|
41
|
Samad A, Kaplan A, Arain M, Attam R,
Jessurun J, Manivel JC and Pambuccian SE: Endoscopic
ultrasound-guided fine-needle aspiration diagnosis of large cell
neuroendocrine carcinoma of the gallbladder and common bile duct:
Report of a case. Diagn Cytopathol. 41:1091–1095. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Abutaka A, El-Matbouly M, Helmy I,
Elmoghazy W, Sulieman I, Ben Gashir M, Soofi M, Khalaf H and
Elaffandi A: Repeat liver resection for pure large cell
neuroendocrine carcinoma of the gallbladder: A favorable outcome.
World J Surg Oncol. 17:1262019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Iype S, Mirza TA, Pmpper DJ, Bhattacharya
S, Feakins RM and Kocher HM: Neuroendocrine tumours of the
gaHbladder: Three cases and a review of the literature. Postgrad
Med J. 85:213–218. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hirose Y, Sakata J, Endo K, Takahashi M,
Saito R, Imano H, Kido T, Yoshino K, Sasaki T and Wakai T: A 0.8-cm
clear cell neuroendocrine tumor G1 of the gallbladder with lymph
node metastasis: A case report. World J Surg Oncol. 16:1502018.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
You YH, Choi DW, Heo JS, Han IW, Choi SH,
Jang KT and Han S: Can surgical treatment be justified for
neuroendocrine carcinoma of the gallbladder? Medicine (Baltimore).
98:e148862019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Sun YW and Liu DJ: Neuroendocrine
carcinoma of the gallbladder. Chin J Pract Surg. 31:265–267.
2011.(In Chinese).
|
|
47
|
Garg PK, Pandey D and Sharma J: The
surgical management of gallbladder cancer. Expert Rev Gastroenterol
Hepatol. 9:155–166. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Machairas N, Paspala A, Frountzas M,
Tsilimigras DI, Moris D, Ntomi V, Tsapralis D and Schizas D: Mixed
adenoneuroendocrine carcinoma (MANEC) of the gallbladder: A
systematic review of outcomes following surgical management. In
Vivo. 33:1721–1726. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Elm'hadi C, Zerrik M, Errihani H and Ichou
M: A long survival woman with primary small-cell carcinoma of the
gallbladder: Role of chemotherapy maintenance. Cureus.
9:e13682017.PubMed/NCBI
|
|
50
|
Shoushtari AN, Bluth MJ, Goldman DA, Bitas
C, Lefkowitz RA, Postow MA, Munhoz RR, Buchar G, Hester RH, Romero
JA, et al: Clinical features and response to systemic therapy in a
historical cohort of advanced or unresectable mucosal melanoma.
Melanoma Res. 27:57–64. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Tani S, Yamagishi S, Fukunaga K, Morita M,
Sonoda T, Murao S, Ikuta S, Kakuno A and Yamanaka N: A case of
disease-free, long survival in a patient with mixed
adenoneuroendocrine carcinoma of the gallbladder treated with
induction CDDP/CPT-11 chemotherapy and resection. Gan To Kagaku
Ryoho. 42:113–117. 2015.(In Japanese). PubMed/NCBI
|
|
52
|
Smith JL and Davis BR: Neuroendocrine
tumor of the gallbladder treated with neoadjuvant chemotherapy and
surgery. Am Surg. 80:318–320. 2014.PubMed/NCBI
|
|
53
|
Elahi F, Ahmadzadeh A, Yadonahzadeh M,
Hassanpour K and Babaei M: Neuroendocrine tumor of the gallbladder.
Arch Iran Med. 16:123–125. 2013.PubMed/NCBI
|
|
54
|
Okuyama Y, Fukui A, Enoki Y, Morishita H,
Yoshida N and Fujimoto S: A large cell neuroendocrine carcinoma of
the gall bladder: Diagnosis with 18FDGPET/CT-guided
biliary cytology and treatment with combined chemotherapy achieved
a long-term stable condition. Jpn J Clin Oncol. 43:571–574. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Inoue D, Ozaka M, Muramatsu Y, Yamada I,
Matsuyama M, Kanda H and Ishii H: Neuroendocrine gallbladder cancer
treated with cisplatin plus irinotecan-A case report. Gan To Kagaku
Ryoho. 41:765–767. 2014.(In Japanese). PubMed/NCBI
|
|
56
|
Barakat MT, Meeran K and Bloom SR:
Neuroendocrine tumours. Endocr Relat Cancer. 11:1–18. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Pavel ME, Hassler G, Baum U, Hahn EG,
Lohmann T and Schuppan D: Circulating levels of angiogenic
cytokines can predict tumour progression and prognosis in
neuroendocrine carcinomas. Clin Endocrinol (Oxf). 62:434–443. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Raymond E, Dahan L, Raoul JL, Bang YJ,
Borbath I, Lombard-Bohas C, Valle J, Metrakos P, Smith D, Vinik A,
et al: Sunitinib malate for the treatment of pancreatic
neuroendocrine tumors. N Engl J Med. 364:501–513. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Yao JC, Shah MH, Ito T, Bohas CL, Wolin
EM, Van Cutsem E, Hobday TJ, Okusaka T, Capdevila J, de Vries EG,
et al: Everolimus for advanced pancreatic neuroendocrine tumors. N
Engl J Med. 364:514–523. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Caplin ME, Pavel M, Cwikla JB, Phan AT,
Raderer M, Sedláčková E, Cadiot G, Wolin EM, Capdevila J, Wall L,
et al: Lanreotide in metastatic enteropancreatic neuroendocrine
tumors. N Engl J Med. 371:224–233. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Igaz P: Efficacy of somatostatin analogues
in the treatment of neuroendocrine tumours based on the results of
recent clinical trials. Orv Hetil. 155:1908–1912. 2014.(In
Hungarian). View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Oberg K, Akerstrom G, Rindi G and Jelic S;
ESMO Guidelines Working Group, : Neuroendocrine
gastroenteropancreatic tumours: ESMO clinical practice guidelines
for diagnosis, treatment and follow-up. Ann Oncol. 21 (Suppl
5):v223–v227. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Lin PC, Lai YC, Lai JI, Hsu SY and Wang
WS: Successful treatment of gallbladder neuroendocrine carcinoma
with combined chemo-radiotherapy: A case report and literature
review. Int J Clin Pharmacol Ther. 49:403–408. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Kwekkeboom DJ, de Herder WW, Kam BL, van
Eijck CH, van Essen M, Kooij PP, Feelders RA, van Aken MO and
Krenning EP: Treatment with the radiolabeled somatostatin analog
[177 Lu-DOTA 0, Tyr3] octreotate: Toxicity, efficacy, and survival.
J Clin Oncol. 26:2124–2130. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Roversi R, Ricci S, Rossi G, Roversi M and
Cavallo G: Locoregional therapy with hypoxic liver perfusion in
malignant neoplasms of the gallbladder. Preliminary experience.
Radiol Med. 94:220–225. 1997.(In Italian). PubMed/NCBI
|
|
66
|
Garin E, Rolland Y, Boucher E, Ardisson V,
Laffont S, Boudjema K, Bourguet P and Raoul JL: First experience of
hepatic radioembolization using microspheres labelled with
yttrium-90 (TheraSphere): Practical aspects concerning its
implementation. Eur J Nucl Med Mol Imaging. 37:453–461. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Kamei K, Shindoh J, Kiya Y, Matsumoto I,
Hashimoto M and Takeyama Y: Conversion surgery after extensive
chemotherapy for stage IV mixed adenoneuroendocrine carcinoma
(MANEC) of the gallbladder: Clinical implications from the patterns
of response and recurrence. Clin J Gastroenterol. Oct 15–2019.(Epub
ahead of print). doi: 10.1007/s12328-019-01053-y. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Salimi Z and Sharafuddin M: Ultrasound
appearance of primary carcinoid tumor of the gallbladder associated
with carcinoid syndrome. J Clin Ultrasound. 23:435–437. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Soga J: Primary endocrinomas (carcinoids
and variant neoplasms) of the gallbladder: A statistical evaluation
of 138 reported cases. J Exp Clin Cancer Res. 22:5–15.
2003.PubMed/NCBI
|
|
70
|
Pantongrag-Brown L, Buetow PC, Carr NJ,
Lichtenstein JE and Buck JL: Calcification and fibrosis in
mesenteric carcinoid tumor: CT findings and pathologic correlation.
AJR Am J Roentgenol. 164:387–391. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Shapera E and Bitting C: Survival: A rare
outcome in large cell neuroendocrine carcinoma of the gallbladder.
Acta Gastroenterol Belg. 82:433–436. 2019.PubMed/NCBI
|
|
72
|
Deshmukh SD, Gulati HK, Gaopande V,
Purandare S and Anand M: Incidental cystic endocrine tumor of the
pancreas: A case report with immunohistochemical study. J Cancer
Res Ther. 8:289–291. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Iwasa S, Morizane C, Okusaka T, Ueno H,
Ikeda M, Kondo S, Tanaka T, Nakachi K, Mitsunaga S, Kojima Y, et
al: Cisplatin and etoposide as first-line chemotherapy for poorly
differentiated neuroendocrine carcinoma of the hepatobiliary tract
and pancreas. Jpn J Clin Oncol. 40:313–318. 2010. View Article : Google Scholar : PubMed/NCBI
|